Abstract
Objective
To identify risk factors for endometrial cancer after benign results of endometrial biopsy or dilatation and curettage (D&C).
Methods
Nested case-control study from Rochester Epidemiology Project data. Among 370 Olmsted County, Minnesota, residents who received an endometrial cancer diagnosis between 1970 and 2008, we identified 90 patients (24.5%) who had previous benign endometrial biopsy or D&C results (no atypical hyperplasia). We compared them with 172 matched controls who had benign endometrial biopsy or D&C results without subsequent endometrial cancer.
Results
Using a multivariable, conditional logistic regression model, we found that oral contraceptive use was protective (odds ratio [OR], 0.18; 95% confidence interval [CI], 0.08-0.45; P<.001), and personal history of colorectal cancer (OR, 4.44; 95% CI, 1.02-19.31; P<.05), endometrial polyp (OR, 4.12; 95% CI, 1.40-12.17; P=.01), and morbid obesity (OR, 3.40; 95% CI, 1.18-9.78; P<.03) were independently associated with subsequent endometrial cancer. Compared with the presence of no risk factor, presence of one and two or more risk factors increased the risk of endometrial cancer by 8.12 (95% CI, 3.08-21.44) and 17.87 (95% CI, 5.57-57.39) times, respectively. Assuming a 2.6% lifetime risk of EC, OR’s of 8.12 and 17.87 for 1 and 2 or more of the 4 afore-mentioned risk factors confer a lifetime risk of approximately 18% and 32%, respectively.
Conclusion
One-fourth of patients with endometrial cancer had previous benign endometrial biopsy or D&C results. Personal history of colorectal cancer, presence of endometrial polyps, and morbid obesity are the strongest risk factors for having endometrial cancer after a benign endometrial biopsy or D&C result, and oral contraceptive use is the strongest protective factor.
Introduction
Endometrial cancer (EC) is the most common malignancy arising from the female reproductive tract in the Western world. In 2012, an estimated 47,130 new cases and 8,010 deaths will be attributed to this disease in the United States (1). Also, the incidence and death rate of EC have increased in the past several decades (1,2). The identification of women who are at increased risk of EC may allow early diagnosis, prevention, and a reduction in the considerable societal burden imposed by EC. In fact, investigators have shown that application of EC screening and preventive strategies to the entire population of women is not cost-effective, while targeting high-risk patients may be an effective option (3-5).
A history of benign breast biopsy is a well-known risk factor for development of breast cancer and is routinely used for risk stratification (6). Twu and Chen (7) and Gull et al (8) reported a higher risk of EC in patients undergoing endometrial biopsy (with a benign diagnosis) when compared with the general population. However, there are no studies that identify and quantify risk or protective factors for subsequent development of EC after a benign result on an index endometrial biopsy or dilatation and curettage (D & C) (EB/DC) in a comprehensive manner. The absence of such studies was confirmed on PubMed and Ovid search engines.
We undertook the present study to identify risk factors for subsequent development of EC after a benign result on index EB/DC, using a partial nested case-control study design.
Methods
Study Sample
This study was approved by the Mayo Clinic and Olmsted Medical Center institutional review boards. The Rochester Epidemiology Project was used to identify patients with EC. The project is a unique research infrastructure (R01 AG034676) that was established in 1966 and has been used to conduct population-based studies among residents of Olmsted County, Minnesota. As part of these resources, the medical records are linked and indexed, and persons in the Olmsted County population with specific diseases may be identified through retrieval of records with pertinent diagnostic codes (9).
With these resources and careful review of the medical records, we identified 370 patients who had received a diagnosis of EC between January 1, 1970, and December 31, 2008, while they were residents of Olmsted County and who had not denied access to their medical records for research purposes.
Cases
Of the 370 patients initially identified with EC, 101 (27%) had a benign EB/DC before diagnosis of EC. From these 101 patients, we excluded 11 patients who had simple or complex atypical hyperplasia in the EB/DC (because of intrinsic high risk of EC), leaving 90 (24.5%) patients for the analysis. Their first benign EB/DC is herein referred to as the index EB/DC.
