Abstract
Zoledronic acid (ZA), a third generation bisphosphonate, has been shown to reduce cell migration, invasion, and metastasis. However, the effects of ZA on the epithelial-mesenchymal transition (EMT), a cellular process essential to the metastatic cascade, remain unclear. Therefore, the effects of ZA on EMT, using triple negative breast cancer cells (TNBC) as a model system, were examined in greater detail. ZA treatment decreased expression of mesenchymal markers N-cadherin, Twist and Snail, and subsequently upregulated expression of E-cadherin. ZA also inhibited cell viability, induced cell cycle arrest and decreased the proliferative capacity of TNBC, suggesting that ZA inhibits viability through reduction of cell proliferation. As EMT has been linked to acquisition of a self-renewal phenotype, the effects of ZA on self-renewal in TNBC were also studied. Treatment with ZA decreased expression of self-renewal proteins BMI-1 and Oct-4, and both prevented and eliminated mammosphere formation. To understand the mechanism of these results, the effect of ZA on established EMT regulator NFκB was investigated. ZA inhibited phosphorylation of RelA, the active subunit of NFκB, at serine 536 and modulated RelA subcellular localization. Treatment with ZA reduced RelA binding to the Twist promoter, providing a direct link between inactivation of NFκB signaling and loss of EMT transcription factor gene expression. Binding of Twist to the BMI-1 promoter was also decreased, correlating modulation of EMT to decreased self-renewal. Based on these results, it is proposed that, through inactivation of NFκB, ZA reverses EMT, which leads to a decrease in self-renewal.
Keywords: zoledronic acid, epithelial mesenchymal transition, self-renewal, triple negativebreast cancer, nuclear factor kappa B
Introduction
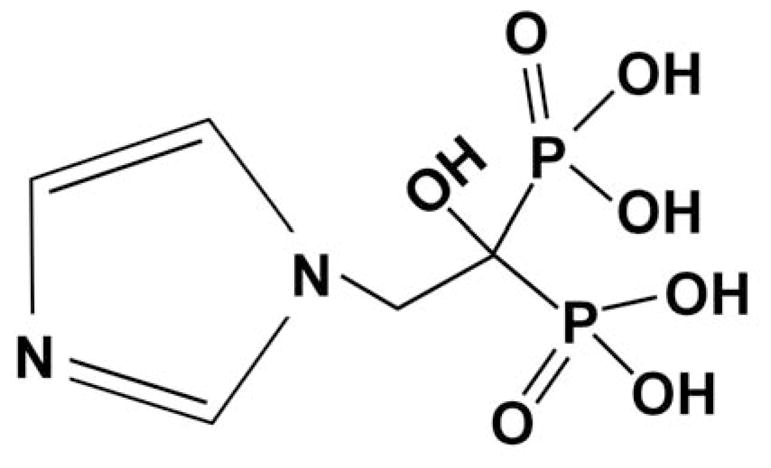
Zoledronic acid (ZA) is a third-generation bisphosphonate originally designed to treat osteoporosis (Figure 1). In addition to its effects on osteoclasteogenesis, clinical studies have shown that treatment with ZA increases disease-free survival (1–4) and inhibits metastatic disease (1) of postmenopausal women diagnosed with breast cancer (BC). Preclinically, treatment with ZA inhibits viability (5–10), migration, and invasion (11, 12) of BC cells. Mechanisms responsible for these effects have yet to be deduced. To date, studies have focused on the effects of ZA on cell motility. There are several steps in the metastatic cascade prior to cell migration and invasion, an example of which is passage through the epithelial-mesenchymal transition (EMT). Here, the effects of ZA on EMT are reported.
Figure 1. Structure of zoledronic acid.
EMT is a cellular process whereby epithelial cells undergo cellular changes, including loss of cell-cell contact and cell polarity, gain of an elongated cell structure, and the ability to move as a single cell, to become more mesenchymal (13, 14). Mesenchymal cells will gain expression of N-cadherin and lose expression of epithelial protein E-cadherin, which correlates with upregulation of EMT transcription factors, Twist and Snail. These act as negative regulators of E-cadherin gene transcription (15–17). Recent literature has also linked EMT to increased self-renewal of cells (18).
Triple negative BC cell lines (TNBC) were used to study the effects of ZA on EMT and self-renewal as they possess many characteristics of a cell which has undergone EMT. These include expression of N-cadherin, Twist, and Snail and the ability to form mammospheres, a measure of self-renewal capability.
The current study hypothesizes that treatment with ZA reverses EMT, which results in decreased mesenchymal characteristics and self-renewal capacity. To address this hypothesis, the effects of ZA on markers of EMT and self-renewal were studied. In addition, the effects of ZA on mechanisms which regulate EMT were examined.
Materials and Methods
Materials
Zoledronic acid was kindly provided by Novartis Pharma (Basel, Switzerland). Dulbecco’s Modified Eagle Medium (DMEM), trypsin/EDTA, penicillin/streptomycin (P/S), dPBS, cell-extraction buffer, and reverse transcriptase reagents (5x reverse transcriptase buffer, DTT, dNTPs, BSA, random primers, and MMLV reverse transcriptase), were purchased from Invitrogen (NY, USA). Fetal bovine serum (FBS) was purchased from Atlanta Biologicals (Lawrenceville, GA). Chromatin immunoprecipitation assay kit, salmon sperm DNA/protein A agarose slurry, and BMI-1 antibody were purchased from Millipore (Billerica, MA). Qiaquick PCR Purification kit, Qiashredder kit, RNeasy kit, RelA siRNA (S100301672), AllStars negative control siRNA, and HiPerfect transfection reagent were purchased from Qiagen (Valencia, CA). DyNAmo SYBR green qPCR mix was purchased from MJ Research (Boston, MA). BCA kit was purchased from Thermo Scientific (Rockford, IL). Caspase-3, N-cadherin, Snail, pRelA(S536), total RelA, GAPDH, and β-actin antibodies were purchased from Cell Signaling (Danvers, MA)PARP-1, Twist-1, RelA (ChIP grade) antibodies and normal rabbit IgG were purchased from Santa Cruz Technologies (Santa Cruz, CA). E-cadherin and Oct-4 antibodies were purchased from abcam (Cambridge, MA). Mammocult media and supplements were purchased from Stem Cell Technologies. Protease (Complete TM) and phosphatase (PhosSTOP) inhibitors were purchased from Roche (Mannheim, Germany). Polyvinylidene fluoride (PVDF) membrane was purchased from Fisher Scientific (Pittsburgh, PA). All other western blotting materials were purchased from BioRad (Hercules, CA). All other reagents were purchased from Sigma-Aldrich (St. Louis, MO).
