Abstract
IκBβ, one of the major IκB proteins, is only partially degraded in response to most extracellular signals. However, the molecular mechanism of this event is unknown. We show here that IκBβ exists in at least two different forms: one that is bound to the NF-κB dimer and the other bound to both NF-κB and κB-Ras, a Ras-like small G protein. Removal of cellular κB-Ras enhances whereas excess κB-Ras blocks induced IκBβ degradation. Remarkably, κB-Ras functions in both GDP- and GTP-bound states, and mutations of the conserved guanine-binding residues of κB-Ras abrogate its ability to block degradation of IκBβ. κB-Ras also directly blocks the in vitro phosphorylation of IκBβ by IKKβ. These observations suggest that IκBβ in the ternary complex is resistant to degradation by most signals. We suggest that specific signals, in addition to those that activate only IKK, are essential for the complete degradation of IκBβ.
The dimeric NF-κB transcription factors are inhibited in quiescent cells through stable association with inhibitor IκBs. A large number of extracellular stimuli transmit signals to relieve this inhibition (2, 17, 38). Almost all of these signals lead to the activation of a specific kinase known as IκB kinase (IKK), which phosphorylates IκB. Phosphorylated IκB proteins are degraded by the sequential actions of ubiquitin ligase and the 26S proteasome releasing free NF-κB (23).
The major IκB proteins, IκBα and IκBβ, resemble each other in both primary sequence and tertiary structure, with the exception of a 40-residue-long insert present within the ankyrin repeat 3 in IκBβ. However, these two proteins exhibit one major functional difference (17, 35, 36). While signal-induced degradation of IκBα is responsible for rapid NF-κB activation, prolonged activation of NF-κB, which is essential for certain biological functions such as T-cell activation, requires IκBβ degradation (1). Several pathological conditions, such as asthma, cystic fibrosis, and viral and bacterial infection, also require prolonged NF-κB activation (3, 4, 10, 19, 22, 29, 33, 35). We do not know why prolonged NF-κB activation requires IκBβ degradation. Two other functional properties of IκBβ distinguish it from IκBα. Unlike IκBα, IκBβ does not fully degrade in response to most stimuli, and IκBβ/NF-κB complexes are exclusively cytoplasmic in resting cells (16, 17, 25, 34). How these two properties contribute to persistent NF-κB activation through IκBβ degradation is not known. A recent report shows that different MEKK kinases recruit IKK to IκBα/NF-κB and IκBβ/NF-κB complexes in tumor necrosis factor alpha (TNF-α)-activated cells (31). This suggests that the compositions of IκBα and IκBβ complexes are different, which may lead to their differential functional properties.
Yeast two-hybrid screening has identified two Ras-like small GTPases, κB-Ras1 and -2, as inhibitors of NF-κB transcriptional activity (14). κB-Ras proteins belong to the Ras family due to their high sequence similarity (5, 6, 39). However, there are some critical differences in the sequences of κB-Ras. In addition to the fact that these two proteins lack lipid modification sites, κB-Ras proteins contain two Ras oncogenic mutations that drastically reduce GTP hydrolysis. The mutations maintain Ras in the constitutively active GTP-bound conformation, converting it to its oncogenic form (5, 6). These differences in sequence suggest that κB-Ras might not function as Ras and other small GTPases.
Although in vivo studies showed that κB-Ras proteins were associated specifically with the IκBβ/p65 complex, in vitro pull-down and transfection experiments suggested that κB-Ras was also able to bind and stabilize IκBα (14). We have shown that κB-Ras1 binds directly to the IκBβ/p65 complex and masks the exposed p65 nuclear localization signal (NLS) (26). We also identified that along with the p65 NLS, the insert of IκBβ is the other primary site of κB-Ras binding.
The focus of this study was to test whether κB-Ras plays a role in the incomplete degradation of IκBβ bound to NF-κB dimers. Our results demonstrate that in quiescent cells, a pool of IκBβ is primarily present in a form that cannot be phosphorylated by active IKK. In vitro experiments reveal that active IKK is unable to phosphorylate IκBβ or IκBβ/NF-κB complexes in the presence of κB-Ras. We further show that IκBβ/NF-κB complexes represent subcomplexes within larger protein complexes, and at least some of these complexes contain κB-Ras. In addition to the insert, κB-Ras also requires the N-terminal signal response region (SRR) of IκBβ for binding. κB-Ras therefore directly masks the signal induced phosphorylation sites (Ser19 and Ser23) located within the SRR of IκBβ. Overexpression of κB-Ras blocks stimulus-dependent degradation of IκBβ, and removal of κB-Ras enhances it. Interestingly, either GTP or GDP is sufficient to function as a cofactor for its inhibitory function in vitro. We suggest that a pool of IκBβ/NF-κB complexes are associated with κB-Ras and respond to extracellular signals differently.
MATERIALS AND METHODS
Materials.
p65 NLS monoclonal antibody was a generous gift from Roche Diagnostics. c-Rel antibody was a gift from Nancy Rice (National Institutes of Health). p65 and IκBβ polyclonal antibodies were purchased from Santa Cruz. κB-Ras antibody was a gift from Sankar Ghosh (Yale University). GTP and GDP were purchased from Sigma Chemicals. Duplex short interfering RNA (siRNA) was purchased from Dharmacon. Single-stranded deoxy oligonucleotides were purchased form Allele Biotechnology, San Diego, Calif.
Protein purification.
The cloning and purification methods used for NF-κB and IκB proteins have been described elsewhere (20). The coding sequence for κB-Ras1 was amplified by using two terminal primers with NdeI and BamHI sites at the 5′ and 3′ ends, respectively. The restricted fragment was inserted into the NdeI- and BamHI-cleaved pET15b vector. Escherichia coli BL21(DE3) cells harboring the expression plasmid were grown and induced with 0.1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) at room temperature. The cell pellet was suspended in a buffer containing 20 mM Tris (pH 7.5), 1 M NaCl, and 2 mM β-mercaptoethanol followed by lysis of cells by sonication. Clear, soluble crude extracts were loaded onto an Ni2+ affinity column (Novagen) and eluted with 200 mM imidazole after extensive washing. Peak fractions containing κB-Ras1 were pooled and further purified by size exclusion chromatography (Superdex 75; Amersham Biosciences). κB-Ras1 was also purified under a denatured condition and subsequently refolded. E. coli cells expressing κB-Ras1 were solubilized in a buffer containing 7 M urea, 1 M NaCl, 20 mM Tris (pH 7.5), and 2 mM β-mercaptoethanol. Cells were lysed by sonication followed by centrifugation to remove cell debris. The clear denatured extract was loaded onto an Ni2+ affinity column equilibrated with lysis buffer. After extensive washing, κB-Ras1 was eluted with 200 mM imidazole. κB-Ras1 was refolded first by diluting the protein to 0.4 mg/ml in the lysis buffer and then subsequently removing urea by dialysis in three steps. Refolded protein was concentrated and subjected to size exclusion chromatography. The elution profile of the protein was same as that of the κB-Ras1 purified with Ni-nitrilotriacetic acid (NTA) and size exclusion chromatography (Superdex 75) under native conditions. The other Ras and Ras-related proteins were expressed in E. coli. Proteins were purified by Ni-NTA and size exclusion chromatography.
Cells, transfection, and extract preparation.
HeLa, Cos-7, and 293 cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, 1 mM glutamine, 100-U/ml penicillin, and 100-μg/ml streptomycin. Transfections were performed with Lipofectamine Plus (Invitrogen). Cytosolic extract of HeLa cells was concentrated by ammonium sulfate precipitation to 75% saturation. The pellet was suspended in chromatography buffer (150 mM KCl, 20 mM Tris [pH 7.5], 1 mM dithiothreitol [DTT]) and loaded onto a Superose 6 column.
