Abstract
Endothelin-1 (ET-1) is unique among a broad range of hyperalgesic agents in that it induces hyperalgesia in rats that is markedly enhanced by repeated mechanical stimulation at the site of administration. Antagonists to the ET-1 receptors, ETA and ETB, attenuated both initial as well as stimulation-induced enhancement of hyperalgesia (SIEH) by endothelin. However, administering antisense oligodeoxynucleotide to attenuate ETA receptor expression on nociceptors attenuated ET-1 hyperalgesia but had no effect on SIEH, suggesting that this is mediated via a non-neuronal cell. Because vascular endothelial cells are both stretch sensitive and express ETA and ETB receptors, we tested the hypothesis that SIEH is dependent on endothelial cells by impairing vascular endothelial function with octoxynol-9 administration; this procedure eliminated SIEH without attenuating ET-1 hyperalgesia. A role for protein kinase Cε (PKCε), a second messenger implicated in the induction and maintenance of chronic pain, was explored. Intrathecal antisense for PKCε did not inhibit either ET-1 hyperalgesia or SIEH, suggesting no role for neuronal PKCε; however, administration of a PKCε inhibitor at the site of testing selectively attenuated SIEH. Compatible with endothelial cells releasing ATP in response to mechanical stimulation, P2X2/3 receptor antagonists eliminated SIEH. The endothelium also appears to contribute to hyperalgesia in two ergonomic pain models (eccentric exercise and hindlimb vibration) and in a model of endometriosis. We propose that SIEH is produced by an effect of ET-1 on vascular endothelial cells, sensitizing its release of ATP in response to mechanical stimulation; ATP in turn acts at the nociceptor P2X2/3 receptor.
Introduction
Endothelins (ETs), a family of polypeptides produced in large part by vascular endothelial cells (Butt et al., 2010; Rodríguez-Pascual et al., 2011), act as potent vasoconstrictors (Uchida et al., 1988; Inoue et al., 1989). Endothelin receptors (i.e., ETA and ETB) are located on nociceptors (Plant et al., 2007; Werner et al., 2010; Laziz et al., 2011), in which endothelin acts to sensitize and activate them (Khodorova et al., 2009b), as well as on vascular endothelial cells to produce their vasoconstrictor effect (Sánchez et al., 2010).
We recently described a pronociceptive effect of endothelin-1 (ET-1), whereby a marked enhancement of endothelin hyperalgesia is produced by repeated testing with threshold noxious mechanical stimulation at the site of administration (Joseph et al., 2011). In the present study, we tested the hypothesis that these two distinct pronociceptive effects of ET-1, primary hyperalgesia and stimulus induced-enhancement of endothelin hyperalgesia (SIEH), are mediated by action on different cells: ET-1 induced primary hyperalgesia by its action on the peripheral terminal of nociceptors and SIEH by its action on vascular endothelial cells, sensitizing them for mechanical stimulus-induced release of a pronociceptive mediator. Given the importance of vasculature in some pain syndromes [e.g., vibration white finger (Stoyneva et al., 2003), intense exercise (Pritchard et al., 1999), and endometriosis (Van Langendonckt et al., 2008)] and that vascular endothelial cells are able to release pronociceptive mediators, such as ATP, in response to mechanical stimulation (Burnstock, 1999), the mechanism proposed here could provide insight into a poorly understood and difficult to treat set of common pain conditions.
Materials and Methods
Animals
Experiments were performed on male Sprague Dawley rats and, for the endometriosis model, female rats (both 200–250 g; Charles River). Animals were housed three per cage, under a 12 h light/dark cycle, in a temperature- and humidity-controlled environment. Food and water were available ad libitum. All behavioral nociceptive testing was performed between 10:00 A.M. and 4:00 P.M. Rats were acclimatized to the experimental area and behavioral procedures before the test. To acclimatize rats to the testing environment, they were brought to the experimental area in their home cages and left in them for 15–30 min, after which they were placed in a restrainer (cylindrical transparent acrylic tubes that have side openings to allow extension of the hindlimbs from the restrainer, for nociceptive testing). Rats were left undisturbed in the restrainer for another 15–30 min before nociceptive testing was started. Nociceptive threshold was defined as the mean of three readings taken at 5 min intervals. All experimental protocols were approved by the University of California, San Francisco Committee on Animal Research and conformed to National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. All efforts were made to minimize the number of animals used and their suffering.
Nociceptive testing
Cutaneous nociception
The nociceptive flexion reflex was quantified with an Ugo Basile Analgesymeter (Stoelting), which applies a linearly increasing mechanical force to the dorsum of the rat's hindpaw. Nociceptive threshold was defined as the force, in grams, at which the rat withdrew its hindpaw. Hyperalgesia was defined as a decrease in mechanical nociceptive threshold, here presented in grams, from baseline paw-withdrawal threshold. Each paw was treated as an independent measure; both paws of the same rat received the same treatment. Each experiment was performed on separate groups of rats. These animals acted as their own controls, with inhibitor injected intradermally into both hindpaws 15 min before the administration of ET-1 and paw-withdrawal thresholds compared before and after drug treatment.
Muscle nociception
Mechanical nociceptive threshold in the gastrocnemius muscle was quantified after hindlimb vibration, eccentric exercise, or implantation and establishment of endometriosis cyst (see below) using a Chatillon digital force transducer (model DFI2; Ametek) (Dina et al., 2008; Khasar et al., 2008; Alvarez et al., 2010). In lightly restrained rats (as described above), a 6-mm-diameter probe, attached to the force transducer, was applied to the skin overlying gastrocnemius muscle to deliver an increasing compression force. This width of probe allows for selective evaluation of muscle pain (vis-à-vis overlying skin pain) (Murase et al., 2010). The nociceptive threshold was defined as the force at which the rat withdrew its hindlimb; results are presented force (in millinewtons) withdrawal thresholds. Each hindlimb (gastrocnemius muscle) is treated as an independent measure, and each experiment is performed on a separate group of rats.
Drugs
The drugs used in this study included the following: ET-1 (100 ng), BQ-123 (2-[(6R,9S,12R,15S)-6-(1H-indol-3-ylmethyl)-9-(2-methylpropyl)-2,5,8,11,14-pentaoxo-12-propan-2-yl-1,4,7,10,13-pentazabicyclo[13.3.0]octadecan-3-yl]acetic acid) (1 μg; ETA receptor antagonist), BQ-788 [(2R)-2-[[(2R)-2-amino-3-(1-methoxycarbonylindol-3-yl)propanoyl]-[(2S)-2-[[(2R,6S)-2,6-dimethylpiperidine-1-carbonyl]amino]-4,4-dimethylpentanoyl]amino]hexanoate] (1 μg; ETB receptor antagonist), TMB-8 [8-(diethylamino)octyl 3,4,5-trimethoxybenzoate] (1 μg; calcium sequestrator), Quin-2 (2-[2-[[8-[bis(carboxymethyl)amino]-6-methoxyquinolin-2-yl]methoxy]-N-(carboxymethyl)-4-methylanilino]acetic acid] (1 μg; calcium chelator), oligomycin and α-lipoic acid [reactive oxygen species (ROS) inhibitors], rotenone (1 μg), and antimycin (1 μg; inhibitors of mitochondrial electron transport chain complexes I and III, respectively), A-317491 (5-[(3-phenoxyphenyl)methyl-[(1S)-1,2,3,4-tetrahydronaphthalen-1-yl]carbamoyl]benzene-1,2,4-tricarboxylic acid) (1 μg; P2X2/3 inhibitor), AF-353 (5-[5-iodo-4-methoxy-2-(1-methylethyl)phenoxy]-2,4-pyrimidinediamine hydrochloride; 1 μg; P2X2/3 receptor antagonist), octoxynol-9 (which functionally impairs vasculature endothelial cell lining (Connor and Feniuk, 1989; Jamal et al., 1992; Sun et al., 1997), oxotremorine and platelet activating factor (all from Sigma-Aldrich), bisindolylmaleimide [BIMM; a nonselective protein kinase C (PKC) inhibitor], and PKCεV1–2 peptide (PKCεI, a selective PKCε-translocation inhibitor; both from Calbiochem, EMD Biosciences). Drug doses used in this study were based on dose–response curves generated in our previous studies (Aley and Levine, 1999; Joseph and Levine, 2006, 2010) or the dose–response curves performed as part of the present study.
ET-1, BQ-123, BQ788, TMB-8, Quin-2, oligomycin, α-lipoic acid, rotenone, antimycin, BIMM, and PKCεI were administered intradermally in a volume of 5 μl using a 30 gauge hypodermic needle attached to a microsyringe (Hamilton) by PE-10 polyethylene tubing. Oxotremorine (0.5 ng in 0.9% sodium chloride vehicle) was injected subcutaneously in a volume of 50 μl. Octoxynol-9 (0.5%, 1 ml/kg) was administered intravenously. All drugs used in this study, except BIMM, were dissolved in saline. BIMM was dissolved in 10% DMSO. All inhibitors were administered 15 min before ET-1 and nociceptive thresholds measured at 15, 20, 25, and 30 min after ET-1 administration. Per se effect of all the inhibitors, including octoxynol-9 and solvents (saline and 10% DMSO), were evaluated separately, and none had significant effect on the basal threshold of the naive rats (data not shown).
Antisense and mismatch oligodeoxynucleotides
PKCε.
