Abstract
Articular cartilage defects are considered a major health problem because articular cartilage has a limited capacity for self-regeneration 1. Untreated cartilage lesions lead to ongoing pain, negatively affect the quality of life and predispose for osteoarthritis. During the last decades, several surgical techniques have been developed to treat such lesions. However, until now it was not possible to achieve a full repair in terms of covering the defect with hyaline articular cartilage or of providing satisfactory long-term recovery 2-4. Therefore, articular cartilage injuries remain a prime target for regenerative techniques such as Tissue Engineering. In contrast to other surgical techniques, which often lead to the formation of fibrous or fibrocartilaginous tissue, Tissue Engineering aims at fully restoring the complex structure and properties of the original articular cartilage by using the chondrogenic potential of transplanted cells. Recent developments opened up promising possibilities for regenerative cartilage therapies.
The first cell based approach for the treatment of full-thickness cartilage or osteochondral lesions was performed in 1994 by Lars Peterson and Mats Brittberg who pioneered clinical autologous chondrocyte implantation (ACI) 5. Today, the technique is clinically well-established for the treatment of large hyaline cartilage defects of the knee, maintaining good clinical results even 10 to 20 years after implantation 6. In recent years, the implantation of autologous chondrocytes underwent a rapid progression. The use of an artificial three-dimensional collagen-matrix on which cells are subsequently replanted became more and more popular 7-9.
MACT comprises of two surgical procedures: First, in order to collect chondrocytes, a cartilage biopsy needs to be performed from a non weight-bearing cartilage area of the knee joint. Then, chondrocytes are being extracted, purified and expanded to a sufficient cell number in vitro. Chondrocytes are then seeded onto a three-dimensional matrix and can subsequently be re-implanted. When preparing a tissue-engineered implant, proliferation rate and differentiation capacity are crucial for a successful tissue regeneration 10. The use of a three-dimensional matrix as a cell carrier is thought to support these cellular characteristics 11.
The following protocol will summarize and demonstrate a technique for the isolation of chondrocytes from cartilage biopsies, their proliferation in vitro and their seeding onto a 3D-matrix (Chondro-Gide, Geistlich Biomaterials, Wollhusen, Switzerland). Finally, the implantation of the cell-matrix-constructs into artificially created chondral defects of a rabbit's knee joint will be described. This technique can be used as an experimental setting for further experiments of cartilage repair.
Keywords: Biomedical Engineering, Issue 75, Medicine, Anatomy, Physiology, Cellular Biology, Molecular Biology, Tissue Engineering, Surgery, Autologous chondrocyte implantation, matrix-assisted, matrix, collagen scaffold, chondral lesion, cartilage, rabbit, experimental, cartilage defects, cartilage repair, regenerative therapy, chondrocytes, cell culture, isolation, transplantation, animal model
Protocol
A. Cartilage Biopsy (Surgery Room; Steps 1-5 in Non-sterile Preparation Room)
Perform a final weight control of the rabbit (New Zealand White rabbit, female, 3.5-4.0 kg body weight, 6 months old) in order to be able to dose drugs properly and to monitor weight subsequent to surgery.
Induce anesthesia to the rabbit by an intravenous injection of 10 mg/kg propofol.
After intubation, maintain anesthesia with 1.5 mg/kg/min propofol and 0.05 mg/kg fentanyl intravenously. Monitor anesthesia by using capnography, pulse oximetry and pulse rate.
Shave the knee to be operated on with an electric clipper and vacuum the fur.
Disinfect the shaved knee thoroughly and cover the rest of the rabbit with a sterile dressing.
Palpate the patella and perform a skin incision medially of the patella.
Open the knee joint by a medial parapatellar arthrotomy under sterile conditions. Try to avoid cutting any small superficial blood vessels.
Displace the patella laterally.
Inspect the knee joint for any anomalies and macroscopic cartilage lesions.
Carefully peel small cartilage pieces (2-3 mm) out of the trochlea ossis femoris with a sterile scalpel (Figure 1).
Immediately place these cartilage biopsies into a 50 ml tube with complete medium (DMEM + 10% fetal calf serum (FCS) + 1% penicillin/streptomycin (Pen/Strep)) to continue with the in vitro culture right after the operation.
