Background: Beclin-1 is one of the essential autophagic proteins.
Results: This study identified a novel complex between breast carcinoma Her2 and Beclin-1 that is disrupted by lapatinib, a Her2-tyrosine kinase inhibitor.
Conclusion: Lapatinib thwarts the reciprocal cross-regulation between Her2 and Beclin-1, impacting cellular autophagy and Her2 signaling.
Significance: The findings elucidate a hitherto unknown association between lapatinib-induced autophagy and the disruption of Her2-Beclin-1 complex.
Keywords: Apoptosis, Autophagy, Breast Cancer, Epidermal Growth Factor Receptor (EGFR), Receptor Tyrosine Kinase, Beclin-1, Her2, Lapatinib
Abstract
Beclin-1 is a key regulator of autophagy that functions in the context of two phase-specific complexes in the initiation and maturation of autophagosomes. Its known interacting proteins include autophagy effectors, Bcl-2 family members, and organelle membrane anchor proteins. Here we report a newly identified interaction between Beclin-1 and the protein tyrosine kinase receptor Her2. We demonstrate that in Her2-expressing breast carcinoma cells that do not succumb to lapatinib, this Her1/2 inhibitor disrupts the cell surface interaction between Her2 and Beclin-1. The data suggest that the ensuing autophagic response is correlatively associated with the release of Beclin-1 from its complex with Her2 and with the subsequent increase in cytosolic Beclin-1. Upon its interaction with Her2, Beclin-1 up-regulates the phosphorylation levels of Her2 and Akt. The Beclin-1 evolutionarily conserved domain is required both for the interaction of Beclin-1 with Her2 and for the increased Her2 and Akt phosphorylation. These findings shed new light on mechanisms involved in lapatinib-mediated autophagy in Her2-expressing breast carcinoma cell lines and in Beclin-1 signaling in these cells.
Introduction
Macroautophagy is a cellular process that directs the trafficking of cytosolic proteins and organelles to lysosomes for degradation (1). The process involves the formation of autophagosomes, double-membrane vesicles that engulf proteins and organelles destined for degradation and recycling. Constitutive levels of autophagy are required for maintaining homeostasis, whereas up-regulation of this process serves to promote cell survival under different conditions of cellular stress, including nutrient deprivation, protein aggregate formation, pathogen infection, endoplasmic reticulum stress, and more (2–4). In recent years, it has become apparent that anticancer cytotoxic drugs that do not culminate in cell death induce autophagy in the treated tumor cells (1). Thus, induction of autophagy has been demonstrated in tumor cells that did not succumb to treatments by radiation (5–8), chemotherapy (9–12), death receptor-targeting cytokines (13–16), and certain anticancer tyrosine kinase inhibitors (TKIs)3 (17, 18). As stress-mediated autophagy promotes the survival of tumor cells under unfavorable conditions, autophagy has been considered a cellular mechanism of drug resistance. Indeed, inhibition of autophagy is being assessed as a therapeutic approach to expand the cell death response to those tumor cells that invoke autophagy to overcome anticancer treatment-mediated stress (9, 19–22).
The current study focuses on autophagy mediated by lapatinib (LP), a small molecule TKI, that targets two members of the EGFR family: EGFR and Her2. The EGFR family has four members, EGFR/Her1, Her2, Her3, and Her4, all of which except Her2 are known to bind ligands. Ligand binding to the extracellular domain of members of this family causes receptor homo- or heterodimerization and tyrosine kinase activation (23). Signal triggering via any of the EGFRs leads to the activation of a network of signaling cascades that are involved in cell proliferation and tumorigenesis. Although ligands for Her2 are not known, it has a key role in EGFR family signaling through its heterodimerization with other family members. LP is an ATP competitor that reversibly binds to the ATP binding pocket in the kinase domain of Her2 or EGFR. It specifically interacts with a non-active conformation of the kinase domain, preventing its activation for the duration of the complex (24).
Recent studies have demonstrated that TKIs (18), including LP (25), induce cytoprotective autophagy in tumor cells that do not succumb to the TKI toxicity. These findings suggest that the inhibition of this cell survival pathway may offer a strategy to overcome multiple molecular mechanisms involved in TKI resistance (26, 27). The molecular mechanisms that give rise to an autophagic response in drug-resistant tumor cells have not yet been fully elucidated.
Beclin-1 is an essential autophagic protein that functions in the context of Complex I (Vps34, Vps15, Atg14) in the signaling events of autophagosome formation and in the context of Complex II (UVRAG (UV radiation resistance-associated gene), Rubicon) in autophagosome maturation (28–30). Beclin-1 is a haploinsufficient tumor suppressor gene whose heterozygous deletion in mice increases the incidence of spontaneous tumors (31). Paradoxically, Beclin-1 is also involved in the tumorigenicity of breast cancer (BrCA) stem cells (32). Additionally, specific phosphorylation of Beclin-1 reverses its tumor suppression function to oncogenesis-driving activity (33).
In the current study, we identified a novel complex between BrCA Her2 and Beclin-1 that is disrupted by LP treatment. Our findings suggest an association between LP-mediated release of Beclin-1 from its sequestration by Her2 and the onset of autophagy in LP-resistant, Her2-expressing BrCA cells. We also obtained evidence that Beclin-1 enhances the phosphorylation level and potentially the signaling capability of Her2. The data further suggest that Beclin-1-mediated cytoprotective autophagy could serve as a complementary therapeutic target in Her2-expressing BrCA cells that utilize distinct mechanisms for LP resistance.
EXPERIMENTAL PROCEDURES
Chemicals and Antibodies
Lapatinib was purchased from LC Laboratories; pepstatin A (PepA) and DAPI were from Sigma; and E64D was from Calbiochem. Antibodies against β-tubulin (sc-9104), Beclin-1 (sc-11427), β-actin (sc-47778), LAMP2 (sc-18822), Her2 (sc-284), p-Her2 (sc-12352), Akt (sc-8312), and p-Akt (sc-7985) were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA); anti-Vps34 (ab5451) was from Abcam; anti-MAP-LC3 antibodies for immunoblotting were from AnaSpec (San Jose, CA); and anti-MAP-LC3 antibody (sc-28266) for immunostaining was from Santa Cruz Biotechnology. The secondary antibodies HRP-conjugated anti-rabbit and anti-mouse IgG were from Thermo Scientific. Alexa Fluor 488- and 647-conjugated anti-rabbit and anti-mouse Ig were from Invitrogen.