Cell Culture, Treatment & Imaging
Cells used for experiments include MDA-MB-231 (gift from Dr. Qun Zhou, University of Maryland, Baltimore, Baltimore, MD), and Hs578t (obtained from ATCC). Cell lines were authenticated by Johns Hopkins University using short tandem repeat profiling in March 2012. Cells were routinely maintained in DMEM, supplemented with 5% FBS and 1% P/S, at 37°C in 5% CO2 and were passaged weekly. For cell treatment, each cell line was seeded and allowed to grow to 70% confluency. Cells were then treated for specified duration with either vehicle (0.9% saline) or treatment (ZA was prepared as 10−2 M stock solution at pH 7.4 and diluted). Cells and mammospheres were imaged using a Canon EOS Rebel T1i at 10x or 4x magnification.
Sample Collection
Cell media was aspirated and remaining attached cells were washed twice with dPBS to remove detached cells from the culture. For western blot and PCR analyses, cells were collected by scraping directly into lysis buffer. For the rest of the experiments attached cells were collected by trypsinization and viability was assayed by trypan blue exclusion. Dead cells were not removed from the sample, but cell numbers were corrected such that the same number of viable cells was used for each experiment.
Trypan Blue Exclusion
Cells were maintained in regular growth medium for the duration of the assay. Cells were seeded into 12-well plates at a density of 20,000 cells/well and allowed to attach overnight. Cells were then treated with individual treatments as specified. Following treatment, media was aspirated, cells were washed with dPBS, and trypsinized. Trypsin was quenched with serum-containing media. 10 μL of cell suspension was mixed with 10 μL trypan blue and total number of viable cells was determined using a BioRad TC10 Automated Cell Counter. Values are expressed as percent change compared to vehicle of total viable cells.
Preparation of Whole Cell Lysates & Cell Fractionation
Whole cell lysates were prepared as previously described (19). For cell fractionation, cells were scraped by rubber policeman in dPBS, washed twice, and centrifuged at 1000 rpm for 5 minutes at 4°C. Pellet was resuspended in 1x hypotonic buffer (20mM Tris-HCl, pH 7.4, 10mM NaCl, 3mM MgCl2) and incubated on ice for 10 minutes. To this was added 25 μL of 10% NP40. Mixture was vortexed for 10 seconds and centrifuged for 10 minutes at 3000 rpm at 4°C. Supernatant was collected as cytoplasmic fraction. The pellet was resuspended in 50 μL complete cell extraction buffer (cell extraction buffer with 1mM PMSF and protease inhibitors), and mixture was vortexed every 10 minutes for 30 minutes. Mixture was centrifuged for 30 minutes at 14,000xg at 4°C. Supernatant was collected as nuclear fraction. Protein concentrations were determined using BCA method.
Western Blotting
Western blotting was performed as previously described (19).
RNA Extraction and Reverse Transcription
RNA was extracted and reverse transcribed as previously described (19).
Polymerase Chain Reaction
mRNA and promoter expression were measured using qPCR as previously described (19). PCR reactions were amplified for 50 cycles with an annealing temperature of 60°C. The following primers were used for analysis: human TWIST-1 forward: 5′-TCAGCCACTGAAAGGAAAGG-3′ and reverse: 5′-CCCTCAGAGGAAGGATGAAA-3″; human Snail forward: 5′-TTCTTCTGCGCTACTGCTGCG-3′ and reverse: 5′-GGGCAGGTATGGAGAGGAAGA-3′ (20), human N-cadherin forward: 5′-AGGGGACCTTTTCCTCAAGA-3′ and reverse: 5′-CTACTGCATGTGCCCTCAAA-3′; human E-cadherin forward: 5′-CGGGAATGCAGTTGAGGATC-3′ and reverse: 5′-AGGATGGTGTAAGCGATGGC-3′ (21); human RelA (accession no. NM_021975) forward: 5′-TAGGCGAGTTATAGCCTCAG-3′ and reverse: 5′-CTGCAGTTTGATGATGAAGA-3″ (22); and human GAPDH forward 5′-AAGGTCGGAGTCAACGGATTTG-3′ and reverse: 5′-CCATGGGTGGAATCATATTGGAA-3′ (23). Values were normalized to GAPDH and are expressed as fold change relative to control treatment.
RNAi
Cells were seeded in 12-well plates at a density of 120,000 cells/1.1mL passage media and were allowed to briefly attach under culture conditions. RelA siRNA (20nM) was mixed with HiPerfect Transfection Reagent (6μL) for 10 minutes at room temperature. Cells were incubated for 72 hours under normal cell culture conditions. Following transfection, cell media was removed and transfected cells were used for future experiments.
Chromatin Immunoprecipitation
Chromatin immunoprecipitation analyses were performed using the Chromatin Immunoprecipitation (ChIP) assay kit per manufacturer’s instructions. Samples were incubated with either 5μg RelA or 5μg Twist antibody overnight at 4°C using end-over-end rotation. In addition, control samples were incubated with 5μg normal rabbit IgG as a negative control. Products resulting from the immunoprecipitation were extracted using the QiaQuick PCR Purification Kit per manufacturer’s instructions. Resulting DNA was used for qPCR. Primers include: human TWIST-1 promoter: forward: 5′-GGGAGGACGAATTGTTAGACC-3′ and reverse: 5′-GGAGGAGGGACTTTTCGAAGTT-3′ and human BMI-1 promoter: forward: 5′-GCAGCCCGCCGAGGCTCG-3′ and reverse: 5′-GGATGCGAGGGGCGGATCC-3′ (24). All primers were amplified for 50 cycles with a 60°C annealing temperature for qPCR. Samples were amplified using negative control primers upstream of the Twist binding site on the BMI-1 promoter (24): forward: 5′-GGTCAAGTACATGTGAC-3′ and reverse: 5′-TCTCCTCTAGCTTGCAG-3′. Values were normalized to each individual input control.
