Abstract
The Behavior Rating Inventory of Executive Function-Adult Version (BRIEF-A) is a questionnaire measure designed to assess executive functioning in everyday life. Analysis of data from the BRIEF-A standardization sample yielded a two-factor solution (labeled Behavioral Regulation and Metacognition). The present investigation employed confirmatory factor analysis (CFA) to evaluate four alternative models of the factor structure of the BRIEF-A self-report form in a sample of 524 healthy young adults. Results indicated that a three-factor model best fits the data: a Metacognition factor, a Behavioral Regulation factor consisting of the Inhibit and Self-Monitor scales, and an Emotional Regulation factor composed of the Emotional Control and Shift scales. The three factors contributed 14%, 19%, and 24% of unique variance to the model, respectively, and a second-order general factor accounted for 41% of variance overall. This three-factor solution is consistent with recent CFAs of the Parent report form of the BRIEF. Furthermore, although the Behavioral Regulation factor score in the two-factor model did not differ between adults with attention-deficit/hyperactivity disorder and a matched healthy comparison group, greater impairment on the Behavioral Regulation factor but not the Emotional Regulation factor was found using the three-factor model. Together, these findings support the multidimensional nature of executive function and the clinical relevance of a three-factor model of the BRIEF-A.
Keywords: Executive function, Factor analysis, Psychometrics, Self-regulation, Neuropsychology, ADHD
Introduction
Executive function is a construct referring broadly to a set of inter-related higher-order cognitive abilities involved in self-regulatory functions that organize, direct, and manage cognitive activities, emotional responses, and overt behaviors (Barkley, 1997, 2011; Gioia, Isquith, & Guy, 2001; Stuss & Alexander, 2000; Stuss & Benson, 1984). The specific processes subsumed under the rubric of executive function remains, however, an active area of scientific inquiry (Jurado & Rosselli, 2007; Stuss & Benson, 1984; Tranel, Anderson, & Benton, 1994). Processes commonly regarded as executive functions include the ability to initiate behaviors; inhibit prepotent responses or competing actions; retain and manipulate information “online” (i.e., working memory); select relevant task goals; plan and organize thoughts and behaviors; think flexibly in order to solve problems or, more generally, to adapt to changes in one's environment; regulate emotions; and monitor and evaluate one's thoughts, emotions, and behaviors. Executive functions, assessed via performance-based and questionnaire measures, have been reported to be associated with multiple aspects of functioning in everyday life in non-clinical and clinical populations such as academic achievement (Weber, Gerber, Turcios, Wagner, & Forbes, 2006), social functioning (Dawson, Shear, & Strakowski, 2012), and behavioral problems (Baird, Silver, & Veague, 2010; Giancola, Godlaski, & Roth, 2012).
Some have argued that executive function is a unitary construct, manifesting in different ways depending on contextual demands (Duncan, Emslie, Williams, Johnson, & Freer, 1996; Garon, Bryson, & Smith, 2008). Several lines of research, however, support the fractionation of executive functions. This has included evidence of discrepant developmental trajectories for different executive functions (Anderson, 2002; Welsh, Pennington, & Grossier, 1991); specific rather than generalized deficits on executive function measures in clinical populations (Godefroy, Cabaret, Petit-Chenal, Pruvo, & Rousseaux, 1999; Mur, Portella, Martinez-Aran, Pifarre, & Vieta, 2007; Nigg et al., 2005); evidence of at least partly distinct neural circuitry subserving different executive functions (Roth, Randolph, Koven, & Isquith, 2006); and typically small to moderate correlations between performance-based tests designed to assess executive functions (Miyake et al., 2000; Nigg et al., 2005).
Factor analytic studies of performance-based tests have also supported a fractionation of executive functions, generally identifying more than one factor or component explaining variability (Busch, McBride, Curtiss, & Vanderploeg, 2005; Klenberg, Korkman, & Lahti-Nuuttila, 2001; Latzman & Markon, 2010). Similar findings have been reported in studies of the Behavior Rating Inventory of Executive Function (BRIEF), a questionnaire measure designed to capture multiple aspects of executive function as manifested in everyday life in children and adolescents (Gioia, Isquith, Guy, & Kenworthy, 2000). Exploratory factor analysis of the eight scales of the parent and teacher forms of the BRIEF revealed a two-factor solution in both typically developing children and a mixed clinical sample (Gioia et al., 2000). The Behavioral Regulation Index or factor reflects the ability to shift cognitive set, modulate emotions and behavior, and exert appropriate inhibitory control. The Metacognition Index or factor measures working memory, the ability to initiate, plan, and organize problem-solving, as well as self-monitoring of behavior.
Gioia, Isquith, Retzlaff, and Espy (2002) subsequently conducted a confirmatory factor analysis (CFA) of the BRIEF parent report form in a mixed clinical sample, using a nine scale version that separated the Monitor scale into a Task-Monitor scale reflecting the monitoring of task-related activities, and a Self-Monitor scale reflecting monitoring of the effects of one’s behavior on others (Gioia & Isquith, 2002). A three-factor solution best fits the data, as opposed to one-, two-, or four-factor models. Although the Metacognition Index remained unchanged, the Behavioral Regulation Index broke down into a Behavioral Regulation factor consisting of the Inhibit and Self-Monitor scales, and an Emotional Regulation factor composed of the Emotional Control and Shift scales. A two-factor rather than one- or three-factor solution for the parent report form was found to be most appropriate in a sample of children and adolescents with intractable epilepsy (Slick, Lautzenhiser, Sherman, & Eyrl, 2006), although this study used the eight- rather than nine-scale model of the BRIEF (i.e., a single Monitor scale). The latter study did, however, find that the Monitor scale loaded equivalently on both the Metacognition Index and Behavioral Regulation Index, supporting the suggestion that this scale does not reflect a unitary construct (Gioia & Isquith, 2002). In a sample of children with traumatic brain injury, a two- rather than one-factor structure involving the original eight scales was reported for the BRIEF parent form, as well as finding that the Inhibit scale loaded more strongly on the Metacognition factor than the expected Behavioral Regulation factor (Donders, DenBraber, & Vos, 2010). The potential of a better fit for a three-factor solution was not examined. Finally, the recent CFAs of the BRIEF parent and teacher forms in a mixed healthy and clinical sample supported a nine-scale, three-factor model of the BRIEF including separate Behavioral Regulation and Emotional Regulation factors (Egeland & Fallmyr, 2010).