Plasmids and site-directed mutagenesis.
All plasmids were constructed by standard recombinant DNA procedures. Site-directed mutagenesis and deletions were performed with the Stratagene Quickchange kit.
In vitro kinase assays.
Baculovirus-expressed, His-tagged IKKβ was purified by nickel affinity chromatography. Ten nanograms of pure IKKβ was used in each kinase reaction. Kinase assays were performed at 30°C for 30 min in buffer containing 20 mM Tris (pH 7.6), 10 mM MgCl2, 2 mM DTT, 20 μM ATP, and 15 μCi of [γ-32P]ATP. For kinase assays, 2 μg of IκBα or IκBβ was mixed with or without 4 μg of p65 homodimer or p50/p65 heterodimer on ice for 30 min. Two or 4 μg of κB-Ras1 was added to the IκBα/NF-κB or IκBβ/NF-κB complexes wherever necessary. Mixtures were incubated on ice for 60 min before kinase assays were performed.
Immunoprecipitation and Western analysis.
Cells were washed three times in phosphate-buffered saline (PBS) buffer. Cytoplasmic extracts were made by lysing cells in buffer containing 1% Triton X-100, 20 mM Tris-HCl (pH 7.6), 200 mM NaCl, 1 mM DTT, and 1 mM phenylmethylsulfonyl fluoride (lysis buffer). One to 2 mg of the extract was mixed with protein A-agarose and primary antibodies and incubated at 4°C overnight. The immunoprecipitates were washed three times in lysis buffer and eluted with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) buffer by heating at 100°C for 5 min. The supernatant was separated by SDS-PAGE (12.5% polyacrylamide). The separated proteins in the gel were transferred to Hybond nitrocellulose membrane (Amersham-Pharmacia Biotech). The membrane was blocked with 5% milk in PBS-Tween buffer and incubated with anti-p65 polyclonal antibody (Santa Cruz Biotechnology) for 1 h at room temperature. The membrane was washed and incubated with horseradish peroxidase-conjugated antirabbit immunoglobulin (Ig) (Santa Cruz Biotechnology). Blots were visualized by use of the ECL enhanced chemiluminescence reagent kit (Amersham-Pharmacia Biotech).
Immunoprecipitations and kinase assays.
293 cell lysates (1.5 mg) were immunoprecipitated with anti-p65 goat polyclonal antibody. Immunoprecipitates were washed four times with lysis buffer and were used as substrates for kinase assays with purified baculovirus-expressed IKKβ.
RNAi experiments.
RNA interference (RNAi) experiments were performed as follows. The sequence of siRNA for the κB-Ras1 was 5′-AAGAUUGCGAAACAAUGGAGG-3′. siRNA (1.0 μg) was transfected into 293 cells in six-well plates with Lipofectamine Plus (Invitrogen). Three days posttransfection, cells were treated with 10 ng of TNF-α for the indicated times. Whole-cell lysates were subjected to SDS-PAGE and Western blot analysis. Six pSUPER siRNA expression vectors, three each for κB-Ras1 and -2, were prepared by inserting duplex oligonucleotides based on the sequence of κB-Ras1 and κB-Ras2 cDNAs between the BglII and HindIII sites of the vector. The sequences of the top strands of these oligonucleotides are listed below. The sequence underlined represents the loop.
The following oligonucleotides were used for pSuper-κB-Ras1: 1 (nucleotides 103 to 121), 5′-GATTGCGAAACAATGGAAGTTCAAGAGACTTCCATTGTTTCGCAATC-3′; 2 (nucleotides 202 to 220), 5′-GGCGTGGAGCTGCCAAAGCTTCAAGAGAGCTTTGGCAGCTCCACGCC-3′; and 3 (nucleotides 388 to 406), 5′-GTGGACGCTGAAGTGGCACTTCAAGAGAGTGCCACTTCAGCGTCCAC-3′. The oligonucleotides for pSuper-κB-Ras2 were as follows: 1 (nucleotides 84 to 102), 5′-CCATGTAGTGGGTTCGGAGTTCAAGAGACTCCGAACCCACTACATGG-3′; 2 (nucleotides 333 to 351), 5′-GGAGGTCACCATCGTGGTCTTCAAGAGAGACCACGATGGTGACCTCC-3′; and 3 (nucleotides 435 to 453), 5′-GCTGTGGGAGGTGTCAGTGTTCAAGAGACACTGACACCTCCCACAGC-3′.
All six of these expression vectors independently worked well as siRNA to knock down κB-Ras1 and -2. The control pSUPER expression vector was prepared by inserting duplex DNA based on the sequence of NEIL-2, a protein involved in DNA repair pathway. The sequence of the top strand of the duplex is 5′-GCTGGCGGGCTGTAGCTTC-3′, located at position +88 of NEIL-2 mRNA. This vector has tested positive for knockdown NEIL-2 expression.
GTP-GDP exchange assays.
GTP-GDP exchange was performed with 2 μg of purified κB-Ras protein incubated for 1 h and 30 min with 1 mM GTP or GDP (Sigma) and 1 mM MgCl2 at 30°C, and this reaction mixture was directly used for the kinase assay.
RESULTS
In vivo association of κB-Ras with IκBβ.
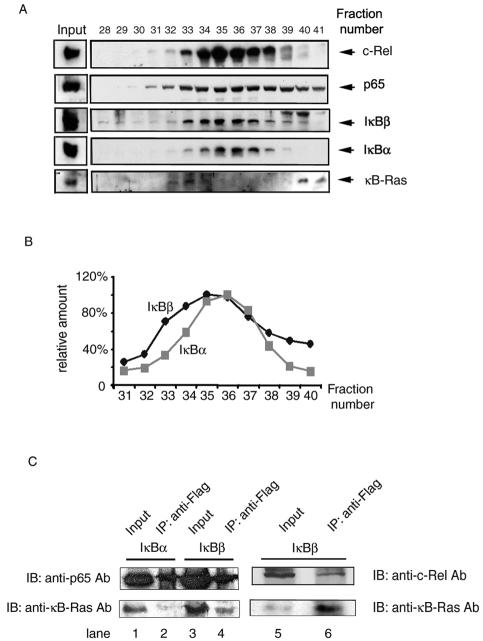
To investigate if cellular IκBβ/NF-κB complexes are associated with κB-Ras, we first size fractionated the cytosolic extracts of HeLa cells by Superose 6 (Amersham-Biosciences) size exclusion chromatography. The proteins in each fraction were separated by SDS-PAGE followed by immunoblotting with IκBβ, IκBα, p65, c-Rel, and κB-Ras antibodies. As expected, IκB and NF-κB proteins are in general present in similar fractions and elute as broad peaks over large volumes (Fig. 1A). A comparison of the elution profiles of IκBβ and IκBα reveals that the IκBβ is shifted toward a larger molecular weight than the IκBα profile (Fig. 1B). It is important to note that each peak represents a convolution of multiple IκB/NF-κB complexes of similar molecular weights, because each of the inhibitors in each fraction is associated with several NF-κB dimers. We observe that κB-Ras elutes in two peaks: one as a high-molecular-weight complex (fractions 32 to 34) and the other as a low-molecular-weight, possibly uncomplexed, free molecule. Although κB-Ras elution does not coincide with the peak fraction of IκBβ, fractions containing κB-Ras also contain substantial amounts of IκBβ. These observations, along with earlier results that κB-Ras associates with IκBβ, suggest that a fraction of IκBβ may specifically bind κB-Ras (8, 14).