Cell Cycle Analysis
Cell media was aspirated and cells were washed twice in dPBS. Attached cells were collected by trypsinization and centrifuged for 3 minutes at 500xg. Cells were counted and resuspended in dPBS at a cell density of 1 million cells/100μL dPBS. Cells were incubated for 30 minutes in hypotonic cell lysis buffer (0.1% sodium citrate, 0.1% Triton X-100) in the presence of 50μg/mL propidium iodide and 25μg/mL ribonuclease A. Following incubation, the cells were analyzed using FlowJo software. The Watson algorithm was used to find the peak and S-phase populations from a univariate distribution curve.
Mammosphere Assay
Cell media was aspirated and cells were washed twice in dPBS. Attached cells were trypsinized and collected by centrifugation at 500xg for 5 minutes at 20°C. Cells were counted and resuspended in mammosphere media (per manufacturer’s instructions; 2.5μg/mL amphoterecin B, and 50μg/mL gentamycin were also added) at a cell density of 10,000 cells/mL. Two milliliters of cell solution was seeded in 6-well ultra-low attachment plates. Cells incubated for one week at 37°C in 5%CO2. Formed mammospheres were counted manually. For secondary mammospheres, mammospheres and media were collected and spun down at 350xg for 5 minutes at 4°C. Media was aspirated and 1mL trypsin-EDTA was added to each mammosphere pellet. The trypsin/pellet was pipetted up and down for approximately 1.5 minutes, and dPBS with 2% FBS was added. Mixture was centrifuged at 350xg for 5 minutes at 4°C. Media was aspirated and cells were resuspended in Mammocult media and counted. 5000 cells were seeded in ultra-low attachment plates and incubated for 1 week at 37°C in 5%CO2. For treated secondary mammospheres, mammospheres formed for 72 hours prior to treatment.
Statistical Analysis
All data are expressed as mean±SEM. p values were calculated using unpaired t test, ANOVA and Tukey’s post hoc analyses using GraphPad Prism 5. p<0.05 was considered significant.
Results
Zoledronic acid induces changes in TNBC cellular morphology
Following treatment with ZA, triple negative BC cell lines (TNBC) MDA-MB-231 and Hs578t underwent morphological changes which occurred in a time-dependent manner. Vehicle treated cells exhibited elongated cell structure, cellular protrusions, and lacked cell-cell contacts (Figure 2a, i & iii). By 48 hours of treatment, protrusions were no longer visible and increased cell-cell contact was observed (Figure 2a, ii & iv). These results suggest that treatment with ZA drives TNBC toward a more epithelial morphology.
Figure 2. Zoledronic acid inhibits mesenchymal phenotype and induces epithelial phenotype.
(a) Cellular morphology of Hs578t (top panels, i–ii) and MDA-MB-231 (bottom panels, iii–iv) following treatment with vehicle (i, iii) or 10μM ZA for 48 hours. Arrows indicate areas of cellular protrusion. Box indicates areas of cell-cell contact. (b) Mesenchymal and epithelial protein expression in MDA-MB-231 (left) or Hs578t (right) following treatment for 48 hours with vehicle, 1, or 10μM ZA. Protein levels were normalized to β-actin. (c–d) Mesenchymal marker mRNA expression in MDA-MB-231 (c) and Hs578t (d) following treatment for 48 hours with vehicle (V) or 10μM ZA (Z). Data are indicative of three independent experiments. p<0.05, ***p<0.001.
Zoledronic acid decreases mesenchymal and increases epithelial marker expression
Protein analyses of mesenchymal and epithelial markers were performed on TNBC following treatment with vehicle, 1μM, or 10μM ZA for 48 hours. Only attached cells were used for analysis. Briefly, following treatment with 10μM ZA, cell media was removed and remaining attached cells were washed with dPBS to remove any additional detached cells. The remaining attached cells were used for the experiment, and exhibited decreased expression of mesenchymal proteins N-cadherin (p<0.05 vs. vehicle in MDA-MB-231; p<0.01 vs. vehicle in Hs578t), Snail (p<0.01 vs. vehicle in MDA-MB-231; p<0.001 vs. vehicle in Hs578t), and Twist (Figure 2, b–c) following both 48 and 72 hours of treatment. These changes were accompanied by a significant upregulation of E-cadherin expression following treatment for 48 hours with 10μM ZA in MDA-MB-231 cells (p<0.05, Figure 2, d). E-cadherin expression could not be detected in either vehicle or ZA treated Hs578t samples, though protein lysates from MCF-7 cells (which express high levels of E-cadherin) were positive for E-cadherin protein expression (Supplemental Figure 1a). Increased CD24 (epithelial marker) cell surface expression was observed in Hs578t following treatment for 48 hours (~40% increase in CD24, Supplemental Figure 1b), confirming an increase in epithelial phenotype.
These results correlated with decreased mRNA expression of mesenchymal markers N-cadherin (p<0.052 vs. vehicle in MDA-MB-231; p<0.04 vs. vehicle in Hs578t), Snail, and Twist (p<0.02 vs. vehicle in Hs578t, Figure 2, c–d), following 48 hours of treatment with 10μM ZA. mRNA expression of epithelial marker E-cadherin was concomitantly upregulated (p<0.03 vs. vehicle in MDA-MB-231; p<0.02 vs. vehicle in Hs578t, Figure 2, c–d). These results signify that ZA reduces expression of mesenchymal markers and increases expression of epithelial markers. Based on these findings, it is concluded that ZA reverses the epithelial-mesenchymal transition.
