Abstract
Objective:
The objective of this secondary analysis was to explore differences in baseline clinical characteristics and opioid replacement therapy treatment outcomes by type (heroin, opioid analgesic [OA], or combined [heroin and OA]) and route (injector or non-injector) of opioid use.
Method:
A total of 1,269 participants (32.2% female) were randomized to receive one of two study medications (methadone or buprenorphine/naloxone [BUP]). Of these, 731 participants completed the 24-week active medication phase. Treatment outcomes were opioid use during the final 30 days of treatment (among treatment completers) and treatment attrition.
Results:
Non-opioid substance dependence diagnoses and injecting differentiated heroin and combined users from OA users. Non-opioid substance dependence diagnoses and greater heroin use differentiated injectors from non-injectors. Further, injectors were more likely to be using at end of treatment compared with non-injectors. OA users were more likely to complete treatment compared with heroin users and combined users. Non-injectors were more likely than injectors to complete treatment. There were no interactions between type of opioid used or injection status and treatment assignment (methadone or BUP) on either opioid use or treatment attrition.
Conclusions:
Findings indicate that substance use severity differentiates heroin users from OA users and injectors from non-injectors. Irrespective of medication, heroin use and injecting are associated with treatment attrition and opioid misuse during treatment. These results have particular clinical interest, as there is no evidence of superiority of BUP over methadone for treating OA users versus heroin users.
Evidence documents differences in the clinical characteristics of individuals with opioid dependence, which vary by the type of opioid used (opioid analgesic [OA] vs. heroin) and route of use (e.g., oral, intravenous). These differences are potentially important factors because they may be associated with clinical outcomes of opioid replacement therapy (ORT).
Compared with heroin users, OA users are more likely to be younger, female, White, and employed and to report pain (Rosenblum et al., 2007). OA users also have fewer attempts at treatment for substance use disorder, have fewer years of opioid use, and are less likely to have drug-related medical complications than heroin users (Moore et al., 2007). Heroin users are more likely to be involved with the legal system (Banta-Green et al., 2009), be older, be African American, have a history of attempts at treatment for substance use disorder, and report using other drugs (Wu et al., 2011). Compared with heroin users, combined (OA and heroin) users are more likely to be White, and compared with OA users, combined users are more likely to have more attempts at treatment for substance use disorder (Moore et al., 2007). Combined users also report more polydrug use and are more likely to have multiple substance use disorder diagnoses, including a lifetime opioid use disorder diagnosis (Wu et al., 2011). Overall, OA users tend to present with a less severe opioid dependence compared with heroin users. Heroin use is often associated with a longer drug-using history compared with OA use, which may partially account for more severe opioid dependence seen in heroin users.
Compared with heroin injectors, non-injectors are more likely to be female (Puigdollers et al., 2004), younger, and employed and have higher levels of education, lower levels of crime, shorter heroin-using histories, fewer symptoms of dependence, fewer attempts at treatment for substance use disorder, and fewer overdoses (Darke et al., 2004). Conversely, compared with non-injectors, heroin injectors are more likely to be homeless, unemployed, long-time users, and younger at first heroin use and to have initiated heroin use through intravenous routes (Neaigus et al., 2001). Injectors are also more likely to report a history of incarceration and arrests (Young and Havens, 2012). In comparison to injectors, non-injectors have a more stable socioeconomic status and fewer adverse effects because of their opioid use. Using opioids in a manner other than oral use seems to put the user at risk for not only medical complications but progression toward more severe opioid dependence as well. Not surprisingly, compared with OA users, heroin users are more likely to have a history of injecting opioids and sharing needles (Brands et al., 2004). However, there are recent reports of opioid users initiating injection use with an OA before any heroin use (Young and Havens, 2012).
Although research has examined the association between treatment outcomes and type of ORT (e.g., buprenorphine/naloxone [BUP], methadone), little information is available regarding this association and type of opioid used or injection status. Examining BUP treatment outcomes, OA users are more likely to complete treatment successfully, remain in treatment longer, have an improved treatment response, and have a higher percentage of opioid-negative urine samples compared with heroin users (Moore et al., 2007). Specifically addressing ORT with methadone maintenance therapy, a history of injecting was associated with ongoing substance use during treatment (Neufeld et al., 2008).
The literature has examined treatment outcomes in methadone maintenance therapy and BUP maintenance therapy by type of opioid used or injection status, but no studies, to our knowledge, have concurrently examined both ORT medications and type and route of opioid use. Because of the potential associations among type of opioid, route of use, and treatment outcomes, particularly relevant with the current high rates of OA use documented in the United States, it is important to better understand these relationships. The objective of this secondary analysis was to examine differences in clinical characteristics and associated treatment outcomes by type and route of opioid use and any potential interactions with ORT treatment assignment in individuals seeking treatment for opioid dependence. These data come from a large, multisite safety trial comparing liver health of participants randomly assigned to 24 weeks of treatment with either methadone or BUP under the auspices of the National Institute on Drug Abuse National Drug Abuse Treatment Clinical Trials Network.
Method
Design
The main study was a randomized, open-label, multi-center, Phase 4 study to assess liver function in participants randomized to medication condition (methadone, BUP). Randomization was stratified within site according to normal versus abnormal screening liver-function test results (see main outcome results, Saxon et al., 2013). The institutional review boards at participating sites approved the study, and participants provided written informed consent.
