Abstract
We previously demonstrated that sodium butyrate is neuroprotective in Huntington's disease (HD) mice and that this therapeutic effect is associated with increased expression of mitogen-activated protein kinase/dual-specificity phosphatase 1 (MKP-1/DUSP1). Here we show that enhancing MKP-1 expression is sufficient to achieve neuroprotection in lentiviral models of HD. Wild-type MKP-1 overexpression inhibited apoptosis in primary striatal neurons exposed to an N-terminal fragment of polyglutamine-expanded huntingtin (Htt171–82Q), blocking caspase-3 activation and significantly reducing neuronal cell death. This neuroprotective effect of MKP-1 was demonstrated to be dependent on its enzymatic activity, being ablated by mutation of its phosphatase domain and being attributed to inhibition of specific MAP kinases (MAPKs). Overexpression of MKP-1 prevented the polyglutamine-expanded huntingtin-induced activation of c-Jun N-terminal kinases (JNKs) and p38 MAPKs, whereas extracellular signal-regulated kinase (ERK) 1/2 activation was not altered by either polyglutamine-expanded Htt or MKP-1. Moreover, mutants of MKP-1 that selectively prevented p38 or JNK binding confirmed the important dual contributions of p38 and JNK regulation to MKP-1-mediated neuroprotection. These results demonstrate additive effects of p38 and JNK MAPK inhibition by MKP-1 without consequence to ERK activation in this striatal neuron-based paradigm. MKP-1 also provided neuroprotection in vivo in a lentiviral model of HD neuropathology in rat striatum. Together, these data extend previous evidence that JNK- and p38-mediated pathways contribute to HD pathogenesis and, importantly, show that therapies simultaneously inhibiting both JNK and p38 signaling pathways may lead to improved neuroprotective outcomes.
Introduction
Huntington's disease (HD) is a hereditary neurodegenerative disorder caused by a polyglutamine-encoding CAG repeat expansion in the huntingtin (HTT) gene (Huntington's Disease Collaborative Research Group, 1993). HD is typified pathologically by intraneuronal huntingtin protein (Htt) inclusion bodies and loss of neurons in the striatum and cerebral cortex (Luthi-Carter, 2007). Clinically, HD often presents as a movement disorder with subtle psychiatric features, although in some individuals the balance of symptoms is inversed (Thu et al., 2010). Despite the long interval since the identification of the mutation, existing therapies remain only palliative, providing great impetus to find ways of preventing striatal and cortical neurodegeneration in HD.
Mitogen-activated protein kinases (MAPKs) are a conserved family of enzymes comprising several subclasses, including extracellular signal-regulated kinase 1/2 (ERK1/2), JNKs, and p38s. MAPKs mediate intracellular signaling responses to external stimuli and regulate diverse cellular activities, including proliferation, differentiation, stress response, neuronal plasticity, and apoptosis (Keshet and Seger, 2010). Activation of MAPKs occurs via multitiered kinase cascades through dual phosphorylation of threonine and tyrosine residues.
Negative regulation of MAPK activity is achieved via dual-specificity phosphatases (DUSPs), also known as MAPK phosphatases (MKPs). Numerous MKPs have coevolved with different substrate specificities and subcellular localizations to achieve diverse cellular functions (Farooq and Zhou, 2004). MKP-1 (DUSP1) is an inducible immediate-early gene expressed in response to stressors, such as heat shock or oxidative damage (Patterson et al., 2009) but can also be upregulated by normal neurotransmitter receptor activation (Horita et al., 2010).
Abnormal MAPK signaling is a known mechanism of neurodegeneration in many human disorders, including HD. Activation of JNK (Liu, 1998; Merienne et al., 2003; Perrin et al., 2009; Reijonen et al., 2010) and p38 (Gianfriddo et al., 2004; Rangone et al., 2004; Reijonen et al., 2010; Wang et al., 2010) has been demonstrated in many HD models and is primarily associated with neurotoxicity, whereas increased ERK activity (Apostol et al., 2006; Roze et al., 2008) has been largely associated with pathways of compensation or neuroprotective intervention in HD (Varma et al., 2007; Lee et al., 2008; Gokce et al., 2009; Scotter et al., 2010; Varma et al., 2010).
Conversely, we have observed early and sustained reduction of MKP-1 expression in R6/2 HD mouse brain (Luthi-Carter et al., 2002). Moreover, sodium butyrate, a histone deacetylase inhibitor that provided significant neuroprotection and increased survival in R6/2 mice, induced upregulation of MKP-1 expression (Ferrante et al., 2003). These results suggested that decreased MKP-1 expression might participate in the hyperactivation of MAPKs in HD and that increasing MKP activity could be neuroprotective.
In this study, we tested the hypothesis that increased MKP-1 levels mitigate the toxicity of polyglutamine-expanded Htt and assessed the molecular mechanisms of MKP-1's neuroprotective effect. These data further corroborated the role of MKP-1 downregulation as a contributor to HD pathogenesis and demonstrated that neuronal MKP-1 activity is ideally adapted for neuroprotection: MKP-1 achieves dual inhibition of JNK and p38 while preserving the activity of ERK, thereby providing a desirable balance of inhibiting toxic MAPK-mediated effects while sparing protective MAPK signaling mechanisms.
Materials and Methods
Microarray analysis.
A total of 10 μg of total RNA from individual R6/2 mouse tissue samples (n = 3 or 4/group, CAG repeat length of 150 ± 7) were processed using the Affymetrix GeneChip One-Cycle Amplification kit, and corresponding cRNA probes were hybridized to MOE430 arrays and analyzed as described by Stack et al. (2007), focusing on differences between saline-treated HD and wild-type mice, and between drug-treated and untreated R6/2 HD mice. The raw microarray data are available in the Gene Expression Omnibus database (GSE1980).
Primary neuronal cultures.
Striatal and cortical tissues were dissected from ganglionic eminences of embryonic day 16 rat embryos and mechanically dissociated with a flame-polished Pasteur pipette. The resulting cultures contain primarily neuronal nuclear antigen (NeuN)-positive neurons and some astrocytes (Zala et al., 2005). Cultures were maintained in Neurobasal medium (Invitrogen) supplemented with B-27 (Invitrogen), 0.5 mm glutamine (Invitrogen), 1× penicillin/streptomycin (Invitrogen), and 150 mm KCl.
cDNAs.
The following plasmids were used to prepare self-inactivating lentiviral expression vectors. cDNAs encoding wild-type and polyglutamine-expanded N-terminal sequences (171 amino acids) of Htt under the control of a TRE promoter (SIN-PGK-htt171–18Q/82Q-WHV) and the tTA transactivator under the control of the mouse PGK (phosphoglycerate kinase) promoter (SIN-PGK-tTA-WHV) have been described previously (Rudinskiy et al., 2009). All MKP-1 constructs consisted of rat cDNAs to which sequence encoding a C-terminal myc tag (EQKLISEEDL) was added. Inactive MKP-1 contained a cysteine to serine mutation at position 258 as previously described (Sun et al., 1993). JNK-specific MKP-1 (MKP-1JNK) consisted of mutation of RRR (three arginines) to MMM (three methionines) at amino acids 53–55 (Slack et al., 2001). We also tested the effect of the mutation of amino acids 53–55 to ASA (alanine-serine-alanine) (Slack et al., 2001), which was less effective at specific JNK1/2/3 inactivation than the triple methionine (MMM) mutant. p38-specific MKP-1 (MKP-1p38) consisted of mutation of RGR (arginine-glycine-arginine) to MGM (methionine-glycine-methionine) at amino acids 72–74 (Tanoue et al., 2002). Mutants with increased protein stability were created by mutation of serine residues to aspartic acid at amino acids 359 and 364 (Brondello et al., 1999) and used in some Western blot experiments. The parallel expression of rat MKP-3 and its CFP and YFP comparison groups was directed by the mouse PGK promoter. The MKP-3 construct included an encoded N-terminal hemagglutinin-tag (YPYDVPDYA).
Lentiviral preparation and infection of primary neurons.
Lentiviral vectors were produced by a four-plasmid system via transfection into human embryonic kidney 239T (HEK 293T) cells as described by Zala et al. (2005). Pelleted virus was resuspended in 1% BSA/PBS and titered by p24 antigen ELISA (RETROtek; Gentaur). Primary cultures were coinfected with Htt (25 ng of p24/ml) and tTA-encoding vectors (40 ng of p24/ml) on DIV1 and MKP-1-encoding vectors (25 ng of p24/ml) were applied on DIV4. Adjustment of the expression of different forms of MKP-1 was performed based on quantitative PCR to achieve equivalent levels.
Antibodies.
Primary antibodies used in this study were as follows: mouse monoclonal anti-c-myc (Ecole Polytechnique Fédérale de Lausanne in-house clone 9E10; ICC, 1:10,000, IHC, 1:500, WB, 1:3000; and Sigma, M4439 clone 9E10; ICC, 1:500, IHC, 1:200, WB, 1:3000), mouse monoclonal anti-HA (Covance, HA.11 clone 16B12; WB, 1:2500), mouse monoclonal anti-Htt (Millipore, MAB5492 clone 2B4; ICC, 1:50, IHC, 1:100, WB, 1:2500), rabbit monoclonal anti-JNK3 (Millipore, 05–893 clone CO5; IHC, 1:200, WB, 1:1000), rabbit polyclonal anti-JNK1/2 (SAPK/JNK) (Cell Signaling Technology, 9252; IHC, 1:500, WB, 1:2000), rabbit polyclonal anti-p38α, β, γ (Cell Signaling Technology, 9212S; IHC, 1:100, WB, 1:1000), rabbit monoclonal anti-phospho p38 (Cell Signaling Technology, 9215S clone 3D7; IHC, 1:100, WB, 1:1000), mouse monoclonal anti-phospho JNK1/2 (Cell Signaling Technology, 9255S clone G9; IHC, 1:1000), rabbit polyclonal anti-p38δ (Cell Signaling Technology, 9214; IHC, 1:100, IHC, 1:1000), rabbit polyclonal anti-phospho JNK1/2/3 (Abcam, ab59196; IHC, 1:200, WB, 1:2000), rabbit polyclonal anti-ERK1/2 (Millipore, 06–182; IHC, 1:100, WB, 1:1000), mouse monoclonal anti-phospho ERK1/2 (Millipore, 05–481 clone 12D4; IHC, 1:100, WB, 1:500), rabbit polyclonal anti-MKP-1 (Santa Cruz Biotechnology, V-15 sc-1199; IHC, 1:100), rabbit polyclonal MKP-1 (Millipore, 07–535; WB, 1:1000), goat polyclonal anti-MKP-3 (Santa Cruz Biotechnology, C-20 sc-8599; ICC, 1:300, IHC, 1:100), mouse monoclonal anti-α-tubulin (Sigma, T5168 clone B-5–1-2; WB, 1:10000), mouse monoclonal anti-neuronal nuclear antigen (NeuN) (Millipore, MAB377 clone A60; ICC, 1:500, IHC, 1:200), rabbit polyclonal anti-DARPP-32 (Millipore Bioscience Research Reagents, AB1656; 1:10,000; Cell Signaling Technology, 2302; 1:200), and mouse monoclonal anti-neuronal class III β-tubulin (Covance, MMS-435P clone TUJ1; ICC, 1:10,000). Secondary antibodies and their concentrations are composed of the following: donkey anti-mouse IRDye800CW (LI-COR Biosciences, 926–32,212; WB-1:20,000), donkey anti-rabbit IRDye680 (LI-COR Biosciences, 926–32223; WB-1:20,000), donkey anti-goat IRDye800CW (LI-COR Biosciences, 926–32214; WB-1:20,000), goat anti-mouse IgG (H+L) Cy3 552 (Jackson ImmunoResearch Laboratories, 115–166-003; ICC, 1:10,000), goat anti-mouse IgG (H+L) AlexaFluor-594 (Invitrogen, A11032; ICC/IHC, 1:500), goat anti-rabbit IgG (H+L) AlexaFluor-488 (Invitrogen, A11008; ICC/IHC, 1:500), and rabbit anti-goat IgG (H+L) AlexaFluor-594 (Invitrogen, A11080; ICC/IHC, 1:500).