TNBC are sensitive to treatment with ZA
Previous groups report that treatment with ZA inhibits cell viability (5–10). Therefore, the effects of ZA on cell viability were assessed following treatment with 10μM ZA in TNBC for 24, 36, 48, 72, and 144 hours. Trypan blue exclusion experiments demonstrate that cell number decreased as early as 24 hours post treatment in both cell lines, but significant changes were not observed until 72 hours of treatment in MDA-MB-231 cells (p<0.01 vs. vehicle in 72 hours, Figure 3, a) or until after 48 hours of treatment in Hs578t cells (p<0.01 vs. vehicle in 48 hours, p<0.001 vs. vehicle in 72 hours, Figure 3, b). Only a small proportion of cells from either cell line remained attached following 144 hours of treatment (p<0.001 vs. vehicle, Figure 3, a–b). Cells which remained attached following treatment for 48 hours with 10μM ZA were assayed for markers of apoptosis (PARP-1 and caspase-3). Resulting experiments demonstrated that neither PARP nor caspase-3 underwent cleavage following treatment (Figure 3c). Based on these results, it is concluded that TNBC are sensitive to treatment with ZA, and those cells which remain attached following treatment are viable and not fated for cell death.
Figure 3. Cells remaining attached in culture following treatment with zoledronic acid are viable and detach after reversal of EMT.
(a–b) Percent viability of MDA-MB-231 (a) and Hs578t (b) cells following treatment with vehicle or 10μM ZA over a time course of 24, 36, 48, 72, or 144 hours. (c) Apoptotic protein expression in MDA-MB-231 (left) and Hs578t (right) following treatment with vehicle or 10μM ZA. Expected cleavage products: PARP: 89kD, caspase-3: 17kD. (d) Mesenchymal and epithelial protein expression in MDA-MB-231 (left) or Hs578t (right) following treatment with vehicle (Veh.) or 10μM ZA for six days. 72 hours after the initial treatment, detached cells were collected (72h float), and cells were retreated. 72 hours after the second treatment, both attached (attach) and detached cells (6d float) were collected. Data are expressed as mean±SEM and are representative of two independent experiments. **p<0.01, ***p<0.001.
ZA reverses EMT prior to cellular detachment
Because such sharp decreases in cell viability were observed following treatment with ZA, it was postulated that the cells which remained attached and became epithelial evade loss of viability. To test this, TNBC were treated for a duration of six days, with detached cells collected after 72 hours (denoted 72h float) or six days (denoted 6d float), and attached cells collected at the end of treatment (denoted attach). These samples were compared to cells treated for six days with vehicle for expression of mesenchymal and epithelial markers. Observed was a mesenchymal to epithelial transition in the detached cells, as evidenced by increased E-cadherin and decreased N-cadherin expression (Figure 3d). Cells which remain attached after six days, [approximately five to ten percent of vehicle treated samples (Figure 3, a–b)], are in transition between mesenchymal and epithelial state (Figure 3d). Taken together, these results suggest that EMT is reversed prior to detachment, and those cells in which EMT is not reversed remain attached and may be resistant to ZA treatment.
Zoledronic acid inhibits cell cycle progression and decreases proliferative capacity
Although western blot assessment of attached cells following treatment with ZA suggested that cells were not fated for cell death, large decreases in viable cells were still observed (Figure 3, a–c). Therefore, it was hypothesized that treatment with ZA may inhibit cell proliferation. To test this hypothesis, TNBC were treated for 48 hours with 10μM ZA, and remaining attached, viable cells were analyzed for changes in cell cycle progression. Both cell lines underwent cell cycle arrest. MDA-MB-231 arrested in G1-phase (p<0.001, Figure 4, a) while Hs578t arrested in S-phase (p<0.001, Figure 4, b). Examination of total cell number following treatment with ZA suggested that cells treated with ZA proliferate at a slower rate when compared to vehicle treated cells. In trypan blue experiments, MDA-MB-231 cells treated with 10μM ZA proliferate to about 65% the amount of the vehicle treated cells (700,000 in ZA treated group compared to 1.1 million in vehicle treated group, Figure 4, c), and Hs578t treated with 10μM ZA proliferate to about 30% the amount of the vehicle treated cells (520,000 in ZA treated group compared to 1.7 million in vehicle treated group, Figure 4, d). This data suggests that treatment with ZA decreases proliferative capacity, which correlates with cell cycle arrest. Taken together, these results suggest that ZA inhibits cell proliferation.
Figure 4. Zoledronic acid inhibits cell cycle progression and decreases proliferative capacity.
(a–b) Cell cycle analysis of MDA-MB-231 (a) and Hs578t (b) following treatment for 48 hours with vehicle or 10μM ZA. (c–d) Total number of viable cells in MDA-MB-231 (c) and Hs578t (d) following treatment with vehicle or 10μM ZA over a time course of 24, 36, 48, 72, or 144 hours. Data are expressed as mean±SEM and are representative of two independent experiments. ***p<0.001.
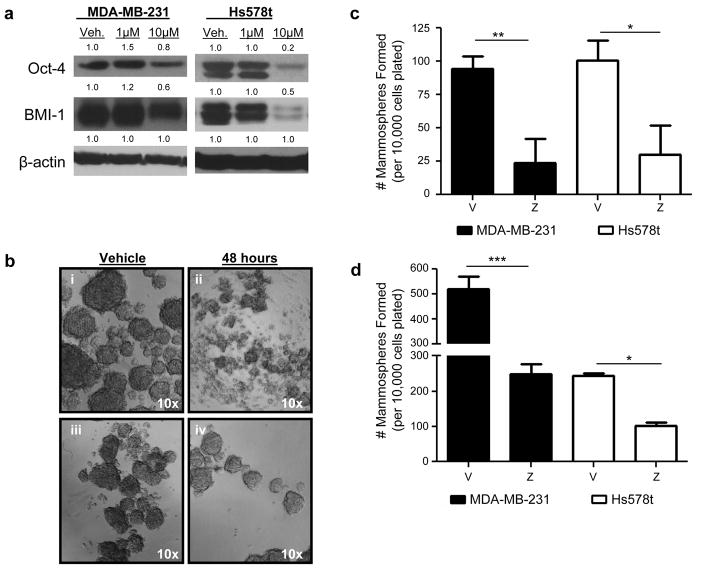
Zoledronic acid inhibits self-renewal capability
Previous findings have suggested that cells which undergo EMT acquire a self-renewal phenotype (18). However, treatment with ZA reverses EMT. Therefore, it was of interest to determine whether treatment with ZA could also inhibit self-renewal capability of TNBC. To study this in greater detail, the effect of ZA on the expression of self-renewal proteins BMI-1 and Oct-4 was examined. Both markers were reduced following treatment with ZA for 48 hours (Figure 5, a). The effects of ZA on TNBC self-renewal capability were studied further using the mammosphere assay. First, the effects of ZA on prevention of mammosphere formation were investigated. TNBC were treated for 48 hours with 10μM ZA under adherent conditions. Following treatment cells that remained attached and viable were collected and seeded in mammosphere culture. Pretreatment with ZA for 48 hours significantly reduced mammosphere formation in both cell lines (MDA-MB-231 p<0.01 vs. vehicle; Hs578t: p<0.05 vs. vehicle, Figure 5, b–c), signifying that treatment with ZA prevents mammosphere formation. The effects of ZA on existing mammospheres prior to ZA treatment were then studied. Secondary mammospheres were generated from both Hs578t and MDA-MB-231 primary mammosphere cultures. Primary mammospheres were serially passaged and were allowed to form for 72 hours. Following this 72 hour period, mammospheres were treated with vehicle or 10μM ZA and allowed to propagate for an additional 7 days in mammosphere culture. Both groups treated with ZA resulted in significantly lower number of mammospheres compared to those treated with vehicle (MDA-MB-231 p<0.001 vs. vehicle; Hs578t: p<0.05 vs. vehicle, Supplemental Figure 2, Figure 5d). Taken together, these data suggest that ZA can reduce mammosphere formation resulting from pretreated adherent cells and existing mammospheres.
