Background: Extracellular acidosis mediates pain and inflammation by activating sensory afferent neurons.
Results: Protons activate and sensitize human TRPA1 in a strongly species-specific manner encoded by transmembrane domains 5 and 6.
Conclusion: Our data identify TRPA1 as an ion channel likely to mediate acid-induced pain in humans.
Significance: Protons are the first known endogenous agonists of TRPA1 with species-specificity for human TRPA1.
Keywords: Acid-sensing Ion Channels (ASIC), Acidosis, Pain, Patch Clamp, TRP Channels, Nociceptor, Species Specificity
Abstract
The surveillance of acid-base homeostasis is concerted by diverse mechanisms, including an activation of sensory afferents. Proton-evoked activation of rodent sensory neurons is mainly mediated by the capsaicin receptor TRPV1 and acid-sensing ion channels. In this study, we demonstrate that extracellular acidosis activates and sensitizes the human irritant receptor TRPA1 (hTRPA1). Proton-evoked membrane currents and calcium influx through hTRPA1 occurred at physiological acidic pH values, were concentration-dependent, and were blocked by the selective TRPA1 antagonist HC030031. Both rodent and rhesus monkey TRPA1 failed to respond to extracellular acidosis, and protons even inhibited rodent TRPA1. Accordingly, mouse dorsal root ganglion neurons lacking TRPV1 only responded to protons when hTRPA1 was expressed heterologously. This species-specific activation of hTRPA1 by protons was reversed in both mouse and rhesus monkey TRPA1 by exchange of distinct residues within transmembrane domains 5 and 6. Furthermore, protons seem to interact with an extracellular interaction site to gate TRPA1 and not via a modification of intracellular N-terminal cysteines known as important interaction sites for electrophilic TRPA1 agonists. Our data suggest that hTRPA1 acts as a sensor for extracellular acidosis in human sensory neurons and should thus be taken into account as a yet unrecognized transduction molecule for proton-evoked pain and inflammation. The species specificity of this property is unique among known endogenous TRPA1 agonists, possibly indicating that evolutionary pressure enforced TRPA1 to inherit the role as an acid sensor in human sensory neurons.
Introduction
Protons interact with and modulate numerous membrane proteins within the peripheral and central nervous system and can act as excitatory or inhibitory cotransmitters regulating neuronal activity. Both ischemia and inflammation are often accompanied by pain, and the activation and sensitization of peripheral sensory neurons in both of these conditions are largely evoked by protons (1, 2). Electrophysiological evidence for a direct proton-evoked activation of sensory neurons was presented decades ago (3), and, meanwhile, the identities of several membrane proteins acting as proton sensors have been identified (4). Acid-sensing ion channels (ASICs)4 are expressed in a large population of sensory neurons and seem to contribute to acute and inflammatory pain (1, 2, 5, 6). ASICs generate transient Na+ currents upon modest extracellular acidification (pH < 7.0), and this response is potentiated by lactate and adenosine triphosphate, which are both released during ischemia (7, 8). Stronger acidosis (pH < 6.3) also activates the capsaicin receptor TRPV1 (9), and more modest acidification sensitizes TRPV1 for activation by other TRPV1 agonists (10). Similar to ASICs, TRPV1 is required for nociceptive and inflammatory pain (1, 9). Taking these properties of ASICs and TRPV1 into account, it is commonly assumed that they are key molecules for proton-evoked activation of sensory neurons and, thus, for proton-evoked pain (4). For obvious reasons, however, cellular studies exploring the mechanisms mediating proton-evoked activation in human sensory neurons are rare.
TRPA1 is known as a receptor for a large variety of exogenous and endogenous irritants and is crucially involved in several types of pain-related behavior in rodents (11, 12). It has been reported that both intracellular acidosis and alkalosis can activate rodent TRPA1 (13–15). Although the study from Wang et al. (14) claims that extracellular acidosis fails to activate rodent TRPA1, a previous study suggested that extracellular protons can evoke a calcium influx through human TRPA1 expressed in HEK-293 cells (16) TRPA1 is indeed subject to a significant species specificity, and several exogenous agonists and antagonists have been shown to elicit different effects on human and rodent TRPA1 (17–22). We therefore asked if extracellular protons interact with TRPA1 in a species-specific manner. By employing patch clamp and ratiometric calcium imaging in combination with site-directed mutagenesis, we obtained data revealing the molecular basis for an unambiguous species specificity of proton-evoked activation of human TRPA1.
EXPERIMENTAL PROCEDURES
cDNA and Transfection Procedures
The plasmids for human TRPA1 (hTRPA1) and hTRPA1-C621S/C641S/C665S (hTRPA1–3C) were provided by Dr. Sven-Eric Jordt (New Haven, CT). Mouse TRPA1 (mTRPA1); the chimeras mTRPA1-hTM5/6 and hTRPA1-mTM5/6; and the mutants hTRPA1-FGFATLIAM hTRPA1-FATL, hTRPA1-IAM, hTRPA1-V875G, and hTRPA1-S873L/T874L were provided by Dr. Ardem Patapoutian (La Jolla, CA). Rat TRPA1 (rTRPA1) was provided by Dr. David Julius (San Francisco, CA). Rhesus monkey TRPA1 (rhTRPA1) and the mutants were provided by Dr. Jun Chen (Abbott Laboratories, IL). All other mutants were generated by site-directed mutagenesis using the QuikChange II XL kit (Agilent Technologies, Santa Clara, CA) with a modified primer design (23). Fidelity of mutagenesis was confirmed by dideoxynucleotide sequencing. Plasmids were transiently expressed in HEK293t cells by using a nanofectin transfection kit according to the instructions of the manufacturer (PAA, Pasching, Austria). To visualize expression for patch clamp experiments, cells were cotransfected with pEGFP-N1 (0.5 μg, Clontech, Palo Alto, CA). After transfection, cells were replated into Petri dishes and used within 12–24 h for patch clamp recordings. Stably expressing hTRPA1-HEK293t cells were established by use of G418 (800 μg/ml). HEK-293t cells were cultured in DMEM (Invitrogen) supplemented with 10% fetal bovine serum (Biochrom, Berlin, Germany), 100 units/ml penicillin, and 100 μg/ml streptomycin (Invitrogen) at 37 °C and 5% CO2.
Electrophysiology
The pipette solution contained 140 mm KCl, 2 mm MgCl2, 5 mm EGTA, and 10 mm HEPES (pH 7.4) and was adjusted with KOH. The external calcium-free solution contained 140 mm NaCl, 5 mm KCl, 2 mm MgCl2, 5 mm EGTA, 10 mm HEPES, and 10 mm glucose (pH 7.4) and was adjusted with tetramethylammonium hydroxide. For calcium-containing experiments we used 140 mm NaCl, 5 mm KCl, 2 mm MgCl2, 10 mm HEPES (or 10 mm MES), 10 mm glucose, and 2 mm CaCl2. The osmolarity of all solutions was adjusted with glucose to 290–300 mosmol/liter. Patch pipettes were fabricated with borosilicate glass (Science Products, Hofheim, Germany) using a conventional puller (DMZ-Universal Puller, Zeitz Instrumente, Martinsried, Germany) and heat-polished to give a pipette resistance of 3–5 MΩ. Only one EGFP-cotransfected fluorescent cell/dish was used for experiments. Test solutions were applied via a gravity-driven perfusion system. Whole cell recordings were performed using a HEKA Electronics USB 10 amplifier combined with Patchmaster software (HEKA Electronics, Lambrecht, Germany). Currents were filtered at 1 kHz and sampled at 2 kHz. Offline analyses were carried out using Fitmaster software (HEKA) and Origin software (Origin 8.5.1 G, Origin Lab, Northampton, MA). Mean values and data for dose response curves are shown as mean ± S.E. Statistical significance was assessed with Student's t test (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
Ca2+ Imaging
For calcium imaging, DRG and HEK cells were stained by the fluorescent dye FURA-2 AM (5 μm) and 0.02% pluronic (both from Invitrogen) for 30 min. After a 15-min washout to allow ester hydrolysis, coverslips were placed in a glass chamber on an Olympus IX71 inverse microscope and superfused constantly with extracellular fluid (145 mm NaCl, 5 mm KCl, 1.25 mm CaCl2, 1 mm MgCl2, 10 mm glucose, 10 mm HEPES). Acetic solutions (pH 5, 6, and 6.4), carvacrol (250 μm), AITC (100 μm), and capsaicin (0.3 μm) were applied using a control unit-operated, seven-channel, gravity-driven common-outlet superfusion system. A 60 mm potassium (DRG cells) or 10 μm ionomycin stimulus (HEK cells) was applied as a control at the end of each experiment. Ca2+ influx was observed by FURA-2 excitation at 340 and 380 nm with a Polychrome V monochromator (Till Photonics). Emission was detected with corresponding filter at 510 nm. Images were exposed for 200 μs at a rate of 1 Hz with a 12-bit CCD camera (Imago Sensicam QE, Till Photonics, Gräfelfing, Germany). Fluorescence absorbance was analyzed with the integrated TILLvisION 4.0.1.3 software (Till Photonics). Ratios were calculated after background subtraction by setting regions of interest. Averaged results are reported as mean ± S.E. of the area under the curve (Δ ratio F340/380 nm).
