Abstract
Purpose.
Ocular complications related to Stevens-Johnson Syndrome (SJS)–Toxic Epidermal Necrolysis (TEN) may persist and progress after resolution of systemic disease. This is thought to be related in part to persistent ocular innate-immune signaling. In this study, our aim was to characterize infiltrative conjunctival cellular profiles during acute (<12 months) and chronic (>12 months) disease.
Methods.
Consecutive patients presenting with SJS-TEN over a 12-month period were followed for 1 year. Detailed clinical examination and conjunctival impression cell recovery was analyzed by flow cytometry for the presence of intraepithelial leukocytes and compared with healthy controls (n = 21).
Results.
Ten patients were recruited of whom six had acute disease and five were classified as TEN (SCORTEN = 1, n = 4). Conjunctival inflammation was graded as absent/mild in a total of nine patients; but despite this, evidence of fornix shrinkage was observed in nine subjects. This inversely correlated with disease duration (P < 0.05). A reduction in percentage of CD8αβ+ T cells compared with controls (80% vs. 57%; P < 0.01) was associated with a corresponding increase in the number/percentage of CD45INTCD11b+CD16+CD14− neutrophils (186 vs. 3.4, P < 0.01, 31% vs. 0.8%, P < 0.001). Neutrophils inversely correlated with disease duration (r = −0.71, P = 0.03), yet there was no absolute change in the CD8αβ+ or neutrophil populations during the study period (P = 1.0).
Conclusions.
These data highlight that a neutrophilic infiltrate is present in mildly inflamed or clinically quiescent conjunctival mucosa in patients with ocular SJS-TEN, where neutrophil numbers inversely correlate with disease duration. Neutrophil persistence endorses the hypothesis of an unresolved innate-inflammatory process that might account for disease progression.
Keywords: SJS, TEN, neutrophils, innate immunity
Ocular Stevens-Johnson Syndrome-Toxic Epidermal Necrolysis is characterized by elevated in conjunctival epithelial CD45INTCD11b+CD16+CD14− neutrophils in acute and chronic disease. Although neutrophils inversely correlate with disease duration, they persist above levels found in healthy subjects.
Introduction
Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) are reaction patterns that have the potential to cause life-threatening mucocutaneous blistering.1,2 TEN is a severe manifestation of SJS characterized by sloughing of epithelial surfaces simulating widespread full-thickness burn injuries, including clinical features such as dehydration, hypovolemia, renal failure, and secondary infection. Mortality is predicted by the severity of systemic features using a grading system called SCORTEN3 and can exceed 90% in those with multiorgan failure.4,5
Ocular complications are related to acute ocular mucosal failure defined by ocular surface epithelial breakdown with ulceration, sloughing of the mucocutaneous junction, fragility of eyelid skin, and infection. In addition, goblet cell loss may lead to mixed aqueous deficient and evaporative dry eye. If the patient survives, on resolution of systemic disease, long-term ocular surface sequelae ensue. These are hallmarked by keratinization, conjunctival fibrosis associated with exacerbations of inflammation, recurrent scleritis, cicatrization and ocular surface failure with blinding infection, keratopathy, or perforation.6,7 In cutaneous SJS-TEN, keratinocyte death is thought to be induced by blister fluid granulysin secreted by CD8+ cytotoxic T cells and CD56+ natural killer cells.8 In ocular disease there is evidence for the presence of CD8+ cytotoxic T cells in the cornea during the acute stages of TEN,9 whilst the persistence of chronic disease is thought to have an innate immune component mediated by toll-like receptor 3.10
Data regarding the cellular profile of the ocular surface submucosa is limited. This is chiefly related to the inability to undertake longitudinal characterization of the cellular and molecular basis of disease without undertaking a substantive tissue biopsy, which can be compounded by potentially adversely affecting ocular disease course and by logistic problems with taking samples in the acute intensive care setting. Ocular surface impression cytology (OSIC) has been proposed as a putative noninvasive technique that samples the superficial layers of the conjunctival epithelium and affords an advantage of repeated longitudinal sampling without affecting the outcomes of clinical disease. OSIC recovered cells can subsequently be analyzed by multicolor flow cytometry,11 such as a detailed analyses of conjunctival epithelial cells or intraepithelial leukocytes. We have recently shown that TCRαβ+CD8αβ+ T cells12 dominate in healthy conjunctival epithelium and that this population of leukocytes is independent of age.
In this study, our aim was to characterize infiltrative cellular profiles within the conjunctival epithelium during acute and chronic SJS-TEN, and to examine whether the OSIC technique could provide a means of objectively monitoring inflammatory processes during the various stages of ocular SJS-TEN.