Clean the defects and rinse them with sterile saline.
Reposition the patella within the trochlea groove.
Wound closure in layers with single button sutures (4-0 Vicryl) and a continuing cutaneous suture (4-0 Monocryl). Use absorbable suture material.
Finally, seal the wound with a spray dressing permeable to water vapor.
B. In vitro Culture
Process the biopsied cartilage pieces as soon as possible after the operation in a sterile laminar flow tissue culture hood.
Wash the sterile harvested cartilage 2x in PBS with subsequent centrifugation at 500 x g for 3 min at RT.
Cut cartilage in pieces of 1 mm3, transfer to a 50 ml falcon and digest them on a shaker for 30 min with 10 ml trypsin-EDTA (0.25%) and 10 ml PBS for 30 min
After 30 min, stop trypsinization by adding complete medium. Then, shake the cartilage pieces for another for 12 hr with Collagenase A (0.21 U/mg) in serum free medium (DMEM + 1% Pen/Strep).
Centrifuge the resulting solution at 170 x g for 3 min at RT.
Resuspend cell pellets in 5 ml complete medium.
Seed the isolated chondrocytes on to 25 cm2 tissue culture flasks in 5 ml complete medium and keep in a humidified tissue culture incubator at 37 °C, 5% CO2.
Grow chondrocytes to a density of 80%.
Release the cells from flasks by washing in PBS 2x before exposing to 0.05% trypsin-EDTA for 3 min. Once chondrocytes detach, stop trypsinization by addition of 10 μl complete medium.
Centrifuge the suspension at 300 x g (3 min) and resuspend pellet in complete medium.
Seed cells in tissue culture flasks (75 cm2) and split at a ratio of 1:3 (∼5,000 cells/cm2) every 5th day or upon 80% confluency.
Determine cell numbers and viability by trypan blue staining.
C. Cell-seeding of the Matrix
Prior to implantation wash and release cells as described above. Perform cell count and transfer 5 x 104 cells into a 1.5 ml microcentrifuge tube. Then centrifuge at 500 x g for 5 min at RT.
Resuspend pellet in 15 μl complete medium for a complete saturation of the matrix.
Cut the bilayer collagen scaffold to the exact dimensions of the trochlear defect by a sterile biopsy punch.
Place matrix into an appropriately sized tissue culture dish (e.g. 24-well plates).
Seed cells onto the porous, cell-adhesive side of the matrix. This deters cell migration away from the matrix.
Leave cell-seeded matrices without further cell culture medium within an incubator for 1 hr to allow full adherence of the chondrocytes.
Then, place cell-matrix-constructs into culturing containers (e.g. 24-well plates) with fresh complete medium. Take care not to wash out any cells off the matrix even though strong adherence is expected.
Now, cell-matrix-constructs can be taken to the surgery room for re-implantation.
D. Matrix-assisted Autologous Chondrocyte Transplantation
Perform parapatellar arthrotomy to the contralateral knee as described above (A. 1-8).
Create two isolated chondral defects in the trochlear groove with a sterile air operating power drill (3.6 mm in diameter).
Clean the defect and rinse with sterile saline.
Attention should be paid not to open the marrow cavity at any time. If necessary, seal the bottom of the defects with electrical cautery against bleeding.
Implant the matrices seeded with the cultured chondrocytes with the porous side face down (Figure 2). Then press-fit into the drill holes and flush the surrounding surface.
Seal the implanted matrices with a little bit of fibrin glue (Tissucol Duo S) to assure secure fixation.
After clotting, relocate the patella within the trochlear groove and apply full range of motion to the knee joint a few times.
Displace the patella laterally once again and check for any event or sign of instable fixation. The matrices must be held still in place.
Replace the patella again and finish the operation with wound closure in layers and spray dressing as described above.
Representative Results
The described surgical technique permits a successful isolation and implantation of autologous chondrocytes into an artificial chondral defect. The experimental setup resulted in a successful integration of the implant into the surrounding cartilage.
