Background: XIAP is involved in cancer cell proliferation.
Results: The deficiency of XIAP E3 ligase inhibits cancer cell anchorage-independent growth, cell cycle transition, and cyclin D1 transcription, which is mediated by its regulation of PP2A catalytic subunit phosphorylation, thereby activating AP-1.
Conclusion: XIAP E3 ligase mediates cyclin D1 transcription via PP2A-regulated AP-1 activation.
Significance: This study suggests that XIAP E3 ligase can serve as a cancer therapeutic target.
Keywords: AP-1 Transcription Factor, Cyclin D1, E3 Ubiquitin Ligase, PP2A, XIAP
Abstract
The X-linked inhibitor of apoptosis protein (XIAP) is a well known potent inhibitor of apoptosis; however, it is also involved in other cancer cell biological behavior. In the current study, we discovered that XIAP and its E3 ligase played a crucial role in regulation of cyclin D1 expression in cancer cells. We found that deficiency of XIAP expression resulted in a marked reduction in cyclin D1 expression. Consistently, cell cycle transition and anchorage-independent cell growth were also attenuated in XIAP-deficient cancer cells compared with those of the parental wild-type cells. Subsequent studies demonstrated that E3 ligase activity within the RING domain of XIAP is crucial for its ability to regulate cyclin D1 transcription, cell cycle transition, and anchorage-independent cell growth by up-regulating transactivation of c-Jun/AP-1. Moreover, we found that E3 ligase within RING domain was required for XIAP inhibition of phosphatase PP2A activity by up-regulation of PP2A phosphorylation at Tyr-307 in its catalytic subunit. Such PP2A phosphorylation and inactivation resulted in phosphorylation and activation of its downstream target c-Jun in turn leading to cyclin D1 expression. Collectively, our studies uncovered a novel function of E3 ligase activity of XIAP in the up-regulation of cyclin D1 expression, providing significant insight into the understanding of the biomedical significance of overexpressed XIAP in cancer development, further offering a new molecular basis for utilizing XIAP E3 ligase as a cancer therapeutic target.
Introduction
The X-linked inhibitor of apoptosis protein (XIAP4; also known as BIRC4) is the best characterized member of the IAP family and a potent inhibitor of the caspase/apoptosis pathway (1). XIAP expression is elevated in many cancer tissues, including prostate (2); acute and chronic leukemia (3, 4); and other types of cancers (5–7) and is closely related to the progression and aggression of malignant cancer (8, 9). Down-regulation of XIAP renders cancer cells more sensitive to apoptotic induction and reduces their tumorigenicity (10, 11). Our most recent studies show that either knock-out or knockdown of XIAP decreases HCT116 cell migration and invasion in vitro, whereas the reconstitutive expression of XIAP in XIAP−/− HCT116 cells restores cancer cell motility (12), demonstrating a novel function of XIAP in the regulation of cancer cell invasion, which is distinctly different from its well characterized antiapoptotic properties.
The structure of XIAP is characterized by three repeats of the baculovirus IAP repeat (BIR) domain at its NH2 terminus and a RING finger domain near its COOH terminus (13). XIAP exerts its antiapoptotic effect by inactivating several key caspases such as caspases 3, 7, and 9 mainly via BIRs (13). The XIAP RING domain has E3 ubiquitin ligase activity and is able to promote the degradation of proteins by marking them with ubiquitin molecules. Through its E3 ligase, the XIAP RING domain activates signaling cascades influencing cell death (14), inflammation (15), and cell migration (16). Our most recent studies have discovered that XIAP RING domain is able to interact with Rho GDP dissociation inhibitor α protein by which XIAP inhibits Rho GDP dissociation inhibitor α SUMOylation at Lys-138, subsequently up-regulating the activity of small Rho GTPase, cancer cell β-actin polymerization, cytoskeleton reorganization, and cell motilities (12, 17). Our studies also find that XIAP E3 ligase mediates HCT116 cell migration via Rho GDP dissociation inhibitor α SUMOylation at Lys-138 (16). Therefore, elucidation of a novel biological function and identification of its functional domains and mechanisms are of great significance for more fully understanding XIAP in cancer cell biological behavior.
Cyclin D1, a key cell cycle regulator, is required for completion of the G1/S transition in normal mammalian cells. Disruption of the cell cycle control mechanism is a common pathway in human cancer development, and aberrant cyclin D1 expression is one of the most common alterations observed in cancer tissues and cells (18–20). Considering that both XIAP and cyclin D1 are overexpressed in many cancer tissues (21), we investigated the potential role of XIAP in the regulation of cyclin D1 expression and the molecular mechanisms underlying the regulation if it does. The results demonstrated that XIAP positively regulated cyclin D1 transcription via its E3 ligase-mediated phosphorylation of serine/threonine protein phosphatase 2A (PP2A) catalytic subunit at Tyr-307 and c-Jun activation.
EXPERIMENTAL PROCEDURES
Cell Culture and Reagents
Human colon cancer cell lines HCT116 wild-type (WT) and XIAP−/− HCT116 cells as well as their transfectants, including HCT116 WT(vector), XIAP−/−(vector), XIAP−/−(HA-XIAP), XIAP−/−(HA-BIRs), XIAP−/−(HA-ΔRING), and XIAP−/−(HA-XIAP H467A), were established in our previously published studies (12, 16, 17). The HCT116 cells and transfectants were cultured in McCoy's 5A medium (Invitrogen) supplemented with 10% FBS and penicillin/streptomycin. All cells were maintained in a humidified incubator at 37 °C with a 5% CO2 humidified atmosphere. Antibodies against, phospho-c-Jun, c-Jun, phospho-JNKs, JNKs, phospho-ERKs, ERKs, phospho-p38, p38, PP2A A subunit, and GAPDH were purchased from Cell Signaling Technology Inc. (Beverly, MA). The antibodies against cyclin D1, cyclin-dependent kinase 4 (CDK4), p65, c-Fos, and Fra1 were bought from Santa Cruz Biotechnology (Santa Cruz, CA). The antibodies against p27 and p50 were purchased from Abcam (Cambridge, MA). The antibody against HA was obtained from Covance Inc. (Princeton, NJ). The antibodies against phospho-PP2A and PP2A C subunit were from Epitomics (Burlingame, CA). The antibody against XIAP was purchased from BD Biosciences.
Plasmids and Stable Cell Transfection
The constructs −963 cyclin D1 promoter-driven luciferase reporter (−963 CD1 Luc) and its AP-1 binding site mutant reporter (−963 AP-1 mut CD1 Luc) were gifts from Dr. Richard G Pestell, Thomas Jefferson University Jefferson Medical College. The plasmid TAM67, a well characterized dominant negative c-Jun mutant (22); cyclin D1 promoter-driven luciferase reporter (23); and AP-1-dependent luciferase reporter (24) were described in our previous studies. Human XIAP- and PP2A catalytic α subunit-specific shRNA constructs were purchased from Open Biosystems (Pittsburgh, PA). For stable transfection, cultures were subjected to blasticidin or hygromycin selection, and cells surviving the antibiotic selection were pooled as stable mass transfectants. These stable transfectants were cultured in the selected antibiotic-free medium for at least two passages before utilization for experiments.
Anchorage-independent Growth Assay
Anchorage-independent growth ability was determined in soft agar as described in our previous studies (25). Briefly, 3 ml of 0.5% agar in basal modified Eagle's medium supplemented with 10% FBS was layered onto each well of 6-well tissue culture plates. Cells (3 × 104 cells) suspended in 1 ml of normal medium were mixed with 2 ml of 0.5% agar in basal modified Eagle's medium supplemented with 10% FBS, and 1 ml of mixture was added into each well on top of the 0.5% agar layer. Plates were incubated at 37 °C in 5% CO2 for 2–3 weeks, and the colonies with more than 32 cells were scored and are presented as colonies/104 cells.