Participants
Individuals were recruited between May 2006 and October 2009 at nine federally licensed opioid treatment programs across the United States. Eligibility criteria included being age 18 or older and meeting criteria for opioid dependence as defined by the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSMIV-TR; American Psychiatric Association, 2000). Medical ineligibility included having an alanine amino transferase or aspartate amino transferase value greater than five times the upper limit of normal or an alkaline phosphatase value greater than three times the upper limit of normal, having an allergy to study drug, being pregnant or lactating, or having a medical/psychiatric condition that would make study participation difficult or unsafe. Participants with a DSMIV-TR diagnosis of current alcohol, benzodiazepine, or other sedative-hypnotic dependence were excluded if they required immediate medical attention. Those with legal or other issues that could result in early termination and those who had recently participated in a treatment study for a substance use disorder were also excluded.
Because of higher dropout in the BUP condition, the initial randomization scheme of 1:1 (BUP:methadone) was changed to 2:1 in December 2007, 18 months after study initiation. Participants completed follow-up assessments through August 2010.
Measures
Measures used in the current secondary analyses included information collected at screening/baseline as well as study outcome data as described below. Demographic information collected included participants’ age, gender, race, and ethnicity.
Clinical characteristics.
Initially, DSM-IV-TR substance use diagnoses were established using the Substance Abuse subsections (Alcohol and Drug) of the Composite International Diagnostic Interview (CIDI Version 2.1; World Health Organization, 1997) (n = 175). The CIDI was replaced with the DSM-IV Checklist (n = 1,094) to reduce length of the assessment battery. The Risk Behavior Survey (Booth et al., 1993) collected drug use histories including substance use and route of use in the previous 30 days. The Fagerström Test for Nicotine Dependence (Heatherton et al., 1991), a six-item self-report questionnaire, assessed severity of nicotine dependence. The Short Form 36-item Health Survey (Ware and Sherbourne, 1992), a self-report instrument, assessed each of eight health concepts: physical functioning, physical role limitations, bodily pain, social functioning, emotional role limitations, general mental health, vitality, and general health perceptions.
Procedures
Eligible participants were stratified by site and liver-function results (normal vs. abnormal) and randomized to either open-label methadone or BUP. Compensation was provided in accordance with local site policies but typically included a maximum of $300 for completing assessments across the entire study.
Treatment
Medication induction and stabilization followed guidelines for methadone and BUP pharmacotherapy for 24 weeks of treatment, followed by a taper, with study completion by Week 32 (see Saxon et al., 2013, for detailed descriptions of the medication phase). Weekly assessments included urine drug screens and adverse event monitoring, and self-reported drug use data were collected every 4 weeks.
Buprenorphine/naloxone.
BUP was provided as a combination sublingual tablet containing both buprenorphine and naloxone. Following a 3-day induction, patients were stabilized on BUP up to 32 mg per day. Recommended dose changes were made in 2 mg to 8 mg increments, with daily doses ranging from 2 mg to 32 mg. The mean maximum daily BUP dose was 22.1 mg (SD = 8.2; Mdn = 24 mg). BUP was provided daily or 3 times weekly in accordance with pertinent federal and state rules about take-home dosing.
Methadone.
Methadone was purchased and obtained directly by each clinical site. Following a 3-day induction, flexible dosing was used during the stabilization period. No specific maximum daily dose was set for methadone. The mean daily maximum methadone dose was 93.2 mg (SD = 42.2; Mdn = 90 mg). Methadone was dispensed daily, although take-home medication was provided for clinic closures, holidays, weekends, and in accordance with clinic policies and procedures.
Statistical analysis
For these analyses, the two groups of interest were defined by opioid type used and injection status. Based on opioid use in the previous 30 days, three opioid-type groups were identified: heroin only, OA only (e.g., oxycodone, hydrocodone), and combined (heroin and OA), and two injection-status groups were identified: injectors and non-injectors.
Our primary outcome measures were abstinence from opioids (heroin and OA) and treatment attrition. Abstinence was defined as self-report of no opioid use in the 30 days before the end of maintenance therapy (i.e., Week 24). Based on attendance records, days in study were calculated and used in the examination of treatment attrition.
Bivariate comparisons were calculated between baseline clinical characteristics and the three opioid-type groups (heroin, OA, and combined), and between the two injectionstatus groups (injector and non-injector) using chi-square tests for categorical variables and two-tailed t tests for continuous variables. Significant predictors from bivariate analyses were included in multivariate analyses for opioid type and injection status. Multinomial logistic regression was used to examine associations of baseline clinical characteristics with the three opioid-type groups. The validity of the independence of irrelevant alternatives assumption for these three groups was confirmed using Hausman (1978) and Small and Hsiao (1985) tests. Subsequently, logistic regression was used to examine associations between baseline clinical characteristics and the two injection status groups.
After examining group differences in baseline clinical characteristics, we examined whether type of opioid and injection status moderated treatment outcomes. The outcomes of interest were abstinence, measured as any opioid use (heroin or OA) in the previous 30 days (yes/no) at end of treatment (i.e., Week 24); and time to dropout, measured in days.