Control Subjects
Using institutional databases, we identified all patients with an EB/DC performed at Mayo Clinic between January 1, 1985 (electronic data not available before 1985), and December 31, 2008, while they were Olmsted County residents, who were age 40 to 85 years and had not denied access to their medical records for research purposes. These patients were cross-referenced with the institution’s medical index to exclude any patient who ever received a diagnosis of EC or atypical hyperplasia. The remaining pool of control subjects with no EC but with a benign EB/DC consisted of 7,994 patients. For each of the 90 EC cases with benign EB/DC, 2 matched control cases with at least as much follow-up after the index EB/DC as the matched case were randomly identified from the control pool. The matching criteria included benign vs hyperplasia diagnosis on the EB/DC, age at EB/DC (within ±8 years), and date of the EB/DC (within ±3 years if the EB/DC was performed after 1985).
For this study, the following definition was used for personal history of hereditary nonpolyposis colorectal cancer (HNPCC)–related malignancy: a personal history of cancer of the pancreas, colon, rectum, ovary, small bowel, stomach, biliary tract, or brain and transitional cancers of the urinary tract. Family history of HNPCC-related malignancy was defined as presence of at least 1 first-degree relative with cancer of the pancreas, colon, rectum, endometrium, ovary, small bowel, stomach, biliary tract, or brain and transitional cancers of the urinary tract. This definition satisfied the need of using an easily assessable and simple variable at the time of the index EB/DC. Also, the inaccuracy of family history in the identification of patients with HNPCC has been demonstrated before (10).
The following definition was used to categorize a polyp: combined epithelial stromal sessile proliferation consisting of glands and fibrotic stroma with large and thick-walled arteries coated by an epithelium surface.
The endometrial biopsy was classified as benign nonspecified when it was defined as benign by the pathologist but was not better characterized and did not fit in the other pathologic categories.
Statistical Analysis
Data were summarized using standard descriptive methods, frequency and percentages for categorical variables, and mean and standard deviation or median and range for continuous variables. For each factor of interest, a separate conditional logistic regression model was fit to evaluate the association between the factor and the case-control status. In addition, a multivariable, conditional logistic model was fit using stepwise and backward variable selection methods. Associations were summarized using the odds ratio (OR) and the corresponding 95% confidence interval (CI). All calculated P values were 2 sided and P values less than .05 were considered statistically significant. Statistical analyses were performed using the SAS version 9.2 software package (SAS Institute Inc; Cary, North Carolina).
Results
The final analysis included 90 cases and 172 controls (8 cases had only 1 control each). Demographic data of the patients are summarized in Table 1. Mean (SD) age at the time of the index EB/DC was 51.8 (11.0) (range, 17.9-81.8) and 51.9 (10.4) (range, 21.5-80.1) years, respectively, among cases and controls. The time interval between the index EB/DC and the diagnosis of EC among cases ranged from 0 to 23.3 years, with a median duration of 6.7 years. Matched controls were selected to have at least as much follow-up as the corresponding cases, with an overall median of 13.8 years from the date of the index EB/DC to the date of last follow-up or hysterectomy.
Table 1.