Cell Cycle Analysis
After treatment, cells were harvested and fixed with 5 ml of ice-cold 80% ethanol overnight. The fixed cells were washed twice with PBS and then suspended in propidium iodide staining solution (50 mg/ml propidium iodide, 10 mg/ml RNase A, and 0.1% Triton X-100) (Sigma) for at least 1 h at 4 °C. The DNA content was determined by flow cytometry using the Epics XL FACS (Beckman Coulter, Miami, FL) and EXPO 32 software as described in previous studies (23).
Cell Proliferation Assay
Confluent monolayers of cells were trypsinized, and 1 × 103 viable cells suspended in 100 μl of medium were added to each well of 96-well plates. After adherence, cells were synchronized by replacing regular culture medium with 0.1% FBS medium and cultured for another 24 h. The proliferation of the cells was determined using the CellTiter-Glo® Luminescent Cell Viability Assay kit (Promega, Madison, WI) with a luminometer (Wallac 1420 Victor2 multipliable counter system) as described in our publications (23, 26).
Western Blot
Cell extracts were prepared with cell lysis buffer (10 mm Tris-HCl, pH 7.4, 1% SDS, and 1 mm Na3VO4), and protein concentrations were determined with a NanoDrop 1000 spectrophotometer (Thermo Scientific, Wilmington, DE). Proteins (30–60 μg) were subjected to SDS-PAGE and subsequently probed with the indicated primary antibodies and alkaline phosphatase-conjugated second antibody (Cell Signaling Technology Inc.) as described in our publications (22, 27). Signals were detected using an enhanced chemifluorescence Western blot system (Model Storm 860, GE Healthcare) as described in our previous publications (28, 29).
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Total RNA was extracted with TRIzol reagent (Invitrogen), and cDNAs were synthesized with the ThermoScript RT-PCR system (Invitrogen). The human cyclin D1 cDNA fragments were amplified by primers 5′-GAG GTC TGC GAG GAA CAG AAG TG-3′ and 5′-GAG GGC GGA TGG AAA TGA ACT TCA-3′, and two oligonucleotides (5′-AGA AGG CTG GGG CTC ATT TG-3′ and 5′-AGG GGC CAT CCA CAG TCT TC-3′) were used as the specific primers to amplify human GAPDH cDNA as a loading control.
Luciferase Reporter Assay
Cells stably transfected with luciferase reporter constructs were seeded into 96-well plates. After the cell density reached 70–80%, cells were treated as indicated in the figure legends and then extracted with luciferase assay lysis buffer (Promega). The luciferase activity was determined with the Dual-Luciferase Reporter Assay System according to the manufacturer's instructions as described (30, 31).
Immunoprecipitation
Total cell lysate was incubated with 2 μg of anti-GFP antibody for 2 h at 4 °C. Protein A/G PLUS-agarose (40 μl) was added into the mixture and incubated with agitation for an additional 4 h at 4 °C as described (28, 32). The immunoprecipitated agarose beads were washed with the cell lysis buffer three times and subjected to Western blot assay.
Statistical Methods
Student's t test was used to determine the significance of differences between different groups from each experiment. The differences were considered significant at p < 0.05.
RESULTS
XIAP and Its E3 Ligase Played an Important Role in Anchorage-independent Growth and Cell Cycle G1/S Progression of Colon Cancer Cells
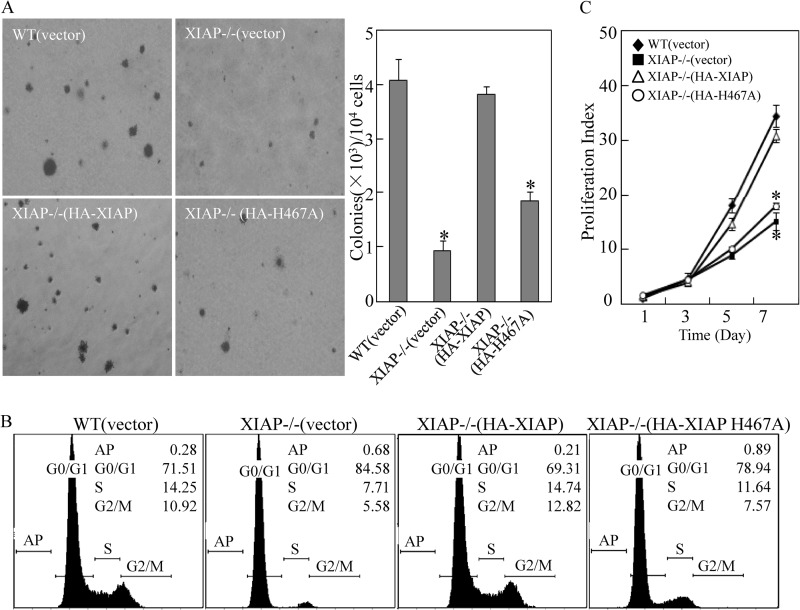
It has been verified that XIAP has additional biological functions that do not rely on its inhibition of apoptosis (12, 33). To delineate the biological significance of XIAP in cancer cell proliferation, HCT116 XIAP-deficient (XIAP−/−) transfectants with XIAP (HA-XIAP) and XIAP H467A, a point mutation resulting in loss of its E3 ubiquitin ligase activity (34), were seeded in soft agar for determination of its anchorage-independent growth capability compared with WT(vector) and XIAP−/−(vector). As shown in Fig. 2A, the expression level of XIAP, HA-XIAP, and its various deletions and point mutation were identified in the stable HCT116 transfectants. The deficiency of XIAP expression in colon cancer cells resulted in a dramatic reduction of anchorage-independent growth capability as compared with that observed with WT(vector) cells (Fig. 1A). The inhibition of size and frequency of colonies in XIAP−/−(vector) cells could be completely restored by reconstituted expression of HA-XIAP in XIAP−/− cells (XIAP−/−(XIAP)), whereas ectopic expression of HA-XIAP H467A in XIAP−/− cells (XIAP−/−(XIAP H467A)) did not show observable restoration (Fig. 1A). These results suggest that XIAP and its E3 ligase activity play an essential role in anchorage-independent growth of HCT116 cells. In view of the association of cell cycle with cancer cell growth, we further explored the effect of XIAP and its E3 ligase on cell cycle progression. Consistent with its effects on anchorage-independent growth, XIAP deficiency resulted in a significant growth arrest at the G0/G1 phase, and the reconstituted HA-XIAP H467A cells displayed a similar G0/G1 alteration, whereas ectopic expression of HA-XIAP restored cell cycle proportions similar to those in WT(vector) cells (Fig. 1B). Furthermore, the results obtained from the cell proliferation assay fully supported the notion that XIAP and its E3 ligase activity played a key role in cancer cell growth (Fig. 1C).
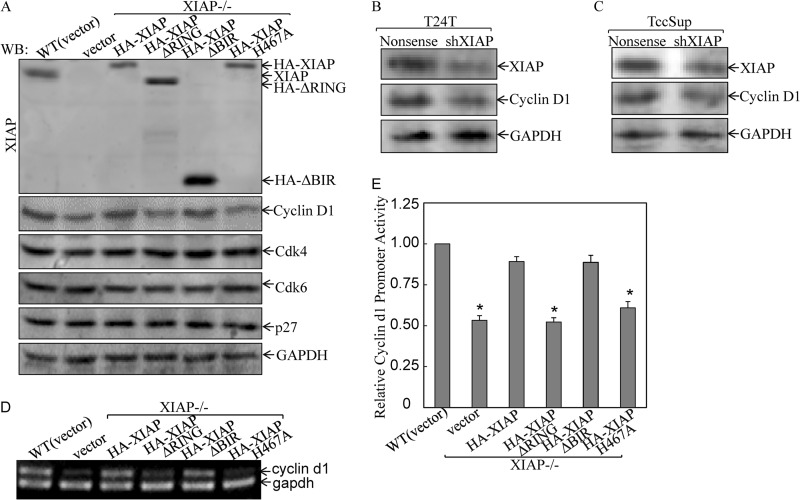
FIGURE 2.