DRG Neuron Cell Culture
Culturing of DRG neurons from C57Bl/6 mice and from congenic TRPA1 as well as TRPV1 knockout mice was performed as described previously (24). Briefly, mice were killed by CO2 inhalation, and DRGs from all levels were excised and transferred to DMEM containing 50 μg/ml gentamicin (Sigma Aldrich, Germany). Following treatment with 1 mg/ml collagenase and 0.1 mg/ml protease for 30 min (both from Sigma Aldrich), ganglia were dissociated using a fire-polished, silicone-coated Pasteur pipette. Isolated cells were transferred onto poly-D-lysine-coated (200 μg/ml, Sigma Aldrich) coverslips and cultured in TNB 100 medium supplemented with TNB 100 lipid protein complex, 100 μg/ml streptomycin, penicillin (all from Biochrom, Berlin, Germany), and mouse NGF (100 ng/ml, Almone Laboratories, Tel Aviv, Israel) at 37 °C and 5% CO2. Cells were used for experiments within 24 h after plating. TRPA1+/− mice were provided by Drs. Kelvin Kwan and David Corey (Harvard, Boston, MA), whereas TRPV1+/− mice were a gift from Dr. John Davis (formerly of Glaxco-Smith-Kline, Harlow, UK). All procedures of this study were approved by the animal protection authorities (local district government, Ansbach, Germany). For transfection of DRG neurons with hTRPA1, DRGs from TRPA1/TRPV1 double knockout mice were used and prepared as described above. Following dissociation of DRGs, neurons were immediately transferred into electroporation cuvettes containing 100 μl of nucleofector solution and 10 μg of hTRPA1 cDNA. Cells were electroporated with an Amaxa nucleofector, program A-033 (Lonza) and plated on coverslips after a 5-min recovery period in Ca2+-free medium. Neurons that underwent the electroporation procedure without added cDNA were used as controls.
Chemicals
Stock solutions of 100 mm carvacrol (Sigma-Aldrich) and 100 mm HC-030031 (Tocris, Bristol, UK) were prepared in DMSO. 1 m L-lactic acid, 100 mm DL-dithiothreitol, 100 mm amiloride, and 100 mm acrolein (Sigma-Aldrich) were dissolved in distilled water. All stock solutions were stored at −20 °C.
RESULTS
Species-specific Activation by Protons of Human TRPA1
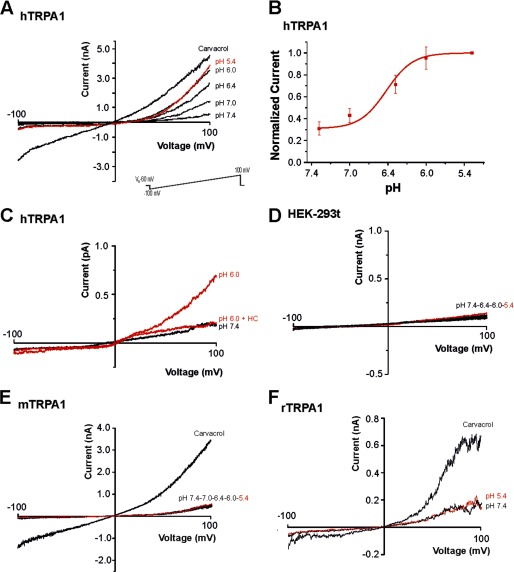
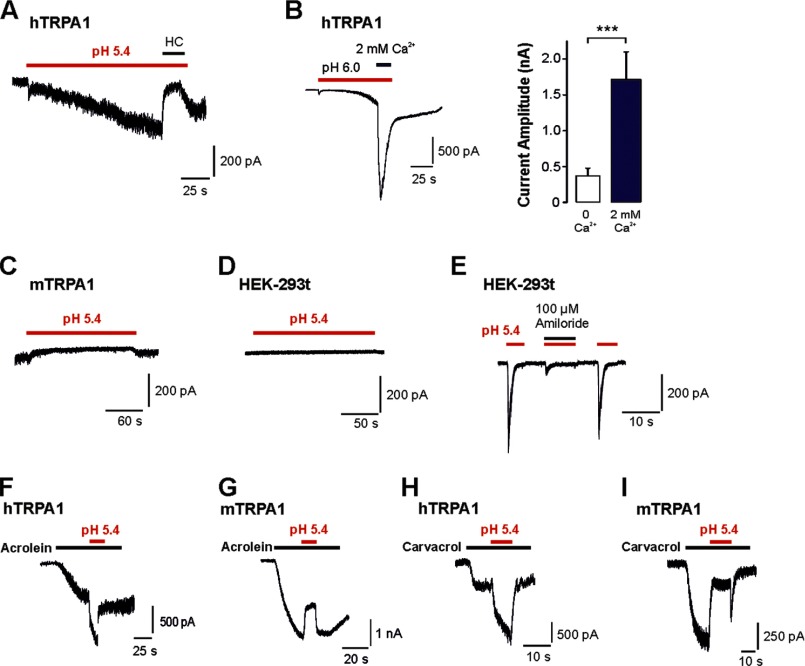
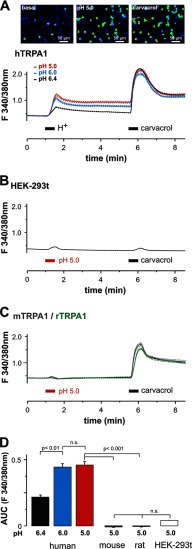
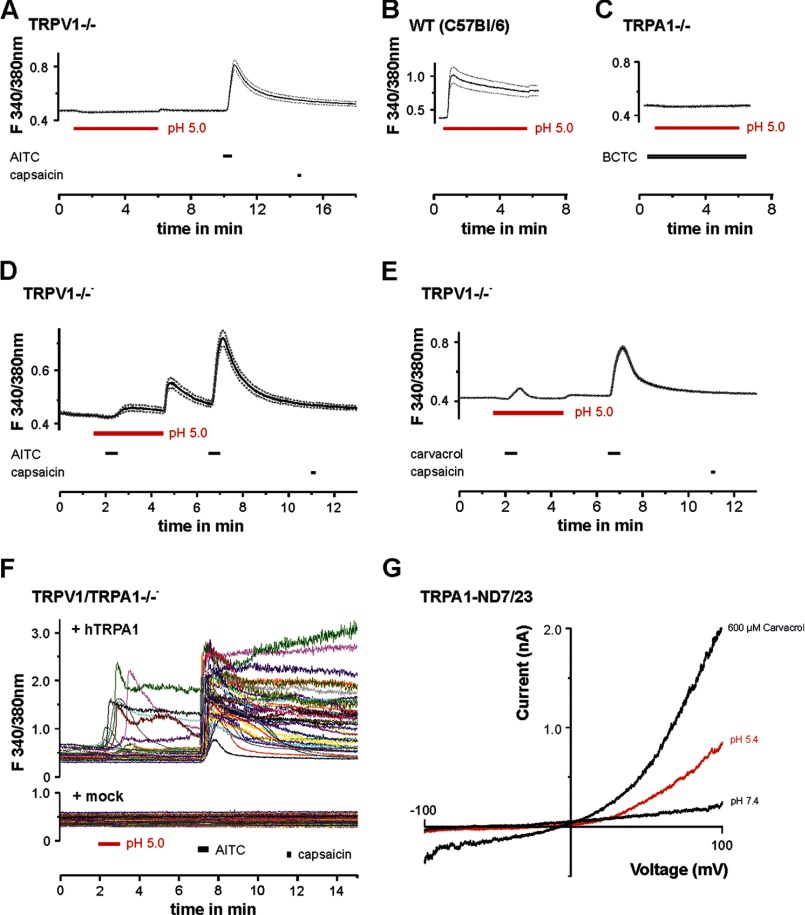
We first asked whether protons evoke different effects on human TRPA1 and rodent TRPA1. HEK-293t cells expressing hTRPA1, mTRPA1, or rTRPA1 were examined by means of whole cell patch clamp recordings. Because of the voltage dependence of TRPA1, currents evoked by most agonists typically exhibit an outward rectification (25). Therefore, the effects of protons on hTRPA1, mTRPA1, rTRPA1, and non-transfected HEK-293t cells were first explored with 500 ms-long voltage ramps from −100 mV to 100 mV (Fig. 1). Acidic solutions with pH 7.4, 7.0, 6.4, 6.0, and 5.4 elicited a concentration-dependent activation of outwardly rectifying membrane currents on hTRPA1 with a threshold around pH 7.0 and a calculated EC50 value of 6.5 ± 0.1 (n = 6; Fig. 1, A and B). These currents were fully reversible and could be reproduced even after several applications of protons (data not shown). As is demonstrated in Fig. 1C, 100 μm of the selective TRPA1 blocker HC030031 completely and reversibly inhibited pH-evoked membrane currents in cells expressing hTRPA1 (n = 6). These proton-evoked membrane currents were never observed in control experiments on non-transfected HEK-293t cells at pH values down to pH 5.4 (Fig. 1D). However, many non-transfected cells generated a prominent outward current when pH 5.0 was applied, i.e. an effect that did not require expression of TRPA1 (data not shown). This current was reported to originate from a constitutively expressed proton-activated outwardly rectifying anion channel activated by very low pH values (26). Therefore, membrane currents were only investigated down to pH 5.4. In contrast to hTRPA1, both mTRPA1 (n = 12, Fig. 1E) and rTRPA1 (n = 6, F) failed to generate membrane currents when acidic solutions down to pH 5.4 were applied. To mimic a more physiological situation, we next examined proton-evoked effects in cells held constantly at −60 mV. In this configuration, protons evoked slowly activating and persistent inward currents in cells expressing hTRPA1 (Fig. 2A). These responses were concentration-dependent (pH 7.0, 43 ± 12 pA, n = 6; pH 6.0, 130 ± 26 pA, n = 5; pH 5.4, 231 ± 58 pA, n = 17) and inhibited by 100 μm HC-030031 (n = 6, Fig. 2A). Another typical property of TRPA1 is a pronounced potentiation by extracellular calcium (27). Accordingly, 2 mm Ca2+-induced an impressive increase of proton-evoked currents through hTRPA1 (615 ± 122%, n = 6, Fig. 2B). Again, HEK-293t cells expressing mTRPA1 (Fig. 2C, n = 13) or rTRPA1 (data not shown) never generated persistent inward currents upon application of acidic solutions down to pH 5.4. Although most non-transfected cells did not produce any proton-evoked inwards currents (Fig. 2D), some non-transfected and transfected cells generated transient inward currents when challenged by acidic solutions (Fig. 2E). These currents were blocked by amiloride (100 μm, Fig. 2E) and have been reported previously to originate from constitutively expressed ASIC1a channels in HEK-293 cells (28). Because of their distinct kinetic properties, ASIC-like currents could easily be isolated from the slow inward currents mediated by hTRPA1. In HEK-293t cells expressing mTRPA1, we noticed that application of pH 5.4 induced a decrease in leak currents (Fig. 2C). Because this effect might be due to an inhibition