Variables Assessed for an Association With Future Development of Endometrial Cancer
| Variable | Controls (n=172) |
Patients (n=90) |
P * |
|---|---|---|---|
| Aat menopause, y | .24 | ||
| No. of patients | 137 | 86 | |
| Mean (SD) | 49.8 (4.3) | 50.1 (5.2) | |
| BMI, kg/m2† | <.04 | ||
| No. of patients | 168 | 75 | |
| Fewer than 25 | 72 (42.9) | 24 (32) | |
| 25-29.9 | 58 (34.5) | 22 (29.3) | |
| 30-34.9 | 22 (13.1) | 9 (12) | |
| 35 or more | 16 (9.5) | 20 (26.7) | |
| Mean (SD) | 27.2 (6.7) | 30.2 (9.1) | |
| Weight,kg† | <.05 | ||
| No. of patients | 168 | 76 | |
| Mean (SD) | 71.9 (18.2) | 79.4 (24.7) | |
| Type of endometrial sampling† | .03 | ||
| Missing | 11 | 4 | |
| Biopsy | 62 (38.5) | 24 (27.9) | |
| D&C | 99 (61.5) | 62 (72.1) | |
| Use of metformin† | .16 | ||
| Missing | 2 | 4 | |
| No | 167 (98.2) | 81 (94.2) | |
| Yes | 3 (1.8) | 5 (5.8) | |
| Use of ET† | .008 | ||
| No | 165 (95.9) | 78 (86.7) | |
| Yes | 7 (4.1) | 12 (13.3) | |
| Use of tamoxifen† | .08 | ||
| Missing | 1 | 3 | |
| No | 170 (99.4) | 83 (95.4) | |
| Yes | 1 (0.6) | 4 (4.6) | |
| No. of pregnancies† | .06 | ||
| Missing | 1 | 4 | |
| 0 | 25 (14.6) | 20 (23.3) | |
| 1 or more | 146 (85.4) | 66 (76.7) | |
| No. of live births† | .008 | ||
| Missing | 1 | 4 | |
| 0 | 27 (15.8) | 25 (29.1) | |
| 1 or more | 144 (84.2) | 61 (70.9) | |
| Use of OCP† | <.001 | ||
| Missing | 4 | 2 | |
| No | 62 (36.9) | 63 (71.6) | |
| Yes | 106 (63.1) | 25 (28.4) | |
| Use of IUD† | ‡ | ||
| No | 172 (100) | 88 (97.8) | |
| Yes | 0 (0) | 2 (2.2) | |
| Family history of HNPCC-related malignancy†,‡ |
.10 | ||
| No | 148 (86.0) | 70 (77.8) | |
| Yes | 24 (14.0) | 20 (22.2) | |
| Personal history of HNPCC- related malignancy†,§ |
<.03 | ||
| No | 165 (95.9) | 79 (87.8) | |
| Yes | 7 (4.1) | 11 (12.2) | |
| Age at menarche, y | .69 | ||
| No. of patients | 71 | 48 | |
| Mean (SD) | 12.9 (1.4) | 12.8 (1.4) | |
| Age at first live birth, y | .36 | ||
| No. of patients | 135 | 55 | |
| Mean (SD) | 25.8 (4.8) | 24.7 (4.9) | |
| Diabetes mellitus† | .21 | ||
| Missing | 1 | 4 | |
| No | 159 (93.0) | 76 (88.4) | |
| Yes | 12 (7.0) | 10 (11.6) | |
| Hypertension† | .70 | ||
| Missing | 1 | 5 | |
| No | 132 (77.2) | 63 (74.1) | |
| Yes | 39 (22.8) | 22 (25.9) | |
| Menopausal status† | .10 | ||
| Missing | 1 | 2 | |
| No | 92 (53.8) | 40 (45.4) | |
| Yes | 79 (45.2) | 48 (54.6) | |
| Use of COX-2† | .19 | ||
| Missing | 2 | 1 | |
| No | 164 (96.5) | 82 (92.1) | |
| Yes | 6 (3.5) | 7 (7.9) | |
| Smoking status† | .89 | ||
| Missing | 0 | 1 | |
| Never | 110 (64.0) | 56 (62.9) | |
| Past or current | 62 (36.0) | 33 (37.1) | |
| Presence of polyp on histologic evaluation† |
<.001 | ||
| Missing | 2 | 5 | |
| No | 162 (95.3) | 66 (76.7) | |
| Yes | 8 (4.7) | 19 (22.3) |
SD, standard deviation; BMI, body mass index; D&C, dilatation and curettage; ET, estrogen therapy; OCP, oral contraceptive pill; IUD, intrauterine device; HNPCC, hereditary nonpolyposis colorectal COX-2, cyclooxygenase 2.
Data are n(%) unless otherwise specified.
Univariable conditional logistic regression model.
Before or at the time of the index endometrial biopsy or D&C.
P value could not be determined in the conditional analysis because none of the controls used an IUD before or at the time of the endometrial biopsy or D&C.