The antisense oligodeoxynucleotide sequence for PKCε, 5′-GCCAGCTCGATCTTGCGCCC-3′ (Invitrogen), was directed against a unique sequence of rat PKCε mRNA. The corresponding GenBank accession number and oligodeoxynucleotide position within the cDNA sequence are NM_017171 and 419–438, respectively. We showed previously that intrathecal administration of antisense oligodeoxynucleotide with this sequence decreases PKCε protein in dorsal root ganglia (Parada et al., 2003b). The mismatch oligodeoxynucleotide sequence, 5′-GCCAGCGCGATCTTTCGCCC-3′, corresponds to the PKCε antisense oligodeoxynucleotide sequence with 2 bases mismatched (denoted by bold).
ETA.
The antisense oligodeoxynucleotide sequence for the ETA receptor, 5′-CGTCCTGTTATGTTGGTCTC-3′ (Invitrogen), was directed against a unique region of the rat ETA mRNA sequence. The corresponding GenBank accession number and oligodeoxynucleotide position within the cDNA sequences are NM_012550 and 252–271, respectively. The mismatch oligodeoxynucleotide sequence, 5′-CTTGCTGTTGTGTTGGTCTG-3′, corresponds to the ETA receptor antisense oligodeoxynucleotide sequence with 7 bases mismatched (denoted by bold).
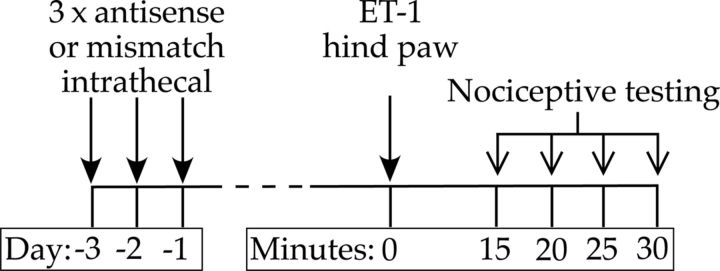
Oligodeoxynucleotides were reconstituted in nuclease-free 0.9% NaCl to a concentration of 10 μg/μl and stored at −20°C until use. Before administration of the oligodeoxynucleotides, rats were anesthetized with 3% isoflurane. An insulin syringe with a 29 gauge needle (Becton Dickinson) was used to deliver the oligodeoxynucleotides. The needle of the syringe was inserted intrathecally on the midline between the fourth and fifth lumbar vertebrae, and its intrathecal location was confirmed by a flicking of the rat's tail (Papir-Kricheli et al., 1987). A dose of 40 μg (20 μl injection volume) of the antisense or mismatch oligodeoxynucleotide was administered, once daily for 3 consecutive days. Figure 1 shows the timing of antisense administration and nociceptive testing.
Figure 1.
Timing of antisense administration and nociceptive testing. Antisense or mismatch oligodeoxynucleotides were administered intrathecally daily for 3 d. On the fourth day, ET-1 was injected intradermally into the hindpaw (time 0), and nociceptive threshold was evaluated at that injection site every 5 min, beginning 15 min after ET-1 administration for 30 min.
Protein extraction and Western blot
To confirm that the change in the nociceptive response associated with antisense treatment for ETA is attributable to a decrease in the peripheral protein expression level of ETA, a Western blot analysis was performed. Saphenous nerves from anesthetized rats were ligated with silk surgical suture (4-0) 1 cm above the knee-level bifurcation of the nerves. A 5 mm section of the saphenous nerve proximal to the ligation was harvested 24 h after the last oligodeoxynucleotide injection and stored at −80°C until additional processing. Protein extraction and protein determination, SDS-PAGE, and Western blot analysis were performed as described previously (Bogen et al., 2008; Summer et al., 2008). ETA immunoreactivity was detected with an affinity-purified rabbit anti-ETA antibody (1:500 dilution, ab85163; Abcam), followed by incubation with an HRP-conjugated donkey anti-rabbit antibody (1:5000 dilution, NA934; GE Healthcare). PKCε immunoreactivity was detected with an affinity-purified rabbit anti-PKCε antibody (1:500 dilution, sc-214; Santa Cruz Biotechnology), followed by incubation with an HRP-conjugated donkey anti-rabbit antibody (GE Healthcare). Immunoreactivity was visualized with enhanced chemiluminescence reagents (Thermo Fisher Scientific), and images were acquired with ChemiImager imaging system and analyzed by computer-assisted densitometry using AlphaEaseFC software (Genetic Technologies). ETA protein levels were normalized with respect to the PKCε level in each sample, and the percentage decrease in ETA expression levels was calculated using the following formula: (normalized density for antisense − normalized density for mismatch)/normalized density for mismatch × 100.
Lesion of vascular endothelium
In the cardiovascular and renal literature, a role of endothelial cells in vascular function has been evaluated, in vivo and in situ, by functionally compromising the endothelial lining, using brief exposure to octoxynol-9. As shown by functional tests and light and electron microscopy, the intravenous or intra-arterial administration of octoxynol-9 functionally impairs the endothelial lining of the vasculature (Randall et al., 1991; McLeod and Piper, 1992; Bourreau et al., 1993; Sun et al., 1997). To evaluate the role of the endothelial cell in SIEH, and preclinical models of pain syndromes, rats received an intravenous injection, through a tail vein, of a 0.5% solution of octoxynol-9 at a volume of 1 ml/kg body weight. In ET-1 experiments, it was injected 15 min later, and the animals were evaluated for ET-1 hyperalgesia and stimulus-induced enhancement of this hyperalgesia. Injection of saline (vehicle for octoxynol-9) served as the control. Rats showed no indication of distress throughout the period of the experiment after administration of octoxynol-9.
Laser Doppler 2D blood flow measurements
To evaluate endothelial cell function at the site of nociceptive testing, we evaluated the response to the muscarinic cholinergic agonist oxotremorine using laser Doppler 2D blood flow measurement; muscarinic agonists cause endothelium-dependent vasodilation by enhancing release of nitric oxide (Baron, 1999), a standard method for determining impairment of endothelial cell function (Clavier et al., 1994; Harukuni et al., 2000). Blood flow was assessed using a Moor LDI2 Laser Doppler Imager (Moor Instruments) equipped with a near-infrared (780 nm) laser, which has the advantage over standard laser Doppler methods in that blood flow in deeper-lying vasculature is also measured. The Doppler imager laser beam was set to scan in a raster pattern over the dorsal surface of the hindpaw; moving blood in the microvasculature causes a Doppler shift that is processed by the integrated software to build up a color-coded image of blood flow. The measurement is noncontact and records perfusion every 90 s, with perfusion at each image position recorded at resolutions of 0.2 mm/pixel; additional analysis was performed using integrated computer software. Rats were anesthetized with isoflurane (3% in oxygen) and placed in a supine position on a heating pad to maintain body temperature at 37°C. Activation of muscarinic cholinergic receptors produces an endothelium-dependent dilation of vascular beds (Furchgott and Zawadzki, 1980). Therefore, to evaluate the functional status of the endothelium, after three baseline scans, oxotremorine (0.5 ng in a volume of 50 μl) was injected subcutaneously into the dorsal surface of the hindpaw in naive controls or 24 h after octoxynol-9 administration. Scanning was resumed to assess changes in blood flow in response to muscarinic stimulation over the next 12 scans (18 min).
Plasma extravasation
As a second measure of impairment of endothelial cell function, we evaluated the effect of octoxynol-9 on endothelium-dependent plasma protein extravasation. Plasma extravasation was evaluated as described previously (Green and Levine, 2005). Rats were anesthetized with sodium pentobarbital (65 mg/kg, i.p.) and were then given a tail vein injection of Evans blue dye (50 mg/kg), which binds stoichiometrically to serum albumin. Vehicle (0.9% saline) was perfused through the knee joint at a constant rate (250 μl/min), and perfusate samples were collected every 5 min. After establishing vehicle baseline levels of plasma extravasation, platelet-activating factor (PAF; 100 nm, which stimulates plasma extravasation in a endothelium-dependent manner) was added to this perfusing fluid (0.9% saline, 1% bovine serum albumin) and remained present in the fluid for the duration of the experiment. We used PAF to evaluate endothelial cell functional integrity in mediating plasma protein extravasation, because we extensively used this compound to evaluate plasma protein extravasation (Green et al., 1993a,b,c,d, 1994, 1995, 1997, 1998; Lo et al., 1999), whereas ET-1 itself has not been validated in this system. Using spectrophotometric measurement (absorbance at 620 nm), samples were then evaluated for Evans blue dye concentration, which is linearly related to protein albumin concentration (Carr and Wilhelm, 1964).
Vibration-induced hyperalgesia
It has been suggested that musculoskeletal pain induced by exposure to vibrating devices used in various occupations may have a vascular component (Ogasawara et al., 1997; Dowd et al., 1998; Mirbod et al., 1999). We demonstrated previously that exposure to vibration produces chronic muscle pain (Chen et al., 2010; Dina et al., 2010). The rat's hindlimb was vibrated with a Digital Vortex Genie II laboratory Vortex mixer (Thermo Fisher Scientific) that has a variable-speed motor with a real-time digital readout of the vibration speed of the head. Rats were anesthetized with 3% isoflurane in oxygen and one hindlimb affixed to the platform with Micropore surgical tape so that the knee and ankle joint angles were both 90°, without rotational torque on the leg. The leg was vibrated at a frequency of 60–80 Hz, with a 5-mm peak-to-peak displacement amplitude. These vibration frequencies are within the ranges that are produced by hand-held power tools (35–150 Hz) (Radwin et al., 1990). In previous studies in the rat, more intense hindlimb vibration at 80 Hz for 5 h daily for 2 d did not cause muscle necrosis (Lundborg et al., 1990; Gudas et al., 1995). In the present experiments, hindlimbs were vibrated once for 15 min.