Methods
Study Population
Ten consecutive patients presenting with SJS-TEN to a tertiary ocular surface diseases service over a 12-month period were recruited and followed for an additional 12 months. Comparisons were made with a cohort of healthy, age-matched controls (n = 21). The study was conducted following ethical approval (Birmingham East, North and Solihull Research Ethics Committee, IOSD 08H1206/165) and the research followed the tenets of the Declaration of Helsinki. Informed consent was obtained from the subjects after explanation of the nature and possible consequences of the study.
Disease Grading and Staging
Data were collected at presentation and at 12 months follow-up. Chronic disease was defined according to Sotozono et al.,13 as persistence of ocular disease for >12 months after disease-onset. Disease activity was based upon the extent of conjunctival inflammation: absent, mild, moderate, or severe (severe defined as being inflamed in all four quadrants, the presence of limbitis and/or conjunctival ulceration).14 Staging of disease was determined both by the staging described by Power et al. (absent, mild, moderate, and severe)15 and also Sotozono et al. (score: 1–39).13 Quantification of lower and upper conjunctival fornix shrinkage was gauged by using a Fornix Depth Measurer (FDM)16 together with counting the number of symblephara. Information regarding therapeutic and surgical intervention was also recorded.
Sample Collection and Laboratory Analysis
Conjunctival ocular surface impression cytology (OSIC) with sterile hydrophilic polyethersulfone filters (Supor; Pall Corporation, Port Washington, NY) were used to collect superficial conjunctival cells as previously described.11,12 Briefly, conjunctival cells were collected from the superior bulbar conjunctiva with autoclaved synthetic membranes (Supor 200; Pall Corporation) divided into two semicircles (measuring 13 × 6.5 mm2 each).11 Conjunctival OSIC was performed with four semicircle membranes per eye and before the application of topical fluorescein drops for clinical examination. Cells were recovered into RPMI1640 supplemented with 1% GPS, 1% HEPES buffer, and 10% heat inactivated fetal calf serum by gentle agitation with a pipette tip for 1 minute. Cell suspensions were transferred to a 1.5 mL Eppendorf tube and centrifuged (400g for 5 minutes) before the supernatant was discarded, and then resuspended in RPMI: 10% HIFCS to a total volume of 200 μL. A total of 100 μL of cells were placed into each well of a 96-well plate for flow-cytometric analysis and stained with fluorochrome-conjugated antibodies identifying T cells and other leukocytes by two nine-color panels (Supplementary Table S1). For dead cell exclusion, a nucleic acid stain (Sytox blue; Invitrogen, Paisley, UK) was added at a dilution of 1/800 to the fluorescence activated cell sorting (FACS) tubes and incubated for 5 minutes prior to running on the flow cytometer.
Flow cytometry was undertaken with a high performance flow cytometer (Dako Cyan ADP; Beckman Coulter, High Wycombe, UK) as previously described.12 Multicolor cytometry compensation was performed using cells or compensation beads individually stained with each fluorochrome conjugated-antibody in order to circumvent spectral overlap by adjusting for false-positives from other fluorochromes. Analysis was undertaken with acquisition and analysis software (Summit 4.3 for Windows; Beckman Coulter).
Statistical Analysis
Nonparametric comparisons were undertaken with the Mann-Whitney U test, Wilcoxon signed rank test and correlations by Spearman's correlation using commercial scientific software (Prism version 5.0 for Macintosh; GraphPad Software, La Jolla, CA). Data were collected on all eyes and comparisons were undertaken between the worst affected eye in patients and arbitrarily the right eye in healthy individuals for cross-sectional analysis. Longitudinal analysis of the same eye was undertaken.
Results
Demographic Information
Of 10 patients (median age 44 [range, 18–67 years], 8 females), seven where of white European descent, two were African Caribbean, and one was South Asian. Six patients presented acutely and four with chronic disease. Five patients were categorized as TEN (Table, Supplementary Fig. S1) and all but one patient had a SCORTEN of 1 (with the exception of patient 10 who had a SCORTEN of 2). Drugs were the most common precipitating agent (n = 7). Nine patients were followed up at 12 months (one patient was unavailable for further follow-up). Visual acuity remained stable throughout the course of the study. All patients were using nonpreserved topical lubricants and 9/10 required topical nonpreserved steroids. Patients with clinical or microbiological evidence of ocular surface infection (based upon a negative conjunctival swab culture, and diagnostic PCR where indicated) were included in this study.
Table.