The BRIEF-Adult version (BRIEF-A) was developed as an extension of the original BRIEF (Gioia et al., 2000) to adults aged 18–90 and has both self- and informant-report forms (Roth, Isquith, & Gioia, 2005). The BRIEF-A has the same nine scales as found in Gioia and Isquith (2002), having Self-Monitor and Task-Monitor scales rather than a single Monitor scale (Table 1). Exploratory factor analysis of the BRIEF-A conducted separately for the two forms, using the normative sample as well as a mixed clinical and healthy adult sample, yielded a two-factor solution (Metacognition factor and Behavioral Regulation factor) consistent with the original version of the measure. This factor structure was invariant across genders and present irrespective of whether younger or older adults were considered.
Table 1.
Description of the nine BRIEF-A scales
| Inhibit | Control impulses; appropriately stop verbal, attentional, physical behavior at the proper time |
| Shift | Move freely from one situation, activity, or aspect of a problem to another as the situation demands; think flexibly to aid problem-solving |
| Emotional control | Modulate one's emotional responses appropriately |
| Self-Monitor | Recognize the effect of one's own behavior on others |
| Initiate | Begin a task or activity without external prompting; independently generate ideas |
| Working memory | Hold information in mind in order to complete a task; stay with, or stick to, an activity |
| Plan/organize | Anticipate future events; set goals; develop steps ahead of time to carry out a task; organize information and behavior to achieve and objective; carry out tasks in a systematic manner |
| Task Monitor | Assess performance during or after finishing a task for mistakes |
| Organization of Materials | Keep workspace and living areas in an orderly manner; keep track of materials needed for tasks |
In the present study, we conducted a CFA of the BRIEF-A self-report form in a large sample of healthy young adults. We compared one-, two-, three-, and four-factor models. A single-factor model was considered in line with the view of executive function as a unitary construct. A two-factor model consistent with the prior exploratory factor analysis of the BRIEF-A was also examined (Roth et al., 2005). We then evaluated whether the BRIEF-A may be better characterized along the line of findings for the original BRIEF, indicating the superiority of a three-factor model that separated the Behavioral Regulation Index into Behavioral Regulation and Emotional Regulation factors (Egeland & Fallmyr, 2010; Gioia et al., 2002). We also considered a four-factor model involving the Behavioral Regulation and Emotion Regulation factors, as well as separating the Metacognition Index into “Internal” and “External” Metacognition factors (Gioia et al., 2002). These latter two factors were conceptualized as reflecting a focus on high-order executive processes such as working memory, initiation, and the ability to plan and organize for problem-solving (Internal) versus attending to one's behavior and environment as reflected by monitoring one's performance on tasks for accuracy and organization of one's work and living space in an orderly manner (External).
Finally, a recent study reported poorer functioning on both the Behavioral Regulation Index and Metacognition Index in adults with attention-deficit/hyperactivity disorder (ADHD) relative to a healthy comparison group, though the difference was larger for the latter index (Rotenberg-Shpigelman, Rapaport, Stern, & Hartmen-Maeir, 2008). We therefore explored in a sample of adults with ADHD whether an alternate model for the BRIEF-A, yielded through the factor analysis indicated above, would provide clinically relevant information beyond that gained from the original two-factor model.
Method
Participants
Participants were 524 (255 men and 269 women) adults between 21 and 35 years of age (M = 23.07; SD = 2.91), recruited as part of larger study on behavior and alcohol use in young adults. They were recruited through advertisements placed in various newspapers and fliers posted around the Lexington, Kentucky metropolitan area. Respondents were initially screened by telephone for inclusion and exclusion criteria. Specific inclusion criteria included being within the age range noted above and ability to read English at least at the fourth grade level. Volunteers were excluded from participation if they reported during an screening interview any past or present drug- or alcohol-related problems, head injury with loss of consciousness and/or requiring medical attention, learning disability, history of severe mental illness (e.g., schizophrenia-spectrum disorder, bipolar disorder), or current or past treatment for a psychiatric disorder. Participants were 87% Caucasian, 10% African American, 1.0% Hispanic, and Other 2.0% (essentially evenly split between men and women). Ninety-two percent of the participants were never married and the sample had an average of 16.2 years of education (SD = 1.98). Written informed consent was obtained according to a protocol approved by the University of Kentucky's Institutional Review Board.
A sample of 19 adults meeting DSM-IV criteria for ADHD (American Psychiatric Association, 2000), referred to our clinics for neuropsychological assessment including the BRIEF-A, was examined to assess the clinical relevance of the factor structures. Within this sample 57.8% met criteria for the inattentive subtype and 42.2% for the combined subtype, and six were receiving medication for ADHD at the time of evaluation (three methylphenidate, two methylphenidate extended-release, and one dextroamphetamine). Eight patients had a history of mood disorder, three generalized anxiety disorder, and one alcohol use disorder. Patients were excluded if they had a history of schizophrenia-spectrum disorder, bipolar disorder, current alcohol or substance use disorder, a neurological disorder, or active medical illness that could affect the central nervous system. Patients were compared with a sample of healthy adults selected pseudorandomly (i.e., matched for age and gender by one of the authors, ASF, without reference to their BRIEF-A scores) from a research database at the Geisel School of Medicine at Dartmouth, ensuring that the data were independent from that used in the factor analysis. All of these participants were between 18 and 35 years of age (M = 25.21; SD = 5.65 for both groups) and Caucasian, and the groups did not differ with respect to gender distribution (% female: ADHD = 36.8, Healthy = 47.4). χ2(1) = 0.42, p = .51. Beck Depression Inventory-II (BDI-II; Beck, Steer, & Brown, 1996) score was available for 12 of the patients (M = 19.17, SD = 9.42) but none of the matched controls.