XIAP E3 ligase regulated cyclin D1 expression at transcription level. A, after synchronization in 0.1% FBS medium, the indicated cells were cultured in 2% FBS medium for another 24 h, and the cell extracts were then subjected to Western blot (WB) as indicated. B and C, T24T and TCCSUP cells were stably transfected with XIAP shRNA (shXIAP), and the stable transfectant extracts were subjected to Western blot as indicated. D, the indicated cells were cultured in 2% FBS for 24 h synchronization. The cells were then used to determine mRNA expression by RT-PCR as described in our previous studies (55). E, cells (1 × 103) stably transfected with cyclin D1 promoter-driven luciferase reporter were seeded into each well of a 96-well plate. After synchronization, cells were cultured in 2% FBS medium for 24 h and then extracted for determination of luciferase activity (27). The results are presented as luciferase activity relative to WT HCT116 cells. The asterisk (*) indicates a significant inhibition of cyclin D1 transcription compared with WT(vector) cells (p < 0.05). Error bars represent S.D.
FIGURE 1.
XIAP and its E3 ligase promoted anchorage-independent growth of colon cancer HCT116 cells. A, HCT116 cell transfectants were seeded in soft agar as described under “Experimental Procedures.” Colonies are expressed as mean ± S.D. from five assays of three independent experiments. The asterisk (*) indicates a significant decrease compared with WT(vector) cells (p < 0.05). B, after synchronization in 0.1% FBS medium for 24 h, cells were cultured in 2% FBS medium for another 24 h and then subjected to cell cycle analysis by flow cytometry as described in our previous studies (23). The results represent one of three independent experiments. C, the proliferation rates were determined in the indicated cells using a CellTiter-Glo Luminescent Cell Viability Assay kit. Results are presented as the mean ± S.D. of the triplicate assays. The asterisk (*) indicates a significant inhibition of proliferation index compared with WT(vector) cells (p < 0.05). Error bars represent S.D.
XIAP RING Domain and Its E3 Ligase Activity Were Required for Cyclin D1 Expression in Cancer Cells
It is known that cyclins, CDKs, and CDK inhibitors are responsible for regulation of G1 to S transition (35). Therefore, we compared protein expression of cyclins and CDKs among various stable transfectants. To exclude the possibility that the altered levels of cyclin D1 were due to differences in cell cycle distribution, cells were synchronized at G0 (24-h serum starvation) followed by release from cell cycle arrest by addition of 2% serum and subsequent inspection of cyclin D1 protein levels. The results showed that deficiency of XIAP repressed expression of cyclin D1 (Fig. 2A). Reconstituted expression of XIAP in XIAP−/− cells completely resumed cyclin D1 protein expression, indicating that XIAP plays an essential role in the mediation of cyclin D1 protein expression. Ectopic expression of XIAP with deletion of all three BIR domains (XIAPΔBIR) could also resume cyclin D1 expression, whereas expression of XIAPΔRING or XIAP H467A did not show observable restoration of cyclin D1 expression in XIAP−/− cells (Fig. 2A), demonstrating that RING domain and its E3 ligase, but not BIRs, were crucial for cyclin D1 expression. Unlike cyclin D1 expression, the expression levels of CDK4, CDK6, and p27 were almost the same across all three cell lines (Fig. 2A). These results suggest that XIAP E3 ligase deficiency directly causes the reduction of cyclin D1 expression rather than affecting other regulators of G0/G1 phase checkpoints. To verify this notion, we stably transfected specific shRNA targeting human XIAP in human bladder cancer T24T and TCCSUP cells, and the stable transfectants were established and identified (Fig. 2, B and C). The results indicated that knockdown of XIAP expression also reduced cyclin D1 expression in both T24T and TCCSUP cancer cells (Fig. 2, B and C), suggesting that XIAP regulation of cyclin D1 expression was not HCT116 cell-specific.
To elucidate the molecular mechanisms underlying the cyclin D1 regulation by XIAP RING domain and its E3 ligase, we evaluated cyclin D1 mRNA levels among various transfectants. Consistent with cyclin D1 protein expression, cyclin D1 mRNA expression was profoundly down-regulated in XIAP−/−(vector), XIAP−/−(XIAPΔRING), and XIAP−/−(XIAP H467A) cells as compared with that in either WT(vector) or XIAP−/−(XIAP) cells (Fig. 2D). Moreover, the results from determination of cyclin D1 promoter-driven luciferase reporter activity indicated that cyclin D1 promoter activity was markedly reduced in XIAP−/−(vector), XIAP−/−(XIAPΔRING), and XIAP−/−(XIAP H467A) cells compared with WT(vector), XIAP−/−(XIAP), and XIAP−/−(XIAPΔBIR) cells (Fig. 2E), indicating that RING domain and its E3 ligase were critical for XIAP regulation of cyclin D1 expression at the transcription level.
c-Jun/AP-1, but Not NFκB, Mediated XIAP Up-regulation of Cyclin D1 Expression
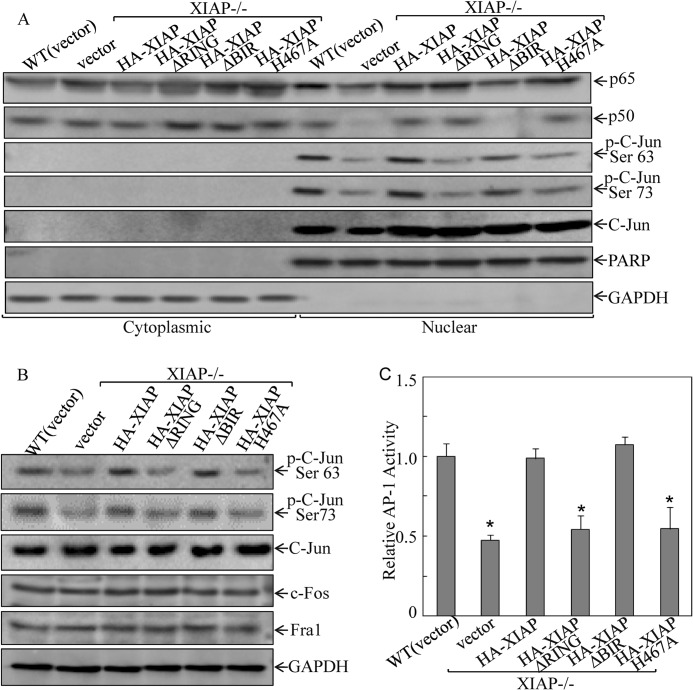
The next experiment was designed to identify the transcription factor responsible for XIAP E3 ligase-mediated cyclin D1 transcription. Because previous studies have demonstrated that NFκB and AP-1 are two key transcription factors in the mediation of cyclin D1 transcription (23, 36, 37) and because BIR domains of XIAP have been reported to be crucial for NFκB activation (38), cytoplasmic and nuclear extracts were prepared, and the nuclear/cytoplasm distribution of NFκB components (p65 and p50) was examined by Western blot in comparison with the distribution of another transcription factor, c-Jun, which is a major component of AP-1, in the transfectants. Consistent with the reported BIR domain regulation of NFκB activation (38), our results showed that XIAP deletion or BIR domain deficiency reduced the nuclear/cytoplasm ratio of either p65 or p50 protein levels in comparison with those in WT cells, whereas RING domain deletion did not show this regulatory effect (Fig. 3A). Very interestingly, XIAP RING domain and its E3 ligase were found to be crucial for c-Jun phosphorylation and activation, although they did not affect c-Jun protein expression and distribution between the nucleus and cytoplasm, and BIR domain was not involved in the XIAP regulation of c-Jun phosphorylation (Fig. 3A). Our results suggest that XIAP expression was essential for its mediation of c-Jun phosphorylation at Ser-63 and Ser-73 rather than regulation of nuclear/cytoplasm distribution and expression of c-Jun protein. During the comparison of cyclin D1 expression with either nuclear c-Jun phosphorylation or NFκB nuclear/cytoplasm distribution among the cells transfected with XIAP and its various deletions, it was noted that cyclin D1 expression and c-Jun phosphorylation at Ser-63/73 were consistently regulated by XIAP and its RING domain and E3 ligase as well, which is distinctly different from BIR domain regulation of p65/p50 nuclear/cytoplasm distribution. Thus, we anticipated that c-Jun phosphorylation at Ser-63/73 mediated by RING domain and its E3 ligase might be associated with cyclin D1 up-regulation, whereas BIR-regulated NFκB is not involved in cyclin D1 transcription. Consistent with our findings of XIAP regulation of c-Jun phosphorylation in nuclear extract, deletion of RING domain or inactivation of E3 ligase by point mutation resulted in loss of their regulation of c-Jun phosphorylation at Ser-63/73 in whole cell extracts without affecting the expression of other AP-1 components, such as c-Fos and Fra1 (Fig. 3B). Given the finding that XIAP RING domain and its E3 ligase regulated c-Jun phosphorylation at Ser-63/73, we examined whether XIAP RING domain and its E3 ligase also modulated AP-1 transactivation. The results showed that deletion of either XIAP or RING domain or point mutation of E3 ligase attenuated AP-1-dependent transactivation, whereas BIR domain deletion showed an AP-1 transactivation similar to that of full-length XIAP (Fig. 3C). Together, our results demonstrate that c-Jun/AP-1 activation and cyclin D1 expression are consistently dependent on RING domain and its E3 ligase, whereas NFκB nuclear translocation is associated with BIR domain. However, XIAP regulates both c-Jun/AP-1 and NFκB in HCT116 cells.