Demographic Information, Etiology, and Therapy During Study
|
Patient |
Ethnicity |
Age |
Sex |
Disease Severity |
Precipitant |
Days Postonset at 0 Months Assessment* |
Topical Steroids During Study Period |
Other Steroids/ Immunosuppression During Study Period |
Surgical Intervention During Study |
| 1 | White | 18 | Female | 10 | Amoxicillin | 114 (Subacute) | Yes | Pulse iv methylprednisolone | Phaco/AMG |
| 2 | African Caribbean | 41 | Female | SJS | Mycoplasma | 168 (Subacute) | Yes | None | None |
| 3 | White | 67 | Female | SJS | Trimethoprim | 16 (Acute) | Yes | None | None |
| 4 | White | 59 | Female | 10 | Phenytoin | 9178 (Chronic) | None | Azathioprine | None |
| 5 | White | 27 | Male | SJS | Unknown | 9 (Acute) | Yes | None | None |
| 6 | African Caribbean | 20 | Male | SJS | Carbamazepine | 1 (Acute) | Yes | None | None |
| 7 | White | 48 | Female | 10 | Unknown | 3657 (Chronic) | Yes | None | None |
| 8 | White | 37 | Female | 10 | Penicillin | 592 (Chronic) | Yes | None | None |
| 9 | Asian | 57 | Female | SJS | Unknown | 2047 (Chronic) | Yes | None | None |
| 10 | White | 46 | Female | 10 | Carbamazepine | 25 (Acute) | Yes | Subtarsal triamcinolone | AMG |
Acute defined as <365 days postonset (subacute defined as 30–364 days); chronic defined as >365 days postonset (Power et al., 199515; Sotozono et al., 200713). AMG, amniotic membrane grafting; iv, intravenous; Phaco, phacoemulsification cataract extraction.
Disease Activity and Staging
Conjunctival inflammation was graded as absent/mild in 9/10 patients (18/20 eyes). At presentation, 9/10 had severe disease according to Power15 versus mild according to the Sotozono score13 (median score of 8/39 [range, 1–21]). Objective evidence of fornix shrinkage using a FDM was seen in 9/10 subjects, and this inversely correlated with disease duration (r = −0.67; P < 0.05).
At 12 months follow-up, all individuals reviewed had mild conjunctival inflammation. Interestingly, all had severe disease according to Power's scoring, while the median Sotozono score remained unchanged (8/39 [range, 1–21]), despite three patients demonstrating progression according to this scale. No patients had pronounced dry eye problems with a median tear breakup time of 7 seconds (4–10). Only one patient each had evidence of lower and upper fornix shrinkage during the course of this study.
Conjunctival Epithelial Leukocyte Populations
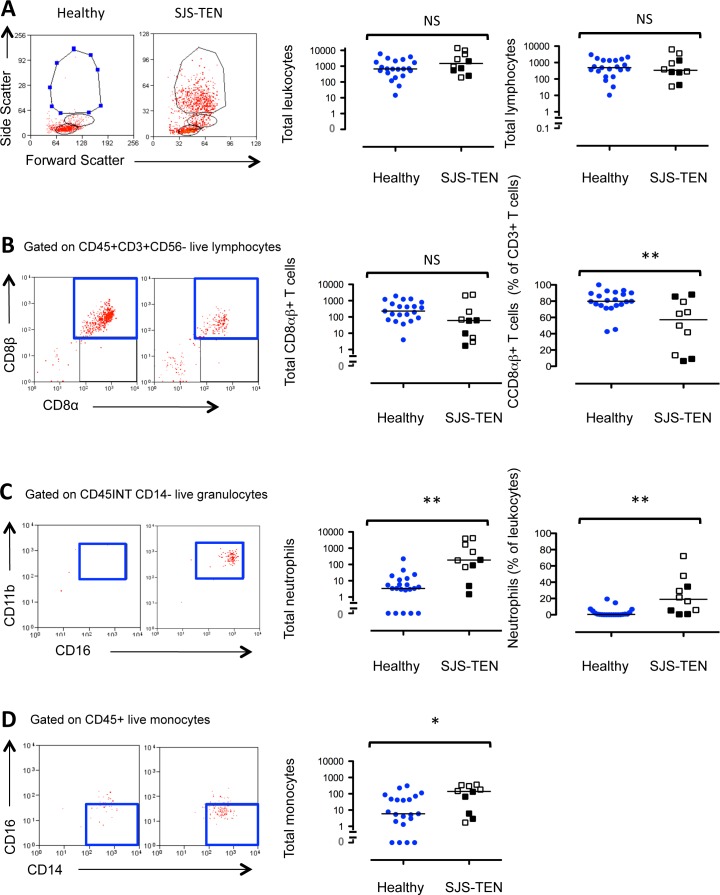
Gating strategies to characterize cellular populations are shown in Figure 1. There were no differences in the total number of conjunctival epithelial leukocytes (P = 0.15) or lymphocytes in SJS-TEN compared with controls (median 337 [range, 35–6383] vs. 488 [10–3026], P = 0.75l; Fig. 2A). No difference between the total number of CD3+CD56− T cells was observed. A reduction in the dominant CD8αβ+ cells (230 vs. 61; P = 0.1) accompanied by a significant reduction in the percentage of CD8αβ+ T cells (57% vs. 80%; P < 0.01; Fig. 2B) and a corresponding elevation of CD8β−CD4− (double negative) T cells (23% vs. 7%; P = 0.11) was seen. No differences were identified in the CD4+ (9% vs. 9%; P = 0.67); CD8αα+ T-cell subsets (1% vs. 3%; P = 0.16); or in other lymphocyte subsets, including NK cells (7.5% vs. 5%; P = 0.45), NKT cells (2% vs. 5.5%; P = 0.28) or CD19+CD20+ B cells (3% vs. 3%; P = 0.72).
