Background: Integrin affinity is controlled by the recruitment of both activators and inactivators on their cytoplasmic domain.
Results: We showed that CamKII phosphorylates the negative regulator ICAP-1α and thereby regulates its interaction with β1 integrins.
Conclusion: This phosphorylation is required for proper focal adhesion dynamics and cell adhesion.
Significance: Our findings support an important role of this pathway in cell adhesion and cell migration.
Keywords: Adhesion, CaMKII, Integrins, Membrane Proteins, Signal Transduction, ICAP-1, Focal Adhesion
Abstract
Focal adhesion turnover during cell migration is an integrated cyclic process requiring tight regulation of integrin function. Interaction of integrin with its ligand depends on its activation state, which is regulated by the direct recruitment of proteins onto the β integrin chain cytoplasmic domain. We previously reported that ICAP-1α, a specific cytoplasmic partner of β1A integrins, limits both talin and kindlin interaction with β1 integrin, thereby restraining focal adhesion assembly. Here we provide evidence that the calcium and calmodulin-dependent serine/threonine protein kinase type II (CaMKII) is an important regulator of ICAP-1α for controlling focal adhesion dynamics. CaMKII directly phosphorylates ICAP-1α and disrupts an intramolecular interaction between the N- and the C-terminal domains of ICAP-1α, unmasking the PTB domain, thereby permitting ICAP-1α binding onto the β1 integrin tail. ICAP-1α direct interaction with the β1 integrin tail and the modulation of β1 integrin affinity state are required for down-regulating focal adhesion assembly. Our results point to a molecular mechanism for the phosphorylation-dependent control of ICAP-1α function by CaMKII, allowing the dynamic control of β1 integrin activation and cell adhesion.
Introduction
In metazoan organisms, cell adhesion to the extracellular matrix is largely mediated by the integrin family and plays a central role in many physiological processes, such as embryonic development, inflammatory response, wound healing, and tissue regeneration (1). These receptors cluster into adhesive sites that connect the microenvironment to actin cytoskeleton and thereby coordinate cell motility. Continuous assembly and disassembly of these adhesive sites is a prerequisite for cell migration (2).
Integrins ectodomains can adopt at least three conformational states: a bent conformation associated with a low affinity state for its extracellular ligand, an incomplete extended form with intermediate affinity for the ligand, and a fully extended conformation corresponding to the ligand-occupied state (3–6). Conformational change from a bent to an extended form leads to integrin activation and is triggered by the recruitment of intracellular integrin partners on the β cytoplasmic domain, such as talin and kindlin (7–13). On the other hand, inhibitors, such as sharpin or integrin cytoplasmic domain-associated protein 1α (ICAP-1α),5 limit the recruitment of those activators (14, 15). Conversely, ICAP-1α is a β1A integrin-specific partner involved in the negative regulation of β1 integrin activity by limiting talin and kindlin-2 recruitment (14, 16–19). More precisely, we demonstrated that ICAP-1α maintains the low affinity state of β1 integrins to slow down focal adhesion (FA) assembly (20). This feature appears to be critical for cells to sense the extracellular matrix surface density and adapt their migratory and adhesive behavior. Despite growing evidence for the role of ICAP-1α during integrin-dependent adhesion, little is known about the signaling pathway controlling ICAP-1α activity. Several putative phosphorylation sites for protein kinases, such as CaMKII, PKC, PKG, and PKA, have been identified within ICAP-1α (19, 21). Although their biological pertinence has not always been accurately verified, previous studies showed that ICAP-1α is phosphorylated upon cell adhesion on fibronectin (FN), suggesting a phosphorylation-dependent regulatory mechanism (18, 19).
Our group and others have illustrated the role of serine/threonine protein kinase CaMKII in integrin-mediated cell adhesion. CaMKII activity promotes FA turnover (22) and cell migration (23, 24). CaMKII is activated following α5β1 integrin engagement and is required for α5β1-dependent cell migration and phagocytosis (25). In turn, CaMKII activation decreases the affinity state of α5β1 integrin and reduces cell spreading on FN (26), suggesting the existence of a CaMKII-dependent feedback loop regulating α5β1 integrin activity. Although the substrate of CaMKII was not clearly identified from those studies, we previously reported that CaMKII inhibition could be overcome by ectopically expressing a mutant form of ICAP-1α in which the threonine 38 of the CaMKII consensus site has been substituted by a non-phosphorylatable alanine (27). This finding suggested that both proteins were involved in the same signaling pathway. To test this hypothesis, we used a combination of β1 integrin and ICAP-1α mutants and showed that CaMKII controls the capability of ICAP-1α to bind to β1 integrin and regulate focal adhesion dynamics. ICAP-1α phosphorylation on threonine 38 by CaMKII released a cryptic binding site for β1 integrin located in the C-terminal part of ICAP-1α. The resulting opened active form of ICAP-1α consequently interacted with the β1A integrin tail and negatively regulated β1 integrin function by slowing down FA turnover.
EXPERIMENTAL PROCEDURES
Proteins, Reagents, and Antibodies
FN was purified from bovine serum as described previously (49). Vitronectin (VN) was purchased from BD Biosciences. KN-93 was purchased from Sigma-Aldrich.
Mouse monoclonal anti-vinculin (hVIN-1) and anti-FLAG M2 magnetic beads were obtained from Sigma-Aldrich. Rat monoclonal anti-β1 integrin 9EG7 was from BD Biosciences. Mouse monoclonal anti-β3 integrin Luc.A5 was from Emfret Analytics (Eibelstadt, Germany). Mouse monoclonal anti-αCaMKII (CBα-2) was from Zymed Laboratories Inc. (ABCYS, Paris, France). Rabbit anti-ICAP-1α was raised in our laboratory as described previously (20, 27). Mouse monoclonal anti-ICAP-1α (9B10H) was prepared using the N-terminal region of ICAP-1α bearing the T38D mutation (amino acids 1–100) as immunogen. AlexaFluor-conjugated goat antibodies were purchased from Invitrogen. Goat anti-mouse and anti-rabbit IgG coupled to HRP were purchased from Bio-Rad and Jackson Immunoresearch Laboratories (Interchim SA, Montluçon, France), respectively, and rabbit TrueBlot HRP anti-rabbit IgG and mouse TrueBlot ULTRA-HRP anti-mouse IgG were from eBiosciences (Paris, France).
Plasmid Constructs
GST, GST-β1A cytodomain, GST-β3 cytodomain, the fragments His-ICAP-1α C- and N-terminal, and the full-length forms ICAP-1αwild-type and ICAP-1αT38A were produced in Escherichia coli BL21 (DE3) using pGEX4T1, pGEX4T1-β1A, pGEX4T1-β3 (gift from M. Schneiller), pET19b-C-terminal ICAP-1α, pET19b-N-terminal ICAP-1wild-type, pET19b-N-terminal ICAP-1T38A, pET19b-N-terminal ICAP-1T38D, pET19b-ICAP-1αwild type, and pET19b- ICAP-1αT38A, respectively. The retroviral vectors used were pCLMFG-eGFP-VASP, pMSCV-mRFP-VASP (gifts from F. Gertler), pBabe-αCaMKIIT286D, pCLMFG-ICAP-1αT38A-IRES-eGFP, and pCLMFG-ICAP-1αT38D-IRES-eGFP. pcDNA4/V5-HisA-FLAG-ICAP-1α was kindly provided by D. A. Marchuk, and SRαCaMKII-T286D was a gift from H. Schulman.
Cell Culture and Retroviral Infection
Osteoblast and NIH3T3 cells were cultured in DMEM, and CHO cells were cultured in αMEM supplemented with 10% FCS (Invitrogen) and 100 units/ml penicillin, 100 μg/ml streptomycin at 37 °C in a 5% CO2-humidified chamber. Immortalized osteoblast cells were generated from β1−/− and Icap-1−/− mice, as described previously (16). β1−/− cells expressing β1wild-type, β1D759A, or β1V787T and Icap-1−/− cells expressing ICAP-1αwild type (Icap-1rescue) were described previously (16) (17). The generation of β1D759A, β1V787T, ICAP-1αT38A, and ICAP-1αT38D mutations was described previously (17, 20, 27).