FIGURE 3.
XIAP and its E3 ligase regulated c-Jun phosphorylation and AP-1 transactivation. A, cells (5 × 105) were seeded into 10-cm dishes. After synchronization, cells were cultured in 2% FBS medium for 12 h and extracted for isolation of cytoplasmic and nuclear fractions according to the protocol of the nuclear/cytosol fractionation kit (Biovision Inc.). The isolated protein fractions were subjected to Western blot for determination of protein expression. B, cells (1 × 105) were seeded into each well of a 6-well plate. After synchronization, cells were cultured in 2% FBS medium for another 12 h, then extracted, and subjected to Western blot. C, cells (1 × 103) stably expressing AP-1-luciferase reporter were seeded into each well of a 96-well plate. After synchronization, cells were cultured in 2% FBS medium for 18 h and then extracted for determination of luciferase activity as described previously (27). The asterisk (*) indicates a significant inhibition of AP-1 activity compared with WT(vector) cells (p < 0.05). Error bars represent S.D. PARP, poly(ADP-ribose) polymerase.
Ectopic Expression of Dominant Negative Mutant c-Jun (TAM67) Abrogated XIAP-regulated Cyclin D1 Expression
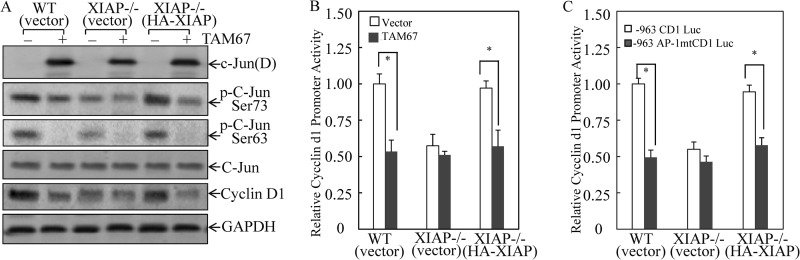
To obtain direct evidence of c-Jun/AP-1 in XIAP regulation of cyclin D1 transcription, we used TAM67, a dominant negative mutant form of c-Jun with a transactivation domain deletion (39). Ectopic expression of TAM67 was identified by detection of expression of the 29-kDa c-Jun mutant (c-Jun(D)), and TAM67 expression exhibited a dramatic inhibition of c-Jun phosphorylation at Ser-73 and impaired c-Jun phosphorylation at Ser-63 in both WT and XIAP−/−(HA-XIAP) cells (Fig. 4A). It was noted that the ectopic expression of TAM67 did not show much inhibition of c-Jun phosphorylation at Ser-73 and Ser-63 in XIAP−/−(vector) cells (Fig. 4A) because deficient XIAP expression in the cells had already resulted in a dramatic reduction of c-Jun phosphorylation at Ser-73 and Ser-63, which was consistent with the results observed in Fig. 3A. Moreover, inhibition of c-Jun phosphorylation by TAM67 also attenuated cyclin D1 protein expression in the same cells (Fig. 4A). Consistent with cyclin D1 protein expression, ectopic TAM67 expression in WT or XIAP−/−(HA-XIAP) cells inhibited cyclin D1 transcription to a level as low as that observed in XIAP−/− cells, suggesting that c-Jun activation is responsible for almost 100% of XIAP-mediated cyclin D1 transcription (Fig. 4B). To further validate the important role of AP-1 in XIAP-mediated cyclin D1 transcription activity, we transfected cyclin D1 promoter-Luc reporter (−963 CD1 Luc) and this reporter with AP-1 binding site mutation (−963 AP-1 mut CD1 Luc) into WT(vector), XIAP−/−(vector), and XIAP−/−(XIAP) cells, respectively. As shown in Fig. 4C, deficient XIAP expression in XIAP−/− cells led to a dramatic reduction of cyclin D1 promoter transcription activity in comparison with that in WT(vector) or XIAP−/−(XIAP) cells that were transfected with wild-type −963 cyclin D1 promoter-luciferase reporter. The XIAP-mediated cyclin D1 promoter transcription activity was completely abolished in WT(vector) and XIAP−/−(XIAP) cells transfected with −963 cyclin D1 promoter with mutated AP-1 binding site (Fig. 4C). These results demonstrate that c-Jun/AP-1 is crucial for XIAP-dependent up-regulation of cyclin D1 transcription.
FIGURE 4.
c-Jun/AP-1 activation mediated by XIAP E3 ligase was crucial for XIAP regulation of cyclin D1 transcription. A, the TAM67 construct or the empty vector was stably transfected into the indicated cells, and stable transfectants were established. After synchronization, cells were cultured in 2% FBS medium for another 24 h and then extracted for Western blot as indicated. B, the cyclin D1 promoter-luciferase reporters were transfected into the indicated cells, and stable transfectants were established. After synchronization, cells were cultured in 2% FBS medium for 24 h and then extracted for determination of luciferase activity. The results are presented as cyclin D1 promoter activity relative to WT(vector) without transfection of TAM67. The asterisk (*) indicates a significant inhibition of cyclin D1 promoter activity compared with control vector transfectant (p < 0.05). C, the cells were transfected with −963 CD1 Luc and −963 AP-1 mut CD1 Luc, respectively, and stable transfectants were established. After synchronization, cells were cultured in 2% FBS medium for 24 h and then extracted for luciferase activity assay. The results are presented as cyclin D1 promoter activity relative to the WT −963 CD1-Luc reporter transfectant. The asterisk (*) indicates a significant inhibition of cyclin D1 promoter activity compared with the WT −963 CD1-Luc reporter transfectant (p < 0.05). Error bars represent S.D.
