Abstract
Mitochondrial translational initiation factor 3 (IF3mt) is a 29 kDa protein that has N-and C-terminal domains, homologous to prokaryotic IF3, connected by a linker region. The homology domains are preceded and followed by short extensions. No information is currently available on the specific residues in IF3mt important for its activity. Based on homology models of IF3mt, mutations were designed in the N-terminal, C-terminal, and linker domains to identify the functionally important regions. Mutation of residues 170–171, and 175 in the C-terminal domain to alanine resulted in a nearly complete loss of activity in initiation complex formation and in the dissociation of mitochondrial 55S ribosomes. However, these mutated proteins bind to the small (28S) subunit of the mammalian mitochondrial ribosome with Kd values similar to the wild-type factor. These mutations appear to lead to a factor defective in the ability to displace the large (39S) subunit of the ribosome from the 55S monosomes in an active process. Other mutations in the N-terminal domain, the linker region, and the C-terminal domain had little or no effect on the ability of IF3mt to promote initiation complex formation on mitochondrial 55S ribosomes. Mutation of residues 247–248 in the C-terminal extension abolished the ability of IF3mt to reduce the binding of fMet-tRNA to the ribosome in the absence of mRNA. The results from this paper suggest that IF3mt plays an active role in initiation of translation.
Over the past several years, understanding of mammalian mitochondria has become of increasing interest as the involvement of these organelles in a variety of diseases has become more apparent. In particular, dysfunctions in mitochondria and mutations in mitochondrial DNA have been linked to genetic diseases, Alzheimers disease, Parkinsons disease, and other age-related neurodegenerative diseases (1). Before the relationship between mitochondria and disease states can be fully understood, a number of fundamental questions about mitochondrial processes, including mitochondrial gene expression, must be answered.
Mammalian mitochondria contain about 16 kilobase pairs of DNA (2). This genetic information encodes two ribosomal RNAs, 22 transfer RNAs, and 13 proteins. The DNA is circular and continuous; it lacks significant non-coding regions. All of the proteins encoded in this genome are hydrophobic membrane proteins that are subunits of either the oligomeric electron transfer complexes or the ATP synthase required for the generation of energy by the cell (2).
Translation of the mRNAs encoded by mitochondrial DNA requires the presence of a protein biosynthetic system that is distinct from that of the cell cytoplasm. Mitochondrial ribosomes are 55S particles that have about half the rRNA content and twice the protein content of bacterial ribosomes (3). Mitochondrial ribosomal subunits have sedimentation coefficients of 28S and 39S, while bacterial ribosomal subunits have sedimentation coefficients of 30S and 50S and form 70S monosomes.
Translation initiation factors have similarities in the bacterial and mitochondrial systems, but several key differences are apparent. Three essential translation initiation factors have been identified in Escherichia coli, while only two have been identified in the mitochondrial system. In E. coli, IF2 promotes the binding of fMet-tRNA to the P-site of the 30S ribosomal subunit and promotes the joining of the 30S and 50S ribosomal subunits (4). Mitochondrial IF2 (IF2mt) appears to have the same fundamental activities found in its bacterial counterpart. IF1 in E. coli is an essential 71 amino acid protein whose exact function is unknown (5). No factor corresponding to IF1 has been identified in mitochondria. However, IF2mt has a 37 amino acid insertion that is believed to function in place of IF1 in translation (6).
In E. coli, IF3 is thought to have a number of roles. These include (1) dissociating of 70S monosomes into subunits by the preferential binding of IF3 to the small ribosomal subunit, (2) promoting the formation of initiation complexes, (3) proofreading initiation complexes by dissociating those with non-canonical start codons, (4) promoting the shift of the mRNA start codon into the P-site of the small subunit, and (5) mediating the codon/anticodon interactions of the initiator tRNA (7–15). Like E. coli IF3, IF3mt stimulates initiation complex formation in part by promoting the dissociation of 55S ribosomes, thereby providing free small subunits for initiation complex formation. IF3mt has an additional role not found in bacteria; it reduces the IF2mt-mediated binding of fMet-tRNA to 28S subunits in the absence of mRNA (16). This observation suggests that mRNA binding normally precedes fMet-tRNA binding in the mitochondrial system.
Following removal of the mitochondrial import signal, IF3mt is a 29 kDa protein composed of three regions that have homology to the bacterial factor: the N-terminal domain, the linker, and the C-terminal domain (Figure 1A). The N-terminal homology domain is preceded by an extension of 31 amino acids, and the C-terminal domain is followed by an extension of 33 amino acids. Most of the functions of E. coli IF3 and IF3mt tested in vitro have been localized to the C-terminal domain. Full length IF3mt is thought to bind on the interface side of the small subunit close to the platform with a Kd of 30 nM (17). The isolated C-terminal domain of IF3mt also has a strong affinity for the 28S subunit and binds with a Kd of 95 nM (17). The isolated N-terminal domain of E. coli IF3 has no detectable binding to the 30S ribosomal subunit (12). This domain of IF3 is thought to increase the affinity of the intact IF3 protein for the 30S subunit by two orders of magnitude. In contrast, the isolated N-terminal domain of IF3mt binds to the 28S subunit with a Kd of 390 nM (17). The N- and C-terminal extensions of IF3mt are not required for binding of the protein to the small subunit, and removal of the extensions has almost no effect on the binding constant (18). However, the C-terminal extension, along with the linker, plays a role in preventing fMet-tRNA binding to the 28S subunit in the absence of mRNA (17).
Figure 1.
Domain organization and model of IF3mt. A. Schematic representation of E. coli IF3 and IF3mt showing the N- and C-terminal homology domains and the linker regions. IF3mt has additional N- and C-terminal extensions not present in the E. coli factor. The leader specifies mitochondrial import and is not present in the constructs used here. B. The 3-D model of IF3mt prepared using Insight II. The N-terminal domain was modeled after the crystal structure of the N-terminal domain of B. stearothermophilus IF3 (PDB coordinates 1TIF (26)), and the C-domain was modeled after the NMR structure of the mouse IF3mt (PDB coordinates 2CRQ, unpublished). The N- and C-terminal extensions are not shown and are predicted to be disordered.
MATERIALS AND METHODS
Materials
Laboratory supplies and chemicals were purchased from Sigma-Aldrich or Fisher Scientific. A rabbit polyclonal primary antibody to the region of IF3mt homologous to the bacterial factors was prepared as previously described (16). Bovine mitochondrial ribosomes (55S), ribosomal subunits (28S and 39S), bovine IF2mt, and yeast [35S]fMet-tRNA were prepared as described (19–21).