of open TRPA1 channels, we further explored this effect by applying pH 5.4 on inward currents induced by acrolein or carvacrol on either hTRPA1 or mTRPA1. Although protons potentiated acrolein-evoked currents of hTRPA1 (+107 ± 37%, n = 6, Fig. 2F), they inhibited currents generated by mTRPA1 (-41 ± 6%, n = 5, G). The same species-specific differences were observed for carvacrol-evoked currents, i.e. potentiation of hTRPA1 (+210 ± 9%, n = 6, Fig. 2H) and inhibition of mTRPA1 (-91 ± 5%, n = 5, I). To substantiate the findings obtained by whole cell patch clamp recordings, we next performed Ca2+ imaging experiments. As demonstrated in Fig. 3A, the acidic solutions pH 6.4, 6.0, and 5.0 evoked an immediate (< 30 s) and only partly reversible increase of [Ca2+]i in a subset of cells responding to carvacrol (i.e. cells expressing hTRPA1; pH 6.4, 21% (31/148); pH 6.0, 45% (63/140); and pH 5.0, 48% (72/150)). In contrast, non-transfected HEK-293t cells (n = 670) and cells expressing mTRPA1 (n = 107) or rTRPA1 (n = 184) never displayed an increase of [Ca2+]i when challenged with pH 5.0 (Fig. 3, B and C). The area under the curve for proton-evoked increases of [Ca2+]i, calculated for the 60-s long application of protons, was significantly larger for hTRPA1 as compared with non-transfected cells and cells expressing mTRPA1 or rTRPA1 (Fig. 3D) (pH 5.0, all p < 0.001, analysis of variance). In contrast, the area under the curve for pH 5.0-evoked responses on mTRPA1- and rTRPA1-expressing cells were not significantly different from the area under the curve calculated for untransfected cells (p > 0.6, analysis of variance). When comparing the data obtained with patch clamp recordings as compared with those obtained with calcium imaging, the only relevant difference seems to be the finding that the majority of cells expressing hTRPA1 failed to evoked a detectable proton-evoked response in Ca2+ imaging experiments. We believe that this discrepancy between the two methods is due to an inhomogeneous transfection efficacy and the fact that protons are rather weak TRPA1 agonists as compared with carvacrol. Although patch clamp experiments only include vital cells displaying a strong expression of EGFP (and, thus, also hTRPA1), calcium imaging experiments covering large populations of cells also include cells with a low expression of hTRPA1. Nevertheless, both methods strongly indicate that hTRPA1, but not rodent TRPA1, can be directly activated by extracellular acidosis. The lack of proton-evoked responses of mTRPA1 is supported by previous studies on mouse DRG neurons, suggesting that TRPV1 is the predominant proton receptor in mouse DRG neurons (29, 30). We wanted to exclude the possibility that an improper functionality of recombinant mTRPA1 in HEK-293t cells is the reason for the lack of proton sensitivity observed in our experiments. We performed Ca2+ imaging experiments on DRG neurons derived from wild-type C56BL/6, TRPV1-null, TRPA1-null, and TRPV1/TRPA1-null mice. If mTRPA1 would mediate proton-evoked responses in DRG neurons, it should be best observed in DRG neurons lacking TRPV1. However, no increase of [Ca2+]i was observed when pH 5.0 was applied for 5 min to TRPV1-null DRG neurons (Fig. 4A, n = 181). As expected, the application of pH 5.0 on wild-type DRG neurons resulted in a large increase of [Ca2+]i in 51% of all neurons (n = 35, Fig. 4B). When the TRPV1 antagonist BCTC was coapplied with pH 5.0, this TRPV1-mediated proton response was blocked completely in neurons derived from TRPA1−/− neurons (n = 118, Fig. 4C). In agreement with our patch clamp data on mTRPA1 in HEK-293t cells, pH 5.0 inhibited TRPA1-mediated responses evoked by both 50 μm AITC (n = 83, Fig. 4D) and 300 μm carvacrol (n = 93, E). These data confirm that extracellular protons inhibit rather than activate mTRPA1 and that this effect is independent of the expression system. A related question was whether proton-evoked activation of hTRPA1 was due to an overexpression in HEK-293t cells. Therefore, DRG neurons derived from TRPV1/TRPA1 double knockout mice were transfected with hTRPA1 by electroporation. This procedure rendered 13% of all neurons sensitive to the TRPA1 agonist AITC (48/379). 21% (10/48) of all AITC-sensitive DRG neurons also responded briskly to the application of pH 5.0 (Fig. 4F). Thus, again, only a fraction of cells expressing hTRPA1 responded to protons. Importantly, both AITC and pH 5.0 did not evoke any increase of [Ca2+]i in DRG neurons in which a sham transfection was performed without hTRPA1 cDNA (n = 108) (Fig. 4F). We also observed hTRPA1-mediated membrane currents being activated by pH 5.4 in transfected neuroblastoma ND7/23 cells (n = 4) (Fig. 4G).
FIGURE 1.
Protons activate hTRPA1. Currents were recorded during a 500-ms voltage ramp from −100 mV to 100 mV in cells held at −60 mV. A, membrane currents evoked by acidic solutions at pH 7.4, 7.0, 6.4, 6.0, and 5.4 and by 300 μm carvacrol on hTRPA1. B, concentration response curve for proton-evoked outward currents through hTRPA1. Peak current amplitudes at 100 mV were determined for all pH values and normalized to the amplitude evoked by pH 5.4. The line represents a fit of the data to the Hill equation. C, proton-evoked currents through hTRPA1 were blocked by the TRPA1 antagonist HC030031 (100 μm). Cells we challenged with pH 6.0 with and without HC030031. D, membrane currents in non-transfected HEK-293t cells challenged with acidic solutions. E and F, membrane currents evoked by acidic solutions and by 300 μm carvacrol in cells expressing mTRPA1 (E) or rTRPA1 (F).