Logistic regression was used to examine whether baseline clinical characteristics, opioid type, or injection status predicted abstinence at end of treatment. In addition, we examined two-way and three-way interactions between treatment assignment (BUP or methadone), opioid type, and injection status. Logistic regression analyses include only participants who completed the 24-week medication phase. Treatment assignment, age, and gender were included as covariates. Finally, Cox regression was used to examine the independent associations between type of opioid or injection status and treatment attrition. Interaction with treatment assignment and either opioid type or injection status on attrition rates was also examined. Treatment assignment, age, and gender were included as covariates.
Results
Participants
A total of 1,920 participants consented; 1,269 met eligibility criteria and were randomized and inducted on study medication (nBUP = 740, nmethadone = 529). Of these, 731 participants completed the 24-week active medication phase (nBUP = 340, nmethadone = 391). Unless otherwise noted, the results reported in these analyses reflect the 1,269 randomized participants for baseline analyses.
Baseline clinical characteristics
Type of opioid.
Group differences by type of opioid (heroin, OA, and combined) are shown in Table 1. Bivariate results and post hoc tests indicated significant differences between the groups on clinical characteristics. Compared with OA users, heroin users were more likely to be older, non-White, Hispanic, cocaine dependent, non–sedative dependent, and injectors. Relative to OA users, combined users were more likely to be older, non-White, Hispanic, cocaine dependent, sedative dependent, and injectors and to report fewer days of OA use. Compared with heroin users, combined users were more likely to be sedative dependent and non-injectors and to report fewer days of heroin use, less bodily pain, and less emotional well-being.
Table 1.
Baseline background and clinical characteristics based on type of opioid used
| Sample based on 30-day use |
||||
| Patient characteristics (n = 1,250)a | Heroin only (n = 693) | OA only (n = 170) | Heroin and OA (n = 387) | p |
| Sociodemographics | ||||
| Female sex, n (%) | 211 (30.4%) | 66 (38.8%) | 123 (31.8%) | N.S. |
| Age, in years, M (SD) | 38.2 (10.9) | 34.4 (10.1) | 37.1 (11.6) | <.001 |
| Race, n (%) | .032 | |||
| White | 471 (68.0%) | 134 (78.8%) | 287 (74.2%) | |
| African American | 75 (10.8%) | 9 (5.3%) | 26 (6.7%) | |
| American Indian | 7 (1.0%) | 2 (1.2%) | 5 (1.3%) | |
| Asian | 7 (1.0%) | 1 (0.6%) | 1 (0.3%) | |
| Pacific Islander | 2 (0.3%) | 1 (0.6%) | 1 (0.3%) | |
| Other | 91 (13.1%) | 9 (5.3%) | 43 (11.1%) | |
| Race not answered | 3 (0.4%) | 2 (1.2%) | 1 (0.3%) | |
| Multiracial | 37 (5.3%) | 12 (7.1%) | 24 (6.2%) | |
| Hispanic, n (%) | 122 (17.6%) | 15 (8.8%) | 66 (17.1%) | .018 |
| Baseline clinical characteristics | ||||
| Non-opioid substance-dependence diagnoses, past year, n (%) | ||||
| Alcohol | 77 (11.1%) | 11 (6.5%) | 44 (11.4%) | N.S. |
| Cannabis | 48 (6.9%) | 12 (7.1%) | 36 (9.3%) | N.S. |
| Cocaine | 174 (25.1%) | 14 (8.2%) | 97 (25.1%) | <.001 |
| Amphetamine | 46 (6.6%) | 11 (6.5%) | 23 (5.9%) | N.S. |
| Sedatives | 30 (4.3%) | 12 (7.1%) | 37 (9.6%) | .003 |
| Days of substance use, past 30 days, M (SD) | ||||
| OAs | – | 27 1 (5.7) | 8.3 (8.8) | <.001 |
| Heroin | 27.7 (5.5) | 23.7 (9.1) | <.001 | |
| Opioid use history, n (%) | ||||
| Ever used heroin | – | 63 (37.1%) | – | – |
| Ever used OA | 422 (60.9%) | – | – | – |
| Injected past 30 days | 570 (82.3%) | 3 (1.8%) | 300 (77.5%) | <.001 |
| Quality of life (SF-36),b M (SD) | ||||
| Physical functioning | 49.5 (9.2) | 49.2 (9.8) | 48.7 (10.0) | N.S. |
| Physical role limitation | 47.2 (11.2) | 46.8 (11.4) | 45.8 (12.1) | N.S. |
| Bodily pain | 46.3 (11.2) | 44.6 (10.7) | 43.4 (11.1) | <.001 |
| General health | 45.2 (9.6) | 44.2 (9.8) | 44.2 (9.3) | N.S. |
| Emotional well-being | 40.5 (11.2) | 38.3 (11.6) | 38.7 (11.8) | .013 |
| Emotional role limitation | 42.9 (13.4) | 41.3 (13.7) | 41.4 (13.7) | N.S. |
| Social functioning | 41.7 (12.1) | 40.2 (11.9) | 40.6 (12.0) | N.S. |
| Energy/vitality | 45.3 (9.2) | 43.8 (9.3) | 44.3 (9.3) | N.S. |
| Fagerström Test for Nicotine Dependence,c | ||||
| total score, M (SD) | 4.3 (2.2) | 4.2 (2.3) | 4.5 (2.3) | N.S. |
Notes: OA = opioid analgesic; N.S. = not significant; SF-36 = Short Form 36-Item Health Survey. t test or chi square.