Procedures
Participants at the University of Kentucky provided demographic data and then completed the BRIEF-A self-report form and the BDI-II (Beck et al., 1996) in addition to a number of other self-report inventories not pertinent to this paper. Participants in the ADHD and the matched healthy group completed the BRIEF-A. The BRIEF-A contains 75 items scored on a three-point Likert scale with higher scores indicating poorer executive function. A minimum fourth grade reading level is required. The BRIEF-A yields an overall score (Global Executive Composite) composed of two index scores, the Behavioral Regulation Index and the Metacognition Index. The Behavioral Regulation index is comprised of four scales (Inhibit, Shift, Emotional Control, and Self-Monitor) and the Metacognition Index is comprised of five scales (Initiate, Working Memory, Plan/Organize, Task Monitor, and Organization of Materials). None of the participants had elevated scores on the three validity scales included in the BRIEF-A (Negativity, Infrequency, and Inconsistency). The BRIEF-A was standardized on 1050 adults between the ages of 18 and 90 sampled to approximate the 2002 U.S. Census proportions with respect to sociodemographic characteristics. The measure has excellent internal consistency (Cronbach α coefficients ranging from 0.93 to 0.96 for the three major indices) and 1-month test–retest reliabilities (ranging from r = .93 to .94 for the three major indices; Roth et al., 2005). There is support for the convergent and discriminant validity of the BRIEF-A (Roth et al., 2005) and its utility has been demonstrated in studies of clinical (Biederman et al., 2011; Chang, Davies, & Gavin, 2009; Garlinghouse, Roth, Isquith, Flashman, & Saykin, 2010; Kumbhani, Roth, Kuck, Flashman, & McAllister, 2010; Rabin et al., 2006) and non-clinical (Christ, Kanne, & Reiersen, 2010; Koven & Thomas, 2010; Rabin, Fogel, & Nutter-Upham, 2010) populations.
Statistical Analyses
The CFA used the mean raw scores for the nine BRIEF-A scales. We tested the four different CFA models examined by Gioia and colleagues (2002) using LISREL 8.8 (Jöreskog & Sörbom, 2004). Model 1 tested whether all nine subscales loaded onto one factor. Model 2 assessed whether Inhibit, Self-Monitor, Emotional Control, and Shift loaded onto a Behavioral Regulation factor and Initiate, Working Memory, Plan/Organize, Organization of Materials, and Task Monitor loaded onto a Metacognition factor. Model 3 extended Model 2 to determine whether the Metacognition Index remained the same but with Inhibit and Self-Monitor loading onto a Behavioral Regulation factor and Emotional Control and Shift scales loading onto an Emotional Regulation factor. Finally, model 4 extended model 3 to examine whether the Behavioral and Emotional Regulation factors would be present 3, as well as whether Initiate, Working Memory, and Plan/Organize would load onto an Internal Metacognition factor and Organization of Materials and Task Monitor would load onto an External Metacognition factor.
Model parameters were estimated using maximum likelihood in LISREL. In addition to the overall χ2 statistic, several overall goodness-of-fit indices were employed to examine the fit of the four different factor models (Marsh, Balla, & McDonald, 1988) with the following “rule-of-thumb” cutoff criteria for well-fitting models: Standardized root mean squared residual (SRMSR) ≤0.08, root mean squared error of approximation (RMSEA) ≤0.06, Tucker–Lewis Index (TLI), and Comparative fit index (CFI) ≥0.95 (see Hu & Benter, 1998, 1999).
Results
Confirmatory Factor Analysis
The mean BDI-II score of the sample was in the “minimal” range (Mean = 6.49, SD = 5.99). The average BRIEF-A scores were all relatively low (Table 2), consistent with those reported for other samples of healthy adults (Kumbhani et al., 2010; Rabin et al., 2010; Roth et al., 2005). The nine BRIEF-A scales were moderately to highly correlated with one another, with coefficients ranging from 0.21 to 0.72 (Table 2).
Table 2.
Descriptive statistics and inter-correlations among the nine BRIEF-A scales
| Mean | SD | Shift | EC | SM | Initiate | WM | P/O | TM | OM | |
|---|---|---|---|---|---|---|---|---|---|---|
| Inhibit | 1.62 | 0.36 | .35 | .40 | .58 | .44 | .64 | .50 | .55 | .37 |
| Shift | 1.41 | 0.34 | .51 | .42 | .50 | .51 | .50 | .49 | .24 | |
| EC | 1.42 | 0.40 | .44 | .35 | .43 | .36 | .35 | .26 | ||
| SM | 1.47 | 0.37 | .38 | .46 | .50 | .51 | .21 | |||
| Initiate | 1.51 | 0.35 | .61 | .70 | .66 | .47 | ||||
| WM | 1.48 | 0.35 | .67 | .67 | .41 | |||||
| P/O | 1.45 | 0.34 | .72 | .57 | ||||||
| TM | 1.54 | 0.36 | .49 | |||||||
| OM | 1.60 | 0.50 |
Notes: Mean scores range from 1–3, with higher scores reflecting worse executive function; EC = Emotional Control; SM = Self-Monitor; WM = Working Memory; P/O = Plan/Organize; TM = Task Monitor; OM = Organization of Materials.
The CFA indicated that all models tested were rejected statistically on the basis of the χ2 statistic and none of the models met the stringent cutoff criterion for RMSEA (Table 3). Note that adjacent models in Table 3 are nested so that they can be compared with one another on the basis of the Δχ2 for their relative fit to the data. For example, the two-factor model is nested within (a special case of) the three-factor model and can be generated from the three-factor model by fixing the correlation between the Behavioral Regulation and Emotional Regulation factors to 1.00 and constraining the correlations between these two factors and the Metacognition factor to be equal. The models that we tested were not all that different from one another in some respects. They all estimated the same number of factor loadings (nine) and uniquenesses (nine). They differed only with respect to the number of factors and correlations among the factors. This is why the global fit indices (i.e., SRMSR, RMSEA, TLI, and CFI) did not differ drastically from model to model. As such, the only remaining means to determine which model best represented the data was to examine the solutions for out-of-bounds parameter estimates and differences in model goodness-of-fit between adjacent, nested models in terms of the Δχ2 test. The Δχ2 test confirmed that the BRIEF-A is not unidimensional (i.e., the two-factor model fit significantly better than did the one-factor model), that the three-factor model improved fit beyond the two-factor model (Table 3), and that adding a fourth factor did not improve the model fit, resulting in an inadmissible solution—the correlation between the Internal and External Metacognition factors was estimated to be 1.02. Thus, the three-factor solution appeared to best represent the data.