Figure 5. Zoledronic acid inhibits self-renewal.
(a) Self-renewal protein expression in MDA-MB-231 (left) or Hs578t (right) following treatment for 48 hours with vehicle, 1, or 10μM ZA. Protein levels were normalized to β-actin. (b) Hs578t (top) and MDA-MB-231 (bottom) mammospheres resulting from cells pretreated for 48 hours with vehicle (i, iii) or 10μM ZA (ii, iv) in adherent conditions. (c) Quantification of mammosphere formation in (b). (d) Quantification of existing mammospheres formed upon serial passage of vehicle treated mammospheres allowed to form 72 hours prior to treatment with vehicle or 10μM ZA. Data are expressed as mean (protein) or mean±SEM (mammospheres). Protein experiments replicated thrice, mammospheres representative of two independent experiments. *p<0.05, ** p<0.01, ***p<0.001.
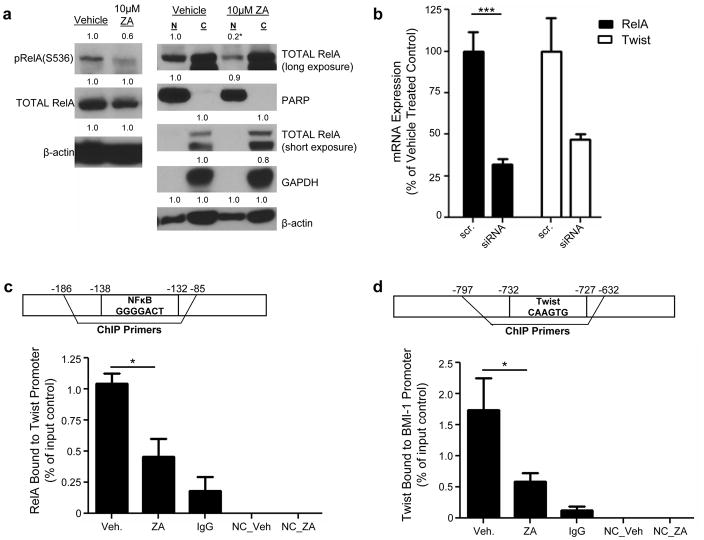
Zoledronic acid has no effect on RelA expression but modulates its cellular localization
Proteins which regulate EMT were investigated as potential mechanisms explaining ZA’s modulation of EMT. The nuclear factor kappa B (NFκB) pathway has been shown to regulate the expression of EMT transcription factors (25, 26). Therefore, it was hypothesized that ZA may modulate EMT through inactivation of NFκB. To determine whether ZA inactivates NFκB in TNBC, the effect on the active subunit of NFκB signaling, RelA, was measured following treatment for 48 hours with 10μM ZA. No significant changes were observed with regard to total RelA protein expression (Figure 6a, supplemental figure 3a). However, decreased phosphorylation of RelA at serine 536 was observed (Figure 6a, supplemental figure 3a). Next, the effect of ZA on RelA cellular localization was studied. Subcellular fractionation experiments were performed on cells treated for 48 hours with 10μM ZA. Observed was a decrease in nuclear RelA localization in both MDA-MB-231 and Hs578t cells (Figure 6a, supplemental figure 3a). Though decreased expression of PARP in the nuclear fraction was observed, equal amounts of lysates were loaded as evidenced by equivalent β-actin levels. Taken together, these experiments suggest that treatment with ZA inactivates NFκB transcriptional activation.
Figure 6. Zoledronic acid inactivates NFκB, and inhibits interactions between RelA and the Twist promoter and Twist and the BMI-1 promoter in MDA-MB-231.
(a, left) Expression of phospho- and total RelA in MDA-MB-231 cells following treatment for 48 hours with vehicle, or 10μM ZA. (a, right) Subcellular fractionation of RelA in MDA-MB-231 cells following treatment for 48 hours with vehicle or 10μM ZA. C: Cytoplasmic, N: Nuclear, PARP: nuclear loading control, GAPDH: cytoplasmic loading control. Protein was normalized to β-actin (total RelA, left; PARP and GAPDH, right), RelA (p-RelA), and PARP or GAPDH (RelA, right). (b) RelA and Twist mRNA expression in MDA-MB-231 following transfection for 72 hours with scramble (scr) or RelA siRNA (siRNA). mRNA levels were normalized to GAPDH. (c, top) Schematic of binding site within gene of interest. (c, bottom) MDA-MB-231 cells were treated for 48 hours with vehicle or 10μM ZA, and interaction between RelA and Twist promoter was measured via chromatin immunoprecipitation (ChIP). (d, top) Schematic of binding site within gene of interest. (d, bottom) MDA-MB-231 cells were treated for 48 hours with vehicle or 10μM ZA and interaction between Twist and BMI-1 promoter was measured via ChIP. H-NC: primers without RelA or Twist binding sites. IgG: samples immunoprecipitated with normal rabbit IgG. Twist and BMI-1 promoter levels were normalized to input controls and are expressed as % bound to promoter. Data are expressed as mean (protein) or mean±SEM (mRNA) and are indicative of three (protein) or two (mRNA, ChIP) independent experiments. *p<0.05, ***p<0.001.