Cell Lines
The human BrCA cell lines MCF7, SKBR3, MDA-MB-361, and BT474 were purchased from ATCC. All experiments were performed with freshly cultured cells from the original shipment. Her2-positive 4T1 and NT-5.1 murine breast carcinoma cells were provided by Dr. Eliezer Gorelik (University of Pittsburgh). 4T1 cells were originally derived from sporadic BrCA in Balb/c mice (34) and were stably transfected with Her2. NT-5.1 cells were derived from Her2/neu transgenic FVB/N mice (35). The tumor cell lines were grown in Dulbecco's modified Eagle's medium containing 10% fetal calf serum, 20 mm l-glutamine, and 100 units/ml each of penicillin and streptomycin. LP-resistant BT474 cells were selected by multiple cycles of increasing LP doses (0.01–3 μm) that were applied during 6–8 weeks in cycles of 3-day treatment and 1 week of recovery.
Cell Survival Assays
Clonogenic assays were performed with methylcellulose-based semisolid medium (MethoCult H4230, StemCell Technologies) according to the manufacturer's protocol. In brief, after treatment, the cells were washed, suspended in MethoCult medium, and cultured in triplicates (300 cells/3 ml) in 35-mm Petri dishes. The cultures were maintained at 37 °C in 5% CO2 for 35 days, and colonies were counted using an inverted microscope and gridded scoring dishes. Cytofluorometric analyses of apoptosis were performed by co-staining with propidium iodide and fluorescein isothiocyanate-annexin V conjugates (BD Biosciences).
Molecular Cloning of Human Beclin-1
Total RNA was isolated from Jurkat T-cells using RNA STAT-60 reagent (Tel-Test, Inc.). Reverse transcription was carried out with 5 μg of total RNA using an oligo(dT)12–18 primer and SuperScript II RNase H− reverse transcriptase (Invitrogen). PCR was performed using the Expand high fidelity PCR system kit (Roche Applied Science). A Beclin-1 amplicon containing its open reading frame (ORF) was generated with the following primer pair that extends from six nucleotides into its 5′-untranslated region (UTR) through the ORF and extends 179 bases into the 3′UTR (forward and reverse): 5′-CGCGGATCCTGAGGGATGGAAGGGTCTAAG-3′ and 5′-ACGCGTCGACGCTCTGGAAAGTATCTGTCAC-3′. The putative full-length Beclin-1 amplicon was size-selected using a 1% agarose gel, and DNA was purified with the QIAquick gel extraction kit (Qiagen). The purified amplicon was digested with the restriction enzymes BamHI and SalI and ligated into a plasmid vector, pcDNA4/TO (Invitrogen), that had been previously digested with BamH1 and XhoI. Following transformation (Escherichia coli TOP 10F′, Invitrogen), plasmids from randomly picked colonies underwent automated DNA sequence analysis (University of Pittsburgh DNA Sequencing Core Facility) to confirm sequence integrity.
Generation of N-terminal 3×FLAG Beclin-1
Utilizing the full-length WT Beclin-1 plasmid described above as a template, we carried out PCR as above with an N-terminal 3×FLAG-encoding forward primer: 5′-CGCGGATCCGCCATGGACTACAAAGACCATGACGGTGATTATAAAGATCATGACATCGATTACAAGGATGACGATGACAAGATGGAAGGGTCTAAGACGT-3′ and the same reverse primer utilized above. The putative N-terminal 3×FLAG-tagged WT amplicon was processed as described above but ligated into the plasmid vector pCR3.1. After transformation (E. coli DH5α, Invitrogen), random colonies were sequenced as above.
Production of N-terminal 3×FLAG Beclin-1 Deletion Mutants
Three N-terminal 3×FLAG Beclin-1 deletion mutants, including ΔBcl-2 binding domain (ΔBBD), Δcoiled-coil domain (ΔCCD), and the Δevolutionarily conserved domain (ΔECD), were generated in a two-step procedure by overlap extension using the PCR method. Using the WT Beclin-1 cDNA clone described previously as a template, PCR was performed in the first step as above with the following sets of forward and reverse primer pairs: ΔBBD (ΔMet-88 through Thr-150), N-terminal 3×FLAG primer from above, and 5′-GACGTTGAGCTGCCTGGCTGGGGGGATGAATCT-3′ and 5′-CCCCCAGCCAGGCAGCTCAACGTCACTGAAAAT-3′ and the reverse primer for WT Beclin-1; ΔCCD (ΔLeu-144 through Val-269), N-terminal 3×FLAG primer and 5′-GGTTGCATTAAAAGTATCTGTGCATTCCTCACAGAG-3′ and 5′-TGCACAGATACTTTTAATGCAACCTTCCACATCTGG-3′ and the reverse primer for WT Beclin-1; ΔECD (ΔAsp-244 through Ser-337), N-terminal 3×FLAG primer and 5′-AGACTCCAGATACAGCTCCAGCTGCTGTCGTTT-3′ and 5′-CAGCTGGAGCTGTATCTGGAGTCTCTGACAGAC-3′ and the reverse primer for WT Beclin-1. In the second round of PCR, 0.5 μl of each cognate PCR reaction was combined using the N-terminal 3×FLAG primer and reverse primer for WT Beclin-1 as the outside primers. All putative deletion mutant amplicons were processed as described for generating the WT Beclin-1 cDNA and ligated into the plasmid vector pCR3.1. After transformation of DH5α cells, random colonies were sequenced as described above to confirm all mutations.
Transfection and RNAi
All siRNAs as well as the matching non-targeting controls were obtained as siGENOME SMARTpool or ON-TARGETplus SMARTpool siRNAs from Dharmacon. These reagents consist of four distinct RNA oligoduplexes per target or non-target. Additional individual Beclin-1 siRNAs and their appropriate negative controls were obtained from Invitrogen. RNAi for each gene included three individual Invitrogen Stealth Select siRNAs and their matching Stealth negative control. All knockdown experiments were repeated with at least two distinct siRNAs per target with similar results. Transfection of siRNA was performed with Oligofectamine according to the manufacturer's transfection protocol (Invitrogen). Transient transfections were carried out with Lipofectamine LTX and Plus reagent (Invitrogen) according to the manufacturer's instructions. Cell treatments were applied 24 h after transfection
Cell Microscopy and Image Acquisition
Confocal images were obtained with an Olympus FluoView 1000 confocal microscope and the companion software FV10-ASW1.6. Images were acquired with the use of the same setting at a resolution of 1020 × 1024 pixels. Morphometric measurements were performed using MetaMorph (Universal Imaging) on at least 50 cells per condition. Endogenous LC3 puncta were monitored by two measures: (i) manual counting of dot number per cell and (ii) determination of cumulative dot area per cell area. Cumulative dot area and cell area were determined by MetaMorph on images where the set threshold eliminated the detection of low or non-puncta staining.