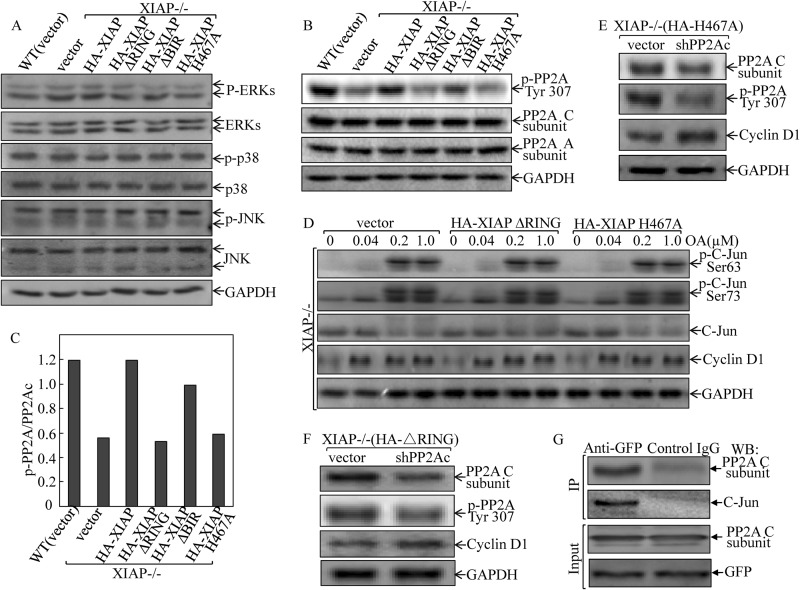
XIAP RING Domain and Its E3 Ligase Regulated c-Jun Phosphorylation via Up-regulating Phosphatase 2A Phosphorylation at Tyr-307
To elucidate the mechanism underlying the regulation of the c-Jun phosphorylation by the XIAP RING domain and its E3 ligase, we next examined whether or not this signaling pathway was the result of c-Jun upstream kinase activation (40). We compared the activation of MAPK family members, including JNKs, p38, and ERKs, among the various XIAP transfectants. The results showed that there was no difference in any of the JNKs, p38, and ERKs among various transfectants (Fig. 5A), indicating that XIAP regulation of c-Jun phosphorylation was not through targeting of its upstream MAPKs. Because c-Jun phosphorylation is also regulated by PP2A (41), we compared the PP2A expression and phosphorylation at Tyr-307. The results did show a consistent requirement of XIAP and its RING domain as well as its E3 ligase for mediation of PP2A phosphorylation at Tyr-307, whereas deletion of BIR domain in XIAP did not show an observable impact on PP2A phosphorylation (Fig. 5, B and C). In contrast, total protein expression levels of A and C subunits of PP2A were comparable in all transfectants (Fig. 5B). Because PP2A phosphorylation at Tyr-307 inhibits PP2A phosphatase activity (42), we therefore anticipated that XIAP expression would attenuate PP2A phosphatase activity on c-Jun phosphorylation by increasing PP2A phosphorylation at Tyr-307 and that RING domain and its E3 ligase are required for this XIAP biological function. To test these notions, we treated XIAP−/−(vector), XIAP−/−(XIAPΔRING), and XIAP−/−(XIAP H467A) cells with PP2A inhibitor okadaic acid to see whether inhibition of PP2A activity could lead to an increase in c-Jun phosphorylation at Ser-63/73 and cyclin D1 expression. The results indicated that inhibition of PP2A activity by okadaic acid led to a marked induction of c-Jun phosphorylation at Ser-63/73 and an increase in cyclin D1 expression in all three cell lines (Fig. 5D). Furthermore, we used shRNA targeting the PP2A catalytic subunit to specifically knock down PP2A expression. The result showed that the knockdown of PP2A expression led to an increase in cyclin D1 expression in XIAP−/−(XIAPΔRING) and XIAP−/−(XIAP H467A) cells (Fig. 5, E and F). In addition, we carried out a co-immunoprecipitation assay to determine whether PP2A could interact with and bind to c-Jun protein. The GFP-c-Jun transfectant was used to carry out the immunoprecipitation assay. As shown in Fig. 5G, the C subunit of PP2A was present in the complex co-immunoprecipitated using anti-GFP antibodies, suggesting that the PP2A C subunit could interact with c-Jun in the intact cells. These results are consistent with previous reports demonstrating that PP2A binds to c-Jun in rat keratinocytes under non-stress condition (43) and regulates c-Jun phosphorylation (41, 44). Taken together, our results strongly indicate that the inhibition of PP2A activity via mediation of its phosphorylation at Tyr-307 by XIAP RING domain and E3 ligase results in increased c-Jun phosphorylation at Ser-63/73 and AP-1 transactivation in turn leading to cyclin D1 transcription and protein expression in XIAP-expressing cancer cells.
FIGURE 5.
XIAP E3 ligase up-regulated protein phosphatase 2A phosphorylation at Tyr-307 and mediated c-Jun phosphorylation at Ser-63/Ser-73. A and B, after synchronization, cells were cultured in 2% FBS medium for 12 h and extracted for Western blot. The results shown in B were quantified using Quantity One software and are presented as the ratio of phospho (p)-PP2A Tyr-307 to PP2A C subunit (C). D, cells (1 × 105) were seeded into each well of 6-well plates. After synchronization, cells were treated with the indicated concentration of okadaic acid (OA) for 8 h in regular medium and then extracted for Western blot. E and F, HCT116 XIAP−/−(XIAPΔRING) and XIAP−/−(XIAP H467A) cells were stably transfected with PP2A catalytic subunit-specific shRNA (shPP2A), and the stable transfectants were cultured in 2% FBS for 24 h after synchronization. The cell extracts were subjected to Western blot (WB) as indicated. G, 293T cells were transfected with the GFP-c-Jun construct. 36 h after transfection, the whole cell lysates were subjected to co-immunoprecipitation (IP) assay using anti-GFP antibody. The immunoprecipitates were subjected to Western blot as indicated.
Ectopic Constitutive Expression of Cyclin D1 Rescued Anchorage-independent Growth and G1/S Phase Transition in XIAP-deficient and E3 Ligase-deficient Colon Cancer Cells
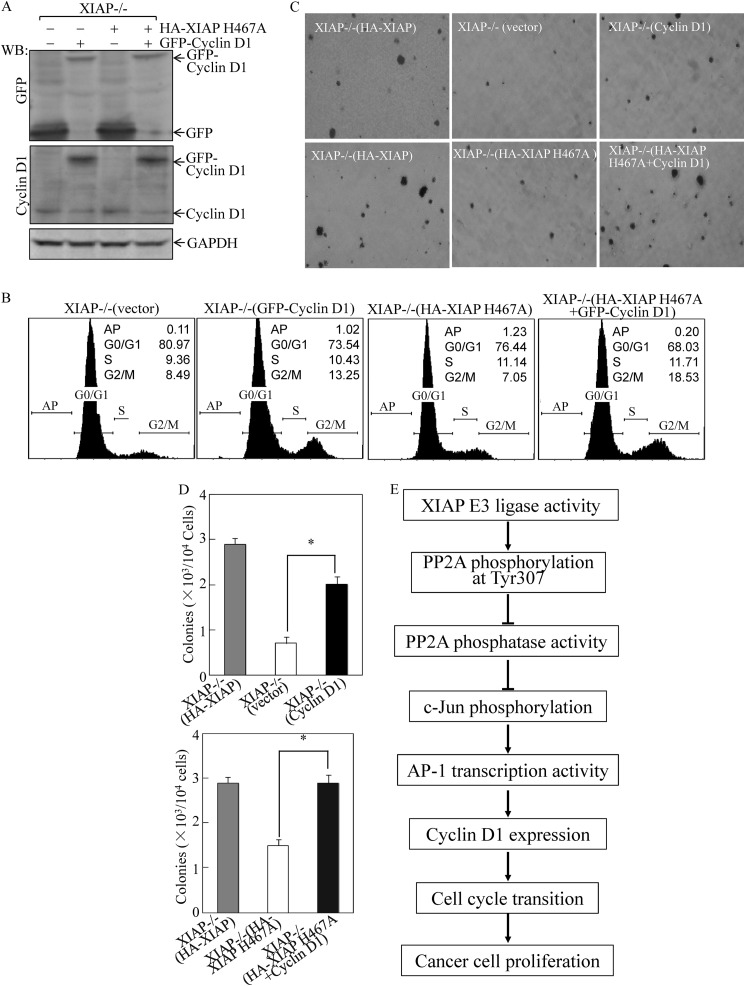
The above results showed that the XIAP-deficient or XIAP E3 ligase-deficient cancer cells failed to exhibit normal anchorage-independent growth and G1/S phase transition with a marked reduction of cyclin D1 protein expression. To test whether the decreased cyclin D1 expression was responsible for attenuation of anchorage-independent growth and cell cycle progression, we transfected GFP-cyclin D1 expression construct into XIAP−/− cells and HA-XIAP H467A transfectants. Ectopic expression of GFP-cyclin D1 in XIAP−/− cells or XIAP−/−(XIAP H467A) cells was demonstrated by Western blot using anti-GFP and anti-cyclin D1 antibodies (Fig. 6A). Ectopic expression of GFP-cyclin D1 in either XIAP−/− cells or XIAP−/−(XIAP H467A) cells promoted cell cycle progression (Fig. 6B) and increased the anchorage-independent growth capability (Fig. 6, C and D) as compared with those observed in their control vector transfectants. In addition, it has been reported that that cyclin D1 expression in human colon cancer cells was a key factor for the growth and tumorigenicity of those colon cancer cells (45). Thus, we conclude that E3 ligase-regulated cyclin D1 expression is crucial for XIAP-mediated cell cycle progression and anchorage-independent growth of cancer cells.