Quantitative real-time PCR.
RNA from rat primary cultures was extracted on the indicated days in vitro using the RNeasy Mini Kit (QIAGEN) and then subsequently precipitated with sodium acetate and ethanol. RNA samples for RT-PCR were prepared as described by Kuhn et al. (2007) for R6/2 mice and as described in Hodges et al. (2006) for human caudate. For knock-in CHL2 mice, RNA was processed with Power SYBR Green RNA to CT (Applied Biosystems, no. 4389986). For all other samples, reverse transcription was performed with the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems), and the resulting cDNA (from 2 ng of total RNA per reaction) was mixed with Power SYBR Green PCR Master Mix (Applied Biosystems) and primers (see next paragraph). Reactions were run with an Applied Biosystems 7900 HT Real-Time PCR System and analyzed with SDS Version 2.3 software.
Primers were designed using Primer Express (Applied Biosystems) and obtained from Microsynth AG. Rat primer sequences were as follows: MKP-1 forward, CTG CTT TGA TCA ACG TCT CG; reverse, AAG CTG AAG TTG GGG GAG AT; MKP-3 forward, ATC TTG AAC GTC ACC CCC AA; reverse, CTC CCC TGC ATT CTC AAA CAG; ERK1 forward, ATT GAC ATC TGG TCT GTG GGC; reverse, CCG GTT GGA GAG CAT CTC AG; ERK2 forward, GCT TTC TCT CCC GCA CAA AA; reverse, TTT GGG AAC AAC CTG TTC CAC; JNK1 forward, GCC AGT CAG GCG AGA GAT TT; reverse, GGA CGC ATC TAT CAC CAG CA; JNK2 forward, GTG ACA GTA AAA GCG ATG GCC; reverse, TTG AGT CTG CCA CTT GCA CAC; JNK3 forward, CTG ATG GAC GCC AAC TTG TG; reverse, CTC GTG GTC CAG CTC CAT CT; p38α forward, CTC GGT GTG TGC TGC TTT TG; reverse, TTC ACT GCC ACA CGA TGT CC; p38β forward, CCT CCT TGG AAG AAT GCT GG; reverse, GCC GCA CTG ACT CTC TGG TC; p38δ forward, TCC AGT CGG AGA TCT TTG CC; reverse, TCA ACA GCA GGA GTT CGC G; and p38γ forward, AGT CGG AGC TGT TTG CCA AG; reverse, GTT TGA GGA GGC GCA ACT CT. Primer sequences for mouse comprised: MKP-1 forward, GGC CAG CTG CTG CAG TTT GAG T; reverse, AGG TGC CCC GGT CAA GGA CA; UbC (reference expression control for CHL2 samples): forward, GAG TTC CGT CTG CTG TGT GA; reverse, CCT CCA GGG TGA TGG TCT TA. Primer sequences for human MKP-1 consisted of the following: forward, CCC GGA GCT GTG CAG CAA ACA; and reverse, ATT TCC ACC GGG CCA CCC TGA.
Western blotting.
Samples were harvested in standard RIPA buffer supplemented with 2 mm EGTA, 2 mm EDTA, 1 mm DTT, and protease inhibitor cocktail (Pierce Chemical), mechanically homogenized with 1.5 ml of pellet mixers (Trefflab/VWR), and centrifuged at 20,000 × g. The supernatants were then separated on a 12.5% SDS polyacrylamide gel and transferred to nitrocellulose. The LI-COR Odyssey system was used for detection. Each biological condition was performed in triplicate (Fig. 5) or duplicate (Fig. 6) in each experiment, and each experiment was performed on two or three separate primary neuronal culture batches. Scion Image (Scion, National Institutes of Health) was used for quantification of blots, and each value represents the mean of means from all experiments (images shown are representative). JNKs 2 and 3 were analyzed together in Figure 6 to accommodate the fact that not all of the three Western blot sets revealed adequate separation of these two bands.
Figure 5.
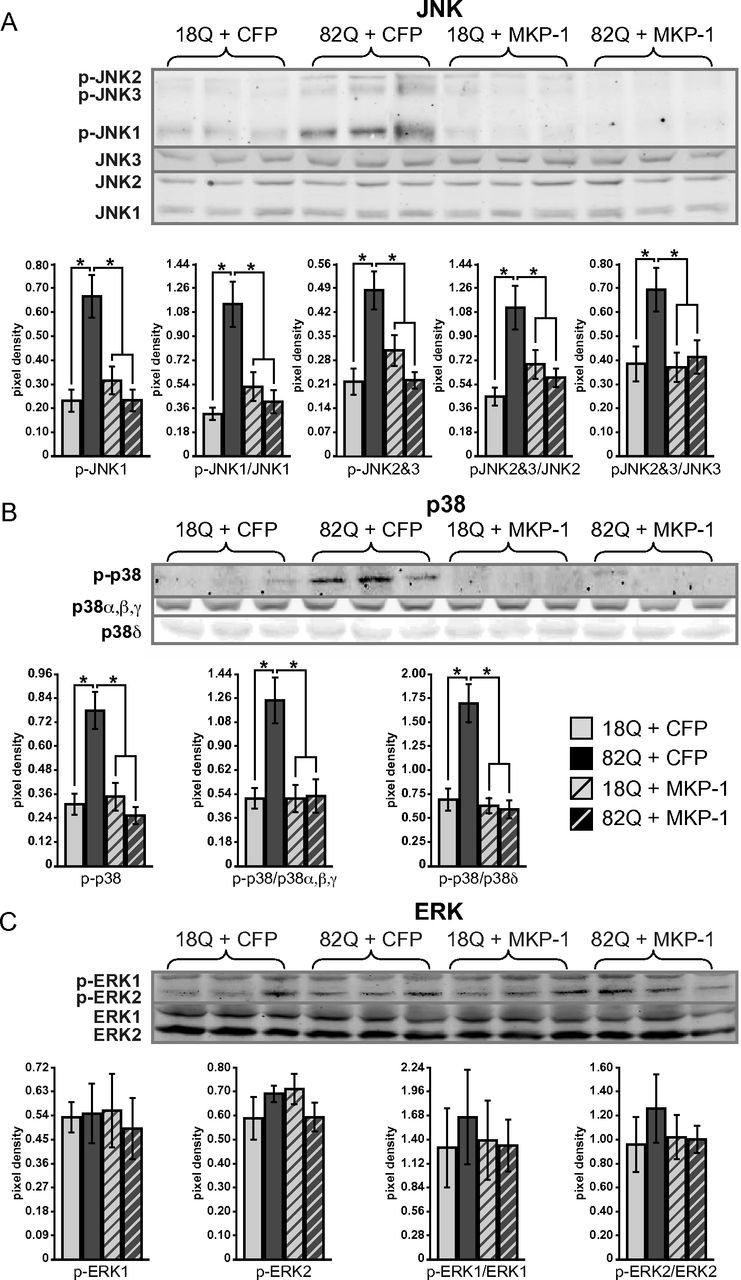
MKP-1 overexpression inhibits a polyglutamine-expanded Htt-conferred activation of JNK and p38, with no effect on ERK phosphorylation. A, Phosphorylation of JNKs 1, 2, and 3 are elevated in striatal cultures coexpressing polyglutamine-expanded Htt and CFP compared with wild-type, which are reduced to wild-type levels by coexpression with MKP-1. Quantification of duplicate blots shows a significant polyglutamine-length-dependent increase in phosphorylation and its inhibition by MKP-1 (after normalization to tubulin loading control or to respective total JNK levels). B, Phosphorylation of p38 is elevated in striatal cultures coexpressing polyglutamine-expanded Htt and CFP compared with wild-type, and is reduced to wild-type levels by coexpression with MKP-1. Quantification of duplicate blots shows a significant polyglutamine-length-dependent increase in phosphorylation and its inhibition by MKP-1 (when normalized to tubulin loading control or to a single band representing total p38 α, β, and γ levels, or the band revealed by an antibody detecting p38δ). C, No change in phosphorylated ERK levels was detected by expression of polyglutamine-expanded Htt or coexpression with MKP-1. *p < 0.05.
Figure 6.
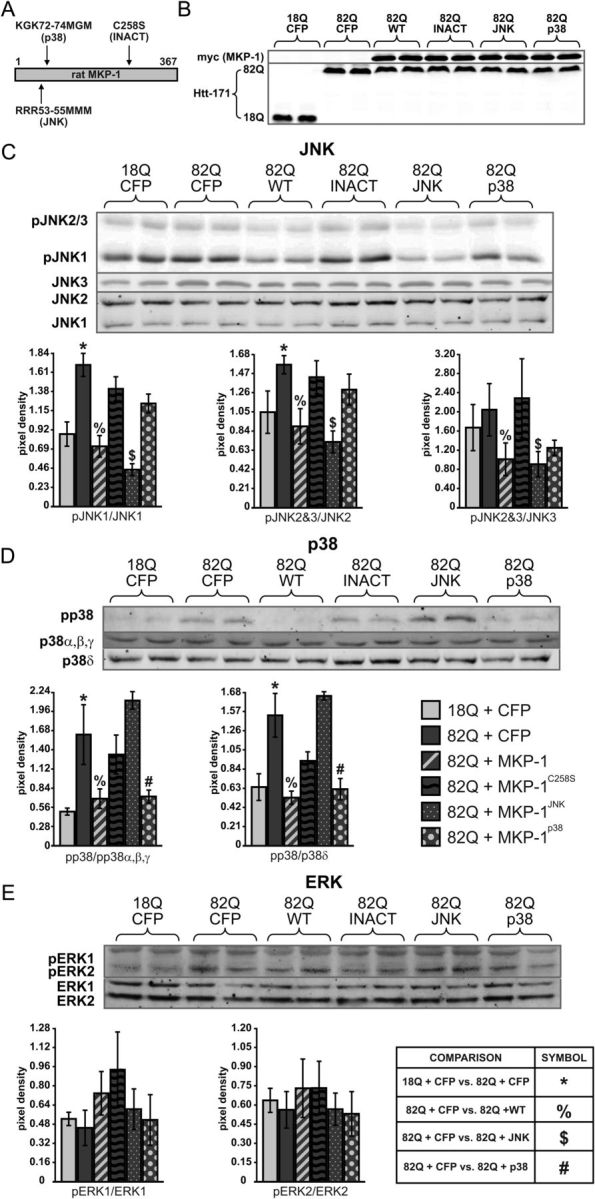
Mutation of MKP-1 creates inactive, JNK-specific, and p38-selective forms. A, Representation of mutations used to create three forms of MKP-1 with varying activity and specificities. B, Control Western blots representing overexpression of MKP-1 and both wild-type and polyglutamine-expanded Htt. C, Western blots and corresponding quantification demonstrating that phosphorylation of JNKs 1, 2, and 3 is elevated in striatal cultures expressing polyglutamine-expanded Htt and CFP and is reduced to wild-type levels by coexpression with wild-type (MKP-1wt) and JNK-specific MKP-1 (MKP-1JNK), but not inactive (MKP-1C258S) or selectively p38-targeting (MKP-1p38) mutants. Quantification of blots was normalized to a tubulin loading control and to total JNK levels. D, Western blots and corresponding quantification demonstrating that phosphorylation of p38 is elevated in striatal cultures expressing polyglutamine-expanded Htt and CFP compared with wild-type and is reduced to wild-type levels by coexpression with wild-type or p38-specific MKP-1, but not inactive or JNK-specific mutants. Quantification of blots was normalized to a tubulin loading control and to a single band representing total p38 α, β, and γ levels, or the band revealed by an antibody detecting p38δ. E, No change in phosphorylated ERK levels was detected by expression of polyglutamine-expanded Htt or coexpression with any of the four MKP-1 forms. Symbols denote p < 0.05 in indicated comparison.
