Abstract
Objective:
This study tested whether the addition of an enhanced continuing care (ECC) intervention that combined in-person and telephone sessions and began in the first week of treatment improved outcomes for cocaine-dependent patients entering an intensive outpatient program (IOP).
Method:
Participants (N = 152) were randomized to IOP treatment as usual (TAU) or IOP plus 12 months of ECC. ECC included cognitive—behavioral therapy elements to increase coping skills, as well as monetary incentives for attendance. It was provided by counselors situated at a separate clinical research facility who did not provide IOP. The primary outcomes measured were (a) cocaine urine toxicology and (b) good clinical outcome, as indicated by abstinence from all drugs and from heavy alcohol use. Secondary outcomes were frequency of abstinent days, cocaine use days, and heavy drinking days. Follow-ups were conducted at 3, 6, 9, and 12 months after baseline.
Results:
Patients in ECC completed a mean of 18 sessions. Contrary to the hypotheses, patients in TAU had better scores on both the cocaine urine toxicology and the good clinical outcome measures than those in ECC, as indicated by significant Group × Time interactions (cocaine urine toxicology, p = .0025; abstinence composite, p = .017). These results were not moderated by substance use before or early in treatment or by IOP attendance. Results with the secondary outcomes also did not favor ECC over TAU.
Conclusions:
Continuing care that is not well integrated with the primary treatment program may interfere in some way with the therapeutic process, particularly when it is implemented shortly after intake.
Wider use of effective continuing care has been recommended for individuals with substance use disorders to increase rates of sustained recoveries and limit the severity and duration of relapses (Dennis and Scott, 2007; Humphreys and Tucker, 2002; McKay, 2009b; Miller and Weisner, 2002). Moreover, continuing care interventions with longer durations and active efforts to deliver the intervention tend to show larger effects (Dennis and Scott, 2012; McKay, 2009a; Scott and Dennis, 2009).
Unfortunately, many patients in treatment for substance use disorders receive little if any continuing care because they experience barriers to participation in clinic-based care after the initial phase of treatment has been completed (McKay, 2009a, 2011). To address this problem, we developed a telephone-based continuing care intervention, referred to as telephone monitoring and counseling (TMC; McKay et al., 2010a). The intervention includes elements found in cognitive–behavioral therapy (CBT), such as regular monitoring of current substance use and other risk and protective factors, identification of upcoming high-risk situations, rehearsal of coping strategies, and linkage to community supports. Prior studies with TMC have found that the intervention produced better drug and alcohol use outcomes than standard care, particularly in patients who continued to use alcohol or other drugs or failed to achieve other goals of early treatment (McKay et al., 2005, 2010b, in press).
However, many patients who might benefit from continuing care never receive it because they drop out of treatment early (McKay, 2011). For these individuals, management of their disorders consists of separate, brief episodes of treatment with essentially no follow-up care. In an effort to address this problem, Dennis, Scott, and colleagues developed the recovery management checkup. In recovery management checkups, patients are assessed every 3 months for periods as long as 2–4 years, and those who are using alcohol or other drugs at dangerous levels are helped to re-engage with treatment. This approach has improved outcomes over standard care in two large randomized studies (Dennis and Scott, 2012; Dennis et al., 2003). Morgenstern and colleagues (2006, 2009) also have shown that providing extended case management for welfare recipients produces better longer-term outcomes than standard care.
In our prior work with telephone-based continuing care, we enrolled intensive outpatient program (IOP) patients after 2–4 weeks of treatment. This has meant that substantial numbers of patients dropped out of IOP before becoming eligible to enter the studies (McKay et al., 2010b). The present study was an initial test of whether we could extend the reach of TMC by offering the intervention to patients immediately following intake to treatment and whether doing so would improve outcomes.
The TMC intervention (McKay et al., 2010a) was modified in anticipation of the challenges inherent in delivering continuing care to a heterogeneous group that included early treatment dropouts. Patients were given $10 for each continuing care session attended in order to promote participation even among those patients who had dropped out of IOP (Van Horn et al., 2011). To capitalize on the potential benefits of providing choice, patients could attend their continuing care sessions either via the telephone or in the clinic. Finally, more systematic efforts were made to link patients to available resources in the community. The modified intervention was referred to as enhanced continuing care (ECC).
Participants were recruited in their first week of IOP and were randomly assigned to receive IOP only or IOP plus ECC, which was delivered for up to 12 months. Substance use and other outcomes were assessed every 3 months for 1 year. The ECC condition was expected to produce better substance use outcomes than IOP alone. These effects were expected to be stronger in patients who had used cocaine or alcohol in the month before IOP intake or during the first month of IOP (McKay et al., 1999, in press) and in those who failed to complete the first 3 weeks of IOP.
Method
Participants
The participants were 152 adults enrolled in publicly funded IOPs in Philadelphia who met criteria for lifetime cocaine dependence according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV; American Psychiatric Association, 1994), and who had used cocaine in the past 6 months. The other eligibility criteria were a willingness to be randomly assigned to a treatment condition, no psychiatric or medical condition that precluded outpatient treatment (i.e., severe dementia, current hallucinations), age between 18 and 65 years, no regular intravenous heroin use within the past 12 months, the ability to read at approximately the fourth-grade level or above, and at least a minimum degree of stability in their living situation (e.g., not living on the street). To facilitate follow-up, participants had to be able to provide the names, addresses, and telephone numbers of at least three contacts. The study was conducted in compliance with the policies of the Institutional Review Boards of the University of Pennsylvania and the Philadelphia Department of Behavioral Health.