For electron microscopy, cells were fixed with 2% paraformaldehyde and 2% glutaraldehyde in 0.1 m phosphate buffer (pH 7.0) followed by 1% OsO4. After dehydration, thin sections were stained with uranyl acetate and lead citrate for observation under a JEM 1011CX electron microscope (JEOL, Peabody, MA). Images were acquired digitally. At least 50 cells per treatment were utilized for quantification.
Immunoblotting and Immunoprecipitation
Cell lysates were prepared with 1% Nonidet P-40, 20 mm Tris-HCl, pH 7.4, 137 mm NaCl, 10% glycerol, 1 mm phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin, and 10 μg/ml aprotinin. The immunoblotting and immunoprecipitation procedures were described in our previous publications (13, 36–38). All immunoprecipitations were controlled by a sham procedure with nonspecific matching immunoglobulin. Quantification of scanned protein bands was performed by the US-SCAN-IT Gel software (Silk Scientific, Inc., Orem, UT).
Determination of Autophagic Flux
Assessment of autophagic flux was performed by quantifications of autophagic markers in the absence or presence of predetermined saturating concentrations of the cathepsin inhibitors E64D and pepstatin A. Autophagic markers included autophagosome number (transmission electron microscopy), LC3 puncta number and relative cell area (confocal microscopy), and LC3-II protein level (immunoblotting).
Statistical Analysis
All immunoblot analyses are representative of at least three experiments. Images are representative of more than 50 cells from at least three repeats. Quantifications by MetaMorph were performed on at least 50 cells per treatment, and the results were confirmed in three independent experiments. Numerical data are presented as means ± S.E. of at least 50 cells used for each quantification. Statistical analysis was performed by GraphPad Prism V software, utilizing the nonparametric test, Mann-Whitney U.
RESULTS
Induction of Cytoprotective Autophagy in LP-treated BrCA Cells
LP is a reversible dual inhibitor of EGFR and Her2 that has also been reported to mediate off-target antitumor activity (39). To investigate the autophagic capability of LP, we utilized BrCA cell lines with amplified expression of Her2 (BT474, SKBR3, and MDA-MB-361) as well as the MCF7 BrCA cell line that expresses low levels of Her2 and EGFR.4 As the majority of the BT474 cells (but not of SKBR3 or MDA-MB-361) are LP-sensitive, we selected by multiple LP treatment cycles a multiclonal LP-resistant cell line that allows the analysis of those cells that do not succumb to the toxic effects of LP. Presumably, such a cell line is composed of multiple clones, each representing an LP resistance mechanism(s) that exists in the WT cell line. The relative resistance to LP of the BT474 selected cells was confirmed by their increased cell survival in a long term clonogenic assay (Fig. 1A). We also determined that cisplatin-selected MCF7 cells (15) were cross-resistant to LP. When freshly treated with LP, the resistant BrCA cell lines, LP-selected BT474 cells and cisplatin-selected MCF7 cells, did not succumb to the drug toxicity, but instead exhibited up-regulation of multiple autophagic markers. In comparison, LP treatment of WT BT474 cells (not preselected for LP resistance) generated a mixed population of apoptotic, necrotic, and autophagic cells (data not shown). As assessed by transmission electron microscopy, LP treatment of preselected, resistant cells induced the formation of autophagosomes and autophagolysosomes (Fig. 1, B and C). In a combined treatment by LP and saturating concentrations of the cathepsin inhibitors, E64D/PepA, there was a further increase in the number of autophagolysosomes and in their size and density, confirming the induction by LP of an autophagic flux (Fig. 1, D and E, results for BT474 are shown). The addition of E64D/PepA changed the appearance of the autophagolysosomes/advanced vesicles (AVds), allowing the distinction between potentially active and inhibited AVds (Fig. 1, D and E).
FIGURE 1.
Transmission electron microscopy evidence for LP-mediated autophagy in LP-resistant BT474 cells. A, increase in clonogenic potential of LP-selected BT474 cells as compared with their WT counterpart and assessed by a long term culture (35 days). The results are means ± S.E. of five plates for each group in one of three experiments with equivalent results. LP-R, LP-resistant. B and C, LP-resistant BT474 cells that were freshly treated with LP (2 μm, 16 h) were assessed by transmission electron microscopy for the presence of autophagosomes and autophagolysosomes. Scale bars: left and middle panels, 2 μm; right, 500 nm; the right panel is an increased magnification of the square area indicated in the middle panel. The arrows indicate autophagosomes. E/P, E64D/PepA. D and E, LP induces autophagic flux in LP-resistant BT474, as indicated by the significantly increased number of AVds in the presence of lysosomal inhibitors. Of note, active and inhibited AVds demonstrate a distinct EM appearance; in panel a, the square shows an active AVd, and in panel b, the square shows an inhibited AVd. The quantification in B and D was performed on at least 30 cells per treatment in three independent experiments. *, p < 0.05; ***, p < 0.0001 (Mann-Whitney U). Results shown in B and D and their corresponding quantification (C and E, respectively) represent two independent experiments.
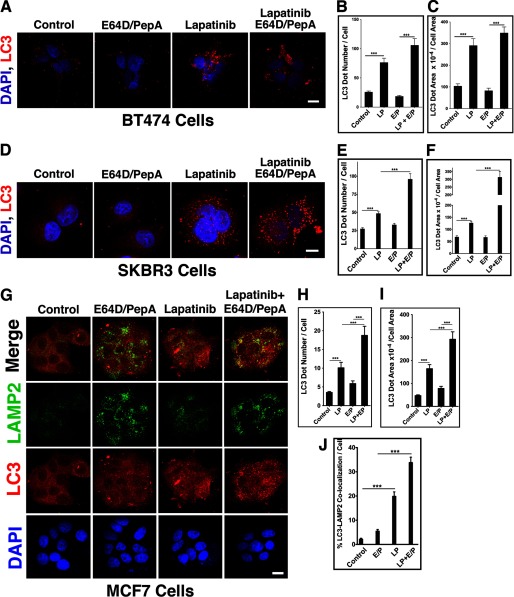
LP-treated BrCA cells, including BT474, SKBR3, and MCF7, exhibited enhanced punctate appearance of endogenous LC3 as assessed by confocal microscopy (Fig. 2) or by quantitative image cytometry (Cellomics ArrayScan, data not shown). The development of an autophagic flux was confirmed by a confocal assessment of endogenous LC3 puncta in BrCA cells treated by LP in the presence E64D/PepA. The autophagic flux was monitored by the increase in the number of endogenous LC3 puncta per cell (Fig. 2, B, E, and H) or by their cumulative area relative to the corresponding cell area (Fig. 2, C, F, and I). The occurrence of an autophagic flux in LP-treated BrCA cells was confirmed by each of the monitoring assays in each of the BrCA cell lines tested. The LP-mediated increase in formation of autophagolysosomes was further demonstrated by increased co-localization of LC3 and LAMP2 puncta (Fig. 2, G and J).