Preparation of mutated derivatives of IF3mt
The sequence for IF3mt containing a 6× histidine tag was previously cloned into the pET21c vector using NdeI and XhoI restriction sites (22). Twelve mutated derivatives, designated IF3mt:1–12 (Table 1), were prepared by site-directed mutagenesis using the primers listed in Table 2 and the QuikChange site-directed mutagenesis protocol (Stratagene). All mutations were verified by DNA sequencing. The mutated plasmids were transformed into E. coli BL21 RIL cells (Stratagene). Cells were grown to an A595 of 0.6 in LB media containing ampicillin and chloramphenicol, at which time the expression of IF3mt was induced using 50 µM isopropyl β-D-1-thiogalactopyranoside. Cells were allowed to induce either overnight at 25 °C or for six h at 37 °C. After induction, cells were lysed and IF3mt was purified using Ni-NTA resin followed by S-sepharose cation exchange chromatography or HPLC purification using a TSKgel SP-5PW cation exchange column as described previously (19). The IF3mt derivatives were tested for structural integrity by comparing the α-helical content obtained using circular dichroism to that of wild-type IF3mt (IF3mt:WT). IF3mt:1 was excluded from CD measurements due to the fact that it failed to separate from its truncated 19 kDa fragment during HPLC purification. All mutated proteins showed little or no change in α-helical character as compared to the wild-type protein.
Table 1.
A. Summary of the mutations prepared in IF3mt.
| Mutation | Location | Residues Mutated |
Residues Changed to |
|---|---|---|---|
| IF3mt:1 | N-domain | KKTKK (66–70) | AATAA |
| IF3mt:2 | N-domain | TSTE (121–124) | AAAA |
| IF3mt:3 | Linker | REMEK (143–147) | AAMAA |
| IF3mt:4 | C-domain | KKK (184–186) | AAA |
| IF3mt:5 | C-domain | HD (170–171) | AA |
| IF3mt:6 | C-domain | K (175) | A |
| IF3mt:7 | C-domain | K (194) | A |
| IF3mt:8 | C-domain | EE (207–208) | AA |
| IF3mt:9 | C-extension | EE (247–248) | AA |
| IF3mt:10 | C-extension | KE (252–253) | AA |
| IF3mt:11 | C-extension | DT (261–262) | AA |
| IF3mt:12 | C-extension | KD (265–266) | AA |
Table 2.
Forward primers used for site-directed mutagenesis of IF3mta
| IF3mt: | Forward Primer |
|---|---|
| 1a | |
| 1b | |
| 1c | |
| 1d | |
| 2 | |
| 3a | |
| 3b | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 |
Mutated nucleotides are highlighted in gray. The reverse primers used were the inverse complements of the forward primers in each case. To make the four amino acid mutations in IF3mt:1, four sequential mutations were required using primers 1a–1d sequentially. To make the four amino acid mutations in IF3mt:3, two sequential mutagenic reactions were carried out using primers 3a and 3b as indicated.
Preparation of polynucleotide phosphorylase
Initiation complex assays were carried out using poly(A,U,G) as the mRNA. Since this polymer is no longer commercially available, it was prepared using polynucleotide phosphorylase. The gene encoding E. coli polynucleotide phosphorylase was previously cloned into the pET11a plasmid in BL21 (DE3) + pLysS cells and was a generous gift from Dr. George H. Jones (Dept. of Biology, Emory University, Atlanta, GA) (23). The enzyme was purified as described (23). To follow the enzyme during purification, activity assays were carried out. Reaction mixtures (100 µL) contained 50 mM Tris-HCl, pH 8.0, 5 mM MgCl2, 5 mM [3H]ADP (7 cpm/pmol), and 20 µL of appropriate fractions. The reactions were incubated at 37 °C for 20 min, at which point 500 µg bovine serum albumin was added and the reactions were precipitated with cold 5 % trichloroacetic acid (24). Following final purification of the enzyme, fractions with ADP polymerization activity were pooled and dialyzed against 50 % ammonium sulfate overnight without stirring at 4 °C.
Synthesis and purification of poly(A,U,G)
A 10 mL reaction containing 50 mM Tris-HCl, pH 8.0, 10 mM MgCl2, 5 mM ADP, pH 7.0, 5 mM UDP, pH 7.0, 5 mM GDP, pH 7.0, and 1 mg polynucleotide phosphorylase was incubated at 37 °C overnight. Following synthesis, the reaction mixture was extracted using phenol (pH 8.0)/chloroform, and the RNA was precipitated with ethanol overnight at −20 °C. RNA was collected by centrifugation at 15,000 rpm in the Sorvall SS-34 rotor for 30 min. The pellets were resuspended in 5 mL sterile H2O and applied to a Sephadex G-50 column (74 cm × 1.1 cm) equilibrated in 3 mM EDTA, pH 8.0, 25 mM Tris-HCl, pH 7.8. The column was developed with 150 mL of the same buffer, and 1 mL fractions were collected at a flow rate of 1 mL/min. UV absorbance was monitored at 260 nm. Fractions containing poly(A,U,G) were pooled and precipitated using 2.5 volumes 100% ethanol overnight at −20 °C. The poly(A,U,G) was collected by centrifugation at 15,000 rpm for 30 min in the SS-34 rotor and stored at −20 °C. Before use, the pellets were dissolved in sterile H2O to a final concentration of 13 µg/µL.
Assay for initiation complex formation on E. coli or mitochondrial ribosomes
The effect of IF3mt and its mutated derivatives on the stimulation of [35S]fMet-tRNA binding to either E. coli 70S or mitochondrial 55S ribosomes was measured using a filter binding assay and scintillation counting as previously described (16,22). In the assay using E. coli ribosomes (100 µL), 2.5–10 pmol IF3mt (0.025–0.1 µM) was incubated with saturating amounts of IF2mt (16 pmol, 0.16 µM), 70S ribosomes (68 µg, 0.3 µM), poly(A,U,G) (5 µg, 0.05 µg/µL), and [35S]fMet-tRNA (6 pmol, 0.06 µM), under the ionic conditions described previously (16). In the assay using mitochondrial 55S ribosomes, 1–4 pmol (0.01–0.04 µM) IF3mt was incubated with saturating amounts of IF2mt (16 pmol, 0.16 µM), 55S ribosomes (8 pmol, 0.08 µM), poly(A,U,G) (5 µg, 0.05 µg/µL), and [35S]fMet-tRNA (6 pmol, 0.06 µM), under the assay conditions described previously (16).