Immunocytochemistry.
For 96-well dishes, cultures were fixed with 4% paraformaldehyde/PBS for 10 min at 4°C and then rinsed three times with PBS. Normal goat serum (10%/PBS) with 0.1% Triton-X was used for blocking followed by incubation in primary antibody diluted in 5% goat serum blocking buffer overnight (∼16 h) at 4°C and three rinses with PBS. Cells were then incubated in secondary antibody diluted in 1% goat serum, Triton-X-free blocking buffer at room temperature for 2 h, followed by three rinses with PBS. All steps during and after secondary antibody incubation were performed with protection from light.
Cells cultured on coverslips were fixed as described in the previous paragraph, followed by incubation in 0.5% NP-40/PBS for 1 min, then 4% paraformaldehyde/PBS for 2 min, and removed directly to blocking solution of 3% BSA/PBS. After a 30 min blocking step, primary antibody diluted in blocking solution was applied for 30 min followed by three rinses with PBS and 30 min incubation with secondary antibody in blocking solution. After three rinses, coverslips were mounted on slides with Immu-mount (Shandon). All steps after the initial fixation were performed at room temperature.
Analyses of neuronal viability by quantification of NeuN- and β-3 tubulin-immunostained cells.
Black 96-well assay plates (Corning, 3603) were imaged using Pathway 835 or 855 High Content Bioimagers (BD Biosciences) yielding composite 2 × 2 images of each culture well. Images were then analyzed using ImageJ (National Institutes of Health) by combining all conditions into a single stack and normalizing the threshold of detection to each intraexperimental control. Size and circularity cutoffs define nuclear regions labeled for NeuN antigen. Total integrated pixel density was used for assessing β-3 tubulin labeling.
3-Nitropropionic (3-NP) acid treatment.
Neurons infected with 25 ng/ml lentivirus encoding CFP or wild-type MKP-1 were treated at DIV14 with 100 μm 3-NP for 24 or 48 h and were assessed for toxicity with NeuN labeling.
MKP-1 silencing.
Primary striatal neurons were plated in a 96-well plate (Corning, black) and infected with tTA/htt171–18Q/82Q and either a negative control shRNA (that hypothetically targets the sequence GCA CUA CCA GCG CUA ACU CAG AUA GUA CU) or one of two MKP-1-targeting shRNAs (shRNA1 targets the MKP-1 sequence GUU CGU GAA GCA GAG GCG GAG UAU UAU CU; shRNA2 targets the MKP-1 sequence GGC ACC UCU ACU ACA ACG GUC UUC AAC UU). Neurons were fixed at DIV32 and assessed for toxicity via NeuN labeling. Total MKP-1 levels were reduced to 69 ± 2% (SEM) (p < 0.0001) after treatment with shRNA1 (sh1) and 45 ± 11% (SEM) (p < 0.0001) after treatment with shRNA2 (sh2) compared with treatment with a scrambled control shRNA, as assessed by real-time PCR (see Quantitative real-time PCR).
Caspase-3 assays.
Primary striatal neurons were plated in a 96-well plate (Corning, black) and infected with tTA/htt171–18Q/82Q and MKP-1. On the fourth day after cell plating, cultures were infected with lentivirus encoding wild-type MKP1 or inactive C258S as described for cytotoxicity assays. Caspase-3 activity was assessed at DIV17 by adding 100 μl/well of Caspase-Glo 3/7 Assay Reagent (Promega) and measuring luminescence on a Tecan GENios Pro (Tecan) after 30 min.
In vivo lentiviral vector injections.
In vivo experiments were performed with ∼200 g adult female Wistar rats (Charles River). Animals had access to food and water ad libitum in temperature-controlled rooms on 12 h day/night cycles. All care and use of animals followed the European Community directive (86/609/EEC), and protocols were approved by the Veterinary Authority of the Canton of Vaud, Switzerland.
Concentrated lentivirus stocks of Htt171–18Q, Htt171–82Q, YFP, CFP, MKP-1, and MKP-3 were produced for in vivo experiments and diluted to 200,000 ng of p24/ml each by thawing on ice and gently resuspending by pipetting. Animals were anesthetized with ketamine (75 mg/kg)/xylazine (10 mg/kg) solution delivered intraperitoneally. Needles (34-gauge blunt-tip) (Phymep) attached to a Hamilton syringe (Hamilton)/polyethylene tubing (Phymep)/pump (Stoelting) system delivered 4 μl of virus, stereotaxically injected to the striatum over 20 min at a rate of 0.2 μl of per minute, after which the needle remained in place for five extra minutes. Stereotaxic coordinates were measured from the bregma (0.5 mm rostral, 3 mm lateral to midline, 5 mm below the skull surface). Wounds were closed with surgical staples (Phymep), which were subsequently removed 2 weeks after injection under isoflurane anesthesia.
After injection, animals were housed for 8 (MKP-3 experiment, n = 6 per group) or 12 weeks (MKP-1 n = 10 per group) before death. An overdose of i.p. sodium pentobarbital was used for death followed by transcardial perfusion with PBS/heparin (0.1%) and then 4% paraformaldehyde in PBS. Brains were removed and postfixed with 4% paraformaldehyde/PBS solution for a minimum of 24 h before transfer to cryoprotection solution (25% sucrose/PBS) for a minimum of 48 h.
Cerebella were removed, brains were frozen to the stage of a sledge microtome (SM2400, Leica Microsystems), and serial coronal sections (25 μm) were sliced and collected across the entire striatum. Sections were stored free-floating in 0.12 μm sodium azide/PBS at 4°C.
Immunohistochemistry.
Striatal sections were removed from storage solution into a 24-well dish (maximum 4 sections/well), rinsed three times in PBS, and then transferred to a blocking solution of 3% BSA/PBS and gently agitated for 1 h at room temperature. Sections were incubated in primary antibody diluted in blocking buffer with gentle rocking overnight at 4°C. After three rinses with PBS, the sections were incubated with secondary antibody in blocking solution for 2 h at room temperature, covered with gentle agitation. For DARPP-32 or NeuN lesion analysis, anti-rabbit 680 and anti-mouse 800 secondary antibodies (from LI-COR) were applied, respectively. After final washes, secondary antibody labeling was visualized using 21 μm resolution scans with the LI-COR Odyssey (Version 1.2) software. Scanned images were analyzed in a blinded fashion and lesion areas were analyzed with ImageJ. For fluorescent images, secondary antibodies were detected by confocal microscopy on a Sp2 inverted microscope (Leica) and imaged at 10× magnification.
Statistics.
Two-group comparisons based on a priori data were analyzed by one-tailed Student's t test. Where data for multiple-comparison tests were first determined to be normally distributed and of equal variance, they were analyzed using ANOVA followed by the Student–Newman–Keuls post hoc test for multiple pairwise comparisons. Datasets that were not normally distributed or of equal variance were analyzed with the Kruskal-Wallis one-way ANOVA on ranks followed by the Student–Newman–Keuls Method. In all cases, a p value of 0.05 was used as the cutoff value for defining significance.
Results
MKP-1 expression correlates with phenotype status in HD systems
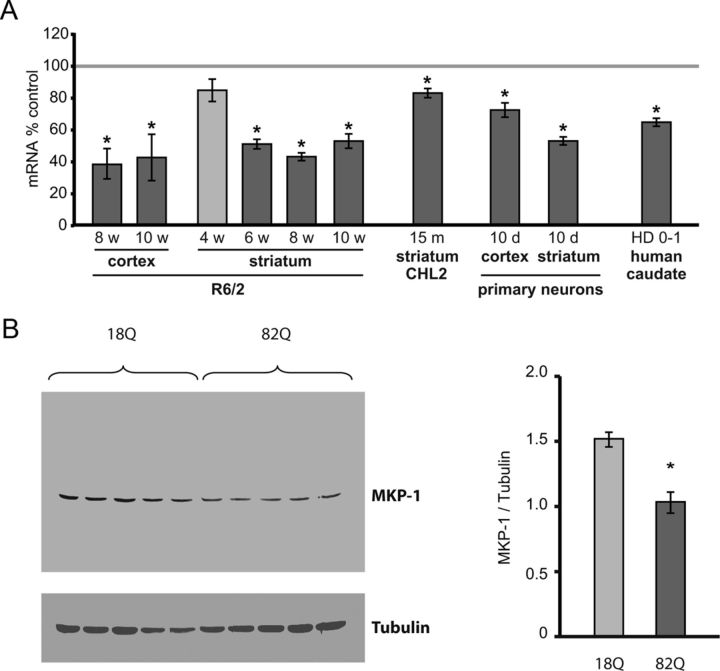
We had previously shown by northern blotting that MKP-1 (also known as 3CH134 or DUSP1) was downregulated in the cerebral cortex of R6/2 HD mice (Luthi-Carter et al., 2002). To extend these findings, we assessed whether or not MKP-1 downregulation occurs more broadly in HD systems. To this end, we first performed real-time PCR on striatum and cortex in an additional cohort of R6/2 animals. These confirmed that MKP-1 expression was significantly decreased at 8 and 10 weeks in the cortex and at 6, 8, and 10 weeks in the striatum (Fig. 1A). A trend toward reduced expression was observed in striatum as early as 4 weeks of age. Reference to the previously published array results of Kuhn et al. (2007) also showed a significant downregulation of MKP-1 in the CHL2 (“knock-in”) mouse model of HD at 22 months (log2 fold-change = −0.855; false discovery rate-corrected p < 0.04); real-time PCR further demonstrated that MKP-1 downregulation occurred in CHL2 animals as early as 15 months (Fig. 1A). We also measured and found significant decreases in MKP-1 expression in primary cortical and striatal neurons exposed to polyglutamine-expanded Htt fragments (Htt171–82Q compared with H171–18Q control), indicating that MKP-1 regulation occurs specifically in Htt-exposed neurons (Fig. 1A). Importantly, decreased MKP-1 expression was also observed in postmortem human HD caudate (pathologic grade 0–1 cases compared with controls) (Fig. 1A), providing evidence of relevance to bona fide HD pathogenesis. A corresponding decrease in MKP-1 protein was observed in Htt171–82Q-exposed striatal cells compared with H171–18Q-exposed controls (Fig. 1B). These data indicate that the downregulation of MKP-1 might contribute to the abnormally high MAPK activation levels previously observed in HD.
Figure 1.
MKP-1 expression is decreased in HD systems. A, mRNA expression is significantly reduced in the striatum and cortex of R6/2 mice; expression of MKP-1 was significantly reduced at 8 (n = 8) and 10 weeks (n = 7) in R6/2 cortex and at 6 (n = 12), 8 (n = 6), and 10 weeks (n = 7) in R6/2 striatum compared with wild-type mice. Decreased MKP-1 mRNA expression was also detected in homozygous CHL2 “knock-in” HD mice at 15 months of age (n = 4). Significantly reduced levels of MKP-1 mRNA were also observed in primary cortical and striatal neurons expressing Htt171–82Q compared with neurons expressing Htt171–18Q (n = 6). Comparison with a noninfected control demonstrates that the expression difference between wild-type and polyglutamine-expanded Htt conditions was not the result of MKP-1 induction in 18Q cells (data not shown). MKP-1 mRNA downregulation was also observed in postmortem human HD Grade 0–1 caudate samples (n = 5) compared with age- and gender-matched controls (n = 5). B, A corresponding decrease of MKP-1 protein is demonstrated in striatal neurons expressing Htt171–82Q compared with striatal neurons expressing Htt171–18Q (n = 5). *p < 0.05 compared with control.