FIGURE 2.
Protons activate inward currents through hTRPA1. Currents were recorded in cells held at −60 mV. A, typical inward current evoked by pH 5.4 in a cell expressing hTRPA1. Note that coapplication of pH 5.4 with 100 μm HC-030031 (HC) resulted in a complete inhibition of the proton-evoked inward current. B, typical current trace displaying a proton-evoked current through hTRPA1 that is potentiated by 2 mm Ca2+. The column bars displays the average peak current amplitudes of currents evoked by pH 6.0 in the presence of 0 and 2 mm Ca2+. Data are expressed as mean ± S.E. C and D, typical responses evoked by application of pH 5.4 in cells expressing mTRPA1 (C) or in non-transfected HEK 293t cells (D). E, ASIC-like transient inward currents evoked by pH 5.4 in a non-transfected cell. Note that coapplication of pH 5.4 with 100 μm amiloride resulted in an almost complete inhibition of the ASIC-like inward current. F–I, current traces displaying the effects of pH 5.4 on acrolein- and carvacrol-evoked inward currents through hTRPA1 (F and H) and mTRPA1 (G and I). pH 5.4 was coapplied with acrolein or carvacrol when inward currents evoked by these agonists had reached a steady state.
FIGURE 3.
Protons evoke calcium influx through hTRPA1 but not through rodent TRPA1. A, upper panel, representative snapshots of hTRPA1-expressing cells loaded with FURA-2 during application of control solution (basal), pH 5.0, and 300 μm carvacrol. Lower panel, average effects of pH 6.4 (n = 148), 6.0 (n = 140), and 5.0 (n = 150) as well as 300 μm carvacrol on intracellular calcium in cells expressing hTRPA1 as determined by ratiometric imaging. Protons and carvacrol were both applied for 30 s. B and C, average effects of pH 5.0 on non-transfected HEK-293t cells (B) and on cells expressing mTRPA1 (C) or rTRPA1 (C) as determined by ratiometric imaging. Note that the minimal effect caused by application of protons and carvacrol in HEK-293t cells is due to an application artifact. D, areas under the curve for the average responses evoked by protons in hTRPA1, mTRPA1, rTRPA1, and non-transfected HEK-293t cells. All data are displayed as mean ± S.E. n.s., not significant.
FIGURE 4.
Proton-evoked calcium influx in mouse DRG neurons depends on TRPV1. A–C, ratiometric imaging of intracellular Ca2+ examined on DRG neurons derived from TRPV1−/− mice (A) (n = 181), wild-type C57Bl/6 mice (B), and TRPA1−/− mice (C). Protons were applied for 5 min. Although protons completely failed to evoke a calcium influx in neurons lacking TRPV1 (A), protons evoked a fast increase of intracellular calcium in wild-type neurons (B). This TRPV1-mediated calcium influx in neurons lacking TRPA1 could be blocked completely by the TRPV1 antagonist BCTC (10 μm) (C). D and E, protons inhibit calcium influx mediated by TRPA1 in TRPV1−/− DRG neurons. Coapplication of pH 5.0 and 100 μm AITC resulted in a suppression of calcium influx, as became obvious by a rebound effect following the washout of protons (D). Protons were applied for 5 min and AITC for 30 s. Coapplication of pH 5.0 and 300 μm carvacrol resulted in a suppression of calcium influx (E). Protons were applied for 5 min and carvacrol for 30 s. Data in A–E are displayed as mean ± S.E. F, original Ca2+ imaging recordings on DRG neurons derived from TRPV1/TRPA1−/− mice. Protons were applied for 60 s, AITC for 30 s, and capsaicin for 20 s. Upper panel, recordings on neurons transfected with hTRPA1. Note that a subpopulation of neurons responding to AITC also responded to pH 5.0. Lower panel, sham-transfected cells failed to respond to both protons and AITC. G, representative membrane currents evoked by pH 5.4 and 600 μm carvacrol in ND7/23 cells expressing hTRPA1. Currents were recorded as described in Fig. 1.
Determinants of Species-specific Actions of Protons on TRPA1
We next asked how the species specificity of proton-evoked effects on TRPA1 is determined. Previous studies showed that the transmembrane (TM) domains 5 and 6 contain important residues for distinct effects of agonists and antagonists on human versus rodent TRPA1 (18, 21). Although rat and mouse TRPA1 share 100% sequence homology within TM domains 5/6, this region contains 17 amino acids that are not identical in human versus rodent TRPA1 (Fig. 5A). Therefore, we examined the effects of protons on the chimeras hTRPA1-mTM5/6 and mTRPA1-hTM5/6 in which the segments encompassing TM5/6 were swapped between hTRPA1 and mTRPA1 (21). The chimera mTRPA1-hTM5/6 generated robust membrane currents evoked by pH 5.4, and these currents were blocked by HC030031 (at −60 mV, 411 ± 159 pA, n = 11, Fig. 5B). When the same cells were investigated with Ca2+ imaging, 14% (164/1169) of all carvacrol-sensitive cells generated a large increase of [Ca2+]i following application of pH 5.0 (Fig. 5C). Although the percentage of mTRPA1-hTM5/6 expressing cells responding to protons was considerably lower as compared with wild-type hTRPA1, these data indicate that the insertion of human TM5/6 into mTRPA1 resulted in a de novo proton sensitivity. Furthermore, mTRPA1-hTM5/6 generated acrolein-evoked inward currents that were not inhibited by pH 5.4 (n = 6, Fig. 5D). In contrast to mTRPA1-hTM5/6, the reverse chimera (hTRPA1-mTM5/6) failed to generate proton-evoked membrane currents (n = 8, Fig. 5E) and an increase of [Ca2+]i (0/228, F). Moreover, potentiation of acrolein-evoked inward currents by pH 5.4 was only marginal (n = 8, Fig. 5G). Thus, the effects elicited by extracellularly applied protons could almost be reversed perfectly by the exchange of TM domains 5/6 between hTRPA1 and mTRPA1. Interestingly, Xiao et al. (21) identified nine of the 17 non-identical amino acids in human and mouse TM domains 5/6 to be collectively relevant for the species-specific effects of menthol on hTRPA1 (activation) and mTRPA1 (inhibition and activation). Because we observed similar differences between hTRPA1 (activation) and mTRPA1 (inhibition) in regard to proton sensitivity, we asked whether these nine amino acids have the same impact on proton sensitivity. The mutant construct hTRPA1-FGFATLIAM, in which these nine amino acids were exchanged to the corresponding residues in mTRPA1, did not generate any proton-evoked membrane currents or proton-evoked increase of [Ca2+]i (data not shown). Consequently, we tried to identify the amino acids rendering hTRPA1-FGFATLIAM proton insensitive by exploring the mutant constructs hTRPA1-FATL and hTRPA1-IAM. Both mutants were completely proton-insensitive in Ca2+ imaging experiments (data not shown), suggesting that more than one residue encodes the proton-insensitive phenotype of hTRPA1-FGFATLIAM and, thus, also for wild-type mTRPA1. On the other hand, it did not exclude the possibility that other residues than those exchanged in hTRPA1-FGFATLIAM might also be required for proton sensitivity. To test this possibility, we examined the relevance of two amino acids that typically interact with protons in other proton-sensitive ion channels. When screening the non-identical amino acids in TM domains 5/6 of hTRPA1 and mTRPA1, we identified Glu-920 and His-933 as possible interaction sites for protons in hTRPA1. We constructed the mutations hTRPA1-E920D and hTRPA1-H933Y but also the reverse mutant constructs mTRPA1-D923E and mTRPA1-Y936H. Both hTRPA1-E920D and hTRPA1-H933Y displayed a preserved proton sensitivity, i.e. protons evoked membrane currents and an increase in [Ca2+]i (data not shown). The mutant mTRPA1-D923E failed to produce an increase of [Ca2+]i when challenged by pH 5.0 (data not shown). The mutant mTRPA1-Y936H was non-functional.
FIGURE 5.