Family history of HNPCC-related malignancy: at least one first-degree relative with cancer of the pancreas, colon, rectum, endometrium, ovary, small bowel, stomach, biliary tract, or brain and transitional cancers of the urinary tract.
Personal history of HNPCC-related malignancy: personal history of cancer of the pancreas, colon, rectum, ovary, small bowel, stomach, biliary tract, or brain or transitional cancers of the urinary tract. In our series, all patients had colorectal cancer.
Among the 262 patients, the index EB/DC was performed as part of the work-up of known nonendometrial disease (eg, uterine fibroids, cervical disease) in 8 patients (3%); by comparison, in 246 patients (94%), the EB/DC was performed because of known endometrial diagnoses documented before the EB/DC (eg, prior endometritis) or symptoms (bleeding) directly referable to the endometrium itself. Two other patients (1%) had an EB/DC for screening, and no indication was documented by the clinician for 6 patients. The method of endometrial sampling for the index EB/DC was available for 247 patients: an endometrial biopsy in 86 (35%) and a D & C in 161 (65%). Patients who underwent a D & C at the time of their index EB/DC were 2.1 times more likely to later have EC than patients who underwent an endometrial biopsy (OR, 2.10; 95% CI, 1.08-4.12; P=.03).
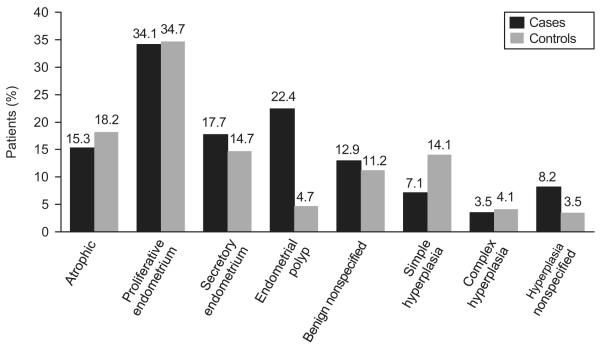
The final pathologic diagnosis of the EB/DC was endometrial hyperplasia without atypia in 16 (19%) of the 85 cases and 37 (22%) of the 170 controls with detailed histologic information. Histologic diagnoses are summarized in Figure 1. Of the 85 cases, 19 (22%) had polyps on EB/DC, compared with only 8 (5%) of the 170 controls (P<.001). In accordance with the study definition, all cases and controls with hyperplasia at the time of the index EB/DC had a diagnosis of hyperplasia without atypia.
Figure 1.
Benign endometrial biopsy or dilatation and curettage diagnosis in patients and control participants. The percentage in each group of cases and controls may exceed 100% because some patients had more than one histologic diagnosis in the same biopsy. Simple hyperplasia, complex hyperplasia, and unspecified hyperplasia are all without atypia, by inclusion criteria. Benign unspecified biopsies were defined as benign by the pathologist but were not better characterized. In addition, they did not fit into the other pathologic categories.
The histologic subtype of the EC that developed after the index EB/DC was endometrioid (78; 87%), serous (2; 2%), mixed (2; 2%), and unknown (8; 9%). The stages of these ECs were as follows: stage I, 81 (90%); stage II, 3 (3%); stage III, 5 (6%); and stage IV, 1 (1%).
Colorectal cancer, within cases and controls, was the predominant cancer in personal and family history of HNPCC-related malignancy. Colon cancer represents 100% (18 patients) of the cancers related to HNPCC in the personal history and 66% (29 patients) in the family history.
On univariable analysis, patient weight and body mass index (BMI), nulliparous status, personal history of HNPCC-related malignancy (100% colorectal cancer), use of unopposed estrogen therapy, D & C, endometrial polyp, and lack of oral contraceptive (OC) use at the time of the benign EB/DC were each identified as being significantly associated with subsequent development of EC (Table 1).
Use of tamoxifen at the time of index EB/DC was almost significantly associated with EC development (P=.08). Among the 172 control subjects, 1 had a history of tamoxifen use, and this patient did not have endometrial polyps present on histologic evaluation. Of the 170 control subjects (1 had missing information) who did not have a history of tamoxifen use, 8 (5%) had polyps present on histologic evaluation. Among the 90 cases, 4 had a history of tamoxifen use and 1 (25%) of these had endometrial polyps. Of the 83 cases (3 had missing information) that did not have a history of tamoxifen use, 17 (21%) had polyps present on histologic evaluation.