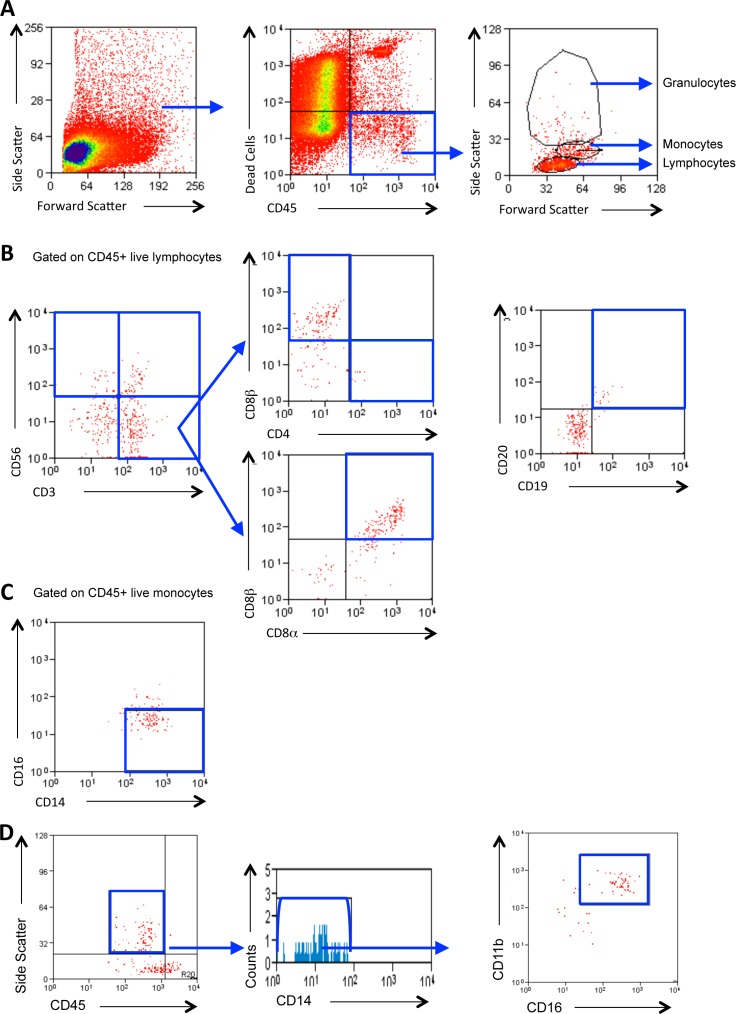
Figure 1.
Gating strategy to determine cellular populations. Representative plots of a subject with SJS-TEN demonstrating the gating strategy used to identify conjunctival leukocytes. Live leukocytes populations (lymphocytes, monocytes, and granulocytes) were identified in conjunctival cells by gating for CD45+ cells that were negative for the dead cell exclusion dye Sytox blue and back-gated to show the forward and side scatter profiles of the CD45+ live cells (A). Lymphocyte subsets were identified according to the expression of CD3 and CD56 in one panel (to discriminate T cells [CD3+CD56−]; natural killer cells [CD56+CD3−]; NKT cells [CD3+CD56+]) and B cells [CD19+CD20+] [B] [CD8+ cell populations shown are TCRαβ+]). Monocytes were further characterized by the presence of CD14 and CD16 (C). Neutrophils were defined as CD45INT, CD14−, CD11b+, and CD16+ granulocytes (D). Cell populations of interest are boxed (blue).
Figure 2.