FIGURE 6.
Ectopic expression of GFP-cyclin D1 restored cell cycle progression and anchorage-independent growth of HCT116 XIAP−/−(vector) and XIAP−/−(XIAP H467A) cells. HCT116 XIAP−/−(vector) and XIAP−/−(XIAP H467A) cells were transfected with the GFP-cyclin D1-expressing plasmid or empty vector, and stable transfectants were established by blasticidin selection. A and B, after synchronization in 0.1% FBS medium, cells were cultured in 2% FBS medium for 24 h. Cells were then extracted for Western blot (WB) as indicated (A) or subjected to flow cytometry analysis (B). C and D, stable transfectants as indicated were subjected to anchorage-independent growth assay in soft agar. The colony formation was photographed (C), and the colonies are expressed as mean ± S.D. from five assays of three-independent experiments (D). The asterisk (*) indicates a significant increase compared with XIAP−/−(vector) or XIAP−/−(XIAP H467A) cells (p < 0.05). Error bars represent S.D. E, the proposed model for XIAP regulation of cancer cell proliferation.
DISCUSSION
Although known for its ability to regulate caspases and apoptosis, XIAP also participates in other diverse cellular functions, including signal activation, copper metabolism, and ubiquitination (46). XIAP is differently up-regulated in many forms of human cancers and confers resistance to chemotherapy-induced cell death (13). Among the IAPs, XIAP is the most potent protein as a cancer therapeutic target. It has been reported that inhibition of XIAP by a chemical inhibitor or small interfering RNA (siRNA) reduces cancer cell growth (47, 48). However, whether these effects of XIAP are direct or rather secondary effects resulting from the induction of other gene expression remains unknown. In the current study, we investigated the role of XIAP in cell cycle progression and demonstrated the activity of XIAP E3 ligase and its downstream signaling components, including PP2A and AP-1, in regulating cyclin D1 expression. We found that the decreased cyclin D1 expression is responsible for the retardation of anchorage-independent growth of XIAP−/− cancer cells. This is the first demonstration that XIAP regulates cancer cell growth via transcriptional positive modulation of cyclin D1 expression.
Cyclin D1 as a cofactor for CDK4 and CDK6 is essential in the transition from G1 phase to S phase. Overexpression of cyclin D1 occurs in one-third or more of colorectal cancers (49, 50). Molecular understanding of cyclin D1 regulation is important in understanding carcinogenic mechanisms of cancer development. Our results showed that knock-out of XIAP in cancer cells decreased cyclin D1 expression, cell cycle transition, and anchorage-independent cell growth. Reconstitutive expression of XIAP in XIAP−/− cells restored these biological effects. D-type cyclins are believed to serve as links between the extracellular environment and the core cell cycle machinery (51, 52). Thus, we anticipate that XIAP might function as a mediator of the signaling pathway between an environmental factor (present in the serum) and the cell cycle machinery by regulating cyclin D1 transcription. Considering that both XIAP and cyclin D1 are overexpressed in many cancer tissues, these results further enable us to explore the potential utilization of XIAP as a target for cancer therapy.
XIAP exerts its antiapoptotic effect by inactivating several key caspases mainly via BIRs (13). In addition, XIAP also contains a RING finger that provides it with ubiquitin ligase (E3) activity. Over the last few years, the E3 ligase activity of XIAP has emerged as a signaling cascade that influences cell death (14), inflammation (15), and migration (16). In this study, we found that expression of XIAP with an E3 ligase-defective point mutant (H467A) in XIAP−/− cells did not rescue decreased cyclin D1 expression, cell cycle transition, or anchorage-independent growth in comparison with the wild-type XIAP. Our results demonstrate a crucial role of E3 ligase in XIAP regulation of malignancy behavior of cancer cells. Thus, the E3 ligase in XIAP RING domain could be a valuable target for cancer therapy.
Identification of the signaling pathways underlying XIAP E3 ligase regulation of cyclin D1 transcription reveals that c-Jun phosphorylation and AP-1 transcription activity are inhibited in XIAP E3 ligase-defective cells. AP-1 is a canonical regulator of transcription of cyclin D1 (37). Deficiency of XIAP E3 ligase activity repressed AP-1 transcriptional activity (Fig. 3C), and the AP-1 binding site mutation could revoke the cyclin D1 up-regulation by XIAP (Fig. 4C). Thus, XIAP E3 ligase may function to activate AP-1 and induce expression of cyclin D1. Expression of cyclin D1 can also be regulated by other transcriptional factors, including NF-κB (23). Although the XIAP BIR domain is related to NF-κB transcription activity, NF-κB is not involved in XIAP-induced transcription of cyclin D1 in cancer cells.
With regard to the mechanism by which XIAP E3 ligase regulates the c-Jun phosphorylation/AP-1 transactivation that subsequently leads to cyclin D1 transcription and expression, we found that the serine/threonine phosphatase PP2A plays an essential role in the mediation of these observed biological effects. Our results show that the XIAP RING domain and E3 ligase are crucial for PP2A phosphorylation at Tyr-307, suggesting that the XIAP RING domain and its E3 ligase are required for XIAP inhibition of PP2A phosphatase activity. Our results also show that the defect of cyclin D1 transcription and expression in XIAP−/−(XIAPΔRING) and XIAP−/−(XIAP H467A) is due to a deficiency of E3 ligase in both cells that results in loss of E3 ligase up-regulation of PP2A phosphorylation at Tyr-307, subsequently leading to the increased PP2A phosphatase activity. Although the molecular mechanism underlying XIAP and its E3 ligase up-regulation of PP2A phosphorylation at Tyr-307 is not understood, there are two possibilities. 1) XIAP may act as an adaptor molecule to recruit a tyrosine phosphatase, thus facilitating the dephosphorylation of PP2A proteins. This scenario would be similar to XIAP E3 ligase facilitating the formation of complexes between TAK1-binding protein 1 and IκB kinase β that enables TGF-β to activate p65/RelA (53). 2) PP2A may be the target of XIAP RING domain-mediated ubiquitination, and this ubiquitination may further affect PP2A phosphorylation at Tyr-307. This would be like Itch, a ubiquitin E3 ligase, that mediates Smad2 ubiquitination and regulates Smad2 phosphorylation independently of changes in Smad2 protein levels (54). Further elucidation of mechanisms underlying XIAP E3 ligase regulation of PP2A phosphorylation at Tyr-307 that are independent of proteasome-dependent degradation is of significance and is a current project underway in our laboratory.
In summary, our current studies elucidate a novel scenario in which the XIAP E3 ligase leads to phosphatase PP2A phosphorylation at Tyr-307, which in turn results in c-Jun phosphorylation at Ser-63/Ser-73 and AP-1 activation, further affecting cyclin D1 transcription and protein expression by which XIAP E3 ligase regulates HCT116 cancer cell cycle progression and anchorage-independent growth. Considering that both XIAP and cyclin D1 are overexpressed in many cancer tissues (21), these results further enable us to explore the potential utilization of XIAP as a target for cancer therapy. Because the biological effects of XIAP are mediated by its E3 ligase, our study also offers valuable insight into the understanding of XIAP-mediated cancer cell anchorage-independent growth, further enabling us to explore the utilization of the E3 ligase of XIAP as a target for cancer therapy.
Acknowledgment
We thank Dr. Richard G. Pestell from Thomas Jefferson University Jefferson Medical College for the generous gift of cyclin D1 luciferase reporters.
This work was supported, in whole or in part, by National Institutes of Health Grants CA112557 from the NCI and ES000260 from the NIEHS. This work was also supported by National Natural Science Foundation of China Grants NSFC81229002 and NSFC9102970.
