Abstract
Many primary sensory neurons are polymodal, responding to multiple stimulus modalities (chemical, thermal, or mechanical), yet each modality is recognized differently. Although polymodality implies that stimulus encoding occurs in higher centers, such as the spinal cord or brain, recent sensory neuron ablation studies find that behavioral responses to different modalities require distinct subpopulations, suggesting the existence of modality-specific labeled lines at the level of the sensory afferent. Here we provide evidence that neurons expressing TRPM8, a cold- and menthol-gated channel required for normal cold responses in mammals, represents a labeled line solely for cold sensation. We examined the behavioral significance of conditionally ablating TRPM8-expressing neurons in adult mice, finding that, like animals lacking TRPM8 channels (Trpm8−/−), animals depleted of TRPM8 neurons (“ablated”) are insensitive to cool to painfully cold temperatures. Ablated animals showed little aversion to noxious cold and did not distinguish between cold and a preferred warm temperature, a phenotype more profound than that of Trpm8−/− mice which exhibit only partial cold-avoidance and -preference behaviors. In addition to acute responses, cold pain associated with inflammation and nerve injury was significantly attenuated in ablated and Trpm8−/− mice. Moreover, cooling-induced analgesia after nerve injury was abolished in both genotypes. Last, heat, mechanical, and proprioceptive behaviors were normal in ablated mice, demonstrating that TRPM8 neurons are dispensable for other somatosensory modalities. Together, these data show that, although some limited cold sensitivity remains in Trpm8−/− mice, TRPM8 neurons are required for the breadth of behavioral responses evoked by cold temperatures.
Introduction
Sensations, such as touch and pain, are mediated by peripheral sensory neurons through activation of modality-specific sensory receptors (Basbaum et al., 2009; Ma, 2010). For temperature, ion channels of the transient receptor potential (TRP) family function as molecular thermo-detectors, providing the receptor potential that initiates signaling to the CNS (Jordt et al., 2003). Unlike the diversity of heat-sensitive TRPV channels, the preponderance of data suggests that TRPM8, the receptor for menthol (the cooling ingredient in mint), functions as the primary mammalian detector of cold (McCoy et al., 2011). TRPM8 channels activate at temperatures below ∼25°C, with currents increasing in magnitude as temperatures decrease (McKemy et al., 2002). Behaviorally, mice lacking TRPM8 channels fail to distinguish warm from cool and poorly avoid noxious cold (Bautista et al., 2007; Colburn et al., 2007; Dhaka et al., 2007; Knowlton et al., 2010). Moreover, cold allodynia (pain caused by innocuous stimuli) associated with inflammatory and neuropathic injury is diminished in these animals (Colburn et al., 2007; Knowlton et al., 2011). Thus, because of its prominent role in cold sensation, TRPM8 and the neurons expressing this receptor are attractive targets in the study of cold and cold pain transduction.
Most sensory neurons are polymodal because they are activated by multiple stimulus modalities (Perl, 1996; Cain et al., 2001), properties suggesting that modality discrimination does not occur in the afferent itself but in higher-order structures (Perl, 2007). Consistent with this view, genetic ablation or silencing of neurons expressing the voltage-gated sodium channel Nav1.8 attenuated thermal, mechanical, and inflammatory pain in mice (Abrahamsen et al., 2008; Liu et al., 2010). However, ablation of polymodal neurons expressing the heat-gated channel TRPV1 diminished thermal pain without altering mechanosensation, whereas ablation of a separate population of non-peptidergic neurons attenuated mechanical pain with no effect on thermal behaviors (Cavanaugh et al., 2009; Mishra and Hoon, 2010; Mishra et al., 2011). These latter results suggest that sensory discrimination does occur, at least in part, at the level of the sensory afferent and that the polymodality inherent in these neurons may not translate to stimulus coding.
Like many classes of sensory nerve fibers, those that are cold sensitive are polymodal. For example, mechanosensitive Aδ and C fibers innervating both hairy and glabrous skin are cold sensitive (Simone and Kajander, 1996, 1997; Cain et al., 2001). Similarly, a cohort of menthol-sensitive neurons respond to capsaicin in vitro (McKemy et al., 2002; Viana et al., 2002; Xing et al., 2006; Hjerling-Leffler et al., 2007), results consistent with the expression of TRPV1 in a subset of TRPM8 neurons (Takashima et al., 2007; Dhaka et al., 2008). However, it is unclear whether this heterogeneity is relevant in coding of somatic stimuli. Here we selectively ablated TRPM8 neurons in vivo, finding that ablated animals are insensitive to both innocuous and noxious cold, show attenuated cold hypersensitivity after injury, and display a loss of cold analgesia after injury. Ablated mice show normal heat sensation, mechanosensation, and proprioception, results suggesting that TRPM8 afferents represent a “labeled line” for cold sensation.
Materials and Methods
Trpm8DTR mice and TRPM8 neuron ablation.
All experiments were approved by the University of Southern California (USC) Institutional Animal Care and Use Committee and performed in accordance with the recommendations of the International Association for the Study of Pain and with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The Trpm8DTR line of transgenic mice was generated as described previously (Takashima et al., 2007). Briefly, a trpm8 bacterial artificial chromosome (BAC) clone (553 H3 of the RPCI.22 BAC library; Invitrogen) was modified by homologous recombination, targeting the simian diphtheria toxin receptor (DTR) transgene [a gift from Dr. R. Lang, Cincinnati Children's Hospital Medical Center, Cincinnati, OH (Jung et al., 2002)] to sequences corresponding to the second coding exon in the trpm8 gene. DNA sequences flanking this site were PCR amplified with PfuUltra HF polymerase (Stratagene) and subcloned into the pLD53.SCA-E-B shuttle vector (a gift from N. Heintz, The Rockefeller University, New York, NY) in which the green fluorescent protein (GFP) cassette was replaced with DTR. Shuttle vector DNA was electroporated into electrocompetent bacteria, and cells in which the transgene was correctly targeted were selected as described previously (Takashima et al., 2007). Modified BAC clones were screened by PCR, and Trpm8DTR–BAC DNA was purified and injected into the pronucleus of fertilized ova at the USC Transgenic Core Facility as described previously (Takashima et al., 2007). Transgenic founder mice were identified by PCR and backcrossed into the C57BL/6 strain out to more than four generations. Animals were maintained on a 12 h light/dark cycle and provided access to food and water ad libitum.
Two consecutive diphtheria toxin (DTx) intraperitoneal injections (50 μg/kg in normal saline; List Biological Laboratories; Sigma) were administered 3 d apart to both transgenic mice and their control littermates aged ≥8 weeks. All behavioral tests were conducted using mice of either sex at least 21 d after initial injection and were performed during the animals' light cycle. All animals were handled extensively before the commencement of behavioral testing and acclimated to the testing room. Animals were housed in their home cages immediately before and after testing and were prehabituated to the behavioral apparatus as appropriate. For most assays, responses were video recorded and later quantified by an experimenter blind to genotype. All results were analyzed using one-way ANOVA tests, followed by Bonferroni's and Tukey's HSD post hoc analyses (Origin graphing software), and all data are graphed as means ± SEM.
Histochemistry.
Immunolabeling was performed on dorsal root (DRG) and trigeminal (TG) ganglia and lumbar spinal cords from transgenic mice as described previously (Takashima et al., 2007). Primary antibodies were diluted in a working solution (1% Triton X-100 in PBS). The antibodies and dilutions used in this study included the following: 1:2000 rabbit anti-GFP (A-11122; Life Technologies), 1:500 chicken anti-GFP (GFP-1020; Aves Labs), 1:500 mouse anti-Nav1.8 (75-166; Neuromab), and 1:1000 rabbit anti-human PGP9.5 (AB1761; Millipore). Labeled sections were incubated overnight at 4°C with primary antibodies, washed, and then incubated 1 h at room temperature with secondary antibodies conjugated to Alexa Fluor-488 or Alexa Fluor-594 (1:1000; Invitrogen). To detect isolectin B4 (IB4) binding, we included 1:2000 Griffonia simplicifolia isolectin GS–IB4–Alexa Fluor-568 (I-21412; Life Technologies) during secondary antibody incubations. Digital images were acquired on a Carl Zeiss AxoImager Z1 with Apotome attachment, and quantification of overlap between GFP expression and that of other neuronal markers was obtained per field, quantified with NIH ImageJ software, and expressed as percentage overlap with the SEM between fields (Takashima et al., 2007, 2010).
Quantitative PCR and microarray.
RNA transcripts were purified from sensory ganglia using the RNAeasy Mini kit (Qiagen). cDNA was made from these extracts from 1 μg of RNA using the iScript cDNA synthesis kit (Bio-Rad) according to the instructions of the manufacturers. Quantitative PCR (qPCR) was performed using a Bio-Rad CFX96 RT-PCR detection system and the SsoFast qPCR kit (Bio-Rad) according to the instructions of the manufacturer. The primers used for TRPM8 transcripts were as follows: M8 forward, 5′-GCTGCCTGAAGAGGAAATTG-3′; M8 reverse, 5′-GCCCAGATGAAGAGAGCTTG-3′. The reference gene used was GAPDH, amplified with the following primer set: GAPDH forward, 5′-TGTAGACCATGTAGTGAGGTCA-3′; GAPDH reverse, 5′-AGGTCGGTGTGAACGGATTTG-3′. For microarray, total RNA was isolated for control and TRPM8 neuron-ablated mice (n = 8 mice per condition) using RNAlater buffer (Qiagen) and then precipitated and purified with the RNeasy Mini kit (Qiagen) with in-column DNA digestion following the instructions of the manufacturer. Microarray analyses comparing control with ablated samples were performed in triplicate and assayed using the GeneChip Mouse Gene 1.0 ST Arrays by the USC/Children's Hospital Los Angeles Affymetrix MicroArray Core Facility.
Acetone evaporative cooling and wet-dog shaking assays.
The acetone evaporation assay was performed as described previously (Knowlton et al., 2011). Briefly, mice were acclimated for 15 min in an elevated chamber with a mesh floor (IITC), a syringe with a piece of rubber tubing attached to the end was filled with acetone, and the plunger was depressed so that a small drop of acetone formed at the tip. The syringe was raised from below, depositing the acetone drop on the paw. Mice were tested with an interstimulation period of 4 min per mouse, alternating paws between stimulations. Responses were video recorded for later quantification by multiple observers blind to the experimental conditions. Behaviors were scored according to the magnitude of the response along the following scale: 0, no response; 1, brief lift, sniff, flick, or startle; 2, jumping, paw shaking; 3, multiple lifts, paw lick; 4, prolonged paw lifting, licking, shaking, or jumping; 5, paw guarding. For wet-dog shakes, mice were injected intraperitoneally with 50 mg/kg body weight of icilin in 1% Tween 80, and then the resulting behaviors were video recorded over a 20 min period.