Whereas MKP-1 is downregulated in HD, it is upregulated by treatment of R6/2 HD mice with sodium butyrate, an HDAC inhibitor that increases survival, improves disease phenotype, and decreases neurodegeneration in these animals (Ferrante et al., 2003). We therefore examined whether MKP-1 levels were improved by other compounds demonstrating therapeutic efficacy. Intriguingly, treatment with the transglutaminase inhibitor cystamine alone (Dedeoglu et al., 2002) or cystamine combined with the chemotherapeutic antibiotic mithramycin (Ferrante et al., 2004) significantly induced MKP-1 in R6/2 HD mice as well (Table 1). The downregulation of MKP-1 in HD and its reversal by treatment with neuroprotective compounds motivated us to assess the potential modulatory role of MKP activity in HD in greater detail.
Table 1.
Increased MKP1/DUSP1 expression resulting from neuroprotective treatments in HD mice as detected by Affymetrix probeset 1448830_at on MOE 430 arrays
| Treatment | Brain region | Log2 FCa | p |
|---|---|---|---|
| Cystamine | Cortex | 0.64 | 4.35E-05 |
| Cystamine | Striatum | 0.60 | 1.60E-04 |
| Cystamine + mithramycin | Cortex | 0.96 | 2.01E-08 |
| Cystamine + mithramycin | Striatum | 0.85 | 4.80E-07 |
a Log2FC refers to log2 fold change of MKP-1 expression.
MKP-1 is neuroprotective against HD pathology in vitro
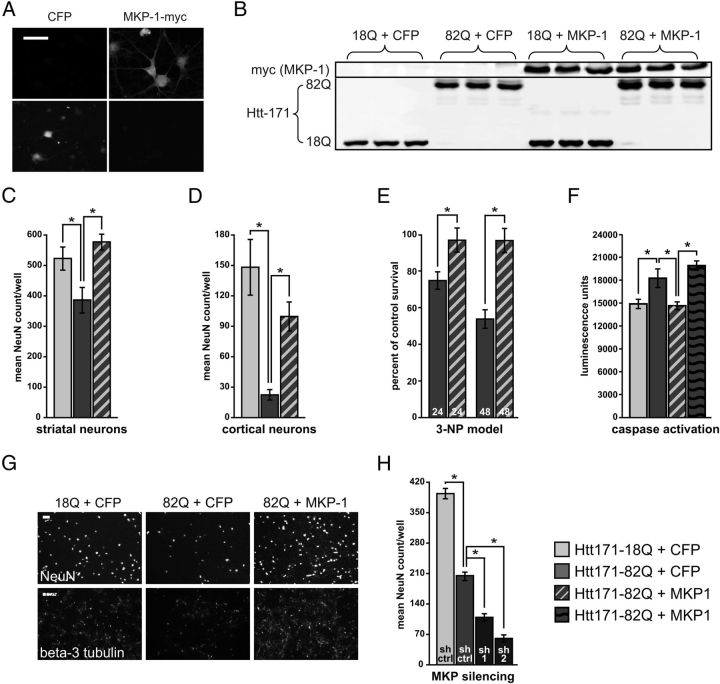
We next produced lentiviral MKP-1 expression vectors to genetically manipulate MKP-1 levels in neurons. Heterologously expressed MKP-1 demonstrated nuclear and cytoplasmic distribution in primary neuronal cells (Fig. 2A). In addition, coexpression of MKP-1 with wild-type (Htt171–18Q) and polyglutamine-expanded (Htt171–82Q) Htt fragments was confirmed by Western blotting (Fig. 2B). Heterologous expression of MKP-1 resulted in significant neuroprotection in primary striatal (Fig. 2C) and cortical (Fig. 2D) neuron models of HD (Rudinskiy et al., 2009; Gambazzi et al., 2010), as assessed by the preservation of NeuN-positive cell counts. MKP-1 also significantly protected against 3-NP toxicity (Fig. 2E). To confirm MKP-1-mediated neuroprotection using a second indicator, we showed that MKP-1 also reversed caspase-3 activation in Htt171–82Q-exposed striatal cells, consistent with its proposed antiapoptotic activity (Magi-Galluzzi et al., 1997) (Fig. 2F). The striking preservation of NeuN- and β-3 (neuronal) tubulin-positive striatal neurons is illustrated in Figure 2G.
Figure 2.
MKP-1 overexpression is neuroprotective in an in vitro model of HD. A, Exogenously expressed, myc-tagged, wild-type MKP-1 is both nuclear and cytoplasmic in primary striatal neurons. Control neurons expressed untagged CFP. Scale bar, 50 μm. B, Control Western blots representing overexpression of MKP-1 and both wild-type and polyglutamine-expanded Htt. C, Coexpression of polyglutamine-expanded Htt with MKP-1 preserved NeuN-positive cells to equivalent or better levels than striatal cultures expressing wild-type Htt at 3 weeks in vitro. D, Significant neuroprotection against polyglutamine-expanded Htt. E, 3-NP toxicity was also evident in primary cortical cultures. F, Wild-type MKP-1, but not a phosphatase-inactive MKP-1 mutant, normalized the polyglutamine-expanded Htt-induced activation of caspase 3 to control levels in striatal neurons. G, Representative images of NeuN and β-3 tubulin labeling of primary striatal cultures illustrate neuroprotection by MKP-1. Scale bars, 400 μm. H, Silencing MKP-1 with two different shRNA sequences significantly exacerbated polyglutamine-expanded Htt toxicity in primary striatal cultures (compared with a control shRNA sequence). *p < 0.05.
To assess whether the downregulation of MKP-1 might contribute to HD-related neurotoxicity, we also evaluated the effect of MKP-1 silencing on the neurodegeneration of Htt171–82Q-exposed striatal cells. Treatment with either of two different MKP-1-targeting shRNAs resulted in the enhancement of Htt171–82Q-induced neurodegeneration (Fig. 2H), supporting that the downregulation of MKP-1 may indeed contribute to HD pathogenesis.
MKP-1 is neuroprotective against HD neuropathology in vivo
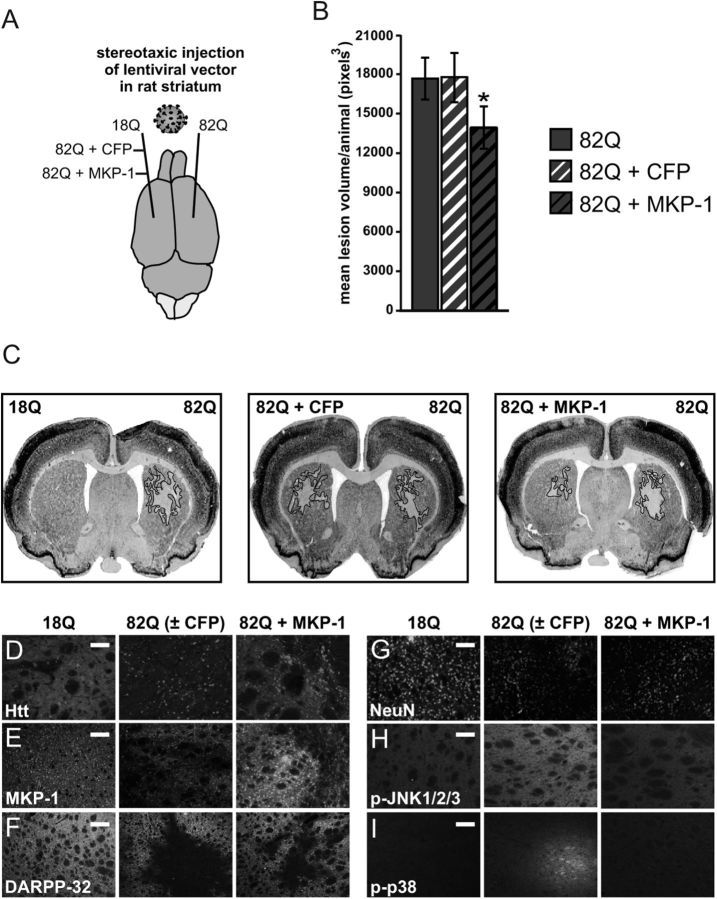
To determine the efficacy of MKP-1 overexpression against polyglutamine-expanded Htt toxicity in vivo, MKP-1 was coexpressed with polyglutamine-expanded Htt (Htt171–82Q) in rat striata by stereotaxic injection of lentiviral expression vectors (Fig. 3A). The volume of polyglutamine-induced neuropathology (de Almeida et al., 2002) in the presence of MKP-1 was compared with that caused by Htt171–82Q expression alone in the opposite hemisphere of the same rats (as delineated by summing NeuN-deficient areas after fluorescent immunohistochemical labeling; Fig. 3B). Coexpression of CFP and Htt171–82Q versus Htt171–82Q alone was tested in a separate set of animals, as a control for nonspecific protein expression effects. The effect of wild-type versus polyglutamine-expanded Htt fragments (Htt171–18Q vs Htt171–82Q) was also compared in a third experimental group to demonstrate the specific toxicity of the expanded polyglutamine-bearing Htt protein. Whereas the Htt171–18Q transprotein was diffusely distributed within targeted cells, polyglutamine-expanded Htt was mostly observed in inclusion bodies (Fig. 3D). MKP-1 overexpression was detected by immunohistochemistry and appeared in both nuclear and non-nuclear compartments, as in primary cultures (Fig. 3E).
Figure 3.
MKP-1 overexpression is neuroprotective in an in vivo model of HD. A, Illustration of conditions used in stereotaxic injection of rat striatum with concentrated lentiviral vectors expressing wild-type or polyglutamine-expanded Htt alone and polyglutamine-expanded Htt together with CFP control or MKP-1. B, Illustration of the process by which the quantification of mean neuropathology volume per animal was performed by measuring lesion area in sections at regular intervals of serially sliced brains. Striata coinjected with polyglutamine-expanded Htt and MKP-1 had significantly smaller mean lesion volume than all four other conditions where polyglutamine-expanded Htt was expressed (n = 10). C, Representative images of NeuN-labeled striata from rats injected as outlined in A, demonstrating discrete areas of polyglutamine neuropathology. D–I, Immunohistochemical images of the MKP-1 effect in the rat lentiviral HD neuropathology model. D, Wild-type Htt was expressed diffusely, but polyglutamine-expanded Htt was detected primarily in inclusion bodies when expressed alone or together with CFP. Htt expression was diffuse around and aggregated within the lesion area in striata coexpressing 82Q + MKP-1. Scale bar, 50 μm. E, MKP-1 overexpression was detected above endogenous levels and was localized to nucleus and cytoplasm. Scale bar, 50 μm. Representative images (F, DARPP-32; and G, NeuN) show example images of the pathologic lesion and its reduction by MKP-1 expression. Scale bars, 200 and 50 μm, respectively. H, I, Immunohistochemistry of pJNK and p-p38, respectively, showing the effect of 82Q and its reversal by MKP-1 coexpression. Scale bars, 200 and 50 μm, respectively. *p < 0.05 compared with both 82Q and 82Q + CFP.