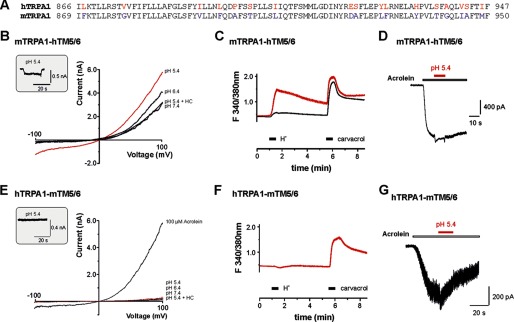
Transmembrane domains 5 and 6 dictate the species-specific effects of protons on TRPA1. A, alignment of TM domains 5/6 from hTRPA1 and mTRPA1. Non-identical amino acids are marked by colored letters. Amino acids involved in the multiple mutant hTRPA1-FGFATLIAM are underlined, and putative binding sites for protons are marked by bold letters. B and E, membrane currents of mTRPA1-hTM5/6 (B) and hTRPA1-mTM5/6 (E) were examined as described in Fig. 1. mTRPA1-hTM5/6 generated prominent proton-evoked inward currents at −60 mV (inset) and ramp currents upon application of pH 6.4 and 5.4. 100 μm HC030031 (HC) blocked outward currents evoked by pH 5.4. In contrast, hTRPA1-mTM5/6 failed to generate both inward currents (inset) and prominent outward currents when challenged by pH 6.4 and 5.4. C and F, typical acrolein-evoked inward currents through mTRPA1-hTM5/6 (C) and hTRPA1-mTM5/6 (F) (n = 8) modified by pH 5.4. C, coapplication of pH 5.4 with 100 μm acrolein resulted in a small potentiation of the acrolein-evoked current in mTRPA1-hTM5/6, and this effect was even smaller in hTRPA1-mTM5/6 (F). Cells were held at −60 mV, and protons were coapplied with acrolein when the inward current evoked by acrolein alone had reached a steady state. D and G, ratiometric calcium imaging on mTRPA1-hTM5/6 (D) and hTRPA1-mTM5/6 (G) expressed in HEK-293t cells. Protons and carvacrol were both applied for 30 s. Note that only a small fraction (14%) of all recorded carvacrol-sensitive cells also responded to pH 5.0 (average trace with the large peak following application of protons). When the mean response of all investigated cells was depicted, no significant proton-evoked effect could be observed (average trace lacking an effect following application of protons). hTRPA1-mTM5/6 completely failed to produce a calcium influx when pH 5.0 was applied but responded briskly upon application of 300 μm carvacrol.
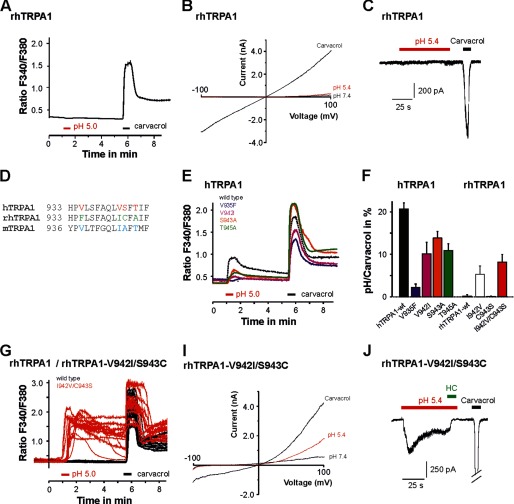
As an alternative approach to identify residues being relevant for proton sensitivity of TRPA1 and to further explore how specific proton-gated activation is for hTRPA1, we next investigated the proton sensitivity of TRPA1 from rhTRPA1. The sequence homology between rhTRPA1 und hTRPA1 is 98%, and a recent report suggests that they display almost identical functional and pharmacological properties (22). Surprisingly, we found that rhTRPA1 failed to produce an increase of [Ca2+]i when challenged by pH 5.0 (0/184, Fig. 6A) and to generate proton-evoked ramp currents or inward currents (n = 9; Fig. 6, B and C). An alignment of TM domains 5/6 from rhTRPA1 and hTRPA1 revealed four non-identical amino acids (Fig. 6D). The exchange of any of these four amino acids in hTRPA1 resulted in a decrease in proton sensitivity as examined with Ca2+ imaging (Fig. 7E). In wild-type hTRPA1, the magnitude of the pH 5.0-evoked calcium response was 21 ± 1% of that observed for the response evoked by carvacrol. This normalized response was reduced to 10 ± 3% for hTRPA1-V942I, 2 ± 1% for hTRPA1-V935F, 14 ± 2% for hTPRA1-S943A, and 11 ± 2% for hTRPA1-T945A (n = 117–224, analysis of variance F(4,1168) = 17, HSD post-hoc tests p < 0.001 versus wild type, Fig. 6F). Although patch clamp experiments on these mutants still revealed small proton-evoked inward currents, we found that the functional expression of hTRPA1-V935F was very poor (data not shown). Therefore, the apparent loss of proton sensitivity found for hTRPA1-V935F might not be specific. To verify that these amino acids indeed encode for proton sensitivity, we next examined if the reverse exchanges in rhTRPA1 results in a gain of proton sensitivity. Among the four single amino acid mutants, Ca2+ imaging experiments revealed that rhTRPA1-I942V conveyed the highest sensitivity to pH 5.0 (5 ± 2% of the carvacrol response, p = 0.003, n = 212, Student's t test compared with the rhTRPA1 wild type, Fig. 6F). Although rhTRPA1-C943S did not display a relevant proton sensitivity in these experiments, the combination of both mutations in rhTRPA1-I942V/C943S displayed the most robust proton sensitivity of all investigated rhTRPA1 mutants (8 ± 2% of the carvacrol response, p = 0.002, n = 270, Student's t test compared with the rhTRPA1 wild type, Fig. 7, F and G). This finding fits the results of the reverse mutants in hTRPA1, where the exchange of both Val-942 and Ser-943 resulted in a significant reduction of proton sensitivity. Even if the percentage of cells expressing rhTRPA1-I942V/C943S displaying proton sensitivity (7%, 20/270) was lower than that found for wild-type hTRPA1, it displayed a clearly changed functional phenotype in regard to proton sensitivity when compared with the completely proton insensitive wild-type rhTRPA1 (Fig. 7G). This finding was supported further by patch clamp experiments. In contrast to wild-type rhTRPA1, rhTRPA1-I942V/C943S generated prominent membrane currents when voltage ramps were applied (Fig. 7I, n = 7). Furthermore, rhTRPA1-I942V/C943S produced prominent inward currents evoked by pH 5.4 in the presence of 2 mm Ca2+ in the cell held at −60 mV (Fig. 7J, n = 5). Taken together, these data prove that the poorly conserved amino acids 942 and 943 are of significant importance for the proton sensitivity of hTRPA1.
FIGURE 6.
Specific residues within TM domains 5/6 dictate the effects of protons on hTRPA1 and rhTRPA1. A, calcium imaging on cells expressing wild-type rhTRPA1 challenged with pH 5.0 and 300 μm carvacrol. B, typical ramp currents elicited in the presence of pH 7.4, pH 5.4, and 300 μm carvacrol in cells expressing rhTRPA1. Currents were examined as described in Fig. 1. C, current trace on a cell expressing rhTRPA1 examined at −60 mV. Note that rhTRPA1 completely failed to respond to pH 5.4, whereas 300 μm carvacrol elicited large inward currents. D, alignment of TM domain 6 from hTRPA1, rhTRPA1, and mTRPA1. Non-identical amino acids are marked by colored letters. E, average calcium imaging responses of cells expressing the hTRPA1 wild type, hTRPA1-V935F, hTRPA1-V942I, hTRPA1-S943A, and hTRPA1-T945A or rhTRPA1-wild type, rhTRPA1-I942V, rhTRPA1-C943S, and rhTRPA1–942V/C943S. Cells were challenged by pH 5.0 and 300 μm carvacrol. F, proton-evoked responses of hTRPA1 mutant constructs, normalized to the response obtained by carvacrol. G, original calcium imaging traces on cells expressing the rhTRPA1 wild type (black) or rhTRPA1–942V/C943S (red), again challenged by protons and carvacrol as described in E. I, representative membrane currents of the mutant rhTRPA1-I942V/C943S, examined as described in Fig. 1. Note that this mutant produces outwardly rectifying current evoked by protons. J, representative proton-evoked (pH 5.4) inward currents in cells expressing wild-type rhTRPA1-I942V/C943S, rhTRPA1-I942V, and rhTRPA1-C943S. Recordings were performed in an extracellular solution containing 2 mm Ca2+. The inward current was blocked by coapplication of 100 μm HC030031 (HC).
FIGURE 7.