The dominant cellular infiltrate in the conjunctival epithelium in SJS-TEN is characterized by an increase in CD45INTCD11b+CD16+CD14− neutrophils. Twenty-one healthy subjects were compared with 10 patients with SJS-TEN (six patients in the acute stages, open square; and 4 in the chronic stages of systemic disease, closed square). Representative flow cytometry plots are shown. Statistical comparisons between healthy and SJS-TEN conjunctival leukocytes and lymphocytes (A), CD45+CD3+CD56−CD8αβ+ T cells ([B], boxed); CD45INTCD11b+CD16+CD14− neutrophil (C); and CD45+CD14+CD16− monocyte (D) populations were undertaken by the Mann-Whitney U test. NS, not significant. *P > 0.05. **P < 0.01).
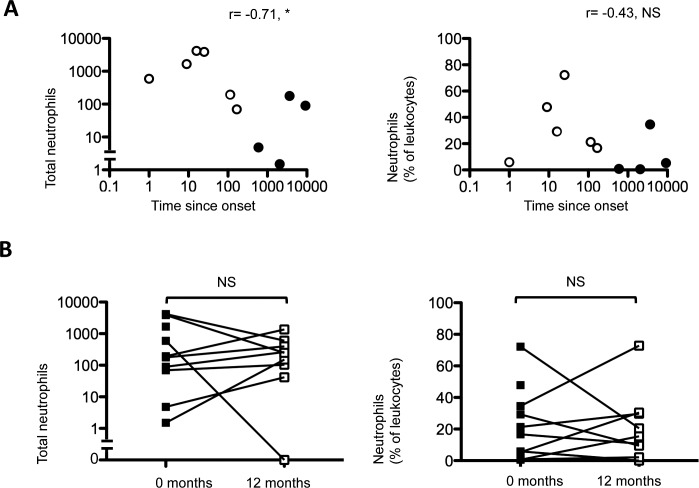
Granulocytes were defined as CD45INTCD11b+CD16+CD14− cells. This gating strategy discriminates neutrophils from other granulocytes (such as basophils and eosinophils) and monocytes.17,18 A significant increase in the number and percentage of neutrophils (186 vs. 3.4, P < 0.01;19% vs. 0.5%, P = 0.001) was seen in SJS-TEN (Fig. 2C). At presentation, however, the elevation in neutrophils were found to inversely correlate with disease duration (r = −0.71, P = 0.03; Fig. 3A). This suggests that neutrophil levels were highest in the acute stages of disease. Of interest, this elevation was maintained over the 12-month study period (P = 1.0), indicating a persistent inflammatory infiltrate (Fig. 3B) compared with healthy participants (247 vs. 3.4, P < 0.01;15% vs. 0.8%, P < 0.001).
Figure 3.
CD45INTCD11b+CD16+CD14− neutrophils are inversely correlated with disease duration but persist with time. CD45INTCD11b+CD16+CD14− neutrophil populations were correlated with disease duration at presentation (0 months; [A]) using Spearman's correlation (*P > 0.05]; **P < 0.05). Nine out of 10 patients with SJS-TEN were sampled at 0 and 12 months (B). Paired analysis of SJS-TEN conjunctival CD45INTCD11b+CD16+CD14− neutrophil populations at 0 (closed squares) and 12 months (open squares) were undertaken by the Wilcoxon signed rank test (P > 0.05; [B]).
The total number and percentage of monocytes was elevated in SJS-TEN (185 vs. 21, P = 0.02; 12% vs. 6%, P = 0.02), and this was attributable to an increase in the number of “classic” CD14+CD16− monocyte subsets19 (140 vs. 6, P = 0.03; Fig. 2D). No change in the total leukocyte (P = 0.82) or lymphocyte populations (P = 0.57) was observed during the course of the study and CD8αβ+ T cell (P = 0.57) and NK cell populations also remained unaltered (P = 1.0).
Discussion
SJS-TEN is an uncommon condition with potentially devastating lifelong ocular surface consequences. Pharmacological or infective agents are frequently cited as triggers, but in many cases, the precipitant remains unidentified.20,21 While systemic disease characteristically resolves, ocular disease may persist or a significant proportion of patients develop severe ocular disease many years after the systemic disease has subsided.6,7 The disease takes the form of a variety of clinical phenotypes such as recurrent inflammatory episodes, ocular surface stem cell failure, or scleritis. Other patients develop a mucous membrane pemphigoid (MMP)–like phenotype, with indistinguishable clinical and immunohistological features from MMP.22 Dysregulated innate immune responses are thought to contribute,23 epithelial expression of HLA-DR correlate with the presence of inflammatory dry eye24; but the underpinning mechanisms for chronic SJS-TEN are largely unknown. No disease-specific biomarkers for prediction of ocular phenotype, prognosis nor for monitoring activity, damage, and progression of disease currently exist.