Quantitation of the binding of IF3mt and its derivatives to mitochondrial 28S subunits using Microcon centrifugation
Ribosome binding reactions were carried out as described (18). The indicated concentration of IF3mt (0.15–1 pmol, 1.5–10 nM) was incubated with 28S subunits (5 pmol, 50 nM) for 20 min at 25 °C. IF3mt bound to 28S subunits was separated from free IF3mt using a Microcon spin column (Millipore). IF3mt was then released from the ribosome by the addition of EDTA to a final concentration of 20 mM. The amount of IF3mt bound to 28S subunits was determined colorimetrically using a dot blot probed with antibodies against IF3mt as described previously (18). A control curve for analysis of the dot blot was prepared by spotting various concentrations of IF3mt (0.5–1.2 pmol, 5–12 nM), 28S (5 pmol, 50 nM), and EDTA (20 mM) directly onto the dot blot nitrocellulose membrane to quantify the change in color versus the amount of protein.
Dissociation of mitochondrial 55S ribosomes by IF3mt and its mutated derivatives
Mitochondrial 55S ribosomes (8 pmol, 0.08 µM) were incubated in a buffer containing 50 mM Tris-HCl, pH 7.6, 5 mM MgCl2, 40 mM KCl, and 1 mM dithiothreitol in the absence or presence of 80 pmol (0.8 µM) IF3mt or its mutated derivatives in 100 µL at 37 °C for 15 min. The samples were placed on ice for 10 min and then loaded onto a 10–30 % sucrose gradient and analyzed as previously described by monitoring the A254 during fractionation of the gradient (18). Each 55S ribosome sample contained some free 39S subunits. Therefore, the percentage of 55S particles was determined by comparing the area of the 55S peak on the absorbance profile to the total area of the 28S, 39S, and 55S peaks combined to account for this contamination. Areas under the peaks were determined using the width of the peak at ½ the maximal height multiplied by the height. To determine the percentage dissociation, the amount of 55S particles in the absence of IF3mt was set to 100% and compared to the amount of 55S particles observed in the presence of IF3mt.
Inhibition of fMet-tRNA binding to 28S subunits by IF3mt in the absence of mRNA
The inhibition of [35S]fMet-tRNA binding to 28S subunits in the absence of mRNA was tested as described (16). Small subunits (6.2 pmol, 0.062 µM) were incubated with saturating amounts of IF2mt (25 pmol, 0.25 µM) and [35S]fMet-tRNA (5 pmol, 0.05 µM) in the presence or absence of IF3mt (0–12 pmol, 0–0.12 µM) or its C-terminal extension mutated derivatives for 15 min at 25 °C in 100 µL reaction mixtures. The amount of [35S]fMet-tRNA remaining bound to the subunits was determined using a nitrocellulose filter binding assay (25).
RESULTS
Design of IF3 mutations
A model of IF3mt was developed using the modeling program Insight II (Figure 1B). The N-terminal domain was modeled after the crystal structure of the Bacillus stearothermophilus IF3 (PDB coordinates 1TIF (26)). This region of IF3 folds into a mixed 4-stranded β-sheet packed against a single helical unit (Helix 1) lying across the sheet and a second α-helix (Helix 2) extending into the linker region. The model of the N-terminus of IF3mt is quite similar to that of the crystal structure of the B. stearothermophilus factor despite the low percent identity (<23 %) between the N-terminal domains. The C-terminal domain was modeled after the NMR structure of the mouse IF3mt (PDB coordinates 2CRQ, to be published) which has been shown to consist of two α-helices (H3 and H4) lying on top of a 4-stranded β-sheet. The sequence of the C-terminal domain of the human factor is 72 % identical to that of mouse IF3mt. The predicted structure of this domain of human IF3mt is quite similar to that of the mouse factor except that one of the β-strands is modeled in two sections due to the presence of an internal proline residue. In the model, the linker region is depicted as being partially α-helical. However, biochemical studies of the linker regions of IF3 from several sources suggest that this region has considerable flexibility 17,27–29). The model does not include the N- and C-terminal extensions, which were predicted to be disordered. In the current work, a number of residues in IF3mt, predicted to lie on the surface of the protein, were mutated, and the effects of these mutations on the ability of IF3mt to promote initiation complex formation and to bind to 28S subunits were examined.
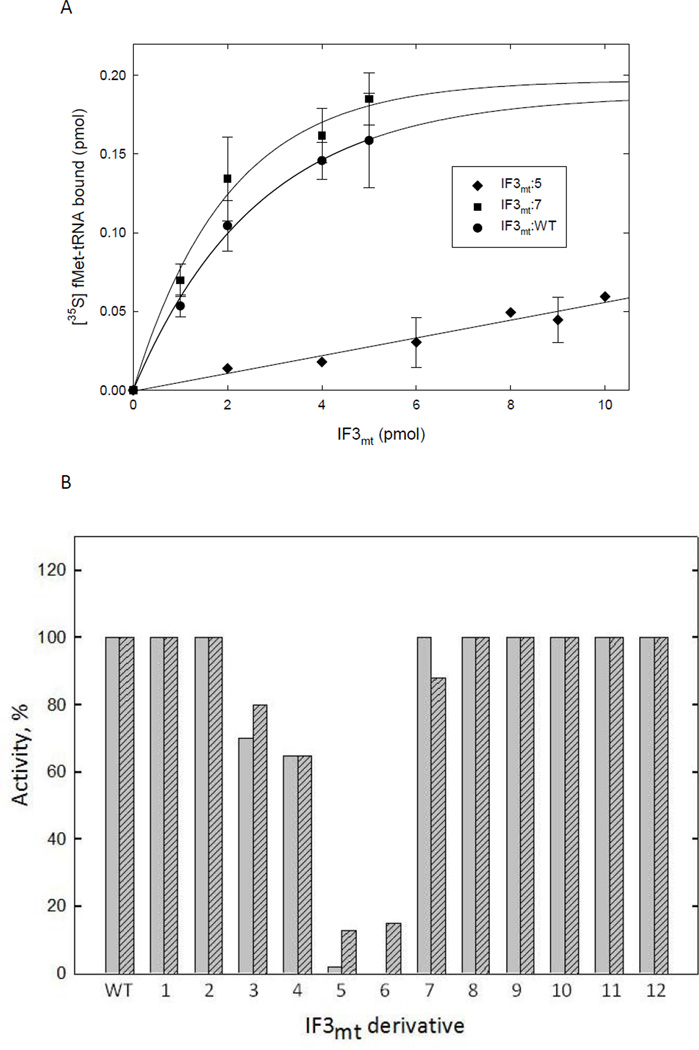
Mitochondrial 55S ribosomes are composed of approximately 2/3 protein and 1/3 RNA. The interface between the 28S and 39S subunits is rich in RNA (3). Significant electrostatic interactions between IF3mt (pI ~10) and the 12S small subunit rRNA are expected to play an important role in the function of this factor. To identify residues in IF3mt potentially involved in binding to the small subunit or in other functions of this factor, clusters of charged amino acids predicted to be exposed on the surface in the model of IF3mt (Figure 1B) were changed to alanine (Figure 2). Twelve different mutated derivatives (IF3mt:1–12) were made in the N- and C-terminal domains, the linker, and the C-terminal extension. The charges of these residues are generally conserved among vertebrate IF3mt, and partially conserved with the mosquito, fruit fly, and bacterial factors (Figure 2A). No mutations were made in the N-terminal extension, because this region is poorly conserved and no known function has been attributed to the N-terminal extension alone.