The participants were on average 42.8 years old (SD = 8.9) and had 11.8 years (SD = 1.7) of education. The majority of participants were male (77%) and African American (82%). The participants had used cocaine on an average of 38.5% (SD = 29.0) of days in the prior 6 months and had drunk alcohol on 24.3% (SD = 30.0) of the days. They averaged 4.4 (SD = 5.0) prior treatments for drug problems.
Intensive outpatient treatment as usual
The IOPs provided approximately 9 hours of 12-step/ abstinence-oriented group counseling per week, and patients could typically attend for up to 3–4 months (McKay et al., 2010b). Patients who completed the IOPs were typically offered 2–3 months of standard outpatient treatment (i.e., one group counseling session per week), although rates of attendance were low. We enrolled 141 participants from one IOP and 11 from a second IOP.
Continuing care treatment condition
Participants had one initial face-to-face session in the first week to orient them to the protocol. They then received brief continuing care sessions for up to 12 months. These 20-minute sessions were offered weekly for the first 8 weeks and every other week for the next 44 weeks. Therefore, the total number of possible scheduled sessions was 30. Participants were given the option of completing sessions in person, rather than over the telephone, if they had difficulty in getting private access to a telephone or preferred a face-to-face format. Each session began with a structured 13-item assessment of current substance use, HIV risk behaviors, and IOP attendance; other risk factors (e.g., craving, low self-efficacy, being in risky situations); and protective factors (e.g., attendance at self-help meetings, participation in other pro-recovery social activities). This assessment was referred to as the progress assessment. A nonconfrontational, motivational interviewing approach was taken in all sessions, particularly with less motivated participants.
The CBT-based counseling was linked to the results of the progress assessment and addressed any anticipated risky situations. Participants identified potential coping strategies and behaviors, with help from the counselor as needed, and these were briefly rehearsed. While participants were still attending IOP, barriers to successful IOP completion were addressed. The intervention also featured a stepped-care component, which included additional telephone or clinic sessions and was triggered when changes to protective and risk factors resulted in a negative shift of 5 points or more on the progress assessment summary score (McKay et al., 2010a). Participants received a $10 gift coupon for each regularly scheduled or stepped-care session attended. The coupons were for discount department stores (Walmart, Target), a drugstore chain, and a local grocery store chain.
The other new components in the intervention focused on increasing linkage to recovery supports in the community and on increased efforts to re-engage patients who dropped out. Study therapists had access to a case manager for consultation on relevant resources as well as direct assistance to patients needing help accessing community resources. A protocol was developed specifying outreach by telephone, mail, and contact with designated locator individuals to be completed for up to a month following a missed appointment, followed by continued attempts to reach the patient throughout the remainder of his or her eligibility for treatment.
Therapists.
Six therapists (four women and two men) delivered ECC. All therapists had prior experience in outpatient treatment for substance use disorders, ranging from 1 to 20 years. Five of the therapists had master’s-level degrees in psychology or social work, and one had a bachelor’s degree. All of the therapists had provided telephone-based continuing care in a prior study (McKay et al., 2010b). All IOP-related services were provided by different counselors, who were employed by the IOPs.
Adherence to treatment protocols.
The ECC sessions were audiotaped to facilitate supervision and to monitor adherence to the protocol. Individual supervision was provided weekly by the study clinical coordinator, and one group supervision session also was held per week. Any deviations from the treatment protocol identified by the clinical coordinator were addressed in the weekly supervision meetings.
Procedures
Recruitment.
Potential participants were screened during their first week in the IOP by study research technicians. Those who were eligible and wanted to participate in the research study completed informed-consent procedures before initiating the baseline assessment procedures. Participants were recruited between January 2010 and February 2011.
Representativeness of the study sample.
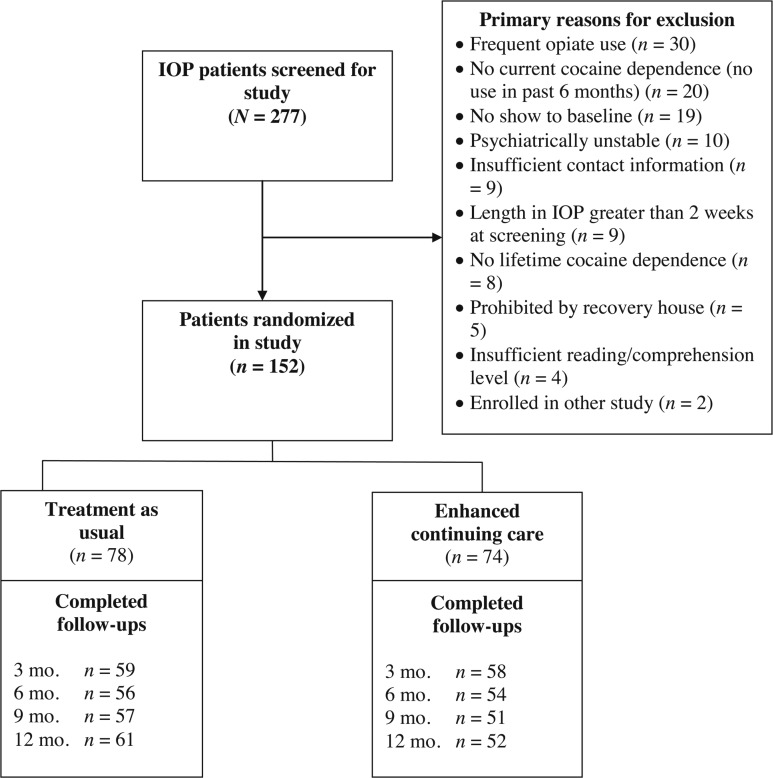
A total of 277 patients were screened at the two IOPs, and, of these, 152 were eligible and willing to participate and were enrolled in the study (Figure 1). The primary reasons for failure to enter the study were frequent opiate use (n = 30), no lifetime cocaine dependence or no cocaine use in the prior 6 months (n = 28), nonattendance at the scheduled baseline assessment (n = 19), psychiatric instability (n = 10), in IOP for more than 2 weeks (n = 9), no contacts (n = 9), living in a halfway house that did not allow participation in research (n = 5), and insufficient reading/comprehension (n = 4). A sample size of 150 was adequate to find medium effect-size differences on primary outcomes between the treatment conditions.