At a molecular level, the initial insult in acute disease is thought to be a cell-mediated phenomenon regulated by the soluble factor granulysin produced by CD8+ T cells and NK+ cells, resulting in severe disruption of epithelial surfaces including the skin.8 While these studies did not examine ocular disease, earlier studies have identified corneal CD8+ T cells with epithelial vacuolation in a patient with TEN,9 and a predominant CD8+ T-cell population in healthy human conjunctival epithelium.12,25 In our current study, we found that conjunctival CD8+ and NK+ populations were not significantly elevated in SJS-TEN compared with healthy controls and this remained unchanged over time. This discrepancy might be explained by the fact that SJS-TEN–induced damage is thought to be mediated by granulysin rather than the extent of cytotoxic cellular infiltrate.8,26 Conversely, both CD14+ monocytes and CD45INTCD11b+CD16+CD14− neutrophils were elevated in SJS-TEN in acute and chronic stages of disease. The precise definition of acute and chronic ocular SJS-TEN has not been universally agreed, so we used a cutoff of >12 months to define chronic disease as described by Sotozono and colleagues.13 An increase in CD14+ monocytes has previously been demonstrated in the skin of patients with SJS-TEN by immunostaining of cryosections. The advantage of undertaking multicolor flow cytometry has been the ability to utilize multiple cell surface markers combined with scatter discrimination, which allowed us to determine both monocyte and neutrophil populations.27 The elevation in neutrophils was inversely correlated with the duration of disease, indicating a higher proportion in those with acute disease. During the study period of 12 months, however, the elevation of neutrophils was maintained with time, indicating a persistent inflammatory response in the absence of culture-positive infection. The presence of mucosal neutrophils may indicate innate inflammatory or complement mediated processes.28 Although recent evidence has suggested a role for neutrophils in dry eye disease states, including extracellular DNA and neutrophil extracellular trap,29 none of our patients had severe dry eye problems that could account for these changes. Moreover, dry eye disease (Sjögren's syndrome, experimental dry models) has been associated with an elevation in CD4+ T cells30,31 rather than CD8+ cells, indicating a disparate disease mechanism driving the two forms of ocular surface disease.
A critical role of neutrophils is to disrupt bacteria by producing elastases and proteinases that in turn could also induce local tissue disruption.28,32 Mice lacking neutrophil elastase have been shown to be resistant to pulmonary fibrosis33 and it is possible that this may provide an important therapeutic target in progressive human conjunctival scarring disease such as SJS-TEN. Furthermore, a persistent elevation in neutrophils may be triggered by disordered innate immune processes and our data supports this hypothesis. This is of particular interest in the context of recurrent ocular inflammation in chronic SJS-TEN,23 where TLR-3 polymorphisms have been linked to a cohort of Japanese patients,10 and the downregulation of both prostaglandin receptors EP3 and EP4, and IL-1α expression in monocytes are all thought to abrogate anti-inflammatory effects in SJS-TEN.34–36 Genetic susceptibility has also been postulated as one of the reasons for abnormal drug reactions in some individuals and a predisposition to SJS in others.23
We accept that OSIC recovers cells from the superficial layers of the epithelium, and will not access the submucosa unless there is frank stromal ulceration. Notwithstanding, it has been used in a number of studies investigating dry eye disease states quantifying reduction of conjunctival goblet cells (MUC5AC) and HLA-DR in patients with TEN and dry eye disease, respectively.37 Other than fundamental CD4, CD8, and CD14 surface expression,38 it has not been used to carry out a detailed interrogation of ocular surface inflammatory cellular infiltrates by multicolor panels and gating strategies as described in our study. The technique has enabled us to highlight that in ocular SJS-TEN, an inflammatory infiltrate with CD45INTCD11b+CD16+CD14− neutrophils is present even in mildly involved or clinically quiescent conjunctival mucosa, indicating the persistence of clinically occult inflammation. There is also a reduction in the percentage of CD8αβ+ cells in the superficial conjunctival epithelium during acute and chronic disease and an elevation in CD14+ monocytes. While neutrophil numbers inversely correlate with disease duration, they persist above levels found in healthy subjects.
The potential to study the presence of neutrophils in early SJS-TEN by OSIC is attractive, not just because of its noninvasive methodology, but because it affords the opportunity to undertake longitudinal sampling in a cohort study. In the absence of both a consistent clinical scoring system and biomarker of ocular disease, this technique combined with multicolor flow cytometry warrants further investigation, enabling us to explore the potential role of neutrophils as putative biomarkers of occult chronic inflammation and its effects on local ocular sequelae.
Supplementary Material
Acknowledgments
Presented in part at the International Ocular Surface Society Meeting, Hollywood, Florida, April 2011, and the annual meeting for the Association for Research in Vision and Ophthalmology, Fort Lauderdale, Florida, May 2011.
Supported by Wellcome Trust, United Kingdom (Clinical Fellowship to GPW), Birmingham Eye Foundation (Registered [UK] Charity 257549). The authors alone are responsible for the content and writing of the paper.