19 of 1,269 reported 0 days of opioid use in past 30 days;
SF-36: 0–100 point scale, lower number = worse;
Fagerström: 0–10 point scale.
Injection status.
Group differences in baseline characteristics by injection status are presented in Table 2. Bivariate results indicated that compared with non-injectors, injectors were more likely to be male, older, Hispanic, and cocaine dependent; to have ever used heroin; and to report fewer days of OA use and more days of heroin use. Non-injectors were significantly more likely to have ever used OA compared with injectors.
Table 2.
Baseline background and clinical characteristics based on injection status
| Sample based on 30-day use |
|||
| Patient characteristics (n = 1,269) | Non-injectors (n = 396) | Injectors (n = 873) | p |
| Sociodemographics | |||
| Female sex, n (%) | 148 (37.4%) | 260 (29.8%) | .007 |
| Age, M (SD) | 35.4(10.4) | 38.3(11.3) | <.001 |
| Race, n (%) | N.S. | ||
| White | 281 (71.0%) | 625 (79.6%) | |
| African American | 45 (11.4%) | 65 (7.4%) | |
| American Indian | 4 (1.0%) | 11 (1.3%) | |
| Asian | 5 (1.3%) | 4 (0.5%) | |
| Pacific Islander | 1 (0.3%) | 3 (0.3%) | |
| Other | 33 (8.3%) | 112 (12.8%) | |
| Race not answered | 2 (0.5%) | 4 (0.5%) | |
| Multiracial | 25 (6.3%) | 49 (5.6%) | |
| Hispanic, n (%) | 50 (12.6%) | 156 (17.9%) | .019 |
| Baseline clinical characteristics | |||
| Non-opioid substance-dependence diagnoses, past year, n (%) | |||
| Alcohol | 36 (9.1%) | 99 (11.3%) | N.S. |
| Cannabis | 32 (8.1%) | 65 (7.4%) | N.S. |
| Cocaine | 65 (16.4%) | 225 (25.8%) | <.001 |
| Amphetamine | 19 (4.8%) | 62 (7.1%) | N.S. |
| Sedatives | 23 (5.8%) | 58 (6.6%) | N.S. |
| Days of substance use, past 30 days, M (SD) | |||
| OAs | 14.1 (13.5) | 2.5 (5.9) | <.001 |
| Heroin | 12.7 (13.8) | 26.7 (6.7) | <.001 |
| Opioid use history, n (%) | |||
| Ever used heroin | 281 (71.0%) | 872 (99.9%) | <.001 |
| Ever used OA | 331 (83.6%) | 665 (76.2%) | .003 |
| Quality of life (SF-36),a M (SD) | |||
| Physical functioning | 48.9 (10.3) | 49.3 (9.2) | N.S. |
| Physical role limitation | 46.8 (11.4) | 46.6 (11.6) | N.S. |
| Bodily pain | 44.9 (11.2) | 45.3 (11.2) | N.S. |
| General health | 44.8 (9.6) | 44.7 (9.6) | N.S. |
| Emotional well-being | 39.4 (11.6) | 39.7 (11.4) | N.S. |
| Emotional role limitation | 41.9 (13.5) | 42.4 (13.5) | N.S. |
| Social functioning | 40.8 (12.2) | 41.3 (12.1) | N.S. |
| Energy/vitality | 44.6 (9.4) | 44.9 (9.2) | N.S. |
| Fagerström Test for Nicotine Dependence,b | |||
| total score, M (SD) | 4.2 (2.3) | 4.4 (2.2) | N.S. |
Notes: N.S. = not significant; OA = opioid analgesic; SF-36 = Short Form 36-item Health Survey. t test or chi square.
SF-36: 0–100 point scale, lower number = worse;
Fagerström: 0–10 point scale.
Multivariate results
The significant bivariate variables were entered as predictors into a multinomial logistic regression model. Relative to heroin users, OA users were significantly more likely to be White (adjusted odds ratio [aOR] = 2.03, 95% confidence interval [CI] [1.17, 3.53]) and less likely to be cocaine dependent (aOR = 0.26, 95% CI [0.13, 0.51]) and injectors (aOR = 0.004, 95% CI [0.00, 0.01]). Compared with combined users, OA users were significantly less likely to be cocaine dependent (aOR = 0.30, 95% CI [0.15, 0.59]) and injectors (aOR = 0.01, 95% CI [0.00, 0.02]). Relative to combined users, heroin users were significantly less likely to be White (aOR = 0.71, 95% CI [0.51, 0.99]), sedative dependent (aOR = 0.46, 95% CI [0.28, 0.78]), and to have pain (aOR = 0.98, 95% CI [0.97, 0.99]) and more likely to be injectors (aOR = 1.39, 95% CI [1.01, 1.92]).
Addressing associations between baseline characteristics and injection status using logistic regression, results indicated that compared with non-injectors, injectors were significantly more likely to be older (aOR = 1.02, 95% CI [1.00, 1.03]), White (aOR = 1.90, 95% CI [1.31, 2.77]), and Hispanic (aOR = 1.89, 95% CI [1.18, 3.00]); to have ever used heroin (aOR = 36.70, 95% CI [4.93, 273.29]) and have ever used OA (aOR = 1.69, 95% CI [1.18, 2.44]); and to report greater number of days of heroin use (aOR = 1.07, 95% CI [1.05, 1.09]) and fewer days of OA use (aOR = 0.96, 95% CI [0.94, 0.98]).