Eccentric exercise
The method used to eccentrically exercise the rat hindlimb (Alvarez et al., 2010) was similar to that described by Kano et al. (2004) and Taguchi et al. (2005). Briefly, isoflurane-anesthetized rats were placed in the supine position, on a heating pad (to maintain body temperature at 37°C), and the right hindpaw was affixed to the foot bracket of the exercise apparatus (model RU-72; NEC Medical Systems) with 3M Micropore surgical paper tape, such that the angle of the knee and ankle joints was ∼90° (with the paw 30° from vertical). The gastrocnemius muscle was stimulated via subcutaneous needle-type electrodes attached to a model DPS-07 stimulator (Dia Medical System) that delivered trains of rectangular pulses (100 Hz, 700 ms, 3 V) every 3 s to give a total of 300 contractions. During these stimulus-induced contractions of the gastrocnemius muscle, the electromotor system rotated the foot to produce extension of the gastrocnemius muscle.
Surgical induction of muscle endometriosis
Our model of surgically induced muscle endometriosis (Alvarez et al., 2012) was adapted from that used to induce peritoneal endometriosis in the rat (Vernon and Wilson, 1985). Rats were anesthetized with a mixture of ketamine hydrochloride and xylazine (80 and 6 mg/kg, s.c., respectively) and maintained with isoflurane (1.5% in 98.5% oxygen). After a midline abdominal anesthetic block performed by injecting 0.25% bupivacaine (0.2 ml, s.c.), under aseptic conditions, a midline incision ∼4 cm in length was performed. After laparotomy, the abdominal cavity was examined and the right uterine horn was identified, exposed, and isolated using a sterilized cotton roll. With the aid of a surgical microscope, the right uterine artery and vein and the uterine vessels from the ovarian artery were ligated at the level of the transition of the uterine horn to the oviduct, with a 5-0 nylon suture. This procedure was repeated 1 cm distally. The uterine horn bounded by these ligatures was sectioned perpendicularly to its axis, and a 1 cm segment was removed and immediately placed in a Petri dish containing 0.9% NaCl. The distal stump of the uterine horn was then tied with 5-0 nylon suture. After confirming hemostasis, the musculature of the abdominal wall was closed with single cross stitches, and the skin incision was closed with horizontal mattress stitches using 5-0 nylon. The excised uterine tissue was measured with a millimeter scale and opened longitudinally; a full-thickness 3 × 3 mm square of uterine tissue was then removed and kept in physiologic saline. To perform the implant, the biceps femoris muscle was exposed by means of a 2 cm skin incision perpendicular to the long axis of the calf. Then, a 1 cm incision was performed in the biceps femoris, allowing exposure of the underlying gastrocnemius muscle. With the aid of a surgical microscope, the square of uterine tissue was sutured to the surface of the gastrocnemius muscle by applying three to four single stitches using 5-0 nylon with the endometrial portion of the uterine tissue contacting the gastrocnemius muscle. After checking for hemostasis, the biceps femoris muscle was closed with single stitches and the skin with single cross stitches using 5-0 nylon. The sham surgical procedure was similar, but the implant sutured to the surface of the gastrocnemius muscle consisted of a 3 × 3 mm square of peritoneal fat instead of uterine tissue. Postoperative recovery was assessed daily. Return of normal estrus cyclicity was found within 1 week of the procedure.
Statistical analyses
The dependent variable in experiments evaluating cutaneous and muscle nociceptive threshold was change in withdrawal threshold in the paw and hindlimb, respectively, from the pretreatment baseline threshold or from that of corresponding controls. Group data are represented as mean ± SEM. Statistical significance was determined by one- or two-way repeated-measures ANOVA, followed by Tukey's or Bonferroni's post hoc test, respectively, or by Student's t test (appropriately mentioned in Results). For nonparametric analysis, Mann–Whitney U test was used. p < 0.05 was considered statistically significant.
Results
ET-1 hyperalgesia and its mechanical stimulus-induced enhancement
Receptors
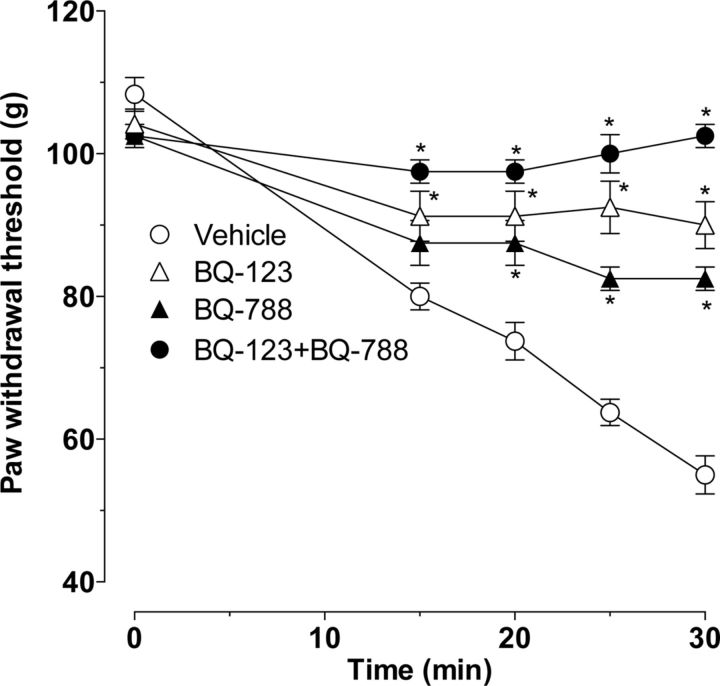
Intradermal administration of ET-1 (100 ng) in the dorsum of the rat hindpaw produced mechanical hyperalgesia, which increased on additional mechanical threshold stimulation at 5 min intervals (SIEH) (Joseph et al., 2011). We studied the effect of endothelin receptor antagonists BQ-123 (ETA antagonist) and BQ-788 (ETB antagonist) on ET-1 induced hyperalgesia and SIEH. Previous (15 min before ET-1) administration of BQ-123 (1 μg) or BQ-788 (1 μg) each alone partially attenuated ET-1-induced mechanical hyperalgesia, whereas coadministration of both antagonists completely eliminated ET-1 hyperalgesia (Fig. 2; p < 0.0001, two-way repeated-measures ANOVA, followed by Bonferroni's post hoc test for both antagonists effect and time points, n = 8). BQ-123 completely attenuated the SIEH (Fig. 2; P = NS, 15 vs 30 min, paired Student's t test, n = 8), whereas BQ-788 administration significantly inhibited SIEH (p < 0.0001, two-way repeated-measures ANOVA, time × treatment interaction), although it was still present (Fig. 2; p < 0.01, 15 vs 30 min, paired Student's t test, n = 8). Of note, neither BQ-123 nor BQ-788, administered alone or in combination, affected baseline nociceptive threshold (data not shown).
Figure 2.
Effect of ETA and ETB receptor antagonists on ET-1-induced mechanical hyperalgesia and stimulus-induced enhancement of ET-1 hyperalgesia. ET-1 (100 ng, i.d., in dorsum of the hindpaw) induced primary mechanical hyperalgesia (15 min time point) that was enhanced by repeated stimulation with a threshold stimulus at the site of nociceptive testing (i.e., SIEH). Local administration of antagonists of the ETA (BQ-123, 1 μg, i.d.) or ETB (BQ-788, 1 μg, i.d.) receptor into the hindpaw 15 min before ET-1 partially attenuated the ET-1 hyperalgesia. BQ-123 completely eliminated SIEH, whereas BQ-788 markedly but not completely inhibited SIEH. Coadministration of both antagonists (BQ-123 + BQ-788 into the hindpaw 15 min before ET-1) completely eliminated both ET-1-induced primary hyperalgesia as well as SIEH (p < 0.0001, for the effect of each inhibitor alone as well as when combined, two-way repeated-measures ANOVA, followed by Bonferroni's post hoc test, n = 6; *p < 0.05 for individual time points).
Second messengers
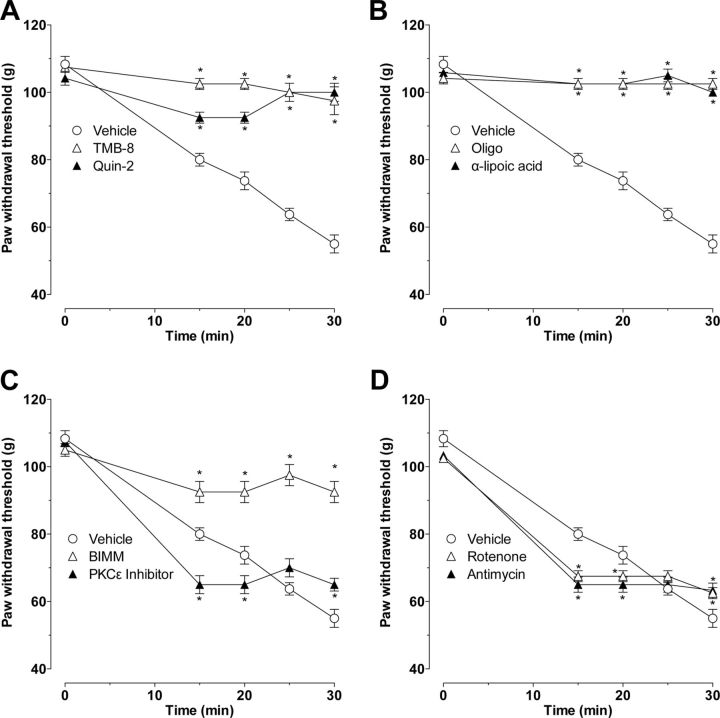
We studied the effect of second-messenger inhibitors on ET-1-induced hyperalgesia and SIEH. The intradermal administration of inhibitors of intracellular calcium signaling [Fig. 3A, TMB-8 (1 μg) and Quin-2 (1 μg)], ROS generation [Fig. 3B, α-lipoic acid (1 μg) and oligomycin (1 μg)], and PKC [Fig. 3C, BIMM (1 μg)] attenuated both ET-1-induced mechanical hyperalgesia and SIEH (p < 0.0001, for all inhibitors and time points, two-way repeated-measures ANOVA, followed by Bonferroni's post hoc test, n = 6).