FIGURE 2.
Altered expression of endogenous LC3 puncta in LP-treated BrCA cells. LP-selected BT474 cells (A–C), SKBR3 cells (D–F), and cisplatin-selected MCF7 cells (G–J) were treated with LP in the absence or presence of E64D/PepA (E/P). Expression patterns for LC3 and LAMP2 were assessed by confocal microscopy (A, D, and G) and quantified by MetaMorph. Scale bars: A and D, 30 μm; G, 50 μm. The LC3 dot number per cell was determined by manual counting (B, E, and H), whereas the cumulative LC3 dot area × 10−4 per cell area was determined by MetaMorph (C, F, and I). The percentage of LC3-LAMP2 co-localization (LC3 over LAMP2) was quantified by MetaMorph. All quantifications were performed on at least 50 cells per treatment in each of three independent experiments with equivalent results; ***, p < 0.0001 (Mann-Whitney U).
Additionally, LP-treated cells exhibited increased conversion of LC3-I to its lipidated form LC3-II, currently the best marker for membrane-associated LC3. The enhanced LC3-II formation was detected in LP-resistant BT474 (WT BT474; not shown), SKBR3, and MCF7 cells, as well as in two Her2-positive mouse mammary cell lines (Fig. 3). An enhanced LC3-II accumulation by a combined LP and E64D/PepA treatment, relative to E64D/PepA alone, confirmed the occurrence of an autophagic flux in these LP-treated cells (Fig. 3).
FIGURE 3.
Induction of autophagic flux as assessed by LC3-II level in LP-treated BrCA cells. A–H, accumulation of LC3-II was tested in the absence or presence of saturating concentrations of E64D/PepA (E/P) (10 μg/ml each). The immune-probed protein bands (A, C, E, and G) were quantified as described under “Experimental Procedures,” and the ratio to control treatment is presented in the corresponding charts (B, D, F, and H). A and B, LP-selected BT474 re-treated with LP (2 μm, 16 h). C and D, LP-treated SKBR3 cells (2 μm, 6 h). E and F, LP-treated cisplatin-resistant MCF7 cells (5 μm, 16 h). G and H, LP-treated 4T1 and NT5.1, Her2-positive murine BrCA cell lines were obtained from Her2 transgenic mouse (2 μm, 16 h). Please note that in certain cell lines, only LC3-II, and not its precursor, LC3-I, is detected.
The cytoprotective nature of the autophagic response to LP was not only demonstrated by their clonogenic survival, but also by the shift of the LP cell response from autophagy to apoptosis upon inhibition of autophagy. Thus, a marked increase in annexin V-positive cells was observed in LP-resistant BrCA cells treated by the combination of LP with Beclin-1 siRNA (Fig. 4, BT474, 26%; SKBR3, 21%; MCF7, 47%). This series of experiments documents the induction of cytoprotective autophagy by LP and the involvement of Beclin-1 in this stress adaptation response of BrCA cells.
FIGURE 4.
Beclin-1 knockdown shifts the response of LP-resistant cells from autophagy to apoptosis. A–F, Beclin-1 knockdown by distinct siRNAs induces LP apoptosis susceptibility in LP-selected BT474 cells (A and B); SKBR3 cells (C and D); and cisplatin-selected MCF7 cells (E and F). B, D, and F, evidence for Beclin-1 RNAi in cells utilized in A, C, and E, respectively. Different Beclin-1 siRNA targeting sequences 1 and 2 are indicated.
Interaction of Beclin-1 with Her2
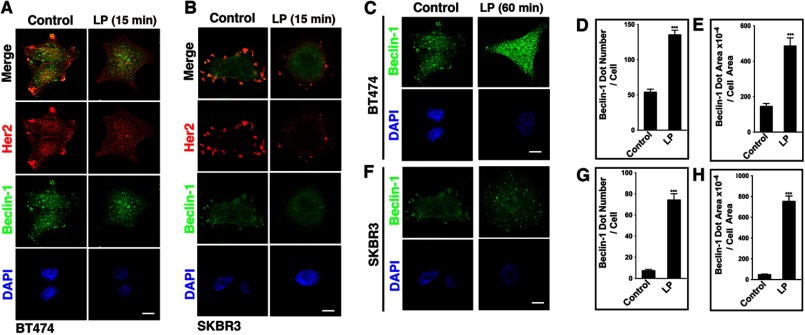
To investigate the autophagic response to LP in Her2-expressing cells, we assessed its effect on the Her2 expression pattern. A significant co-localization between Her2 and Beclin-1 was detected at the cell surface of both BT474 and SKBR3 cells (Fig. 5, A and B). However, within 15 min of exposure to LP, both BT474 and SKBR3 cells lost the surface expression of Her2 and its surface co-localization with Beclin-1. An extended treatment by LP revealed a significant cytosolic accumulation of Beclin-1 puncta (Fig. 5, C–H). Thus, LP treatment disturbed the cell surface co-localization of Her2 and Beclin-1 and concomitantly induced the formation of cytosolic Beclin-1 vesicles.
FIGURE 5.
LP disrupts the constitutive co-localization of cell surface Her2 and Beclin-1. A and B, LP-selected BT474 cells (A) and SKBR3 cells (B) were treated with LP (2 μm, 15 min) and assessed by confocal microscopy for the expression of Her2, Beclin-1, and DAPI. Scale bars: 10 μm. C–H, cellular accumulation of Beclin-1 dots in LP-treated BT474 cells (C–E) and in LP-treated SKBR3 cells (F–H). Quantification was performed by MetaMorph, and at least 50 cells were individually assessed for each group in three independent experiments with similar results. ***, p < 0.0001 (Mann-Whitney U).
To characterize the nature of the interaction between Her2 and Beclin-1, we assessed by immunoprecipitation their potential presence within the same complex. Co-immunoprecipitation of Beclin-1 and Her2 confirmed the existence of such a complex between either endogenously expressed Her2 and Beclin-1 or endogenous Her2 and transfected Beclin-1 (Fig. 6, A–C). In confirmation of the results obtained by immunostaining, LP treatment significantly disrupted the Her2-Beclin-1 complex, as demonstrated by a reduction in their co-immunoprecipitation in the presence of this inhibitor (Fig. 6, D and E).
FIGURE 6.