RelA knockdown inhibits Twist mRNA expression
To determine the role of the NFκB pathway plays on EMT transcription factor expression in TNBC, RNAi strategies were used to decrease RelA expression in TNBC. Using this strategy, RelA mRNA expression was reduced approximately 70% in both MDA-MB-231 and Hs578t (Figure 6b, supplemental figure 3b). Following RelA knockdown, levels of Twist mRNA were measured. Twist was downregulated as compared to scramble control (p<0.07 in MDA-MB-231, Twist: p<0.01 in Hs578t; Figure 6b, supplemental figure 3b), signifying that RelA regulates Twist mRNA expression in TNBC.
Zoledronic acid reduces interactions between RelA and the Twist promoter and Twist and the BMI-1 promoter
To confirm transcriptional regulation of RelA on Twist expression, ChIP analyses were performed on both Hs578t and MDA-MB-231 cell lines treated with 10μM ZA for 48 hours. In vehicle treated cells, the Twist promoter interacted with RelA, confirming transcriptional regulation of Twist by RelA in both cell lines (Figure 6c, supplemental figure 3c). Furthermore, treatment with ZA reduced this interaction (p<0.05 vs. vehicle in 231, Figure 6c, supplemental figure 3c). To determine whether the modulation of EMT played a role in decreased self-renewal, the regulation of BMI-1 by Twist, which is an established regulator of BMI-1 expression (24), was studied via ChIP. Twist interacted with the BMI-1 promoter (Figure 6d, supplemental figure 3d), and that treatment with ZA inhibited this interaction (MDA-MB-231 p<0.05 vs. vehicle; Figure 6d, supplemental figure 3d). Based on these findings, it is concluded that ZA decreases RelA transcriptional control of Twist gene expression, leading to decreased Twist expression. Furthermore, treatment with ZA decreases Twist transcriptional control of BMI-1 gene expression, leading to decreased BMI-1 expression.
Discussion
Zoledronic acid (ZA) has shown promise as a BC therapeutic, as evidenced by its effects on increased disease-free survival (1–4), decreased metastatic disease (1), and inhibition of cell viability (5–10), invasion, and migration (11, 12) preclinically. Prior to this study, the mechanisms responsible for decreased migration, invasion, and metastatic disease were not well studied. The novel findings of these studies indicate that ZA can reverse EMT and decrease self-renewal capacity of TNBC. Results of these studies are the first to show loss of interaction between nuclear factor kappa B (NFκB) active subunit RelA and the Twist promoter, providing evidence which correlates the loss of RelA activation to modulation of EMT. Additionally, these studies are the first to show that treatment with ZA inhibits Twist binding to the BMI-1 promoter, providing evidence which correlates modulation of EMT to decreased self-renewal. Finally, these studies demonstrate that treatment with ZA inhibits the self-renewal capabilities of TNBC, as evidenced by decreased expression of self-renewal proteins and both prevention and elimination of mammosphere formation.
Treatment with ZA led to a decrease in mesenchymal characteristics, including loss of N-cadherin, Twist, and Snail mRNA and protein expression, as well as a loss of mesenchymal cell morphology. These results are consistent with those of other groups that have reported ZA’s effects on the expression of proteins involved in metastatic disease. For example, ZA has been shown to have differential effects on expression and activation of matrix metalloproteinases (27, 28), required for the escape of cells from a primary tumor site. Furthermore, ZA has also been reported to inactivate and downregulate both αVβ3 and αVβ5 integrins (29, 30), which are involved in cell interaction with extracellular matrices. Also observed was an increase in epithelial characteristics, including increases in cell-cell contact, E-cadherin mRNA and protein expression, and CD24 cell surface expression. These findings are novel and are reported for the first time in the current studies.
Examination of the timeline of events between reversal of EMT and detachment unveiled that cells gain epithelial characteristics prior to detachment. However, a small proportion of cells remain attached even after long exposure to ZA. These cells remain viable and maintain mesenchymal characteristics, suggesting that some cells are innately resistant to treatment with ZA. This may explain why some cells do not undergo cell cycle arrest and/or why some cells maintain the ability to self-renew. Regardless, it is concluded that treatment with ZA reverses EMT prior to inducing cellular detachment. Furthermore, attached cells in which EMT has been reversed are not fated for cell death, suggesting that decreases in cell proliferation, and not induction of apoptosis, lead to reduction in cell viability.
The conclusion that ZA inhibits cell viability through inhibition of cell proliferation and not induction of apoptosis is corroborated by the effect of ZA on cell cycle progression, which show arrest in both G1 (MDA-MB-231) and S (Hs578t) phase of the cell cycle. These effects on cell cycle progression agree with previous results, which illustrate that treatment with ZA inhibits cell cycle in S-phase (31–33). Although arrest of cells in G1 has been observed (34–36), the mechanism explaining this finding remains unclear. Proliferative capacity of the cells was also reduced, further substantiating these findings.
In addition to its effects on EMT, treatment with ZA led to decreases in self-renewal capacity. Regulators of EMT were studied as possible leads. One such molecule is Twist, which promotes EMT by inhibiting expression of E-cadherin and increasing self-renewal through upregulation of BMI-1, a protein involved in keeping cells undifferentiated through inhibition of chromatin remodeling (24). The current study found a decrease in Twist expression at both the protein and mRNA level, which correlates with increased E-cadherin expression, decreased BMI-1 expression, and decreased interaction with the BMI-1 promoter. These results are supported by previous reports which have shown that silencing of Twist using RNAi strategies can reverse EMT and decrease metastasis (17).