Figure 3.
Effect of second-messenger inhibitors on ET-1-induced mechanical hyperalgesia and stimulus-induced enhancement of ET-1 hyperalgesia. ET-1-induced hyperalgesia and SIEH were both significantly attenuated by local pretreatment with inhibitors of intracellular calcium (A, TMB-8 and Quin-2, both 1 μg, i.d.), ROS generation (B, α-lipoic acid and oligomycin, both 1 μg, i.d.), and PKC (C, BIMM, 1 μg, i.d.) (p < 0.0001 for all, 2-way repeated-measures ANOVA, followed by Bonferroni's post hoc test, n = 6). In contrast, neither the selective PKCε inhibitor (C, PKCεI, 1 μg, i.d.) nor inhibitors of mitochondrial electron transport chain complexes I (D, rotenone, 1 μg, i.d.) and III (antimycin, 1 μg, i.d.) attenuated ET-1-induced mechanical hyperalgesia (C, D, n = 6), whereas each inhibited SIEH (C, PKCε, p = 0.001; D, rotenone, p = 0.01 and antimycin, p = 0.01, two-way repeated-measures ANOVA, followed by Bonferroni's post hoc test, n = 6; *p < 0.05 for individual time points). Administration of inhibitors alone did not affect nociceptive threshold (data not shown).
However, intradermal administration of a selective inhibitor of PKCε [Fig. 3C, PKCεI (1 μg)] and inhibitors of mitochondrial electron transport chain complexes I and III [Fig. 3D, rotenone (1 μg) and antimycin (1 μg)] did not attenuate ET-1-induced mechanical hyperalgesia, but each alone did inhibit SIEH (Fig. 3C, PKCεI, p = 0.001; D, rotenone, p = 0.01 and antimycin, p = 0.01; two-way repeated-measures ANOVA, followed by Bonferroni's post hoc test, n = 8 for each treatment group).
We next administered antisense oligodeoxynucleotide intrathecally to attenuate PKCε levels in sensory neurons (Parada et al., 2003a,b). Unlike intradermal injection of the PKCε inhibitor, which selectively prevented SIEH, intrathecal administration of the oligodeoxynucleotide antisense to PKCε affected neither ET-1-induced hyperalgesia nor SIEH. The effect of PKCε antisense (n = 8) was not different from the control (PKCε mismatch, n = 6; Fig. 4A).
Figure 4.
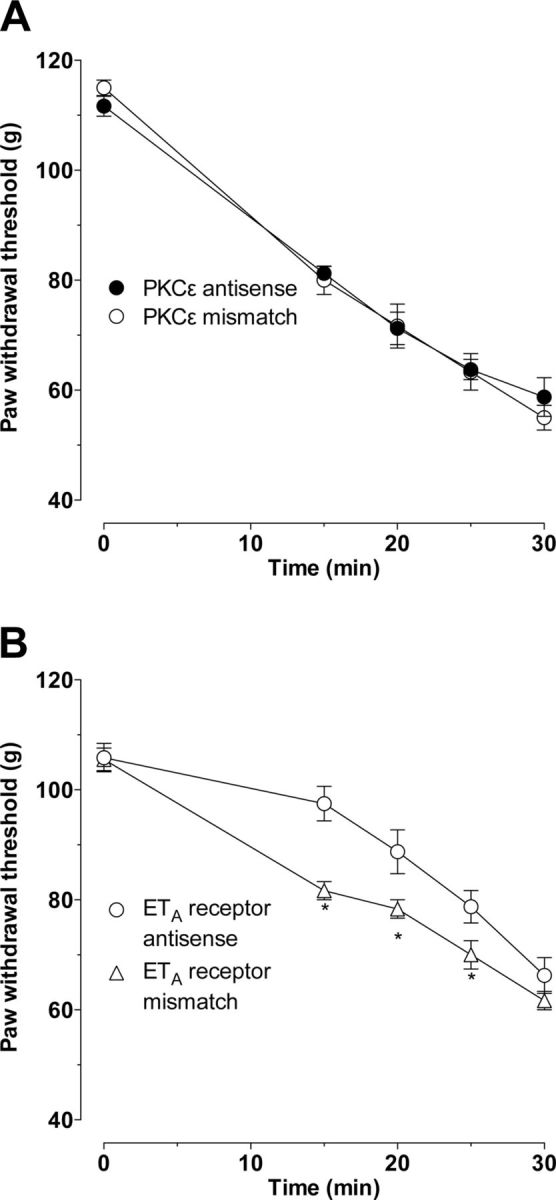
Effect of intrathecal PKCε and endothelin ETA receptor antisense on ET-1-induced mechanical hyperalgesia and stimulus-induced enhancement of ET-1 hyperalgesia. Intrathecal PKCε antisense (40 μg/20 μl) administered once daily for 3 consecutive days did not affect ET-1-induced hyperalgesia or SIEH (A, n = 6). Intrathecal administration of antisense to the ETA receptor attenuated ET-1-induced hyperalgesia without affecting the SIEH (B, p = 0.0001 for all, two-way repeated-measures ANOVA, followed by Bonferroni's post hoc test, n = 8; *p < 0.05 for individual time points).
Site of action of ET-1 to induce SIEH
To test the hypothesis that SIEH is produced by an action of ET-1 on ET receptors on the primary afferent nociceptor, we administered antisense oligodeoxynucleotide intrathecally to attenuate the ETA receptor level in sensory neurons. Importantly, when antisense is administered intrathecally, sensory neurons are the only cell in the skin exposed to it. Intrathecal administration of oligodeoxynucleotide antisense sequence to mRNA for the ETA receptor attenuated ET-1-induced hyperalgesia without affecting the SIEH. ETA receptor mismatch oligodeoxynucleotide treatment had no effect on ET-1 hyperalgesia (Fig. 4B, p < 0.0001, two-way repeated-measures ANOVA, followed by Bonferroni's post hoc test, n = 8).
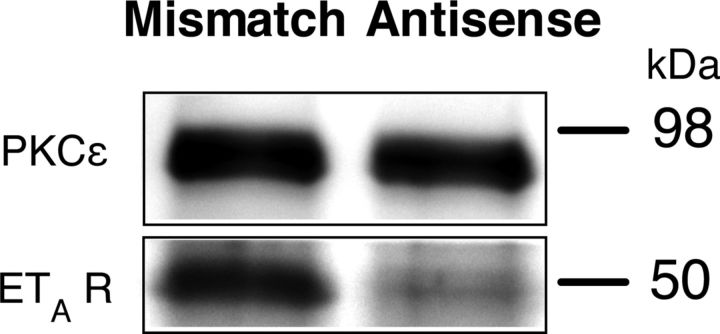
To confirm attenuation of ETA receptor in dorsal root ganglion neurons, we performed Western blot analysis on peripheral nerves of rats treated with oligodeoxynucleotide antisense to ETA mRNA and a mismatch oligodeoxynucleotide control. Antisense oligodeoxynucleotide to ETA mRNA significantly attenuated the level of ETA protein in the peripheral nerve when compared with mismatch treated controls (Fig. 5, p < 0.05, Mann–Whitney U test).
Figure 5.
Western blot analysis for the effect of antisense on ETA receptor. Western blot analysis demonstrated downregulation of the expression of peripheral ETA receptor by intrathecal antisense for ETA mRNA. Protein extracts derived from the saphenous nerve of rats treated with intrathecal antisense oligodeoxynucleotide for ETA mRNA for 3 consecutive days demonstrate a 47 ± 8% decrease in the ETA immunoreactivity compared with those rats treated with mismatch oligodeoxynucleotide (p < 0.05, Mann–Whitney U test, n = 6 for antisense- and mismatch-treated rats). PKCε, used as a housekeeping gene in this analysis, has the calculated molecular weight of ∼84 kDa (according to UniProtKB/Swiss-Prot database entry P09216), whereas the calculated molecular weight of ETA is ∼48 kDa (according to UniProtKB/Swiss-Prot database entry P26684).