Hotplate/cold plate assay.
Animals were placed on a plate chilled to 0°C or heated to either 48 or 52°C, and then their responses were video recorded. For cold, latencies to forepaw flinching and licking or wringing were later quantified, whereas for heat, latencies to hindpaw shaking or licking were determined. For cold, mice were tested with an extended cutoff time of 2 min, whereas cutoff times for animals tested at 48 and 52°C were at 60 and 30 s, respectively.
Two-temperature choice assay.
The two-temperature choice test was performed as described previously (Daniels and McKemy, 2010; Knowlton et al., 2010). Briefly, animals were acclimated to the chamber for 5 min with both plates set to 30°C. Next the animals were given a choice between the control plate (30°C) and the test plate, which was set to temperatures ranging from 5 to 50°C. During this 5 min testing period, animals were video-recorded from above. Another 30−30°C acclimation trial was conducted, and then a final test trial was recorded but with plate temperature orientation reversed. Behavior was quantified from videos using custom software (MouseChaser) written in C++ that analyzed each video frame (30 frames/s), extracted the position of the mouse, and saved it to a log file. The method for tracking the mouse in the video uses the feature-space-based mean-shift algorithm with color histogram as the feature (Comaniciu et al., 2000). The initial position of the mouse on the first video frame was specified manually by entering the x and y positions of the top-left vertex of a square defining a 50 × 50 pixel patch on the mouse body as shown in Figure 4A. The red, green, and blue values of each of the 50 × 50 pixels in the patch were computed. Each channel's pixels were averaged producing a signature color-based feature from which subsequent tracking was performed. On each subsequent frame of the video, 50 × 50 pixel patches around the starting position were analyzed, and a Euclidean distance measure in feature was computed between each candidate patch (cp) and the target patch (tp) defined in the first frame as follows:
where rtp and rcp are the mean red pixel values for tp and cp, respectively, and similarly g and b are the mean values for the green and blue channels, respectively. For each iteration, eight nearby patches were analyzed, and the cp with the smallest d measure was chosen as the next location of the center of the mouse. The log file stored by the program contained x and y locations of the mouse over the period of the experiment, which were plotted as a two-dimensional histogram. The two-dimensional space of the image was binned into 5-pixel-wide bins, and each time the mouse center was estimated to be at a particular x,y location, the corresponding bin was incremented. The resulting x,y matrices were normalized to the number of mice in the dataset, and the resulting data were graphed as pseudocolored heat maps to visualize plate location. When tested on the same videos, the software provided comparable results with human observers who measured the time the mouse spent on each plate using a timer.
Figure 4.
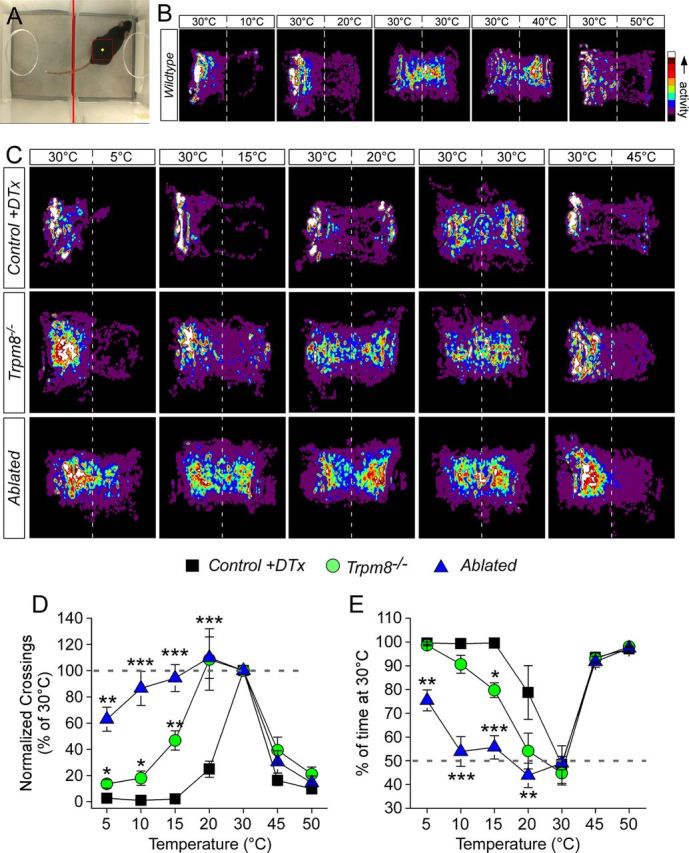
Mouse mobility on the two-temperature choice assay is altered in absence of TRPM8 channels and neurons. A, The MouseChaser tracking software was developed to analyze mouse movement on the two-temperature choice assay. Screen capture from software after annotation of video with position of mouse. The red square is an estimate of the mouse's position with the yellow diamond marking the center of which the x and y coordinates are logged over the course of the video. B, Movements of wild-type mice in the two-temperature choice chamber, with the control surface held constant at 30°C and the test at temperatures from 10 to 50°C, were monitored over a 5 min period. Pseudocolored exploration plots show qualitatively equal activity on both surfaces set to 30°C, with reduced movement to and on the test surface as it was either heated or cooled. C, Comparative analysis of mobility from noxious cold (5°C) to noxious heat (45°C) for control, Trpm8−/−, and ablated mice. D, Avoidance of the test surface was measured as the number of times the animal crossed between surfaces, with control mice showing decreased mobility as the temperature deviated from 30°C. Both Trpm8−/− and ablated mice avoided noxious heat but, unlike controls, did not avoid 20 versus 30°C (**p < 0.01, n = 6–12). Trpm8−/− mice did avoid cold as temperatures dropped below 20°C but not to the same extent as control mice (*p < 0.05). However, ablated mice showed no avoidance behaviors until test plate temperatures of 5°C. E, Preference for warmth was determined by quantifying the time spent on the 30°C surface, with all genotypes showing strong preference for warmth over painful heat (p < 0.001 compared with test at 30°C). Control and Trpm8−/− mice showed warm preference at temperatures ≤15°C (p < 0.001 compared with test at 30°C), whereas ablated animals showed no preference between 10 and 20°C (p > 0.05 compared with test at 30°C) and moderate preference at 5°C (p < 0.01 compared with test at 30°C). Preference behaviors for ablated mice were different from control and Trpm8−/− animals at all temperatures below 20°C (**p < 0.01, ***p < 0.001).
Hargreaves radiant heat assay.
Mice were placed in plastic chambers on a glass surface heated to 32°C through which radiant heat was focused on the hindpaw (IITC), and the latency to a paw withdrawal was determined as the average of three trials per animal taken at 10 min intervals (Hargreaves et al., 1988). To avoid tissue damage, a cutoff latency of 20 s was used.
Grip-strength test and accelerating rotarod.
Animals' grip strength limits were measured using a grip-strength monitor (Chantillon) fitted with a triangular grip wire (Yoo and Ko, 2011). The animal was suspended by the tail in front of the wire and allowed to grip it while the experimenter gently pulled the mouse backward. The maximum force exerted by the mouse at time of release was recorded and averaged over five trials. General coordination was assessed on a rotarod device (Letica Scientific Instruments) while it was slowly turning at four rotations per minute. When the animal was oriented in the correct direction and stably maintaining its grip on the rod, the device was activated to maximum speed (40 rpm) over 60 s. Time to drop was averaged over five trials.
Dynamic stroke assay.
A cotton-tipped swab was puffed up until it was approximately three times its original volume (Garrison et al., 2012). Animals were acclimated on an elevated mesh platform for 15 min, and then the cotton swab was quickly (<1 s) swiped across the plantar surface of each hindpaw. Removal or shaking of the hindpaw was counted as a positive response, and the percentage of responses were calculated over 10 trials, five per foot, alternating paws, and at least 30 s apart.
Electronic von Frey.
Stimulation with an electronic von Frey apparatus (IITC) was performed as described previously (Knowlton et al., 2011). Briefly, animals were acclimated to an elevated mesh platform for 15 min. The apparatus was fitted with a semi-flexible tip and raised to the plantar surface of the hindpaw. The force at which the mouse removed the paw was measured, and average paw-withdrawal threshold was measured either per mouse or per hindlimb (depending on experiment) from five trials per hindlimb, alternating paws, with 4 min between trials.
Pain models.
As described previously (Knowlton et al., 2011), inflammation was induced by injection of 20 μl of complete Freund's adjuvant (CFA) into the hindpaw unilaterally. Mice were tested at 2 d after injection. For neuropathic pain, the chronic constriction injury (CCI) model was used (Bennett and Xie, 1988). Briefly, animals were anesthetized with 5% isoflurane for induction and then 3% isoflurane for maintenance. The surface of the right hindlimb was shaved and sterilized. A 2 cm incision was made in the skin, and the muscles overlaying the sciatic nerve were gently retracted. Three loose ligatures of 6-0 chromic gut were placed around the nerve proximal to the trifurcation site. The muscles were replaced, and the wound was closed with VetBond tissue adhesive (3M) and swabbed with topical antibiotic. Animals were monitored for infection and proper wound healing and were tested on day 7 or later after surgery.
Cooling analgesia.
Between 10 and 14 days after CCI surgery, animals were tested for paw-withdrawal thresholds with the electronic von Frey apparatus as described above. Animals were then placed in a shallow (∼0.5 cm deep) water bath held at 17°C for 5 min. Mice were then retested every 5 min with the electronic von Frey for a total of 60 min.