Figure 1.
Consort diagram. Note: Participants who died during the course of the study or asked to withdraw from the study: treatment as usual = 0, telephone monitoring and counseling = 4. IOP = intensive outpatient program; mo. = month.
Randomization procedures.
A blocked randomization scheme, using blocks of 16, was used to yield a balanced allocation of participants to the two treatment conditions. The study statistician generated the sequences. The assignments were placed in envelopes by the study coordinator, and the study staff members were blinded to the randomization sequence until the point of treatment assignment.
Assessments.
Baseline assessments were completed in Week 1 of IOP. Follow-up assessments were conducted at 3, 6, 9, and 12 months. Participants received $50 for the baseline assessment and $50 each for the four follow-up sessions. All study interviews were conducted by experienced research personnel who were blind to the study hypotheses but not to the treatment condition. The material gathered at follow-ups, informal comments made by participants, and the length of the follow-up precluded maintaining a cadre of interviewers unaware of the treatment-condition assignment.
Measures
Psychiatric diagnoses.
The Structured Clinical Interview for DSM-IV (SCID; First et al., 1996) was administered at baseline to determine cocaine and alcohol substance use disorder diagnoses and to rule out any psychiatric disorders that would preclude study participation.
Self-reported cocaine and alcohol use.
Timeline Followback (TLFB; Sobell et al., 1979) calendar assessment techniques were used to gather self-reports of cocaine and alcohol use during the 6 months preceding baseline and during the 12-month follow-up. In studies with patients who had drug use disorders, TLFB reports of days of cocaine use were highly correlated with urine toxicology results (Ehrman and Robbins, 1994; Fals-Stewart et al., 2000). In alcoholic samples, TLFB reports of percentage days abstinent have correlated .80 or better with collateral reports (Maisto et al., 1979; Stout et al., 1989). In cases where participants missed one or more follow-ups but then completed a subsequent follow-up, data from the missing follow-ups were obtained at the next follow-up via the TLFB calendar method.
Demographic information and problem severity.
The Addiction Severity Index (ASI; McLellan et al., 1980) was used to gather demographic data and information on lifetime substance use, prior addiction treatment, and problem severity at baseline in seven areas of functioning (i.e., medical, employment, drug use, alcohol use, legal, family/social, and psychiatric). The ASI has demonstrated adequate to good internal consistency, test-retest, and interrater reliabilities (McLellan et al., 1985).
Urine toxicology.
Urine samples were obtained at baseline and at each follow-up point to provide a more objective measure of the use of cocaine and other drugs (e.g., amphetamines, opiates, barbiturates, benzodiazepines, and tetrahydrocannabinol [THC]). The samples were tested with a homogeneous enzyme immunoassay method with established cutoffs for positive drug results.
Outcome measures.
The primary outcome measures were cocaine urine toxicology results and a dichotomous measure of good overall substance use outcomes (i.e., “abstinence composite”). To be considered abstinent on this measure in a given 3-month segment of the follow-up, the participant had to have (a) no self-reported cocaine use, (b) no positive drug urine toxicology tests, and (c) no heavy alcohol use (i.e., five or more drinks per day for men, four or more drinks per day for women).
The measures were operationalized in the following manner: Information from the TLFB was considered first. Any cocaine use or heavy alcohol use reported on the TLFB generated a nonabstinent score for that follow-up period. Next, urine toxicology data were considered in participants who had provided those data at the follow-up point and who reported no cocaine use or heavy drinking on the TLFB. A positive urine toxicology result on any of the five substances placed the participant in the nonabstinent category for that period. Other than cocaine, cannabis was the most frequently detected drug in the urine samples (12%–18% positive across the four assessment points). The use of amphetamines (0% positive), barbiturates (0%–3%), benzodiazepines (3%–8%), and opiates (6%–7%) was less common. Three secondary outcomes from the TLFB also were included: percentage of days abstinent, percentage of days with cocaine use, and percentage of days with heavy alcohol use.
Moderator measures.
The first moderators were dichotomous indicators of whether participants had 1 or more days of cocaine use (yes: n = 91) and 1 or more days of alcohol use (yes: n = 64) during the month before baseline on the TLFB (i.e., current use before treatment). The next moderator was a dichotomous indicator of whether participants had a cocaine-positive urine result at baseline or reported any cocaine or alcohol use in the first 4 weeks of treatment on the TLFB (i.e., use during the first month of IOP) (yes: n = 44). The final moderator was a dichotomous indicator of whether the patient dropped out of IOP before completing 10 IOP sessions (yes: n = 39), which represented 3–4 weeks of treatment.
Follow-up rates
The follow-up rates at each follow-up point were as follows: 3 months, 78%; 6 months, 73%; 9 months, 73%; 12 months, 76%. When TLFB data from subsequent follow-ups were used to backfill prior missing follow-ups, the TLFB follow-up rates were 3 months, 88%; 6 months, 84%; 9 months, 81%. The two treatment conditions did not differ significantly on follow-up rates at any point.