Role of vascular endothelial cells
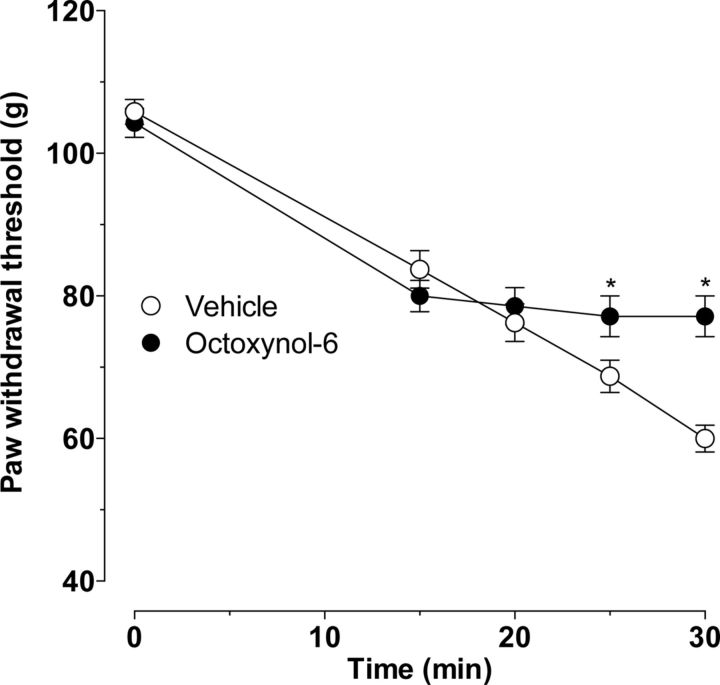
To test the hypothesis that the cell at which ET-1 acts to induce SIEH is the vascular endothelial cell, we functionally impaired the vascular endothelium by intravenous administration of octoxynol-9 (1 ml/kg of a 0.5% solution in saline), a procedure that rapidly impairs endothelial cell function but leaves the cells in the blood vessel that underlie them intact and functional (Connor and Feniuk, 1989; Jamal et al., 1992). This procedure abolished SIEH (Fig. 6, p < 0.0001, two-way ANOVA, followed by Bonferroni's post hoc test, n = 8) without affecting ET-1-induced mechanical hyperalgesia, supporting the hypothesis of a role for the endothelium.
Figure 6.
Effect of octoxynol-9 on ET-1-induced mechanical hyperalgesia and SIEH. Intravenous administration of octoxynol-9 abolished SIEH (p < 0.0001, two-way ANOVA, followed by Bonferroni's post hoc test; *p < 0.05 for individual time points) without affecting ET-1-induced mechanical hyperalgesia in the skin (n = 8).
Effect of octoxynol-9 on oxotremorine-induced increase in blood flow
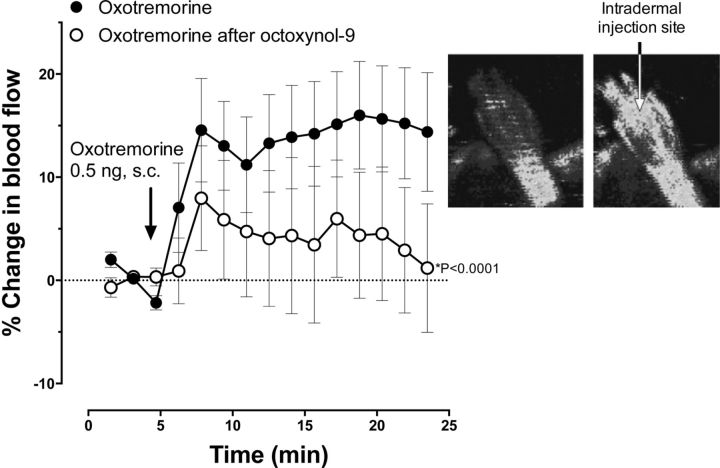
To confirm that octoxynol-9 had impaired endothelial function, we evaluated oxotremorine-induced vasodilation, a standard measure of endothelial function (Clavier et al., 1994; Harukuni et al., 2000). After subcutaneous injection of the muscarinic agonist oxotremorine (0.5 ng/50 μl), there was an increase in cutaneous blood flow. However, in rats that had been pretreated with octoxynol-9 (1 ml/kg of a 0.5% solution in saline, i.v.), oxotremorine-induced increased cutaneous blood flow was markedly attenuated [Fig. 7, p < 0.0001; F = 17.29, DFn = 1, DFd = 570, two-way repeated-measures ANOVA, n = 23 (control) and n = 17 (octoxynol-9)].
Figure 7.
Oxotremorine induced an increase in blood flow attenuated by treatment with octoxynol-9. Time course for changes in hindpaw blood flow produced by oxotremorine. Increased cutaneous blood flow after subcutaneous administration of oxotremorine (0.5 ng) into the hindpaw was significantly attenuated in rats that had been treated with octoxynol-9 (intravenously) 24 h before blood flow assessment (F = 17.29, DFn = 1, DFd = 570; p < 0.0001, repeated-measures ANOVA). The Moor Laser Doppler Imager data analysis software processed time series data images during experiments via an analog-to-digital converter in the imaging system. Inset, Scans immediately before and 15 min after oxotremorine administration in one representative rat showing increased blood flow on the dorsal surface of a rat hindpaw. Blood flow is quantified by a Moor Laser Doppler Imager as flux, which is a measure proportional to the product of the average speed of the blood cells and their concentration. Hindpaws were scanned (15 scans over 23.5 min), and the flux for the whole hindpaw was measured.
Effect of octoxynol-9 on PAF-induced plasma extravasation
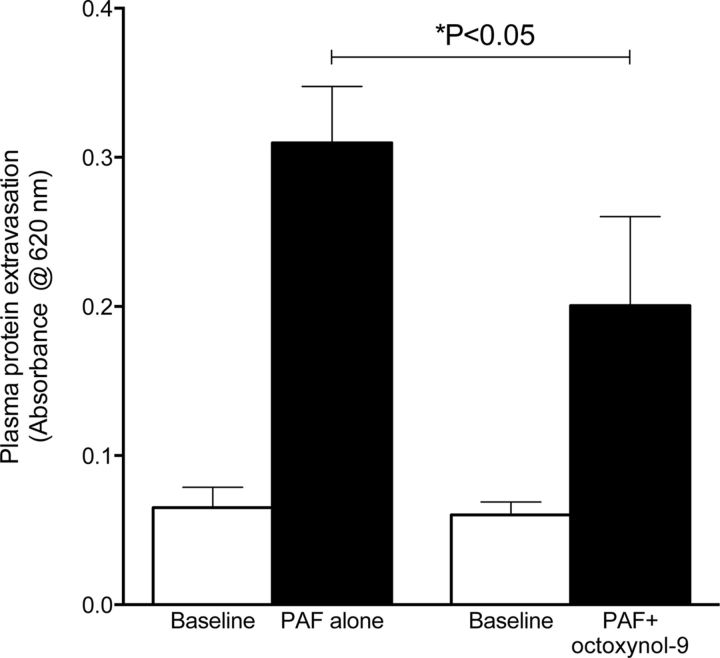
As a second measure of loss of an endothelial function, we evaluated the effect of octoxynol-9 on the endothelium-dependent (Braquet et al., 1987) plasma protein extravasation induced by PAF (Mulder and Colditz, 1993; Miao et al., 1996; Cheng et al., 2009). We used PAF to evaluate endothelial cell functional integrity in mediating plasma protein extravasation, because we extensively used this compound to evaluate plasma protein extravasation (Green et al., 1993a,b,c,d, 1994, 1995, 1997, 1998; Lo et al., 1999), whereas ET-1 itself has not been studied with respect to this function. The knee joint of the anesthetized rat was cross-perfused with saline, to which PAF was then added. When PAF-induced plasma extravasation was established, octoxynol-9 was administered intravenously (0.5%, 1 ml/kg). The administration of octoxynol-9 attenuated PAF-induced plasma extravasation (Fig. 8, p < 0.05 unpaired Student's t test, n = 10). Of note, although the magnitude of attenuation of plasma extravasation appears less than the attenuation of SIEH, the increased plasma extravasation (attributable to action of PAF on PAF receptors) is via a different mechanism than that of SIEH (action of endothelin or an endothelin-derived hyperalgesic mediator on the nociceptor). Thus, there is no a priori reason to predict same sensitivity to the effects of octoxynol-9.
Figure 8.
Effect of octoxynol-9 on platelet activating factor-induced plasma protein extravasation. Perfusion of PAF through the knee joint induces an increase in plasma protein extravasation. Intravenous administration of octoxynol-9 significantly attenuated PAF-induced plasma extravasation (p < 0.05, unpaired Student's t test, both groups n = 10).
Role of endothelial cells in models of vascular pain syndrome
Because tonic release of mediators from endothelial cells has been implicated in vascular diseases (Pate et al., 2010; Mironidou-Tzouveleki et al., 2011; Triggle et al., 2012), we evaluated their role in a model of a pain syndrome associated with vascular dysfunction, induced by exposure to ergonomic vibration (Palmer and Collin, 1993; Gemne, 1997; Dowd et al., 1998; Mirbod et al., 1999). We also evaluated the contribution of the endothelium to two other disease models in which a vascular pathology has been suggested to contribute to pain: eccentric exercise and endometriosis, in which a profusion of fine blood vessels issuing from the surrounding epimysium is evident on the surface of the endometrial cyst (Alvarez et al., 2012). In the two ergonomic pain models, hindlimb vibration and hindlimb eccentric exercise, administration of octoxynol-9 significantly attenuated the mechanical hyperalgesia [percentage reduction in nociceptive threshold: 40 ± 2.9% (vibration, n = 9) vs 3 ± 1.9% (vibration + octoxynol-9, n = 9); 35 ± 2.5% (eccentric exercise, n = 7) vs 8.4 ± 1.7% (eccentric exercise + octoxynol-9, n = 10), both p < 0.0001, unpaired Student's t test]. In the endometriosis model, administration of octoxynol-9 also markedly attenuated the hyperalgesia [1235 ± 83 mN (endometriosis model, n = 7) vs 2154 ± 93 mN (endometriosis + octoxynol-9, n = 7), p < 0.0001, paired Student's t test].