Table 3.
Model goodness of fit
| Model | df | χ2 | SRMSR | RMSEA (90% CI) | TLI | CFI |
|---|---|---|---|---|---|---|
| 1. One factor | 27 | 316.09* | 0.065 | 0.140 (0.13–0.16) | 0.92 | 0.94 |
| Models 1 versus 2 | 1 | 71.47* | ||||
| 2. Two factor | 26 | 242.62* | 0.055 | 0.120 (0.10–0.13) | 0.93 | 0.95 |
| Models 2 versuss 3 | 2 | 46.41* | ||||
| 3. Three factor | 24 | 196.21* | 0.047 | 0.110 (0.10–0.13) | 0.94 | 0.96 |
| Models 3 versus 4 | 3 | 8.34 | ||||
| 4. Four factor | 21 | 187.87* | 0.046 | 0.120 (0.10–0.13) | 0.94 | 0.96 |
Notes: df = model degrees of freedom; SRMSR = standardized root mean squared residual; RMSEA = root mean squared error of approximation; 90% CI = 90% confidence interval for RMSEA; TLI = Tucker–Lewis Index, CFI = comparative fit index.
*p <.01.
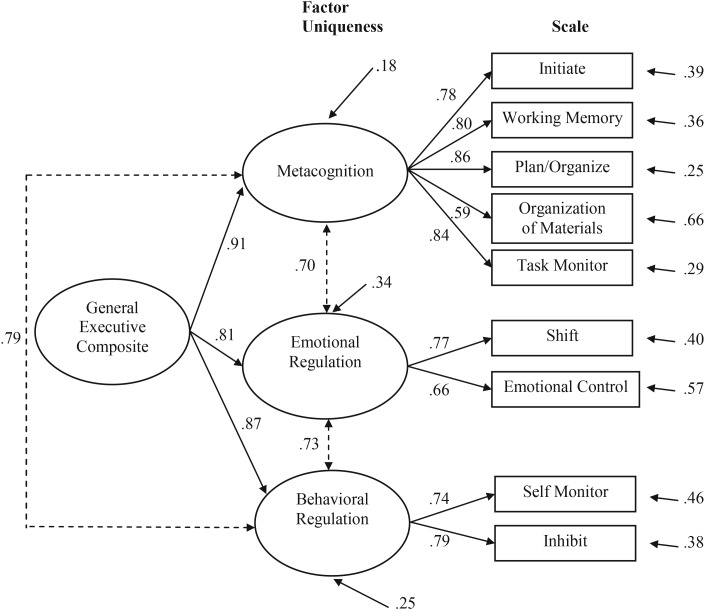
Figure 1 displays the parameter estimates for the three-factor model. All factor loadings were relatively high and statistically significant with the Metacognition, Emotional Regulation, and Behavioral Regulation factors accounting for 61%, 52%, and 58% of the variance in their respective scales, respectively. The three factors contributed 14%, 19%, and 24% of unique variance to the model, respectively, and a second-order general factor accounted for 41% of variance overall. The factors were also highly inter-related, with correlations ranging from .70 to .79. This suggested that the three first-order factors (FOFs) could also be summarized in terms of their loadings on a single general second-order factor (SOF), labeled the General Executive Composite, as is shown in Fig. 1. Note that the FOF and the SOF model are not differentiable statistically from one another, but are merely alternative plausible representations of the same latent correlational structure.
Fig. 1.
Single general SOF.
Factor Scores in Adult ADHD
Table 4 presents BRIEF-A mean factor scores for the ADHD and the healthy comparison group. Since the factor analysis indicated that the three-factor solution is preferable to the other alternate models tested here, we restricted group comparisons to scores for the two- and three-factor models. Independent sample t-test indicated significantly worse functioning on the Metacognition factor in the ADHD group irrespective of model—t(1) = 4.39, p = .001, d = 1.42. In the two-factor model, the group difference for the Behavioral Regulation factor was only at the level of a trend—t(1) = 1.83, p = .08, d = 0.59. In contrast, analysis of the three-factor model revealed poorer executive functioning in the ADHD group as reflect by the Behavioral Regulation factor—t(1) = 2.18, p = .04, d = 0.71—but not the Emotional Regulation factor—t(1) = 1.19, p = .24, d = 0.39. In the subset of patients with BDI-II scores, greater depressed mood was associated with significantly worse executive function on the Metacognition (r = .76, p = .004) and Emotional Regulation (r = .68, p = .02) factors, a trend for greater difficulty on the Behavioral Regulation factor in the two-factor model (r = .55, p = .07), but was unrelated to score on the Behavioral Regulation factor in the three-factor model (r = .30, p = .34).
Table 4.
Mean (SD) for BRIEF-A factor scores healthy adult and ADHD groups
| BRIEF-A | Healthy adult (N = 19) | ADHD (N = 19) |
|---|---|---|
| Two-factor structure | ||
| Metacognition factor | 1.59 (0.42) | 2.13 (0.33) |
| Behavioral regulation factor | 1.57 (0.37) | 1.80 (0.40) |
| Three-factor structure | ||
| Metacognition factor | 1.59 (0.42) | 2.13 (0.33) |
| Behavioral Regulation factor | 1.54 (0.35) | 1.84 (0.50) |
| Emotional Regulation factor | 1.59 (0.44) | 1.75 (0.40) |
Notes: BRIEF-A = Behavior Rating Inventory of Executive Function-Adult Version; ADHD = Attention-Deficit/Hyperactivity Disorder.
Discussion
The present study investigated the factor structure of the BRIEF-A in order to determine which of four competing models best fit the data obtained from a large sample of young adults. Results showed that a three-factor model fits the data better than either the one-, two-, or four-factor models tested. The structure of the Metacognition factor was found to be the same as that obtained in an exploratory factor analysis of the BRIEF-A, being composed of the Initiate, Working Memory, Plan/Organize, Task Monitor, and Organization of Materials scales (Roth et al., 2005). In contrast, the originally unitary Behavioral Regulation Index separated into a Behavioral Regulation factor consisting of the Inhibit and Self-Monitor scales and an Emotional Regulation factor composed of the Emotional Control and Shift scales. This separation of the Behavioral Regulation Index into two factors, and the scale composition of those factors, is consistent with the results of CFAs of the nine-scale version of the original BRIEF that was designed for use with children and adolescents (Egeland & Fallmyr, 2010; Gioia & Isquith, 2002).