The cell types generated by retroviral infection were as follows: Icap-1−/− cells expressing either ICAP-1αwild type, ICAP-1αT38A, or ICAP-1αT38D alone or in combination with mRFP-VASP; Icap-1−/− and Icap-1+/+ cells expressing CaMKIIαT286D as well as β1wild-type, β1D759A, and β1V787T osteoblasts expressing either CaMKIIαT286D, mRFP-VASP, or eGFP-VASP. Retroviral supernatants were obtained from Phoenix cells transfected with pCLMFG or pBabe vectors and were incubated with cells in 20% FCS-DMEM supplemented with 4 μg/ml Polybrene (Sigma-Aldrich) for 12 h at 37 °C. Cells expressing ICAP-1α mutants were selected by FACS based on eGFP expression level, cells expressing CaMKIIαT286D were selected in the presence of 5 μg/ml puromycin, and cells expressing mRFP-VASP or eGFP-VASP were checked by epifluorescence microscopy.
Cell Treatment and Immunofluorescence
Osteoblast cells were trypsinized, treated with 1 mg/ml soybean trypsin inhibitor (Sigma-Aldrich), washed in PBS, and incubated in serum-free DMEM containing 5% BSA for 30 min at 37 °C. To inhibit CaMKIIα activity, cells in suspension were treated with 10 μm KN-93 for 1 h at 37 °C. CaMKIIαT286D-expressing cells or KN-93-treated cells were seeded for either 2 or 16 h at 37 °C on glass coverslips coated with either 10 μg/ml FN or 5 μg/ml VN in FN-free FCS/DMEM-supplemented medium. Cells were then fixed with a 3% paraformaldehyde, 2% sucrose solution for 10 min; permeabilized with 0.2% Triton X-100 for 10 min; blocked with PBS, 3% BSA for 30 min; and incubated with primary antibodies for 45 min at 37 °C. After washes in PBS, 3% BSA, 0.05% Tween 20, AlexaFluor-conjugated goat secondary antibodies were added for 45 min at 37 °C. Cells were finally mounted in Mowiol solution and imaged on an apotome microscope (Carl Zeiss S.A.S., Le Pecq, France).
In Vitro Pull-down Assays
CHO cells were transfected with the expression vectors pcDNA3.1-ICAP-1αwild-type, pcDNA3.1-ICAP-1αT38A, or pcDNA3.1-ICAP-1αT38D using Exgen500 (Euromedex, Souffelweyersheim, France). After 24 h, cells were lysed in a 1% Nonidet P-40, 10% glycerol, 20 mm Tris pH 8, 137 mm NaCl buffer with protease inhibitors (Roche Applied Science) supplemented or not with phosphatase inhibitor mixture (Sigma-Aldrich). For alkaline phosphatase treatment, cell lysates were incubated with calf intestinal alkaline phosphatase (0.06 unit/μl, Euromedex) for 25 min at 37 °C. Recombinant proteins GST, GST-β1A cytodomain, and GST-β3 cytodomain were produced in E. coli BL21 (DE3) and purified using glutathione-coupled Sepharose beads (GE Healthcare). Cell lysates were incubated with glutathione-Sepharose beads coupled to GST proteins for 2 h at 4 °C. Beads were then washed twice in cell lysis buffer and twice in PBS. Beads were resuspended with reduced sample buffer, and proteins were separated by 12% SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes for Western blotting. Immunological detection was achieved with rabbit anti-ICAP-1 antibody and HRP-conjugated anti-rabbit IgG secondary antibody followed by chemiluminescence revelation (ECL, GE Healthcare).
Co-immunoprecipitation
CHO cells and Icap-1−/− osteoblasts were singly transfected or co-transfected with expression vector pcDNA4V5HisA-FLAG-ICAP-1α or/and SRαCaMKIIT286D using Exgen500 and Lipofectamine 2000 reagent (Invitrogen), respectively. 24 h after transfection, cells were incubated with 3 mm cross-linker di-tert-butyl peroxide (Thermo Fisher Scientific) in culture medium containing 25 mm HEPES for 30 min at 37 °C (50). Cross-linker was quenched with 20 mm Tris-HCl, and cells were lysed with CSK plus buffer (10 mm HEPES, 50 mm NaCl, 20 mm Tris-HCl, 150 mm sucrose, 3 mm MgCl2, 1 mm MnCl2, 0.5% Triton X-100, 2 mm Na3VO4, protease inhibitors, and phosphatase inhibitor mixtures). Cell lysates were incubated with anti-FLAG M2 magnetic beads or with control mouse IgG coupled to Protein G magnetic beads for 3 h at 4 °C. After washing twice with CSK plus buffer and twice with 50 mm Tris, pH 7.5, 150 mm NaCl solution, beads were incubated with 100 mm DTT in Tris-NaCl solution. Proteins were eluted from magnetic beads with reduced sample buffer, separated by 4–20% SDS-PAGE (Bio-Rad), and transferred on PVDF membranes (Bio-Rad). Revelation of proteins was realized with rabbit polyclonal antibody anti-ICAP-1 and mouse monoclonal antibody anti-CaMKIIα and secondary rabbit TrueBlot-HRP and mouse TrueBlot ULTRA-HRP antibodies (eBioscience), respectively, followed by chemiluminescence revelation (ECL, GE Healthcare).
Solid Phase Assay
The interaction between the N- and C-terminal regions of ICAP-1α was carried out using a solid phase assay. Briefly, a 96-well microplate (Maxisorp, Nunc (Dutscher, Brumath, France)) was coated with either 10 μg of recombinant C-terminal ICAP-1α or BSA for 16 h at 4 °C and blocked with a 3% BSA, PBS solution for 1 h at room temperature. After washes with a 3% BSA, 0.01% Tween 20, PBS solution, increased amounts of either recombinant N-terminal ICAP-1wild-type, N-terminal ICAP-1T38A, or N-terminal ICAP-1T38D (0–400 ng) were added for 1 h at 37 °C. After washes in 3% BSA, 0.01% Tween 20, PBS solution, the monoclonal antibody 9B10H directed against the N-terminal part of ICAP-1 was added for 1 h at 37 °C, and then an HRP-conjugated secondary anti-mouse antibody was added for 45 min at 37 °C. After washes in 3% BSA, 0.01% Tween 20, PBS solution, the presence of N-terminal ICAP-1 was detected by adding ABTS reagent and quantified by measuring absorbance at 405 nm.
Microinjection
NIH3T3 cells were seeded for 16 h at 37 °C onto 10 μg/ml FN-coated glass coverslips. Microinjections were carried out with a micromanipulator model 5171 connected to an Eppendorf microinjector unit (Transjector 5246). The cells were microinjected with a KCl (120 mm), HEPES (10 mm), pH 7, solution containing 100 μm tetramethyl rhodamine-dextran amine (Mr 3000; Molecular Probes, Interchim) and 1 mg/ml purified recombinant fragment C-terminal ICAP-1α alone or in combination with either N-terminal ICAP-1T38A or N-terminal ICAP-1T38D. 30 min after microinjection, cells were fixed and immunostained for vinculin localization using a mouse anti-vinculin antibody and an Alexa488-conjugated anti-mouse IgG antibody. Cells were imaged using a wide field epifluorescence microscope (Olympus Provis AX70), and each rhodamine-positive cell was used to count for FA-positive or -negative.
CaMKII Phosphorylation Assay
The CaMKII phosphorylation assay was performed using CaMKII extracted from rat brain. Brains were homogenized in extraction buffer (50 mm Tris-HCl, pH 7.5, 10% ethylene glycol, 250 mm sucrose, 4 mm EDTA, 2 mm EGTA, 1 mm DTT, protease inhibitors). After centrifugation at 100,000 × g for 45 min at 4 °C, the lysate was clarified with a non-immune IgG2A serum coupled to protein A-conjugated Sepharose beads (GE Healthcare) for 2 h at 4 °C. Then supernatant was incubated with mouse monoclonal anti-αCaMKII (CBα-2) for 1 h at 4 °C, and CaMKII was purified using the protein A-coupled Sepharose beads. After 1 h at 4 °C, beads were washed once in extraction buffer and twice in kinase assay buffer (10 mm Tris-HCl, pH 7.4, 150 mm NaCl, 10 mm MgCl2, 0.5 mm DTT). Then the phosphorylation reaction was performed in the presence of 1 mg/ml N-terminal ICAP-1 fragment, 100 μm ATP, 2.5 μCi of [γ-32P]ATP, 100 μm CaCl2, and 10 μg/ml calmodulin. The reaction was stopped after 30 min at 37 °C by adding reducing Laemmli sample buffer, and samples were run on 10% SDS-PAGE. The gel was stained with Coomassie Blue and then digitized and dried before the autoradiography was processed at −80 °C. For control experiments, phosphorylation reactions were performed without either calmodulin or N-terminal ICAP-1 or in the presence of 1 mm EGTA.