Data analyses
Differences between the two conditions at baseline were evaluated with one-way analyses of variance (ANOVAs) (continuous measures) and chi-square tests (categorical measures). Treatment differences in the number of days on which IOP was attended also were evaluated with one-way ANOVAs.
Generalized estimating equations (SAS PROC GEN-MOD) were used to compare the continuing care conditions on the binary urine toxicology and abstinence composite outcome measures at 3, 6, 9, and 12 months in intent-to-treat analyses. For the continuous secondary outcomes, generalized estimating equations with a Poisson distribution were used. Time was modeled as a continuous and as a categorical factor with four levels. In the treatment condition main-effect analyses, the independent variables were treatment condition, time, and Treatment × Time interactions. Significant Treatment Condition × Time interactions were hypothesized, in which the effects favoring ECC over TAU would increase over the course of the follow-up. The covariates in the analyses were (a) days of cocaine use before baseline (both outcomes) and (b) baseline cocaine urine toxicology result (cocaine urine toxicology outcome only). Site was not included as a covariate because it was not significant in preliminary analyses, and an inspection of data plots indicated that results were similar at each site. For the moderator analyses, the moderator term and the interaction of the moderator and the treatment condition were added to the model.
Results
Comparison of treatment conditions at baseline
Participants in the two treatment conditions were compared on 22 demographic, diagnostic, treatment, and problem severity variables assessed at baseline. These data are presented in Table 1. Although the conditions did not differ significantly on any variable, scores on psychiatric severity, days of alcohol use, and living with someone using alcohol or other drugs were somewhat worse in the ECC than in the TAU condition. However, because including these variables as covariates in the analyses did not influence any of the results, they were not included.
Table 1.
Characteristics of sample at baseline
| Variable | TAU (n = 78) | ECC (n = 74) | F or χ2 | P |
| Demographics | ||||
| Race | 2.00 | .37 | ||
| African American, % (n) | 85.9 (67) | 77.0 (57) | ||
| White, % (n) | 7.7 (6) | 12.2 (9) | ||
| Other (Hispanic), % (n) | 6.4 (5) | 10.8 (8) | ||
| Gender, % male (n) | 71.8 (56) | 82.4 (61) | 2.40 | .12 |
| Age, in years, M (SD) | 43.4 (8.8) | 42.3 (9.0) | 0.29 | .59 |
| Education, in years, M (SD) | 11.8 (1.7) | 11.8 (1.7) | 0.27 | .60 |
| Substance use | ||||
| Prior treatments for drug, M (SD) | 4.2 (4.1) | 4.7 (5.8) | 0.51 | .48 |
| Years of regular cocaine use, M (SD) | 17.3 (8.9) | 17.8 (9.1) | 0.30 | .59 |
| Years of regular alcohol use, M (SD) | 17.8 (12.3) | 17.2 (12.0) | 0.04 | .84 |
| Substance use in prior 6 months | ||||
| % days cocaine, M (SD) | 37.0 (30.3) | 41.2 (27.7) | 1.02 | .31 |
| % days alcohol, M (SD) | 20.8 (29.4) | 28.9 (30.6) | 1.25 | .27 |
| Current diagnosis before treatment | ||||
| Cocaine dependence, % (n) | 70.0 (53) | 68.9 (51) | 0.00 | .99 |
| Alcohol dependence, % (n) | 26.9 (21) | 32.4 (24) | 1.96 | .38 |
| Current substance use | ||||
| Any cocaine in prior 30 days, % (n) | 61.5 (48) | 59.5 (44) | 0.07 | .79 |
| Any alcohol in prior 30 days, % (n) | 43.6 (34) | 41.9 (31) | 0.05 | .83 |
| ASI drug composite, M (SD) | 0.16 (0.10) | 0.16 (0.10) | 0.00 | .99 |
| ASI alcohol composite, M (SD) | 0.21 (0.23) | 0.21 (0.26) | 0.31 | .58 |
| Other problem severities | ||||
| Living with person using alcohol | ||||
| or other drugs % (n) | 38.5 (30) | 50.0 (37) | 2.05 | .15 |
| ASI composites, M (SD) | ||||
| Medical | 0.42 (0.33) | 0.33 (0.37) | 0.02 | .90 |
| Employment | 0.90 (0.17) | 0.90 (0.16) | 0.07 | .79 |
| Legal | 0.09 (0.18) | 0.11 (0.17) | 0.33 | .57 |
| Family/social | 0.19 (0.24) | 0.17 (0.22) | 0.90 | .34 |
| Psychiatric | 0.23 (0.22) | 0.29 (0.25) | 3.10 | .08 |
Notes: TAU = treatment as usual; ECC = enhanced continuing care; ASI = Addiction Severity Index.
Participation in intensive outpatient program treatment
Data on IOP attendance were available from 120 participants. Participants attended IOP on an average of 18 days (range: 0–38), with no difference between the two continuing care conditions (TAU: M = 18.4, SD = 14.0; ECC: M = 17.8, SD = 12.4), F(1, 118) = .05, p = .82.
Participation in enhanced continuing care
Sixty-nine of 74 participants (93%) completed the orientation session and were eligible to receive ECC. The mean number of ECC sessions received by participants who completed their orientations was 18.0 (SD = 11.7). The mean duration of sessions was 23.8 minutes (SD = 13.4). The majority of sessions were completed in person rather than over the telephone (67%). Participants in ECC who had completed an orientation earned an average of 18.3 vouchers (SD = 11.2).
Adherence to treatment manuals
All sessions were recorded, and 10% were randomly selected and scored for adherence to the manuals. We used a 12-item checklist that had been developed in our prior study (McKay et al., 2010) and was modified to capture the new components of the intervention. Ratings were performed by the clinical supervisor. The items were rated on 3-point scales (i.e., not done, partially done, completely done).