The nuclear factor kappa B (NFκB) pathway has been implicated in the regulation of EMT transcription factors, including Twist (25, 26). While previous studies with ZA (29) and alendronate (37), another bisphosphonate, have demonstrated differential effects of bisphosphonates on NFκB signaling, no work has been performed in BC cell lines. When treated with ZA, a loss in phosphorylation of RelA at serine 536 was observed. Previous reports suggest that phosphorylation at this site occurs independently of canonical NFκB signaling (38). This noncanonical phosphorylation is in accordance with other findings which report no effect of ZA on canonical NFκB activation (29). The interaction between RelA and the Twist promoter was also decreased following treatment with ZA, providing a direct link between ZA treatment and the inhibition of Twist expression. Although further investigation is required to determine the inhibitory effects of ZA on NFκB activity, current findings suggest that RelA inhibition plays a role in Twist downregulation.
This study is the first to report that treatment with ZA can reverse EMT and decrease self-renewal. These findings imply that treatment with ZA may be able to inhibit metastasis by both preventing cells from leaving their primary site and abrogating their ability to repopulate at a secondary site. The findings reported by this study support the use of ZA as a treatment for BC, as it may prove beneficial in the prevention of metastatic disease.
Supplementary Material
Acknowledgments
Financial Support:
A.Schech: NIH R01 CA62483
A. Kazi: NIH R01 CA62483
R. Gilani: NIH R01 CA62483
A. Brodie: NIH R01 CA62483
Grant Support:
Work was funded by grant awarded to AH Brodie: NIH R01 CA62483.
Abbreviations
- ZA
zoledronic acid
- BC
breast cancer
- EMT
epithelial-mesenchymaltransition
- TNBC
triple negative breast cancer cell lines
- NFκB
nuclear factor kappa B
Footnotes
Conflict of Interest Statement:
Amanda J. Schech, Armina A. Kazi, Rabia A. Gilani, and Angela H. Brodie have no conflicts of interest to disclose.
References
- 1.Gnant M, Mlineritsch B, Schippinger W, Luschin-Ebengreuth G, Postlberger S, Menzel C, et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009;360:679–91. doi: 10.1056/NEJMoa0806285. [DOI] [PubMed] [Google Scholar]
- 2.Eidtmann H, de Boer R, Bundred N, Llombart-Cussac A, Davidson N, Neven P, et al. Efficacy of zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: 36-month results of the ZO-FAST study. Ann Oncol. 2010;21:2188–94. doi: 10.1093/annonc/mdq217. [DOI] [PubMed] [Google Scholar]
- 3.Gnant M, Mlineritsch B, Stoeger H, Luschin-Ebengreuth G, Heck D, Menzel C, et al. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-month follow-up from the ABCSG-12 randomised trial. Lancet Oncol. 2011;12:631–41. doi: 10.1016/S1470-2045(11)70122-X. [DOI] [PubMed] [Google Scholar]
- 4.Coleman RE, Marshall H, Cameron D, Dodwell D, Burkinshaw R, Keane M, et al. Breast-cancer adjuvant therapy with zoledronic acid. N Engl J Med. 2011;365:1396–405. doi: 10.1056/NEJMoa1105195. [DOI] [PubMed] [Google Scholar]
- 5.Fromigue O, Lagneaux L, Body JJ. Bisphosphonates induce breast cancer cell death in vitro. J Bone Miner Res. 2000;15:2211–21. doi: 10.1359/jbmr.2000.15.11.2211. [DOI] [PubMed] [Google Scholar]
- 6.Senaratne SG, Pirianov G, Mansi JL, Arnett TR, Colston KW. Bisphosphonates induce apoptosis in human breast cancer cell lines. Br J Cancer. 2000;82:1459–68. doi: 10.1054/bjoc.1999.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jagdev SP, Coleman RE, Shipman CM, Rostami-H A, Croucher PI. The bisphosphonate, zoledronic acid, induces apoptosis of breast cancer cells: Evidence for synergy with paclitaxel. Br J Cancer. 2001;84:1126–34. doi: 10.1054/bjoc.2001.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Senaratne SG, Mansi JL, Colston KW. The bisphosphonate zoledronic acid impairs ras membrane [correction of impairs membrane] localisation and induces cytochrome c release in breast cancer cells. Br J Cancer. 2002;86:1479–86. doi: 10.1038/sj.bjc.6600297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verdijk R, Franke HR, Wolbers F, Vermes I. Differential effects of bisphosphonates on breast cancer cell lines. Cancer Lett. 2007;246:308–12. doi: 10.1016/j.canlet.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 10.Almubarak H, Jones A, Chaisuparat R, Zhang M, Meiller TF, Scheper MA. Zoledronic acid directly suppresses cell proliferation and induces apoptosis in highly tumorigenic prostate and breast cancers. J Carcinog. 2011;10:2. doi: 10.4103/1477-3163.75723. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Boissier S, Ferreras M, Peyruchaud O, Magnetto S, Ebetino FH, Colombel M, et al. Bisphosphonates inhibit breast and prostate carcinoma cell invasion, an early event in the formation of bone metastases. Cancer Res. 2000;60:2949–54. [PubMed] [Google Scholar]
- 12.Denoyelle C, Hong L, Vannier JP, Soria J, Soria C. New insights into the actions of bisphosphonate zoledronic acid in breast cancer cells by dual RhoA-dependent and -independent effects. Br J Cancer. 2003 May 19;88:1631–40. doi: 10.1038/sj.bjc.6600925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guarino M, Rubino B, Ballabio G. The role of epithelial-mesenchymal transition in cancer pathology. Pathology. 2007;39:305–18. doi: 10.1080/00313020701329914. [DOI] [PubMed] [Google Scholar]