Alternatively, for ICAP-1WT and its T38A mutant, ICAP-1 phosphorylation was assayed using recombinant CaMKII. Briefly, before use, CaMKII (New England Biolabs, Evry, France) was activated by autophosphorylation as follows. CaMKII (500 units) was first incubated in the presence of ATP (200 μm), calmodulin (1.2 μm), and CaCl2 (2 mm) for 10 min at 30 °C, and then 5 μl of recombinant protein His-ICAP-1 immobilized on nickel-nitrilotriacetic acid beads were added in the presence 3 μCi of [γ-32P]ATP, MgCl2, and CaCl2 for 1 h at 30 °C. The reaction was stopped by adding 5× Laemmli buffer and heating at 95 °C for 10 min. CaMKII-mediated ICAP-1 phosphorylation was visualized on an SDS-polyacrylamide gel after autoradiography and Coomassie Blue staining.
Time Lapse Videomicroscopy
Time lapse recordings were processed on osteoblast cells (β1−/− or Icap-1−/−) expressing either eGFP-VASP or mRFP-VASP together with ICAP-1αwild-type, ICAP-1αT38A, ICAP-1αT38D, β1wild-type, β1D759A, or β1V787T. Cells treated or not with 10 μm KN-93 were trypsinized, blocked using a soybean trypsin inhibitor-containing solution (1 mg/ml), washed in PBS, and resuspended in serum-free DMEM containing 5% BSA. After 1 h at 37 °C, cells were seeded in LabTek I chambers (Nunc) coated with 1 μg/ml FN. Cells were allowed to spread in FN-free FCS/DMEM supplemented or not with 10 μm KN-93 for 2 or 16 h, and time lapse recordings were assessed as described previously (20). Briefly, records were done in a 5% CO2 humidified atmosphere at 37 °C using an inverted microscope (Axiovert 200 M, Carl Zeiss) and a live cell imaging plan apochromat ×63, numerical aperture 1.2 water immersion objective (Carl Zeiss). For each condition, 20 cells were randomly chosen, and images were taken simultaneously every 4 min using a motorized stage and a cooled CCD camera (CoolSNAP HQ2; Roper Scientific). Photobleaching events were minimized with a FluoArc system and an ND75 filter (Carl Zeiss), which reduce the excitation light of a 100-watt mercury lamp. FAs located at the leading edge were selectively chosen, and their turnover was quantified using Metamorph software (Molecular Devices, Sunnyvale, CA) as described previously (20). Briefly, mature FAs were manually outlined as well as their surrounding cytoplasmic area, and adhesions were manually tracked from their nucleation to their disassembly. The mean fluorescence intensity in the adhesion area was subtracted from the cytoplasmic background value. From these data, a shaped bell curve denoting FA turnover was obtained, allowing the measurements of FA assembly and disassembly rates as well as FA lifetime.
Image Analysis
Cells were imaged on a nonlinear optics LSM510 inverted confocal and biphoton laser-scanning microscope equipped with a ×63, numerical aperture 1.4 oil immersion plan apochromat objective (Carl Zeiss, Inc.). The images were manually thresholded, and FAs were automatically selected using MetaMorph software. Number and surface of β1 integrin-, β3 integrin-, and vinculin-containing FAs were quantified. Experiments were performed in triplicate, and at least 15 cells were analyzed per condition.
RESULTS
CaMKII Negatively Regulates β1 Integrin-dependent FA Assembly and Turnover Upstream of ICAP-1α
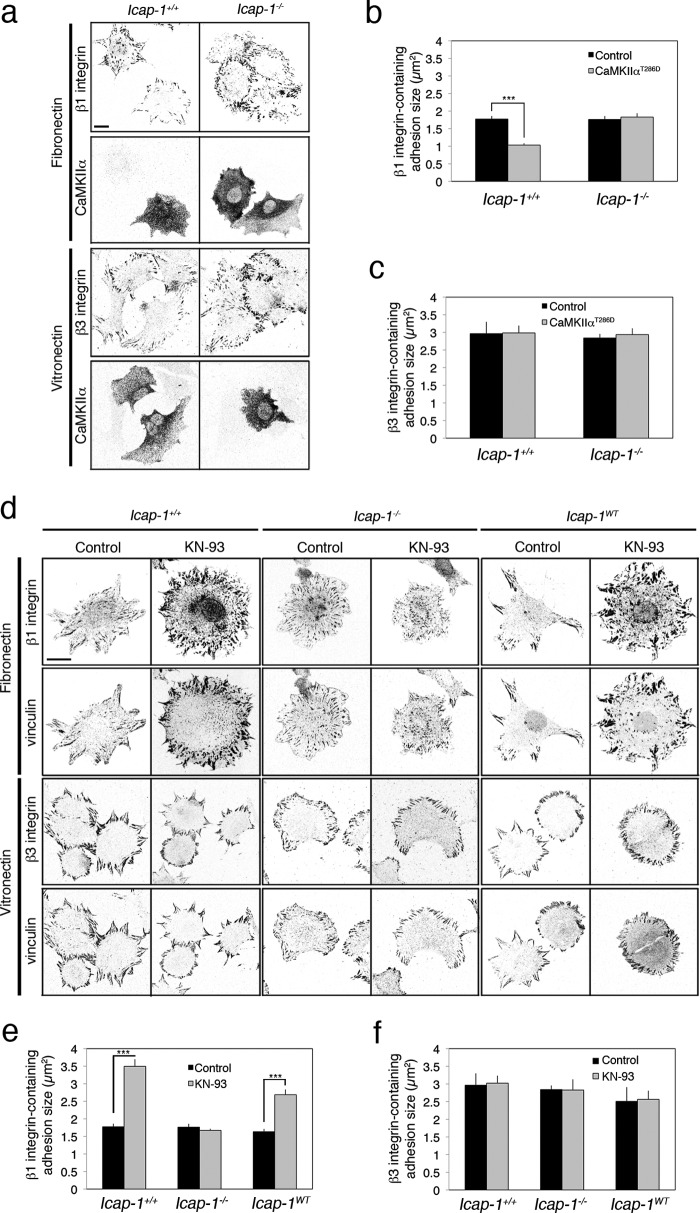
Immortalized osteoblast cells derived from Icap-1 knock-out or control mice were used to analyze whether the inhibition or activation of CaMKII could differentially affect ICAP-1α-dependent FA formation and dynamics (16). The constitutively active mutant αCaMKIIT286D (28–30) was stably expressed into Icap-1+/+ and Icap-1−/− osteoblast cells. Increasing CaMKII activity leads to a strong reduction in FA size in control cells spread on FN (a β1 integrin-dependent matrix) (Fig. 1, a and b). In contrast, on a VN matrix (to engage β3 integrin) or in Icap-1 null background, αCaMKIIT286D did not mediate any deleterious effect on FA growth, suggesting that CaMKII acts on β1 integrin-specific adhesion sites through ICAP-1α (Fig. 1, a and c). Consistent with those data, pharmacological inhibition of CaMKII activity with KN-93 leads to a rapid (within 2 h) increase in FA size in Icap-1+/+ cells spread on FN but had no effect on VN or in Icap-1−/− cells (Fig. 1, d–f). Re-expression of ICAP-1α in Icap-1−/− cells restored the effect of KN-93 treatment on FA growth observed in Icap-1+/+ cells, indicating that CaMKII controls β1 integrin-dependent adhesion complex formation in an ICAP-1α-dependent manner (Fig. 1, d–f).
FIGURE 1.