Figure 2.
Mutations in IF3mt. A. Conservation of the charged residues mutated in IF3mt:1–8. Positively charged residues are shown in dark gray and negatively charged residues are shown in light gray. The numbers above each cluster of residues indicates the IF3mt derivative containing mutations of those residues. B. stearothermophilus IF3 does not have residues corresponding to IF3mt:1. These residues are present in E. coli IF3 as KRVQT. B. Sequence conservation of IF3mt:9–12 among the vertebrate lineage. Positively charged residues are shown in dark gray and negatively charged residues are shown in light gray. C. Model of IF3mt showing IF3mt:1–8. The N-terminal domain is shown in red, the linker is in green, and the mutated residues are in blue. IF3mt:1 could not be modeled based on the crystal structure of B. stearothermophilus because these residues are not present in this IF3 nor are they present in the NMR structure of the N-domain of E. coli IF3 (38). IF3mt:1 has been placed in the model at the N-terminus of the protein for illustrative purposes. The C-terminal homology domain is shown in blue and the mutated residues are shown in orange. Part of the C-terminal extension is shown in yellow.
The N-terminal domain of E. coli IF3 does not bind independently to 30S subunits (12). In contrast, the N-terminal domain of IF3mt binds 28S subunits with a Kd of 390 ± 60 nM (17). Two clusters of residues in the N-terminal domain were mutated (Figure 2). The selected residues were fairly well conserved among the mammalian factors (Figure 2A) but less conserved with the IF3mt of other organisms. IF3mt:1 is located five residues into the N-terminal domain of IF3mt in a region where no structural information is available. Its predicted location is shown in the model (Figure 2C). This region was selected for mutagenesis since the first six residues of E. coli IF3 are important for the function of this factor (30). IF3mt:2 is also located in the N-terminal domain in a loop between two β-strands facing away from the linker region. IF3mt:3 is located in a predicted α-helical region in the IF3mt linker. IF3mt:4–6 are located in the C-terminal domain of IF3mt in an α-helix (Helix 3) predicted previously to be essential for the function of the E. coli protein (31). IF3mt:7 is also in the C-terminal domain at the tip of a β- strand (corresponding to β-strand 7 in the prokaryotic factors) in the center of the domain. IF3mt:8 is located behind IF3mt:7 on the opposite face of the C-terminal domain (Figure 2C). IF3mt:9–12 are located in the C-terminal extension of IF3mt, where no structural data is currently available. A summary of the IF3mt mutations is shown in Table 1.
The mutated proteins were purified and tested for structural integrity by measuring the α-helical content using circular dichroism. The activities of the derivatives in promoting fMet-tRNA binding to E. coli and mitochondrial ribosomes were tested. Proteins that showed reduced activity in promoting initiation complex formation were further tested for their abilities to dissociate mitochondrial 55S ribosomes. Those unable to dissociate 55S ribosomes were tested for their abilities to bind to mitochondrial 28S small subunits.
Mutations in the N-terminal domain and linker
IF3mt derivatives with mutations in the N-terminal domain and linker were tested for their abilities to promote fMet-tRNA binding to both E. coli and mitochondrial ribosomes in the presence of IF2mt. This assay primarily measures the ribosomal subunit dissociation activity of this factor (22). A representative graph showing initiation complex formation as a function of increasing amounts IF3mt is shown in Figure 3A. IF3mt:1 and IF3mt:2 showed activity similar to the unmutated factor (IF3mt:WT) on both E. coli and mitochondrial ribosomes (Figure 3B), indicating that these residues are not important for the activity of this protein. IF3mt:3, located in the linker region of the protein, showed a slight reduction in activity in initiation complex formation on both E. coli and mitochondrial ribosomes (Figure 3B). The slight loss of activity for IF3mt:3 was further explored in an assay that measures the ability of the protein to dissociate mitochondrial 55S ribosomes as described below. IF3mt:3 showed the same subunit dissociation pattern as the wild-type protein in this assay, which indicates that it did not have any significant defect in this function (Figure 4D).
Figure 3.
Effect of mutated derivatives of IF3mt on initiation complex formation. A. Effect of IF3mt:WT, IF3mt:7, and IF3mt:5 on initiation complex formation on mitochondrial 55S ribosomes. [35S]fMet-tRNA binding to mitochondrial 55S particles was tested in the presence of saturating amounts of IF2mt using IF3mt:WT (●), IF3mt:7 (■), or IF3mt:5 (◆). A blank representing the amount of [35S]fMet-tRNA bound to ribosomes in the absence of IF3mt (~0.1 pmol) was subtracted from each value. B. Summary of the activities of IF3mt:1–12 in initiation complex formation. This assay primarily measures ribosome dissociation. The activity of IF3mt:WT was set to 100 % and the mutated derivatives were compared to that value using the linear regions of the dose response curves. The activity on 70S ribosomes is shown in gray while activity on mitochondrial ribosomes is in striped gray. Derivatives identical to wild-type within error are shown as 100 %.
Figure 4.