The following 4 variables were identified as independently associated with subsequent development of EC on the basis of a multivariable conditional logistic regression model using variable selection methods: OC use before or at EB/DC (protective factor) (OR, 0.18; 95% CI, 0.08-0.45; P<.001), presence of polyp on histologic evaluation (OR, 4.12; 95% CI, 1.40-12.17; P=.01), personal history of HNPCC-related malignancy (100% colorectal cancer) (OR, 4.44; 95% CI, 1.02-19.31; P<.05) and BMI of 35 or more (OR, 3.40; 95% CI, 1.18-9.78; P<.03).
We stratified patients on the basis of the number of the 4 independent predictors (BMI ≥35, no OC use, endometrial polyps, and personal history of HNPCC-related malignancy). Overall, 86 patients had 1 risk factor, 39 patients had 2, and 7 had 3. Patients with at least 1 risk factor had an 8.12 times higher risk of EC than women without risk factors. The presence of 2 or more risk factors increased the risk of cancer by 17.87 times (Table 2). Based on the SEER rates from 2007-2009, there is a 2.6% lifetime risk of EC, in US women (11). Assuming this lifetime risk estimate is applicable to the cohort of women with a benign EB/DC with none of the 4 aforementioned risk factors, OR’s of 8.12 and 17.87 for 1 and 2 or more of the 4 aforementioned risk factors confer a lifetime risk of approximately 18% and 32%, respectively.
Table 2.
Estimated Risk of Endometrial Cancer Developing After an Index Endometrial Biopsy or Dilatation and Curettage, Based on Number of Risk Factors
| No. of Risk Factors* | Odds Ratio (95% CI) | P |
|---|---|---|
| None (n=102) | 1 (reference) | |
| 1 (n=86) vs none | 8.12 (3.08-21.44) | <.001 |
| 2 or more (n=46) vs none | 17.87 (5.57-57.39) | <.001 |
CI, confidence interval.
Risk factors are: 1) lack of use of oral contraceptives; 2) personal history of hereditary nonpolyposis colorectal cancer–related- malignancy (in our series, all patients had colorectal cancer); 3) presence of endometrial polyp at the time of the index endometrial biopsy or dilatation and curettage; and 4) body mass index 35 kg/m2 or higher.
Discussion
In this study, we evaluated risk factors associated with EC development after benign EB/DC. The EB/DC gives pathologic information about the endometrium and is also a surrogate for identifying women at increased risk of EC. This notion is supported by our data depicting that approximately 25% of women with EC in our population had already had an EB/DC in the past showing benign findings (excluding atypical hyperplasia). The cohort of women who undergo EB/DC is inherently a high-risk subgroup compared with the rest of the population. These women have had multiple-layer filtering in terms of their risk stratification by virtue of their history and examination, with an increased probability of EC development.
In our population, the median time to EC was 6.7 years after an index EB/DC. This long period provides a potential window of opportunity in which surveillance programs can target the identification of women who might have had a benign EB/DC and might have 1 or more risk factors identified in our study (eg, no OC use, personal history of HNPCC-related malignancy (colorectal cancer), presence of polyp on EB/DC, morbid obesity). Although some of the risk factors found to be of significance in our study had been already implicated for the development of EC (12,13), the uniqueness of our findings is that we identified those risk factors at a time when several steps can be undertaken to address EC prevention. We suggest that after such high-risk women are identified, a closer follow-up may be performed to address screening and preventive actions. Steps that can be taken include risk modification (such as weight loss), routine serial pelvic ultrasonographic scans, targeted endometrial sampling, administration of OC, or prophylactic hysterectomy. However, we lack prospective demonstration of the cost-effectiveness of the above suggestions.