Treatment outcomes
Continued opioid use.
Any opioid use (heroin or OA) at end of treatment (use or no use) was compared with baseline characteristics and opioid-type and injection-status groups (Table 3). As noted above, 731 participants completed the 24-week assessments and are included in these analyses. Bivariate results indicated that those who were African American were more likely to be using opioids, and those who were cannabis dependent were less likely to be using opioids at end of treatment. Those who reported ever using heroin with a greater number of days of heroin use at baseline were more likely to be using opioids, and those who reported a greater number of days of OA use at baseline were less likely to be using opioids at end of treatment. Injectors were more likely to be using opioids at end of treatment compared with non-injectors. Among the opioid-type groups, OA users were less likely to be using at end of treatment compared with heroin and combined users.
Table 3.
Comparison of baseline characteristics by any opioid use at end of treatment
| Sample based on 30-day use |
|||
| Patient characteristics (n = 705)a | No use (n = 415) | Use (n = 290) | p |
| Sociodemographics | |||
| Female sex, n (%) | 142 (34.2%) | 84 (29.0%) | N.S. |
| Age, M (SD) | 38.6 (11.2) | 38.3 (11.5) | N.S. |
| Race, n (%) | .014 | ||
| White | 311 (74.9%) | 198 (68.3%) | |
| African American | 32 (7.7%) | 40 (13.8%) | |
| American Indian | 5 (1.2%) | 0 (0.0%) | |
| Asian | 1 (0.2%) | 3 (1.0%) | |
| Pacific Islander | 0 (0.0%) | 0 (0.0%) | |
| Other | 37 (8.9%) | 33 (11.4%) | |
| Race not answered | 3 (0.7%) | 0 (0.0%) | |
| Multiracial | 26 (6.3%) | 16 (5.5%) | |
| Hispanic, n (%) | 60 (14.5%) | 46 (15.9%) | N.S. |
| Baseline clinical characteristics | |||
| Non-opioid substance-dependence diagnoses, past year, n (%) | |||
| Alcohol | 50 (12.0%) | 25 (8.6%) | N.S. |
| Cannabis | 37 (8.9%) | 14 (4.8%) | .039 |
| Cocaine | 88 (21.2%) | 65 (22.4%) | N.S. |
| Amphetamine | 33 (8.0%) | 19 (6.6%) | N.S. |
| Sedatives | 29 (7.0%) | 15 (5.2%) | N.S. |
| Days of substance use, past 30 days, M (SD) | |||
| OAs | 7.8 (11.6) | 4.4 (8.7) | <.001 |
| Heroin | 19.7 (12.8) | 24.8 (9.5) | <.001 |
| Opioid use history, n (%) | |||
| Ever used heroin | 356 (85.8%) | 277 (95.5%) | <.001 |
| Ever used OA | 338 (81.4%) | 230 (79.3%) | N.S. |
| Injected past 30 days | 247 (59.5%) | 220 (75.9%) | <.001 |
| Type of opioid used, n (%) | <.001 | ||
| Heroin | 203 (50.5%) | 171 (59.0%) | |
| OA | 82 (20.4%) | 23 (7.9%) | |
| Heroin and OA | 117 (29.1%) | 96 (33.1%) | |
| Fagerström Test for Nicotine Dependence,b | |||
| total score, M (SD) | 4.5 (2.3) | 4.3 (2.1) | N.S. |
| In study, treatment assignment | N.S. | ||
| Methadone, n (%) | 222 (53.5%) | 153 (52.8%) | |
| BUP, n (%) | 193 (46.5%) | 137 (47.2%) | |
Notes: N.S. = not significant; OA = opioid analgesic; BUP = buprenorphine/naloxone. t test or chi square.
26 of 731 did not complete the Risk Behavior Survey at Week 24;
Fagerström: 0–10 point scale.
Controlling for treatment assignment, age, and gender, those who were cannabis dependent were significantly less likely to be using opioids at end of treatment (aOR = 0.48, 95% CI [0.25, 0.92]), and compared with non-injectors, injectors were significantly more likely to be using opioids at end of treatment (aOR = 1.98, 95% CI [1.41, 2.80]). Last, there were no statistically significant two-way or three-way interactions among treatment assignment, type of opioid, and injection status on opioid use at end of treatment. In other words, there was no effect of treatment by type of opioid used or injection status on opioid use outcome among treatment completers. In examining the three-way interaction, only three OA users reported injection. To determine the impact of these two small cell sizes on the model, a sensitivity analysis was performed (collapsing the OA injectors with the non-injectors for BUP and methadone by omitting those interaction terms from the model); results were unchanged.
Treatment attrition.