- XIAP
- X-linked inhibitor of apoptosis protein
- AP-1
- activator protein 1
- CDK
- cyclin-dependent kinase
- PP2A
- protein phosphatase 2A
- IAP
- inhibitor of apoptosis protein
- BIR
- baculovirus IAP repeat
- SUMO
- small ubiquitin-like modifier.
REFERENCES
- 1. Srinivasula S. M., Ashwell J. D. (2008) IAPs: what's in a name? Mol. Cell 30, 123–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Krajewska M., Krajewski S., Banares S., Huang X., Turner B., Bubendorf L., Kallioniemi O. P., Shabaik A., Vitiello A., Peehl D., Gao G. J., Reed J. C. (2003) Elevated expression of inhibitor of apoptosis proteins in prostate cancer. Clin. Cancer Res. 9, 4914–4925 [PubMed] [Google Scholar]
- 3. Byrd J. C., Kitada S., Flinn I. W., Aron J. L., Pearson M., Lucas D., Reed J. C. (2002) The mechanism of tumor cell clearance by rituximab in vivo in patients with B-cell chronic lymphocytic leukemia: evidence of caspase activation and apoptosis induction. Blood 99, 1038–1043 [DOI] [PubMed] [Google Scholar]
- 4. Akyurek N., Ren Y., Rassidakis G. Z., Schlette E. J., Medeiros L. J. (2006) Expression of inhibitor of apoptosis proteins in B-cell non-Hodgkin and Hodgkin lymphomas. Cancer 107, 1844–1851 [DOI] [PubMed] [Google Scholar]
- 5. Kluger H. M., McCarthy M. M., Alvero A. B., Sznol M., Ariyan S., Camp R. L., Rimm D. L., Mor G. (2007) The X-linked inhibitor of apoptosis protein (XIAP) is up-regulated in metastatic melanoma, and XIAP cleavage by phenoxodiol is associated with carboplatin sensitization. J. Transl. Med. 5, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nagi C., Xiao G. Q., Li G., Genden E., Burstein D. E. (2007) Immunohistochemical detection of X-linked inhibitor of apoptosis in head and neck squamous cell carcinoma. Ann. Diagn. Pathol. 11, 402–406 [DOI] [PubMed] [Google Scholar]
- 7. Nemoto T., Kitagawa M., Hasegawa M., Ikeda S., Akashi T., Takizawa T., Hirokawa K., Koike M. (2004) Expression of IAP family proteins in esophageal cancer. Exp. Mol. Pathol. 76, 253–259 [DOI] [PubMed] [Google Scholar]
- 8. Fong W. G., Liston P., Rajcan-Separovic E., St Jean M., Craig C., Korneluk R. G. (2000) Expression and genetic analysis of XIAP-associated factor 1 (XAF1) in cancer cell lines. Genomics 70, 113–122 [DOI] [PubMed] [Google Scholar]
- 9. Yang L., Cao Z., Yan H., Wood W. C. (2003) Coexistence of high levels of apoptotic signaling and inhibitor of apoptosis proteins in human tumor cells: implication for cancer specific therapy. Cancer Res. 63, 6815–6824 [PubMed] [Google Scholar]
- 10. Holcik M., Gibson H., Korneluk R. G. (2001) XIAP: apoptotic brake and promising therapeutic target. Apoptosis 6, 253–261 [DOI] [PubMed] [Google Scholar]
- 11. Tong Q. S., Zheng L. D., Wang L., Zeng F. Q., Chen F. M., Dong J. H., Lu G. C. (2005) Downregulation of XIAP expression induces apoptosis and enhances chemotherapeutic sensitivity in human gastric cancer cells. Cancer Gene Ther. 12, 509–514 [DOI] [PubMed] [Google Scholar]
- 12. Liu J., Zhang D., Luo W., Yu Y., Yu J., Li J., Zhang X., Zhang B., Chen J., Wu X. R., Rosas-Acosta G., Huang C. (2011) X-linked inhibitor of apoptosis protein (XIAP) mediates cancer cell motility via Rho GDP dissociation inhibitor (RhoGDI)-dependent regulation of the cytoskeleton. J. Biol. Chem. 286, 15630–15640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schimmer A. D., Dalili S., Batey R. A., Riedl S. J. (2006) Targeting XIAP for the treatment of malignancy. Cell Death Differ. 13, 179–188 [DOI] [PubMed] [Google Scholar]
- 14. Suzuki Y., Nakabayashi Y., Takahashi R. (2001) Ubiquitin-protein ligase activity of X-linked inhibitor of apoptosis protein promotes proteasomal degradation of caspase-3 and enhances its anti-apoptotic effect in Fas-induced cell death. Proc. Natl. Acad. Sci. U.S.A. 98, 8662–8667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gyrd-Hansen M., Meier P. (2010) IAPs: from caspase inhibitors to modulators of NF-κB, inflammation and cancer. Nat. Rev. Cancer 10, 561–574 [DOI] [PubMed] [Google Scholar]
- 16. Liu J., Zhang D., Luo W., Yu J., Li J., Yu Y., Zhang X., Chen J., Wu X. R., Huang C. (2012) E3 ligase activity of XIAP RING domain is required for XIAP-mediated cancer cell migration, but not for its RhoGDI binding activity. PLoS One 7, e35682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yu J., Zhang D., Liu J., Li J., Yu Y., Wu X. R., Huang C. (2012) RhoGDI SUMOylation at Lys-138 increases its binding activity to Rho GTPase and its inhibiting cancer cell motility. J. Biol. Chem. 287, 13752–13760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cordon-Cardo C. (1995) Mutations of cell cycle regulators. Biological and clinical implications for human neoplasia. Am. J. Pathol. 147, 545–560 [PMC free article] [PubMed] [Google Scholar]
- 19. Fu M., Wang C., Li Z., Sakamaki T., Pestell R. G. (2004) Minireview: cyclin D1: normal and abnormal functions. Endocrinology 145, 5439–5447 [DOI] [PubMed] [Google Scholar]
- 20. Knudsen K. E., Diehl J. A., Haiman C. A., Knudsen E. S. (2006) Cyclin D1: polymorphism, aberrant splicing and cancer risk. Oncogene 25, 1620–1628 [DOI] [PubMed] [Google Scholar]
- 21. Che Y., Ye F., Xu R., Qing H., Wang X., Yin F., Cui M., Burstein D., Jiang B., Zhang D. Y. (2012) Co-expression of XIAP and cyclin D1 complex correlates with a poor prognosis in patients with hepatocellular carcinoma. Am. J. Pathol. 180, 1798–1807 [DOI] [PubMed] [Google Scholar]
- 22. Zhang D., Song L., Li J., Wu K., Huang C. (2006) Coordination of JNK1 and JNK2 is critical for GADD45α induction and its mediated cell apoptosis in arsenite responses. J. Biol. Chem. 281, 34113–34123 [DOI] [PubMed] [Google Scholar]
- 23. Ouyang W., Ma Q., Li J., Zhang D., Liu Z. G., Rustgi A. K., Huang C. (2005) Cyclin D1 induction through IκB kinase β/nuclear factor-κB pathway is responsible for arsenite-induced increased cell cycle G1-S phase transition in human keratinocytes. Cancer Res. 65, 9287–9293 [DOI] [PubMed] [Google Scholar]
- 24. Wang J., Ouyang W., Li J., Wei L., Ma Q., Zhang Z., Tong Q., He J., Huang C. (2005) Loss of tumor suppressor p53 decreases PTEN expression and enhances signaling pathways leading to activation of activator protein 1 and nuclear factor κB induced by UV radiation. Cancer Res. 65, 6601–6611 [DOI] [PubMed] [Google Scholar]
- 25. Luo W., Liu J., Li J., Zhang D., Liu M., Addo J. K., Patil S., Zhang L., Yu J., Buolamwini J. K., Chen J., Huang C. (2008) Anti-cancer effects of JKA97 are associated with its induction of cell apoptosis via a Bax-dependent and p53-independent pathway. J. Biol. Chem. 283, 8624–8633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang D., Li J., Wu K., Ouyang W., Ding J., Liu Z. G., Costa M., Huang C. (2007) JNK1, but not JNK2, is required for COX-2 induction by nickel compounds. Carcinogenesis 28, 883–891 [DOI] [PubMed] [Google Scholar]