Role of purinergic agonists and the P2X2/3 receptor in stimulus-induced enhancement of endothelin hyperalgesia
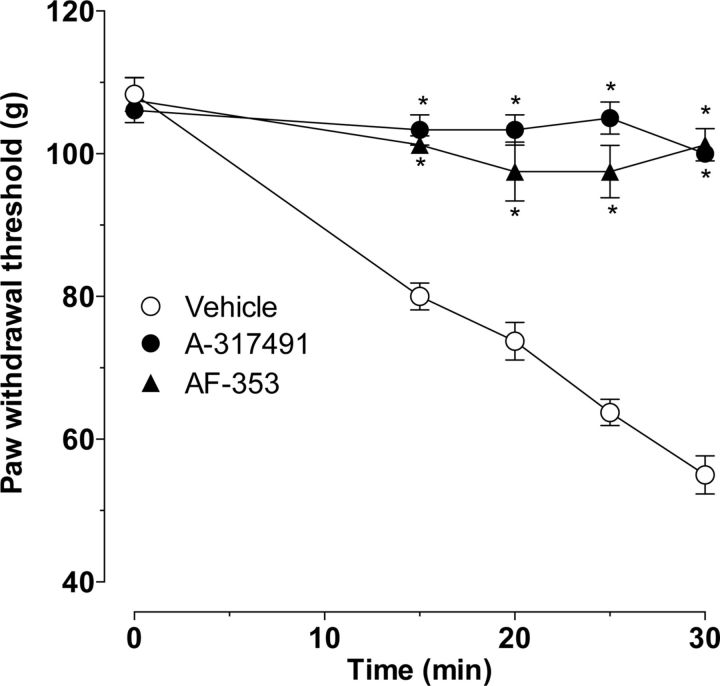
Treatment with ETA receptor antisense attenuated ET-1-induced hyperalgesia, without affecting SIEH. Because endothelial cells contain endothelin receptors and release ATP in response to mechanical stimulation (Milner et al., 1990), which can act on P2X2/3 receptors on nociceptors to sensitize them (Hester et al., 1995; Ford, 2012), we tested the effect of a P2X2/3 inhibitor (A-317491) on SIEH. When ET-1 was administered 15 min after the administration of A-317491 (1 μg, i.d.), stimulus-induced hyperalgesia was abolished (Fig. 9, p < 0.0001, two-way ANOVA, followed by Bonferroni's post hoc test, n = 6). A second inhibitor at P2X2/3 receptors, AF-353 (Gever et al., 2010), also inhibited SIEH (Fig. 9B, p < 0.0001, two-way ANOVA, followed by Bonferroni's post hoc test, n = 8).
Figure 9.
Effect of P2X2/3 inhibitors on ET-1-induced mechanical hyperalgesia and SIEH. At 15 min before intradermal ET-1, rats received vehicle or one of two P2X2/3 inhibitors (A-317491 or AF-353) intradermally. Both A-317491 and AF-353 significantly inhibited ET-1-induced hyperalgesia and SIEH compared with vehicle-treated controls (p < 0.001, two-way ANOVA, n = 6 for A-317491, n = 8 for AF-353; *p < 0.05 for individual time points).
Discussion
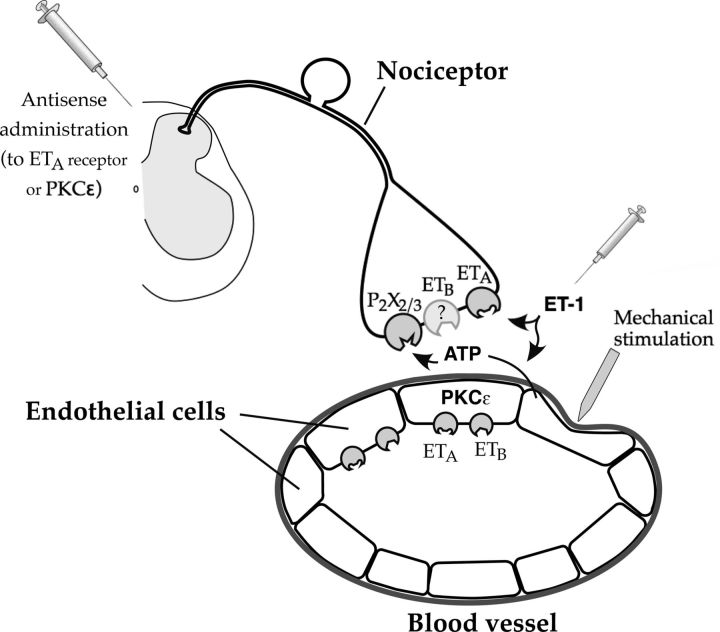
The intradermal administration of ET-1 produces mechanical hyperalgesia, which is further enhanced by repeated testing with a threshold nociceptive intensity mechanical stimulus, which we referred to as SIEH, a phenomenon not produced by multiple other pronociceptive mediators (Joseph et al., 2011). In the experimental protocol used to study ET-1 hyperalgesia, SIEH is produced by a mechanical stimulus of the intensity of the mechanical nociceptive threshold (Joseph et al., 2011), an intensity that decreases with each subsequent stimulus application. How such a mild intensity noxious stimulus produces progressive enhancement of hyperalgesia has not been elucidated. The markedly different time course of ET-1 hyperalgesia and SIEH (Joseph et al., 2011) led us to test the hypothesis that these two pronociceptive effects of ET-1 are mediated by different mechanisms, present on different cells in the skin; ET-1-induced hyperalgesia is mediated by its action on ETA receptors on some type of the primary afferent nociceptors (Werner et al., 2010; Laziz et al., 2011) as well as on ETB receptors, on nociceptors or a non-neuronal cell, whereas SIEH is mediated via ET receptors on a non-nociceptor cell at the site of injection in the skin.
ETA and ETB receptor antagonists each, alone, only partially inhibited ET-1 hyperalgesia, coadministration of both antagonists was required to completely eliminate ET-1 hyperalgesia, and dual block of both receptor subtypes is needed to eliminate ET-1 actions, as has been observed in other tissues (Fukuroda et al., 1996). The ETA antagonist eliminated SIEH, whereas the ETB receptor antagonist markedly but not completely attenuated SIEH.
The cells that contain the ETA and ETB receptors and the downstream signaling pathways mediating the sensitization of the nociceptor underlying the mechanical hyperalgesia induced by activation of each ET receptor remain to be established. Attenuation of ETA receptor on nociceptors markedly inhibited primary ET-1 hyperalgesia but had no effect on SIEH, whereas the ETA receptor antagonist alone completely prevented SIEH, leading to the suggestion that primary hyperalgesia is mediated via ETA receptors on nociceptors but SIEH is mediated via endothelin receptors on the endothelial cell. Of note, although we detected a decrease in ETA receptor expression in peripheral nerve after administration of antisense oligodeoxynucleotide to ETA mRNA (Fig. 5), we were unable to detect a change in ETB protein in the peripheral nerve after administration of antisense oligodeoxynucleotide to ETB mRNA (data not shown). It is also possible that endothelin may have action on other cell types in the periphery that contribute to nociception. For example, gene products of glial cells, such as Schwann cells, have been hypothesized to play a role in nerve injury and sensory nerve function (Campana, 2007; Gosselin et al., 2010), whereas a role for immune cells (e.g., neutrophils, macrophages, T cells) in acute and chronic inflammatory and neuropathic pain is well established (Scholz and Woolf, 2007; Chiu et al., 2012; Guillot et al., 2012). Other cell types, such as keratinocytes (Khodorova et al., 2003; Gopinath et al., 2005) and mast cells (Parada et al., 2001; Jankowski and Koerber, 2010), are also implicated in peripheral nociceptive mechanisms. It is likely that actions of mediators, released from these cell types, on nociceptors in the periphery contribute to regulating nociceptor function in chronic pain. Of note, although ETB receptors are present on many cell types at the site of nociceptive testing, we cannot exclude the possibility that BQ-788 might also have some antagonistic actions on ETA receptors because we do not know BQ-788 concentration at the receptor level; BQ-788 has ∼300-fold selectivity for ETB (porcine coronary artery smooth muscle cells, IC50 of 288 nm at ETA receptors vs 0.9 nm at ETB receptors) (Okada and Nishikibe, 2002). This contrasts with 20,000-fold selectivity of BQ-123 for ETA receptors (Russell and Davenport, 1996).
Because the signaling pathways activated by ET-1 are complex, involving action at two receptors (i.e., ETA and ETB), both of which have been implicated in nociceptor function (Khodorova et al., 2009a), and signal via several second messengers (Fellner and Arendshorst, 2007; Khodorova et al., 2009b), to distinguish the pronociceptive mechanisms mediating ET-1 hyperalgesia and SIEH, we used inhibitors of a panel of second messengers, albeit far from covering all second messengers implicated in nociceptor sensitization. Using this approach, we identified two general classes of inhibitors, namely those that inhibit both ET-1 hyperalgesia and stimulus-induced enhancement of ET-1 hyperalgesia (i.e., ETA and ETB receptor antagonists and inhibitors of intracellular calcium, ATP synthase, ROS generation, and nonselective PKC) and those that only inhibited SIEH (i.e., inhibitors of PKCε and mitochondrial electron transport chain complexes I and III). Although this evidence is indirect, these studies provide support for the hypothesis that ET-1-induced hyperalgesia and its enhancement by mechanical stimulation are mediated by action of ET-1 at two different cell types in the skin.