A Her2-Beclin-1 complex demonstrates two-way co-immunoprecipitation (IP), which is disrupted by LP treatment. A, SKBR3 cells were transfected with Beclin-1 or a matching vector and subjected to IP by control IgG, anti-Beclin-1, or anti-Her2 antibodies. A slight shift detected in the gel migration of pelleted Beclin-1 relative to the input may relate to unknown post-translational modifications of the Her2-interacting Beclin-1. B and C, BT474 cells were transfected with Beclin-1 or a matching vector and subjected to Beclin-1 IP (B) or Her2 IP (C). The ratio between the pellet and the input loading is 3:1. In C, the input and the pellets were run on the same gel, but different exposure times of the chemiluminescence reaction are shown. D and E, LP treatment disrupts the complex between Her2 and Beclin-1 in BT474 cells (D) and in NT5.1 cells (E). Following treatments by LP (2 μm, 15 min) or vehicle control, the cells were subjected to Her2 IP. F, control IP with non-immune rabbit IgG precipitates neither Her2 nor Beclin-1 in lysates of the indicated cell lines. The asterisks indicate unidentified protein bands.
To map the potential Beclin-1 interaction site(s) with Her2, we generated 3×FLAG (N terminus)-tagged WT Beclin-1 and its corresponding deletion mutants, including a deletion of the coiled-coil domain (Beclin-1ΔCCD), of the Bcl-2 binding domain (Beclin-1ΔBBD), and of the evolutionarily conserved domain (Beclin-1ΔECD). These 3×FLAG-tagged Beclin-1 variants were transfected into BT474 or SKBR3 cells and subjected to immunoprecipitation by either anti-FLAG or anti-Her2 antibodies. In both cell lines, the deletion of the Beclin-1 ECD inhibited the immunoprecipitation of the Her2-Beclin-1 complex (Fig. 7, A–C). As the interaction between Beclin-1 and Her2 is present under basal conditions, but disrupted by LP concomitant with its autophagy induction, these findings suggest that LP interferes with sequestration of Beclin-1 by Her2.
FIGURE 7.

The ECD of Beclin-1 is required for its interaction with Her2. BT474 or SKBR3 cells were transfected (Tx.) with 3×FLAG WT Beclin-1 or with the indicated deletion mutants of Beclin-1 (ΔCCD, ΔBBD, and ΔECD), each with an N-terminal 3×FLAG tag. A–C, the cells were then subjected to IP of endogenous Her2 (A and C) or of an exogenous 3×FLAG Beclin-1 variant (B). The asterisks indicate unidentified protein bands.
Impact of Beclin-1 on Her2 Signaling
To investigate the impact of Beclin-1 on Her2 signaling, we transfected Beclin-1 into three Her2-expressing BrCA cell lines, including MDA-MB-361, SKBR3, and BT474, and assessed the phosphorylation levels of Her2 and Akt in the presence of LP or a control vehicle. Relative to the control vector, Beclin-1 overexpression increased the phosphorylation levels of both Her2 and Akt (Fig. 8, A, C, and E, lane 1′ versus lane 1) in each of the tested Her2-expressing BrCA cell lines (Fig. 8, A–F). Moreover, in cells overexpressing Beclin-1, the negative impact of LP treatment on the expression levels of p-Her2 or p-Akt was attenuated (Fig. 8, A, C, and E, lanes 2′ and 3′ versus lanes 2 and 3). This attenuation may be related to an increased phosphorylation level in the presence of Beclin-1, to a reduced access of LP to Her2 in the presence of Beclin-1, or to a combination of these impacts. Although Beclin-1 appears to reduce the access of LP to Her2 (based on attenuated Her2 dephosphorylation by LP in the presence of Beclin-1), LP effectively disrupts the Her2-Beclin-1 complex, and overexpression of Beclin-1 does not provide protection against LP to LP-sensitive cells. The increased phosphorylation of Her2 and Akt was not observed when Beclin-1ΔECD was transfected into MDA-MB-361, SKBR3, or BT474 cells (Fig. 8, G and H). Likewise, the attenuation of LP dephosphorylation activity on p-Her2 or p-Akt was not detected with the ECD-deficient Beclin-1. These findings suggest that the ECD of Beclin-1 is required for the interaction between Beclin-1 and Her2, for the impact of Beclin-1 on the signaling capability of Her2, and for attenuating the dephosphorylation activity of LP on either p-Her2 or p-Akt.
FIGURE 8.
Increased expression of Beclin-1, but not Beclin-1ΔECD, enhances the phosphorylation levels of Her2 and Akt and reduces the access of LP to Her2. A–F, MDA-MB-361 cells (A and B), SKBR3 cells (C and D), and WT BT474 cells (E and F) were transfected with vector control or WT Beclin-1 and treated with LP (6 h, 1 or 2.5 μm (A–D) and 0.25 or 0.5 μm) (E and F). The cell lysates were assessed by immunoblotting for the expression of the indicated proteins. Results of one representative experiment of three performed for each cell line are shown. The expression levels of p-Her2 and p-Akt were quantified, and the percentages of increases in Beclin-1 transfected cells versus vector-transfected cells are presented in the corresponding charts (B, D, and F). G and H, the Beclin-1 ECD domain is required for the observed Her2 and Akt phosphorylation changes. MDA-MB-361 cells (G), SKBR3 cells (G), and WT BT474 cells (H) were transfected with vector control or Beclin-1ΔECD and treated with LP, as described above for the corresponding cell line. The asterisks indicate unidentified protein bands.
DISCUSSION
The current study describes a heretofore unknown interaction between Beclin-1 and Her2. The data suggest that the Her2-Beclin-1 complex is present at the cell surface of Her2-expressing BrCA cells; that the Her2-Beclin-1 complex is disrupted by LP treatment, which concomitantly induces adaptive autophagy in BrCA cells that are resistant to the death-inducing effect of this TKI; and that the interaction with Beclin-1 increases the phosphorylation of Her2 and attenuates the Her2 dephosphorylation activity of LP. These findings further suggest that under basal conditions, Beclin-1 may impact the Her2 signaling network. The disruption of this Her2-Beclin-1 complex may contribute to the cell autophagic response by both interfering with the Her2 survival signaling (cellular stress) in combination with the release of Beclin-1 from potential sequestration.
There is a significant dichotomy between LP-sensitive and LP-resistant BrCA with regard to LP-mediated autophagy. BrCA cells that are LP-sensitive are being eliminated in response to LP treatment by various mechanisms of cell death. In contrast, BrCA cells that respond in autophagy carry primary resistance to LP, potentially mediated by specific mutations in their Her2 kinase domain. Such an autophagic response is cytoprotective, and potentially targetable, as it facilitates the adaption of the cell to the stress mediated by the drug, independent of the primary resistance mechanism.