After 12 weeks in vivo, the chondral defect was filled by repair tissue with a homogenous and intact surface, which reduced shear stress and damage to the implant (Figure 4). Moreover, no hypertrophy or calcification of the implant was seen. The repair tissue showed a stiff and solid quality, which was comparable to the healthy surrounding cartilage tissue. This was an important aspect because increased load on the adjacent cartilage due to insufficient biomechanical properties of the implant would be a risk for premature degeneration. Moreover, a graft delamination has not occurred. Having cut the membrane to the same size of the defect preoperatively, any fissuring or clefts between implant and surrounding cartilage were avoided.
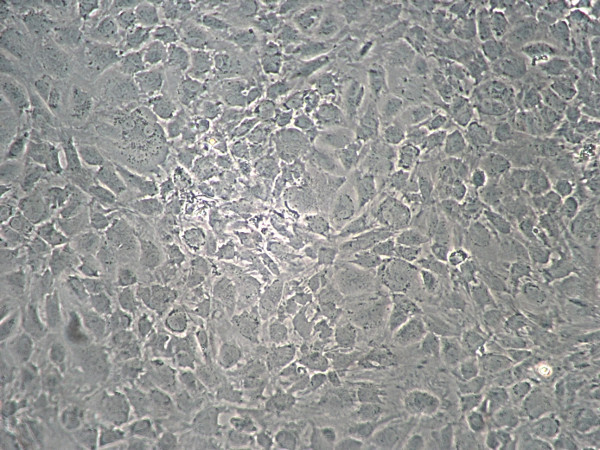 Figure 1. Monolayer of chondrocytes primary culture at 80% confluency.
Figure 1. Monolayer of chondrocytes primary culture at 80% confluency.
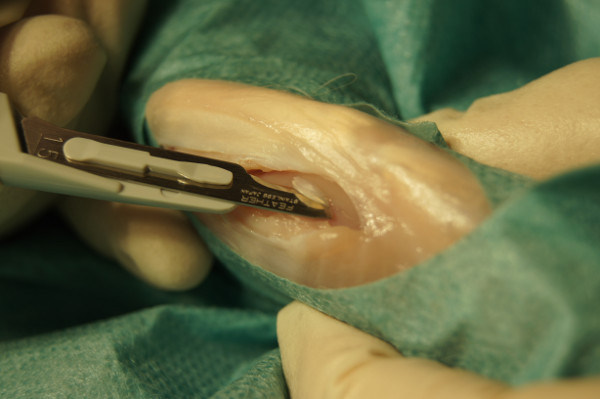 Figure 2. Taking a cartilage biopsy out of the femoral trochlea groove.
Figure 2. Taking a cartilage biopsy out of the femoral trochlea groove.
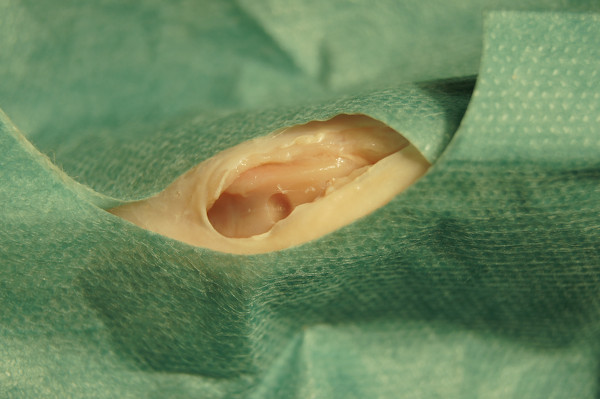 Figure 3. Artificially created trochlear cartilage defect.
Figure 3. Artificially created trochlear cartilage defect.
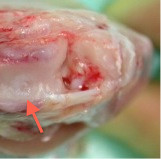 Figure 4. Opened knee joint 12 weeks after implantation of a membrane seeded with autologous chondrocytes into a pre-drilled chondral defect (red arrow).
Figure 4. Opened knee joint 12 weeks after implantation of a membrane seeded with autologous chondrocytes into a pre-drilled chondral defect (red arrow).
Discussion
The presented protocol provides an established 9,12,13 and easily reproducible technique to isolate autologous chondrocytes for subsequent proliferation and re-implantation into artificially created cartilage defects in rabbit knees. The use of autologous chondrocytes for remodeling and repair of articular cartilage lesions is already in clinical use providing satisfying long-term results 6.