CaMKIIα negatively regulates the formation of β1 integrin-dependent adhesion complexes in an ICAP-1α-dependent manner. a, Icap-1+/+ and Icap-1−/− osteoblasts expressing or not the constitutively activated CaMKIIαT286D and spread on FN (10 μg/ml) or on VN (5 μg/ml) for 2 h were immunostained to visualize β1 integrin (using 9EG7 antibody) and CaMKIIα. Scale bar, 10 μm. b and c, quantification of β1 (b) and β3 (c) integrin-containing FA size in Icap-1+/+ and Icap-1−/− osteoblasts expressing or not the constitutively activated CaMKIIαT286D. ***, p < 0.0001. d, Icap-1+/+, Icap-1−/−, and Icap-1WT osteoblasts spread on FN (10 μg/ml) or on VN (5 μg/ml) and treated or not with KN-93 (10 μm) for 2 h were immunostained to visualize vinculin and either activated β1 integrin (using 9EG7 antibody, for FN coating) or β3 integrin (for VN coating). Scale bar, 10 μm. e and f, quantification of β1 (e) and β3 (f) integrin-containing FA size in Icap-1+/+, Icap-1−/−, and Icap-1WT osteoblasts treated or not with KN-93 (10 μm) for 2 h. ***, p value < 0.0001. Error bars, S.D.
To characterize how CaMKII activity influences the dynamics of FN-induced adhesion complexes in Icap-1+/+ and Icap-1−/− cells, live cell imaging was performed using cells expressing an eGFP-tagged VASP widely used as an adhesion marker. Because FA dynamics largely depend on their relative location within the cells (leading edges or trailing end) (31), the measurement of adhesion dynamics was assessed on FAs located at the cell front. Four different parameters of FA dynamics were measured: assembly and disassembly rates, the steady state duration, and the total lifetime. Consistent with an increased growth upon KN-93 treatment, the dynamics of adhesion complexes in Icap-1+/+ cells were decreased by short term inhibition of CaMKII. The assembly rate was faster, whereas the disassembly rate was lower, and the resulting steady state as well as the lifetime were longer (Fig. 2, a and b). Again, none of those dynamic parameters appeared to be affected in Icap-1-deficient cells, indicating that CaMKII functions through an ICAP-1α-dependent pathway (Fig. 2b). We previously showed that Icap-1 deficiency promotes the formation of centrally located β1 integrin-containing focal adhesions (20). To identify whether CaMKII also constrains the assembly and distribution of adhesion complexes, control and Icap-1−/− cells were treated overnight with KN-93 to measure focal adhesion distribution and dynamics in a permanently inhibited CaMKII cell context. Inhibition of CaMKII with KN93 in control cells stimulated the formation of β1 integrin-positive FAs, mimicking the Icap-1 null phenotype (Fig. 2, c and d). Moreover, the high number of FAs in Icap-1 null cells was not further affected upon CaMKII inhibition. Quantification of focal adhesion assembly and disassembly rates as well as lifetime showed that long term CaMKII inhibition accelerated focal adhesion assembly without modifying the disassembly rate, thereby leading to an overall reduction in adhesion lifetime (Fig. 2e). Interestingly, CaMKII inhibition in control cells reproduced the adhesion dynamics of Icap-1−/− cells. Here again, KN-93 treatment in Icap-1−/− cells did not further modify FA dynamics. These data indicated that CaMKII negatively regulated FA assembly and turnover upstream of ICAP-1α.
FIGURE 2.
CaMKIIα regulates FA dynamics upstream of ICAP-1α. a, time lapse sequences of eGFP-VASP-expressing osteoblasts treated or not with KN-93 (10 μm). The arrows indicate the turnover of a single adhesion complex. Scale bar, 5 μm. b, Icap-1+/+ and Icap-1−/− osteoblasts expressing eGFP-VASP were treated or not with KN-93 (10 μm) for 2 h, and focal adhesion dynamics were recorded. Four parameters were measured: assembly and disassembly rates, adhesion steady state duration, and total lifetime. At least 20 cells and 10 FAs in each cell type were recorded. ***, p < 0.0001. c, Icap-1+/+ and Icap-1−/− osteoblasts spread on FN (10 μg/ml) and treated or not with KN-93 (10 μm) for 16 h were immunostained to visualize vinculin and activated β1 integrin (using 9EG7 antibody). Scale bar, 10 μm. d, quantification of FA numbers in Icap-1+/+ and Icap-1−/− osteoblasts spread on FN (10 μg/ml) and treated or not with KN-93 (10 μm) for 16 h. ***, p value < 0.0001. e, quantification of FA dynamics in Icap-1+/+, Icap-1−/−, and Icap-1WT osteoblasts expressing either eGFP- or mRFP-VASP and treated or not with KN-93 (10 μm) for 16 h. Three parameters were measured: assembly and disassembly rates and the adhesion lifetime. At least 20 cells and 10 FAs in each cell type were recorded. ***, p < 0.0001. Error bars, S.D.
CaMKII-mediated Down-regulation of β1A Integrin Activation Requires ICAP-1α Binding onto the β1A Tail
We previously showed that ICAP-1α reduced FA assembly by restraining β1 integrin in a low affinity state for its extracellular ligand (20). On the other hand, we also previously demonstrated that the disruption of the salt bridge between the α and β1 subunits by the β1D759A mutation reproduced the FA pattern and dynamics observed upon ICAP-1α loss. To identify whether CaMKII regulates FA formation and dynamics by controlling the β1 integrin affinity state, CaMKII activity was either increased using the active form of CaMKIIα (αCaMKIIT286D) or inhibited upon KN-93 treatment in β1−/− osteoblasts expressing the β1D759A preactivated mutant. Although the expression of αCaMKIIT286D affected FA growth in control cells (β1wild-type), no significant difference was observed in β1D759A-expressing cells (Fig. 3, a and b). As shown previously, short term treatment of KN93 (2 h) increased FA size in control cells, whereas β1D759A expression rendered FAs insensitive to CaMKII inhibition (Fig. 3, c and d). As shown previously (20), quantification of FA assembly and disassembly rates as well as lifetime in eGFP-VASP-expressing β1WT and β1D759A cells showed an increased FA assembly rate together with a reduced lifetime in β1D759A cells compared with β1wild-type cells, mimicking Icap-1 loss (Fig. 3e). Long term inhibition of CaMKII (overnight) accelerated adhesion assembly and reduced lifetime in β1wild-type cells to a similar extent as in β1D759A cells. Again, KN-93 did not significantly modify FA turnover in β1D759A cells, indicating that the expression of a preactivated β1 integrin phenocopied the effect of CaMKII inhibition and ICAP-1α loss. These findings suggested that CaMKII controls β1 integrin activation state and thereby FA assembly and turnover.
FIGURE 3.
CaMKIIα regulates FA formation and dynamics through ICAP-1α binding to β1 cytodomain. a, β1wild-type, β1D759A, and β1V787T osteoblast cells expressing CaMKIIαT286D mutant were labeled with anti-activated β1 integrin and anti-CaMKIIα antibodies. Scale bar, 10 μm. b, quantification of β1 integrin-containing FA size in β1wild-type, β1D759A, and β1V787T osteoblast cells expressing or not the constitutively activated CaMKIIαT286D. ***, p < 0.0001. c, β1wild-type, β1D759A, and β1V787T osteoblast cells were treated or not with 10 μm KN-93 for 2 h and immunostained to visualize vinculin and activated β1 integrin. Scale bar, 10 μm. d, quantification of β1 integrin-containing FA size in β1wild-type, β1D759A, and β1V787T osteoblast cells treated or not with KN-93 (10 μm) for 2 h. ***, p < 0.0001. e, quantification of FA assembly and disassembly rates and total lifetime in β1wild-type, β1D759A, and β1V787T osteoblast cells expressing eGFP-VASP and treated or not with 10 μm KN-93 for 16 h. ***, p value < 0.0001. Error bars, S.D.
Having shown that FA dynamics controlled by CaMKII also depended on ICAP-1α and integrin activation, it remained to clarify the molecular bases for this mechanism. First, we addressed the question of whether the ICAP-1α-dependent regulation of FA assembly and turnover required a direct binding of ICAP-1α to β1A integrin. ICAP-1α binding to the β1A integrin cytoplasmic domain requires the membrane-distal NPKY motif as well as two valine residues at positions −2 and −5 from the asparagine 792 (human β1A chain) (32, 33). Point mutation of valine 787 into threonine has been reported to selectively interfere with ICAP-1α binding onto β1A without modifying the recruitment of other integrin partners, such as kindlins (17). Expression of the β1V787T mutant recapitulated the phenotype of FA dynamics in β1D759A cells: an accelerated assembly and a shorter lifetime (Fig. 3e). Similarly to what we observed in β1D759A cells, CaMKII activation (using the CaMKIIαT286D mutant) or inhibition (with KN-93) in β1V787T-expressing cells did not affect FA growth, in contrast to control cells (Fig. 3, a–d). Altogether, these findings indicate that CaMKII controls FA assembly via a direct binding of ICAP-1α to β1A integrin.