FIG. 1.
(A) Fractionation of IκBβ/NF-κB complexes. The presence of c-Rel, p65, IκBβ, IκBα, and κB-Ras in the fractions was confirmed by separation of proteins by SDS-PAGE and immunoblotting. Anti-c-Rel, anti-IκBβ(C-20), anti-IκBα, and anti-p65(C-20) antibodies (Ab), respectively, were used for immunoblotting (IB). (B) Graphic presentation of IκBα and IκBβ amounts across the chromatographic fractions shown in panel A. (C) κB-Ras associates with IκBβ in vivo. Cytosolic extract from Jurkat T cells expressing Flag-IκBβ or IκBα were immunoprecipitated (IP), followed by immunoblotting with anti-κB-Ras, anti-p65, or anti-c-Rel antibodies. These results show that Flag-IκBβ associates with p65, c-Rel, and κB-Ras. Flag-IκBα associates with p65 and c-Rel but not with κB-Ras.
To confirm the in vivo association between IκBβ and κB-Ras, we have done coimmunoprecipitation (co-IP) experiments (Fig. 1C). Because the small Ig subunit and κB-Ras are of similar molecular weights and the quality of κB-Ras antibody is poor, it is difficult to convincingly show an association between κB-Ras and IκB by using polyclonal antibodies in immunoprecipitation experiments. To circumvent this problem, we have made Jurkat cells stably expressing IκBα or IκBβ fused to a Flag M2 peptide. Cytosolic extracts were precipitated with anti-Flag monoclonal antibody followed by Western blotting with p65 and IκBα or IκBβ. As expected, our results showed that p65 associates with both IκBα and IκBβ, whereas κB-Ras is associated primarily with IκBβ. immunoprecipitation also revealed associations between c-Rel, IκBβ, and κB-Ras.
κB-Ras1 blocks phosphorylation of IκBβ by IKKβ.
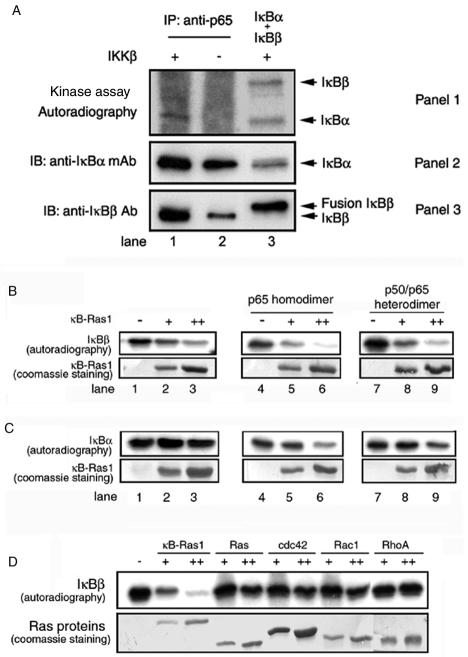
It is known that in contrast to complete degradation of IκBα, IκBβ only partially degrades in response to most stimuli. We wanted to investigate whether inefficient IκBβ degradation is due to its inability to be phosphorylated by IKK. To test this, we immunoprecipitated IκB/p65 complexes by using anti-p65 activation domain antibody and tested the ability of IκBα and IκBβ in the immunoprecipitation complex for phosphorylation by IKKβ (Fig. 2A, panel 1, lane 1). In parallel, we have performed phosphorylation reaction on an equimolar mixture of purified free IκBα and IκBβ and analyzed the products. We observed that both of these pure E. coli-derived proteins are efficiently phosphorylated by IKKβ (lane 3). The immunoprecipitation complex is a mixture of all IκB/p65 complexes, including the IκBα/p65 and IκBβ/p65 complexes. The presence of IκBα and IκBβ in the immunoprecipitation complex is revealed by immunoblotting (Fig. 2A, panels 2 and 3, lanes 1 and 2). Because E. coli-derived pure IκBβ is a poly-histidine fusion protein, it migrates as a slightly-higher-molecular-weight protein compared to native IκBβ (Fig. 2A, panel 3, compare lanes 1 to 2 versus lane 3). The addition of active baculovirus-derived IKKβ to the immunoprecipitation complex under appropriate phosphorylation reaction conditions and subsequent autoradiography clearly reveal a band at a position corresponding to IκBα (Fig. 2A, panel 1, lane 1). In contrast, we did not observe a clear radiolabeled band from the immunoprecipitation complex that corresponds to IκBβ (panel 1, lane 1). In the absence of IKKβ, no specific phosphorylation was observed, suggesting that the phosphorylation is IKKβ specific (panel 1, lane 2). These results suggest that the IKK phosphorylation sites on IκBα in the IκBα/p65 complexes are free, but the homologous sites on IκBβ might not be free in the IκBβ/NF-κB complexes. However, we cannot rule out the possibility that the differential phosphorylation efficiency might be due to a smaller amount of IκBβ in the pull-down reaction.
FIG. 2.
(A) Phosphorylation sites of IκBβ are blocked in vivo. IκB/p65 complexes were immunoprecipitated (IP) from the extract of 293 cells. Immunoprecipitated complexes were subjected to phosphorylation by pure (panel 1, lane 1) or mock (panels 1, lane 2) IKKβ. In parallel, a mixture of equimolar amounts of IκBα and IκBβ was subjected to phosphorylation by pure IKKβ (panel 1, lane 3). The reaction products were identified by separation of proteins by SDS-PAGE followed by autoradiography. The presence of IκBα and IκBβ in the immunoprecipitate or pure IκBα and IκBβ is shown by the Western blot (panels 2 and 3). Ab, antibody; mAb, monoclonal antibody; IB, immunoblotting. (B) Inhibition of IκBβ phosphorylation by κB-Ras in vitro. Pure IκBβ (1.5 μg) was subjected to phosphorylation by IKKβ, and the products were resolved by SDS-PAGE, transferred to membrane, and exposed to phosphorimaging. Phosphorylated IκBβ is shown in the absence (top panel, lane 1) or presence of 2 or 4 μg of pure κB-Ras1 (lanes 2 and 3). The effects of p65 homodimer (top panel, lanes 4 to 6) and p50/p65 heterodimer (top panel, lanes 7 to 9) on phosphorylation inhibition are shown in the absence (lanes 4 and 7) or the presence of κB-Ras1 (lanes 5 and 6 and 8 and 9). The bottom panel shows Coomassie staining of κB-Ras1 of the same blot used for the autoradiography. (C) Phosphorylation of IκBα by IKKβ. IκBα (top panel, lanes 1 to 3), IκBα/p65 complex (lanes 4 to 6), and IκBα/p50/p65 (lanes 7 to 9) were subjected to phosphorylation in the absence (top panel, lanes 1, 4, and 7) or presence (lanes 2 and 3, 5 and 6, and 8 and 9) of increasing amounts of κB-Ras. The bottom panel shows Coomassie staining of the same blot. Only the κB-Ras portion of the blot is shown. (D) Phosphorylations of IκBβ in the presence or absence of κB-Ras1, H-Ras, cdc42, Rac1, and RhoA (top panel). The bottom panel shows the Coomassie staining of the Ras proteins used in the reaction.