Major problems as for example periosteal hypertrophy and calcification, graft delamination or donor site morbidity occurred with the first and second generation of autologous chondrocyte implantation 14. Therefore researchers have developed techniques using exogenous bioresorbable materials to deliver chondrocytes to the defect site. The positive effect of three-dimensional culture system on the maintenance of the chondrocytic characteristics has been repeatedly demonstrated, for example when using agarose 15, poly - dioxanon and polyglactin 16, polyesters poly(L-lactide) (PLLA) and poly(D,L-lactide-coglycolide) (PLGA) 17, fibrin 12, hyaluronan 18, alginate 19, collagen 20 or collagen-matrices 9.
The bilayer collagen membrane Chondro-Gide used in this study has been successfully applied in several preclinical studies before 9,21,22 and is already part of clinical applications 21,23. The bilayer structure of the collagen membrane offers a stable microenvironment for chondrocyte integration and proliferation, allows for an even distribution of the cells within the matrix and eliminates the possibility of cell leakage as it is seen in conventional ACI 11. By use of this MACT technique, the transplanted chondrocytes are locally retained with maintained viability. Matrix-assisted autologous chondrocyte transplantation leads to the synthesis of cartilage-like regeneration tissue and is clinically applicable 21.
The described animal model is well accepted in the experimental treatment of articular cartilage lesions 11,24,25. However, the translation to clinical routine of possible results of experiments using the presented technique is difficult for several reasons. In an animal model it is almost impossible to achieve non or partial weight-bearing in the first days/weeks after surgery which would be generally recommended to allow the implanted cells to start with early integration processes. Full weight bearing immediately after surgery could potentially harm the transplant and therefore might influence the outcome. But this setting was similar in all animal models comparable 9,12. Experiments with larger animals, more closely resembling the human situation, are warranted in further animal studies.
In summary, however, an established experimental animal model as it is described above provides a basis for further experimental studies of cartilage repair and might even facilitate the realization and performance of complex study settings. Experimental examinations with growth factors, gene induction and changing matrix properties using this animal model might be helpful to improve the established clinical settings.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This project was funded by the German Research Association (DFG, HE 4578/3-1).
References
- Albrecht C, et al. Gene expression and cell differentiation in matrix-associated chondrocyte transplantation grafts: a comparative study. Osteoarthritis Cartilage. 2011;19:1219–1227. doi: 10.1016/j.joca.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Pridie KH. A method of resurfacing osteoarthritic knee joints. J. Bone Joint Surg. Br. 1959;41:618–619. [Google Scholar]
- Johnson LL. Arthroscopic abrasion arthroplasty historical and pathologic perspective: present status. Arthroscopy. 1986;2:54–69. doi: 10.1016/s0749-8063(86)80012-3. [DOI] [PubMed] [Google Scholar]
- Steadman JR, Rodkey WG, Singelton SB, Briggs KK. Microfracture technique for full-thickness chondral defects: technique and clinical result. Operat. Tech. Orthop. 1997;7:300–304. [Google Scholar]
- Brittberg M, et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- Peterson L, Vasiliadis HS, Brittberg M, Lindahl A. Autologous chondrocyte implantation: a long-term follow-up. Am. J. Sports Med. 2010;38:1117–1124. doi: 10.1177/0363546509357915. [DOI] [PubMed] [Google Scholar]
- Nehrer S, et al. Chondrocyte-seeded collagen matrices implanted in a chondral defect in a canine model. Biomaterials. 1998;19:2313–2328. doi: 10.1016/s0142-9612(98)00143-4. [DOI] [PubMed] [Google Scholar]