As expected, no NeuN-delineated neuropathology was observed in striata expressing wild-type Htt171–18Q, and no significant difference was observed between the mean lesion size in rats expressing Htt171–82Q on one side and Htt171–82Q + CFP on the other (Fig. 3B). In accordance with the in vitro data, polyglutamine-expanded Htt-induced lesion size was significantly reduced by coexpression of MKP-1, both compared with the opposite hemisphere of the same rats (without MKP-1) (Fig. 3B) and to the mean lesion volume of all other cohorts in which Htt171–82Q was expressed (data not shown). No significant difference was observed between any of the other three conditions in which polyglutamine-expanded Htt was expressed (data not shown). Other immunohistochemical indicators also confirmed the functional effects and neuroprotection of enhanced MKP-1 expression. Measurements of both DARPP-32- and NeuN-poor areas gave equivalent results, both indicating significant neuroprotection (Fig. 3F, G). Qualitatively, increased levels of phosphorylated JNK1, 2, and 3 and p38s were apparent in the neurons at the edge of lesion areas in tissue expressing polyglutamine-expanded Htt alone, but not in the same regions of striata coexpressing MKP-1 with polyglutamine-expanded Htt (Fig. 3H, I) (see also below). In contrast, no qualitative change in phosphorylated ERK1/2 was observed in any condition (data not shown).
MKP-3 overexpression is not neuroprotective in vitro or in vivo
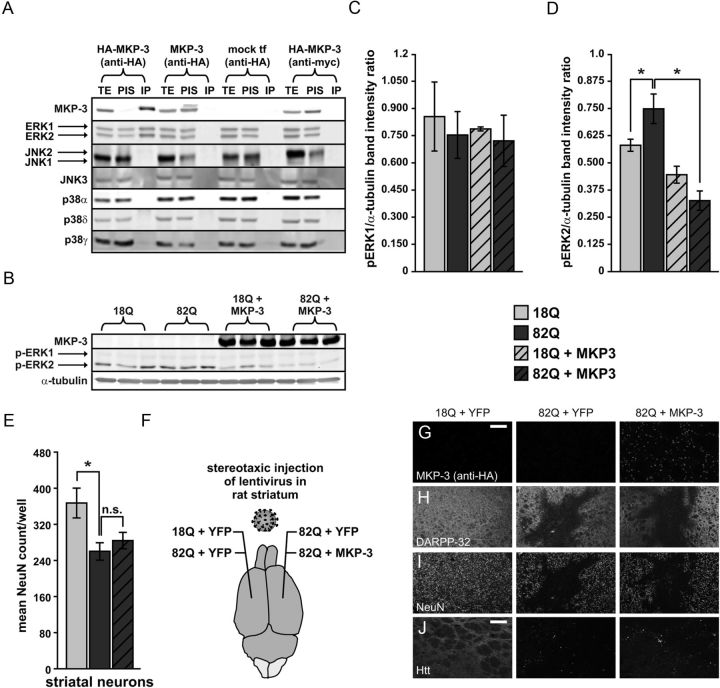
To assess the possible selectivity of the neuroprotective effect of MKP-1, we also examined whether another MKP, MKP-3/DUSP6, would yield similar results. MKP-3 has been previously shown to be an ERK-selective phosphatase; and consistent with these previous data (Muda et al., 1996), we observed that that ERK associates with MKP-3 by coimmunoprecipitation (Fig. 4A, B). In contrast, no binding to either JNKs or p38s was observed under the same conditions (Fig. 4A). As expected, heterologous MKP-3 expression also decreased ERK2 phosphorylation (Fig. 4C, D). In contrast to MKP-1, however, MKP-3 was not neuroprotective in primary neurons exposed to Htt171–82Q (Fig. 4E). We also assessed whether MKP-3 could have a neuroprotective effect in vivo, and again no significant decrease in polyglutamine-expanded Htt-induced neuropathology was observed (Fig. 4,F, H–J). Immunohistochemical labeling for MKP-3 confirmed heterologous overexpression and cytoplasmic localization in vivo (Fig. 4G) and in vitro (data not shown), thereby ruling out failure of expression as an explanation for its lack of neuroprotection. These results show that MKP-1 has a unique profile of enzymatic and neuroprotective activities compared with MKP-3.
Figure 4.
MKP-3 overexpression is not neuroprotective in models of HD. A, Coimmunoprecipitation of MKP-3 with ERK1 and 2, but not JNKs or p38s. TE, Total extract; PIS, after immunoprecipitation supernatant; IP, immunoprecipitate. B, Western blot demonstrating ERK2 dephosphorylation by MKP-3 overexpression in primary striatal neurons expressing wild-type and polyglutamine-expanded Htt. C, Quantification of ERK1. D, ERK2 phosphorylation from Western blots represented in B. E, MKP-3 has no effect on the polyglutamine-induced reduction of NeuN-positive cell counts in primary striatal neurons. n.s., Not significant. F, Illustration of conditions used in stereotaxic injections of rat striatum with concentrated lentiviral vectors expressing wild-type or polyglutamine-expanded Htt fragments alone or coinjection of Htt171–82Q fragments with YFP control or MKP-3 (n = 6). G–J, Immunohistochemical images showing the lack of effect of MKP-3 in the lentiviral rat HD neuropathology model. Mean volume of DARPP-32-depleted striatum, compared with opposite hemispheres coexpressing polyglutamine-expanded Htt with YFP (9869 ± 1338 pixels3 for 82Q + YFP right hemisphere and 10614 ± 1767 pixels3 for 82Q + MKP-3 left hemisphere). G, Heterologously expressed MKP-3 was detected in the cytoplasm. H, Polyglutamine length-dependent loss of DARPP-32 labeling in the injected region of the striatum was not altered by MKP-3 overexpression. I, Labeling with NeuN shows a similar effect to that revealed with DARPP-32. J, Wild-type Htt was expressed diffusely, but polyglutamine-expanded Htt was found in inclusion bodies when coexpressed with YFP or MKP-3. Scale bars, 200 μm. *p < 0.05.
MKP-1-mediated neuroprotection is phosphatase activity-dependent and occurs through direct regulation of JNKs and p38s
The next goal of our studies was to gain a better understanding of how MKP-1 achieves neuroprotection. Thus, we examined whether its effect was mediated by negative regulation of MAPKs, either individually or in combination. The known substrates of MKP-1 include all three major MAPK families: JNKs and p38s (Franklin and Kraft, 1995; Groom et al., 1996) as well as ERKs (Slack et al., 2001). We thus assessed to what extent blocking the actions of these specific MAPKs would mediate the neuroprotection that we had observed.
Exposure of primary striatal neurons to polyglutamine-expanded Htt171–82Q fragments conferred significant (∼2-fold) activation of JNKs 1, 2, and 3 (as assessed by JNK phosphorylation), compared with neurons exposed to wild-type Htt171–18Q (Fig. 5A). Conversely, concomitant MKP-1 overexpression in polyglutamine-expanded Htt171–82Q neurons significantly blocked the polyglutamine-induced phosphorylation of all three JNK species (Fig. 5A). Normalization of p-JNK levels to the expression of their respective total isoform expressions indicated that increased phosphorylation was not a result of JNK gene induction or reduced JNK protein degradation (Fig. 5A). A similar effect was observed for p38, the phosphorylation of which was also increased (∼3.5-fold) by polyglutamine-expanded Htt and diminished by MKP-1 (Fig. 5B). In contrast, there were no changes in ERK1/2 activation under these conditions (Fig. 5C). Stable expression of JNKs, p38s, and ERKs across all conditions was also confirmed with real-time quantitative PCR (data not shown). These data showed that the neuroprotective activity of MKP-1 correlated with the prevention of polyglutamine-expanded Htt-induced JNK and p38 activation.
We next tested whether the neuroprotective effects of MKP-1 were dependent on its intact enzymatic activity. To address this issue, we first created and tested a phosphatase-inactive form of MKP-1 (Fig. 6A) through mutation of the cysteine residue at position 258 to serine (Sun et al., 1993). We also assessed whether the MKP-1 effects were mediated by direct dephosphorylation of JNKs and/or p38s by using MKP-1 mutations that selectively abolish JNK or p38 binding. Mutation of three arginines at amino acids 53–55 to methionines has been previously described to abolish p38 binding and yield a form of MKP-1 that can only dephosphorylate JNKs (Slack et al., 2001) (Fig. 6A). Similarly, mutation of two arginines at amino acids 72 and 74 has been shown to have a reduced ability to bind JNK, thus resulting in a selectively p38-targeting form of the enzyme (Tanoue et al., 2002) (Fig. 6A). Heterologous protein expression of all MKP-1 species was confirmed for each condition (Fig. 6B). As expected, the selective JNK-binding form of MKP-1 resulted in a significant inhibition of 82Q-mediated JNK activation. Moreover, JNK dephosphorylation was not observed by heterologous expression of the inactive or p38-preferring MKP-1 mutants (Fig. 6C). In addition, selective p38-binding forms of MKP-1 decreased the p38 phosphorylation induced by polyglutamine-expanded Htt expression, whereas inactive and JNK-selective mutants had no significant effect (Fig. 6D). Quantitatively, the selectivity of both substrate-selective mutants was observed to be ≥85% (i.e., ≤15% of residual dephosphorylation of the nontargeted substrate was observed) (Fig. 6). None of the treatments had a significant impact on ERK1/2 activation (Fig. 6E).
Next, we assessed the functional contributions of MKP-1 phosphatase activity, as well as JNK and p38 kinase inhibition, on MKP-1-mediated neuroprotection. As expected, the phosphatase-inactive form of MKP-1 exhibited no significant neuroprotective activity against polyglutamine-expanded Htt toxicity (Fig. 7A). In contrast, both the JNK-specific (Fig. 7B) and p38-specific (Fig. 7C) mutants were significantly neuroprotective, yet in each case, they were significantly less protective than wild-type MKP-1 (Fig. 7B, C). These data show that the full extent of neuroprotection mediated by MKP-1 relies on both JNK and p38 inhibition. As such, these results indicate a role for MKP-1 in modulating HD neurotoxicity and provide evidence that the combinatorial inhibition of JNK and p38 kinases is a rational strategy for HD therapy.
Figure 7.