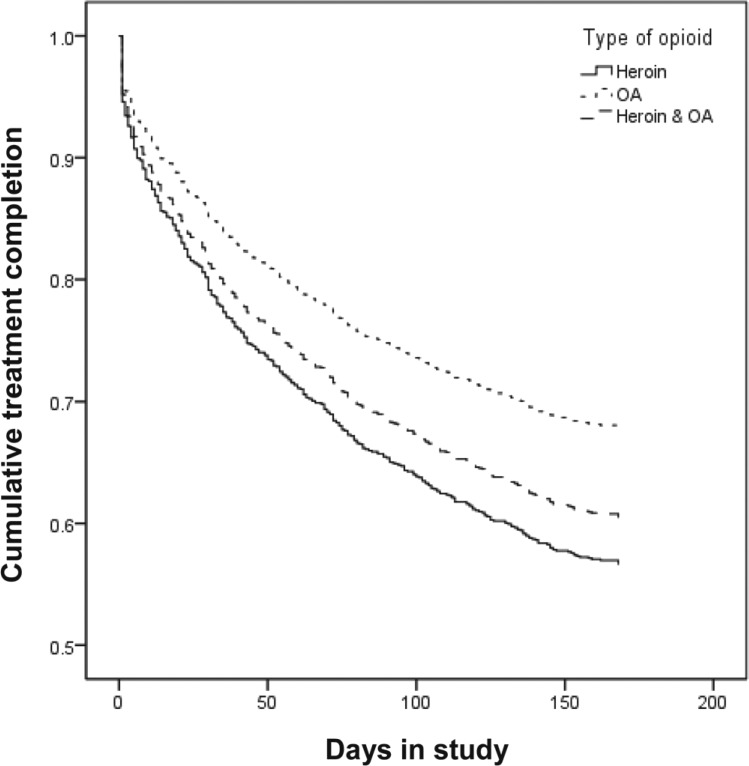
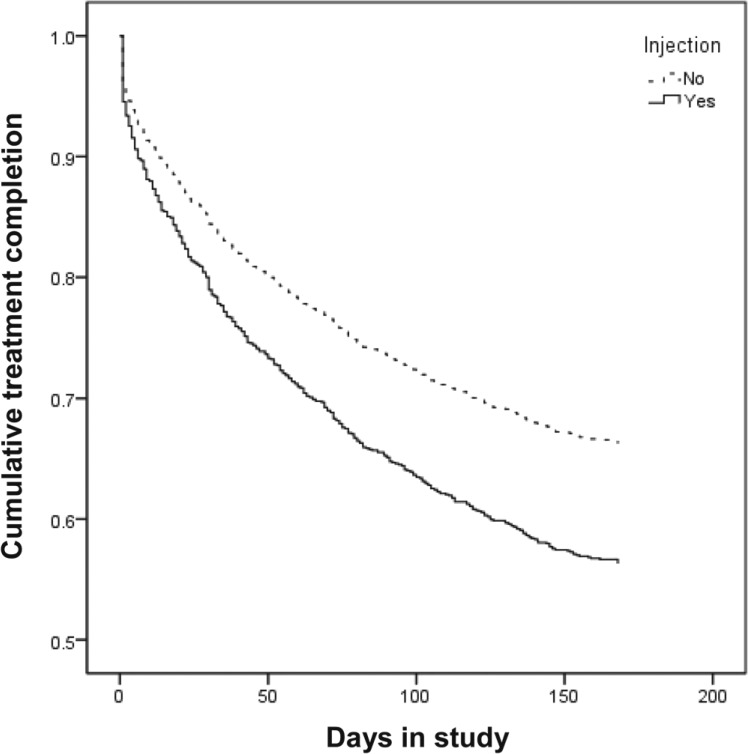
BUP participants were significantly less likely to complete treatment compared with those on methadone (OR = 0.38, 95% CI [0.31, 0.46]). Because of this significant difference in attrition rates, treatment assignment was included as a covariate in all Cox regressions; age and gender were also included as covariates in the examinations of differential attrition rates for type of opioid and injection status. Results indicated that those who were older were significantly less likely to complete treatment (aOR = 0.98, 95% CI [0.97, 0.99]). As shown in Figure 1, compared with OA users, heroin users were significantly less likely to complete treatment (aOR = 0.56, 95% CI [0.40, 0.77]). Similarly, compared with OA users, combined users were also significantly less likely to complete treatment (aOR = 0.66, 95% CI [0.46, 0.94]). There was no significant difference between heroin users and combined users in treatment attrition. As seen in Figure 2, non-injectors were significantly more likely than injectors to complete treatment (aOR = 1.61, 95% CI [1.29, 2.02]).
Figure 1.
Treatment attrition by type of opioid. OA = opioid analgesic.
Figure 2.
Treatment attrition by injection status.
Furthermore, there were no significant two-way interactions between treatment assignment and opioid type or treatment assignment and injection status on attrition. In other words, there were similar factors influencing rates of attrition for those on BUP and those on methadone concerning type of opioid used or injection status.
Discussion
The purpose of this secondary analysis was to compare baseline clinical characteristics of opioid users and explore whether type of opioid used and injection status are associated with subsequent response to ORT (either methadone or BUP), with both opioid use at end of treatment and attrition as measures of treatment outcome. As a whole, this study extends prior findings by including combined opioid users and injection status in examining treatment response to BUP and methadone in a single study.