Phosphorylation of ICAP-1α at Threonine 38 Regulates FA Dynamics in a CaMKII-dependent Manner
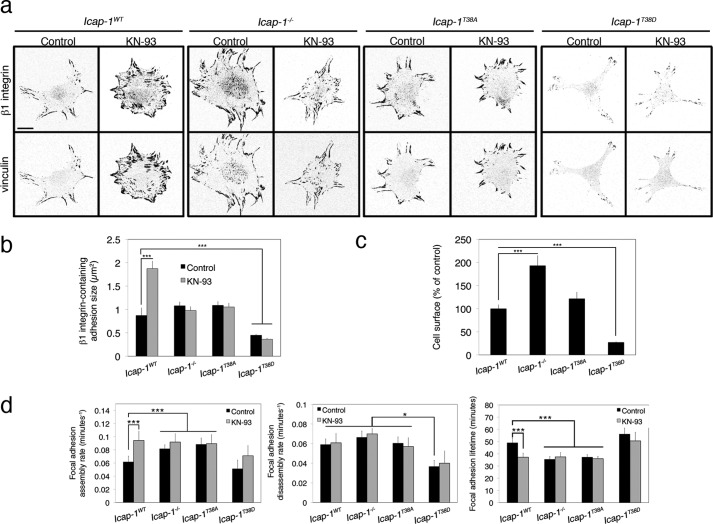
We previously reported that CaMKII regulates α5β1-dependent cell adhesion through a predicted CaMKII phosphorylation site on ICAP-1 threonine 38 (27). To identify whether this putative CaMKII phosphorylation site found in ICAP-1α might participate in FA dynamics, the phosphomimetic T38D and a non-phosphorylatable T38A mutant form of ICAP-1 (27) were expressed in Icap-1 null cells, respectively, and FA formation and dynamics were analyzed. Whereas the expression of ICAP-1αT38A had no significant effect on FAs formation, the ICAP-1αT38D mutant strongly reduced it, suggesting that phosphorylation of threonine 38 negatively regulates FA formation (Fig. 4, a and b). Consequently, ICAP-1αT38D cells were less spread compared with control cells (Fig. 4c). CaMKII inhibition by KN-93 did not significantly modify FA formation in cells expressing both ICAP-1α phosphomutants (Fig. 4, a and b). As previously, we conducted dynamic analysis of FA behavior in cells expressing ICAP-1αwild-type, ICAP-1αT38A, or ICAP-1αT38D. Whereas ICAP-1α loss accelerated FA assembly rate and consequently shortened lifetime, the few remaining adhesions in ICAP-1αT38D cells displayed much slower dynamics with decreased assembly and disassembly rates, leading to a longer lifetime (Fig. 4d). Conversely, ICAP-1αT38A cells displayed a faster assembly phase and a reduced lifetime similar to Icap-1−/− cells or control cells after inhibition of CaMKII, indicating that the deleterious effect of ICAP-1α Thr-38 phosphorylation on adhesion formation depends on CaMKII activity.
FIGURE 4.
CaMKIIα-mediated down-regulation of FA formation and turnover requires ICAP-1α threonine 38 phosphorylation. a, Icap-1−/−, Icap-1rescue, Icap-1T38A, and Icap-1T38D osteoblasts were treated or not with 10 μm KN-93 for 2 h and immunostained to visualize vinculin and activated β1 integrin. Scale bar, 10 μm. b, quantification of β1 integrin-containing FA size in Icap-1−/−, Icap-1rescue, Icap-1T38A, and Icap-1T38D osteoblasts treated or not with KN-93 (10 μm) for 2 h. ***, p < 0.0001. c, quantification of the surface of Icap-1−/−, Icap-1rescue, Icap-1T38A, and Icap-1T38D osteoblasts. ***, p < 0.0001. d, quantification of FA assembly and disassembly rates and total lifetime in Icap-1−/−, Icap-1rescue, Icap-1T38A, and Icap-1T38D osteoblasts expressing eGFP- or mRFP-VASP and treated or not with 10 μm KN-93 for 16 h. ***, p < 0.0001. Error bars, S.D.
Threonine 38 Phosphorylation Disrupts ICAP-1α N- to C-terminal Interaction and Controls Its Binding to β1 Integrins
ICAP-1α regulates FA dynamics by directly interacting with β1 integrins, and this regulation is at least partly dependent on the phosphorylation status of the threonine 38. Next, we investigated whether ICAP-1α phosphorylation is a prerequisite to its binding to β1 integrins. We used an in vitro pull-down assay to estimate the capability of the wild-type form of ICAP-1α to interact with the β1A or β3 integrin cytoplasmic tail, either in the presence of phosphatase inhibitors or alkaline phosphatase. As shown in Fig. 5a (left), whereas phosphatase inhibitors promoted the specific ICAP-1α binding onto the β1A cytoplasmic tail, alkaline phosphatase treatment strongly reduced it. Subsequently, we investigated the binding capability of the T38A or T38D ICAP-1α mutants to the cytoplasmic tail of β1A, using the β3 integrin tail as a negative control. ICAP-1αT38D and ICAP-1αwild-type displayed a stronger interaction with β1 cytoplasmic tail compared with ICAP-1αT38A (Fig. 5a, left). Those data are highly suggestive that ICAP-1α recruitment onto the β1A tail is regulated by its phosphorylation status. We previously demonstrated that ICAP-1α association with β1 integrins prevents the recruitment of integrin activators such as kindlins, thereby maintaining integrins in their low affinity state for extracellular ligands (17). As shown in Fig. 5b, the expression of ICAP-1T38D reduced kindlin-2 binding on the β1 integrin tail, indicating that the threonine 38 phosphorylation state modulates kindlin-2 association with integrins and their subsequent activation. ICAP-1α is composed of an N-terminal moiety (amino acids 1–58), which is devoid of any structural motif but enriched in serine and threonine residues and encompasses several potential phosphorylation sites (including the CaMKII consensus site), and a C-terminal domain (amino acids 59–200), which folds into a PTB motif containing the β1 integrin binding site (32, 34) (Fig. 7). To test whether ICAP-1α activity may be regulated by a head to tail interaction, we used a solid phase assay to probe this putative intramolecular interaction. Indeed, a specific and dose-dependent interaction was detected between the N-terminal (residues 1–99) and C-terminal (residues 100–200) moieties of ICAP-1α (Fig. 5c). To check whether this interaction could be modulated by the Thr-38 phosphorylation, the ability of the N-terminal region of ICAP-1α containing either a T38A or T38D mutation to interact with the C-terminal domain was measured. Although the T38A mutation did not affect the interaction between the N- and the C-terminal domains of ICAP-1, the phosphomimetic mutation of the threonine by an aspartic acid residue abolished it (T38D) (Fig. 5c).
FIGURE 5.
ICAP-1α exists in a close, inactive conformation opened upon threonine 38 phosphorylation. a, pull-down assays between ICAP-1α and β1A integrin cytodomain. Left, CHO cell lysates expressing ICAP-1αwild-type and treated with phosphatase inhibitors or alkaline phosphatase were incubated with either GST alone or fused to the cytoplasmic tail of β1A or β3 integrin. Alternatively (bottom), CHO cell lysates expressing either ICAP-1αwild-type, ICAP-1αT38A, or ICAP-1αT38D mutants were incubated with GST alone or GST-integrin tail fusion proteins. Bound fraction of ICAP-1α was revealed by immunoblotting. b, pull-down assays between kindlin-2 and β1A integrin cytodomain. HEK 293 cells were transiently transfected with pcDNA3.1-ICAPwt or pcDNA3.1-ICAPT38D, respectively, and kindlin-2 associated with β1A and β3 integrin tails fused to GST or GST alone were visualized by Western blotting. c, solid phase assay of the intramolecular interaction between N- and C-terminal ICAP-1α. Increased amounts of either N-terminal ICAP-1wild-type, N-terminal ICAP-1T38A, or N-terminal ICAP-1T38D (1–400 ng) were added to C-terminal ICAP-1 (10 μg) coated onto a 96-well microplate. After washes, the interaction between ICAP-1 fragments was revealed with an anti-ICAP-1 antibody (9B10H) that recognizes the N-terminal region. As a negative control, BSA-coated wells were used to estimate the nonspecific binding of N-terminal ICAP-1. Error bars, S.D. of three independent experiments. d, cells were co-microinjected with the recombinant C-terminal ICAP-1 (residues 100–200) and dextran rhodamine alone or with N-terminal ICAP-1 bearing the Thr-38 phosphomimetic mutations (amino acids 1–99). Cells were fixed and stained for vinculin to visualize adhesion sites. Numbers of cells devoid or not of FAs were counted, and FA disruption efficiency was expressed as a percentage of total cells.