Unfortunately, our case-control design and the lack of abstracted clinical information on the total Olmsted County population who had benign endometrial biopsy between 1970 and 2008 do not allow the precise estimation of the risk of developing EC after benign EB/DC in our population. For this reason, we opted to use the SEER database for estimating a baseline risk. According to the SEER database, the lifetime risk of EC in US women is 2.6% (11). Similarly, it has been reported that the overall risk of development of EC after benign endometrial sample for post-menopausal bleeding is 2.7% (8), and this is increased to 3.5% in presence of an endometrial polyp (14). The above estimations have been utilized for baseline risk of EC in patients with benign EB/DC, and no risk factors. However, lifetime risks may change according to the age of the patients and presence of risk factors, and our percentages are only an approximation based on general population data.
In HNPCC syndrome, the lifetime risk of EC may be as high as 60% (15). Our observation that personal history of HNPCC-related malignancy (colorectal cancer) is a strong risk factor for EC developing after a benign EB/DC is consistent with the above-mentioned data.
The presence of an endometrial polyp has already been described as a risk factor for EC (16,17). We demonstrated that the presence of an endometrial polyp in an index EB/DC increases the risk of EC development by 4.2 times compared with other benign diagnoses.
Our study was not designed to identify any association between tamoxifen and endometrial polyps. However, despite the small numbers, results of our statistical analysis seem to favor a direct association between endometrial polyp and future EC, independent of tamoxifen use.
We observed that OC use before the benign EB/DC was associated with more than 6-times-lower likelihood of EC. Similar findings in the general population were reported by other investigators (18,19): estimated protection with use of OCs ranged from 20% with 1 year of use to 80% with 10 years. Interestingly, the protective role of OCs may not be observed in patients with a personal history of HNPCC-related malignancy, because HNPCC-related EC usually occurs independently of hormonal stimulation (20).
Patients with morbid obesity at the time of benign EB/DC are at increased risk of EC. It is known that obese patients have more bioavailable estrogens; therefore, they are more vulnerable to EC type I (21,22). Since obesity is a modifiable risk factor, preventive strategies and lifestyle changes may have an important effect in this population.
In our study, we excluded patients with atypical hyperplasia for the following reasons: first, 40% to 50% of these patients have concomitant EC (23-24) at the time of diagnosis of atypical hyperplasia. Second, atypical hyperplasia is a well-recognized risk factor for and precursor of EC and has been extensively studied (25). Third, most women (80%) (26) with a diagnosis of atypical hyperplasia may undergo hysterectomy as a primary and preventive treatment.
In contrast to studies that report equal or better diagnostic efficacy of D & C (27), we observed that D & C was more likely to be associated with subsequent EC when compared with Pipelle biopsy. This divergent observation may be related to the time course of our study, which included early years when office endometrial biopsy was not routinely performed. In later years, selection bias of high-risk patients having D&C may explain the discrepancy.
The limitations of our study include its retrospective nature; the lack of detailed information about progesterone use and history of infertility; the long observation time period, and the reliance on data collected from a geographically limited area, with relatively homogeneous populations that have reasonably easy access to medical care. Also, controls and cases were selected from slightly different time periods because of the unavailability of EB/DC electronic data before 1985. However, we report a long follow-up period, our data collection was comprehensive for the population under study, and we monitored all residents of Olmsted County. Hence, the selection bias in our study is likely minimal. Moreover, the resources from the Rochester Epidemiology Project provide accurate patient history and a foundation for population-based studies with comprehensive disease, follow-up, and outcome information. Although, as seen in previous studies, the interpretation of an EB/DC (28) may vary depending on pathologists, our study is strengthened by a robust central pathology review by specialized gynecologic pathologists.
In summary, we have shown that approximately one-fourth of women with EC had a previous benign EB/DC. Considering the cohort of women who have benign EB/DC findings, we observed colorectal cancer, presence of a benign endometrial polyp in the sample, and morbid obesity are strong risk factors for future development of EC, whereas use of OCs is protective. These data can guide clinicians and patients for efficient and targeted use of diagnostic or preventive strategies for EC.
Footnotes
Financial Disclosure: The authors did not report any potential conflicts of interest.
Presented at the Society of Gynecologic Oncologists 42nd Annual Meeting on Womens’ Cancer, March 5-9, 2011, Orlando, Florida.