- Frenkel SR, Toolan B, Menche D, Pitman MI, Pachence JM. Chondrocyte transplantation using a collagen bilayer matrix for cartilage repair. J. Bone. Joint Surg. Br. 1997;79:831–836. doi: 10.1302/0301-620x.79b5.7278. [DOI] [PubMed] [Google Scholar]
- Salzmann GM, et al. The dependence of autologous chondrocyte transplantation on varying cellular passage, yield and culture duration. Biomaterials. 2011;32:5810–5818. doi: 10.1016/j.biomaterials.2011.04.073. [DOI] [PubMed] [Google Scholar]
- Frohlich M, Malicev E, Gorensek M, Knezevic M, Kregar Velikonja N. Evaluation of rabbit auricular chondrocyte isolation and growth parameters in cell culture. Cell Biol. Int. 2007;31:620–625. doi: 10.1016/j.cellbi.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Willers C, Chen J, Wood D, Xu J, Zheng MH. Autologous chondrocyte implantation with collagen bioscaffold for the treatment of osteochondral defects in rabbits. Tissue Eng. 2005;11:1065–1076. doi: 10.1089/ten.2005.11.1065. [DOI] [PubMed] [Google Scholar]
- Vogt S, et al. The influence of the stable expression of BMP2 in fibrin clots on the remodelling and repair of osteochondral defects. Biomaterials. 2009;30:2385–2392. doi: 10.1016/j.biomaterials.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Ueblacker P, et al. In vivo analysis of retroviral gene transfer to chondrocytes within collagen scaffolds for the treatment of osteochondral defects. Biomaterials. 2007;28:4480–4487. doi: 10.1016/j.biomaterials.2007.06.027. [DOI] [PubMed] [Google Scholar]
- Marlovits S, Zeller P, Singer P, Resinger C, Vecsei V. Cartilage repair: generations of autologous chondrocyte transplantation. Eur. J. Radiol. 2006;57:24–31. doi: 10.1016/j.ejrad.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- Rudert M, Hirschmann F, Wirth CJ. Growth behavior of chondrocytes on various biomaterials. Orthopade. 1999;28:68–75. doi: 10.1007/s001320050323. [DOI] [PubMed] [Google Scholar]
- Hsu SH, et al. Evaluation of biodegradable polyesters modified by type II collagen and Arg-Gly-Asp as Tissue Engineering scaffolding materials for cartilage regeneration. Artificial Organs. 2006;30:42–55. doi: 10.1111/j.1525-1594.2006.00179.x. [DOI] [PubMed] [Google Scholar]
- Brun P, Cortivo R, Zavan B, Vecchiato N, Abatangelo G. In vitro reconstructed tissues on hyaluronan-based temporary scaffolding. J. Mater. Sci. Mater. Med. 1999;10:683–688. doi: 10.1023/a:1008960413362. [DOI] [PubMed] [Google Scholar]
- Domm C, Fay J, Schunke M, Kurz B. Redifferentiation of dedifferentiated joint cartilage cells in alginate culture. Effect of intermittent hydrostatic pressure and low oxygen partial pressure. Orthopade. 2000;29:91–99. doi: 10.1007/s001320050015. [DOI] [PubMed] [Google Scholar]
- Kimura T, Yasui N, Ohsawa S, Ono K. Chondrocytes embedded in collagen gels maintain cartilage phenotype during long-term cultures. Clin. Orthop. Relat. Res. 1984. pp. 231–239. [PubMed]
- Kon E, et al. Second-generation autologous chondrocyte implantation: results in patients older than 40 years. Am. J. Sports Med. 2011;39:1668–1675. doi: 10.1177/0363546511404675. [DOI] [PubMed] [Google Scholar]
- Gavenis K, Schmidt-Rohlfing B, Mueller-Rath R, Andereya S, Schneider U. In vitro comparison of six different matrix systems for the cultivation of human chondrocytes. In Vitro Cell Dev. Biol. Anim. 2006;42:159–167. doi: 10.1290/0511079.1. [DOI] [PubMed] [Google Scholar]
- Niemeyer P, et al. Characteristic complications after autologous chondrocyte implantation for cartilage defects of the knee joint. Am. J. Sports Med. 2008;36:2091–2099. doi: 10.1177/0363546508322131. [DOI] [PubMed] [Google Scholar]
- Tay LX, et al. Treatment outcomes of alginate-embedded allogenic mesenchymal stem cells versus autologous chondrocytes for the repair of focal articular cartilage defects in a rabbit model. The American Journal of Sports Medicine. 2012;40:83–90. doi: 10.1177/0363546511420819. [DOI] [PubMed] [Google Scholar]
- Brittberg M, Nilsson A, Lindahl A, Ohlsson C, Peterson L. Rabbit articular cartilage defects treated with autologous cultured chondrocytes. Clin. Orthop. Relat. Res. 1996. pp. 270–283. [DOI] [PubMed]