Effect of IF3mt and its mutated derivates on the dissociation of mitochondrial 55S ribosomes. Fractionation profiles of mitochondrial 55S ribosomes after centrifugation on a 10– 30 % sucrose gradient. Mitochondrial 55S ribosomes (8 pmol) were incubated as described in Materials and Methods in the absence (A) or presence of 80 pmol IF3mt (B) or its mutated derivative IF3mt:6 (C) and subsequently subjected to centrifugation on a 10–30 % sucrose density gradient. Gradients were fractionated while monitoring the A254. D. Percentage of mitochondrial 55S ribosomes remaining after the addition of IF3mt:WT or its mutated derivatives as measured by sucrose density gradient centrifugation.
Mutations in the C-terminal domain
The C-terminal domain of IF3mt with the linker binds to the small ribosomal subunit with nearly the same affinity as the full-length protein and shows the same activity as the full length protein in stimulating fMet-tRNA binding to mitochondrial ribosomes (17). IF3mt derivatives with mutations in the C-terminal domain were tested for activity in initiation complex formation on E. coli and mitochondrial ribosomes. IF3mt:7 and IF3mt:8 had the same activity as the wild-type protein (Figure 3). IF3mt:4 showed slightly reduced activity, but still maintained its ability to dissociate 55S ribosomes as well as the wild-type protein (Figure 4). However, IF3mt:5 and IF3mt:6 had almost no activity in promoting initiation complex formation on E. coli ribosomes, and had significantly reduced activity on mitochondrial ribosomes (Figure 3).
Since neither IF3mt:5 nor IF3mt:6 were active in stimulating initiation complex formation, an assay that basically measures ribosome dissociation, their abilities to promote the dissociation of 55S ribosomes was examined directly by sucrose density centrifugation. Both proteins were deficient in dissociation activity (Figure 4). IF3mt:5 dissociated 55S ribosomes by only ~15 %, compared to just over 50 % dissociation with the same level of IF3mt:WT. IF3mt:6 had essentially no activity in the ribosome dissociation assay.
One possible explanation for the lack of activity observed with IF3mt:5 and IF3mt:6 is that these mutated proteins are unable to bind 28S subunits, and, therefore, are unable to effectively prevent the interaction of the 28S and 39S ribosomal subunits. To test this possible explanation directly, the binding of IF3mt and its mutated derivatives to 28S subunits was measured using Microcon-100 centrifugation followed by immunological detection of IF3mt on a dot blot apparatus (18). This method takes advantage of the observation that, in the absence of 28S subunits, IF3mt passes through the membrane while IF3mt bound to 28S subunits does not. IF3mt:WT binds to 28S subunits with a Kd of 35 ± 13 nM (Table 2). As indicated in Figure 5, IF3mt:6 binds 28S subunits with a Kd of 45 ± 17 nM and IF3mt:5 binds to 28S subunits with a Kd of 19 ± 11 nM. Since both mutated proteins show the same binding to 28S subunits as IF3mt:WT, the lack of activity of the mutated proteins in initiation complex formation and in the dissociation of 55S ribosomes into the 28S and 39S subunits cannot be attributed to a defect in their abilities to bind the small ribosomal subunit.
Figure 5.
Binding of IF3mt:6 to 28S subunits. Binding assays were performed using a Microcon spin column as described (18). Inset: Calibration curve of IF3mt: WT using a colorimetric assay and a dot blot apparatus. This curve was used to determine the amount of IF3mt bound to mitochondrial 28S ribosomes in the Microcon centrifugation assay as described in Materials and Methods.
Mutations in the C-terminal extension
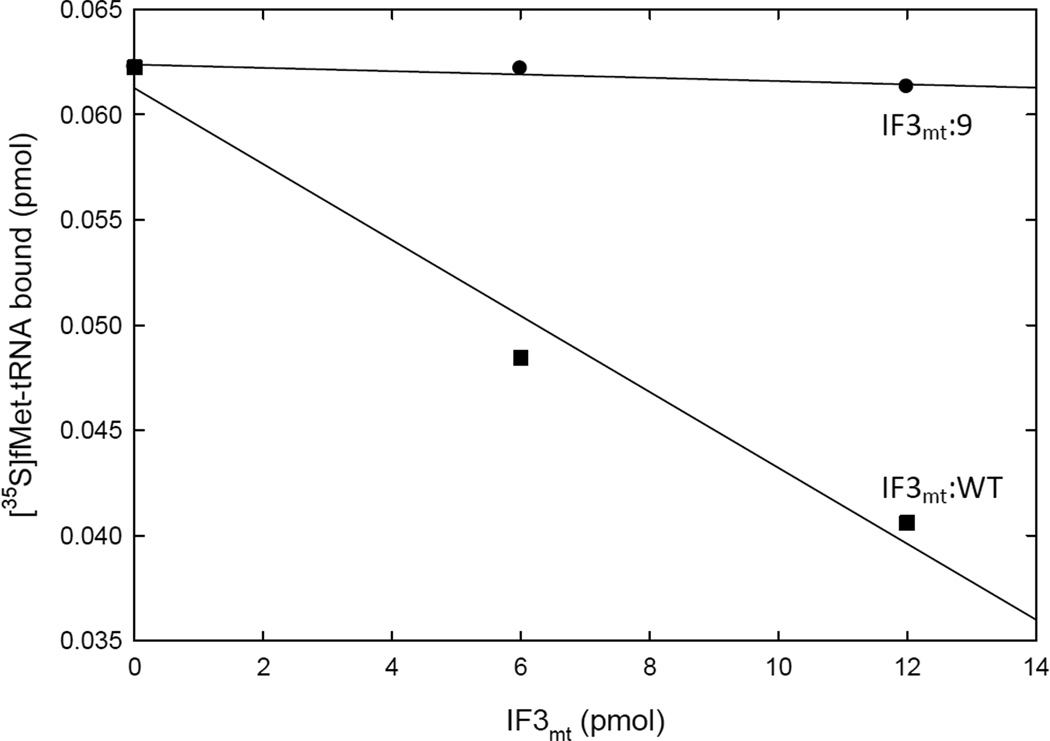
One unusual property of IF3mt is that its C-terminal extension is thought to play an important role in decreasing the amount of fMet-tRNA bound to mitochondrial 28S subunits in the absence of mRNA. This observation suggests that mRNA binding should precede fMet-tRNA binding in the mitochondrial system (16). No structural data is currently available for the C-terminal extension of IF3mt. Modeling of this region indicated that it is probably disordered in solution. Regions of highly charged amino acids (acidic and basic) conserved in vertebrate IF3mt lineages were selected for mutation to alanine residues (Figure 2). All of these derivatives were as active as IF3mt:WT in promoting initiation complex formation on both E. coli and mitochondrial ribosomes (Figure 3). As indicated in Figure 6, IF3mt:WT inhibits the binding of fMet-tRNA to 28S subunits in the absence of mRNA. IF3mt:5 and IF3mt:6 in the C-terminal domain, along with IF3mt:10, IF3mt:11, and IF3mt:12 in the C-terminal extension showed normal activity in this assay (data not shown). However, IF3mt:9, also in the C-terminal extension, was unable to reduce the amount of fMet-tRNA bound to ribosomes in the absence of mRNA (Figure 6). This observation pinpoints a region of the C-terminal extension that is critical for inhibiting initiation complex formation before the mRNA is bound. Interestingly, the linker region of IF3mt also appears to have a role in preventing fMet-tRNA binding in the absence of mRNA (17).