FIGURE 7.
Model of the signaling pathway regulating ICAP-1α function in the dynamics of β1 integrin-mediated FAs. a, schematic representation of ICAP-1. b, ICAP-1 phosphorylation controls β1 integrin activation. In the cytoplasm, ICAP-1α adopts a bent conformation in which the β1 integrin binding site (IBS) is masked by an intramolecular interaction between the N- and the C-terminal moieties. Phosphorylation of threonine 38 by CaMKIIα disrupts this association, releases the PTB domain, and promotes ICAP-1α association with the β1A cytoplasmic tail. ICAP-1α maintains low affinity of β1 integrin to slow down FA assembly and turnover. Illustrated proteins are not to scale.
Next, we investigated the functional effect of ICAP-1α intramolecular association on FA assembly. A complementation strategy was used to investigate whether the co-injection of the N-terminal moiety of ICAP-1α prevented the deleterious effect on FA formation induced by the C-terminal domain (14) (Fig. 5d). As previously published (14), microinjection of the ICAP-1α C-terminal moiety alone led to the disappearance of FA sites in 66% of microinjected cells as visualized by vinculin staining. Co-injection of the ICAP-1T38A N-terminal moiety together with the C-terminal part of the protein strongly blocked its effect on FA disassembly, with only 27% of microinjected cells showing disrupted adhesion structures. On the other hand, co-injection of the ICAP-1T38D N terminus with the C terminus did not significantly modify FA disassembly induced by the C-terminal fragment (76% versus 66%, respectively). These results suggested that the N-terminal region masks the integrin binding site when ICAP-1α is unphosphorylated on threonine 38.
ICAP-1α Is a Substrate of CaMKIIα in Vitro
The finding that ICAP-1α phosphorylation on threonine 38, a residue located within a putative CaMKII consensus site, and ICAP-1α binding onto β1 integrin were required for CaMKII-mediated down-regulation of FA assembly and turnover suggested that ICAP-1α was directly regulated by CaMKII. To further demonstrate that ICAP-1α is a direct target of CaMKII, we carried out co-immunoprecipitation experiments after expression of one or both proteins (ICAP-1α and/or CaMKIIα) using standard lysis conditions (radioimmune precipitation assay or glycerol buffer). Under those conditions, we obtained non-reproducible co-immunoprecipitations (2 positives over 5 tentatives), probably due to a labile and transient interaction that has often been reported between protein kinases and their substrates. Therefore, we decided to express into CHO cells ICAP-1α either alone or with CaMKIIαT286D and to use a cross-linking reagent before immunoenrichment to stabilize the interaction. Indeed, treating the cells before lysis with a cell-permeable cross-linker greatly increased the level and efficiency of co-immunoprecipitation of ICAP-1α with CaMKIIα (ectopically or endogenously expressed) (Fig. 6a). Such experiments were also performed in Icap-1−/− osteoblasts and gave similar results (data not shown). Next, an in vitro phosphorylation assay using immunoprecipitated CaMKIIα and the purified recombinant ICAP-1α N-terminal moiety in the presence of Ca2+ and calmodulin (two co-factors essential for CaMKII activity) revealed that ICAP-1α can be phosphorylated by CaMKII (Fig. 6b), confirming that ICAP-1α is an actual substrate of CaMKII. To directly visualize the phosphorylation of the Thr-38 residue by CaMKII, we performed comparative in vitro CaMKII phosphorylation assays with wild-type ICAP-1α or its mutant (ICAP-1T38A). Indeed, we showed that the T38A mutation strongly diminished the ability of CaMKII to phosphorylate ICAP-1 compared with the wild-type form of ICAP-1 (Fig. 6c). Altogether, these results indicated that CaMKII phosphorylates ICAP-1α on threonine 38.
FIGURE 6.

In vitro phosphorylation of ICAP-1α by CaMKIIα. a, ICAP-1α association with αCaMKII was analyzed by immunoprecipitation of FLAG-ICAP-1α in CHO cells expressing or not expressing αCaMKIIT286D. Interaction between CaMKII and ICAP was analyzed after immunoprecipitation of FLAG-ICAP-1, and the presence of CaMKII was detected by Western blotting analysis using polyclonal CaMKII antibody. FLAG-ICAP co-immunoprecipitated ectopically expressed CaMKII (left) or endogenous CaMKII (middle), whereas control immunoprecipitation (when FLAG-ICAP was not expressed) did not immunoprecipitate CaMKII as expected (right). b, CaMKIIα immunoprecipitated from rat brain and recombinant N-terminal ICAP-1 (residues 1–99) were incubated with calmodulin, Ca2+, and [γ-32P]ATP. ICAP-1 phosphorylation was revealed by autoradiography. EGTA was added as a negative control of CaMKII-dependent ICAP-1 phosphorylation. c, schematic representation of ICAP-1α N- and C-terminal structures illustrating ICAP-1 head domain with the kinase phosphorylation sites and the PTB domain containing the β1A integrin binding site. c, His-ICAP-1wild-type or mutated form T38A, immobilized on nickel-nitrilotriacetic acid beads, was incubated with purified CaMKII in the presence of [γ-32P]ATP. GST protein immobilized on glutathione-Sepharose beads was used as control. CaMKII-induced phosphorylation was analyzed by autoradiography (top). The amount of recombinant proteins was determined with Coomassie staining (bottom).
DISCUSSION
In this study, we dissected the signaling pathway regulating the function of ICAP-1α, a negative regulator of β1 integrin activation. We showed that ICAP-1α phosphorylation by CaMKII enabled its interaction with the β1A integrin cytosolic tail by allowing the accessibility to the PTB domain and thereby regulating FA dynamics (Fig. 7).
Regulation of the capability of proteins to interact with effectors by unmasking cryptic binding sites through a phosphorylation-dependent process has been identified in several adhesion proteins, such as vinculin (35, 36), talin (37, 38), and ERM (ezrin, radixin, and moesin) proteins (39, 40). Recently, the ICAP-1α structure has been solved, and in line with the previous hypothesized model, ICAP-1α contains a PTB domain (which starts at residue 58) that is preceded by a presumably unstructured N-terminal part enriched in serine and threonine residues and containing most of the phosphorylation sites (32, 34). Our findings suggested that this region might block the accessibility of the PTB domain to the β1A integrin tail, thereby regulating ICAP-1α function. Phosphorylation at threonine 38 releases the cis-interaction and promotes ICAP-1α binding to β1A integrin. Although our study focused on CaMKII phosphorylation of ICAP-1α threonine 38, at this stage, we cannot rule out that phosphorylation at residues other than threonine 38 may also be involved in the regulation of ICAP-1α activity as well as the involvement of other protein kinases, such as PKC, PKG, or PKA. Indeed, a phosphomapping of Krit-1-associated ICAP-1α in the U2OS cell line did not identify the threonine 38, whereas a high throughput MS analysis of phosphorylated proteins has identified Thr-38 as a phosphorylation site (41, 42). This suggests the existence of other redundant phosphorylation sites or an integrin-specific regulation of ICAP-1α by CaMKII. Interestingly, in a very recent study (34), it has been proposed that Krit1 antagonizes the inactivating effect of ICAP-1α toward β1 integrins. This observation added a new way to regulate ICAP-1α inhibitory function and could be complementary to the phosphorylation control because ICAP-1α/Krit1 interaction is Thr-38 phosphorylation-independent.