Publisher: To expedite proof approval, send proof via scipubs@mayo.edu.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012 Jan-Feb;62(1):10–29. doi: 10.3322/caac.20138. Epub 2012 Jan 4. [DOI] [PubMed] [Google Scholar]
- 2.Weiss NS, Szekely DR, Austin DF. Increasing incidence of endometrial cancer in the United States. N Engl J Med. 1976 Jun 3;294(23):1259–62. doi: 10.1056/NEJM197606032942303. [DOI] [PubMed] [Google Scholar]
- 3.Havrilesky LJ, Maxwell GL, Myers ER. Cost-effectiveness analysis of annual screening strategies for endometrial cancer. Am J Obstet Gynecol. 2009 Jun;200(6):640.e1–8. doi: 10.1016/j.ajog.2009.02.022. Epub 2009 Apr 19. [DOI] [PubMed] [Google Scholar]
- 4.Kwon JS, Lu KH. Cost-effectiveness analysis of endometrial cancer prevention strategies for obese women. Obstet Gynecol. 2008 Jul;112(1):56–63. doi: 10.1097/AOG.0b013e31817d53a4. [DOI] [PubMed] [Google Scholar]
- 5.Leslie KK, Thiel KW, Yang S. Endometrial cancer: potential treatment and prevention with progestin-containing intrauterine devices. Obstet Gynecol. 2012 Feb;119(2 Pt 2):419–20. doi: 10.1097/AOG.0b013e3182444f15. [DOI] [PubMed] [Google Scholar]
- 6.Hartmann LC, Sellers TA, Frost MH, Lingle WL, Degnim AC, Ghosh K, et al. Benign breast disease and the risk of breast cancer. N Engl J Med. 2005 Jul 21;353(3):229–37. doi: 10.1056/NEJMoa044383. [DOI] [PubMed] [Google Scholar]
- 7.Twu NF, Chen SS. Five-year follow-up of patients with recurrent postmenopausal bleeding. Zhonghua Yi Xue Za Zhi (Taipei) 2000 Aug;63(8):628–33. [PubMed] [Google Scholar]
- 8.Gull B, Karlsson B, Milsom I, Granberg S. Can ultrasound replace dilation and curettage? A longitudinal evaluation of postmenopausal bleeding and transvaginal sonographic measurement of the endometrium as predictors of endometrial cancer. Am J Obstet Gynecol. 2003 Feb;188(2):401–8. doi: 10.1067/mob.2003.154. [DOI] [PubMed] [Google Scholar]
- 9.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996 Mar;71(3):266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 10.Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P, et al. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer) N Engl J Med. 2005 May 5;352(18):1851–60. doi: 10.1056/NEJMoa043146. [DOI] [PubMed] [Google Scholar]
- 11.Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER Cancer Statistics Review, 1975-2009. National Cancer Institute; Bethesda, MD: 2009. Vintage. Populations. http://seer.cancer.gov/csr/1975_2009_pops09/, based on November 2011 SEER data submission, posted to the SEER web site, April 2012. [Google Scholar]
- 12.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008 Feb 16;371(9612):569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 13.Parazzini F, Negri E, La Vecchia C, Benzi G, Chiaffarino F, Polatti A, et al. Role of reproductive factors on the risk of endometrial cancer. Int J Cancer. 1998 Jun 10;76(6):784–6. doi: 10.1002/(sici)1097-0215(19980610)76:6<784::aid-ijc2>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 14.Armenia CS. Sequential relationship between endometrial polyps and carcinoma of the endometrium. Obstet Gynecol. 1967;30(4):524–9. [PubMed] [Google Scholar]