Correlations among the three BRIEF-A factors were significant, however, indicating considerable interactions among components of executive function. Nonetheless, the results of the present analyses, as well as those of the BRIEF designed for children (Egeland & Fallmyr, 2010; Gioia & Isquith, 2002) and other questionnaire measures of executive function (Chaytor & Schmitter-Edgecombe, 2007), are not consistent with a model emphasizing a unitary, general executive construct. Rather, the observation of separate factors of metacognition, behavioral regulation (including inhibitory control), and emotional regulation meshes well with models of executive function emphasizing fractionation of executive function into at least partially distinct components each have important roles in self-regulation (Barkley, 1997; Miyake et al., 2000).
The presence of separate Behavioral Regulation and Emotional Regulation factors in the BRIEF-A is consistent with a growing body of evidence, indicating at least partly distinct neural substrates for these two forms of self-regulation (Kompus, Hugdahl, Ohman, Marklund, & Nyberg, 2009; Mohanty et al., 2007). In addition, the two factors appear to overlap conceptually, to some extent, with theoretical models of executive function that argue for a unique role of emotion regulation, such as that of Zelazo & Müller (2002) proposing the presence “cool” cognitive and “hot” affective aspects of executive control. Thus, further research is warranted to assess the reliability and validity of the Behavioral Regulation and Emotional Regulation factors.
The ability to monitor one's own behavior is vital for problem-solving, accurate completion of tasks (e.g., homework, job-related tasks), and interacting in a socially appropriate manner. In this study, the BRIEF-A Task-Monitor and Self-Monitor scales loaded differentially on the Metacognition and Behavioral Regulation factors, a finding that was also obtained in prior studies using the BRIEF to examine parent and teacher reports of executive functioning in children (Egeland & Fallmyr, 2010; Gioia et al., 2000), as well as an exploratory factor analysis of the BRIEF-A (Roth et al., 2005). These findings indicate that monitoring is not a unitary construct, but rather varies depending on the nature of the information being monitored and the context in which monitoring tasks place. Our findings also appear to be consistent with functional neuroimaging research and studies of patients with acquired brain lesions, which together suggest at least a partial dissociation between the neural correlates of different types of monitoring, though they involve richly interconnected prefrontal regions. Accurate monitoring of performance on behavioral tasks, reflected in the BRIEF-A Task-Monitor scale, has been most consistently associated with the anterior cingulate gyrus (Swick & Turken, 2002; van Veen & Carter, 2002). In contrast, the ability to judge the appropriateness of one’s behavior in social contexts, reflected in the BRIEF-A Self-Monitor scale, is more commonly associated with integrity of ventral prefrontal cortical regions such as the orbitofrontal cortex (Beer, John, Scabini, & Knight, 2006; Viskontas, Possin, & Miller, 2007, but see also Turken & Swick, 2008). Recent research demonstrating a relationship between an event-related potential correlate of the awareness of having committed an error during a behavioral task and the Task Monitor, but not Self-Monitor scale in adults with ADHD (Chang et al., 2009), provides additional support for the distinctiveness of the two monitoring scales. Further research examining the BRIEF-A Task-Monitor and Self-Monitor scales in clinical populations, including those with lesions in different regions within the prefrontal cortex, will be important to determine the consistency with which these two scales can be dissociated, as well as their clinical relevance.
In addition to the CFAs in healthy young adults, we examined BRIEF-A scores based on the two- and three-factor models in a sample of young adults with ADHD. Our patient group reported greater difficulty on the Metacognition factor than a matched group of healthy adults, consistent with a prior study on adult ADHD (Rotenberg-Shpigelma et al., 2008). The Behavioral Regulation factor showed only a trend toward being worse in the ADHD group when examined in the two-factor model. In contrast, analysis of the three-factor model revealed that adult ADHD is associated with poorer scores on the Behavioral Regulation factor (reflecting inhibitory control and monitoring of social behavior) but not Emotional Regulation factor (reflecting control of emotions and cognitive flexibility). Furthermore, a differential pattern of correlations was observed between self-reported depression and the BRIEF-A factor scores. Mood was unrelated to Behavioral Regulation in the three-factor model but showed a trend to be associated with Behavioral Regulation in the two-factor model and was highly correlated with the Emotional Regulation factor. These findings support the clinical relevance of the three-factor model and indicate that the current BRIEF-A Behavioral Regulation Index score, although of demonstrated usefulness in a variety of clinical studies (Garcia-Molina, Tormos, Bernabeu, Junque, & Roig-Rovira, 2012; Reid, Karim, McCrory, & Carpenter, 2010; Rotenberg-Shpigelma et al., 2008), may mask meaningful problems with more specific aspects of executive functioning.
Together, the findings suggest that reliance on use of the Global Executive Composite score, representing the overall integrity of executive functions as measured by the BRIEF-A, might obscure more specific relationships between executive functioning in everyday life (as reflected by index/factor scores or individual scale scores) and clinical problems, neuropsychological test performance, or other salient variables. Indeed, previous studies have demonstrated the importance of examining the BRIEF at the level of the indexes/factor scores or at the level of individuals scales in a variety of pediatric (Brown et al., 2008; Mahone et al., 2002) and adult (Christ et al., 2010; Kumbhani et al., 2010; Schroeder & Kelley, 2008) populations.