To better understand whether κB-Ras plays a direct role in the regulation of phosphorylation of IκBβ by IKK, we carried out in vitro phosphorylation reactions with IκBβ in the presence and absence of pure recombinant κB-Ras1. The products of the phosphorylation reactions were analyzed by SDS-PAGE followed by phosphorimaging. The extent of inhibition increased with increasing concentration of κB-Ras1. We observed that phosphorylation of free IκBβ by IKKβ was significantly inhibited in the presence of κB-Ras1 (Fig. 2B, top left panel). The inhibition was more pronounced when IκBβ is in complex with the p65 homodimer or the p50/p65 heterodimer (compare lanes 3, 6, and 9). This is consistent with our earlier observation that κB-Ras1 most likely contacts the NF-κB NLS and thereby is more effective in inhibition of phosphorylation by forming a more stable complex in the presence of NF-κB (26). In contrast to IκBβ, phosphorylation of IκBα either in its free form or in complexes with NF-κB is only marginally affected by the presence of κB-Ras1 (Fig. 2C, top panel). This result indicates that κB-Ras1 does not block IKK and that the inhibition is specific to IκBβ. Overall, the reduced level of IKK phosphorylation of IκBβ in the immunoprecipitation complex and in vitro complex suggests that κB-Ras at least partially masks the phosphorylation sites of IκBβ.
There is ample evidence in the literature suggesting that other small GTPases, such as Ras, Rho, and cdc42, are also capable of modulating NF-κB activation (27, 30). It is possible that some of these Ras and Ras-related proteins alter NF-κB activation by directly inhibiting IκBβ phosphorylation. To test this, we used H-Ras, Rac1, RhoA, and cdc42 to see if they could inhibit phosphorylation of IκBβ by IKK (Fig. 2D). We observed that the inhibitory activity is specific to κB-Ras; other small G proteins failed to inhibit phosphorylation of IκBβ. It is likely that these GTPases are unable to bind IκBβ. These results suggest that Ras and other GTPases modulate NF-κB activation pathways differently.
Removal of κB-Ras from cells enhances the rate of signal-induced degradation of IκBβ.
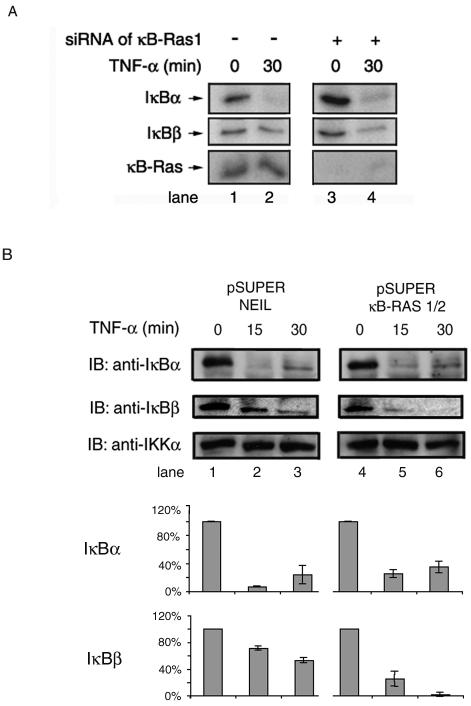
The above experiments suggest that blockade of the IκBβ SRR by κB-Ras might be responsible, at least in part, for the incomplete degradation of IκBβ in induced cells. To test this, we selectively removed κB-Ras1 from cells by RNAi and observed the effect of inducers on IκBβ degradation (11, 12). We transfected siRNA directed against κB-Ras1, and the transfected cells were induced with TNF-α (Fig. 3). We observed that selective removal of κB-Ras1 renders IκBβ more susceptible to degradation by TNF-α. After 30 min of TNF treatment, more than 50% of IκBβ is degraded in κB-Ras-deficient cells compared to a <10% reduction in wild-type cells (Fig. 3A, middle panel, compare lanes 2 and 4). In contrast, we found that removal of κB-Ras1 has little effect on IκBα degradation, because the degradation pattern of IκBα remained unchanged (Fig. 3A, top panel). We further tested TNF-α-induced degradation of IκBβ in cells expressing siRNA for both κB-Ras1 and -2 in an expression vector. HeLa cells were transfected with pSUPER expressing a control siRNA and combined κB-Ras1/κB-Ras 2 vectors, and after 24 h, cells were treated with TNF-α (7). As expected, no change in IκBα degradation was observed, but significant amounts of IκBβ were degraded after 30 min of TNF-α treatment in cells expressing siRNA for κB-Ras1 or -2 (middle panel, lanes 3 and 6). Whereas RNAi experiments conducted with an RNA duplex against only κB-Ras1 did not generate complete degradation of IκBβ, pSUPER vector-transfected cells expressing siRNA against both κB-Ras1 and -2 showed complete degradation of IκBβ upon stimulation. These experiments clearly demonstrate the importance of κB-Ras in regulating induced degradation of IκBβ.
FIG. 3.
siRNA-treated 293 cells are susceptible to IκBβ degradation in response to inducers. (A) 293 cells transfected with siRNA directed against κB-Ras1 were induced with TNF-α after 48 h of growth for the indicated period of time. (B) HeLa cells were transfected with pSUPER vector expressing siRNA for κB-Ras1/2 or siRNA for NEIL-2 as a control. Transfected cells were treated with TNF-α after 24 h of growth for the indicated period of time.
κB-Ras uses the unique insert and the N-terminal SRR of IκBβ for complex formation.
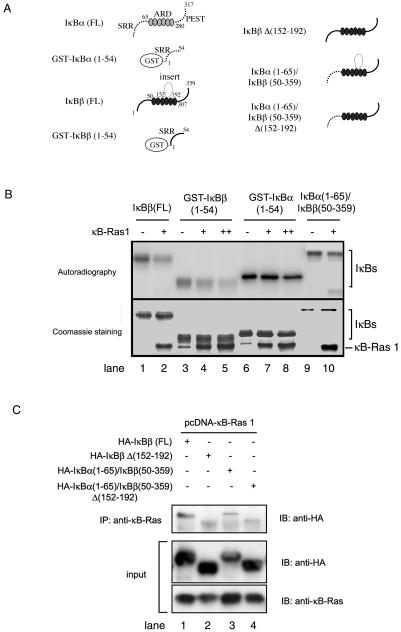
We have shown that the insert of IκBβ plays a significant role in κB-Ras1 binding (8). In light of the above experiments, we suspected that the SRR of IκBβ might be important for this binding. We generated different deletion mutants of IκBβ and IκBα/IκBβ hybrid molecules based on their structure to ensure that the structural integrity of these molecules was intact (Fig. 4A). The association between κB-Ras1 and IκBβ was tested by inhibition of phosphorylation of E. coli-expressed and -purified IκBβ mutants. Figure 4B shows the effect of IKK phosphorylation of these mutants in vitro. The phosphorylation of GST-IκBβ(1-54) was significantly reduced in the presence of κB-Ras1. In contrast, phosphorylation of GST-IκBα(1-54) by IKKβ is not reduced in the presence of κB-Ras1. Also, importantly, κB-Ras1 did not efficiently block the phosphorylation of a hybrid protein containing the SRR of IκBα and the ankyrin repeat domain (ARD) and PEST of IκBβ: IκBα(1-65)/IκBβ(50-359). These results strongly suggest that the SRR of IκBβ, but not IκBα, is directly involved in κB-Ras1 binding. We could not test the effect of the carboxy-terminal PEST in κB-Ras binding in vitro due to aggregation of the PEST-deleted IκBβ protein.
FIG. 4.