Of all the second-messenger inhibitors tested, only those that inhibited PKCε and mitochondrial electron transport chain complexes I and III selectively inhibited SIEH. Of note in this regard, PKCε can affect activity in the mitochondrial electron transport chain (Nowak et al., 2004; Costa and Garlid, 2008), and it has been shown, in other cells, that PKCε can translocate to mitochondria (Joseph and Levine, 2006; Ferrari and Levine, 2010) in which it can phosphorylate proteins, including members of the mitochondrial electron transport chain complex (Costa and Garlid, 2008). Because endothelial cells can be activated by mechanical stimuli, such as shear stress (Binti et al., 2011; Hennig et al., 2011), signaling via the mitochondrial electron transport chain in endothelial cells (Ali et al., 2004; Zhang et al., 2005) can lead to the release of a number of pronociceptive mediators (e.g., prostaglandins, ATP, nitric oxide, ET-1, platelet-derived growth factor, interleukin-1, interleukin-6, and ROS) (Corl et al., 2008; Iwata et al., 2010; Laskin et al., 2010), and mechanical stimulation of the endothelial cell releases ATP (Milner et al., 1990), the endothelial cell is a compelling candidate for mediating SIEH.
To address the role of the vascular endothelial cell in SIEH, we used an approach that has been used in the cardiovascular and renal literature to study endothelial cell-dependent mechanisms, functionally impairing the vascular endothelium by brief exposure to octoxynol-9, leaving underlying cells intact but functionally impaired (Connor and Feniuk, 1989; Jamal et al., 1992). In the present experiments, octoxynol-9 treatment completely eliminated SIEH but had no effect on ET-1-induced hyperalgesia. To confirm loss of endothelial cell function at the site of nociceptive testing, we evaluated its function in the skin of the hindpaw by evaluating a key signature endothelial cell function, muscarinic cholinergic agonist-induced vasodilatation (Furchgott and Zawadzki, 1980; Komori and Suzuki, 1987; Medhora et al., 2001), which is lost in the setting of endothelial cell dysfunction. This experiment provides direct support for the hypothesis that ET-1 acts on endothelial cells lining cutaneous blood vessels to produce SIEH. As a second test of the loss of endothelial function, we evaluated the effect of octoxynol-9 on PAF-induced plasma protein extravasation. Octoxynol-9 reversed PAF-induced plasma protein extravasation. We have not excluded the possibility that octoxynol-9 can also have action at cell types other than endothelial cells. However, our observations regarding the ability of octoxynol-9 to attenuate stimulation of plasma protein extravasation and blood flow, vibration- and eccentric exercise-induced hyperalgesia, as well as SIEH are consistent with the ability of octonoxyl-9 to disrupt endothelial cell function.
Because endothelial cells contain ETA and ETB receptors (Abraham and Dashwood, 2008; Piechota et al., 2010; Bkaily et al., 2011) and stimulation of endothelial cells by mechanical stress stimulates the release of ATP (Milner et al., 1990), we tested the hypothesis that ATP functions as a mediator of SIEH. In support of this hypothesis, we found that SIEH was markedly inhibited by two selective P2X2/3 inhibitors. Based on the results of these studies, we suggest that SIEH is mediated by release of ATP from endothelial cells in cutaneous blood vessels (P2X receptor is expressed in rat vascular primary afferents, e.g., innervating mesenteric arteries; Kirkup et al., 1999) and that ongoing hyperalgesia is not necessary for ET-1 to induce hyperalgesia (i.e., ET-1 can “directly” produce mechanical hyperalgesia by this mechanism). It is also possible that endothelial cells could release a substance that enhances P2X3 signaling in nociceptors indirectly.
Mechanical-stretch induces release of ATP from endothelial cells (Bodin and Burnstock, 2001), and although it has not been established that endothelin increases the amount of ATP released from endothelial cells by mechanical stimulation, mechanosensitive ATP release is closely correlated with calcium levels (Boudreault and Grygorczyk, 2004) and ET-1 dose dependently increases intracellular free calcium levels in endothelial cells (Avedanian et al., 2010). Furthermore, activation of endothelin receptors has been shown to enhance stimulated (but not basal) release of ATP from some cell types (Mutafova-Yambolieva and Westfall, 1995). Thus, we believe the proposed mechanism to be well supported by the literature. Furthermore, although a straightforward interpretation of our observations is that ATP is released from endothelial cells, it is also possible that endothelial cells could release a substance that enhances P2X3 signaling in nociceptors indirectly.
It was somewhat unexpected that inhibition of PKCε did not affect ET-1 hyperalgesia while BIMM completely eliminated it, especially because ET receptors can signal via PKCε in the heart and arterial smooth muscle cells (Nelson et al., 2008; Cheng et al., 2009; Rainbow et al., 2009) and PKCε is present in nociceptors in which its activation can produce sensitization (Parada et al., 2003a; Parada et al., 2003b) as well as contribute to the nociceptor sensitization produced by activation of some of their G-protein-coupled receptors (Khasar et al., 1999, 2008), whereas other pronociceptive mediators sensitize nociceptors by PKCε-independent second-messenger signaling pathways (Pate et al., 2010; Toya and Malik, 2012; Triggle et al., 2012). That BIMM, a nonselective PKC inhibitor (Jang et al., 2008), did attenuate ET-1-induced hyperalgesia does suggest that a non-ε isoform of PKC contributes to ET-1-induced nociceptor sensitization and mechanical hyperalgesia. Finally, because tonic release of endothelial cell mediators has been implicated in several diseases (Pate et al., 2010; Toya and Malik, 2012; Triggle et al., 2012), we tested the hypothesis that these cells contributed to ongoing pain in a model of a vascular pain syndrome, vibration-induced muscle hyperalgesia, as well as two other models, eccentric exercise and endometriosis, in which the vasculature may play a role. In support of this endothelium hypothesis, the hyperalgesia produced by vibration or eccentric exercise of the hindlimb, or the establishment of an endometriosis-like lesion, could each be reversed by intravenous octoxynol-9. How important endothelial cell function is in other pain syndromes that are associated with vascular dysfunction remains to be evaluated.
In summary, to distinguish the mechanisms underlying ET-1-induced mechanical hyperalgesia from those mediating SIEH, we evaluated the differential effect of peripherally administered ET receptor antagonists and inhibitors of second messengers implicated in peripheral pain mechanisms on these two ET-1-induced pronociceptive phenomena. Based on these studies, we propose that stimulus-induced enhancement of ET-1 hyperalgesia is produced by its action on vascular endothelial cells, sensitizing them to mechanical stimulation-induced release of ATP; thus ATP, in turn, acts at P2X2/3 receptors on nociceptors (Fig. 10). More generally, the importance of endothelial cells in other pain syndromes remains to be evaluated.
Figure 10.
Schematic figure showing hypothesized mechanism of endothelin hyperalgesia and SIEH. This figure demonstrates the proposed mechanisms by which ET-1 produces primary mechanical hyperalgesia and SIEH. ET-1-induced hyperalgesia is mediated by its action on ETA receptors on primary afferent nociceptors (Laziz et al., 2010; Werner et al., 2010) as well as on ETB receptors on nociceptors or a non-neuronal cell in the skin, whereas SIEH is mediated via ET receptors on a non-nociceptor cell in the skin, the endothelial cell. Based on these studies, we propose that stimulus-induced enhancement of ET-1 hyperalgesia is produced by its action on vascular endothelial cells, sensitizing release of ATP by mechanical stimulation; thus ATP, in turn, acts at P2X2/3 receptors on nociceptors.
Footnotes
This work was supported by National Institutes of Health.