The present findings should be interpreted within the context of the limitations of this study. First, the factor analysis sample consisted of generally well educated young adults and thus it remains unknown whether the three-factor model would also provide the best fit in other populations. Although exploratory factor analysis of the BRIEF-A indicated that older and younger adults did not differ with respect to the two-factor structure reported (Roth et al., 2005), it is possible that age differences could be observed in the three-factor model. Furthermore, while at most small effects on BRIEF-A scores have been reported in relation to gender, race/ethnicity, and educational level (effect sizes being generally 0.01 or less; Roth et al., 2005), it remains an empirical question whether such variables impact the factor structure of the measure. Similarly, it will be important to determine whether the factor structure is invariant in clinical populations, especially given that greater variability in scores may be seen in clinical than healthy samples, thus potentially affecting factor loadings. In addition, although exploratory factor analyses indicated consistent factor structures for the BRIEF-A Self- and Informant-Report forms, the present results are based on factor analysis of only the Self-Report form. Thus, although the Self- and Informant-Report forms are essentially identical, it remains unknown whether the three-factor model is generalizable to informant report form of the measure. In addition, we discovered in post hoc analyses that BRIEF-A scores were positively skewed and some were also kurtotic. Asymptotic distribution-free estimators are now available for non-normal data (Browne, 1984; Jöreskog & Sörbom, 2004) but they require much larger sample sizes (in the 1,000 s; see Cortina, Chen & Dunlap, 2001; Hu, Bentler, & Kano, 1992) than was available here. Rather, we used normal theory maximum likelihood estimators that have been shown to be robust to at least modest violations of the multivariate normality assumption (Chou, Bentler, & Satorra, 1991; Cortina et al., 2001). Still, this violation may be responsible for some of the models' imperfect fit to the data (e.g., RMSEAs > 0.08). Our sample of adults with ADHD was relatively small and represented a clinical convenience sample rather than a carefully selected, comorbidity-free research sample. Furthermore, a subset of the patients were medicated for the disorder, although prior work (Roth et al., 2005) has indicated that treatment with methylphenidate improves scores on the BRIEF-A in adults with ADHD, and thus medication status is unlikely to account for the differences observed between the patient and matched control groups. Nonetheless, further investigation into the clinical sensitivity of the three-factor model of the BRIEF-A in this population is needed.
Overall, with these caveats in mind, this study supports a multidimensional model of executive function, consistent with the design of the BRIEF-A and its forerunner the BRIEF. It should be noted, however, that the clinical use of the three-factor model is limited at this time by the lack of appropriate normative data. Furthermore, as noted above, additional research is required to ensure the reliability and the utility of the three-factor model prior to its application in clinical contexts. Nonetheless, the present findings provide a promising avenue for examining components of executive function as manifested in everyday life.
Conflict of Interest
PKI and RMR are co-authors of the BRIEF-A and receive royalties from the publisher.
Funding
This work was supported by grants from the National Institute on Alcohol Abuse and Alcoholism (R01-AA-11691 to PRG) and the National Center for Research Resources to PRG.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: Author; 2000. revised, [Google Scholar]
- Anderson P. Assessment and development of executive function (EF) during childhood. Child Neuropsychology. 2002;8(2):71–82. doi: 10.1076/chin.8.2.71.8724. [DOI] [PubMed] [Google Scholar]
- Baird A. A., Silver S. H., Veague H. B. Cognitive control reduces sensitivity to relational aggression among adolescent girls. Social Neuroscience. 2010;5(5–6):519–532. doi: 10.1080/17470911003747386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley R. A. ADHD and the nature of self-control. New York: The Guilford Press; 1997. [Google Scholar]
- Barkley R. A. Executive functioning in everyday life. New York: The Guilford Press; 2011. [Google Scholar]
- Beck A. T., Steer R. A., Brown G. K. Beck Depression Inventory-II (BDI-II) San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- Beer J. S., John O. P., Scabini D., Knight R. T. Orbitofrontal cortex and social behavior: Integrating self-monitoring and emotion–cognition interactions. Journal of Cognitive Neuroscience. 2006;18(6):871–879. doi: 10.1162/jocn.2006.18.6.871. [DOI] [PubMed] [Google Scholar]
- Biederman J., Mick E., Fried R., Wilner N., Spencer T. J., Faraone S. V. Are stimulants effective in the treatment of executive function deficits? Results from a randomized double blind study of OROS-methylphenidate in adults with ADHD. European Neuropsychopharmacology. 2011;21(7):508–515. doi: 10.1016/j.euroneuro.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Brown T. M., Ris M. D., Beebe D., Ammerman R. T., Oppenheimer S. G., Yeates K. O., et al. Factors of biological risk and reserve associated with executive behaviors in children and adolescents with spina bifida myelomeningocele. Child Neuropsychology. 2008;14(2):118–134. doi: 10.1080/09297040601147605. [DOI] [PubMed] [Google Scholar]
- Browne M. W. Asymptotically distribution-free methods for the analysis of covariance structures. British Journal of Mathematical and Statistical Psychology. 1984;37:62–83. doi: 10.1111/j.2044-8317.1984.tb00789.x. [DOI] [PubMed] [Google Scholar]
- Busch R. M., McBride A., Curtiss G., Vanderploeg R. D. The components of executive functioning in traumatic brain injury. Journal of Clinical and Experimental Neuropsychology. 2005;27(8):1022–1032. doi: 10.1080/13803390490919263. [DOI] [PubMed] [Google Scholar]
- Chang W.-P., Davies P. L., Gavin W. J. Error monitoring in college students with attention-deficit/hyperactivity disorder. Journal of Psychophysiology. 2009;23:113–125. [Google Scholar]