Results
Conditional ablation of TRPM8-lineage neurons
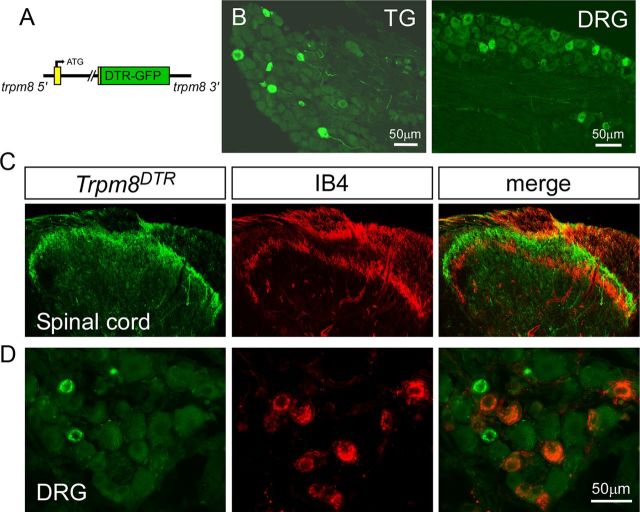
Using a previously developed transgenic strategy (Takashima et al., 2007), we created BAC transgenic mouse lines in which expression of DTR, a fusion with GFP (Saito et al., 2001; Jung et al., 2002), is driven by the trpm8 transcriptional promoter (Fig. 1A). These animals (referred to as Trpm8DTR mice) displayed normal Mendelian genetics and were phenotypically identical to their nontransgenic littermates. Transgene expression was analyzed by GFP fluorescence and immunoreactivity, and we observed robust labeling in a minor subset of small-diameter neurons within both the DRG and TG [DRG, 11.5 ± 0.4% of PGP9.5-labeled neurons (n = 102), 23.1 ± 0.5 μm average diameter (n = 117); TG, 16.2 ± 1.3% (n = 475), 19.3 ± 0.2 μm (n = 406); Fig. 1B]. As with our previously characterized line of GFP reporter mice [Trpm8GFP, JAX Stock #020650 (The Jackson Laboratory), C57BL/6-Tg(Trpm8-EGFP)1Dmck/J] that were generated using the same BAC targeting strategy (Takashima et al., 2007, 2010), DTR expression (as determined by GFP immunoreactivity) was observed in axon terminals in the spinal cord dorsal horn in the Trpm8DTR line (Fig. 1C). GFP-expressing axons terminated dorsal to non-peptidergic IB4-labeled axons and in the most superficial laminar layers, a termination pattern consistent with thermosensitive afferent fibers. In DRG and TG, GFP expression overlapped with a number of afferent markers but was not found in small-diameter neurons that bind IB4 (Fig. 1D), data consistent with previous reports on the distribution of TRPM8 expression in neurochemically distinct neurons (Peier et al., 2002; Takashima et al., 2007, 2010; Hayashi et al., 2009).
Figure 1.
Development of the Trpm8DTR mouse line. A, A complementary DNA transgene for the simian DTR, a GFP fusion protein, was inserted into the trpm8 BAC clone at sequences corresponding to the second coding exon. B, Transgene expression (GFP-expression) in TG and DRG neurons. C, DTR-expressing central projections (green) were localized to the outermost lamina of the dorsal horn of the lumbar spinal cord and did not overlap with those binding to IB4 (red). D, Immunolabeling revealed no transgene expression in non-peptidergic neurons that bind IB4.
The simian DTR has a >105 higher affinity for DTx than the rodent ortholog, and systemic injections of subtoxic doses of toxin in mice selectively kills the genetically targeted cell type (Fig. 2A) (Palmiter, 2001; Saito et al., 2001). Thus, mice are allowed to develop normally into adulthood, and cell death is initiated at a specific experimental time point. Following established methods (Chen et al., 2005; Cavanaugh et al., 2009), we developed a DTx injection protocol (two intraperitoneal injections of 50 μg/kg DTx separated by 3 d) that led to significant reductions in TRPM8 transcripts in DRG of DTx-injected Trpm8DTR animals by 7 d after the first injection, as determined by qPCR (Fig. 2B; one injection, 12.6 ± 3.8% of control; two injections, 0.18 ± 0.04% of control, p < 0.0001, n = 5). Similarly, we observed a complete loss of GFP expression in DRG from DTx-treated Trpm8DTR animals (Fig. 2C,D) but not in DTx-injected Trpm8GFP mice (Fig. 2E). Ablation was specific for TRPM8 neurons because there was no significant change in the number of neurons expressing other neurochemical markers. For example, the number of IB4-positive cells in sensory ganglia from DTx-injected Trpm8DTR mice versus control animals was unchanged [TG and DRG: 36.0 ± 1.3% ablated (n = 4919 cells); 33.1 ± 1.6% control (n = 3927 cells), p > 0.05, n = 2–3 mice each].
Figure 2.
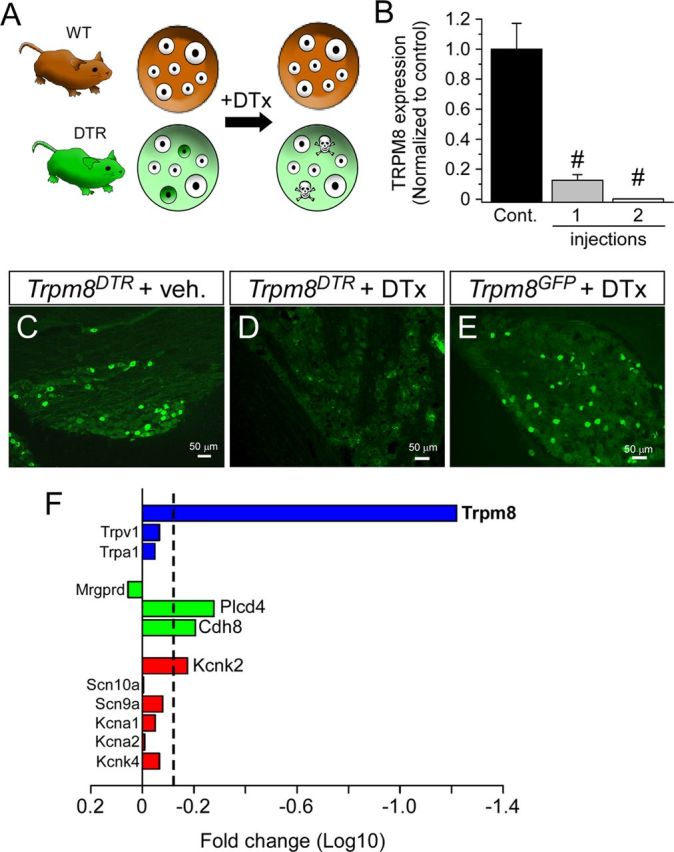
Selective ablation of TRPM8-expressing sensory neurons. A, Schematic of the TRPM8 neuron ablation protocol. Adult mice, both Trpm8DTR (DTR) and control littermates (WT), were given two intraperitoneal injections of 50 μg/kg DTx, which selectively ablated only targeted neurons (green cells). B, One week after injection of a single dose of DTx, the expression of TRPM8 transcripts was reduced to 12.6 ± 3.8% of control and then to 0.18 ± 0.04% if two injections were performed (n = 5, #p < 0.0001), as measured by qPCR. GFP-like immunoreactivity was abolished in Trpm8DTR mice injected with DTx (D) compared with vehicle-injected Trpm8DTR mice (C) or control Trpm8GFP mice (E) subjected to the same DTx injection protocol as in D. F, Comparative analysis of RNA transcripts isolated from control and ablated DRG was performed by microarray and plotted as the fold reduction (log10) in expression between the two conditions. Dashed line indicates the 1.5-fold reduction threshold for significant reduction in expression.
To further confirm the specificity of TRPM8 neuron ablation, we used microarrays and compared transcript expression in TG and DRG isolated from DTx-injected Trpm8DTR and control animals (DTx-injected transgene-negative littermates of Trpm8DTR mice). Ganglia were dissected and total RNA purified separately for each sample (n = 8 each condition). Before combining samples, qPCR was performed on each to ensure that Trpm8 transcript levels were reduced to <1% (0.8 ± 0.5%) in DTx-injected Trpm8DTR animals. In this screen, TRPM8 transcript expression was reduced 21.8-fold, in addition to other genes whose products are known to express with TRPM8, including cadherin-8, PLCδ4, and TREK-1 (Fig. 2F) (Suzuki et al., 2007; Daniels et al., 2009; Descoeur et al., 2011). Expression of other transcripts known not to colocalize with TRPM8, such as TRPA1 and Mrgprd, were unaffected by TRPM8 neuron ablation (Story et al., 2003; Zylka et al., 2005; Cavanaugh et al., 2009). Thus, using this animal model and the DTx injection protocol described, we set out to determine the necessity of neurons within the TRPM8 lineage in mouse somatosensory behaviors.
Mice lacking TRPM8 neurons are insensitive to innocuous cool and cold mimetics
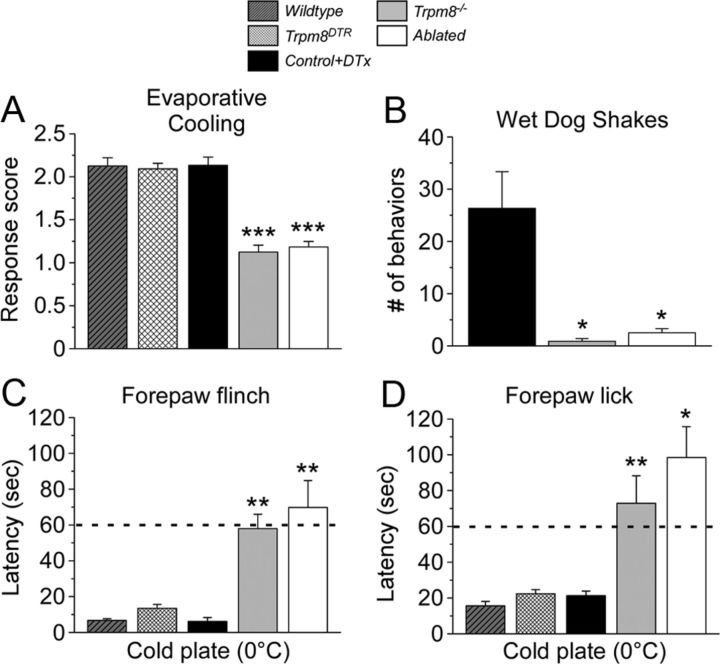
We tested DTx-injected Trpm8DTR mice (termed ablated) and their injected control transgene-negative littermates in a variety of behavioral assays, all performed after a recovery period of greater than 3 weeks after DTx injection to fully resolve cell ablation. In addition, to control for any effects of transgene expression and assess differences in mice lacking TRPM8 neurons to those lacking functional TRPM8 channels (Trpm8−/−), we also examined un-injected Trpm8DTR mice along with wild-type and Trpm8−/− mice in these assays (Bautista et al., 2007; Knowlton et al., 2010). First, we assessed responses to innocuous cool using the acetone evaporative cooling assay, a stimulus applied to the mouse hindpaw that transiently reduces surface skin temperature to ∼17°C (Bautista et al., 2007; Colburn et al., 2007; Knowlton et al., 2011). Flinch, lick, and guarding behaviors were scored on a 5-point scale, with wild-type and un-injected Trpm8DTR mice exhibiting similar scores of 2.1 ± 0.1 and 2.1 ± 0.1, respectively (n = 8–12 animals, p > 0.05; Fig. 3A), demonstrating that transgene expression had no effect on cold behaviors. DTx injection did not alter cold-induced behaviors because like scores were obtained from control littermates (2.1 ± 0.1), whereas both Trpm8−/− and ablated animals showed little to no response to acetone (1.1 ± 0.1 Trpm8−/−, 1.2 ± 0.1 ablated; n = 8–12, p < 0.001 compared with control) (Fig. 3A). In this assay, a score of 1 indicates a brief lift or sniff of the stimulated paw—responses similar to if a droplet of room temperature water were applied. These data show that response to innocuous cool requires intact TRPM8 afferents, as well as functional TRPM8 channels.