Overall, the ECC treatment was provided in a manner consistent with the protocol. Seven items received a completely done rating in more than 80% of sessions coded. Three additional items were rated completely done in more than 70% of sessions coded. The two items that captured use of resources in the community received lower scores. On the item assessing the degree to which the therapist addressed progress toward the patient’s social network goals, 52.5% of the sessions were scored as completely done, and 37.7 were scored as partially done. On the item assessing specific referrals to community resources or to the in-house case manager, only 10% of the sessions were rated as completely done and another 15% as partially done. This item represented new referrals in response to an identified need rather than follow-up on existing referrals and, therefore, was not expected to be completed in all sessions. The case manager’s contact log indicated that she had at least one contact with 20 of the 69 participants who completed orientation.
Relation of treatment attendance to outcome
Intensive outpatient program.
The number of sessions attended was not related to urine toxicology outcomes (TAU: p = .17; ECC: p = .73), χ2(1) = 1.27, p = .26, but was positively related to good clinical outcome (TAU: p = .01; ECC: p = .005), χ2(1) = 5.15, p = .02.
Continuing care.
The number of ECC sessions attended was negatively related to urine toxicology (p = .005) and positively related to good clinical outcome (p = .01). The odds of having a cocaine-free urine result and a good clinical outcome were 2.35 and 2.15 times higher, respectively, for those who completed at least one continuing care session in a 3-month period compared with those who completed no sessions.
Treatment condition main effects
Cocaine urine toxicology.
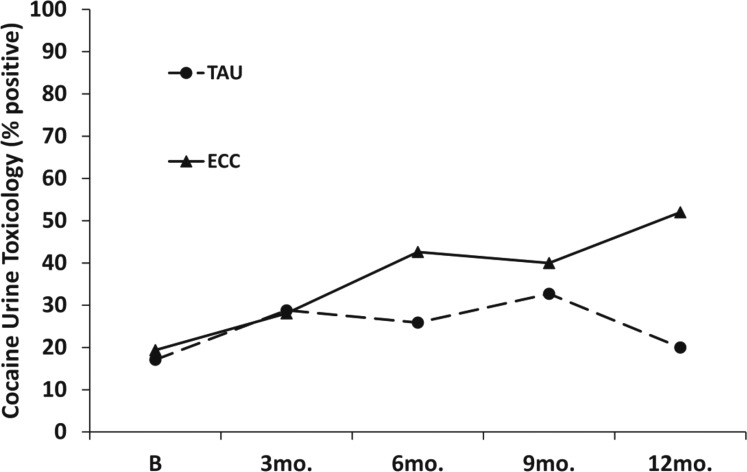
Contrary to what had been hypothesized, rates of cocaine-positive urine samples during follow-up were higher in the ECC than in the TAU participants (Figure 2). This difference increased over the course of the follow-up, generating a significant Treatment Condition x Time interaction, χ2(1) = 9.11, p = .0025. By 12 months, the rate of cocaine-positive urine samples was 32 percentage points higher in the ECC than in the TAU subjects (52% vs. 20%).
Figure 2.
Cocaine urine toxicology outcomes. TAU = intensive outpatient program treatment as usual; ECC = enhanced continuing care; B = baseline; mo. = month. Analyses controlled for days of cocaine use in the 30 days before baseline and baseline cocaine urine toxicology results. The Continuing Care Condition x Time interaction was significant, χ2(1) = 9.1 1, p = .0025.
Good clinical outcomes.
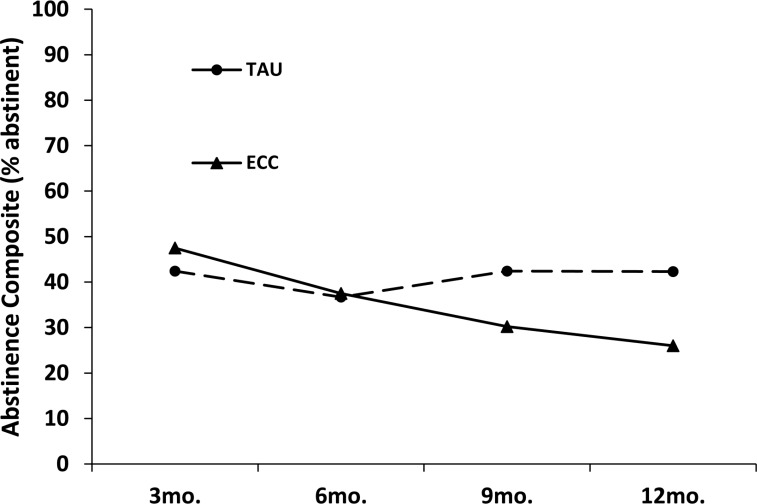
Rates of good clinical outcome were slightly higher in the ECC than in the TAU condition at 3 months (47% vs. 42%), but at 9 and 12 months the TAU was superior to the ECC condition (Figure 3). By 12 months, good clinical outcome rates were 17 percentage points higher in the TAU than in the ECC participants (43% vs. 26%). This pattern of results produced a significant Treatment Condition x Time interaction, χ2(1) = 5.56, p = .018.
Figure 3.
Good clinical outcomes on the abstinence composite measure. TAU = intensive outpatient program treatment as usual; ECC = enhanced continuing care; mo. = month. Analyses controlled for days of cocaine use in the 30 days before baseline. The Continuing Care Condition × Time interaction was significant, χ2(1) = 5.56, p = .018.
Secondary outcomes.