- Chaytor N., Schmitter-Edgecombe M. Fractionation of the dysexecutive syndrome in a heterogeneous neurological sample: Comparing the Dysexecutive Questionnaire and the Brock Adaptive Functioning Questionnaire. Brain Injury. 2007;21(6):615–621. doi: 10.1080/02699050701426949. [DOI] [PubMed] [Google Scholar]
- Chou C. P., Bentler P., Satorra A. Scaled test statistics and robust standard errors for non-normal data in covariance structure analysis: A Monte Carlo study. British Journal of Mathematical and Statistical Psychology. 1991;44:347–357. doi: 10.1111/j.2044-8317.1991.tb00966.x. [DOI] [PubMed] [Google Scholar]
- Christ S. E., Kanne S. M., Reiersen A. M. Executive function in individuals with subthreshold autism traits. Neuropsychology. 2010;24(5):590–598. doi: 10.1037/a0019176. [DOI] [PubMed] [Google Scholar]
- Cortina J. M., Chen G., Dunlap W. P. Testing interaction effects in LISREL: Examination and illustration of available procedures. Organizational Research Methods. 2001;4:324–360. [Google Scholar]
- Dawson E. L., Shear P. K., Strakowski S. M. Behavior regulation and mood predict social functioning among healthy young adults. Journal of Clinical and Experimental Neuropsychology. 2012;34(3):297–305. doi: 10.1080/13803395.2011.639297. [DOI] [PubMed] [Google Scholar]
- Donders J., DenBraber D., Vos L. Construct and criterion validity of the Behaviour Rating Inventory of Executive Function (BRIEF) in children referred for neuropsychological assessment after paediatric traumatic brain injury. Journal of Neuropsychology. 2010;4:197–209. doi: 10.1348/174866409X478970. [DOI] [PubMed] [Google Scholar]
- Duncan J., Emslie H., Williams P., Johnson R., Freer C. Intelligence and the frontal lobes: The organization of goal directed behavior. Cognitive Psychology. 1996;30:257–303. doi: 10.1006/cogp.1996.0008. [DOI] [PubMed] [Google Scholar]
- Egeland J., Fallmyr Ø. Confirmatory factor analysis of BRIEF: Support for a distinction between emotional and behavioural regulation. Child Neuropsychology. 2010;16:326–337. doi: 10.1080/09297041003601462. [DOI] [PubMed] [Google Scholar]
- Garcia-Molina A., Tormos J. M., Bernabeu M., Junque C., Roig-Rovira T. Do traditional executive measures tell us anything about daily-life functioning after traumatic brain injury in Spanish-speaking individuals? Brain Injury. 2012;26(6):864–874. doi: 10.3109/02699052.2012.655362. [DOI] [PubMed] [Google Scholar]
- Garlinghouse M. A., Roth R. M., Isquith P. K., Flashman L. A., Saykin A. J. Subjective rating of working memory is associated with frontal lobe volume in schizophrenia. Schizophrenia Research. 2010;120:71–75. doi: 10.1016/j.schres.2010.02.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garon N., Bryson S. E., Smith I. M. Executive function in preschoolers: A review using an integrative framework. Psychological Bulletin. 2008;134(1):31–60. doi: 10.1037/0033-2909.134.1.31. [DOI] [PubMed] [Google Scholar]
- Giancola P. R., Godlaski A. J., Roth R. M. Identifying component-processes of executive functioning that serve as risk factors for the alcohol-aggression relation. Psycholology of Addictive Behaviors. 2012;26(2):201–211. doi: 10.1037/a0025207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioia G. A., Isquith P. K. Two faces of monitor: They self and thy task. Journal of the International Neuropsychological Society. 2002;8:229. [Google Scholar]
- Gioia G. A., Isquith P. K., Guy S. C. Assessment of executive function in children with neurological impairments. In: Simeonsson R., Rosenthal S., editors. Psychological and developmental assessment. New York: The Guilford Press; 2001. pp. 317–356. [Google Scholar]
- Gioia G. A., Isquith P. K., Guy S. C., Kenworthy L. BRIEF: Behavior Rating Inventory of Executive Function. Lutz, Florida: Psychological Assessment Resources; 2000. [Google Scholar]
- Gioia G. A., Isquith P. K., Retzlaff P. D., Espy K. A. Confirmatory factor analysis of the Behavior Rating Inventory of Executive Function (BRIEF) in a clinical sample. Child Neuropsychology. 2002;8(4):249–257. doi: 10.1076/chin.8.4.249.13513. [DOI] [PubMed] [Google Scholar]
- Godefroy O., Cabaret M., Petit-Chenal V., Pruvo J.-P., Rousseaux M. Control functions of the frontal lobes: Modularity of the central-supervisory system? Cortex. 1999;35:1–20. doi: 10.1016/s0010-9452(08)70782-2. [DOI] [PubMed] [Google Scholar]
- Hu L., Benter P. M. Fit indices in covariance structure modeling: Sensitivity to underparameterized model misspecification. Psychological Methods. 1998;3:424–453. doi:10.1037/1082-989X.3.4.424. [Google Scholar]
- Hu L., Bentler P. M. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- Hu L. T., Bentler P. M., Kano Y. Can test statistics in covariance structure analysis be trusted? Psychological Bulletin. 1992;112:351–362. doi: 10.1037/0033-2909.112.2.351. [DOI] [PubMed] [Google Scholar]
- Jöreskog K. G., Sörbom D. LISREL 8.80. Chicago: Scientific Software International; 2004. [Google Scholar]
- Jurado M. B., Rosselli M. The elusive nature of executive functions: A review of our current understanding. Neuropsychology Review. 2007;17(3):213–233. doi: 10.1007/s11065-007-9040-z. [DOI] [PubMed] [Google Scholar]
- Klenberg L., Korkman M., Lahti-Nuuttila P. Differential development of attention and executive functions in 3- to 12-year-old Finnish children. Developmental Neuropsychology. 2001;20:407–428. doi: 10.1207/S15326942DN2001_6. [DOI] [PubMed] [Google Scholar]
- Kompus K., Hugdahl K., Ohman A., Marklund P., Nyberg L. Distinct control networks for cognition and emotion in the prefrontal cortex. Neuroscience Letters. 2009;467(2):76–80. doi: 10.1016/j.neulet.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Koven N. S., Thomas W. Mapping facets of alexithymia to executive dysfunction in daily life. Personality and Individual Differences. 2010;49:24–28. [Google Scholar]
- Kumbhani S., Roth R. M., Kuck C. L., Flashman L. A., McAllister T. W. Non-clinical obsessive compulsive symptoms and executive functions in schizophrenia. Journal of Neuropsychiatry and Clinical Neurosciences. 2010;22:304–312. doi: 10.1176/appi.neuropsych.22.3.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latzman R. D., Markon K. E. The factor structure and age-related factorial invariance of the Delis-Kaplan Executive Function System (D-KEFS) Assessment. 2010;17(2):172–184. doi: 10.1177/1073191109356254. [DOI] [PubMed] [Google Scholar]