- 27. Ouyang W., Luo W., Zhang D., Jian J., Ma Q., Li J., Shi X., Chen J., Gao J., Huang C. (2008) PI-3K/Akt pathway-dependent cyclin D1 expression is responsible for arsenite-induced human keratinocyte transformation. Environ. Health Perspect. 116, 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Song L., Li J., Zhang D., Liu Z. G., Ye J., Zhan Q., Shen H. M., Whiteman M., Huang C. (2006) IKKβ programs to turn on the GADD45α-MKK4-JNK apoptotic cascade specifically via p50 NF-κB in arsenite response. J. Cell Biol. 175, 607–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Song L., Li J., Ye J., Yu G., Ding J., Zhang D., Ouyang W., Dong Z., Kim S. O., Huang C. (2007) p85α acts as a novel signal transducer for mediation of cellular apoptotic response to UV radiation. Mol. Cell. Biol. 27, 2713–2731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li J., Chen H., Tang M. S., Shi X., Amin S., Desai D., Costa M., Huang C. (2004) PI-3K and Akt are mediators of AP-1 induction by 5-MCDE in mouse epidermal Cl41 cells. J. Cell Biol. 165, 77–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang C., Ma W. Y., Dawson M. I., Rincon M., Flavell R. A., Dong Z. (1997) Blocking activator protein-1 activity, but not activating retinoic acid response element, is required for the antitumor promotion effect of retinoic acid. Proc. Natl. Acad. Sci. U.S.A. 94, 5826–5830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huang C., Ma W. Y., Maxiner A., Sun Y., Dong Z. (1999) p38 kinase mediates UV-induced phosphorylation of p53 protein at serine 389. J. Biol. Chem. 274, 12229–12235 [DOI] [PubMed] [Google Scholar]
- 33. Dubrez-Daloz L., Dupoux A., Cartier J. (2008) IAPs: more than just inhibitors of apoptosis proteins. Cell Cycle 7, 1036–1046 [DOI] [PubMed] [Google Scholar]
- 34. Yang Y., Fang S., Jensen J. P., Weissman A. M., Ashwell J. D. (2000) Ubiquitin protein ligase activity of IAPs and their degradation in proteasomes in response to apoptotic stimuli. Science 288, 874–877 [DOI] [PubMed] [Google Scholar]
- 35. Sherr C. J. (1994) G1 phase progression: cycling on cue. Cell 79, 551–555 [DOI] [PubMed] [Google Scholar]
- 36. Shaulian E., Karin M. (2001) AP-1 in cell proliferation and survival. Oncogene 20, 2390–2400 [DOI] [PubMed] [Google Scholar]
- 37. Zhang D., Li J., Gao J., Huang C. (2009) c-Jun/AP-1 pathway-mediated cyclin D1 expression participates in low dose arsenite-induced transformation in mouse epidermal JB6 Cl41 cells. Toxicol. Appl. Pharmacol. 235, 18–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lu M., Lin S. C., Huang Y., Kang Y. J., Rich R., Lo Y. C., Myszka D., Han J., Wu H. (2007) XIAP induces NF-κB activation via the BIR1/TAB1 interaction and BIR1 dimerization. Mol. Cell 26, 689–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cooper S. J., MacGowan J., Ranger-Moore J., Young M. R., Colburn N. H., Bowden G. T. (2003) Expression of dominant negative c-jun inhibits ultraviolet B-induced squamous cell carcinoma number and size in an SKH-1 hairless mouse model. Mol. Cancer Res. 1, 848–854 [PubMed] [Google Scholar]
- 40. Karin M. (1995) The regulation of AP-1 activity by mitogen-activated protein kinases. J. Biol. Chem. 270, 16483–16486 [DOI] [PubMed] [Google Scholar]
- 41. Al-Murrani S. W., Woodgett J. R., Damuni Z. (1999) Expression of I2PP2A, an inhibitor of protein phosphatase 2A, induces c-Jun and AP-1 activity. Biochem. J. 341, 293–298 [PMC free article] [PubMed] [Google Scholar]
- 42. Chen J., Parsons S., Brautigan D. L. (1994) Tyrosine phosphorylation of protein phosphatase 2A in response to growth stimulation and v-src transformation of fibroblasts. J. Biol. Chem. 269, 7957–7962 [PubMed] [Google Scholar]
- 43. O'Shaughnessy R. F., Welti J. C., Sully K., Byrne C. (2009) Akt-dependent Pp2a activity is required for epidermal barrier formation during late embryonic development. Development 136, 3423–3431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Alberts A. S., Deng T., Lin A., Meinkoth J. L., Schönthal A., Mumby M. C., Karin M., Feramisco J. R. (1993) Protein phosphatase 2A potentiates activity of promoters containing AP-1-binding elements. Mol. Cell. Biol. 13, 2104–2112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Arber N., Doki Y., Han E. K., Sgambato A., Zhou P., Kim N. H., Delohery T., Klein M. G., Holt P. R., Weinstein I. B. (1997) Antisense to cyclin D1 inhibits the growth and tumorigenicity of human colon cancer cells. Cancer Res. 57, 1569–1574 [PubMed] [Google Scholar]
- 46. Eckelman B. P., Salvesen G. S., Scott F. L. (2006) Human inhibitor of apoptosis proteins: why XIAP is the black sheep of the family. EMBO Rep. 7, 988–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang R., Li B., Wang X., Lin F., Gao P., Cheng S. Y., Zhang H. Z. (2009) Inhibiting XIAP expression by RNAi to inhibit proliferation and enhance radiosensitivity in laryngeal cancer cell line. Auris Nasus Larynx 36, 332–339 [DOI] [PubMed] [Google Scholar]
- 48. Ma J. J., Chen B. L., Xin X. Y. (2009) XIAP gene downregulation by small interfering RNA inhibits proliferation, induces apoptosis, and reverses the cisplatin resistance of ovarian carcinoma. Eur. J. Obstet. Gynecol. Reprod. Biol. 146, 222–226 [DOI] [PubMed] [Google Scholar]
- 49. Arber N., Hibshoosh H., Moss S. F., Sutter T., Zhang Y., Begg M., Wang S., Weinstein I. B., Holt P. R. (1996) Increased expression of cyclin D1 is an early event in multistage colorectal carcinogenesis. Gastroenterology 110, 669–674 [DOI] [PubMed] [Google Scholar]
- 50. Palmqvist R., Rutegârd J. N., Bozoky B., Landberg G., Stenling R. (2000) Human colorectal cancers with an intact p16/cyclin D1/pRb pathway have up-regulated p16 expression and decreased proliferation in small invasive tumor clusters. Am. J. Pathol. 157, 1947–1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sherr C. J., Roberts J. M. (2004) Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 18, 2699–2711 [DOI] [PubMed] [Google Scholar]
- 52. Kozar K., Sicinski P. (2005) Cell cycle progression without cyclin D-CDK4 and cyclin D-CDK6 complexes. Cell Cycle 4, 388–391 [DOI] [PubMed] [Google Scholar]
- 53. Neil J. R., Tian M., Schiemann W. P. (2009) X-linked inhibitor of apoptosis protein and its E3 ligase activity promote transforming growth factor-β-mediated nuclear factor-κB activation during breast cancer progression. J. Biol. Chem. 284, 21209–21217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bai Y., Yang C., Hu K., Elly C., Liu Y. C. (2004) Itch E3 ligase-mediated regulation of TGF-β signaling by modulating smad2 phosphorylation. Mol. Cell 15, 825–831 [DOI] [PubMed] [Google Scholar]
- 55. Zhang J., Ouyang W., Li J., Zhang D., Yu Y., Wang Y., Li X., Huang C. (2012) Suberoylanilide hydroxamic acid (SAHA) inhibits EGF-induced cell transformation via reduction of cyclin D1 mRNA stability. Toxicol. Appl. Pharmacol. 263, 218–224 [DOI] [PMC free article] [PubMed] [Google Scholar]