- 14.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–8. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–9. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- 16.Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 17.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–39. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schech AJ, Nemieboka BE, Brodie AH. Zoledronic acid inhibits aromatase activity and phosphorylation: Potential mechanism for additive zoledronic acid and letrozole drug interaction. J Steroid Biochem Mol Biol. 2012;132:195–202. doi: 10.1016/j.jsbmb.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiao W, Miyazaki K, Kitajima Y. Inverse correlation between E-cadherin and snail expression in hepatocellular carcinoma cell lines in vitro and in vivo. Br J Cancer. 2002;86:98–101. doi: 10.1038/sj.bjc.6600017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sitarz R, Leguit RJ, de Leng WW, Morsink FH, Polkowski WP, Maciejewski R, et al. Cyclooxygenase-2 mediated regulation of E-cadherin occurs in conventional but not early-onset gastric cancer cell lines. Cell Oncol. 2009;31:475–85. doi: 10.3233/CLO-2009-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee UJ, Choung SR, Prakash KV, Lee EJ, Lee MY, Kim YJ, et al. Dual knockdown of p65 and p50 subunits of NF-kappaB by siRNA inhibits the induction of inflammatory cytokines and significantly enhance apoptosis in human primary synoviocytes treated with tumor necrosis factor-alpha. Mol Biol Rep. 2008;35:291–8. doi: 10.1007/s11033-007-9084-4. [DOI] [PubMed] [Google Scholar]
- 23.Synthesis-gene.com [Homepage on the internet] Shanghai: ShineGene Molecular Biotech Inc; [cited 2011 Jun 12]. Available from: http://www.synthesisgene.com/tools/Housekeeping-Gene-Primers.pdf. [Google Scholar]
- 24.Yang MH, Hsu DS, Wang HW, Wang HJ, Lan HY, Yang WH, et al. Bmi1 is essential in Twist1-induced epithelial-mesenchymal transition. Nat Cell Biol. 2010;12:982–92. doi: 10.1038/ncb2099. [DOI] [PubMed] [Google Scholar]
- 25.Pham CG, Bubici C, Zazzeroni F, Knabb JR, Papa S, Kuntzen C, et al. Upregulation of twist-1 by NF-kappaB blocks cytotoxicity induced by chemotherapeutic drugs. Mol Cell Biol. 2007;27:3920–35. doi: 10.1128/MCB.01219-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li CW, Xia W, Huo L, Lim SO, Wu Y, Hsu JL, et al. Epithelial-mesenchymal transition induced by TNF-alpha requires NF-kappaB-mediated transcriptional upregulation of Twist1. Cancer Res. 2012;72:1290–300. doi: 10.1158/0008-5472.CAN-11-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teronen O, Heikkila P, Konttinen YT, Laitinen M, Salo T, Hanemaaijer R, et al. MMP inhibition and downregulation by bisphosphonates. Ann N Y Acad Sci. 1999;878:453–65. doi: 10.1111/j.1749-6632.1999.tb07702.x. [DOI] [PubMed] [Google Scholar]
- 28.Montague R, Hart CA, George NJ, Ramani VA, Brown MD, Clarke NW. Differential inhibition of invasion and proliferation by bisphosphonates: Anti-metastatic potential of zoledronic acid in prostate cancer. Eur Urol. 2004;46:389, 401. doi: 10.1016/j.eururo.2004.04.022. discussion 401–2. [DOI] [PubMed] [Google Scholar]
- 29.Bezzi M, Hasmim M, Bieler G, Dormond O, Ruegg C. Zoledronate sensitizes endothelial cells to tumor necrosis factor-induced programmed cell death: Evidence for the suppression of sustained activation of focal adhesion kinase and protein kinase B/Akt. J Biol Chem. 2003;278:43603–14. doi: 10.1074/jbc.M308114200. [DOI] [PubMed] [Google Scholar]
- 30.Bellahcene A, Chaplet M, Bonjean K, Castronovo V. Zoledronate inhibits alphavbeta3 and alphavbeta5 integrin cell surface expression in endothelial cells. Endothelium. 2007;14:123–30. doi: 10.1080/10623320701347187. [DOI] [PubMed] [Google Scholar]
- 31.Romani AA, Desenzani S, Morganti MM, La Monica S, Borghetti AF, Soliani P. Zoledronic acid determines S-phase arrest but fails to induce apoptosis in cholangiocarcinoma cells. Biochem Pharmacol. 2009;78:133–41. doi: 10.1016/j.bcp.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 32.Okamoto S, Kawamura K, Li Q, Yamanaka M, Yang S, Fukamachi T, et al. Zoledronic acid produces antitumor effects on mesothelioma through apoptosis and S-phase arrest in p53-independent and ras prenylation-independent manners. J Thorac Oncol. 2012;7:873–82. doi: 10.1097/JTO.0b013e31824c7d43. [DOI] [PubMed] [Google Scholar]
- 33.Li XY, Lin YC, Huang WL, Lin W, Wang HB, Lin WZ, et al. Zoledronic acid inhibits human nasopharyngeal carcinoma cell proliferation by activating mitochondrial apoptotic pathway. Med Oncol. 2012 Jun 23; doi: 10.1007/s12032-012-0281-1. [DOI] [PubMed] [Google Scholar]
- 34.Lee MV, Fong EM, Singer FR, Guenette RS. Bisphosphonate treatment inhibits the growth of prostate cancer cells. Cancer Res. 2001;61:2602–8. [PubMed] [Google Scholar]
- 35.Tenta R, Pitulis N, Tiblalexi D, Consoulas C, Katopodis H, Konstantinidou E, et al. Mechanisms of the action of zoledronic acid on human MG-63 osteosarcoma cells. Horm Metab Res. 2008;40:737–45. doi: 10.1055/s-2008-1078753. [DOI] [PubMed] [Google Scholar]
- 36.Di Salvatore M, Orlandi A, Bagala C, Quirino M, Cassano A, Astone A, et al. Anti-tumour and anti-angiogenetic effects of zoledronic acid on human non-small-cell lung cancer cell line. Cell Prolif. 2011;44:139–46. doi: 10.1111/j.1365-2184.2011.00745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inoue R, Matsuki NA, Jing G, Kanematsu T, Abe K, Hirata M. The inhibitory effect of alendronate, a nitrogen-containing bisphosphonate on the PI3K-akt-NFkappaB pathway in osteosarcoma cells. Br J Pharmacol. 2005;146:633–41. doi: 10.1038/sj.bjp.0706373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sasaki CY, Barberi TJ, Ghosh P, Longo DL. Phosphorylation of RelA/p65 on serine 536 defines an I{kappa}B{alpha}-independent NF-{kappa}B pathway. J Biol Chem. 2005;280:34538–47. doi: 10.1074/jbc.M504943200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.