Not surprisingly, clinical differences emerged between our three types of opioid users. Heroin and combined users exhibited the more severe clinical presentation (i.e., more likely to have additional non-opioid substance dependence diagnoses and to be injectors). As has been reported previously (Nielsen et al., 2012), exclusive OA users did report greater pain and emotional distress; however, these factors did not differentiate the three groups in multivariate analyses. Rather, substance use severity is most salient. As with the results reported above, substance use severity rather than other clinical factors differentiates injectors from non-injectors. Compared with non-injectors, injectors are more likely to have used heroin and with greater frequency. Taken together, these findings support the assertion that heroin use and injecting are markers of clinical severity that may affect treatment response.
Indeed, lifetime heroin use predicts poorer treatment outcome compared with OA use. This finding supports previous reports indicating that heroin users are less likely to have successful treatment outcomes compared with OA users (Banta-Green et al., 2009; Brands et al., 2004; Moore et al., 2007). For example, one study found that any heroin use increased risk of poor treatment response compared with no heroin use in a sample that was primarily dependent on OAs (Weiss et al., 2011). As expected (Neufeld et al., 2008), injection status predicts poor treatment response to methadone and BUP treatment. The poorer response associated with heroin use and history of injection in this report is irrespective of maintenance medication received. Notably, this study appears to be the first to directly compare methadone and BUP treatment responses for these groups. This suggests that, although other factors may drive medication selection (e.g., availability and patient preference), there is no evidence to indicate a differential outcome for methadone or BUP.
Similar to treatment response, there were significant differences in attrition indicating poor treatment retention for heroin users and injectors. Consistent with previous findings (Brands et al., 2004; Moore et al., 2007), heroin users are less likely to complete treatment compared with OA users. Similarly, injectors are less likely to complete treatment compared with non-injectors. These are particularly important findings as retention in treatment is the best predictor of clinical outcomes (Corsi et al., 2009; Hutchinson et al., 2000; Weiss et al., 2011). Finally, as with opioid use at end of treatment, these results were irrespective of treatment assignment.
It is important to note that our results indicate no significant clinical advantage for BUP versus methadone in treating OA versus heroin users or non-injectors versus injectors for those who complete treatment. This is particularly interesting because, in the absence of empirical evidence, there may be a perception that OA users will respond more favorably to BUP. Our findings suggest that there is no superior treatment for OA users; receiving either ORT increases the probability of attaining a successful outcome. However, there may be other factors to consider when deciding on an ORT (e.g., provision through an individual health care provider, as is possible with BUP, versus through a structured methadone clinic). This is particularly important given the higher attrition rate observed in BUP patients compared with methadone patients. However, the reasons for this dropout do not appear to be related to type of opioid or injection status.
As with any secondary analysis, there are limitations. Opioid type, injection status, and opioid use at end of treatment were based on self-report. Except for treatment assignment, these analyses considered baseline characteristics. In-treatment factors may have also affected outcome. Information regarding treatment response, defined for these analyses as opioid use in the previous 30 days, was only available for individuals who attended their final treatment visit at Week 24. Although the Risk Behavior Survey is a self-report instrument, evidence indicates that self-report is a reliable and valid measure of drug behaviors (Darke, 1998). Finally, there was a higher dropout rate in the BUP compared with the methadone treatment condition.
Conclusion
To our knowledge, this is the first report that examines the role of type and route of opioid use on both methadone and BUP outcomes directly. It is also the first study to enable a direct comparison between BUP and methadone for OA users. The findings reported here add to the literature a large-scale examination of differences in treatment outcomes for heroin versus OA users and for injectors versus non-injectors. Of particular interest is the finding that BUP does not appear to be superior to methadone for treating OA users.
In total, these findings indicate that heroin use and injecting are significantly associated with poor treatment outcomes regardless of ORT medication, and this may be attributed to the progression in the disease state observed in heroin users and injectors. Clinically, this suggests that programs offering maintenance therapies may benefit from targeting retention efforts on heroin and injection users. Potential interventions could include assuring that medication doses are optimized (Strain et al., 1999), using contingency management (Peirce et al., 2006), or directing interventions to specific problem areas of individual patients (McLellan et al., 1993).
Footnotes
This research was supported by National Institute on Drug Abuse Clinical Trials Network Grants U10 DA020024 (to Madhukar H. Trivedi), U10 DA13045 (to Walter Ling), 5 U10 DA013714-08 (to Dennis Donovan), U10 DA13036 (to Dennis McCarty), and K23 DA02297 (to Jennifer S. Potter).