Figure 3.
Ablation of TRPM8 afferents abolishes acute cold behaviors. A, Evaporative cooling induced by application of a droplet of acetone to the hindpaw evokes stereotyped behaviors from wild-type, un-injected Trpm8DTR, and control mice as determined on a 5-point score scale (see Materials and Methods; Knowlton et al., 2011). The response scores of Trpm8−/− and ablated mice were significantly reduced from control to 1.1 ± 0.1 and 1.2 ± 0.1 (n = 12, ***p < 0.001), respectively, and were not different from one another (p > 0.05). B, Intraperitoneal icilin injections (50 mg/kg body weight) induced robust wet-dog shakes in control but were absent in both Trpm8−/− and ablated mice (*p < 0.05 each compared with control). The latency to nocifensive flinching (C) and licking/wringing (D) behaviors of the forepaw when mice were placed on a 0°C metal plate was recorded. The average latency to flinch and licks in control mice was 6.2 ± 2.1 and 21.3 ± 2.5 s, respectively (n = 6), and similar to that observed in wild-type and Trpm8DTR mice (p > 0.05). Latencies of Trpm8−/− and ablated mice were significantly higher compared with control at 58.0 ± 7.9 s (n = 10) and 69.8 ± 15.0 s (n = 10) for flinching, respectively, and 72.9 ± 15.4 and 98.5 ± 17.1 s for licking, respectively (*p < 0.05, **p < 0.01). Dashed line marks the standard 60 s cutoff time point.
The super-cooling cold-mimetic icilin elicits forceful TRPM8-dependent shaking movements, characterized as wet-dog shakes, when given systemically (Wei and Seid, 1983; Colburn et al., 2007; Dhaka et al., 2007), as well as evokes nocifensive flinching behaviors after intraplantar paw injections in the mouse (Knowlton et al., 2010). These behaviors are thought to be attributable to the cooling effects of icilin (Tse and Wei, 1986), and we tested whether the shivering-like behaviors required intact TRPM8 afferents. Injection of icilin into the intraperitoneal cavity of control mice induced 26.3 ± 7.0 (n = 6) shakes during the 20 min observation period, whereas Trpm8−/− animals exhibited little to no shivering-like behaviors (0.9 ± 0.5 shakes, n = 8, p < 0.05 compared with control) (Fig. 3B), as reported previously (Dhaka et al., 2007). Ablation of TRPM8 afferents also abolished icilin-induced wet-dog shakes with the number of behaviors (2.5 ± 0.8, n = 12, p < 0.05 compared with control) trending higher but not statistically different from Trpm8−/− mice (p > 0.05; Fig. 3B). Together with the evaporative cooling results, these data show that animals lacking TRPM8-expressing neurons exhibit profound impairments in their ability to acutely respond to cold temperatures and chemical cold mimetics.
Novel assays reveal the necessity of TRPM8 channels and neurons in detecting noxious cold
One of the great difficulties in assessing noxious cold-evoked behaviors is that rodents are essentially unperturbed when exposed to these temperatures, unlike the robust hindpaw lifting, flinching, and licking behaviors observed when animals are placed on a surface heated above 45°C. This is most evident with traditional measures of hindpaw response latencies in mice placed on a 0°C cold plate ranging from 5 to 200 s in several independent reports (Bautista et al., 2006, 2007; Colburn et al., 2007; Dhaka et al., 2007). Our analyses observed that wild-type C57BL/6 mice rarely show nocifensive behaviors with their hindpaws under these conditions, generally assuming a posture primarily limiting contact with the cold surface to only the hindpaws (W.M.K., R.P., E.K.L., D.D.McK., unpublished observations). However, additional examination revealed two robust and reproducible behaviors performed by every animal tested, both involving the forepaws. The first presented as a shivering or shaking motion of both forepaws, termed forepaw flinching, which occurred shortly after wild-type animals are placed on the cold surface. This is followed by a second behavior involving a wringing or licking of the forepaw, suggestive of intense irritation.
Thus, we turned our analysis to mouse forepaw behaviors when placed on a 0°C cold plate to determine whether ablation of TRPM8 neurons alters responses to noxious cold. In control animals, we observed a latency to forepaw flinch of 6.2 ± 2.2 s (Fig. 3C, n = 6), similar (p > 0.05) to that of wild-type (6.8 ± 0.9 s, n = 8) and Trpm8DTR (13.5 ± 2.2 s, n = 8) mice. In contrast, both Trpm8−/− and ablated mice had significantly longer flinch latencies of 58.0 ± 7.9 and 69.8 ± 15.0 s, respectively (Fig. 3C; n = 10, p < 0.01 compared with control). Similarly, latencies to lick were 15.6 ± 2.5, 22.4 ± 2.3, and 21.3 ± 2.5 s for wild-type, Trpm8DTR, and control mice (n = 6–16), respectively, and significantly longer in Trpm8−/− and ablated animals at 72.0 ± 15.4 (p < 0.01 compared with control) and 98.5 ± 17.1 s (p < 0.05 compared with control), respectively (Fig. 3D, n = 10). It should be noted that cutoff times to limit exposure to such potentially tissue-damaging stimuli are typically near 60 s (Cavanaugh et al., 2009; Mishra et al., 2011), a time exceeded to observe any behavior in Trpm8−/− and ablated animals. Therefore, it is unclear whether these animals are responding only after prolonged and profound cooling or to possible tissue damage induced by exposure to noxious cold temperatures. Regardless, the impairment observed in ablated mice compared with control demonstrates these animals' inability to appropriately respond to painfully cold temperatures.
TRPM8 neuron-ablated animals show decreased cold discrimination
In another test of the necessity of TRPM8 neurons in cold behaviors, we determined whether ablated animals have deficits in temperature discrimination using the two-temperature choice assay (Bautista et al., 2007; Colburn et al., 2007; Dhaka et al., 2007; Daniels and McKemy, 2010; Knowlton et al., 2010). Mice were permitted to freely explore two neighboring surfaces, with one set to a preferred temperature of 30°C (Moqrich et al., 2005) and the other set to temperatures ranging from 50 to 5°C. We developed a mouse tracking software system (MouseChaser) to monitor these movements in an unbiased manner (Fig. 4A; see Materials and Methods). Mouse movement was monitored for 5 min, and then averaged activity was plotted as pseudo-colored “heat maps” (n = 6–16 animals) when given the choice of 30°C or a range of temperatures from 50 to 5°C. Analysis of wild-type mouse movement demonstrates that animals restricted exploration to the control plate as test plate temperatures diverged to either cold or hot extremes (Fig. 4B). Careful examination of these data showed that wild-type mice appear to avoid noxious cold (10°C) even more robustly than noxious heat (50°C). When control, Trpm8−/−, and ablated mouse movement was analyzed in this manner, we observed similar activity between all genotypes when the test plate was at 30 and 45°C, with all animals showing less activity on the 45°C surface (Fig. 4C). Comparable behaviors were observed for control animals at 20°C, with progressively lower activity on the test surface as temperatures advanced toward 5°C. The activity of Trpm8−/− and ablated mice on the 20°C surface was indistinguishable from that observed at 30°C, whereas Trpm8−/− animals did show reduced movements at 5°C, although not as robust as controls. However, ablated mice showed more activity on the 5°C surface than control and Trpm8−/− mice, indicating either reduced aversion to this noxious cold stimulus or lessened preference for warmth.
We quantified this behavior as described previously (Knowlton et al., 2010), first examining the number of transitions (crossings) from the control (30°C) to the test surface, behaviors indicative of thermal avoidance. For example, when the test surface was set to 30°C, mice of all genotypes freely transitioned between surfaces (Fig. 4D). However, when the test surface is set to 50°C, a highly noxious heat temperature, control, Trpm8−/−, and ablated mice showed significantly reduced mobility and avoided the test surface, demonstrating that mice lacking TRPM8 channels and neurons are able to detect a noxious heat stimulus. Similarly, when the test plate is cooled as little as 10°C from the preferred temperature of 30°C, control animals (n = 6) avoided the cold surface with the number of crossings at 20°C decreasing to 24.8 ± 6.1% of those at 30°C (Fig. 4D, p < 0.001). However, both Trpm8−/− (n = 8) and ablated (n = 12) mice did not discriminate warm from cold with the number of crossings at 108.5 ± 23.5 and 109.9 ± 15.8%, respectively, of those at 30°C when the test surface was held at 20°C (p > 0.05). As the test surface was cooled further, Trpm8−/− mice did exhibit decreased mobility but still crossed more than control mice, even at noxious cold temperatures (13.6 ± 3.2% for Trpm8−/− and 2.6 ± 2.6% for control at 10°C, p < 0.05; Fig. 4D). However, ablated animals displayed an even greater deficit in cold-avoidance behaviors, crossing at a rate similar to when both plates were at 30°C even when the test plate was cooled to 10°C (86.5 ± 13.0% of 30°C, p = 0.3), and only showed decreased mobility at 5°C (63.0 ± 9.2% of the crossings at 30°C, p < 0.01), results consistent with the heat map analysis (Fig. 4C).
We also analyzed behaviors in terms of preference for one temperature over another by quantifying the percentage of the recording period that the animal was on the control surface (Bautista et al., 2007; Colburn et al., 2007; Dhaka et al., 2007; Knowlton et al., 2010). When both surfaces were set at 30°C, animals of all genotypes showed no preference for either surface but did show a strong preference for the control surface when the test plate was heated (45–50°C; Fig. 4E). When the test surface was set to an innocuous cool temperature (20°C), control animals spent a significant percentage of the 5 min observation period on the control side (78.7 ± 11.3% of the recording period, p < 0.05; Fig. 4E), indicating either a strong preference for the warmth or avoidance of the cold, motivations that cannot be differentiated in this type of analysis (Knowlton and McKemy, 2011; McCoy et al., 2011). Furthermore, when the test plate was cooled into the noxious cold range, control mice showed an even more robust preference for warmth (99.3 ± 0.6% when test plate at 10°C, p < 0.001). As we and others have shown (Bautista et al., 2007; Colburn et al., 2007; Dhaka et al., 2007; Knowlton et al., 2010), Trpm8−/− mice were unable to discriminate between 30 and 20°C, spending 54.2 ± 7.5% of the recording period on the control plate (p < 0.001 vs control at 20°C; p > 0.05 vs at 30°C), a behavior that was phenocopied by ablated animals who spent 43.9 ± 5.0% of the observation period on the control surface (p < 0.001 vs control at 20°C; p > 0.05 vs 30°C; Fig. 4E). Thus, consistent with the evaporative cooling assays (Fig. 3A), mice lacking TRPM8 channels and neurons cannot detect temperatures in the innocuously cool range.