There was a significant Treatment Condition × Time interaction on the percentage of days abstinent, χ2(3) = 8.11, p = .04, in which the ECC group had more days of abstinence than the TAU condition at 6 months, with no difference at other points. An interaction at the level of a trend was obtained on the percentage of days of cocaine use, χ2(3) = 7.43, p = .06, in which the frequency of cocaine use was lower in the ECC than in the TAU participants at 6 months but higher at months 9 and 12. Finally, there were no treatment effects on the percentage of days of heavy drinking (ps > .45).
Moderator analyses
Pre- and early treatment cocaine or alcohol use.
Sixty percent of the sample reported at least 1 day of cocaine use in the 30 days before baseline, and 42% reported at least 1 day of alcohol use. Limiting the analyses to patients who had used any cocaine or alcohol in the 30 days before baseline still produced significant Treatment Condition × Time interactions with the cocaine urine toxicology and abstinence composite outcomes that favored TAU over ECC. We also limited the analyses to those who had used cocaine on at least 3 days in the 30 days before baseline (n = 73, or 48% of the sample), but this still did not reduce the magnitude of the effect favoring TAU over ECC on both outcomes. Given these results, there was no need to perform statistical tests for moderator effects with the full sample.
With regard to substance use early in IOP, 18% of the sample provided a cocaine-positive urine sample at baseline. Seventeen percent of the sample reported cocaine use in the first month of treatment, and 14% reported alcohol use during that period. A total of 44 participants (29%) either had a cocaine-positive urine result at baseline or reported cocaine or alcohol use in the first month of treatment. An examination of outcomes in these 44 participants yielded results very similar to those obtained in the full sample. Therefore, moderator analyses were not performed.
Early dropout from intensive outpatient program.
These analyses were conducted with the 120 participants for whom IOP attendance data were available. The interaction of IOP sessions attended and the continuing care condition was not significant with either outcome: cocaine urine toxicology, χ2(1) = 0.14, p = .71; good clinical outcome, χ2(1) = 0.00, p = .95. Analyses also were done with only those participants who received fewer than 10 IOP sessions (n = 39). An examination of the data indicated that the same general results that had been obtained with the full sample were found in the early dropouts—outcomes were better in TAU than in ECC, although not significantly so with the reduced sample size.
Discussion
The goal of this study was to determine whether providing ECC to all patients, rather than just to those who remained engaged in IOP for 3–4 weeks, would improve substance use outcomes in the treatment of cocaine dependence. Based on prior research (McKay et al., 1999, in press), ECC was expected to be particularly effective for participants who had used cocaine or alcohol in the month before intake or during the first month of IOP. We also predicted that ECC would be more effective for those who dropped out before completing 10 sessions of IOP, because these patients might be most in need of extended disease management approaches (McKay, 2011).
However, the results of the study indicated that adding the ECC intervention to IOP did not improve substance use outcomes over IOP alone. In fact, ECC produced worse scores on both primary outcomes, as indicated by significant Treatment Condition × Time effects, and ECC was not superior to IOP alone on the secondary substance use outcomes. Moreover, there was no evidence of the predicted moderator and subgroup effects favoring ECC over IOP alone.
Given prior findings with TMC (McKay et al., 2010b, in press) and other interventions that provide extended recovery support (Dennis and Scott, 2012; McKay, 2009b; Morgenstern et al., 2006), these negative results are surprising. However, there are several potential explanations for the findings. First, most patients had stopped or greatly reduced their cocaine use in the month before treatment, and less than 30% showed evidence of cocaine use in the first month of IOP. In another recent study with cocaine-dependent patients, telephone continuing care was not effective for participants who were able to achieve abstinence from cocaine immediately before or during the first 3 weeks of IOP. In these participants, IOP-only treatment actually produced better outcomes than IOP plus telephone continuing care, although the differences were not statistically significant (McKay et al., in press).
It is conceivable that, for patients who are responding well to IOP, adding what is essentially a parallel treatment delivered by a different counselor at a different facility interferes in some way with the therapeutic process in the IOP. Providing this separate intervention to patients from the beginning of IOP, rather than only to those who complete 3–4 weeks of IOP (McKay et al., 2010b, in press), may exacerbate this problem. Patients may feel less invested in the IOP, despite efforts to actively support continued IOP participation in the continuing care sessions. On the other hand, the continuing care conditions did not differ on days of IOP attendance, which suggests that ECC did not decrease IOP attendance.
Another possibility is that the combination of the 12-step-oriented IOP and the individual CBT-ECC sessions may have led to some confusion in the patients. In the Cocaine Collaborative Study, the combination of 12-step-oriented group counseling and individual cognitive therapy was not as effective as the condition in which the group and individual sessions were both 12-step oriented (Crits-Christoph et al., 1999). In the TMC/ECC interventions, every effort was made to make the CBT-based intervention compatible with 12-step-oriented treatment. Moreover, confusion resulting from different orientations does not appear to have been an issue in our prior work with telephone-based continuing care interventions, which all yielded positive effects (McKay et al., 2005, 2010b, in press). However, in these studies, the continuing care interventions were offered only to patients who had completed 2–4 weeks of IOP.
The addition of ECC to IOP also may have resulted in overtreatment. Although there has been little prior research on the potential negative implications of excessive treatment, there is some indication that it can lead to worse outcomes. One study found that, among patients with substance use disorders who received residential care when they were appropriate for IOP, rates of no-shows were higher in women, those with supportive family environments, and those with anxiety disorders (Angarita et al., 2007). A recent meta-analysis found that promoting patients’ autonomy was associated with greater intrinsic motivation and autonomous self-regulation and promoted better mental and physical health outcomes (Ng et al., 2012). These studies raise the possibility that providing ECC to patients who had just begun IOP could undermine autonomy and self-efficacy to some degree, thereby interfering with the recovery process.