Figure 6.
IF3mt-mediated inhibition of fMet-tRNA binding to mitochondrial 28S subunits in the absence of mRNA. Mitochondrial 28S subunits were incubated with saturating amounts of IF2mt and [35S]fMet-tRNA in the presence or absence of IF3mt:WT or its C-terminal extension mutated derivative IF3mt:9 as described (16). The amount of [35S]fMet-tRNA remaining bound to the filter in the presence of IF3mt:WT (■) or IF3mt:9 (●) was determined using a nitrocellulose filter binding assay.
The C-terminal extension is predicted to emerge from the C-terminal domain near the linker region according to the model (Figure 1B). The linker, and, thus, the C-terminal extension, may span the region between the platform, where the C-terminal domain is thought to bind, and the head of the small subunit. The linker may be positioned toward the mRNA binding channel as predicted in the model of McCutcheon et al. (13). A transient association between of these regions of IF3mt with the mRNA channel near the P-site may permit this factor to distinguish prematurely formed initiation complexes.
DISCUSSION
Comparison of mutational effects in IF3mt and E. coli IF3
Both NMR (32) and mutational analysis (31,33) indicate that there are a number of contacts between E. coli IF3 and the small subunit in the C-terminal domain. These interactions appear to span much of Helix 3, the loop regions, and portions of Helix 4. Key differences between IF3mt and the E. coli factor become apparent when the extensive battery of mutations made in made in Arg residues in E. coli IF3 are compared to those presented here in the mitochondrial factor (Figure 7). In E. coli IF3, the most drastic effects on the activity of the factor in initiation complex formation measured by ribosomal subunit dissociation were caused by a mutation in the center of Helix 4 in the C-terminal domain (31). This mutation also caused significant reductions in binding of the mutated protein to E. coli 30S ribosomal subunits, suggesting that the defect in initiation arises from a weaker interaction with the small subunit. However, IF3mt:8, located in Helix 4 in the C-terminal domain of IF3mt, was able to stimulate initiation complex formation as well as the wild-type factor. This observation suggests that residues in Helix 4 may play a less important role in IF3mt than in E. coli IF3.
Figure 7.
Effects of mutations in the C-terminal domain of E. coli IF3 and IF3mt. A. Mutated residues in the C-terminal domain of E. coli IF3 and their effects on activity in initiation complex formation on E. coli ribosomes. The activity remaining was based on 70S dissociation, one of several assays carried out with these mutated derivatives of E. coli IF3. The most drastic effects on activity are seen with the mutation in the center of the α-helix (Helix 4) on the back of the protein. B. Mutated residues in the C-terminal domain of IF3mt and the effects of these mutations on activity in initiation complex formation on mitochondrial ribosomes. The most drastic effects are seen with the two mutations located at the base of Helix 3.
In the mitochondrial factor, the most drastic effects were seen with two mutations in Helix 3 (IF3mt:5 and IF3mt:6, Figure 7). A mutation made in the center of this helix in E. coli IF3 (R112S) had only a modest effect on initiation complex formation (31), and the mutated derivative still retained >60 % of the activity observed with the wild-type factor. In the mitochondrial factor, the mutations in Helix 3 are located closer to the base of the helix rather than in the center. The side chains of these residues are predicted to be shifted approximately 90 degrees towards the front of the protein when compared to the side chain of the mutated residues in E. coli IF3. IF3mt derivatives containing these mutations do not promote initiation complex formation, but are still able to bind to mitochondrial 28S subunits. Thus, residues near to the bottom of Helix 3 are not predicted to be in direct contact with the 28S subunits. Rather, they may define a surface of IF3mt that contacts the 39S subunit during the dissociation of the 55S ribosomes into subunits.
Model for IF3mt-mediated dissociation of 55S ribosomes
Mutation of residues 170–171 and residue 175 in Helix 3 of the C-terminal domain of IF3mt drastically reduced the activity of IF3mt in promoting initiation complex formation. This assay basically measures the ability of IF3mt to dissociate 55S ribosomes. Sucrose density gradient centrifugation confirmed the idea that these mutations inactivate IF3mt in ribosomal subunit dissociation. The most logical explanation for these results is that the mutated proteins fail to bind mitochondrial 28S subunits and, therefore, cannot prevent 39S joining. However, both of these mutated derivatives of IF3mt bind to 28S subunits as well as the wild-type factor (Table 3). This observation indicates that an alternative problem must underlie the loss of activity in these mutated IF3mt derivatives. One possible explanation is that there are two distinct functionally important surfaces of IF3mt. The first surface would function as a dissociation interface and may interact with one or both of the ribosomal subunits. The second surface would function as a binding interface between the factor and the 28S subunit.
Table 3.
Binding of IF3mt and its mutated derivatives to 28S mitochondrial ribosomesa
| IF3mt | Kd (nM) |
|---|---|
| IF3mt:WT | 35 ± 13 |
| IF3mt:5 | 19 ± 11 |
| IF3mt:6 | 45 ± 17 |
Kd values of IF3mt:WT and its mutated derivatives binding to 28S mitochondrial ribosomes as determined by Microcon centrifugation.
Recent work has indicated that the position of the C-terminal domain of E. coli IF3 blocks bridge B2b of the 70S ribosome, preventing the association of the large and small subunits when IF3 is present (14). Bridge B2b involves 16S rRNA nucleotide 794 (34), which is in proximity to the proposed IF3 binding site on the 30S subunit (8,35). Cryo-electron microscopy studies of mammalian mitochondrial ribosomes (3) indicates that this intersubunit bridge is one of several conserved contacts between the two subunits. IF3mt is expected to bind to 28S subunits in a manner similar to the binding of E. coli IF3 to 30S subunits. It is likely that residues 170–171 and 175 play an essential role in allowing IF3mt to block the formation of bridge B2b. Mutation of these residues could impede the blocking this intersubunit bridge by IF3mt, preventing these mutated forms of IF3mt from effectively competing with the 39S subunit for binding to the 28S subunit.