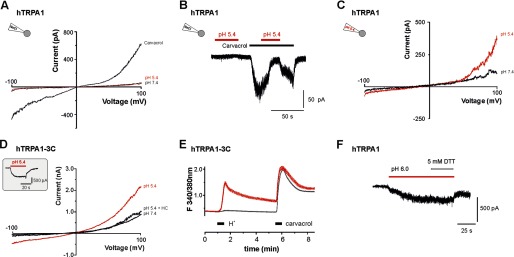
Activation of hTRPA1 by protons is not mediated by an interaction with intracellular cysteines. A and B, protons (pH 5.4) fail to activate and sensitize membrane currents through hTRPA1 when examined in cell-attached recordings. Ramp currents evoked by 500 ms long voltage ramps from −100 to 100 mV revealed outwardly rectifying currents evoked by 300 μm carvacrol but not by protons (A). Protons also failed to potentiate carvacrol-evoked currents recorded in the cell-attached mode (B). Protons were coapplied with carvacrol when the current evoked by carvacrol alone had reached a steady state. C, typical ramp current on hTRPA1 recorded in whole cell mode with an intracellular pH 5.4. Ramp currents were activated as described in Fig. 1. Note that pH 5.4 gates hTRPA1, although an intracellular acidosis is already established. D and E, current traces of hTRPA1-C621S/C641S/C665S (hTRP1–3C) evoked by pH 5.4 and 300 μm carvacrol. Ramp currents and inward currents (inset) through hTRPA1–3C could be evoked by pH 5.4. Note that 100 μm HC030031 completely blocked the membrane currents induced by pH 5.4 (D). E, average responses of hTRPA1–3C to application of pH 5.0 (30 s) and carvacrol (100 μm, 30 s) as examined by calcium imaging. Note that only a small fraction (6%) of all recorded carvacrol-sensitive cells also responded to pH 5.0 (average trace with the large peak following application of protons). When the mean response of investigated cells was depicted, no significant proton-evoked effect could be observed (average trace lacking an effect following application of protons). F, proton-evoked inward current that is not reduced by 5 mm DTT. DTT was applied together with pH 6.0 when inward currents evoked by pH 6.0 alone had reached a steady state.
Protons Activate hTRPA1 via an Extracellular Mechanism
Now we had identified TM domains 5/6 as an important region and the amino acids Val-942 and Ser-943 as important determinants for proton sensitivity of hTRPA1. However, the data did not allow us to postulate a molecular mechanism for proton-evoked activation of hTRPA1. Studies on both ASICs and TRPV1 have shown that the mechanism for proton-evoked activation can be complex and involve several residues throughout the channel protein (31–34). Thus, an unbiased screen for putative interaction sites for protons on hTRPA1 would go beyond the scope of this study. However, it was demonstrated recently that weak acids activate rodent TRPA1 by inducing an intracellular acidosis and that protons directly activate rodent TRPA1 when applied onto the cytosolic side (14, 15). On the other hand, Takahashi et al. (16) demonstrated that hTRPA1 produces a proton-evoked increase of [Ca2+]i and suggested that the N-terminal cysteines 414 and 421 are required for this effect. As extracellularly applied protons can cross the membrane to induce intracellular acidosis (36), we explored the possibility that the exclusive activation of hTRPA1 by protons is due to an intracellular acidification and a successive modification of N-terminal cysteines. We first performed cell-attached patch clamp recordings on HEK-293t cells expressing hTRPA1. This configuration allows to record channels that are isolated within the pipette orifice and, thus, not confronted with the acidic solution from the extracellular side. Although 300 μm carvacrol evoked a robust response, extracellular protons failed to gate hTRPA1 in this configuration (n = 5, Fig. 7A). Moreover, carvacrol-evoked responses were inhibited by protons in this configuration (n = 8, Fig. 7B). If an intracellular acidification would be decisive for activation of hTRPA1 by protons applied from the extracellular site, a pre-existing intracellular acidosis might inhibit this effect by desensitization. Therefore, whole cell recordings with an acidic pipette solution (pH 5.4) were performed. Extracellularly applied protons (pH 5.4) still evoked small but reproducible currents in these experiments (n = 6, Fig. 7C). We also explored the proton sensitivity of the mutant construct hTRPA1-C621S/C641S/C665S (hTRPA1–3C), which is known to be insensitive to several electrophilic agonists (36, 37). hTRPA1–3C displayed a preserved proton sensitivity in patch clamp experiments (pH 5.4, 235 ± 52 pA, n = 7, Fig. 7D). Cells expressing hTRPA1–3C also responded to protons when examined with Ca2+ imaging (Fig. 7E). However, the percentage of hTRPA1–3C-expressing cells (6%, 117/1944) responding to pH 5.0 was considerably lower than for cells expressing wild-type hTRPA1. Finally, the reducing agent DTT (5 mm), which is able to reverse electrophilic cysteine interactions, did not inhibit proton-evoked currents of hTRPA1 (n = 6, Fig. 7F). Taken together, these experiments did not support the possibility that proton-evoked activation of hTRPA1 is mediated by an intracellular mechanism involving oxidation or modification of N-terminal cysteines.
DISCUSSION
In this study, we identified TRPA1 as a transduction molecule for activation of human sensory neurons by protons. This property of hTRPA1 is subject to a striking species specificity, and our data indicate that several residues within transmembrane domains 5 and 6 encode this specificity. To our knowledge, protons are the first identified endogenous TRPA1 agonists that display this exclusive species specificity. Distinct properties of TRPA1 from different species have already been acknowledged to complicate the development of TRPA1-modifying therapeutics (17, 22). Thus, our data not only unravel the yet unrecognized property of hTRPA1 as a sensor for extracellular acidosis but also strengthen the notion that the species of TRPA1 matters.
Early electrophysiological experiments document excitatory effects of protons on rodent DRG neurons (3, 38) and C-fibers (39). In rat and mouse DRG neurons, protons evoke a fast transient current followed by a slow sustained current (30, 38). Although the transient current is generated by ASICs, TRPV1 generates the sustained current (29, 30). Moreover, rodent ASIC3 generates a sustained current that was suggested to contribute to persistent activation of sensory neurons (40). Although several other membrane molecules in sensory neurons are modulated by protons, ASICs and TRPV1 are regarded as the principle transducers driving proton-evoked activation of sensory neurons (4). Interestingly, Baumann et al. (41, 42) identified a delayed, slowly activating and inactivating proton-evoked inward current in about 50% of cultured human DRG neurons. This current has never been reported in rodent sensory neurons, but its properties accurately describe the proton-evoked currents we observe on hTRPA1. As to the suggested ability of ASIC3 to generate a sustained current mediating persistent pain (40), Delaunay et al. (43) recently demonstrated that human ASIC3 does not generate a sustained current during acidosis. Unfortunately, we do not have access to vital human DRG neurons, which would allow us to verify our findings. However, we show that hTRPA1 generates proton-evoked responses when expressed in mouse DRG neurons lacking both mTRPA1 and TRPV1. As was already described in the first study on TRPV1-null mice, we could also confirm that mouse DRG neurons lacking TRPV1 fail to respond to pH 5.0 when examined with Ca2+ imaging (29). Thus, mTRPA1 fails to respond to protons even if expressed in native DRG neurons, and the proton sensitivity of hTRPA1 observed in HEK-293t cells is not due to the non-neuronal expression system.
It is important to note that the relevance of proton-evoked activation of hTRPA1 found in our study is yet to be determined in more physiological models on human volunteers. However, such studies would require a specific TRPA1 blocker permitted for human use, and we are not aware of any such substances. Interestingly, a continuous infusion of acidic buffer (pH 5.2) into the forearm skin of healthy volunteers evokes a sustained pain driven by a non-adapting excitation of nociceptors (44, 45). ASICs were shown to undergo inactivation during sustained acidosis (46). Similarly, TRPV1 displays a strong tachyphylaxis upon prolonged activation (47). Thus, it is possible that TRPA1 drives this proton-evoked, non-adapting activation of nociceptors in human skin. In similar human pain models, pain induced by moderate acidosis was found to be reduced by amiloride but enhanced by the NO donor glyceryl trinitrate (48, 49, 50). Because ASICs are blocked by amiloride and potentiated by NO, the authors of these studies concluded that ASICs are likely to be predominant transducers for acid-evoked pain in human skin. More recent reports demonstrated that the actions of both these substances are not specific for ASICs, i.e. high concentrations of amiloride inhibit TRPA1 (51), and NO activates TRPA1 (52). Together with these reports, our cellular data suggest that TRPA1 might also act as a transducer for proton-evoked pain in humans.