κB-Ras1 uses the SRR and insert of IκBβ for binding. (A) Schematic representation of IκBα, IκBβ, and various mutants used in these experiments. The domain boundaries of wild-type IκBα and IκBβ are indicated. (B) The top panel shows inhibition of phosphorylation of full-length and truncated IκBβ proteins. Lanes 2, 4, 5, 7, 8, and 10 show the efficiency of phosphorylation of full-length IκBβ and mutants in the presence of increasing amounts of pure κB-Ras. Lanes 1, 3, 6, and 9 indicate phosphorylation in the absence of κB-Ras1. The bottom panel shows Coomassie staining of the same blot used for phosphorimaging. (C) Co-IP of IκBβ and κB-Ras from COS cells transfected with HA-κB-Ras1 and wild-type or mutant IκBβ. Immunoprecipitation was done with anti-κB-Ras antibody immunoblotted (IB) with antihemagglutinin (HA) antibody. The top panel shows that wild-type IκBβ forms complex with κB-Ras (lane 1). A hydrid IκB protein containing the SRR of IκBα and the rest of IκBβ binds poorly with κB-Ras (lane 3). Both wild-type IκBβ and hybrid IκBβ with the insert deleted do not bind κB-Ras (lanes 2 and 4). The bottom panels show the expression of IκBβ and κB-Ras.
To further confirm the role of the N-terminal SRR of IκBβ in κB-Ras binding, we performed co-IP experiments using extracts of cells transfected with κB-Ras1 and various IκBβ expression vectors. We have already shown that IκBβΔ(152-192) did not associate with κB-Ras (8). Here we show that binding of a hybrid IκB, IκBα(1-65)/IκBβ(50-359), to κB-Ras was significantly lower than the binding of wild-type IκBβ (Fig. 4C, compare lanes 1 and 3). This suggests that in addition to the insert of IκBβ, the N terminus also plays a role in κB-Ras binding. When the insert of IκBβ was removed from the hybrid molecule, the resulting truncated hybrid molecule, IκBα(1-65)/IκBβ(50-359)Δ(152-192), failed to bind κB-Ras1.
Overexpression of wild-type κB-Ras1 blocks IκBβ degradation.
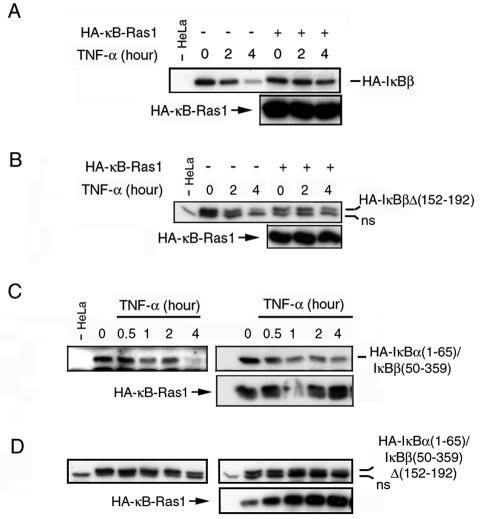
The above results prompted us to examine the effect of overexpression of κB-Ras on IκBβ degradation. We tested the effect of κB-Ras1 on inducer-dependent IκBβ degradation in HeLa cells. HeLa cells transfected with mammalian vectors expressing IκBβ and p65, in the presence or absence of κB-Ras1, were treated with TNF-α. We observed that κB-Ras1 blocked TNF-α-induced degradation of wild-type IκBβ (Fig. 5A).
FIG. 5.
κB-Ras 1 protects IκBβ degradation. (A) HeLa cells cotransfected with hemagglutinin (HA)-tagged IκBβ and κB-Ras1 or empty vector were grown for 2 days followed by treatment with TNF-α for the indicated period of time. Similar experiments were done with three different IκBβ mutants: HA-IκBβΔ152-192 (B), HA-IκBα(1-65)/IκBβ(50-359) (C), and HA-IκBα (1-65)/IκBβ(50-359)Δ(152-192). ns, nonspecific binding.
We tested the mutant IκBβ proteins for protection against degradation by κB-Ras1 in TNF-α-induced HeLa cells (Fig. 5B to D). We observed that the protective effect of κB-Ras1 is only minimal on IκBβΔ(152-192) (Fig. 5B). As expected the hybrid IκBα(1-65)/IκBβ(50-359) molecule was not protected as well as the wild-type IκBβ (Fig. 5C). κB-Ras1 could not protect IκBα(1-65)/IκBβ(50-359)Δ(152-192) at all (Fig. 5D). These results indicated that κB-Ras1 was unable to block the degradation of hydrid or deletion mutants of IκBβ in which the binding sites were removed. These experiments suggested that κB-Ras proteins are able to associate with IκBβ/NF-κB complexes and protect IκBβ from signal-dependent degradation. Altogether, these results suggest that κB-Ras acts at the level of IKK phosphorylation in the signaling process.
Both GDP- and GTP-bound forms of κB-Ras1 inhibit IκBβ phosphorylation.
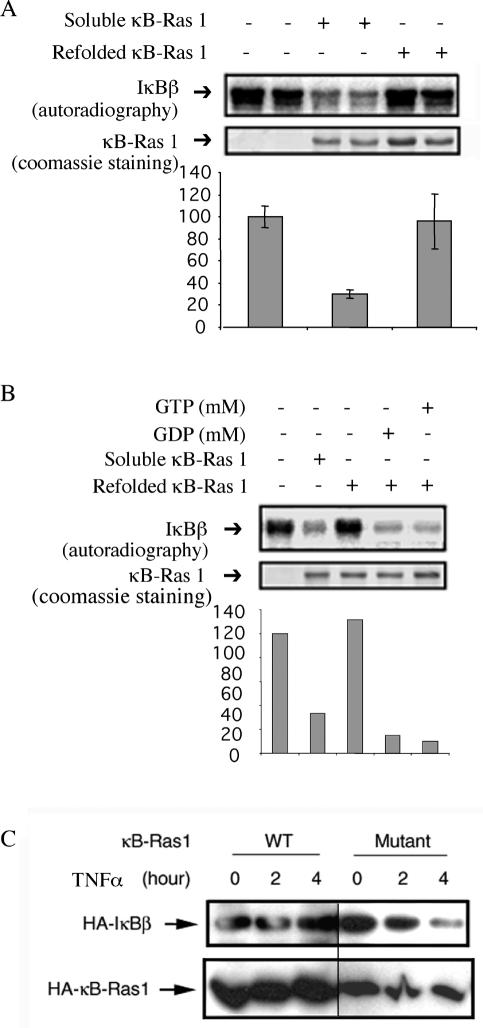
We wanted to examine the relationship between κB-Ras activity and guanine (G)-nucleotide binding states. κB-Ras1 preparations used in all previous in vitro assays are proteins expressed in E. coli in soluble forms. To convincingly prove if both GDP and GTP or only one form of κB-Ras acts as an inhibitor of IκBβ phosphorylation, it is essential to prepare homogeneous κB-Ras loaded with either GDP or GTP. In Ras and most related small GTPases, E. coli-expressed proteins are loaded with GDP (37). In vitro nucleotide exchange reactions are required to prepare homogeneous preparations of GDP- or GTP-bound G proteins. Interestingly, the conventional in vitro GTP/GDP exchange reaction does not work for κB-Ras1 (data not shown) (21). We prepared κB-Ras1 by purifying denatured protein and subsequently refolding it under native conditions. The refolded protein was further purified by size exclusion chromatography. We observed that although the refolded protein behaved similarly to the soluble form of κB-Ras1 chromatographically, it failed to block IκBβ phosphorylation by IKK (Fig. 6A). This observation suggested that lack of the nucleotide in refolded κB-Ras may have rendered it inactive. Indeed, we observed that GTP was present in κB-Ras1 purified under native conditions, whereas refolded κB-Ras1 did not contain any GTP or GDP (data not shown). The refolded protein can be loaded with either GDP or GTP in the presence of MgCl2. Nucleotide-loaded κB-Ras1 was tested for phosphorylation of IκBβ by IKK. Figure 6B shows that both the GDP- and GTP-bound forms of κB-Ras1 efficiently block phosphorylation of IκBβ by IKK.