Previous reports, including our own, analyzing preference behaviors have shown that Trpm8−/− animals are partial for warmth as temperatures enter into the noxious cold range of below 15°C (Bautista et al., 2007; Dhaka et al., 2007; Knowlton et al., 2010). Other cold-detection mechanisms have been suggested to account for this preference for warmth at noxious cold, chief among these the irritant receptor TRPA1, a channel implicated previously in noxious cold detection (Story et al., 2003). However, we have shown that these preference behaviors are retained in mice deficient in both TRPM8 and TRPA1 channels (Knowlton et al., 2010). Thus, it is not clear whether this behavior indicates the existence of TRPM8-independent cold-detection mechanisms at cold extremes or a robust attraction to the warmth that predominates even in the absence of input from cold fibers (Knowlton et al., 2010; McCoy et al., 2011). Here we also observe warm preference in Trpm8−/− mice at temperatures ≤15°C, with responses similar to control mice at the lowest temperatures tested [90.6 ± 3.8% (p > 0.05) and 98.6 ± 0.3% (p > 0.05) at 10 and 5°C, respectively]. Moreover, control mice showed similar preference behaviors as wild-type and un-injected Trpm8DTR mice (data not shown). However, animals lacking TRPM8 neurons failed to show any preference for warmth at moderately noxious cold temperatures, spending 55.7 ± 4.9 and 53.9 ± 6.2% of the recording period on the control surface when the test was held at 15 and 10°C, respectively (p < 0.001 vs control at 15 and 10°C; p > 0.05 vs 30°C; Fig. 4E). It was not until the test plate was cooled to 5°C did we observe warm preference in ablated mice (75.4 ± 4.4%, p < 0.001 vs ablated at 30°C) but to levels that still were significantly different from behaviors of control or Trpm8−/− animals at this temperature (p < 0.001).
TRPM8 neuron-ablated animals have a selective loss of acute cold behaviors
We next tested whether TRPM8-expressing neurons contribute to other somatosensory modalities or whether they represent a sensory labeled line for only cold (Ma, 2012). The two-temperature choice assay described above shows that animals lacking TRPM8 neurons can accurately discriminate warmth from noxious heat (Fig. 4). Furthermore, we tested the latency to exhibit a nocifensive response (hindpaw shaking and licking) on a hotplate in ablated and control animals. When placed on a 48 or 52°C hotplate, ablated mice responded with latencies of 14.2 ± 1.3 and 8.9 ± 1.2 s (n = 12), respectively, responses that were not significantly different from those of control animals (13.5 ± 0.8 s at 48°C and 8.5 ± 0.8 s at 52°C) or the number of other genotypes tested, including wild-type, Trpm8−/−, and un-injected Trpm8DTR mice (p > 0.05, n = 6–12; Fig. 5A,B). In addition, we observed no difference in latency to paw withdrawal from a radiant heat source in Trpm8−/− or ablated mice compared with control (p > 0.05; Fig. 6C,D).
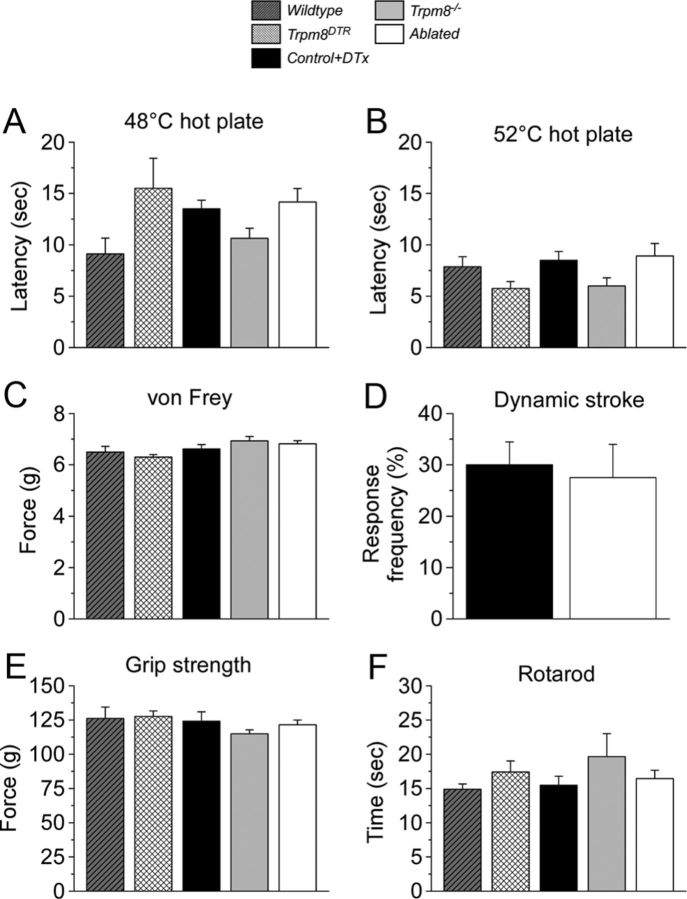
Figure 5.
Heat, mechanical, and proprioceptive behaviors do not require TRPM8 neurons. Latencies to hindpaw flinching on a hotplate were the same at (A) 48°C and (B) 52°C in all genotypes tested (p > 0.05, n = 6–12). C, Mildly noxious mechanical stimulation of the hindpaw with the electronic von Frey apparatus yielded similar paw-withdrawal thresholds in all genotypes (n = 6–12, p > 0.05). D, Dynamic stroke of the hindpaw with a puffed-up cotton swab yielded similar response frequencies in both control and ablated mice (n = 8–11, p > 0.05). E, The average maximum grip strengths were similar across all genotypes, as was the latency to drop from an accelerating rotarod (F) (n = 6–27, p > 0.05).
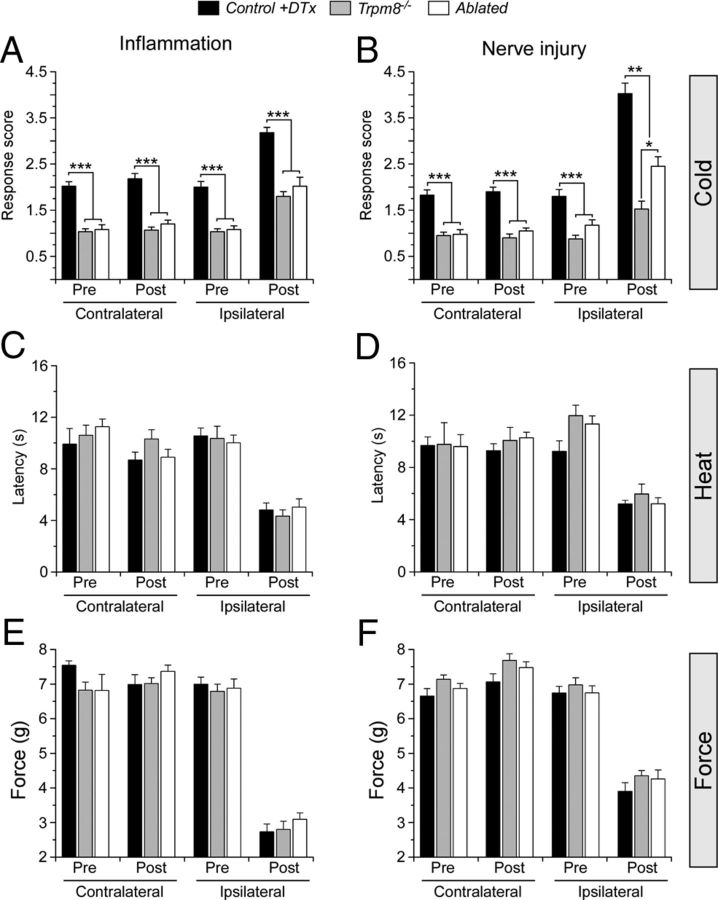
Figure 6.
TRPM8 neuron ablation selectively diminishes inflammation- and nerve injury-induced cold allodynia. A, Inflammation significantly increased ipsilateral, but not contralateral, response scores to evaporative cooling in control mice by 2 d after CFA injection (n = 10). Both Trpm8−/− (n = 6) and ablated mice (n = 10) showed significantly lower sensitization compared with controls (***p < 0.001) but were significantly increased over baseline and the contralateral side (p < 0.01). There were no significant differences in sensitization between Trpm8−/− and ablated mice (p > 0.05). B, After induction of irritation to the sciatic nerve (CCI), control mice showed an increase in ipsilateral scores, whereas contralateral scores remained unchanged (n = 8). Both Trpm8−/− and ablated mice (n = 8 each) showed significantly lower sensitization compared with controls and were significantly increased over baseline and the contralateral side (p < 0.01). Unlike CFA, ablated mice showed a significant increase in nocifensive behaviors compared with control mice (*p < 0.05, **p < 0.01, ***p < 0.001). In both the CFA (C) and CCI (D) models, the significant (p < 0.001) decrease in latencies to hindpaw lift from a radiant heat source were identical between all genotypes tested (p > 0.05). Similarly, mechanical thresholds were increased on the ipsilateral side (p < 0.001) but were indistinguishable (p > 0.05) between all genotypes before and after injury in both pain models (E, F).
To determine whether TRPM8 neuron ablation altered mechanosensation or proprioception, we tested paw-withdrawal thresholds with the electronic von Frey assay, an indicator of low-threshold mechanical nociception. As with noxious heat, neither control nor ablated animals differed in the force required to induce a paw withdrawal (6.6 ± 0.2 g for control, 6.8 ± 0.1 g for ablated), similar to forces that evoked withdrawals in Trpm8−/−, wild-type, and un-injected Trpm8DTR mice (p > 0.05, n = 6–12; Fig. 5C). To test light mechanosensation, we used the dynamic stroke assay (Garrison et al., 2012), again finding no differences between the responses of control and ablated animals to this stimulus. Ablated animals responded to 27.5 ± 6.5% (n = 8) of the stimulation trials, with control mice responding to 30 ± 4.5% of the trials (p > 0.05, n = 11; Fig. 5D). For proprioception and motor coordination, we tested each genotype on the grip strength and accelerating rotarod assays, again finding no differences between ablated animals and the various other genotypes tested (Fig. 5E,F). Control animals had a maximum grip strength of 124.1 ± 6.8 g and a fall latency of 15.5 ± 1.3 s in the rotarod assay (n = 6), whereas ablated animals had a maximum grip strength of 121.4 ± 3.5 g (p > 0.05) and a latency to fall of 16.4 ± 1.2 s (p > 0.05) on the rotarod (n = 27). Together with the cold behavior results, these data suggest that TRPM8 neurons are primarily dedicated cold sensors. Moreover, although a small subset of TRPV1 neurons are depleted in TRPM8 neuron-ablated animals, these mice retain normal heat sensation. Similarly, cold fibers have been shown to be mechanosensitive in nerve recordings, but TRPM8 afferents are dispensable for normal acute mechanosensation.