- Mahone E. M., Cirino P. T., Cutting L. E., Cerrone P. M., Hagelthorn K. M., Hiemenz J. R., et al. Validity of the Behavior Rating Inventory of executive function in children with ADHD and/or Tourette syndrome. Archives of Clinical Neuropsychology. 2002;17:643–662. [PubMed] [Google Scholar]
- Marsh H. W., Balla J. R., McDonald R. P. Goodness-of-fit indexes in confirmatory factor analysis: The effect of sample size. Psychological Bulletin. 1988;103:391–410. [Google Scholar]
- Miyake A., Friedman N. P., Emerson M. J., Witzki A. H., Howerter A., Wager T. D. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: A latent variable analysis. Cognitive Psychology. 2000;41(1):49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Mohanty A., Engels A. S., Herrington J. D., Heller W., Ho M.-H. R., Banich M. T., et al. Differential engagement of anterior cingulate cortex subdivisions for cognitive and emotional function. Psychophysiology. 2007;44(3):343–351. doi: 10.1111/j.1469-8986.2007.00515.x. [DOI] [PubMed] [Google Scholar]
- Mur M., Portella M. J., Martinez-Aran A., Pifarre J., Vieta E. Persistent neuropsychological deficit in euthymic bipolar patients: Executive function as a core deficit. Journal of Clinical Psychiatry. 2007;68(7):1078–1086. doi: 10.4088/jcp.v68n0715. [DOI] [PubMed] [Google Scholar]
- Nigg J. T., Stavro G., Ettenhofer M., Hambrick D. Z., Miller T., Henderson J. M. Executive functions and ADHD in adults: Evidence for selective effects on ADHD symptom domains. Journal of Abnormal Psychology. 2005;114(4):706–717. doi: 10.1037/0021-843X.114.3.706. [DOI] [PubMed] [Google Scholar]
- Rabin L. A., Fogel J., Nutter-Upham K. E. Academic procrastination in college students: The role of self-reported executive function. Journal of Clinical and Experimental Neuropsychology. 2010;33(3):344–357. doi: 10.1080/13803395.2010.518597. [DOI] [PubMed] [Google Scholar]
- Rabin L. A., Roth R. M., Isquith P. K., Wishart H. A., Nutter-Upham K. E., Pare N., et al. Self and informant reports of executive function in mild cognitive impairment and older adults with cognitive complaints. Archives of Clinical Neuropsychology. 2006;21:721–732. doi: 10.1016/j.acn.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Reid R. C., Karim R., McCrory E., Carpenter B. N. Self-reported differences on measures of executive function and hypersexual behavior in a patient and community sample of men. International Journal of Neuroscience. 2010;120:120–127. doi: 10.3109/00207450903165577. [DOI] [PubMed] [Google Scholar]
- Rotenberg-Shpigelman S., Rapaport R., Stern A., Hartmen-Maeir A. Content validity and internal consistency reliability of the Behavior Rating Inventory of Executive Function - Adult Version (BRIEF-A) in Israeli adults with attention-deficit/hyperactivity disorder. Israeli Journal of Occupational Therapy. 2008;17(2):77–96. [Google Scholar]
- Roth R. M., Isquith P. K., Gioia G. A. Behavior Rating Inventory of Executive Function - Adult Version (BRIEF-A) Lutz, FL: Psychological Assessment Resources; 2005. [Google Scholar]
- Roth R. M., Randolph J. J., Koven N. S., Isquith P. K. Neural substrates of executive functions: Insights from functional neuroimaging. In: Dupri J. R., editor. Focus on neuropsychology research. New York: Nova Science; 2006. pp. 1–36. [Google Scholar]
- Schroeder V. M., Kelley M. L. The influence of family factors on the executive functioning of adult children of alcoholics in college. Family Relations. 2008;57:404–414. [Google Scholar]
- Slick D. J., Lautzenhiser A., Sherman E. M. S., Eyrl K. Frequency of scale elevations and factor structure of the Behavior Rating Inventory of Executive Function (BRIEF) in children and adolescents with intractable epilepsy. Child Neuropsychology. 2006;12(3):181–189. doi: 10.1080/09297040600611320. [DOI] [PubMed] [Google Scholar]
- Stuss D. T., Alexander M. P. Executive functions and the frontal lobes: A conceptual view. Psychological Research. 2000;63(3–4):289–298. doi: 10.1007/s004269900007. [DOI] [PubMed] [Google Scholar]
- Stuss D. T., Benson D. F. Neuropsychological studies of the frontal lobes. Psychological Bulletin. 1984;95:3–28. [PubMed] [Google Scholar]
- Swick D., Turken A. U. Dissociation between conflict detection and error monitoring in the human anterior cingulate cortex. Proceedings of the National Academy of Sciences of the USA. 2002;99(25):16354–16359. doi: 10.1073/pnas.252521499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranel D., Anderson S. W., Benton A. L. Development of the concept of “executive function” and its relationship to the frontal lobes. In: Boller F., Grafman J., editors. Handbook of neuropsychology. Vol. 9. Amsterdam: Elsevier Science; 1994. pp. 125–148. [Google Scholar]
- Turken A. U., Swick D. The effect of orbitofrontal lesions on the error-related negativity. Neuroscience Letters. 2008;441(1):7–10. doi: 10.1016/j.neulet.2008.05.115. [DOI] [PubMed] [Google Scholar]
- van Veen V., Carter C. S. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiology and Behavior. 2002;77(4–5):477–482. doi: 10.1016/s0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- Viskontas I. V., Possin K. L., Miller B. L. Symptoms of frontotemporal dementia provide insights into orbitofrontal cortex function and social behavior. Annals of the New York Academy of Sciences. 2007;1121:528–545. doi: 10.1196/annals.1401.025. [DOI] [PubMed] [Google Scholar]
- Weber D. P., Gerber E. B., Turcios V. Y., Wagner E. R., Forbes P. W. Executive functioning and performance on high-stakes testing in children from urban schools. Developmental Neuropsychology. 2006;29:459–477. doi: 10.1207/s15326942dn2903_5. [DOI] [PubMed] [Google Scholar]
- Welsh M. C., Pennington B. F., Grossier D. B. A normative-developmental study of executive function: A window on prefrontal function in children. Developmental Neuropsychology. 1991;7:199–230. [Google Scholar]
- Zelazo P. D., Müller U. Executive function in typical and atypical development. In: Goswami U., editor. Handbook of childhood cognitive development. Oxford, England: Blackwell; 2002. pp. 445–469. [Google Scholar]