Recent studies have linked Beclin-1 to multiple cellular processes, including development, tumorigenesis, immunity, endocytosis, cytokinesis, adaptation to stress, and signal transduction (40, 41). Although many of the identified functions may well relate to its role in autophagosome formation and maturation, newly identified Beclin-1-interacting proteins suggest its involvement in non-autophagic functions as well.
The newly identified interaction of Beclin-1 with Her2 suggests a novel regulatory role for Beclin-1 in Her2 signal transduction. The involvement of Beclin-1 in receptor signaling has been previously indicated by its interaction with membrane receptors or their adaptors (42–45). Thus, in a similar fashion to the induction of autophagy by the Her2 antagonist LP, it has been demonstrated that the inositol 1,4,5-trisphosphate receptor (IP3R) interacts with Beclin-1 and that xestospongin B, an IP3R antagonist, induces autophagy by disrupting the IP3R-Beclin-1 complex (44). Estrogen receptor α (ERα) is another BrCA receptor that co-localizes and co-immunoprecipitates with Beclin-1 (42). Binding of Beclin-1 to ERα has been shown to inhibit the ERα growth response to receptor agonists. Other receptors, including the δ2 glutamate receptor (GluRδ2) (45) and Toll-like receptors (TLRs) (43) bind to specific adaptors that mediate their interaction with Beclin-1. Particularly, the interaction of GluRδ2 with Beclin-1 is bridged by nPIST, a binding protein to the receptor C terminus, and the link between Beclin-1 and TLRs is mediated by MyD88 and TRIF (TIR-domain-containing adapter-inducing interferon-β), TLR adaptors that potentially induce autophagy by altering the cellular level of the inhibitory complex of Beclin-1 with Bcl-2. Although the exact mechanism(s) by which these Beclin-1 complexes impact autophagy are not completely elucidated, they may relate to the cellular level of Beclin-1 that was previously established as a trigger mechanism for autophagy (46). The Beclin-1-dependent initiation of autophagy may be determined by the level of sequestered Beclin-1 versus its free form or by the balance between Beclin-1 complexes with either positive or negative autophagic regulators. In any event, the sequestration of Beclin-1 by multiple signaling complexes underscores the involvement of Beclin-1 in non-autophagic cellular processes. It also provides an explanation for the involvement of Beclin-1-dependent autophagy in a broad array of cellular processes; such autophagy is potentially induced by the disruption of Beclin-1 pre-existing complexes with different cellular proteins.
The increased phosphorylation of Her2 appears to be dependent on its interaction with Beclin-1 as the Beclin-1 ΔECD mutant that loses its ability to interact with Her2 also loses its phosphorylation effect on Her2. However, it remains unclear how Beclin-1 overexpression did not increase the expression level of the Her2-Beclin-1 complex yet increased the phosphorylation of Her2 in a manner that appears to be dependent on the physical interaction between the proteins. It is plausible that the complex level depends on the availability of a currently unknown component(s), and therefore its expression level remains unchanged despite overexpression of Beclin-1. However, overexpression of Beclin-1 may contribute to the complex stability, facilitating the Beclin-1-dependent Her2 phosphorylation. Alternatively, it is possible that the Her2 phosphorylation is Beclin-1-dependent (and thus, increases with Beclin-1 overexpression), but is independent of the interaction of Her2 with Beclin-1. In such a case, the Beclin-1 ΔECD mutant loses its capability of inducing Her2 phosphorylation because of changes in the protein that are not directly related to its ability to interact with Her2.
The Beclin-1-mediated increase in the Her2 phosphorylation level suggests that Beclin-1 contributes to the signaling and potentially, the oncogenic activity of Her2. These findings are inconsistent with the established tumor suppressor activity of Beclin-1. The tumor suppressor activity of Beclin-1 has been deduced from the monoallelic deletion of Beclin-1 in multiple cancers, including BrCA, and from the increased incidence of spontaneous tumors in heterozygous BECN+/− mice (31, 47). Beclin-1 tumor suppression may relate to its autophagic role, yet autophagy may both enhance and inhibit the tumorigenesis process. Thus, autophagic degradation of damaged cellular components generated by genetic alterations and chromosomal instability plays an anti-tumorigenesis role, whereas the cytoprotective function of autophagy may have the opposite effect of enhanced tumorigenesis (48, 49). Furthermore, in contrast to established BrCA tumors where tumorigenesis is associated with reduced Beclin-1 expression, tumorigenesis of BrCA stem cells has been demonstrated to be Beclin-1-dependent (32). In addition, specific Akt phosphorylation of Beclin-1 shifts its activity from suppression to progression of the oncogenic process (33). Thus, the tumor suppressor activity of Beclin-1 may depend on the phase of the tumor development, specific Beclin-1 phosphorylation, and the overall network of Beclin-1 cellular interactions.
This study is the first to report the interaction between Beclin-1 and a member of the EGFR family. Although Beclin-1 has been identified as an essential participant in the EGFR endocytic pathway (50), the exact mechanism that underlies its involvement in the EGFR degradation remains unknown. The potential involvement of pre-existing complexes between Beclin-1 and other EGFR members and the cross-talk between their signaling networks and the autophagic response will be the focus of future studies.
This work was supported, in whole or in part, by National Institutes of Health Grants RO1 CA134776 (to H. R.). This work was also supported by Department of Defense Grant W81XWH-12-1-0228 (to H. R.) and The Pittsburgh Foundation (to J. H.).
J. Han and H. Rabinowich, unpublished results.
- TKI
- tyrosine kinase inhibitor
- LP
- lapatinib
- EGFR
- epidermal growth factor receptor
- BBD
- Bcl-2 binding domain
- CCD
- coiled-coil domain
- ECD
- evolutionarily conserved domain
- PepA
- pepstatin A
- LC3
- light chain 3
- IP3R
- inositol 1,4,5-trisphosphate receptor
- TLR
- Toll-like receptor
- ERα
- estrogen receptor α
- AVd
- advanced vesicle
- IP
- immunoprecipitation
- p
- phospho.