Disclosure: G.P. Williams, None; P.J. Tomlins, None; A.K. Denniston, None; H.S. Southworth, None; S. Sreekantham, None; S.J. Curnow, None; S. Rauz, None
References
- 1. Wojnarowska FT, Briggaman RA. Management of Blistering Diseases. London: Chapman and Hall Medical; 1990. [Google Scholar]
- 2. Saw VP, Dart JK. Ocular mucous membrane pemphigoid: diagnosis and management strategies. Ocul Surf. 2008; 6: 128–142 [DOI] [PubMed] [Google Scholar]
- 3. Bastuji-Garin S, Fouchard N, Bertocchi M, Roujeau JC, Revuz J, Wolkenstein P. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000; 115: 149–153 [DOI] [PubMed] [Google Scholar]
- 4. Schopf E, Stuhmer A, Rzany B, Victor N, Zentgraf R, Kapp JF. Toxic epidermal necrolysis and Stevens-Johnson syndrome. An epidemiologic study from West Germany. Arch Dermatol. 1991; 127: 839–842 [DOI] [PubMed] [Google Scholar]
- 5. Bastuji-Garin S, Rzany B, Stern RS, Shear NH, Naldi L, Roujeau JC. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993; 129: 92–96 [PubMed] [Google Scholar]
- 6. Foster CS, Fong LP, Azar D, Kenyon KR. Episodic conjunctival inflammation after Stevens-Johnson syndrome. Ophthalmology. 1988; 95: 453–462 [DOI] [PubMed] [Google Scholar]
- 7. De Rojas MV, Dart JK, Saw VP. The natural history of Stevens Johnson syndrome: patterns of chronic ocular disease and the role of systemic immunosuppressive therapy. Br J Ophthalmol. 2007; 91: 1048–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chung WH, Hung SI, Yang JY, et al. Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nat Med. 2008; 14: 1343–1350 [DOI] [PubMed] [Google Scholar]
- 9. Williams GP, Mudhar HS, Leyland M. Early pathological features of the cornea in toxic epidermal necrolysis. Br J Ophthalmol. 2007; 91: 1129–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ueta M, Sotozono C, Inatomi T, et al. Toll-like receptor 3 gene polymorphisms in Japanese patients with Stevens-Johnson syndrome. Br J Ophthalmol. 2007; 91: 962–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brignole-Baudouin F, Ott AC, Warnet JM, Baudouin C. Flow cytometry in conjunctival impression cytology: a new tool for exploring ocular surface pathologies. Exp Eye Res. 2004; 78: 473–481 [DOI] [PubMed] [Google Scholar]
- 12. Williams GP, Denniston AK, Oswal KS, et al. The dominant human conjunctival epithelial CD8alphabeta+ T cell population is maintained with age but the number of CD4+ T cells increases. Age (Dordr). 2012; 34: 1517–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sotozono C, Ang LP, Koizumi N, et al. New grading system for the evaluation of chronic ocular manifestations in patients with Stevens-Johnson syndrome. Ophthalmology. 2007; 114: 1294–1302 [DOI] [PubMed] [Google Scholar]
- 14. Elder MJ, Bernauer W. Monitoring of activity and progression in cicatrising conjunctivitis. In: Bernauer W, Dart JKG, Elder MJ. eds Cicatrising Conjunctivitis. Basel, Switzerland: Karger; 1997: 111–122 [DOI] [PubMed] [Google Scholar]
- 15. Power WJ, Ghoraishi M, Merayo-Lloves J, Neves RA, Foster CS. Analysis of the acute ophthalmic manifestations of the erythema multiforme/Stevens-Johnson syndrome/toxic epidermal necrolysis disease spectrum. Ophthalmology. 1995; 102: 1669–1676 [DOI] [PubMed] [Google Scholar]
- 16. Williams GP, Saw VP, Saeed T, et al. Validation of a fornix depth measurer: a putative tool for the assessment of progressive cicatrising conjunctivitis. Br J Ophthalmol. 2011; 95: 842–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stelzer GT, Shults KE, Loken MR. CD45 gating for routine flow cytometric analysis of human bone marrow specimens. Ann N Y Acad Sci. 1993; 677: 265–280 [DOI] [PubMed] [Google Scholar]
- 18. Gopinath R, Nutman TB. Identification of eosinophils in lysed whole blood using side scatter and CD16 negativity. Cytometry. 1997; 30: 313–316 [PubMed] [Google Scholar]
- 19. Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol. 2007; 81: 584–592 [DOI] [PubMed] [Google Scholar]