Whereas PKC inhibition does not alter the ability of α5β1 integrin to bind FN (26), PKA has been shown to be involved in αvβ3 and α5β1 cross-talk by regulating VASP phosphorylation (43). Determination of whether PKA also controls ICAP-1α phosphorylation and function requires more work, but this could be an attractive hypothesis to explain how β3 integrins negatively cross-talk with β1 integrins.
In this study, we have accumulated a large body of evidence to show that CaMKII and ICAP-1α regulate focal adhesion dynamics in a linear pathway. CaMKII function in the control of cell adhesion has been demonstrated in several previous reports. CaMKII inhibition accelerates cell spreading on FN, whereas its constitutive activation decreases cell adhesion and destabilizes FAs (22, 26). CaMKII activity is largely controlled by an increase in cytoplasmic calcium content within the cell (44). Interestingly, a close relationship between integrins and calcium channels has been shown. Whereas α5β1 integrin engagement with FN induces a release of intracellular calcium stores, αvβ3 integrin binding to VN strongly reduces the intracellular calcium flux (45, 46). Moreover, αvβ3 and α5β1 engagement differentially regulates CaMKII activity. CaMKII activation upon α5β1 integrin ligation is inhibited by αvβ3 integrin binding to VN (25). Our present work opens the possibility that ICAP-1α plays an important role in integrin cross-talk and could provide the missing link between CaMKII and β1 integrin. In line with this, CaMKII activity was shown to be concentrated at the leading edge of cells, and this spatial distribution promotes cell motility and polarization (47). ICAP-1α is also localized in lamellipodia during the early stage of cell spreading together with β1 integrin (48). Thus, CaMKII activation at the leading edge may limit ICAP-1α activity to the lamellipodia, where adhesion assembly initially takes place.
ICAP-1α was previously reported to restrict the recruitment of talin and kindlin-2 onto β1 tail and to reduce FA assembly by maintaining the β1 integrin low affinity state (14, 20). Interestingly, CaMKII inhibition and ICAP-1α depletion as well as blocking ICAP-1α interaction onto the β1 tail strongly reproduced the ex vivo phenotype of the preactivated mutant β1D759A (20). CaMKII-induced ICAP-1α binding to β1 integrin might keep this integrin in its inactive form by limiting kindlin and talin recruitment and subsequently reducing the initiation of FA assembly (Fig. 7). Clearly, more work is still needed to further clarify the precise interplay between talin, kindlins, and ICAP-1α as well as the biological significance of such a dynamic process.
Acknowledgments
We thank R. Fässler for providing β1 integrin floxed mice, M. Humphries for critical reading, and A. Grichine for technical assistance in microscopy.
This work was supported by a Pro-INSERM grant, Ligue contre le Cancer, and INCa (to D. B.) and Ligue contre le Cancer (équipe labelisé 2010) (to C. A.-R.).
- ICAP
- integrin cytoplasmic domain-associated protein
- FN
- fibronectin
- VN
- vitronectin
- FA
- focal adhesion
- CaMKII
- calcium and calmodulin-dependent protein kinase of type II
- eGFP
- enhanced green fluorescent protein
- mRFP
- monomeric red fluorescent protein
- PTB
- phosphotyrosine binding
- VASP
- vasodilator-stimulated phosphoprotein.
REFERENCES
- 1. Hynes R. O. (2002) Integrins. Bidirectional, allosteric signaling machines. Cell 110, 673–687 [DOI] [PubMed] [Google Scholar]
- 2. Lauffenburger D. A., Horwitz A. F. (1996) Cell migration. A physically integrated molecular process. Cell 84, 359–369 [DOI] [PubMed] [Google Scholar]
- 3. Xiong J. P., Stehle T., Diefenbach B., Zhang R., Dunker R., Scott D. L., Joachimiak A., Goodman S. L., Arnaout M. A. (2001) Crystal structure of the extracellular segment of integrin α Vβ3. Science 294, 339–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shimaoka M., Takagi J., Springer T. A. (2002) Conformational regulation of integrin structure and function. Annu. Rev. Biophys. Biomol. Struct. 31, 485–516 [DOI] [PubMed] [Google Scholar]
- 5. Takagi J., Petre B. M., Walz T., Springer T. A. (2002) Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell 110, 599–611 [DOI] [PubMed] [Google Scholar]
- 6. Mould A. P., Humphries M. J. (2004) Regulation of integrin function through conformational complexity. Not simply a knee-jerk reaction? Curr. Opin. Cell Biol. 16, 544–551 [DOI] [PubMed] [Google Scholar]
- 7. Calderwood D. A., Yan B., de Pereda J. M., Alvarez B. G., Fujioka Y., Liddington R. C., Ginsberg M. H. (2002) The phosphotyrosine binding-like domain of talin activates integrins. J. Biol. Chem. 277, 21749–21758 [DOI] [PubMed] [Google Scholar]
- 8. Bouaouina M., Lad Y., Calderwood D. A. (2008) The N-terminal domains of talin cooperate with the phosphotyrosine binding-like domain to activate β1 and β3 integrins. J. Biol. Chem. 283, 6118–6125 [DOI] [PubMed] [Google Scholar]
- 9. Kloeker S., Major M. B., Calderwood D. A., Ginsberg M. H., Jones D. A., Beckerle M. C. (2004) The Kindler syndrome protein is regulated by transforming growth factor-β and involved in integrin-mediated adhesion. J. Biol. Chem. 279, 6824–6833 [DOI] [PubMed] [Google Scholar]
- 10. Shi X., Ma Y. Q., Tu Y., Chen K., Wu S., Fukuda K., Qin J., Plow E. F., Wu C. (2007) The MIG-2/integrin interaction strengthens cell-matrix adhesion and modulates cell motility. J. Biol. Chem. 282, 20455–20466 [DOI] [PubMed] [Google Scholar]
- 11. Moser M., Bauer M., Schmid S., Ruppert R., Schmidt S., Sixt M., Wang H. V., Sperandio M., Fässler R. (2009) Kindlin-3 is required for β2 integrin-mediated leukocyte adhesion to endothelial cells. Nat. Med. 15, 300–305 [DOI] [PubMed] [Google Scholar]
- 12. Moser M., Legate K. R., Zent R., Fässler R. (2009) The tail of integrins, talin, and kindlins. Science 324, 895–899 [DOI] [PubMed] [Google Scholar]
- 13. Moser M., Nieswandt B., Ussar S., Pozgajova M., Fässler R. (2008) Kindlin-3 is essential for integrin activation and platelet aggregation. Nat. Med. 14, 325–330 [DOI] [PubMed] [Google Scholar]
- 14. Bouvard D., Vignoud L., Dupé-Manet S., Abed N., Fournier H. N., Vincent-Monegat C., Retta S. F., Fassler R., Block M. R. (2003) Disruption of focal adhesions by integrin cytoplasmic domain-associated protein-1 α. J. Biol. Chem. 278, 6567–6574 [DOI] [PubMed] [Google Scholar]
- 15. Rantala J. K., Pouwels J., Pellinen T., Veltel S., Laasola P., Mattila E., Potter C. S., Duffy T., Sundberg J. P., Kallioniemi O., Askari J. A., Humphries M. J., Parsons M., Salmi M., Ivaska J. (2011) SHARPIN is an endogenous inhibitor of β1-integrin activation. Nat. Cell Biol. 13, 1315–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bouvard D., Aszodi A., Kostka G., Block M. R., Albigès-Rizo C., Fässler R. (2007) Defective osteoblast function in ICAP-1-deficient mice. Development 134, 2615–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brunner M., Millon-Frémillon A., Chevalier G., Nakchbandi I. A., Mosher D., Block M. R., Albigès-Rizo C., Bouvard D. (2011) Osteoblast mineralization requires beta1 integrin/ICAP-1-dependent fibronectin deposition. J. Cell Biol. 194, 307–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chang D. D., Wong C., Smith H., Liu J. (1997) ICAP-1, a novel β1 integrin cytoplasmic domain-associated protein, binds to a conserved and functionally important NPXY sequence motif of β1 integrin. J. Cell Biol. 138, 1149–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang X. A., Hemler M. E. (1999) Interaction of the integrin β1 cytoplasmic domain with ICAP-1 protein. J. Biol. Chem. 274, 11–19 [DOI] [PubMed] [Google Scholar]