Proton-evoked membrane currents through hTRPA1 displayed a pronounced outward rectification and were blocked by HC030031 and potentiated by extracellular calcium, i.e. current properties that are commonly observed for many TRPA1 agonists. Unlike electrophilic TRPA1 agonists, protons do not seem to interact with the N-terminal cysteines Cys-621, Cys-641, and Cys-665 to gate hTRPA1 (36, 37). Because a specific interaction between protons and cysteines cannot be expected, this finding might seem trivial. However, Takahashi et al. (16) suggested that the cytoplasmic cysteines 414 and 421 are required for activation of hTRPA1 by protons and, thus, we had good reasons to investigate this possibility. It is also important to note that all of the above mentioned N-terminal cysteines are conserved in human, rodent, and rhesus monkey TRPA1. Taking into account the exclusive activation of hTRPA1 by extracellular protons, it seems reasonable that protons target other residues than the N-terminal cysteines to gate TRPA1. Although protons can rapidly permeate through the membrane to induce an intracellular acidosis (35), we also argue that protons are likely to target extracellularly located residues to activate hTRPA1. This notion is in part on the basis of our experiments in the cell-attached mode by which protons completely failed to activate or potentiate hTRPA1. Furthermore, both human and rodent TRPA1 are activated by intracellular protons (14, 15). This property more or less excludes an intracellular mechanism for the specific activation of hTRPA1 by extracellular protons. When it comes to identifying putative binding sites for protons on TRPA1, studies on ASICs and TRPV1 have shown that more than one amino residue can be involved. Although several interaction sites for protons have been identified on both ASICs and TRPV1, the exact mechanisms for activation by protons are still to be defined (31–34). Our data strongly suggest that the architecture of the transmembrane domains 5 and 6 dictate the pronounced species specificity of proton sensitivity of TRPA1. The complete exchange of TM domains 5/6 or mutations within this region rendered both mTRPA1 and rhTRPA1 proton-sensitive or hTRPA1 proton-insensitive. It is clear that more than one of the non-identical amino acids within TM domains 5/6 of mouse, rhesus monkey, and human TRPA1 encode for these effects. In case of hTRPA1, the reduced or abolished proton sensitivity of the constructs hTRPA1-mTM5/6, hTRPA1-FGFATLIAM, hTRPA1-FATL, hTRPA1-IAM, hTRPA1-S943A, and hTRPA1-V942I demonstrates that the mechanisms enabling activation by protons is rather fragile. On the other hand, our data do not allow us to postulate a defined motif other than TM domains 5/6, which renders rodent TRPA1 proton-sensitive. The characterization of rhTRPA1 allowed us to draw more definitive conclusions in regard to which amino acids are crucial for proton sensitivity. As rhTRPA1 and hTRPA1 share 97% sequence homology and display almost identical pharmacological properties (22), we were surprised to observe that wild-type rhTRPA1 is completely proton-insensitive. We can conclude that the identities of the residues 942 and 943 can essentially dictate whether or not rhTRPA1 is activated by extracellular acidosis. The double mutant rhTRPA1-I942V/C943S displayed a robust proton sensitivity as compared with an absolute lack of proton sensitivity found for wild-type rhTRPA1. However, no rhTRPA1 mutant construct displayed a proton sensitivity that was comparable with that of hTRPA1. This finding might reflect a generally smaller chemical sensitivity of rhTRPA1 as compared with hTRPA1 (32) but might as well indicate that further residues within or outside TM domains 5/6 also have an impact on the proton sensitivity of hTRPA1.
In this study, we identified human TRPA1 as a receptor for extracellular protons and argue that TRPA1 is likely to play a significant role in the surveillance system that monitors the acid-base homeostasis in humans. TRPA1 is the principal receptor for numerous endogenous algogenic substances that accumulate during tissue injury, inflammation, and metabolic dysregulation. An interstitial acidosis is regarded to contribute to several types of pain. Thus, activation and sensitization of TRPA1 by protons might be an important event in peripheral pain processing. Future studies that circumvent the challenges arising from the species specificity of this property are required to further elucidate the relevance of the acid sensitivity of hTRPA1.
Acknowledgments
We thank Mrs. Heike Bürger (Anesthesiology, Hannover), Mrs. Iwona Izydorzyk and Mrs. Birgit Vogler (Physiology, Erlangen) for assistance, and Mr. Andreas Niesel (Neurology, Hannover) for technical support.
This study was supported by an internal grant of the Hannover Medical School (to J. d. l. R.) and by German Research Association Grant LA2740/2-1 (to A. L.).
- ASIC
- acid-sensing ion channel
- hTRPA1
- human hTRPA1
- mTRPA1
- mouse TRPA1
- rTRPA1; rhTRPA1
- rhesus monkey TRPA1
- DRG
- dorsal root ganglion
- AITC
- allyl isothiocyanate
- pA
- picoampere
- TM
- transmembrane
- TRP
- transient receptor potential
- BCTC
- 4-(3-chloro-2-pyridinyl)-N-[4-(1,1-dimethylethyl)phenyl]-1-piperazinecarboxamide.
REFERENCES
- 1. Basbaum A. I., Bautista D. M., Scherrer G., Julius D. (2009) Cellular and molecular mechanisms of pain. Cell 139, 267–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reeh P. W., Kress M. (2001) Molecular physiology of proton transduction in nociceptors. Curr. Opin. Pharmacol 1, 45–51 [DOI] [PubMed] [Google Scholar]
- 3. Krishtal O. A., Pidoplichko V. I. (1981) Receptor for protons in the membrane of sensory neurons. Brain Res. 214, 150–154 [DOI] [PubMed] [Google Scholar]
- 4. Holzer P. (2009) Acid-sensitive ion channels and receptors. Handb. Exp. Pharmacol. 194, 283–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Deval E., Gasull X., Noël J., Salinas M., Baron A., Diochot S., Lingueglia E. (2010) Acid-sensing ion channels (ASICs). Pharmacology and implication in pain. Pharmacol. Ther. 128, 549–558 [DOI] [PubMed] [Google Scholar]
- 6. Deval E., Noël J., Lay N., Alloui A., Diochot S., Friend V., Jodar M., Lazdunski M., Lingueglia E. (2008) ASIC3, a sensor of acidic and primary inflammatory pain. EMBO J. 27, 3047–3055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Birdsong W. T., Fierro L., Williams F. G., Spelta V., Naves L. A., Knowles M., Marsh-Haffner J., Adelman J. P., Almers W., Elde R. P., McCleskey E. W. (2010) Sensing muscle ischemia. Coincident detection of acid and ATP via interplay of two ion channels. Neuron 68, 739–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Immke D. C., McCleskey E. W. (2001) Lactate enhances the acid-sensing Na+ channel on ischemia-sensing neurons. Nat. Neurosci. 4, 869–870 [DOI] [PubMed] [Google Scholar]
- 9. Caterina M. J., Schumacher M. A., Tominaga M., Rosen T. A., Levine J. D., Julius D. (1997) The capsaicin receptor. A heat-activated ion channel in the pain pathway. Nature 389, 816–824 [DOI] [PubMed] [Google Scholar]
- 10. Tominaga M., Caterina M. J., Malmberg A. B., Rosen T. A., Gilbert H., Skinner K., Raumann B. E., Basbaum A. I., Julius D. (1998) The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 21, 531–543 [DOI] [PubMed] [Google Scholar]
- 11. Nilius B., Prenen J., Owsianik G. (2011) Irritating channels. The case of TRPA1. J. Physiol. 589, 1543–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Patapoutian A., Tate S., Woolf C.J. (2009) Transient receptor potential channels. Targeting pain at the source. Nat. Rev. Drug. Discov. 8, 55–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fujita F., Uchida K., Moriyama T., Shima A., Shibasaki K., Inada H., Sokabe T., Tominaga M. (2008) Intracellular alkalization causes pain sensation through activation of TRPA1 in mice. J. Clin. Invest. 118, 4049–4057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Y. Y., Chang R. B., Allgood S. D., Silver W. L., Liman E. R. (2011) A TRPA1-dependent mechanism for the pungent sensation of weak acids. J. Gen. Physiol. 137, 493–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang Y. Y., Chang R. B., Liman E. R. (2010) TRPA1 is a component of the nociceptive response to CO2. J. Neurosci. 30, 12958–12963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Takahashi N., Mizuno Y., Kozai D., Yamamoto S., Kiyonaka S., Shibata T., Uchida K., Mori Y. (2008) Molecular characterization of TRPA1 channel activation by cysteine-reactive inflammatory mediators. Channels 2, 287–298 [DOI] [PubMed] [Google Scholar]