FIG. 6.
GDP or GTP binding is essential for κB-Ras1 function. (A) Autoradiogram of phosphorylation of IκBβ by IKKβ in the presence of κB-Ras 1 purified as a soluble or refolded protein (top panel). Coomassie staining of the same membrane shows the quality of κB-Ras 1 preparations (bottom panel). (B) Refolded κB-Ras 1 was used for nucleotide loading with GDP and GTP. IκBβ was subjected to phosphorylation in the presence of nucleotide-loaded κB-Ras1. (C) Effect of a mutant κB-Ras1 in protecting IκBβ degradation in HeLa cells. HeLa cells were transfected with vectors expressing wild-type (WT) IκBβ and wild-type or mutant κB-Ras followed by TNF treatment for indicated times. Degradation of IκBβ was monitored by Western blotting with antihemagglutinin (HA) antibody.
Sequence comparison between κB-Ras and other GTPases reveal that all critical G-box residues that have been shown to contact G nucleotides in Ras and other GTPases are identical in κB-Ras (39). To further prove whether G-nucleotide binding is important for κB-Ras activity, we generated a mutant κB-Ras1 protein in which the guanine-binding residue Thr38 was mutated to Ala. HeLa cells were transfected with expression vectors containing mutant κB-Ras1 proteins followed by TNF-α treatment for different times. We observed that this mutant did not block IκBβ degradation effectively (Fig. 6C). These results suggest that G-nucleotide binding is essential for κB-Ras1 function.
DISCUSSION
One of the fundamental questions about cellular signaling is how different members of a protein family impart specificity in signal transduction pathways. With respect to NF-κB-mediated regulation of gene expression, it is now known from knockout experiments that all five members of the NF-κB family play distinct, albeit overlapping roles. Whereas promoter accessibility, combinational dimer formation, DNA binding affinity, and posttranslational modification contribute to achieving such specificity, inhibition of NF-κB dimers by IκB family proteins is perhaps the most important mechanism of NF-κB regulation. How different IκB proteins regulate each NF-κB dimer is not known.
A pool of IκBβ/NF-κB complexes associate with κB-Ras.
In most cells, almost equal amounts of NF-κB dimers are associated with IκBα and IκBβ. However, IκBβ is less sensitive to several extracellular signals (16, 17). The biochemical mechanism for this lack of stimulus-dependent complete IκBβ degradation has not been addressed. Whereas incomplete degradation of IκBβ can result from defects at any one of the multiple steps required for degradation, we suspected that defects in IκBβ phosphorylation by IKK were principally responsible. Using a series of biochemical experiments, we have shown that there are two pools of IκBβ in quiescent cells: one present as a binary complex with NF-κB and the other as a ternary complex with both NF-κB and κB-Ras. Our in vitro experiments reveal that IκBα/NF-κB and IκBβ/NF-κB binary complexes are fully competent substrates for IKK phosphorylation. When HeLa cells are induced with TNF-α, the kinetics of IκBα and IκBβ degradations are different. We believe that IκBβ of the ternary complex is more resistant to stimulus-dependent degradation. Reduction or elimination of κB-Ras by RNAi showed enhanced degradation of IκBβ. We suggest that IκBβ of the IκBβ/NF-κB complex is degraded quickly in wild-type cells, while IκBβ in the ternary complex remains resistant to degradation. The latter pool becomes susceptible only after the removal of κB-Ras by siRNA.
The question that remains unanswered is whether a specific NF-κB dimer dictates the formation of IκBβ binary and ternary complexes. Although NF-κB subunit-dependent ternary complex formation may appear unlikely based on our current results, which show that κB-Ras binds primarily through IκBβ, we cannot entirely rule out this possibility. Under physiological conditions, κB-Ras may have a higher affinity for a specific IκBβ/NF-κB complex compared to other binary complexes. Depending on the cellular concentrations of these proteins and in the presence of competing binding partners, a high-affinity ternary complex may predominate. In vitro binding experiments reveal that IκBα binds NF-κB p50/p65 heterodimer more strongly than the homodimers of p65 and c-Rel or the p50/c-Rel heterodimer (C. Phelps and G. Ghosh, unpublished results). IκBβ, on the other hand, binds all of these dimers more weakly than IκBα (25). It is possible that IκBβ displays increased variations in binding affinity and specificity in the presence of κB-Ras (i.e., IκBβ may preferentially bind to a specific dimer in the presence of κB-Ras). Future experiments will be needed to resolve whether the κB-Ras/IκBβ/NF-κB ternary complex requires specific NF-κB dimers.
A second important question is how the sequestration of IκBβ/NF-κB complexes are regulated. It has been shown by us and others that all IκBβ/NF-κB complexes are cytoplasmic. However, in this study, we have shown that κB-Ras binds to only a fraction of these IκBβ/NF-κB complexes and masks their apparently exposed NF-κB NLS. What regulates the cytoplasmic sequestration of the rest of the IκBβ/NF-κB complexes that do not bind to κB-Ras? At this point, we do not know the answer to this question. It is possible that IκBβ in the binary complex might be modified, which causes the masking of the second NLS. Yet another possibility is that this second pool of IκBβ/NF-κB complex is not actually binary but interacts with another protein that blocks only the second NLS, but unlike κB-Ras does not prevent phosphorylation of IκBβ.
Unique sequence and structural features of IκBβ explain its functional differences.
Although the presence of highly homologous ankyrin repeats and the PEST sequence of IκBα and IκBβ masks the clear differences in their sequences, it is the nonhomologous segments that are the key to the formation of a larger complex nucleated by IκBβ. Results presented in this paper indicate that κB-Ras1 interacts with IκBβ by using two different segments of IκBβ: the insert and the SRR. The insert is absent in IκBα, and the SRR and PEST are not completely homologous in IκBα and IκBβ. The most critical of these segments for κB-Ras1 binding is the insert. The insert, which is not a part of the relatively rigid ARD structure, is also present in Cactus, the Drosophila homolog of IκB proteins (15, 24). It is possible that Cactus interacts with Drosophila κB-Ras in a manner similar to IκBβ.
Our results also reveal that the SRR of IκBβ, but not IκBα, is able to make direct contact with κB-Ras1. The SRR of IκBα is different in sequence from the SRR of IκBβ. The SRR is important for IKK phosphorylation and recognition by the E3RS/β-TRCP ubiquitination machinery. However, a stringent sequence requirement for these two functions is restricted only to a small, highly homologous segment of 16 amino acids. Outside of this small homologous region, the SRR sequences of the two IκB proteins are different. We do not, however, know if the IKK-phosphorylatable serines (Ser19 and Ser23) are directly involved in κB-Ras binding or are occluded from phosphorylation because of indirect blockade of IKKβ recognition.
κB-Ras1 is an unusual small G protein.
κB-Ras proteins are somewhat unusual members of the Ras family of GTPases in that they contain the constitutively active oncogenic mutations G12L and Q61L in Ras (5). Because of the similarity to oncogenic Ras mutations within κB-Ras sequence, it was originally thought that native κB-Ras exists in cells in a constitutively active form. While it still might be true that κB-Ras binds GTP in vivo and acts as a constitutively active form of GTPase, several observations indicate that κB-Ras proteins are clearly different from other GTPases. First, we observed that both GDP- and GTP-bound forms of κB-Ras are equally potent in blocking phosphorylation of IκBβ by IKKβ in vitro (Fig. 6B). This suggests that even if other accessory proteins induce GTP hydrolysis, the hydrolyzed product GDP would remain bound and maintain its activity in IκBβ binding. Second, κB-Ras functions as an inhibitor of signal transduction (i.e., signals are terminated through κB-Ras). In contrast, oncogenic Ras proteins sustain signals by continuously binding to effector molecules. It is therefore likely that κB-Ras proteins represent a different paradigm in cell signaling.