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders, text revision. 4th ed. Washington, DC: Author; 2000. [Google Scholar]
- Banta-Green CJ, Maynard C, Koepsell TD, Wells EA, Donovan DM. Retention in methadone maintenance drug treatment for prescription-type opioid primary users compared to heroin users. Addiction. 2009;104:775–783. doi: 10.1111/j.1360-0443.2009.02538.x. [DOI] [PubMed] [Google Scholar]
- Booth RE, Watters JK, Chitwood DD. HIV risk-related sex behaviors among injection drug users, crack smokers, and injection drug users who smoke crack. American Journal of Public Health. 1993;83:1144–1148. doi: 10.2105/ajph.83.8.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brands B, Blake J, Sproule B, Gourlay D, Busto U. Prescription opioid abuse in patients presenting for methadone maintenance treatment. Drug and Alcohol Dependence. 2004;73:199–207. doi: 10.1016/j.drugalcdep.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Corsi KF, Lehman WK, Booth RE. The effect of methadone maintenance on positive outcomes for opiate injection drug users. Journal of Substance Abuse Treatment. 2009;37:120–126. doi: 10.1016/j.jsat.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darke S. Self-report among injecting drug users: A review. Drug and Alcohol Dependence. 1998;51:253–263. doi: 10.1016/s0376-8716(98)00028-3. discussion 267–268. [DOI] [PubMed] [Google Scholar]
- Darke S, Hetherington K, Ross J, Lynskey M, Teesson M. Non-injecting routes of administration among entrants to three treatment modalities for heroin dependence. Drug and Alcohol Review. 2004;23:177–183. doi: 10.1080/095952304100017044163. [DOI] [PubMed] [Google Scholar]
- Hausman JA. Specification tests in econometrics. Econometrica. 1978;46:1251–1271. [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hutchinson SJ, Taylor A, Gruer L, Barr C, Mills C, Elliott L. One-year follow-up of opiate injectors treated with oral methadone in a GP-centred programme. Addiction. 2000;95:1055–1068. doi: 10.1046/j.1360-0443.2000.95710557.x. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Arndt IO, Metzger DS, Woody GE, O’Brien CP. The effects of psychosocial services in substance abuse treatment. Journal of the American Medical Association. 1993;269:1953–1959. [PubMed] [Google Scholar]
- Moore BA, Fiellin DA, Barry DT, Sullivan LE, Chawarski MC, O’Connor PG, Schottenfeld RS. Primary care office-based buprenorphine treatment: Comparison of heroin and prescription opioid dependent patients. Journal of General Internal Medicine. 2007;22:527–530. doi: 10.1007/s11606-007-0129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neaigus A, Miller M, Friedman SR, Hagen DL, Sifaneck SJ, Ildefonso G, des Jarlais DC. Potential risk factors for the transition to injecting among non-injecting heroin users: A comparison of former injectors and never injectors. Addiction. 2001;96:847–860. doi: 10.1046/j.1360-0443.2001.9668476.x. [DOI] [PubMed] [Google Scholar]
- Neufeld K, King V, Peirce J, Kolodner K, Brooner R, Kidorf M. A comparison of 1-year substance abuse treatment outcomes in community syringe exchange participants versus other referrals. Drug and Alcohol Dependence. 2008;97:122–129. doi: 10.1016/j.drugalcdep.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S, Hillhouse M, Mooney L, Fahey J, Ling W. Comparing buprenorphine induction experience with heroin and prescription opioid users. Journal of Substance Abuse Treatment. 2012;43:285–290. doi: 10.1016/j.jsat.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce JM, Petry NM, Stitzer ML, Blaine J, Kellogg S, Satterfield F, Li R. Effects of lower-cost incentives on stimulant abstinence in methadone maintenance treatment: A National Drug Abuse Treatment Clinical Trials Network study. Archives of General Psychiatry. 2006;63:201–208. doi: 10.1001/archpsyc.63.2.201. [DOI] [PubMed] [Google Scholar]
- Puigdollers E, Domingo-Salvany A, Brugal MT, Torrens M, Alvarós J, Castillo C, Vázquez JM. Characteristics of heroin addicts entering methadone maintenance treatment: Quality of life and gender. Substance Use & Misuse. 2004;39:1353–1368. doi: 10.1081/ja-120039392. [DOI] [PubMed] [Google Scholar]
- Rosenblum A, Parrino M, Schnoll SH, Fong C, Maxwell C, Cleland CM, Haddox JD. Prescription opioid abuse among enrollees into methadone maintenance treatment. Drug and Alcohol Dependence. 2007;90:64–71. doi: 10.1016/j.drugalcdep.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Saxon AJ, Ling W, Hillhouse M, Thomas C, Hasson A, Ang A, Jacobs P. Buprenorphine/naloxone and methadone effects on laboratory indices of liver health: A randomized trial. Drug and Alcohol Dependence. 2013;128:71–76. doi: 10.1016/j.drugalcdep.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small KA, Hsiao C. Multinomial logit specification tests. International Economic Review. 1985;26:619–627. [Google Scholar]
- Strain EC, Bigelow GE, Liebson IA, Stitzer ML. Moderate- vs high-dose methadone in the treatment of opioid dependence: A randomized trial. Journal of the American Medical Association. 1999;281:1000–1005. doi: 10.1001/jama.281.11.1000. [DOI] [PubMed] [Google Scholar]
- Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Medical Care. 1992;30:473–483. [PubMed] [Google Scholar]
- Weiss RD, Potter JS, Fiellin DA, Byrne M, Connery HS, Dickinson W, Ling W. Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: A 2-phase randomized controlled trial. Archives of General Psychiatry. 2011;68:1238–1246. doi: 10.1001/archgenpsychiatry.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Composite International Diagnostic Interview – Version 2.1. Geneva, Switzerland: Author; 1997. [Google Scholar]
- Wu LT, Woody GE, Yang C, Blazer DG. How do prescription opioid users differ from users of heroin or other drugs in psychopathology: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Journal of Addiction Medicine. 2011;5:28–35. doi: 10.1097/ADM.0b013e3181e0364e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AM, Havens JR. Transition from first illicit drug use to first injection drug use among rural Appalachian drug users: A cross-sectional comparison and retrospective survival analysis. Addiction. 2012;107:587–596. doi: 10.1111/j.1360-0443.2011.03635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]