Contribution of TRPM8 neurons to cold hypersensitivity
We next examined inflammatory and neuropathic pain behaviors in control, Trpm8−/−, and ablated animals. To test inflammatory pain, we performed unilateral hindpaw injections of CFA, a condition that induces robust cold allodynia that is primarily dependent on expression of functional TRPM8 channels (Colburn et al., 2007; Knowlton et al., 2011). Using the evaporative cooling assay, we observed a significant increase in cold behaviors in control mice in the inflamed (ipsilateral) paw, with response scores increasing from 2.0 ± 0.1 before CFA injection to 3.2 ± 0.1 2 d later (p < 0.01, n = 10; Fig. 6A). In comparison, responses in the un-inflamed (contralateral) paw were unchanged (2.0 ± 0.1 before injection to 2.2 ± 0.1 after injection, p > 0.05). Interestingly, both Trpm8−/− (n = 6) and ablated mice (n = 10) also showed significant increases in acetone score from baseline at 2 d after injection, although responses at both time points were significantly lower than controls. Trpm8−/− mice increased from 1.0 ± 0.1 to 1.8 ± 0.1, with ablated similarly increasing from 1.1 ± 0.1 to 2.0 ± 0.2 (Fig. 6A, p < 0.01), post-injury values that were indistinguishable between these two genotypes (p = 0.4). However, both Trpm8−/− and ablated mice failed to develop the intense hypersensitivity observed in control mice (p < 0.001). This inability of inflammation to induce cold allodynia was not attributable to a general lack of sensitization because there were no differences between the three groups in the development of heat (n = 5–7 each genotype, p > 0.05; Fig. 6C) or mechanical hypersensitivity (n = 6 each genotype, p > 0.05; Fig. 6E).
We also examined neuropathic pain induced by CCI of the sciatic nerve (Bennett and Xie, 1988), a nerve injury model leading to cold allodynia that is also attributed to TRPM8 (Colburn et al., 2007; Knowlton et al., 2011; Su et al., 2011). Similar to inflammation, irritation of the sciatic nerve induced a large increase in nocifensive behaviors to evaporative cooling in control mice (n = 8), with response scores increasing from 1.8 ± 0.1 before to 4.0 ± 0.2 by 1 week after surgery (p < 0.001; Fig. 6B). In both Trpm8−/− and ablated animals (n = 8), cold allodynia was significantly muted in the CCI model compared with control but was again increased over baseline measurements or that observed in the contralateral paw (p < 0.05). However, unlike the results of inflammation, there was a significant difference in nocifensive responses to cooling between Trpm8−/− and ablated animals with response scores of 1.5 ± 0.2 and 2.5 ± 0.2, respectively (Fig. 6B, p < 0.05). These results suggest that cold allodynia primarily requires expression of TRPM8 but that ablation of TRPM8-expressing afferents only partially suppresses neuropathic cold hypersensitivity. TRPM8 protein levels in rat DRG have been shown to increase after nerve injury (Su et al., 2011). Moreover, TRPM8-like immunoreactivity was observed ectopically in nociceptors that do not express the channel under nonpathological control conditions in the CCI model in rats (Proudfoot et al., 2006). However, we failed to observe TRPM8 expression by qPCR in ipsilateral or contralateral DRG from ablated animals 14 d after surgery (data not shown), results demonstrating that this phenotype was not attributable to incomplete TRPM8 neuron ablation. Furthermore, heat (n = 5–7 each genotype) and mechanical (n = 6 each genotype) thresholds were decreased after nerve injury and indistinguishable between mice of all three genotypes (p > 0.05; Fig. 6D,F).
TRPM8 cells are required for cooling-mediated analgesia
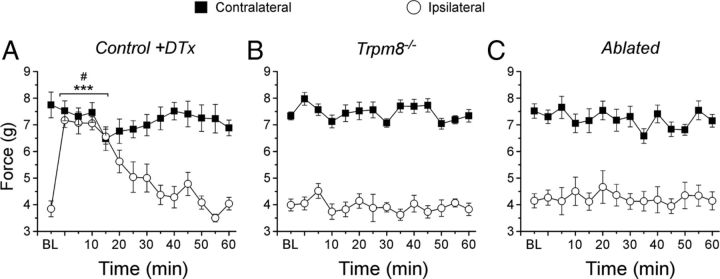
Cooling and menthol are used medicinally to alleviate acute and chronic pain (Bini et al., 1984; Eccles, 1994; Green and McAuliffe, 2000). In a rodent model of neuropathic pain, mild cooling (16–18°C) produces transient analgesia to mechanical and nociceptive stimuli (Proudfoot et al., 2006; Dhaka et al., 2007). Although these elegant studies strongly implicated TRPM8, the dependence of cooling analgesia on expression of functional TRPM8 channels in chronic pain models was not conclusively determined, nor is it clear whether these effects depend on intact cold afferent signaling. To test this, we used the CCI model of neuropathic pain in control, Trpm8−/−, and ablated mice and examined mechanical hypersensitivity 7–10 d after surgery. Thresholds for all genotypes were lowered on the ipsilateral side compared with those recorded on the contralateral paw (n = 6–8, p < 0.001; Fig. 7A–C), and there were no significant differences between the three genotypes (p > 0.05), consistent with that described previously (Fig. 6F). To induce cooling analgesia, mice were placed for 5 min in a thermally controlled water-filled chamber, held at 17°C, in which the water just covered the animals' paws (Proudfoot et al., 2006). Immediately after cessation of cooling, we measured sensitivity, observing no significant differences (p > 0.05) in response thresholds on the contralateral paw in all three genotypes, demonstrating that the cooling had no effect on normal mechanosensation (Fig. 7A–C). However, in control animals, ipsilateral thresholds were significantly different (p < 0.001) from baseline measurements immediately after cooling, changes that were maintained for up to 20 min before returning to baseline by ∼40 min after cooling (Fig. 7A). Moreover, the ipsilateral paw-withdrawal thresholds were not significantly different from contralateral thresholds (p > 0.05 for the period 0–15 min after cooling). However, this cooling-mediated analgesia was completely absent in Trpm8−/− and ablated animals, showing no changes in paw-withdrawal thresholds after cooling, maintaining similar thresholds throughout the course of the experiment (n = 8; Fig. 7B,C). These data confirm previous reports on the involvement of TRPM8 in cooling-induced pain relief (Proudfoot et al., 2006; Dhaka et al., 2007) but show for the first time the absence of analgesia in Trpm8−/− mice in a neuropathic pain model, as well as demonstrate that this pain-relief mechanism requires signaling via TRPM8 afferent nerve fibers.
Figure 7.
Mice lacking TRPM8 or neurons are deficient in cooling-induced analgesia. A, In a neuropathic pain model (CCI), control mice exhibited increased sensitivity to mechanical stimuli that was temporally alleviated by cooling the affected paw (17°C for 5 min). Before cooling [baseline (BL)], ipsilateral thresholds were significantly lowered from that recorded contralateral (p < 0.001). After cooling, ipsilateral thresholds were significantly increased over baseline (3.8 ± 0.3 g at baseline compared with 7.1 ± 0.4 g at 5 min after cooling; ***p < 0.001) and indistinguishable from contralateral thresholds (7.7 ± 0.5 g at baseline compared with 7.3 ± 0.3 g at 5 min after cooling, #p > 0.05). Trpm8−/− (B) and ablated (C) mice showed decreased ipsilateral mechanical thresholds in the CCI model (p < 0.001) similar to controls and were unaffected by cooling.
Discussion
Polymodality of sensory afferents suggests that stimulus encoding does not occur in the afferent itself but is deciphered in higher centers, such as the spinal cord or brain (Perl, 2007; Basbaum et al., 2009). Consistent with this hypothesis, ablation of DRG neurons expressing Nav1.8 led to deficits in mechanical, cold, and inflammatory pain (Abrahamsen et al., 2008). Capsaicin-sensitive neurons are also polymodal, responding to both thermal and mechanical stimuli in nerve recordings (Cain et al., 2001). However, chemical ablation of capsaicin-sensitive central projections of DRG neurons causes deficits in painful heat sensitivity but has no effect on mechanical responses (Cavanaugh et al., 2009). Similar results were observed after genetic ablation of neurons within the TRPV1 lineage (McKemy, 2011; Mishra et al., 2011). Furthermore, genetic ablation of neurons expressing the Mas-related G-protein-coupled receptors Mrgprd attenuated mechanical pain yet had no effect on thermal behaviors (Cavanaugh et al., 2009). These results imply the presence of so-called labeled lines, at least at the level of the afferent nerve fiber, that exist as non-overlapping neuronal populations carrying information pertaining to a single somatosensory modality to the CNS (Cavanaugh et al., 2009; Ma, 2010).
Here we provide evidence that, like TRPV1 and Mrgprd-expressing afferents, TRPM8 neurons also represent a labeled line, in this case mediating cold and cold pain. Like many afferent types, TRPM8 neurons are molecularly heterogeneous; however, the functional significance of this heterogeneity is uncertain (Takashima et al., 2007). For example, approximately one-half of these cells are also capsaicin-sensitive and express TRPV1 (McKemy et al., 2002; Viana et al., 2002; Babes et al., 2004; Hjerling-Leffler et al., 2007; Takashima et al., 2007, 2010). Thus, subsets of neurons are both heat and cold sensitive, yet the significance of their activation is unknown. We observed no defects in heat sensing in Trpm8DTR mice given DTx, yet only a small fraction of TRPV1-expressing neurons (∼10%) were ablated in these animals, suggesting that the presence of other intact TRPV1 afferents were likely sufficient to elicit appropriate behaviors. Moreover, in both inflammatory and neuropathic pain models, ablation of TRPM8 neurons had no effect on thermal hyperalgesia. Similarly, many cold-sensitive neurons are also mechanosensitive (Simone and Kajander, 1996, 1997), yet we observed no deficits in acute mechanical responses in ablated mice, nor were there differences in mechanical pain after injury, further supporting the select role of TRPM8 neurons in cold transduction.