The fact that κB-Ras1 binds to IκBβ in both its GDP- and GTP-bound states suggests that this protein may not assume two different conformations. We suggest that κB-Ras1 is not a molecular switch like other small G proteins. We have not been able to show if the κB-Ras/GDP complex is a physiologic entity and functions as an inhibitor of IκBβ phosphorylation by IKK in vivo. Based on its simple role as an inhibitory adapter molecule, there is no reason for κB-Ras to not use both GDP and GTP as cofactors to carry out its function in vivo. G nucleotides contacting residues in all four G-boxes in κB-Ras proteins (both 1 and 2), however, are invariant, and alteration of conserved G-nucleotide binding residues or the removal of G nucleotide by a denaturation-renaturation process makes κB-Ras inactive (Fig. 6C). These results indicate that G-nucleotide binding is essential for κB-Ras function.
At least some small G protein families contain members that are GTP hydrolysis-deficient (13, 28). Exactly how they function is unclear. It is possible that like κB-Ras, these non-GTPase small G proteins are functionally distinct from the prototypical members. These proteins simply bind G nucleotides as a cofactor for their cellular activities and do not undergo GTP-GDP exchange cycles.
Conclusions.
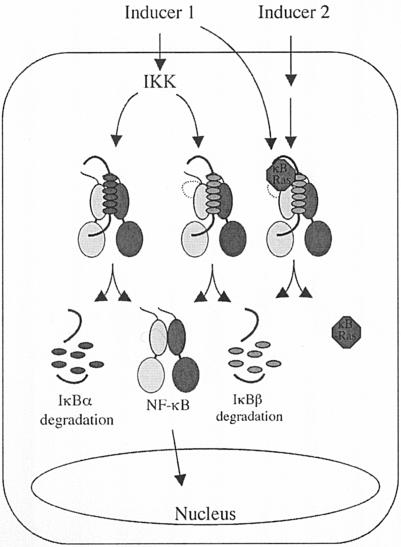
We have demonstrated the biochemical mechanism of the origin of differential degradation of IκBα and IκBβ in response to inducers. We suggest that a pool of IκBβ/NF-κB complex is bound to κB-Ras as a ternary complex. IκBβ of this ternary complex is not degradable by most inducers (Fig. 7). It is likely that a specific NF-κB dimer is a part of this ternary complex. Further biochemical purification of these complexes is necessary to determine the composition of these complexes.
FIG. 7.
Model of NF-κB activation from the IκBα- and IκBβ-bound states. IκBα and a pool of IκBβ complexes are present as binary complexes with NF-κB. These IκBs are rapidly degraded by inducers such as TNF-α. A second pool of IκBβ remains as a ternary complex with NF-κB and κB-Ras. Release of an NF-κB dimer from IκBβ from the second pool requires compound signals.
Recent results have shown the importance of specific ligands such as BAFF and lymphotoxin-β in activating p52 in a cell-type-specific manner (9, 32, 40). Similarly, costimulation of T cells by anti-CD3 and anti-CD28 is essential for interleukin-2 expression through the activation of c-Rel homodimer (1, 18, 41). In summary, to understand how specific inducers activate specific NF-κB dimers, it is important to know how these dimers are inhibited by IκB proteins. Future biochemical dissection of these inhibited complexes will unravel that puzzle.
Acknowledgments
We thank Tom Huxford, Rasmi Talwar, and Anu K. Moorthy for critically reading the manuscript; Sankar Ghosh and Tapas Hazra for reagents and helpful discussion; and Chun Wu for helpful suggestions. We also thank Anu K. Moorthy for help with HeLa cytoplasmic extracts, Michael Karin and Mireille Delhase for discussion and reagents, and Ju Chen for support.
This research was supported by grants from the National Cancer Institute, the Cystic Fibrosis Foundation, and the Human Frontier Science Program.
REFERENCES
- 1.Attar, R. M., H. Macdonald-Bravo, C. Raventos-Suarez, S. K. Durham, and R. Bravo. 1998. Expression of constitutively active IκBβ in T cells of transgenic mice: persistent NF-κB activity is required for T-cell immune responses. Mol. Cell. Biol. 18:477-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldwin, A. S. 1996. The NF-kappaB and IkappaB proteins: new discoveries and insights. Annu. Rev. Immunol. 14:649-683. [DOI] [PubMed] [Google Scholar]
- 3.Bitko, V., and S. Barik. 1998. Persistent activation of RelA by respiratory syncytial virus involves protein kinase C, underphosphorylated IκBβ, and sequestration of protein phosphatase 2A by the viral phosphoprotein. J. Virol. 72:5610-5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blackwell, T. S., A. A. Stecenko, and J. W. Christman. 2001. Dysregulated NF-kappaB activation in cystic fibrosis: evidence for a primary inflammatory disorder. Am. J. Physiol. 281:L69-L70. [DOI] [PubMed] [Google Scholar]
- 5.Boguski, M. S., and F. McCormick. 1993. Proteins regulating ras and its relatives. Nature 366:643-654. [DOI] [PubMed] [Google Scholar]
- 6.Bourne, H. R., D. A. Sanders, and F. McCormick. 1990. The GTPase superfamily: a conserved switch for diverse cell functions. Nature 348:125-132. [DOI] [PubMed] [Google Scholar]
- 7.Brummelkamp, T. R., R. Bernards, and R. Agami. 2002. A system for stable expression of short interfering RNAs in mammalian cells. Science 296:550-553. [DOI] [PubMed] [Google Scholar]
- 8.Chen, Y., J. Wu, and G. Ghosh. 2003. κB-Ras binds to the unique insert within the ankyrin repeat domain of Ikappa Bbeta and regulates cytoplasmic retention of Ikappa Bbeta/NF-kappa B complexes. J. Biol. Chem. 278:23101-23106. [DOI] [PubMed] [Google Scholar]
- 9.Claudio, E., K. Brown, S. Park, H. Wang, and U. Siebenlist. 2002. BAFF-induced NEMO-independent processing of NF-kB2 in maturing B cells. Nat. Immunol. 3:958-965. [DOI] [PubMed] [Google Scholar]
- 10.DeLuca, C., L. Petropoulos, D. Zmeureanu, and J. Hiscott. 1999. Nuclear IkappaBbeta maintains persistent NF-kappaB activation in HIV-1-infected myeloid cells. J. Biol. Chem. 274:13010-13016. [DOI] [PubMed] [Google Scholar]
- 11.Elbashir, S. M., J. Harborth, W. Lendeckel, A. Yalcin, K. Weber, and T. Tuschl. 2001. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411:494-498. [DOI] [PubMed] [Google Scholar]
- 12.Elbashir, S. M., W. Lendeckel, and T. Tuschl. 2001. RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev. 15:188-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erdman, R. A., K. E. Shellenberger, J. H. Overmeyer, and W. A. Maltese. 2000. Rab24 is an atypical member of the Rab GTPase family: deficient GTPase activity, GDP dissociation inhibitor interaction, and prenylation of Rab24 expressed in cultured cells. J. Biol. Chem. 275:3848-3856. [DOI] [PubMed] [Google Scholar]
- 14.Fenwick, C., S.-Y. Na, R. E. Voll, H. Zhong, S.-Y. Im, J. W. Lee, and S. Ghosh. 2000. A subclass of Ras proteins that regulate the degradation of IkappaB. Science 287:869-873. [DOI] [PubMed] [Google Scholar]