Incentivizing participation in ECC might again have undermined patient motivation or interfered with other elements of the therapeutic process. In a recent study by Litt et al. (2009), adding incentives for completing between-session tasks to a treatment designed to increase social support produced worse drinking outcomes than the same treatment without incentives and produced lower self-efficacy. In another recently completed study, we found that incentivizing continuing care attendance led to a large increase in the number of sessions attended but not to added effectiveness relative to continuing care without incentives (McKay et al., in press; Van Horn et al., 2011).
The factors discussed here that may have produced worse outcomes in ECC—distraction or confusion stemming from participation in a concurrent intervention from the start of treatment, differences between the orientations of the two interventions, overtreatment, and incentives for attendance— may have been accentuated by the fact that most of the ECC sessions occurred in person rather than over the telephone. In prior research where TMC was used to augment standard care and positive results were obtained (McKay et al., 2010b, in press), more than 65% of the sessions were conducted over the telephone.
Finally, despite the fact that ECC produced worse outcomes than IOP only, the number of sessions attended in ECC was positively related to the two primary outcomes. This finding probably reflects the self-selection bias commonly found in treatment studies, in which more motivated or responsive patients go to more sessions and have better outcomes, regardless of the overall efficacy of the intervention.
Study strengths and limitations
The study had a number of strengths, including a randomized design, inclusion of patients from a “real-world” publicly funded addiction-treatment program, documented adherence to the treatment manual (Carroll et al., 2000), the availability of both self-report and biological outcome data, four outcome assessments over a 12-month period, and a good follow-up rate. At the same time, the study had several limitations. As has been discussed, the continuing care intervention was delivered by counselors who were not located in the IOP and were not involved in the delivery of IOP services, and the theoretical orientations of ECC (i.e., CBT) and the IOP (i.e., 12-step) were not the same. The component of ECC that involved specific referrals to community resources or to the in-house case manager was not implemented as fully as was intended. Finally, the results may not generalize to other treatment populations, and we did not have data on the characteristics of those who declined participation in the study.
Conclusions
Evidence continues to accrue that providing extended recovery support to patients with substance use disorders produces better long-term outcomes, particularly for higher risk patients (Dennis and Scott, 2012; McKay et al., 2011, McKay et al., in press; Morgenstern et al., 2006, 2009). However, the negative findings in this study suggest that more treatment is not always good, and may in fact be counterproductive if not well integrated with the primary treatment program with regard to staffing, location, and theoretical orientation of the interventions.
Acknowledgments
The authors thank Oubah Abdalla, Rachel Chandler, Daniel Herd, Tyrone Thomas, and Sarah Weiss, who contributed to the development of the intervention and delivered it in the study. We also thank Northeast Treatment Centers and the Presbyterian Medical Center, which served as the clinical sites for this study, for collaborating with us on this research and on a number of prior studies.
Footnotes
This research was supported by National Institute on Drug Abuse Grants RC1 DA029062 and K24 DA029062. Additional support was provided by the Medical Research Service and the Substance Use Disorder Quality Enhancement Research Initiative (SUD-QUERI) of the Department of Veterans Affairs.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: Author; 1994. [Google Scholar]
- Angarita GA, Reif S, Pirard S, Lee S, Sharon E, Gastfriend DR. No-show for treatment in substance abuse patients with comorbid symptomatology: Validity results from a controlled trial of the ASAM Patient Placement Criteria. Journal of Addiction Medicine. 2007;1:79–87. doi: 10.1097/ADM.0b013e3180634c1d. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Nich C, Sifry RL, Nuro KF, Frankforter TL, Ball SA, Rounsaville BJ. A general system for evaluating therapist adherence and competence in psychotherapy research in the addictions. Drug and Alcohol Dependence. 2000;57:225–238. doi: 10.1016/s0376-8716(99)00049-6. [DOI] [PubMed] [Google Scholar]
- Crits-Christoph P, Siqueland L, Blaine J, Frank A, Luborsky L, Onken LS, Beck AT. Psychosocial treatments for cocaine dependence: National Institute on Drug Abuse Collaborative Cocaine Treatment Study. Archives of General Psychiatry. 1999;56:493–502. doi: 10.1001/archpsyc.56.6.493. [DOI] [PubMed] [Google Scholar]
- Dennis M, Scott CK. Managing addiction as a chronic condition. Addiction Science & Clinical Practice. 2007;4:45–55. doi: 10.1151/ascp074145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis ML, Scott CK. Four-year outcomes from the early re-intervention (ERI) experiment using Recovery Management Checkups (RMCs) Drug and Alcohol Dependence. 2012;121:10–17. doi: 10.1016/j.drugalcdep.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M, Scott CK, Funk R. An experimental evaluation of recovery management checkups (RMC) for people with chronic substance use disorders. Evaluation and Program Planning. 2003;26:339–352. doi: 10.1016/S0149-7189(03)00037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ. Reliability and validity of 6-month timeline reports of cocaine and heroin use in a methadone population. Journal of Consulting and Clinical Psychology. 1994;62:843–850. doi: 10.1037//0022-006x.62.4.843. [DOI] [PubMed] [Google Scholar]