- 17. Chen J., Kym P. R. (2009) TRPA1. The species difference. J. Gen. Physiol. 133, 623–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen J., Zhang X. F., Kort M. E., Huth J. R., Sun C., Miesbauer L. J., Cassar S. C., Neelands T., Scott V. E., Moreland R. B., Reilly R. M., Hajduk P. J., Kym P. R., Hutchins C. W., Faltynek C. R. (2008) Molecular determinants of species-specific activation or blockade of TRPA1 channels. J. Neurosci. 28, 5063–5071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fischer M. J., Leffler A., Niedermirtl F., Kistner K., Eberhardt M., Reeh P. W., Nau C. (2010) The general anesthetic propofol excites nociceptors by activating TRPV1 and TRPA1 rather than GABAA receptors. J. Biol. Chem. 285, 34781–34792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leffler A., Lattrell A., Kronewald S., Niedermirtl F., Nau C. (2011) Activation of TRPA1 by membrane permeable local anesthetics. Mol. Pain 7, 1346–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xiao B., Dubin A. E., Bursulaya B., Viswanath V., Jegla T. J., Patapoutian A. (2008) Identification of transmembrane domain 5 as a critical molecular determinant of menthol sensitivity in mammalian TRPA1 channels. J. Neurosci. 28, 9640–9651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bianchi B. R., Zhang X. F., Reilly R. M., Kym P. R., Yao B. B., Chen J. (2012) Species comparison and pharmacological characterization of human, monkey, rat, and mouse TRPA1 channels. J. Pharmacol. Exp. Ther. 341, 360–368 [DOI] [PubMed] [Google Scholar]
- 23. Liu H., Naismith J. H. (2008) An efficient one-step site-directed deletion, insertion, single and multiple-site plasmid mutagenesis protocol. BMC Biotechnol. 8, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eberhardt M. J., Filipovic M. R., Leffler A., de la Roche J., Kistner K., Fischer M. J., Fleming T., Zimmermann K., Ivanovic-Burmazovic I., Nawroth P. P., Bierhaus A., Reeh P. W., Sauer S. K. (2012) Methylglyoxal activates nociceptors through transient receptor potential channel A1 (TRPA1). A possible mechanism of metabolic neuropathies. J. Biol. Chem. 287, 28291–28306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Samad A., Sura L., Benedikt J., Ettrich R., Minofar B., Teisinger J., Vlachova V. (2011) The C-terminal basic residues contribute to the chemical- and voltage-dependent activation of TRPA1. Biochem. J. 433, 197–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lambert S., Oberwinkler J. (2005) Characterization of a proton-activated, outwardly rectifying anion channel. J. Physiol. 567, 191–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang Y. Y., Chang R. B., Waters H. N., McKemy D. D., Liman E. R. (2008) The nociceptor ion channel TRPA1 is potentiated and inactivated by permeating calcium ions. J. Biol. Chem. 283, 32691–32703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gunthorpe M. J., Smith G. D., Davis J. B., Randall A. D. (2001) Characterisation of a human acid-sensing ion channel (hASIC1a) endogenously expressed in HEK293 cells. Pflugers Arch. 442, 668–674 [DOI] [PubMed] [Google Scholar]
- 29. Caterina M. J., Leffler A., Malmberg A. B., Martin W. J., Trafton J., Petersen-Zeitz K. R., Koltzenburg M., Basbaum A. I., Julius D. (2000) Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288, 306–313 [DOI] [PubMed] [Google Scholar]
- 30. Leffler A., Mönter B., Koltzenburg M. (2006) The role of the capsaicin receptor TRPV1 and acid-sensing ion channels (ASICS) in proton sensitivity of subpopulations of primary nociceptive neurons in rats and mice. Neuroscience 139, 699–709 [DOI] [PubMed] [Google Scholar]
- 31. Jordt S. E., Tominaga M., Julius D. (2000) Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc. Natl. Acad. Sci. U.S.A. 97, 8134–8139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ryu S., Liu B., Yao J., Fu Q., Qin F. (2007) Uncoupling proton activation of vanilloid receptor TRPV1. J. Neurosci. 27, 12797–12807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sherwood T. W., Frey E. N., Askwith C. C. (2012) Structure and activity of the acid-sensing ion channels. Am. J. Physiol. Cell Physiol. 303, C699–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang S., Poon K., Oswald R. E., Chuang H. H. (2010) Distinct modulations of human capsaicin receptor by protons and magnesium through different domains. J. Biol. Chem. 285, 11547–11556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Andersson D. A., Chase H. W., Bevan S. (2004) TRPM8 activation by menthol, icilin, and cold is differentially modulated by intracellular pH. J. Neurosci. 24, 5364–5369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hinman A., Chuang H. H., Bautista D. M., Julius D. (2006) TRP channel activation by reversible covalent modification. Proc. Natl. Acad. Sci. U.S.A. 103, 19564–19568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Macpherson L. J., Dubin A. E., Evans M. J., Marr F., Schultz P. G., Cravatt B. F., Patapoutian A. (2007) Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 445, 541–545 [DOI] [PubMed] [Google Scholar]
- 38. Bevan S., Yeats J. (1991) Protons activate a cation conductance in a sub-population of rat dorsal root ganglion neurones. J. Physiol. 433, 145–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Steen K. H., Reeh P. W., Anton F., Handwerker H. O. (1992) Protons selectively induce lasting excitation and sensitization to mechanical stimulation of nociceptors in rat skin, in vitro. J. Neurosci. 12, 86–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yagi J., Wenk H. N., Naves L. A., McCleskey E. W. (2006) Sustained currents through ASIC3 ion channels at the modest pH changes that occur during myocardial ischemia. Circ. Res. 99, 501–509 [DOI] [PubMed] [Google Scholar]
- 41. Baumann T. K., Burchiel K. J., Ingram S. L., Martenson M. E. (1996) Responses of adult human dorsal root ganglion neurons in culture to capsaicin and low pH. Pain 65, 31–38 [DOI] [PubMed] [Google Scholar]
- 42. Baumann T. K., Chaudhary P., Martenson M. E. (2004) Background potassium channel block and TRPV1 activation contribute to proton depolarization of sensory neurons from humans with neuropathic pain. Eur. J. Neurosci. 19, 1343–1351 [DOI] [PubMed] [Google Scholar]
- 43. Delaunay A., Gasull X., Salinas M., Noël J., Friend V., Lingueglia E., Deval E. (2012) Human ASIC3 channel dynamically adapts its activity to sense the extracellular pH in both acidic and alkaline directions. Proc. Natl. Acad. Sci. U.S.A. 109, 13124–13129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Steen K. H., Issberner U., Reeh P. W. (1995) Pain due to experimental acidosis in human skin. Evidence for non-adapting nociceptor excitation. Neurosci. Lett. 199, 29–32 [DOI] [PubMed] [Google Scholar]
- 45. Steen K. H., Wegner H., Reeh P. W. (1999) The pH response of rat cutaneous nociceptors correlates with extracellular (Na+) and is increased under amiloride. Eur. J. Neurosci. 11, 2783–2792 [DOI] [PubMed] [Google Scholar]
- 46. Alvarez de la Rosa D., Zhang P., Shao D., White F., Canessa C. M. (2002) Functional implications of the localization and activity of acid-sensitive channels in rat peripheral nervous system. Proc. Natl. Acad. Sci. U.S.A. 99, 2326–2331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mohapatra D. P., Wang S. Y., Wang G. K., Nau C. (2003) A tyrosine residue in TM6 of the vanilloid receptor TRPV1 involved in desensitization and calcium permeability of capsaicin-activated currents. Mol. Cell Neurosci. 23, 314–324 [DOI] [PubMed] [Google Scholar]
- 48. Cadiou H., Studer M., Jones N. G., Smith E. S., Ballard A., McMahon S. B., McNaughton P. A. (2007) Modulation of acid-sensing ion channel activity by nitric oxide. J. Neurosci. 27, 13251–13260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jones N. G., Slater R., Cadiou H., McNaughton P., McMahon S. B. (2004) Acid-induced pain and its modulation in humans. J. Neurosci. 24, 10974–10979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ugawa S., Ueda T., Ishida Y., Nishigaki M., Shibata Y., Shimada S. (2002) Amiloride-blockable acid-sensing ion channels are leading acid sensors expressed in human nociceptors. J. Clin. Invest. 110, 1185–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Banke T. G. (2011) The dilated TRPA1 channel pore state is blocked by amiloride and analogues. Brain Res. 1381, 21–30 [DOI] [PubMed] [Google Scholar]
- 52. Miyamoto T., Dubin A. E., Petrus M. J., Patapoutian A. (2009) TRPV1 and TRPA1 mediate peripheral nitric oxide-induced nociception in mice. PLoS ONE 4, e7596. [DOI] [PMC free article] [PubMed] [Google Scholar]