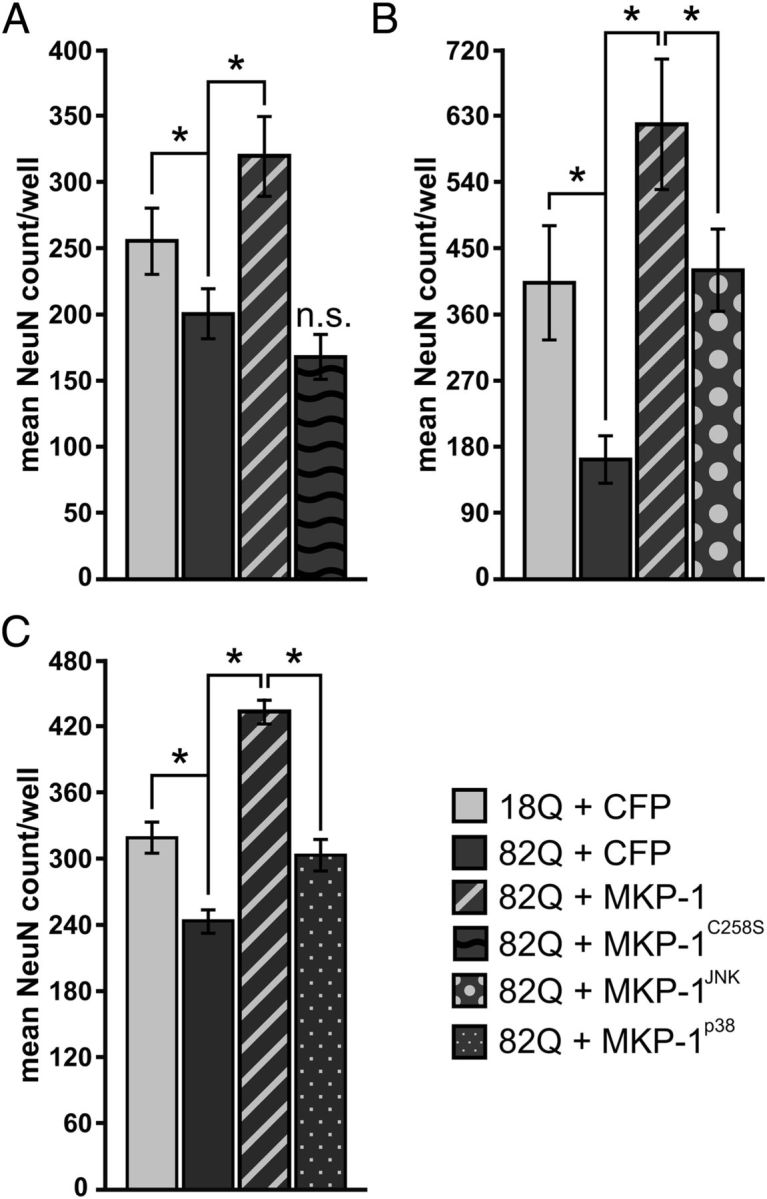
Neuroprotection by MKP mutants selectively targeting JNK or p38 occurs to a lesser extent than wild-type MKP-1 in primary neuron HD models. A, Phosphatase-deficient MKP-1 (MKP-1C258S) has no effect on polyglutamine-expanded Htt toxicity in striatal neurons. n.s., Not significant. B, A JNK-specific mutant of MKP-1 (MKP-1JNK). C, A p38-selective mutant of MKP-1 (MKP-1p38) each significantly preserves NeuN-positive cell counts in primary striatal neurons exposed to polyglutamine-expanded Htt fragments but to a lower extent than that yielded by expression of wild-type MKP-1. *p < 0.05.
Discussion
In this report, we provide evidence that MKP-1 expression can positively modulate neuronal survival in models of HD. This effect was shown to be attributable to dual JNK and p38 inhibition, as demonstrated by the fact that selective inactivation by MKP-1 of either JNK or p38 alone yielded partial rescue of neuronal survival. Blocking both pathways simultaneously provided greater effects, resulting in complete rescue of primary striatal neurons from polyglutamine-expanded Htt toxicity. These data are consistent with previous studies implicating MAPK activation as a modulator in HD pathogenesis. Whereas activation of ERKs, JNKs, and p38s can occur in response to polyglutamine-expanded Htt toxicity, JNK- and p38-mediated pathways have generally been regarded as contributing to HD pathogenesis and ERK activation has been ascribed a compensatory, prosurvival role (see below, Discussion).
The involvement of JNKs in polyglutamine-expanded Htt-mediated toxicity has been supported by a number of previous reports (Liu, 1998; Garcia et al., 2002; Merienne et al., 2003; Charvin et al., 2005; Apostol et al., 2006; Perrin et al., 2009; Reijonen et al., 2010). The work by our group has demonstrated JNK hyperactivation in a lentiviral model of HD. Moreover, we have shown that reversal of this activation and diminution of Htt toxicity could be achieved through overexpression of dominant negative MEKK1 and the active domain of the scaffold protein JIP-1/IBI (Perrin et al., 2009). Neuroprotection via JNK inhibition has also been reported for Mixed Lineage Kinase inhibitors CEP-11004 and CEP-1347 (Apostol et al., 2008) and the direct JNK inhibitor SP-600125, which reduced dopamine-dependent aggravation of polyglutamine-expanded Htt toxicity in primary striatal cultures (Charvin et al., 2005). The present data therefore confirm previous studies, supporting a significant role for JNK in mediating Htt toxicity and providing a rationale for targeting JNK as a novel HD therapeutic strategy.
Although the involvement of p38 in HD pathogenesis has been studied previously, its precise role has remained more enigmatic. Gianfriddo et al. (2004) first demonstrated activation of p38 in the striatum of R6/2 mice, where it was hypothesized to mediate HD pathogenesis. A proapoptotic effect of p38 has also been reported, showing a p38-induced translocation of Bax from the cytosol to mitochondria in a malonate model of HD (Gomez-Lazaro et al., 2007). In addition, the GSK3 inhibitor HSB-13 prevented p38 activation in a 3-NP model of HD in rats, where it also reduced striatal degeneration and improved behavioral performance (Wang et al., 2010). In contrast, p38 activation has also been linked to the positive regulation of the serum/glucocorticoid-induced kinase (Rangone et al., 2004), which has been shown to diminish Htt toxicity by direct phosphorylation of Htt at serine 421. One possible explanation of these findings is that p38 could regulate both proapoptotic and compensatory pathways in HD depending on which p38 substrates become engaged. Of particular relevance, the shortest N-terminal polyglutamine-expanded Htt fragments (such as the ones represented by the models used in our study) do not contain the p38-activated serum/glucocorticoid-induced kinase phosphorylation site and thus would not be subject to neuroprotection via this mechanism (Rangone et al., 2004).
The potential involvement of ERK as a modulator of HD neurotoxicity has also been studied previously. Importantly, the neuroprotective role of brain-derived neurotrophic factor in HD (Zuccato et al., 2001, 2005) is thought to be mediated in part by activation of ERK1/2 (Gokce et al., 2009). ERK activation has also been attributed to mediate the neuroprotective effects of metabolic inhibitors (Varma et al., 2007) and granulocyte-colony stimulating factor (Lee et al., 2008) in HD. A compensatory role for endogenous ERK1/2 activation has also been observed in polyglutamine-expanded Htt fragment-expressing PC12 cells (Apostol et al., 2006). Although MKP-1 has been shown to dephosphorylate ERKs under some circumstances (Slack et al., 2001), we did not observe any effect on ERK1/2 in our studies. Nonetheless, diminishing ERK phosphorylation via MKP-3 did not exacerbate the toxicity of polyglutamine-expanded Htt, suggesting that although ERK activation promotes survival, reducing it from baseline does not produce any major adverse effects (with the caveat that the extent to which other ERKs compensate for ERK1/2 inactivation remains unknown).
One possibility not addressed by the present study is to what extent other MAPKs (or other potential substrates of MKP-1) may be regulated by MKP-1 in a disease-modifying way. For example, little is known about ERK5 activation in models of neurodegenerative disease, yet the fact that it has been linked to both Akt activation (Finegan et al., 2009) and the regulation of apoptosis (Liu et al., 2003) may be impetus for such work (Obara and Nakahata, 2010).
The possible role of other DUSPs in HD is another potentially interesting question. In the process of the current studies, we also evaluated the possible dysregulation of other DUSPs/MKPs in HD model systems. This showed that MKP-1/DUSP1, MKP-3/DUSP6, and MKP-6/DUSP14 were the only brain-expressed DUSPs to be significantly downregulated in R6/2 mice (by quantitative PCR analysis, R. Moser and R. Luthi-Carter, unpublished) and that MKP-1 was the only significantly downregulated DUSP in CHL2 brain (in the published microarray analyses of Kuhn et al. (2007)). Although we think that the current evidence points to MKP-1 as the most relevant MKP/DUSP contributor to HD pathogenesis, we have not ruled out a possible contribution of DUSP14, which is reported to be a JNK- and ERK-selective enzyme (Bai et al., 2004; Marie-Claire et al., 2008).
The complete rescue of polyglutamine-expanded Htt-expressing striatal neurons by MKP-1 required the dual inhibition of JNK and p38. This provides a specific rationale for simultaneous treatment with JNK and p38 inhibitors (Cuny, 2009). Moreover, we hypothesize that multipartite MAPK-targeted therapy could also include an agent capable of activating the ERK1/2 pathway (Varma et al., 2007) to achieve additional benefit.
In addition to the possibility of modulating MAPKs directly, our data reveal the potential for treatment with compounds that stimulate MKP-1 expression. Increased expression of MKP-1 in the CNS has been primarily associated with positive outcomes. The Chao group (Jeanneteau et al., 2010) has recently demonstrated that MKP-1 is upregulated by and mediates positive effects of brain-derived neurotrophic factor via negative regulation of JNK activation. This is a particularly interesting link to HD because brain-derived neurotrophic factor has shown beneficial effects in several HD preclinical studies (Bemelmans et al., 1999; Kells et al., 2004; Zala et al., 2005; Zuccato et al., 2005). MKP-1 upregulation in the CNS has also been associated with reduced inflammation via endocannabinoid-mediated microglial expression (Eljaschewitsch et al., 2006), inhibition of TNFα stimulated calcitonin gene-related peptide secretion (Bowen et al., 2006), improved sensory neuron functioning (Horita et al., 2010), and increased ischemic tolerance (Kawahara et al., 2004). Reduced levels of MKP-1 have been demonstrated in several neurological conditions, including cognitive impairment in diabetes mellitus (Zhou et al., 2007), cerebral hypoxia (Mishra and Delivoria-Papadopoulos, 2004), and hippocampal and cortical neurons undergoing excitotoxicity (Choi et al., 2006).
However, while showing the benefit of JNK and p38 inhibition and addressing the concern of abrogating ERK-dependent survival pathways, our data do not evaluate whether MKP-1 activation may lead to other adverse consequences. One concern regarding MKP activation is its potential oncogenicity (Lin et al., 2008; Haagenson and Wu, 2010). On the other hand, this may be somewhat counterbalanced by the evidence that two of the MKP-1-inducing, neuroprotective compounds discussed in the present study (sodium butyrate and mithramycin) are anti-cancer chemotherapeutics, and that MKP-1 activation in brain has also been shown to diminish (rather than enhance) brain tumor invasiveness (Jan et al., 2009). A second concern about adverse MKP-1-mediated effects stems from a previous report showing that exposure to chronic stress induced MKP-1 expression and resulted in depressive behaviors, both of which were normalized by treatment with the selective serotonin reuptake inhibitor antidepressant fluoxetine (Duric et al., 2010). Although this correlation does not predict whether MKP-1 activity would antagonize the therapeutic benefit of antidepressants, this issue needs to be explored because depression is a common clinical feature of HD.
To our knowledge, the present study is first to show additive effects of simultaneously targeting both JNK and p38 pathways to achieve neuroprotection. Given that JNK and p38 activation has also been implicated in the pathogenesis of other neurodegenerative conditions, such as Alzheimer's and Parkinson's diseases (Borsello and Forloni, 2007; Karunakaran et al., 2008; Munoz and Ammit, 2010), our data provide a strong rationale for examining the therapeutic potential of dual JNK/p38 inhibitor administration or enhancing MKP-1 expression in relevant models of additional neurodegenerative disorders.
Footnotes
This work was supported by the Ecole Polytechnique Fédérale de Lausanne, the Swiss National Science Foundation, the National Institutes of Health (Grants NS045242, NS066912, and NS058793), and the College of Medicine, Biological Sciences and Psychology of the University of Leicester. Microarrays were processed at the Harvard Medical School Biopolymers Facility. We thank Maria de Fatima Rey, Lely Feletti, Marc Forrest, Dr. Valerie Perrin, Philippe Colin, Christel Sadeghi, Ewa Jaworska, Dr. Nicoleta Moisoi, Dr. Nikita Rudinskiy, Dr. Darren Moore, Dr. Jean-Réné Cardinaux, and Diego Chiappe for their invaluable assistance.
The authors declare no competing financial interests.