- 15.Geisler, R., A. Bergmann, Y. Hiromi, and C. Nuesslein-Volhard. 1992. cactus, a gene involved in dorsoventral pattern formation of Drosophila, is related to the IkappaB gene family of vertebrates. Cell 71:613-621. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh, S., and M. Karin. 2002. Missing pieces in the NF-kappaB puzzle. Cell 109:S81-S96. [DOI] [PubMed] [Google Scholar]
- 17.Ghosh, S., M. J. May, and E. B. Kopp. 1998. NF-kappaB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 16:225-260. [DOI] [PubMed] [Google Scholar]
- 18.Harhaj, E. W., S. B. Maggirwar, L. Good, and S.-C. Sun. 1996. CD28 mediates a potent costimulatory signal for rapid degradation of IκBβ which is associated with accelerated activation of various NF-κB/Rel heterodimers. Mol. Cell. Biol. 16:6736-6743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hiscott, J., H. Kwon, and P. Genin. 2001. Hostile takeovers: viral appropriation of the NF-kappaB pathway. J. Clin. Investig. 107:143-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huxford, T., S. Malek, and G. Ghosh. 2000. Preparation and crystallization of dynamic NF-kappa B · IkappaB complexes. J. Biol. Chem. 275:32800-32806. [DOI] [PubMed] [Google Scholar]
- 21.John, J., R. Sohmen, J. Feuerstein, R. Linke, A. Wittinghofer, and R. S. Goody. 1990. Kinetics of interaction of nucleotides with nucleotide-free H-ras p21. Biochemistry 29:6058-6065. [DOI] [PubMed] [Google Scholar]
- 22.Johnson, D. R., I. Douglas, A. Jahnke, S. Ghosh, and J. S. Pober. 1996. A sustained reduction in I-kappa-B-beta may contribute to persistent NF-kappa-B activation in human endothelial cells. J. Biol. Chem. 271:16317-16322. [DOI] [PubMed] [Google Scholar]
- 23.Karin, M., and Y. Ben-Neriah. 2000. Phosphorylation meets ubiquitination: the control of NF-kappaB activity. Annu. Rev. Immunol. 18:621-663. [DOI] [PubMed] [Google Scholar]
- 24.Kidd, S. 1992. Characterization of the Drosophila cactus locus and analysis of interactions between cactus and dorsal proteins. Cell 71:623-635. [DOI] [PubMed] [Google Scholar]
- 25.Malek, S., Y. Chen, T. Huxford, and G. Ghosh. 2001. IkappaBbeta, but not IkappaBalpha, functions as a classical cytoplasmic inhibitor of NF-kappaB dimers by masking both NF-kappaB nuclear localization sequences in resting cells. J. Biol. Chem. 276:45225-45235. [DOI] [PubMed] [Google Scholar]
- 26.Malek, S., D. B. Huang, T. Huxford, S. Ghosh, and G. Ghosh. 2003. X-ray crystal structure of an Ikappa Bbeta/NF-kappa B p65 homodimer complex. J. Biol. Chem. 278:23094-23100. [DOI] [PubMed] [Google Scholar]
- 27.Montaner, S., R. Perona, L. Saniger, and J. C. Lacal. 1998. Multiple signalling pathways lead to the activation of the nuclear factor kappaB by the Rho family of GTPases. J. Biol. Chem. 273:12779-12785. [DOI] [PubMed] [Google Scholar]
- 28.Nobes, C. D., I. Lauritzen, M.-G. Mattei, S. Paris, A. Hall, and P. Chardin. 1998. A new member of the Rho family, Rnd1, promotes disassembly of actin filament structures and loss of cell adhesion. J. Cell Biol. 141:187-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palmer, G. H., J. Machado, P. Fernandez, V. Heussler, T. Perinat, and D. A. E. Dobbelaere. 1997. Parasite-mediated nuclear factor kappaB regulation in lymphoproliferation caused by Theileria parva infection. Proc. Natl. Acad. Sci. USA 94:12527-12532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perona, R., S. Montaner, L. Saniger, I. Sanchez-Perez, R. Bravo, and J. C. Lacal. 1997. Activation of the nuclear factor-kappa-B by Rho, CDC42, and Rac-1 proteins. Genes Dev. 11:463-475. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt, C., B. Peng, Z. Li, G. M. Sclabas, S. Fujioka, J. Niu, M. Schmidt-Supprian, D. B. Evans, J. L. Abbruzzese, and P. J. Chiao. 2003. Mechanisms of proinflammatory cytokine-induced biphasic NF-kappaB activation. Mol. Cell 12:1287-1300. [DOI] [PubMed] [Google Scholar]
- 32.Senftleben, U., Y. Cao, G. Xiao, F. R. Greten, G. Krahn, G. Bonizzi, Y. Chen, Y. Hu, A. Fong, S.-C. Sun, and M. Karin. 2001. Activation by IKKalpha of a second, evolutionary conserved, NF-kappaB signaling pathway. Science 293:1495-1499. [DOI] [PubMed] [Google Scholar]
- 33.Stecenko, A. A., G. King, K. Torii, R. M. Breyer, R. Dworski, T. S. Blackwell, J. W. Christman, and K. L. Brigham. 2001. Dysregulated cytokine production in human cystic fibrosis bronchial epithelial cells. Inflammation 25:145-155. [DOI] [PubMed] [Google Scholar]
- 34.Tam, W. F., and R. Sen. 2001. IkappaB family members function by different mechanisms. J. Biol. Chem. 276:7701-7704. [DOI] [PubMed] [Google Scholar]
- 35.Thompson, J. E., R. J. Phillips, H. Erdjument-Bromage, P. Tempst, and S. Ghosh. 1995. I-kappa-B-beta regulates the persistent response in a biphasic activation of NF-kappa-B. Cell 80:573-582. [DOI] [PubMed] [Google Scholar]
- 36.Tran, K., M. Merika, and D. Thanos. 1997. Distinct functional properties of IκBα and IκBβ. Mol. Cell. Biol. 17:5386-5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tucker, J., G. Sczakiel, J. Feuerstein, J. John, R. S. Goody, and A. Wittinghofer. 1986. Expression of p21 proteins in Escherichia coli and stereochemistry of the nucleotide-binding site. EMBO J. 5:1351-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verma, I. M., K. Stevenson, E. W. Schwarz, D. Van Antwerp, and S. Miyamoto. 1995. Rel/NF-kappa-B/I-kappa-B family: intimate tales of association and dissociation. Genes Dev. 9:2723-2735. [DOI] [PubMed] [Google Scholar]
- 39.Vetter, I. R., and A. Wittinghofer. 2001. The guanine nucleotide-binding switch in three dimensions. Science 294:1299-1304. [DOI] [PubMed] [Google Scholar]
- 40.Xiao, G., E. W. Harhaj, and S.-C. Sun. 2001. NF-kappaB-inducing kinase regulates the processing of NF-kappaB2 p100. Mol. Cell 7:401-409. [DOI] [PubMed] [Google Scholar]
- 41.Zhou, X.-Y., Y. Yashiro-Ohtani, M. Nakahira, W. R. Park, R. Abe, T. Hamaoka, M. Naramura, H. Gu, and H. Fujiwara. 2002. Molecular mechanism underlying differential contribution of CD28 versus non-CD28 costimulatory molecules to IL-2 promoter activation. J. Immunol. 168:3847-3854. [DOI] [PubMed] [Google Scholar]