There are two current models to explain the action of IF3mt in ribosomal subunit dissociation (Figure 8). In the passive model, IF3mt binds to 28S subunits after the subunits dissociate transiently (Figure 8A, passive model). In this model, IF3mt acts more as an anti-association factor than a dissociation factor (36). In the active model, an equilibrium exists between 55S ribosomes and a transient 28S:IF3:39S complex (Figure 8B, active step 1). This transient complex is in equilibrium with free 39S subunits and 28S subunits bound to IF3mt (active step 2). The active model suggests that IF3mt plays a more direct role in dissociating 55S ribosomes than simply binding to 28S subunits that are present at equilibrium. IF3mt: 5 and IF3mt:6, though they bind well to 28S subunits (active step 1), are deficient in dissociating 55S ribosomes (active step 2). This observation suggests that a transient 28S:IF3:39S complex formed by these mutated derivatives fails to dissociate into its component subunits. This idea is supposed by recent kinetic data, which suggest that in E. coli, IF3 may remain associated with the 30S subunit that is partially bound to the 50S (14), and by recent cryo-electron microscopy data, which tentatively suggests that IF3 may not be released from 70S particles until the hydrolysis of GTP by IF2 has occurred (37). The present results suggest that IF3mt plays an active role rather than a passive role in ribosomal subunit dissociation in the mitochondrial translational system.
Figure 8.
Models for the mechanism of IF3mt in the dissociation of mitochondrial ribosomes. A. In the passive model, the subunits are in equilibrium with the 55S monosome (passive step 1). IF3mt binds to free 28S subunits, preventing reassociation with the 39S subunit (passive step 2). B. In the active model, IF3mt interacts with the 55S particle, forming a transient 28S:IF3mt:39S complex (active step 1), which then dissociates into a 28S:IF3mt complex and free 39S subunits (active step 2).
Acknowledgements
We thank Brenda Temple (Univ. North Carolina) for help with the molecular modeling. We thank Emdadul Haque, Christie Jones (Univ. North Carolina) for helpful discussions.
Abbreviations
- IF3
translation initiation factor 3
- IF3mt
human mitochondrial translation initiation factor 3
- IF1
translation initiation factor 1
- IF2
translation initiation factor 2
- IF2mt
bovine mitochondrial translation initiation factor 2
Footnotes
This study was supported NIH grant GM32734.
REFERENCES
- 1.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 2.Scheffler I. Mitochondria. New York: Wiley-Liss, Inc; 1999. [Google Scholar]
- 3.Sharma MR, Koc EC, Datta PP, Booth TM, Spremulli LL, Agrawal RK. Structure of the mammalian mitochondrial ribosome reveals an expanded functional role for its component proteins. Cell. 2003;115:97–108. doi: 10.1016/s0092-8674(03)00762-1. [DOI] [PubMed] [Google Scholar]
- 4.Gualerzi C, Pon C. Initiation of mRNA translation in prokaryotes. Biochemistry. 1990;29:5881–5889. doi: 10.1021/bi00477a001. [DOI] [PubMed] [Google Scholar]
- 5.Cummings H, Hershey J. Translation initiation factor IF-1 is essential for cell viability in Escherichia coli . J. Bacteriol. 1994;176:198–205. doi: 10.1128/jb.176.1.198-205.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaur R, Grasso D, Datta PP, Krishna PDV, Das G, Spencer A, Agrawal RK, Spremulli L, Varshney U. A single mammalian mitochondrial translation initiation factor functionally replaces two bacterial factors. Mol. Cell. 2008;29:180–190. doi: 10.1016/j.molcel.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dottavio-Martin D, Suttle DP, Ravel JM. The effects of initiation factors IF-1 and IF-3 on the dissociation of Escherichia coli 70 S ribosomes. FEBS Lett. 1979;97:105–110. doi: 10.1016/0014-5793(79)80062-9. [DOI] [PubMed] [Google Scholar]
- 8.Dallas A, Noller HF. Interaction of translation initiation factor 3 with the 30S ribosomal subunit. Mol. Cell. 2001;8:855–864. doi: 10.1016/s1097-2765(01)00356-2. [DOI] [PubMed] [Google Scholar]
- 9.Paci M, Pon C, Lammi M, Gualerzi C. Structure-function relationship in Escherichia coli translational initiation factors. Characterization of IF-3 by high resolution 1H NMR spectroscopy. J. Biol. Chem. 1984;259:9628–9634. [PubMed] [Google Scholar]
- 10.La Teana A, Gualerzi C, Brimacombe R. From stand-by to decoding site. Adjustment of the mRNA on the 30 S subunit under the influence of the initiation factors. RNA. 1995;1:772–782. [PMC free article] [PubMed] [Google Scholar]
- 11.Antoun A, Pavlov MY, Lovmar M, Ehrenberg M. How initiation factors tune the rate of initiation of protein synthesis in bacteria. EMBO J. 2006;25:2539–2550. doi: 10.1038/sj.emboj.7601140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petrelli D, LaTeana A, Garofalo C, Spurio R, Pon CL, Gualerzi CO. Translation initiation factor IF3: two domains, five functions, one mechanism? EMBO J. 2001;20:4560–4569. doi: 10.1093/emboj/20.16.4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCutcheon J, Agrawal R, Philips SM, Grassucci R, Gerchman S, Clemons WM, Ramakrishnan V, Frank J. Location of translational initiation factor IF3 on the small ribosomal subunit. Proc. Natl. Acad. Sci. U.S.A. 1999;96:4301–4306. doi: 10.1073/pnas.96.8.4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fabbretti A, Pon CL, Hennelly SP, Hill W, Lodmell J, Gualerzi CO. The real-time path of translation factor IF3 onto and off the ribosome. Mol. Cell. 2007;25:285–296. doi: 10.1016/j.molcel.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Laursen BS, Sorensen HP, Mortensen KK, Sperling-Petersen HU. Initiation of protein synthesis in bacteria. Microbiol. Mol. Biol. Rev. 2005;69:101–123. doi: 10.1128/MMBR.69.1.101-123.