- 15.Aarnio M, Sankila R, Pukkala E, Salovaara R, Aaltonen LA, de la Chapelle A, et al. Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int J Cancer. 1999 Apr 12;81(2):214–8. doi: 10.1002/(sici)1097-0215(19990412)81:2<214::aid-ijc8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 16.Cohen I. Endometrial polyps in pre-and postmenopausal women: factors associated with malignancy. Maturitas. 2008 Jan 20;59(1):99–100. doi: 10.1016/j.maturitas.2007.08.005. Epub 2007 Dec 26. [DOI] [PubMed] [Google Scholar]
- 17.Lev-Sagie A, Hamani Y, Imbar T, Hurwitz A, Lavy Y. The significance of intrauterine lesions detected by ultrasound in asymptomatic postmenopausal patients. BJOG. 2005 Mar;112(3):379–81. doi: 10.1111/j.1471-0528.2004.00444.x. [DOI] [PubMed] [Google Scholar]
- 18.Kiley J, Hammond C. Combined oral contraceptives: a comprehensive review. Clin Obstet Gynecol. 2007 Dec;50(4):868–77. doi: 10.1097/GRF.0b013e318159c06a. [DOI] [PubMed] [Google Scholar]
- 19.Schlesselman JJ. Risk of endometrial cancer in relation to use of combined oral contraceptives: a practitioner’s guide to meta-analysis. Hum Reprod. 1997 Sep;12(9):1851–63. doi: 10.1093/humrep/12.9.1851. [DOI] [PubMed] [Google Scholar]
- 20.Zighelboim I, Goodfellow PJ, Gao F, Gibb RK, Powell MA, Rader JS, et al. Microsatellite instability and epigenetic inactivation of MLH1 and outcome of patients with endometrial carcinomas of the endometrioid type. J Clin Oncol. 2007 May 20;25(15):2042–8. doi: 10.1200/JCO.2006.08.2107. [DOI] [PubMed] [Google Scholar]
- 21.Kulie T, Slattengren A, Redmer J, Counts H, Eglash A, Schrager S. Obesity and women’s health: an evidence-based review. J Am Board Fam Med. 2011 Jan-Feb;24(1):75–85. doi: 10.3122/jabfm.2011.01.100076. [DOI] [PubMed] [Google Scholar]
- 22.Kaaks R, Lukanova A, Kurzer MS. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiol Biomarkers Prev. 2002 Dec;11(12):1531–43. [PubMed] [Google Scholar]
- 23.Trimble CL, Kauderer J, Zaino R, Silverberg S, Lim PC, Burke JJ, 2nd, et al. Concurrent endometrial carcinoma in women with a biopsy diagnosis of atypical endometrial hyperplasia: a Gynecologic Oncology Group study. Cancer. 2006 Feb 15;106(4):812–9. doi: 10.1002/cncr.21650. [DOI] [PubMed] [Google Scholar]
- 24.Silverberg SG. Problems in the differential diagnosis of endometrial hyperplasia and carcinoma. Mod Pathol. 2000 Mar;13(3):309–27. doi: 10.1038/modpathol.3880053. [DOI] [PubMed] [Google Scholar]
- 25.Lacey JV, Jr, Ioffe OB, Ronnett BM, Rush BB, Richesson DA, Chatterjee N, et al. Endometrial carcinoma risk among women diagnosed with endometrial hyperplasia: the 34-year experience in a large health plan. Br J Cancer. 2008 Jan 15;98(1):45–53. doi: 10.1038/sj.bjc.6604102. Epub 2007 Nov 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pennant S, Manek S, Kehoe S. Endometrial atypical hyperplasia and subsequent diagnosis of endometrial cancer: a retrospective audit and literature review. J Obstet Gynaecol. 2008 Aug;28(6):632–3. doi: 10.1080/01443610802355817. [DOI] [PubMed] [Google Scholar]
- 27.Leitao MM, Jr., Kehoe S, et al. Comparison of D&C and office endometrial biopsy accuracy in patients with FIGO grade 1 endometrial adenocarcinoma. Gynecol Oncol. 2009;113(1):105–108. doi: 10.1016/j.ygyno.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 28.Zaino RJ, Kauderer J, Trimble CL, Silverberg SG, Curtin JP, Lim PC, et al. Reproducibility of the diagnosis of atypical endometrial hyperplasia: a Gynecologic Oncology Group study. Cancer. 2006 Feb 15;106(4):804–11. doi: 10.1002/cncr.21649. [DOI] [PubMed] [Google Scholar]