- Fals-Stewart W, O’Farrell TJ, Freitas TT, McFarlin SK, Ruti-gliano P. The timeline followback reports of psychoactive substance use by drug-abusing patients: Psychometric properties. Journal of Consulting and Clinical Psychology. 2000;68:134–144. doi: 10.1037//0022-006x.68.1.134. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1996. Structured Clinical Interview for DSM-IV Axis I Disorders—patient edition (SCID-I/P, version 2.0) [Google Scholar]
- Humphreys K, Tucker JA. Toward more responsive and effective intervention systems for alcohol-related problems. Addiction. 2002;97:126–132. doi: 10.1046/j.1360-0443.2002.00004.x. [DOI] [PubMed] [Google Scholar]
- Litt MD, Kadden RM, Kabela-Cormier E, Petry NM. Changing network support for drinking: Network support project 2-year follow-up. Journal of Consulting and Clinical Psychology. 2009;77:229–242. doi: 10.1037/a0015252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisto SA, Sobell LC, Sobell MB. Comparison of alcoholics’ self-reports of drinking behavior with reports of collateral informants. Journal of Consulting and Clinical Psychology. 1979;47:106–112. [PubMed] [Google Scholar]
- McKay JR. Continuing care research: What we have learned and where we are going. Journal of Substance Abuse Treatment. 2009a;36:131–145. doi: 10.1016/j.jsat.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay JR. Washington, DC: American Psychological Association; 2009b. Treating substance use disorders with adaptive continuing care. [Google Scholar]
- McKay JR. Continuing care and recovery. In: Kelly J, White W, editors. Addiction recovery management: Theory, research, and practice. New York, NY: Springer-Science; 2011. pp. 163–186. [Google Scholar]
- McKay JR, Alterman AI, Cacciola JS, O’Brien CP, Koppenhaver JM, Shepard DS. Continuing care for cocaine dependence: Comprehensive 2-year outcomes. Journal of Consulting and Clinical Psychology. 1999;67:420–427. doi: 10.1037//0022-006x.67.3.420. [DOI] [PubMed] [Google Scholar]
- McKay JR, Lynch KG, Shepard DS, Pettinati HM. The effectiveness of telephone-based continuing care for alcohol and cocaine dependence: 24-month outcomes. Archives of General Psychiatry. 2005;62:199–207. doi: 10.1001/archpsyc.62.2.199. [DOI] [PubMed] [Google Scholar]
- McKay JR, Van Horn DHA, Morrison R. Center City, MN: Hazelden Foundation Press; 2010a. Telephone continuing care for adults. [Google Scholar]
- McKay JR, Van Horn DHA, Lynch KG, Ivey M, Cary MS, Drapkin M, et al. An adaptive approach for identifying cocaine dependent patients who benefit from extended continuing care. Journal of Consulting and Clinical Psychology. doi: 10.1037/a0034265. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay JR, Van Horn DHA, Oslin D, Ivey M, Drapkin M, Covi-ello D, Yu G, Lynch KG. Extended telephone-based continuing care for alcohol dependence: 24 month outcomes and subgroup analyses. Addiction. 2011;106:1760–1769. doi: 10.1111/j.1360-0443.2011.03483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay JR, Van Horn DHA, Oslin DW, Lynch KG, Ivey M, Ward K, Coviello DM. A randomized trial of extended telephone-based continuing care for alcohol dependence: Within-treatment substance use outcomes. Journal of Consulting and Clinical Psychology. 2010b;78:912–923. doi: 10.1037/a0020700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Cacciola J, Griffith J, Evans F, Barr HL, O’Brien CP. New data from the Addiction Severity Index. Reliability and validity in three centers. Journal of Nervous and Mental Disease. 1985;173:412–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Woody GE, O’Brien CP. An improved diagnostic evaluation instrument for substance abuse patients: The Addiction Severity Index. Journal of Nervous and Mental Disease. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- Miller WR, Weisner C. Integrated care. In: Miller WR, Weisner CM, editors. Changing substance abuse through health and social systems. New York, NY: Kluwer Academic/Plenum; 2002. pp. 243–253. [Google Scholar]
- Morgenstern J, Blanchard KA, McCrady BS, McVeigh KH, Morgan TJ, Pandina RJ. Effectiveness of intensive case management for substance-dependent women receiving temporary assistance for needy families. American Journal of Public Health. 2006;96:2016–2023. doi: 10.2105/AJPH.2005.076380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenstern J, Hogue A, Dauber S, Dasaro C, McKay JR. A practical clinical trial of coordinated care management to treat substance use disorders among public assistance beneficiaries. Journal of Consulting and Clinical Psychology. 2009;77:257–269. doi: 10.1037/a0014489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng JYY, Ntoumanis N, Thøgersen-Ntoumani C, Deci EL, Ryan RM, Duda JL, Williams GC. Self-determination theory applied to health contexts: A meta-analysis. Perspectives on Psychological Science. 2012;7:325–340. doi: 10.1177/1745691612447309. [DOI] [PubMed] [Google Scholar]
- Scott CK, Dennis ML. Results from two randomized clinical trials evaluating the impact of quarterly recovery management checkups with adult chronic substance users. Addiction. 2009;104:959–971. doi: 10.1111/j.1360-0443.2009.02525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Maisto SA, Sobell MB, Cooper AM. Reliability of alcohol abusers’ self-reports of drinking behavior. Behaviour Research and Therapy. 1979;17:157–160. doi: 10.1016/0005-7967(79)90025-1. [DOI] [PubMed] [Google Scholar]
- Stout RL, Beattie MC, Longabaugh R, Noel N. Factors affecting correspondence between patient and significant other reports of drinking [Abstract] Alcoholism: Clinical and Experimental Research. 1989;13:336. [Google Scholar]
- Van Horn DH, Drapkin M, Ivey M, Thomas T, Domis SW, Abdalla O, McKay JR. Voucher incentives increase treatment participation in telephone-based continuing care for cocaine dependence. Drug and Alcohol Dependence. 2011;114:225–228. doi: 10.1016/j.drugalcdep.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]