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhargava K, Spremulli LL. Role of the N- and C-terminal extensions on the activity of mammalian mitochondrial translational initiation factor 3. Nucleic Acids Res. 2005;33:7011–7018. doi: 10.1093/nar/gki1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haque M, Spremulli LL. Roles of the N- and C-terminal domains of mammalian mitochondrial initiation factor 3 in protein biosynthesis. J. Mol. Biol. 2008;384:929–940. doi: 10.1016/j.jmb.2008.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haque ME, Grasso D, Spremulli LL. The interaction of mammalian mitochondrial translational initiation factor 3 with ribosomes: Evolution of terminal extensions in IF3mt . Nucleic Acids Res. 2008;36:589–597. doi: 10.1093/nar/gkm1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grasso DG, Christian BE, Spencer AC, Spremulli LL. Over-expression and purification of mitochondrial translational initiation factor 2 and initiation factor 3. Meth. Enzymol. Translation Initiation: Reconstituted Systems and Biophysical Methods. 2007:59–78. doi: 10.1016/S0076-6879(07)30004-9. [DOI] [PubMed] [Google Scholar]
- 20.Spremulli LL. In: Methods Mol. Biol. Mitochondria, Practical Protocols. Leister D, Herrmann J, editors. Totowa, NJ: Humana Press; 2007. pp. 265–275. [Google Scholar]
- 21.Graves M, Spremulli LL. Activity of Euglena gracilis chloroplast ribosomes with prokaryotic and eukaryotic initiation factors. Arch. Biochem. Biophys. 1983;222:192–199. doi: 10.1016/0003-9861(83)90516-7. [DOI] [PubMed] [Google Scholar]
- 22.Koc EC, Spremulli LL. Identification of mammalian mitochondrial translational initiation factor 3 and examination of its role in initiation complex formation with natural mRNAs. J. Biol. Chem. 2002;277:35541–35549. doi: 10.1074/jbc.M202498200. [DOI] [PubMed] [Google Scholar]
- 23.Jones GH, Symmons MF, Hankins JS, Mackie GA. Overexpression and purification of untagged polynucleotide phosphorylases. Protein Expression Purif. 2003;32:202–209. doi: 10.1016/j.pep.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Spencer AC, Heck AH, Takeuchi N, Watanabe K, Spremulli LL. Characterization of the human mitochondrial methionyl-tRNA synthetase. Biochemistry. 2004;43:9743–9754. doi: 10.1021/bi049639w. [DOI] [PubMed] [Google Scholar]
- 25.Liao H-X, Spremulli LL. Initiation of protein synthesis in animal mitochondria: Purification and characterization of translational initiation factor 2. J. Biol. Chem. 1991;266:20714–20719. [PubMed] [Google Scholar]
- 26.Biou V, Shu F, Ramakrishnan V. X-ray crystallography shows that translational initiation factor IF3 consists of two compact α/β domains linked by an α-helix. EMBO J. 1995;14:4056–4064. doi: 10.1002/j.1460-2075.1995.tb00077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moreau M, de Cock E, Fortier P-L, Garcia C, Albaret C, Blanquet S, Lallemand J-Y, Dardel F. Heteronuclear NMR studies of E. coli translation initiation factor IF3. Evidence that the inter-domain region is disordered in solution. J. Mol. Biol. 1997;266:15–22. doi: 10.1006/jmbi.1996.0756. [DOI] [PubMed] [Google Scholar]
- 28.Hua Y, Raleigh D. On the global architecture of initiation factor IF3: a comparative study of the linker regions from the Escherichia coli protein and the Bacillus stearothermophilus protein. J. Mol. Biol. 1998;278:871–878. doi: 10.1006/jmbi.1998.1736. [DOI] [PubMed] [Google Scholar]
- 29.de Cock E, Springer M, Dardel F. The interdomain linker of Escherichia coli initiation factor IF3: a possible trigger of translation initiation specificity. Mol. Microbiol. 1999;32:193–202. doi: 10.1046/j.1365-2958.1999.01350.x. [DOI] [PubMed] [Google Scholar]
- 30.Lammi M, Pon C, Gualerzi C. The NH2-terminal cleavage of Escherichia coli translational initiation factor 3: A mechanism to control the intracellular level of the factor. FEBS Lett. 1987;215:115–121. doi: 10.1016/0014-5793(87)80124-2. [DOI] [PubMed] [Google Scholar]
- 31.Petrelli D, Garofalo C, Lammi M, Spurio R, Pon CL, Gualerzi CO, Teana AL. Mapping the active sites of bacterial translation initiation factor IF3. J. Mol. Biol. 2003;331:541–556. doi: 10.1016/s0022-2836(03)00731-9. [DOI] [PubMed] [Google Scholar]
- 32.Sette M, Spurio R, VanTilborg P, Gualerzi C, Boelens R. Identification of the ribosome binding sites of translation initiation factor IF3 by multidimensional heteronuclear NMR spectroscopy. RNA. 1999;5:82–92. doi: 10.1017/s1355838299981487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bellis D, Liveris D, Goss D, Ringquist S, Schwartz I. Structure-function analysis of Escherichia coli translation initiation factor 3: Tyrosine 107 and lysine 110 are required for ribosome binding. Biochemistry. 1992;31:11984–11990. doi: 10.1021/bi00163a005. [DOI] [PubMed] [Google Scholar]
- 34.Liiv A, O'Connor M. Mutations in the intersubunit bridge regions of 23S rRNA. J. Biol. Chem. 2006;281:29850–29862. doi: 10.1074/jbc.M603013200. [DOI] [PubMed] [Google Scholar]
- 35.Tapprich W, Goss D, Dahlberg A. Mutation at position 791 in Escherichia coli 16 S ribosomal RNA affects processes involved in the initiation of protein synthesis. Proc. Natl. Acad. Sci. U.S.A. 1989;86:4927–4931. doi: 10.1073/pnas.86.13.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naaktgeboren N, Roobol K, Voorma HO. The effect of initiation factor IF-1 on the dissociation of 70S ribosomes of Escherichia coli . Eur. J. Biochem. 1977;72:49–56. doi: 10.1111/j.1432-1033.1977.tb11223.x. [DOI] [PubMed] [Google Scholar]
- 37.Allen GS, Zavialov A, Gursky R, Ehrenberg M, Frank J. The cryo-EM structure of a translation initiation complex from Escherichia coli . Cell. 2005;121:703–712. doi: 10.1016/j.cell.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 38.Garcia C, Fortier P-L, Blanquet S, Lallemand J-Y, Dardel F. 1H and 15N resonance assignments and structure of the N-terminal domain of Escherichia coli initiation factor 3. Eur. J. Biochem. 1995;228:395–402. [PubMed] [Google Scholar]