References
- Apostol BL, Illes K, Pallos J, Bodai L, Wu J, Strand A, Schweitzer ES, Olson JM, Kazantsev A, Marsh JL, Thompson LM. Mutant huntingtin alters MAPK signaling pathways in PC12 and striatal cells: ERK1/2 protects against mutant huntingtin-associated toxicity. Hum Mol Genet. 2006;15:273–285. doi: 10.1093/hmg/ddi443. [DOI] [PubMed] [Google Scholar]
- Apostol BL, Simmons DA, Zuccato C, Illes K, Pallos J, Casale M, Conforti P, Ramos C, Roarke M, Kathuria S, Cattaneo E, Marsh JL, Thompson LM. CEP-1347 reduces mutant huntingtin-associated neurotoxicity and restores BDNF levels in R6/2 mice. Mol Cell Neurosci. 2008;39:8–20. doi: 10.1016/j.mcn.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Bai L, Yoon SO, King PD, Merchant JL. ZBP-89-induced apoptosis is p53-independent and requires JNK. Cell Death Differ. 2004;11:663–673. doi: 10.1038/sj.cdd.4401393. [DOI] [PubMed] [Google Scholar]
- Bemelmans AP, Horellou P, Pradier L, Brunet I, Colin P, Mallet J. Brain-derived neurotrophic factor-mediated protection of striatal neurons in an excitotoxic rat model of Huntington's disease, as demonstrated by adenoviral gene transfer. Hum Gene Ther. 1999;10:2987–2997. doi: 10.1089/10430349950016393. [DOI] [PubMed] [Google Scholar]
- Borsello T, Forloni G. JNK signalling: a possible target to prevent neurodegeneration. Curr Pharm Des. 2007;13:1875–1886. doi: 10.2174/138161207780858384. [DOI] [PubMed] [Google Scholar]
- Bowen EJ, Schmidt TW, Firm CS, Russo AF, Durham PL. Tumor necrosis factor-alpha stimulation of calcitonin gene-related peptide expression and secretion from rat trigeminal ganglion neurons. J Neurochem. 2006;96:65–77. doi: 10.1111/j.1471-4159.2005.03524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brondello JM, Pouysségur J, McKenzie FR. Reduced MAP kinase phosphatase-1 degradation after p42/p44MAPK-dependent phosphorylation. Science. 1999;286:2514–2517. doi: 10.1126/science.286.5449.2514. [DOI] [PubMed] [Google Scholar]
- Charvin D, Vanhoutte P, Pagès C, Borrelli E, Borelli E, Caboche J. Unraveling a role for dopamine in Huntington's disease: the dual role of reactive oxygen species and D2 receptor stimulation. Proc Natl Acad Sci U S A. 2005;102:12218–12223. doi: 10.1073/pnas.0502698102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi BH, Hur EM, Lee JH, Jun DJ, Kim KT. Protein kinase Cδ-mediated proteasomal degradation of MAP kinase phosphatase-1 contributes to glutamate-induced neuronal cell death. J Cell Sci. 2006;119:1329–1340. doi: 10.1242/jcs.02837. [DOI] [PubMed] [Google Scholar]
- Cuny GD. Kinase inhibitors as potential therapeutics for acute and chronic neurodegenerative conditions. Curr Pharm Des. 2009;15:3919–3939. doi: 10.2174/138161209789649330. [DOI] [PubMed] [Google Scholar]
- de Almeida LP, Ross CA, Zala D, Aebischer P, Déglon N. Lentiviral-mediated delivery of mutant huntingtin in the striatum of rats induces a selective neuropathology modulated by polyglutamine repeat size, huntingtin expression levels, and protein length. J Neurosci. 2002;22:3473–3483. doi: 10.1523/JNEUROSCI.22-09-03473.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedeoglu A, Kubilus JK, Jeitner TM, Matson SA, Bogdanov M, Kowall NW, Matson WR, Cooper AJ, Ratan RR, Beal MF, Hersch SM, Ferrante RJ. Therapeutic effects of cystamine in a murine model of Huntington's disease. J Neurosci. 2002;22:8942–8950. doi: 10.1523/JNEUROSCI.22-20-08942.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duric V, Banasr M, Licznerski P, Schmidt HD, Stockmeier CA, Simen AA, Newton SS, Duman RS. A negative regulator of MAP kinase causes depressive behavior. Nat Med. 2010;16:1328–1332. doi: 10.1038/nm.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eljaschewitsch E, Witting A, Mawrin C, Lee T, Schmidt PM, Wolf S, Hoertnagl H, Raine CS, Schneider-Stock R, Nitsch R, Ullrich O. The endocannabinoid anandamide protects neurons during CNS inflammation by induction of MKP-1 in microglial cells. Neuron. 2006;49:67–79. doi: 10.1016/j.neuron.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Farooq A, Zhou MM. Structure and regulation of MAPK phosphatases. Cell Signal. 2004;16:769–779. doi: 10.1016/j.cellsig.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Ferrante RJ, Kubilus JK, Lee J, Ryu H, Beesen A, Zucker B, Smith K, Kowall NW, Ratan RR, Luthi-Carter R, Hersch SM. Histone deacetylase inhibition by sodium butyrate chemotherapy ameliorates the neurodegenerative phenotype in Huntington's disease mice. J Neurosci. 2003;23:9418–9427. doi: 10.1523/JNEUROSCI.23-28-09418.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante RJ, Ryu H, Kubilus JK, D'Mello S, Sugars KL, Lee J, Lu P, Smith K, Browne S, Beal MF, Kristal BS, Stavrovskaya IG, Hewett S, Rubinsztein DC, Langley B, Ratan RR. Chemotherapy for the brain: the antitumor antibiotic mithramycin prolongs survival in a mouse model of Huntington's disease. J Neurosci. 2004;24:10335–10342. doi: 10.1523/JNEUROSCI.2599-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegan KG, Wang X, Lee EJ, Robinson AC, Tournier C. Regulation of neuronal survival by the extracellular signal-regulated protein kinase 5. Cell Death Differ. 2009;16:674–683. doi: 10.1038/cdd.2008.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin CC, Kraft AS. Constitutively active MAP kinase kinase (MEK1) stimulates SAP kinase and c-Jun transcriptional activity in U937 human leukemic cells. Oncogene. 1995;11:2365–2374. [PubMed] [Google Scholar]
- Gambazzi L, Gokce O, Seredenina T, Katsyuba E, Runne H, Markram H, Giugliano M, Luthi-Carter R. Diminished activity-dependent brain-derived neurotrophic factor expression underlies cortical neuron microcircuit hypoconnectivity resulting from exposure to mutant huntingtin fragments. J Pharmacol Exp Ther. 2010;335:13–22. doi: 10.1124/jpet.110.167551. [DOI] [PubMed] [Google Scholar]
- Garcia M, Vanhoutte P, Pages C, Besson MJ, Brouillet E, Caboche J. The mitochondrial toxin 3-nitropropionic acid induces striatal neurodegeneration via a c-Jun N-terminal kinase/c-Jun module. J Neurosci. 2002;22:2174–2184. doi: 10.1523/JNEUROSCI.22-06-02174.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianfriddo M, Melani A, Turchi D, Giovannini MG, Pedata F. Adenosine and glutamate extracellular concentrations and mitogen-activated protein kinases in the striatum of Huntington transgenic mice: selective antagonism of adenosine A2A receptors reduces transmitter outflow. Neurobiol Dis. 2004;17:77–88. doi: 10.1016/j.nbd.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Gokce O, Runne H, Kuhn A, Luthi-Carter R. Short-term striatal gene expression responses to brain-derived neurotrophic factor are dependent on MEK and ERK activation. PLoS One. 2009;4:e5292. doi: 10.1371/journal.pone.0005292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lazaro M, Galindo MF, Melero-Fernandez de Mera RM, Fernandez-Gómez FJ, Concannon CG, Segura MF, Comella JX, Prehn JH, Jordan J. Reactive oxygen species and p38 mitogen-activated protein kinase activate Bax to induce mitochondrial cytochrome c release and apoptosis in response to malonate. Mol Pharmacol. 2007;71:736–743. doi: 10.1124/mol.106.030718. [DOI] [PubMed] [Google Scholar]
- Groom LA, Sneddon AA, Alessi DR, Dowd S, Keyse SM. Differential regulation of the MAP, SAP and RK/p38 kinases by Pyst1, a novel cytosolic dual-specificity phosphatase. EMBO J. 1996;15:3621–3632. [PMC free article] [PubMed] [Google Scholar]
- Haagenson KK, Wu GS. Mitogen activated protein kinase phosphatases and cancer. Cancer Biol Ther. 2010;9:337–340. doi: 10.4161/cbt.9.5.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges A, Strand AD, Aragaki AK, Kuhn A, Sengstag T, Hughes G, Elliston LA, Hartog C, Goldstein DR, Thu D, Hollingsworth ZR, Collin F, Synek B, Holmans PA, Young AB, Wexler NS, Delorenzi M, Kooperberg C, Augood SJ, Faull RL, Olson JM, Jones L, Luthi-Carter R. Regional and cellular gene expression changes in human Huntington's disease brain. Hum Mol Genet. 2006;15:965–977. doi: 10.1093/hmg/ddl013. [DOI] [PubMed] [Google Scholar]
- Horita H, Wada K, Rivas MV, Hara E, Jarvis ED. The dusp1 immediate early gene is regulated by natural stimuli predominantly in sensory input neurons. J Comp Neurol. 2010;518:2873–2901. doi: 10.1002/cne.22370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntington's Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes: the Huntington's Disease Collaborative Research Group. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- Jan HJ, Lee CC, Lin YM, Lai JH, Wei HW, Lee HM. Rosiglitazone reduces cell invasiveness by inducing MKP-1 in human U87MG glioma cells. Cancer Lett. 2009;277:141–148. doi: 10.1016/j.canlet.2008.11.033. [DOI] [PubMed] [Google Scholar]
- Jeanneteau F, Deinhardt K, Miyoshi G, Bennett AM, Chao MV. The MAP kinase phosphatase MKP-1 regulates BDNF-induced axon branching. Nat Neurosci. 2010;13:1373–1379. doi: 10.1038/nn.2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunakaran S, Saeed U, Mishra M, Valli RK, Joshi SD, Meka DP, Seth P, Ravindranath V. Selective activation of p38 mitogen-activated protein kinase in dopaminergic neurons of substantia nigra leads to nuclear translocation of p53 in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice. J Neurosci. 2008;28:12500–12509. doi: 10.1523/JNEUROSCI.4511-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara N, Wang Y, Mukasa A, Furuya K, Shimizu T, Hamakubo T, Aburatani H, Kodama T, Kirino T. Genome-wide gene expression analysis for induced ischemic tolerance and delayed neuronal death following transient global ischemia in rats. J Cereb Blood Flow Metab. 2004;24:212–223. doi: 10.1097/01.WCB.0000106012.33322.A2. [DOI] [PubMed] [Google Scholar]
- Kells AP, Fong DM, Dragunow M, During MJ, Young D, Connor B. AAV-mediated gene delivery of BDNF or GDNF is neuroprotective in a model of Huntington disease. Mol Ther. 2004;9:682–688. doi: 10.1016/j.ymthe.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Keshet Y, Seger R. The MAP kinase signaling cascades: a system of hundreds of components regulates a diverse array of physiological functions. Methods Mol Biol. 2010;661:3–38. doi: 10.1007/978-1-60761-795-2_1. [DOI] [PubMed] [Google Scholar]