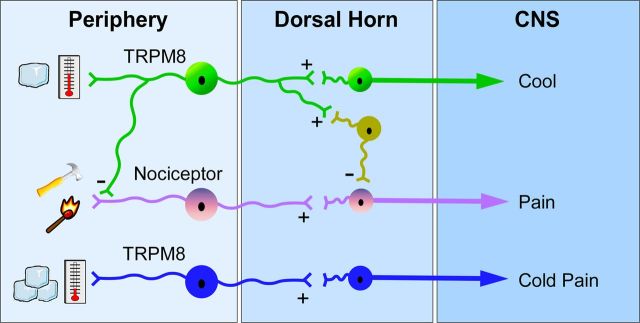
TRPM8 is required for multiple forms of cold signaling, including innocuous cool, noxious cold, cold allodynia, and cooling analgesia (Proudfoot et al., 2006; Colburn et al., 2007; Knowlton et al., 2011). This is a striking range of phenotypes considering that neurons within the Trpm8 lineage make up ∼10% of the afferent population and suggests the existence of subpopulations that mediate distinct cold percepts (Xing et al., 2006; Madrid et al., 2009; McCoy et al., 2011). Ablated and Trpm8−/− mice showed little to no noxious cold-evoked responses in our analysis of forepaw flinching and licking behaviors on a 0°C cold plate. These mice were also unresponsive to innocuous cooling in the evaporative cooling and two-temperature choice assays. Together, these data suggest the presence of TRPM8 in isolated and discrete innocuous cool fibers and cold nociceptors that signal these perceptually distinct temperatures centrally (Fig. 8). Additionally, the deficiencies in injury-evoked cold allodynia and lack of cooling-induced analgesia in Trpm8−/− and ablated mice further show the complexity of TRPM8 in cold signaling. These data suggest that cold nociceptors expressing TRPM8 become sensitized after injury and advocates for a population of cold sensors that provides pain relief under mild cooling. It will be interesting to determine whether these two seemingly paradoxical behaviors are coded by different cohorts within the overall TRPM8-expressing population of sensory neurons or whether this effect is attributable to signal processing at the level of the afferent, spinal cord, or beyond (Fig. 8).
Figure 8.
Model for TRPM8 neuronal function. Our data suggest the presence of TRPM8 neurons that mediate innocuous cool (green) and likely provide inhibitory input, either through spinal inhibitory interneurons (yellow) or directly to nociceptors (pink) to induce cooling analgesia. Cold nociceptors (blue) likely also express TRPM8 and provide input on noxious cold temperatures that drive thermal-avoidance behaviors.
Our data show that cold avoidance and thermal preference depends on both TRPM8 channels and neurons but that conditional ablation of TRPM8 afferents in the adult mouse leads to more profound deficits in cold behaviors compared with animals in which the trpm8 gene is rendered inactive embryonically. These results suggest the presence of TRPM8-independent transduction mechanisms in TRPM8 neurons, thereby providing cold sensitivity in the absence of TRPM8 channels. Other molecules have been linked to cold transduction, including TRPA1, the only other putative cold transducer (Story et al., 2003), and channels involved in signal propagation, such as Kv1 and two-pore (K2P) potassium channels that may work as excitability brakes (Madrid et al., 2009; Noël et al., 2009), and Nav1.8, which has biophysical properties that suggest that it functions as the lone impulse generator at cold temperatures (Zimmermann et al., 2007). Decreased expression of K+ conductances is known to decrease cold thresholds, thereby increasing the number of cold-sensitive neurons and cold excitability during cold neuropathies (Madrid et al., 2009; Noël et al., 2009; Descoeur et al., 2011). Nav1.8 neurons play an important role in inflammatory pain and are needed for cold pain but not innocuous cold detection (Abrahamsen et al., 2008), and approximately one-half of menthol-sensitive DRG neurons express Nav1.8 (Shields et al., 2012). Consistent with these functional results, we find that 40.2 ± 7.3% of GFP-expressing TRPM8 neurons in the Trpm8GFP mouse line are also immunoreactive for Nav1.8 (n = 1859 cells; R.P. and D.D.McK., unpublished observations). These results suggest that Nav1.8 may serve in noxious cold excitability in the absence of TRPM8 channels in Trpm8−/− mice, but that when all TRPM8 neurons, including those expressing Nav1.8, are ablated, responses to noxious cold are attenuated. Last, recent evidence suggests that TRPA1 has a limited role, if any, in acute nonpathological cold sensation but is a key molecule in nociceptor hypersensitivity (Caspani and Heppenstall, 2009; del Camino et al., 2010; Knowlton et al., 2010). Thus, the incomplete abrogation of cold allodynia in Trpm8−/− and ablated mice suggests that, although TRPM8 is important for cold hypersensitivity, additional molecular and cellular mechanisms also contribute significantly and account for the residual cold sensitivity we observe after injury in both TRPM8-deficient genotypes.
We find that ablated animals were phenotypically identical to Trpm8−/− mice in many respects, including responses to evaporative cooling, cold-plate nocifensive behaviors, and cooling-induced analgesia. However, there were some significant differences between Trpm8−/− and ablated mice in the two-temperature choice assay and with neuropathic nerve injury. The latter is also intriguing because we did not observe any differences in inflammatory responses between the two genotypes. In rats, Proudfoot et al. (2006) reported altered TRPM8 expression in the CCI model, observing increased channel expression in A-fibers. Similarly, Su et al. (2011) presented evidence for increased TRPM8 protein expression in affected rat DRG by day 4 after injury, expression that peaked by day 10, a time course that matches the evolution of cold allodynia. Because suitable antibodies detecting mouse TRPM8 are lacking, we used qPCR to determine whether TRPM8 transcript expression increased in ablated animals after injury but did not detect any expression. Therefore, it is unclear why ablated animals had increased cold sensitivity over Trpm8−/− mice in the CCI model, results that warrant additional investigation.
There were some striking differences between ablated mice and Trpm8−/− animals in the two-temperature choice assay, with the former showing little to no ability to detect cold well into the noxious range. Indeed, it was not until test plate temperatures were lowered to 5°C that ablated mice showed any evidence of either cold avoidance or warm preference. As we and others have reported, Trpm8−/− mice do show robust cold avoidance and warm preference starting at 15°C but never to levels observed in control animals (Bautista et al., 2007; Dhaka et al., 2007; Knowlton et al., 2010). These data suggest that, although TRPM8 is important for cold sensing in the noxious range, other mechanisms also contribute but intriguingly may be localized to TRPM8 neurons. As described above, Kv1, K2P, and Nav1.8 channels have been implicated in cold transduction. Differential expression of potassium channels are proposed to make cold- and menthol-sensitive neurons amenable to activation by cold (Viana et al., 2002; Madrid et al., 2009) and segregate them into two loosely distinguished populations: those responding to innocuous cool temperatures having high TRPM8 and low Kv1 expression, whereas those activated by lower, noxious temperatures expressing low levels of TRPM8 and high levels of Kv1 channels (Madrid et al., 2009; McCoy et al., 2011). This model predicts that cold may be able to activate these cells even in the absence of TRPM8 activity (Viana et al., 2002; Madrid et al., 2009), a posit supported by our data. Last, mice in which neurons in the Nav1.8 lineage are ablated failed to respond on a 0°C cold plate but retained normal responses in the acetone evaporative cooling assay, results suggesting a cellular separation of innocuous cool and noxious cold sensing (Abrahamsen et al., 2008). These intriguing observations suggest that Nav1.8 (and perhaps Kv1 and K2P) channels are differentially expressed in a subset of TRPM8 neurons and provides distinct neuronal populations the ability to respond to innocuous versus noxious cold.
Footnotes
This study was supported by National Institutes of Health Grants NS054069 and NS078530 (D.D.McK.). We thank C. P. Ko for assistance with rotarod and grip strength assays, C. Stucky and S. Garrison for assistance with the dynamic stroke assay, R. Lang for the DTR–GFP construct, Y. Takashima, K. Renduchintala, R. Romanu, and C. Messinger for histology assistance, and J. Teng for assistance with behavior quantification.