REFERENCES
- 1. Kroemer G., Mariño G., Levine B. (2010) Autophagy and the integrated stress response. Mol. Cell 40, 280–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moreau K., Luo S., Rubinsztein D. C. (2010) Cytoprotective roles for autophagy. Curr. Opin. Cell Biol. 22, 206–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mariño G., Madeo F., Kroemer G. (2011) Autophagy for tissue homeostasis and neuroprotection. Curr. Opin. Cell Biol. 23, 198–206 [DOI] [PubMed] [Google Scholar]
- 4. Shen S., Kepp O., Kroemer G. (2012) The end of autophagic cell death? Autophagy 8, 1–3 [DOI] [PubMed] [Google Scholar]
- 5. Ito H., Daido S., Kanzawa T., Kondo S., Kondo Y. (2005) Radiation-induced autophagy is associated with LC3, and its inhibition sensitizes malignant glioma cells. Int. J. Oncol. 26, 1401–1410 [PubMed] [Google Scholar]
- 6. Apel A., Herr I., Schwarz H., Rodemann H. P., Mayer A. (2008) Blocked autophagy sensitizes resistant carcinoma cells to radiation therapy. Cancer Res. 68, 1485–1494 [DOI] [PubMed] [Google Scholar]
- 7. Chaachouay H., Ohneseit P., Toulany M., Kehlbach R., Multhoff G., Rodemann H. P. (2011) Autophagy contributes to resistance of tumor cells to ionizing radiation. Radiother. Oncol. 99, 287–292 [DOI] [PubMed] [Google Scholar]
- 8. Chen Y. S., Song H. X., Lu Y., Li X., Chen T., Zhang Y., Xue J. X., Liu H., Kan B., Yang G., Fu T. (2011) Autophagy inhibition contributes to radiation sensitization of esophageal squamous carcinoma cells. Dis. Esophagus 24, 437–443 [DOI] [PubMed] [Google Scholar]
- 9. Amaravadi R. K., Yu D., Lum J. J., Bui T., Christophorou M. A., Evan G. I., Thomas-Tikhonenko A., Thompson C. B. (2007) Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J. Clin. Invest. 117, 326–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Levy J. M., Thorburn A. (2011) Targeting autophagy during cancer therapy to improve clinical outcomes. Pharmacol. Ther. 131, 130–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li J., Hou N., Faried A., Tsutsumi S., Takeuchi T., Kuwano H. (2009) Inhibition of autophagy by 3-MA enhances the effect of 5-FU-induced apoptosis in colon cancer cells. Ann. Surg. Oncol. 16, 761–771 [DOI] [PubMed] [Google Scholar]
- 12. Livesey K. M., Tang D., Zeh H. J., Lotze M. T. (2009) Autophagy inhibition in combination cancer treatment. Curr. Opin. Investig. Drugs 10, 1269–1279 [PubMed] [Google Scholar]
- 13. Han J., Hou W., Goldstein L. A., Lu C., Stolz D. B., Yin X. M., Rabinowich H. (2008) Involvement of protective autophagy in TRAIL resistance of apoptosis-defective tumor cells. J. Biol. Chem. 283, 19665–19677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Herrero-Martín G., Høyer-Hansen M., García-García C., Fumarola C., Farkas T., López-Rivas A., Jäättelä M. (2009) TAK1 activates AMPK-dependent cytoprotective autophagy in TRAIL-treated epithelial cells. EMBO J. 28, 677–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hou W., Han J., Lu C., Goldstein L. A., Rabinowich H. (2008) Enhancement of tumor-TRAIL susceptibility by modulation of autophagy. Autophagy 4, 940–943 [DOI] [PubMed] [Google Scholar]
- 16. Mills K. R., Reginato M., Debnath J., Queenan B., Brugge J. S. (2004) Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is required for induction of autophagy during lumen formation in vitro. Proc. Natl. Acad. Sci. U.S.A. 101, 3438–3443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gorzalczany Y., Gilad Y., Amihai D., Hammel I., Sagi-Eisenberg R., Merimsky O. (2011) Combining an EGFR directed tyrosine kinase inhibitor with autophagy-inducing drugs: a beneficial strategy to combat non-small cell lung cancer. Cancer Lett. 310, 207–215 [DOI] [PubMed] [Google Scholar]
- 18. Han W., Pan H., Chen Y., Sun J., Wang Y., Li J., Ge W., Feng L., Lin X., Wang X., Wang X., Jin H. (2011) EGFR tyrosine kinase inhibitors activate autophagy as a cytoprotective response in human lung cancer cells. PLoS One 6, e18691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Amaravadi R. K., Lippincott-Schwartz J., Yin X. M., Weiss W. A., Takebe N., Timmer W., DiPaola R. S., Lotze M. T., White E. (2011) Principles and current strategies for targeting autophagy for cancer treatment. Clin. Cancer Res. 17, 654–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bellodi C., Lidonnici M. R., Hamilton A., Helgason G. V., Soliera A. R., Ronchetti M., Galavotti S., Young K. W., Selmi T., Yacobi R., Van Etten R. A., Donato N., Hunter A., Dinsdale D., Tirrò E., Vigneri P., Nicotera P., Dyer M. J., Holyoake T., Salomoni P., Calabretta B. (2009) Targeting autophagy potentiates tyrosine kinase inhibitor-induced cell death in Philadelphia chromosome-positive cells, including primary CML stem cells. J. Clin. Invest. 119, 1109–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Calabretta B., Salomoni P. (2011) Inhibition of autophagy: a new strategy to enhance sensitivity of chronic myeloid leukemia stem cells to tyrosine kinase inhibitors. Leuk. Lymphoma 52, Suppl. 1, 54–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Janku F., McConkey D. J., Hong D. S., Kurzrock R. (2011) Autophagy as a target for anticancer therapy. Nat. Rev. Clin. Oncol. 8, 528–539 [DOI] [PubMed] [Google Scholar]
- 23. Holbro T., Hynes N. E. (2004) ErbB receptors: directing key signaling networks throughout life. Annu. Rev. Pharmacol. Toxicol. 44, 195–217 [DOI] [PubMed] [Google Scholar]
- 24. Shan Y., Eastwood M. P., Zhang X., Kim E. T., Arkhipov A., Dror R. O., Jumper J., Kuriyan J., Shaw D. E. (2012) Oncogenic mutations counteract intrinsic disorder in the EGFR kinase and promote receptor dimerization. Cell 149, 860–870 [DOI] [PubMed] [Google Scholar]