- 20. Millon-Frémillon A., Bouvard D., Grichine A., Manet-Dupé S., Block M. R., Albiges-Rizo C. (2008) Cell adaptive response to extracellular matrix density is controlled by ICAP-1-dependent β1-integrin affinity. J. Cell Biol. 180, 427–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bouvard D., Millon-Fremillon A., Dupe-Manet S., Block M. R., Albiges-Rizo C. (2006) Unraveling ICAP-1 function. Toward a new direction? Eur. J. Cell Biol. 85, 275–282 [DOI] [PubMed] [Google Scholar]
- 22. Easley C. A., 4th, Brown C. M., Horwitz A. F., Tombes R. M. (2008) CaMK-II promotes focal adhesion turnover and cell motility by inducing tyrosine dephosphorylation of FAK and paxillin. Cell Motil. Cytoskeleton 65, 662–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bilato C., Curto K. A., Monticone R. E., Pauly R. R., White A. J., Crow M. T. (1997) The inhibition of vascular smooth muscle cell migration by peptide and antibody antagonists of the αvβ3 integrin complex is reversed by activated calcium/calmodulin- dependent protein kinase II. J. Clin. Invest. 100, 693–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lundberg M. S., Curto K. A., Bilato C., Monticone R. E., Crow M. T. (1998) Regulation of vascular smooth muscle migration by mitogen-activated protein kinase and calcium/calmodulin-dependent protein kinase II signaling pathways. J. Mol. Cell Cardiol. 30, 2377–2389 [DOI] [PubMed] [Google Scholar]
- 25. Blystone S. D., Slater S. E., Williams M. P., Crow M. T., Brown E. J. (1999) A molecular mechanism of integrin crosstalk. αvβ3 suppression of calcium/calmodulin-dependent protein kinase II regulates α5β1 function. J. Cell Biol. 145, 889–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bouvard D., Molla A., Block M. R. (1998) Calcium/calmodulin-dependent protein kinase II controls α5β1 integrin-mediated inside-out signaling. J. Cell Sci. 111, 657–665 [DOI] [PubMed] [Google Scholar]
- 27. Bouvard D., Block M. R. (1998) Calcium/calmodulin-dependent protein kinase II controls integrin α5β1-mediated cell adhesion through the integrin cytoplasmic domain associated protein-1α. Biochem. Biophys. Res. Commun. 252, 46–50 [DOI] [PubMed] [Google Scholar]
- 28. Bejar R., Yasuda R., Krugers H., Hood K., Mayford M. (2002) Transgenic calmodulin-dependent protein kinase II activation. Dose-dependent effects on synaptic plasticity, learning, and memory. J. Neurosci. 22, 5719–5726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bach M. E., Hawkins R. D., Osman M., Kandel E. R., Mayford M. (1995) Impairment of spatial but not contextual memory in CaMKII mutant mice with a selective loss of hippocampal LTP in the range of the θ frequency. Cell 81, 905–915 [DOI] [PubMed] [Google Scholar]
- 30. Rotenberg A., Mayford M., Hawkins R. D., Kandel E. R., Muller R. U. (1996) Mice expressing activated CaMKII lack low frequency LTP and do not form stable place cells in the CA1 region of the hippocampus. Cell 87, 1351–1361 [DOI] [PubMed] [Google Scholar]
- 31. Ballestrem C., Hinz B., Imhof B. A., Wehrle-Haller B. (2001) Marching at the front and dragging behind. Differential αVβ3-integrin turnover regulates focal adhesion behavior. J. Cell Biol. 155, 1319–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chang D. D., Hoang B. Q., Liu J., Springer T. A. (2002) Molecular basis for interaction between Icap1 α PTB domain and β1 integrin. J. Biol. Chem. 277, 8140–8145 [DOI] [PubMed] [Google Scholar]
- 33. Degani S., Balzac F., Brancaccio M., Guazzone S., Retta S. F., Silengo L., Eva A., Tarone G. (2002) The integrin cytoplasmic domain-associated protein ICAP-1 binds and regulates Rho family GTPases during cell spreading. J. Cell Biol. 156, 377–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu W., Draheim K. M., Zhang R., Calderwood D. A., Boggon T. J. (2013) Mechanism for KRIT1 release of ICAP1-mediated suppression of integrin activation. Mol. Cell 49, 719–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Johnson R. P., Craig S. W. (1994) An intramolecular association between the head and tail domains of vinculin modulates talin binding. J. Biol. Chem. 269, 12611–12619 [PubMed] [Google Scholar]
- 36. Johnson R. P., Craig S. W. (1995) F-actin binding site masked by the intramolecular association of vinculin head and tail domains. Nature 373, 261–264 [DOI] [PubMed] [Google Scholar]
- 37. Goksoy E., Ma Y. Q., Wang X., Kong X., Perera D., Plow E. F., Qin J. (2008) Structural basis for the autoinhibition of talin in regulating integrin activation. Mol. Cell 31, 124–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Martel V., Racaud-Sultan C., Dupe S., Marie C., Paulhe F., Galmiche A., Block M. R., Albiges-Rizo C. (2001) Conformation, localization, and integrin binding of talin depend on its interaction with phosphoinositides. J. Biol. Chem. 276, 21217–21227 [DOI] [PubMed] [Google Scholar]
- 39. Bretscher A., Gary R., Berryman M. (1995) Soluble ezrin purified from placenta exists as stable monomers and elongated dimers with masked C-terminal ezrin-radixin-moesin association domains. Biochemistry 34, 16830–16837 [DOI] [PubMed] [Google Scholar]
- 40. Martin M., Andréoli C., Sahuquet A., Montcourrier P., Algrain M., Mangeat P. (1995) Ezrin NH2-terminal domain inhibits the cell extension activity of the COOH-terminal domain. J. Cell Biol. 128, 1081–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Huttlin E. L., Jedrychowski M. P., Elias J. E., Goswami T., Rad R., Beausoleil S. A., Villén J., Haas W., Sowa M. E., Gygi S. P. (2010) A tissue-specific atlas of mouse protein phosphorylation and expression. Cell 143, 1174–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kim J., Sherman N. E., Fox J. W., Ginsberg M. H. (2011) Phosphorylation sites in the cerebral cavernous malformations complex. J. Cell Sci. 124, 3929–3932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Worth D. C., Hodivala-Dilke K., Robinson S. D., King S. J., Morton P. E., Gertler F. B., Humphries M. J., Parsons M. (2010) αvβ3 integrin spatially regulates VASP and RIAM to control adhesion dynamics and migration. J. Cell Biol. 189, 369–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. De Koninck P., Schulman H. (1998) Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science 279, 227–230 [DOI] [PubMed] [Google Scholar]
- 45. Wu X., Mogford J. E., Platts S. H., Davis G. E., Meininger G. A., Davis M. J. (1998) Modulation of calcium current in arteriolar smooth muscle by αvβ3 and α5β1 integrin ligands. J. Cell Biol. 143, 241–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sjaastad M. D., Lewis R. S., Nelson W. J. (1996) Mechanisms of integrin-mediated calcium signaling in MDCK cells. Regulation of adhesion by IP3- and store-independent calcium influx. Mol. Biol. Cell 7, 1025–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mercure M. Z., Ginnan R., Singer H. A. (2008) CaM kinase II δ2-dependent regulation of vascular smooth muscle cell polarization and migration. Am. J. Physiol. Cell Physiol. 294, C1465–C1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fournier H. N., Dupé-Manet S., Bouvard D., Lacombe M. L., Marie C., Block M. R., Albiges-Rizo C. (2002) Integrin cytoplasmic domain-associated protein 1α (ICAP-1α) interacts directly with the metastasis suppressor nm23-H2, and both proteins are targeted to newly formed cell adhesion sites upon integrin engagement. J. Biol. Chem. 277, 20895–20902 [DOI] [PubMed] [Google Scholar]
- 49. Engvall E., Ruoslahti E. (1977) Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int. J. Cancer 20, 1–5 [DOI] [PubMed] [Google Scholar]
- 50. Humphries J. D., Byron A., Bass M. D., Craig S. E., Pinney J. W., Knight D., Humphries M. J. (2009) Proteomic analysis of integrin-associated complexes identifies RCC2 as a dual regulator of Rac1 and Arf6. Sci. Signal. 2, ra51. [DOI] [PMC free article] [PubMed] [Google Scholar]