References
- Abrahamsen B, Zhao J, Asante CO, Cendan CM, Marsh S, Martinez-Barbera JP, Nassar MA, Dickenson AH, Wood JN. The cell and molecular basis of mechanical, cold, and inflammatory pain. Science. 2008;321:702–705. doi: 10.1126/science.1156916. [DOI] [PubMed] [Google Scholar]
- Babes A, Zorzon D, Reid G. Two populations of cold-sensitive neurons in rat dorsal root ganglia and their modulation by nerve growth factor. Eur J Neurosci. 2004;20:2276–2282. doi: 10.1111/j.1460-9568.2004.03695.x. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Bini G, Cruccu G, Hagbarth KE, Schady W, Torebjörk E. Analgesic effect of vibration and cooling on pain induced by intraneural electrical stimulation. Pain. 1984;18:239–248. doi: 10.1016/0304-3959(84)90819-4. [DOI] [PubMed] [Google Scholar]
- Cain DM, Khasabov SG, Simone DA. Response properties of mechanoreceptors and nociceptors in mouse glabrous skin: an in vivo study. J Neurophysiol. 2001;85:1561–1574. doi: 10.1152/jn.2001.85.4.1561. [DOI] [PubMed] [Google Scholar]
- Caspani O, Heppenstall PA. TRPA1 and cold transduction: an unresolved issue? J Gen Physiol. 2009;133:245–249. doi: 10.1085/jgp.200810136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, Anderson DJ. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci U S A. 2009;106:9075–9080. doi: 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Kohno K, Gong Q. Conditional ablation of mature olfactory sensory neurons mediated by diphtheria toxin receptor. J Neurocytol. 2005;34:37–47. doi: 10.1007/s11068-005-5046-8. [DOI] [PubMed] [Google Scholar]
- Colburn RW, Lubin ML, Stone DJ, Jr, Wang Y, Lawrence D, D'Andrea MR, Brandt MR, Liu Y, Flores CM, Qin N. Attenuated cold sensitivity in TRPM8 null mice. Neuron. 2007;54:379–386. doi: 10.1016/j.neuron.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Comaniciu D, Ramesh V, Meer P. Real-time tracking of non-rigid objects using mean shift. Proceed 2000 IEEE Conf Comput Vision Pattern Recognition. 2000;2:142–149. [Google Scholar]
- Daniels RL, McKemy DD. Design and construction of a two-temperature preference behavioral assay for undergraduate neuroscience laboratories. J Undergrad Neurosci Educ. 2010;9:A51–A56. [PMC free article] [PubMed] [Google Scholar]
- Daniels RL, Takashima Y, McKemy DD. Activity of the neuronal cold sensor TRPM8 is regulated by phospholipase C via the phospholipid phosphoinositol 4,5-bisphosphate. J Biol Chem. 2009;284:1570–1582. doi: 10.1074/jbc.M807270200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Camino D, Murphy S, Heiry M, Barrett LB, Earley TJ, Cook CA, Petrus MJ, Zhao M, D'Amours M, Deering N, Brenner GJ, Costigan M, Hayward NJ, Chong JA, Fanger CM, Woolf CJ, Patapoutian A, Moran MM. TRPA1 contributes to cold hypersensitivity. J Neurosci. 2010;30:15165–15174. doi: 10.1523/JNEUROSCI.2580-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descoeur J, Pereira V, Pizzoccaro A, Francois A, Ling B, Maffre V, Couette B, Busserolles J, Courteix C, Noel J, Lazdunski M, Eschalier A, Authier N, Bourinet E. Oxaliplatin-induced cold hypersensitivity is due to remodelling of ion channel expression in nociceptors. EMBO Mol Med. 2011;3:266–278. doi: 10.1002/emmm.201100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron. 2007;54:371–378. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Dhaka A, Earley TJ, Watson J, Patapoutian A. Visualizing cold spots: TRPM8-expressing sensory neurons and their projections. J Neurosci. 2008;28:566–575. doi: 10.1523/JNEUROSCI.3976-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles R. Menthol and related cooling compounds. J Pharm Pharmacol. 1994;46:618–630. doi: 10.1111/j.2042-7158.1994.tb03871.x. [DOI] [PubMed] [Google Scholar]
- Garrison SR, Dietrich A, Stucky CL. TRPC1 contributes to light-touch sensation and mechanical responses in low-threshold cutaneous sensory neurons. J Neurophysiol. 2012;107:913–922. doi: 10.1152/jn.00658.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BG, McAuliffe BL. Menthol desensitization of capsaicin irritation. Evidence of a short-term anti-nociceptive effect. Physiol Behav. 2000;68:631–639. doi: 10.1016/s0031-9384(99)00221-8. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Kondo T, Ishimatsu M, Yamada S, Nakamura K, Matsuoka K, Akasu T. Expression of the TRPM8-immunoreactivity in dorsal root ganglion neurons innervating the rat urinary bladder. Neurosci Res. 2009;65:245–251. doi: 10.1016/j.neures.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Hjerling-Leffler J, Alqatari M, Ernfors P, Koltzenburg M. Emergence of functional sensory subtypes as defined by transient receptor potential channel expression. J Neurosci. 2007;27:2435–2443. doi: 10.1523/JNEUROSCI.5614-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordt SE, McKemy DD, Julius D. Lessons from peppers and peppermint: the molecular logic of thermosensation. Curr Opin Neurobiol. 2003;13:487–492. doi: 10.1016/s0959-4388(03)00101-6. [DOI] [PubMed] [Google Scholar]
- Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer EG, Littman DR, Lang RA. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton WM, McKemy DD. TRPM8: from cold to cancer, peppermint to pain. Curr Pharm Biotechnol. 2011;12:68–77. doi: 10.2174/138920111793937961. [DOI] [PubMed] [Google Scholar]
- Knowlton WM, Bifolck-Fisher A, Bautista DM, McKemy DD. TRPM8, but not TRPA1, is required for neural and behavioral responses to acute noxious cold temperatures and cold-mimetics in vivo. Pain. 2010;150:340–350. doi: 10.1016/j.pain.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton WM, Daniels RL, Palkar R, McCoy DD, McKemy DD. Pharmacological blockade of TRPM8 ion channels alters cold and cold pain responses in mice. PLoS One. 2011;6:e25894. doi: 10.1371/journal.pone.0025894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Abdel Samad O, Zhang L, Duan B, Tong Q, Lopes C, Ji RR, Lowell BB, Ma Q. VGLUT2-dependent glutamate release from nociceptors is required to sense pain and suppress itch. Neuron. 2010;68:543–556. doi: 10.1016/j.neuron.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q. Labeled lines meet and talk: population coding of somatic sensations. J Clin Invest. 2010;120:3773–3778. doi: 10.1172/JCI43426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q. Population coding of somatic sensations. Neurosci Bull. 2012;28:91–99. doi: 10.1007/s12264-012-1201-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrid R, de la Peña E, Donovan-Rodriguez T, Belmonte C, Viana F. Variable threshold of trigeminal cold-thermosensitive neurons is determined by a balance between TRPM8 and Kv1 potassium channels. J Neurosci. 2009;29:3120–3131. doi: 10.1523/JNEUROSCI.4778-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy DD, Knowlton WM, McKemy DD. Scraping through the ice: uncovering the role of TRPM8 in cold transduction. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1278–R1287. doi: 10.1152/ajpregu.00631.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKemy DD. A spicy family tree: TRPV1 and its thermoceptive and nociceptive lineage. EMBO J. 2011;30:453–455. doi: 10.1038/emboj.2010.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- Mishra SK, Hoon MA. Ablation of TrpV1 neurons reveals their selective role in thermal pain sensation. Mol Cell Neurosci. 2010;43:157–163. doi: 10.1016/j.mcn.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SK, Tisel SM, Orestes P, Bhangoo SK, Hoon MA. TRPV1-lineage neurons are required for thermal sensation. EMBO J. 2011;30:582–593. doi: 10.1038/emboj.2010.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, Spencer KS, Andahazy M, Story GM, Patapoutian A. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science. 2005;307:1468–1472. doi: 10.1126/science.1108609. [DOI] [PubMed] [Google Scholar]
- Noël J, Zimmermann K, Busserolles J, Deval E, Alloui A, Diochot S, Guy N, Borsotto M, Reeh P, Eschalier A, Lazdunski M. The mechano-activated K+ channels TRAAK and TREK-1 control both warm and cold perception. EMBO J. 2009;28:1308–1318. doi: 10.1038/emboj.2009.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. Interrogation by toxin. Nat Biotechnol. 2001;19:731–732. doi: 10.1038/90770. [DOI] [PubMed] [Google Scholar]
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- Perl ER. Cutaneous polymodal receptors: characteristics and plasticity. Prog Brain Res. 1996;113:21–37. doi: 10.1016/s0079-6123(08)61079-1. [DOI] [PubMed] [Google Scholar]
- Perl ER. Ideas about pain, a historical view. Nat Rev Neurosci. 2007;8:71–80. doi: 10.1038/nrn2042. [DOI] [PubMed] [Google Scholar]
- Proudfoot CJ, Garry EM, Cottrell DF, Rosie R, Anderson H, Robertson DC, Fleetwood-Walker SM, Mitchell R. Analgesia mediated by the TRPM8 cold receptor in chronic neuropathic pain. Curr Biol. 2006;16:1591–1605. doi: 10.1016/j.cub.2006.07.061. [DOI] [PubMed] [Google Scholar]
- Saito M, Iwawaki T, Taya C, Yonekawa H, Noda M, Inui Y, Mekada E, Kimata Y, Tsuru A, Kohno K. Diphtheria toxin receptor-mediated conditional and targeted cell ablation in transgenic mice. Nat Biotechnol. 2001;19:746–750. doi: 10.1038/90795. [DOI] [PubMed] [Google Scholar]
- Shields SD, Ahn HS, Yang Y, Han C, Seal RP, Wood JN, Waxman SG, Dib-Hajj SD. Na(v)1.8 expression is not restricted to nociceptors in mouse peripheral nervous system. Pain. 2012;153:2017–2030. doi: 10.1016/j.pain.2012.04.022. [DOI] [PubMed] [Google Scholar]
- Simone DA, Kajander KC. Excitation of rat cutaneous nociceptors by noxious cold. Neurosci Lett. 1996;213:53–56. doi: 10.1016/0304-3940(96)12838-x. [DOI] [PubMed] [Google Scholar]
- Simone DA, Kajander KC. Responses of cutaneous A-fiber nociceptors to noxious cold. J Neurophysiol. 1997;77:2049–2060. doi: 10.1152/jn.1997.77.4.2049. [DOI] [PubMed] [Google Scholar]
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- Su L, Wang C, Yu YH, Ren YY, Xie KL, Wang GL. Role of TRPM8 in dorsal root ganglion in nerve injury-induced chronic pain. BMC Neurosci. 2011;12:120. doi: 10.1186/1471-2202-12-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki SC, Furue H, Koga K, Jiang N, Nohmi M, Shimazaki Y, Katoh-Fukui Y, Yokoyama M, Yoshimura M, Takeichi M. Cadherin-8 is required for the first relay synapses to receive functional inputs from primary sensory afferents for cold sensation. J Neurosci. 2007;27:3466–3476. doi: 10.1523/JNEUROSCI.0243-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima Y, Daniels RL, Knowlton W, Teng J, Liman ER, McKemy DD. Diversity in the neural circuitry of cold sensing revealed by genetic axonal labeling of transient receptor potential melastatin 8 neurons. J Neurosci. 2007;27:14147–14157. doi: 10.1523/JNEUROSCI.4578-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima Y, Ma L, McKemy DD. The development of peripheral cold neural circuits based on TRPM8 expression. Neuroscience. 2010;169:828–842. doi: 10.1016/j.neuroscience.2010.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse SY, Wei ET. Inhibition of the shake response in rats by adenosine and 2-chloroadenosine. Psychopharmacology (Berl) 1986;90:322–326. doi: 10.1007/BF00179184. [DOI] [PubMed] [Google Scholar]
- Viana F, de la Peña E, Belmonte C. Specificity of cold thermotransduction is determined by differential ionic channel expression. Nat Neurosci. 2002;5:254–260. doi: 10.1038/nn809. [DOI] [PubMed] [Google Scholar]
- Wei ET, Seid DA. AG-3–5: a chemical producing sensations of cold. J Pharm Pharmacol. 1983;35:110–112. doi: 10.1111/j.2042-7158.1983.tb04279.x. [DOI] [PubMed] [Google Scholar]
- Xing H, Ling J, Chen M, Gu JG. Chemical and cold sensitivity of two distinct populations of TRPM8-expressing somatosensory neurons. J Neurophysiol. 2006;95:1221–1230. doi: 10.1152/jn.01035.2005. [DOI] [PubMed] [Google Scholar]
- Yoo YE, Ko CP. Treatment with trichostatin A initiated after disease onset delays disease progression and increases survival in a mouse model of amyotrophic lateral sclerosis. Exp Neurol. 2011;231:147–159. doi: 10.1016/j.expneurol.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Zimmermann K, Leffler A, Babes A, Cendan CM, Carr RW, Kobayashi J, Nau C, Wood JN, Reeh PW. Sensory neuron sodium channel Nav1.8 is essential for pain at low temperatures. Nature. 2007;447:855–858. doi: 10.1038/nature05880. [DOI] [PubMed] [Google Scholar]
- Zylka MJ, Rice FL, Anderson DJ. Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to mrgprd. Neuron. 2005;45:17–25. doi: 10.1016/j.neuron.2004.12.015. [DOI] [PubMed] [Google Scholar]