- 20. Roujeau JC, Kelly JP, Naldi L, et al. Medication use and the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. N Engl J Med. 1995; 333: 1600–1607 [DOI] [PubMed] [Google Scholar]
- 21. French LE. Toxic epidermal necrolysis and Stevens Johnson syndrome: our current understanding. Allergol Int. 2006; 55: 9–16 [DOI] [PubMed] [Google Scholar]
- 22. Chan LS, Soong HK, Foster CS, Hammerberg C, Cooper KD. Ocular cicatricial pemphigoid occurring as a sequela of Stevens-Johnson syndrome. JAMA. 1991; 266: 1543–1546 [PubMed] [Google Scholar]
- 23. Ueta M, Kinoshita S. Ocular surface inflammation mediated by innate immunity. Eye Contact Lens. 2010; 36: 269–281 [DOI] [PubMed] [Google Scholar]
- 24. Baudouin C, Brignole F, Pisella PJ, De Jean MS, Goguel A. Flow cytometric analysis of the inflammatory marker HLA DR in dry eye syndrome: results from 12 months of randomized treatment with topical cyclosporin A. Adv Exp Med Biol. 2002; 506: 761–769 [DOI] [PubMed] [Google Scholar]
- 25. Hingorani M, Metz D, Lightman SL. Characterisation of the normal conjunctival leukocyte population. Exp Eye Res. 1997; 64: 905–912 [DOI] [PubMed] [Google Scholar]
- 26. Quinn AM, Brown K, Bonish BK, et al. Uncovering histologic criteria with prognostic significance in toxic epidermal necrolysis. Arch Dermatol. 2005; 141: 683–687 [DOI] [PubMed] [Google Scholar]
- 27. Tohyama M, Watanabe H, Murakami S, et al. Possible involvement of CD14+ CD16+ monocyte lineage cells in the epidermal damage of Stevens-Johnson syndrome and toxic epidermal necrolysis. Br J Dermatol. 2012; 166: 322–330 [DOI] [PubMed] [Google Scholar]
- 28. Neutrophils Nathan C. and immunity: challenges and opportunities. Nat Rev Immunol. 2006; 6: 173–182 [DOI] [PubMed] [Google Scholar]
- 29. Sonawane S, Khanolkar V, Namavari A, et al. Ocular surface extracellular DNA and nuclease activity imbalance: a new paradigm for inflammation in dry eye disease. Invest Ophthalmol Vis Sci. 2012; 53: 8253–8263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stern ME, Gao J, Schwalb TA, et al. Conjunctival T-cell subpopulations in Sjogren's and non-Sjogren's patients with dry eye. Invest Ophthalmol Vis Sci. 2002; 43: 2609–2614 [PubMed] [Google Scholar]
- 31. Niederkorn JY, Stern ME, Pflugfelder SC, et al. Desiccating stress induces T cell-mediated Sjögren's Syndrome-like lacrimal keratoconjunctivitis. J Immunol. 2006; 176: 3950–3957 [DOI] [PubMed] [Google Scholar]
- 32. Witko-Sarsat V, Rieu P, Descamps-Latscha B, Lesavre P, Halbwachs-Mecarelli L. Neutrophils: molecules, functions and pathophysiological aspects. Lab Invest. 2000; 80: 617–653 [DOI] [PubMed] [Google Scholar]
- 33. Chua F, Dunsmore SE, Clingen PH, et al. Mice lacking neutrophil elastase are resistant to bleomycin-induced pulmonary fibrosis. Am J Pathol. 2007; 170: 65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ueta M, Sotozono C, Yokoi N, Inatomi T, Kinoshita S. Prostaglandin E receptor subtype EP3 expression in human conjunctival epithelium and its changes in various ocular surface disorders. PLoS One. 2011; 6: e25209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ueta M, Sotozono C, Yamada K, Yokoi N, Inatomi T, Kinoshita S. Expression of prostaglandin E receptor subtype EP4 in conjunctival epithelium of patients with ocular surface disorders: case-control study. BMJ Open. 2012; 2:e001330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ueta M. Innate immunity of the ocular surface and ocular surface inflammatory disorders. Cornea. 2008; 27 (suppl 1): S31–S40 [DOI] [PubMed] [Google Scholar]
- 37. Lopez-Garcia JS, Rivas Jara L, Garcia-Lozano CI, Conesa E, de Juan IE, Murube del Castillo J. Ocular features and histopathologic changes during follow-up of toxic epidermal necrolysis. Ophthalmology. 2011; 118: 265–271 [DOI] [PubMed] [Google Scholar]
- 38. Barabino S, Montaldo E, Solignani F, Valente C, Mingari MC, Rolando M. Immune response in the conjunctival epithelium of patients with dry eye. Exp Eye Res. 2010; 91: 524–529 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.