- Kuhn A, Goldstein DR, Hodges A, Strand AD, Sengstag T, Kooperberg C, Becanovic K, Pouladi MA, Sathasivam K, Cha JH, Hannan AJ, Hayden MR, Leavitt BR, Dunnett SB, Ferrante RJ, Albin R, Shelbourne P, Delorenzi M, Augood SJ, Faull RL, Olson JM, Bates GP, Jones L, Luthi-Carter R. Mutant Huntington's effects on striatal gene expression in mice recapitulate changes observed in human Huntington's disease brain and do not differ with mutant huntingtin length or wild-type huntingtin dosage. Hum Mol Genet. 2007;16:1845–1861. doi: 10.1093/hmg/ddm133. [DOI] [PubMed] [Google Scholar]
- Lee ST, Park JE, Kim DH, Kim S, Im WS, Kang L, Jung SH, Kim MW, Chu K, Kim M. Granulocyte-colony stimulating factor attenuates striatal degeneration with activating survival pathways in 3-nitropropionic acid model of Huntington's disease. Brain Res. 2008;1194:130–137. doi: 10.1016/j.brainres.2007.11.058. [DOI] [PubMed] [Google Scholar]
- Lin YM, Jan HJ, Lee CC, Tao HY, Shih YL, Wei HW, Lee HM. Dexamethasone reduced invasiveness of human malignant glioblastoma cells through a MAPK phosphatase-1 (MKP-1) dependent mechanism. Eur J Pharmacol. 2008;593:1–9. doi: 10.1016/j.ejphar.2008.06.111. [DOI] [PubMed] [Google Scholar]
- Liu L, Cavanaugh JE, Wang Y, Sakagami H, Mao Z, Xia Z. ERK5 activation of MEF2-mediated gene expression plays a critical role in BDNF-promoted survival of developing but not mature cortical neurons. Proc Natl Acad Sci U S A. 2003;100:8532–8537. doi: 10.1073/pnas.1332804100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YF. Expression of polyglutamine-expanded Huntingtin activates the SEK1-JNK pathway and induces apoptosis in a hippocampal neuronal cell line. J Biol Chem. 1998;273:28873–28877. doi: 10.1074/jbc.273.44.28873. [DOI] [PubMed] [Google Scholar]
- Luthi-Carter R. Huntington's and other polyglutamine diseases: many effects of single gene mutations. Drug Discov Today. 2007;4:111–119. [Google Scholar]
- Luthi-Carter R, Hanson SA, Strand AD, Bergstrom DA, Chun W, Peters NL, Woods AM, Chan EY, Kooperberg C, Krainc D, Young AB, Tapscott SJ, Olson JM. Dysregulation of gene expression in the R6/2 model of polyglutamine disease: parallel changes in muscle and brain. Hum Mol Genet. 2002;11:1911–1926. doi: 10.1093/hmg/11.17.1911. [DOI] [PubMed] [Google Scholar]
- Magi-Galluzzi C, Mishra R, Fiorentino M, Montironi R, Yao H, Capodieci P, Wishnow K, Kaplan I, Stork PJ, Loda M. Mitogen-activated protein kinase phosphatase 1 is overexpressed in prostate cancers and is inversely related to apoptosis. Lab Invest. 1997;76:37–51. [PubMed] [Google Scholar]
- Marie-Claire C, Benturquia N, Lundqvist A, Courtin C, Noble F. Characteristics of dual specificity phosphatases mRNA regulation by 3,4-methylenedioxymethamphetamine acute treatment in mice striatum. Brain Res. 2008;1239:42–48. doi: 10.1016/j.brainres.2008.08.050. [DOI] [PubMed] [Google Scholar]
- Merienne K, Helmlinger D, Perkin GR, Devys D, Trottier Y. Polyglutamine expansion induces a protein-damaging stress connecting heat shock protein 70 to the JNK pathway. J Biol Chem. 2003;278:16957–16967. doi: 10.1074/jbc.M212049200. [DOI] [PubMed] [Google Scholar]
- Mishra OP, Delivoria-Papadopoulos M. Effect of hypoxia on the expression and activity of mitogen-activated protein (MAP) kinase-phosphatase-1 (MKP-1) and MKP-3 in neuronal nuclei of newborn piglets: the role of nitric oxide. Neuroscience. 2004;129:665–673. doi: 10.1016/j.neuroscience.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Muda M, Boschert U, Dickinson R, Martinou JC, Martinou I, Camps M, Schlegel W, Arkinstall S. MKP-3, a novel cytosolic protein-tyrosine phosphatase that exemplifies a new class of mitogen-activated protein kinase phosphatase. J Biol Chem. 1996;271:4319–4326. doi: 10.1074/jbc.271.8.4319. [DOI] [PubMed] [Google Scholar]
- Munoz L, Ammit AJ. Targeting p38 MAPK pathway for the treatment of Alzheimer's disease. Neuropharmacology. 2010;58:561–568. doi: 10.1016/j.neuropharm.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Obara Y, Nakahata N. The signaling pathway leading to extracellular signal-regulated kinase 5 (ERK5) activation via G-proteins and ERK5-dependent neurotrophic effects. Mol Pharmacol. 2010;77:10–16. doi: 10.1124/mol.109.060236. [DOI] [PubMed] [Google Scholar]
- Patterson KI, Brummer T, O'Brien PM, Daly RJ. Dual-specificity phosphatases: critical regulators with diverse cellular targets. Biochem J. 2009;418:475–489. doi: 10.1042/BJ20082234. [DOI] [PubMed] [Google Scholar]
- Perrin V, Dufour N, Raoul C, Hassig R, Brouillet E, Aebischer P, Luthi-Carter R, Déglon N. Implication of the JNK pathway in a rat model of Huntington's disease. Exp Neurol. 2009;215:191–200. doi: 10.1016/j.expneurol.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Rangone H, Poizat G, Troncoso J, Ross CA, MacDonald ME, Saudou F, Humbert S. The serum- and glucocorticoid-induced kinase SGK inhibits mutant huntingtin-induced toxicity by phosphorylating serine 421 of huntingtin. Eur J Neurosci. 2004;19:273–279. doi: 10.1111/j.0953-816x.2003.03131.x. [DOI] [PubMed] [Google Scholar]
- Reijonen S, Kukkonen JP, Hyrskyluoto A, Kivinen J, Kairisalo M, Takei N, Lindholm D, Korhonen L. Downregulation of NF-κB signaling by mutant huntingtin proteins induces oxidative stress and cell death. Cell Mol Life Sci. 2010;67:1929–1941. doi: 10.1007/s00018-010-0305-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roze E, Betuing S, Deyts C, Marcon E, Brami-Cherrier K, Pagès C, Humbert S, Mérienne K, Caboche J. Mitogen- and stress-activated protein kinase-1 deficiency is involved in expanded-huntingtin-induced transcriptional dysregulation and striatal death. FASEB J. 2008;22:1083–1093. doi: 10.1096/fj.07-9814. [DOI] [PubMed] [Google Scholar]
- Rudinskiy N, Kaneko YA, Beesen AA, Gokce O, Régulier E, Déglon N, Luthi-Carter R. Diminished hippocalcin expression in Huntington's disease brain does not account for increased striatal neuron vulnerability as assessed in primary neurons. J Neurochem. 2009;111:460–472. doi: 10.1111/j.1471-4159.2009.06344.x. [DOI] [PubMed] [Google Scholar]
- Scotter EL, Goodfellow CE, Graham ES, Dragunow M, Glass M. Neuroprotective potential of CB1 receptor agonists in an in vitro model of Huntington's disease. Br J Pharmacol. 2010;160:747–761. doi: 10.1111/j.1476-5381.2010.00773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack DN, Seternes OM, Gabrielsen M, Keyse SM. Distinct binding determinants for ERK2/p38alpha and JNK MAP kinases mediate catalytic activation and substrate selectivity of MAP kinase phosphatase-1. J Biol Chem. 2001;276:16491–16500. doi: 10.1074/jbc.M010966200. [DOI] [PubMed] [Google Scholar]
- Stack EC, Del Signore SJ, Luthi-Carter R, Soh BY, Goldstein DR, Matson S, Goodrich S, Markey AL, Cormier K, Hagerty SW, Smith K, Ryu H, Ferrante RJ. Modulation of nucleosome dynamics in Huntington's disease. Hum Mol Genet. 2007;16:1164–1175. doi: 10.1093/hmg/ddm064. [DOI] [PubMed] [Google Scholar]
- Sun H, Charles CH, Lau LF, Tonks NK. MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell. 1993;75:487–493. doi: 10.1016/0092-8674(93)90383-2. [DOI] [PubMed] [Google Scholar]
- Tanoue T, Yamamoto T, Nishida E. Modular structure of a docking surface on MAPK phosphatases. J Biol Chem. 2002;277:22942–22949. doi: 10.1074/jbc.M202096200. [DOI] [PubMed] [Google Scholar]
- Thu DC, Oorschot DE, Tippett LJ, Nana AL, Hogg VM, Synek BJ, Luthi-Carter R, Waldvogel HJ, Faull RL. Cell loss in the motor and cingulate cortex correlates with symptomatology in Huntington's disease. Brain. 2010;133:1094–1110. doi: 10.1093/brain/awq047. [DOI] [PubMed] [Google Scholar]
- Varma H, Cheng R, Voisine C, Hart AC, Stockwell BR. Inhibitors of metabolism rescue cell death in Huntington's disease models. Proc Natl Acad Sci U S A. 2007;104:14525–14530. doi: 10.1073/pnas.0704482104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma H, Yamamoto A, Sarantos MR, Hughes RE, Stockwell BR. Mutant huntingtin alters cell fate in response to microtubule depolymerization via the GEF-H1-RhoA-ERK pathway. J Biol Chem. 2010;285:37445–37457. doi: 10.1074/jbc.M110.125542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Ankati H, Akubathini SK, Balderamos M, Storey CA, Patel AV, Price V, Kretzschmar D, Biehl ER, D'Mello SR. Identification of novel 1,4-benzoxazine compounds that are protective in tissue culture and in vivo models of neurodegeneration. J Neurosci Res. 2010;88:1970–1984. doi: 10.1002/jnr.22352. [DOI] [PubMed] [Google Scholar]
- Zala D, Benchoua A, Brouillet E, Perrin V, Gaillard MC, Zurn AD, Aebischer P, Déglon N. Progressive and selective striatal degeneration in primary neuronal cultures using lentiviral vector coding for a mutant huntingtin fragment. Neurobiol Dis. 2005;20:785–798. doi: 10.1016/j.nbd.2005.05.017. [DOI] [PubMed] [Google Scholar]
- Zhou J, Wang L, Ling S, Zhang X. Expression changes of growth-associated protein-43 (GAP-43) and mitogen-activated protein kinase phosphatase-1 (MKP-1) and in hippocampus of streptozotocin-induced diabetic cognitive impairment rats. Exp Neurol. 2007;206:201–208. doi: 10.1016/j.expneurol.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Zuccato C, Ciammola A, Rigamonti D, Leavitt BR, Goffredo D, Conti L, MacDonald ME, Friedlander RM, Silani V, Hayden MR, Timmusk T, Sipione S, Cattaneo E. Loss of huntingtin-mediated BDNF gene transcription in Huntington's disease. Science. 2001;293:493–498. doi: 10.1126/science.1059581. [DOI] [PubMed] [Google Scholar]
- Zuccato C, Liber D, Ramos C, Tarditi A, Rigamonti D, Tartari M, Valenza M, Cattaneo E. Progressive loss of BDNF in a mouse model of Huntington's disease and rescue by BDNF delivery. Pharmacol Res. 2005;52:133–139. doi: 10.1016/j.phrs.2005.01.001. [DOI] [PubMed] [Google Scholar]