- 25. Huang H. L., Chen Y. C., Huang Y. C., Yang K. C., Pan H. y., Shih S. P., Chen Y. J. (2011) Lapatinib induces autophagy, apoptosis, and megakaryocytic differentiation in chronic myelogenous leukemia K562 cells. PloS one 6, e29014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mizushima N., Levine B., Cuervo A. M., Klionsky D. J. (2008) Autophagy fights disease through cellular self-digestion. Nature 451, 1069–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen Y., Klionsky D. J. (2011) The regulation of autophagy – unanswered questions. J. Cell Sci. 124, 161–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Itakura E., Kishi C., Inoue K., Mizushima N. (2008) Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG. Mol. Biol. Cell 19, 5360–5372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Matsunaga K., Saitoh T., Tabata K., Omori H., Satoh T., Kurotori N., Maejima I., Shirahama-Noda K., Ichimura T., Isobe T., Akira S., Noda T., Yoshimori T. (2009) Two Beclin 1-binding proteins, Atg14L and Rubicon, reciprocally regulate autophagy at different stages. Nat. Cell Biol. 11, 385–396 [DOI] [PubMed] [Google Scholar]
- 30. Zhong Y., Wang Q. J., Li X., Yan Y., Backer J. M., Chait B. T., Heintz N., Yue Z. (2009) Distinct regulation of autophagic activity by Atg14L and Rubicon associated with Beclin 1-phosphatidylinositol-3-kinase complex. Nat. Cell Biol. 11, 468–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Qu X., Yu J., Bhagat G., Furuya N., Hibshoosh H., Troxel A., Rosen J., Eskelinen E. L., Mizushima N., Ohsumi Y., Cattoretti G., Levine B. (2003) Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J. Clin. Invest. 112, 1809–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gong C., Bauvy C., Tonelli G., Yue W., Deloménie C., Nicolas V., Zhu Y., Domergue V., Marin-Esteban V., Tharinger H., Delbos L., Gary-Gouy H., Morel A. P., Ghavami S., Song E., Codogno P., Mehrpour M. (2013) Beclin 1 and autophagy are required for the tumorigenicity of breast cancer stem-like/progenitor cells. Oncogene 32, 2261–2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang R. C., Wei Y., An Z., Zou Z., Xiao G., Bhagat G., White M., Reichelt J., Levine B. (2012) Akt-mediated regulation of autophagy and tumorigenesis through Beclin 1 phosphorylation. Science 338, 956–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pulaski B. A., Ostrand-Rosenberg S. (2001) Mouse 4T1 breast tumor model. in Current Protocols in Immunology (Coligan J. E., Bierer B. E., Margulies D. H., Shevach E. M., Strober W., eds), John Wiley & Sons, Inc., New York: [DOI] [PubMed] [Google Scholar]
- 35. Reilly R. T., Gottlieb M. B., Ercolini A. M., Machiels J. P., Kane C. E., Okoye F. I., Muller W. J., Dixon K. H., Jaffee E. M. (2000) HER-2/neu is a tumor rejection target in tolerized HER-2/neu transgenic mice. Cancer Res. 60, 3569–3576 [PubMed] [Google Scholar]
- 36. Han J., Goldstein L. A., Hou W., Froelich C. J., Watkins S. C., Rabinowich H. (2010) Deregulation of mitochondrial membrane potential by mitochondrial insertion of granzyme B and direct Hax-1 cleavage. J. Biol. Chem. 285, 22461–22472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Han J., Goldstein L. A., Hou W., Gastman B. R., Rabinowich H. (2010) Regulation of mitochondrial apoptotic events by p53-mediated disruption of complexes between antiapoptotic Bcl-2 members and Bim. J. Biol. Chem. 285, 22473–22483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Han J., Goldstein L. A., Hou W., Rabinowich H. (2007) Functional linkage between NOXA and Bim in mitochondrial apoptotic events. J. Biol. Chem. 282, 16223–16231 [DOI] [PubMed] [Google Scholar]
- 39. Lainey E., Thépot S., Bouteloup C., Sébert M., Adès L., Tailler M., Gardin C., de Botton S., Baruchel A., Fenaux P., Kroemer G., Boehrer S. (2011) Tyrosine kinase inhibitors for the treatment of acute myeloid leukemia: delineation of anti-leukemic mechanisms of action. Biochem. Pharmacol. 82, 1457–1466 [DOI] [PubMed] [Google Scholar]
- 40. He C., Levine B. (2010) The Beclin 1 interactome. Curr. Opin. Cell Biol. 22, 140–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wirawan E., Vande Walle L., Kersse K., Cornelis S., Claerhout S., Vanoverberghe I., Roelandt R., De Rycke R., Verspurten J., Declercq W., Agostinis P., Vanden Berghe T., Lippens S., Vandenabeele P. (2010) Caspase-mediated cleavage of Beclin-1 inactivates Beclin-1-induced autophagy and enhances apoptosis by promoting the release of proapoptotic factors from mitochondria. Cell Death Dis. 1, e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. John S., Nayvelt I., Hsu H. C., Yang P., Liu W., Das G. M., Thomas T., Thomas T. J. (2008) Regulation of estrogenic effects by beclin 1 in breast cancer cells. Cancer Res. 68, 7855–7863 [DOI] [PubMed] [Google Scholar]
- 43. Shi C. S., Kehrl J. H. (2008) MyD88 and Trif target Beclin 1 to trigger autophagy in macrophages. J. Biol. Chem. 283, 33175–33182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vicencio J. M., Ortiz C., Criollo A., Jones A. W., Kepp O., Galluzzi L., Joza N., Vitale I., Morselli E., Tailler M., Castedo M., Maiuri M. C., Molgó J., Szabadkai G., Lavandero S., Kroemer G. (2009) The inositol 1,4,5-trisphosphate receptor regulates autophagy through its interaction with Beclin 1. Cell Death Differ. 16, 1006–1017 [DOI] [PubMed] [Google Scholar]
- 45. Yue Z., Horton A., Bravin M., DeJager P. L., Selimi F., Heintz N. (2002) A novel protein complex linking the δ2 glutamate receptor and autophagy: implications for neurodegeneration in lurcher mice. Neuron 35, 921–933 [DOI] [PubMed] [Google Scholar]
- 46. Liang X. H., Jackson S., Seaman M., Brown K., Kempkes B., Hibshoosh H., Levine B. (1999) Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 402, 672–676 [DOI] [PubMed] [Google Scholar]
- 47. Yue Z., Jin S., Yang C., Levine A. J., Heintz N. (2003) Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc. Natl. Acad. Sci. U.S.A. 100, 15077–15082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dikic I., Johansen T., Kirkin V. (2010) Selective autophagy in cancer development and therapy. Cancer Res. 70, 3431–3434 [DOI] [PubMed] [Google Scholar]
- 49. Mathew R., White E. (2011) Autophagy in tumorigenesis and energy metabolism: friend by day, foe by night. Curr. Opin. Genet. Dev. 21, 113–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Thoresen S. B., Pedersen N. M., Liestøl K., Stenmark H. (2010) A phosphatidylinositol 3-kinase class III sub-complex containing VPS15, VPS34, Beclin 1, UVRAG, and BIF-1 regulates cytokinesis and degradative endocytic traffic. Exp. Cell Res. 316, 3368–3378 [DOI] [PubMed] [Google Scholar]