References
- Abraham D, Dashwood M. Endothelin: role in vascular disease. Rheumatology (Oxford) 2008;47(Suppl 5):v23–v24. doi: 10.1093/rheumatology/ken282. [DOI] [PubMed] [Google Scholar]
- Aley KO, Levine JD. Role of protein kinase A in the maintenance of inflammatory pain. J Neurosci. 1999;19:2181–2186. doi: 10.1523/JNEUROSCI.19-06-02181.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali MH, Pearlstein DP, Mathieu CE, Schumacker PT. Mitochondrial requirement for endothelial responses to cyclic strain: implications for mechanotransduction. Am J Physiol Lung Cell Mol Physiol. 2004;287:L486–L496. doi: 10.1152/ajplung.00389.2003. [DOI] [PubMed] [Google Scholar]
- Alvarez P, Levine JD, Green PG. Eccentric exercise induces chronic alterations in musculoskeletal nociception in the rat. Eur J Neurosci. 2010;32:819–825. doi: 10.1111/j.1460-9568.2010.07359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez P, Chen X, Hendrich J, Irwin JC, Green PG, Giudice LC, Levine JD. Ectopic uterine tissue as a chronic pain generator. Neuroscience. 2012;225:269–282. doi: 10.1016/j.neuroscience.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avedanian L, Riopel J, Bkaily G, Nader M, D'Orleans-Juste P, Jacques D. ETA receptors are present in human aortic vascular endothelial cells and modulate intracellular calcium. Can J Physiol Pharmacol. 2010;88:817–829. doi: 10.1139/Y10-057. [DOI] [PubMed] [Google Scholar]
- Baron AD. Vascular reactivity. Am J Cardiol. 1999;84:25J–27J. doi: 10.1016/s0002-9149(99)00354-9. [DOI] [PubMed] [Google Scholar]
- Binti MD, Isa K, Kawasaki N, Ueyama K, Sumii T, Kudo S. Effects of cold exposure and shear stress on endothelial nitric oxide synthase activation. Biochem Biophys Res Commun. 2011;412:318–322. doi: 10.1016/j.bbrc.2011.07.092. [DOI] [PubMed] [Google Scholar]
- Bkaily G, Avedanian L, Al-Khoury J, Provost C, Nader M, D'Orléans-Juste P, Jacques D. Nuclear membrane receptors for ET-1 in cardiovascular function. Am J Physiol Regul Integr Comp Physiol. 2011;300:R251–R263. doi: 10.1152/ajpregu.00736.2009. [DOI] [PubMed] [Google Scholar]
- Bodin P, Burnstock G. Evidence that release of adenosine triphosphate from endothelial cells during increased shear stress is vesicular. J Cardiovasc Pharmacol. 2001;38:900–908. doi: 10.1097/00005344-200112000-00012. [DOI] [PubMed] [Google Scholar]
- Bogen O, Joseph EK, Chen X, Levine JD. GDNF hyperalgesia is mediated by PLCgamma, MAPK/ERK, PI3K, CDK5 and Src family kinase signaling and dependent on the IB4-binding protein versican. Eur J Neurosci. 2008;28:12–19. doi: 10.1111/j.1460-9568.2008.06308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreault F, Grygorczyk R. Cell swelling-induced ATP release is tightly dependent on intracellular calcium elevations. J Physiol. 2004;561:499–513. doi: 10.1113/jphysiol.2004.072306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourreau JP, Banijamali HS, Challice CE. Modification of excitation-contraction coupling in cat ventricular myocardium following endocardial damage. Can J Physiol Pharmacol. 1993;71:254–262. doi: 10.1139/y93-040. [DOI] [PubMed] [Google Scholar]
- Braquet P, Touqui L, Shen TY, Vargaftig BB. Perspectives in platelet-activating factor research. Pharmacol Rev. 1987;39:97–145. [PubMed] [Google Scholar]
- Burnstock G. Release of vasoactive substances from endothelial cells by shear stress and purinergic mechanosensory transduction. J Anat. 1999;194:335–342. doi: 10.1046/j.1469-7580.1999.19430335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt M, Dwivedi G, Blann A, Khair O, Lip GY. Endothelial dysfunction: methods of assessment and implications for cardiovascular diseases. Curr Pharm Des. 2010;16:3442–3454. doi: 10.2174/138161210793563383. [DOI] [PubMed] [Google Scholar]
- Campana WM. Schwann cells: activated peripheral glia and their role in neuropathic pain. Brain Behav Immun. 2007;21:522–527. doi: 10.1016/j.bbi.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr J, Wilhelm DL. The evaluation of increased vascular permeability in the skin of guinea-pigs. Aust J Exp Biol Med Sci. 1964;42:511–522. doi: 10.1038/icb.1964.48. [DOI] [PubMed] [Google Scholar]
- Chen X, Green PG, Levine JD. Neuropathic pain-like alterations in muscle nociceptor function associated with vibration-induced muscle pain. Pain. 2010;151:460–466. doi: 10.1016/j.pain.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng YS, Dai DZ, Dai Y. Isoproterenol disperses distribution of NADPH oxidase, MMP-9, and pPKCepsilon in the heart, which are mitigated by endothelin receptor antagonist CPU0213. Acta Pharmacol Sin. 2009;30:1099–1106. doi: 10.1038/aps.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu IM, von Hehn CA, Woolf CJ. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat Neurosci. 2012;15:1063–1067. doi: 10.1038/nn.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavier N, Kirsch JR, Hurn PD, Traystman RJ. Effect of postischemic hypoperfusion on vasodilatory mechanisms in cats. Am J Physiol. 1994;267:H2012–H2018. doi: 10.1152/ajpheart.1994.267.5.H2012. [DOI] [PubMed] [Google Scholar]
- Connor HE, Feniuk W. Influence of the endothelium on contractile effects of 5-hydroxytryptamine and selective 5-HT agonists in canine basilar artery. Br J Pharmacol. 1989;96:170–178. doi: 10.1111/j.1476-5381.1989.tb11797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corl CM, Gandy JC, Sordillo LM. Platelet activating factor production and proinflammatory gene expression in endotoxin-challenged bovine mammary endothelial cells. J Dairy Sci. 2008;91:3067–3078. doi: 10.3168/jds.2008-1066. [DOI] [PubMed] [Google Scholar]
- Costa AD, Garlid KD. Intramitochondrial signaling: interactions among mitoKATP, PKCepsilon, ROS, and MPT. Am J Physiol Heart Circ Physiol. 2008;295:H874–H882. doi: 10.1152/ajpheart.01189.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dina OA, Levine JD, Green PG. Muscle inflammation induces a protein kinase Cepsilon-dependent chronic-latent muscle pain. J Pain. 2008;9:457–462. doi: 10.1016/j.jpain.2008.01.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dina OA, Joseph EK, Levine JD, Green PG. Mechanisms mediating vibration-induced chronic musculoskeletal pain analyzed in the rat. J Pain. 2010;11:369–377. doi: 10.1016/j.jpain.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd PM, Goldsmith PC, Chopra S, Bull HA, Foreman JC. Cutaneous responses to endothelin-1 and histamine in patients with vibration white finger. J Invest Dermatol. 1998;110:127–131. doi: 10.1046/j.1523-1747.1998.00096.x. [DOI] [PubMed] [Google Scholar]
- Fellner SK, Arendshorst W. Endothelin-A and -B receptors, superoxide, and Ca2+ signaling in afferent arterioles. Am J Physiol Renal Physiol. 2007;292:F175–F184. doi: 10.1152/ajprenal.00050.2006. [DOI] [PubMed] [Google Scholar]
- Ferrari LF, Levine JD. Alcohol consumption enhances antiretroviral painful peripheral neuropathy by mitochondrial mechanisms. Eur J Neurosci. 2010;32:811–818. doi: 10.1111/j.1460-9568.2010.07355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford AP. In pursuit of P2X3 antagonists: novel therapeutics for chronic pain and afferent sensitization. Purinergic Signal. 2012;8:3–26. doi: 10.1007/s11302-011-9271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuroda T, Ozaki S, Ihara M, Ishikawa K, Yano M, Miyauchi T, Ishikawa S, Onizuka M, Goto K, Nishikibe M. Necessity of dual blockade of endothelin ETA and ETB receptor subtypes for antagonism of endothelin-1-induced contraction in human bronchi. Br J Pharmacol. 1996;117:995–999. doi: 10.1111/j.1476-5381.1996.tb16688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gemne G. Diagnostics of hand-arm system disorders in workers who use vibrating tools. Occup Environ Med. 1997;54:90–95. doi: 10.1136/oem.54.2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gever JR, Soto R, Henningsen RA, Martin RS, Hackos DH, Panicker S, Rubas W, Oglesby IB, Dillon MP, Milla ME, Burnstock G, Ford AP. AF-353, a novel, potent and orally bioavailable P2X3/P2X2/3 receptor antagonist. Br J Pharmacol. 2010;160:1387–1398. doi: 10.1111/j.1476-5381.2010.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath P, Wan E, Holdcroft A, Facer P, Davis JB, Smith GD, Bountra C, Anand P. Increased capsaicin receptor TRPV1 in skin nerve fibres and related vanilloid receptors TRPV3 and TRPV4 in keratinocytes in human breast pain. BMC Womens Health. 2005;5:2. doi: 10.1186/1472-6874-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin RD, Suter MR, Ji RR, Decosterd I. Glial cells and chronic pain. Neuroscientist. 2010;16:519–531. doi: 10.1177/1073858409360822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green PG, Levine JD. Sexual dimorphism in the effect of nonhabituating stress on neurogenic plasma extravasation. Eur J Neurosci. 2005;21:486–492. doi: 10.1111/j.1460-9568.2005.03872.x. [DOI] [PubMed] [Google Scholar]
- Green PG, Luo J, Hammond ER, Levine JD. Trypsin enhances sympathetic neuron-dependent plasma extravasation in the rat knee joint. Neurosci Lett. 1993a;158:117–119. doi: 10.1016/0304-3940(93)90626-v. [DOI] [PubMed] [Google Scholar]
- Green PG, Luo J, Heller P, Levine JD. Modulation of bradykinin-induced plasma extravasation in the rat knee joint by sympathetic co-transmitters. Neuroscience. 1993b;52:451–458. doi: 10.1016/0306-4522(93)90171-b. [DOI] [PubMed] [Google Scholar]
- Green PG, Luo J, Heller PH, Levine JD. Further substantiation of a significant role for the sympathetic nervous system in inflammation. Neuroscience. 1993c;55:1037–1043. doi: 10.1016/0306-4522(93)90317-9. [DOI] [PubMed] [Google Scholar]
- Green PG, Luo J, Heller PH, Levine JD. Neurogenic and non-neurogenic mechanisms of plasma extravasation in the rat. Neuroscience. 1993d;52:735–743. doi: 10.1016/0306-4522(93)90422-c. [DOI] [PubMed] [Google Scholar]
- Green PG, Luo J, Heller PH, Levine JD. Effect of E-type prostaglandins on bradykinin-induced plasma extravasation in the knee joint of the rat. Eur J Pharmacol. 1994;252:127–132. doi: 10.1016/0014-2999(94)90587-8. [DOI] [PubMed] [Google Scholar]
- Green PG, Miao FJ, Jänig W, Levine JD. Negative feedback neuroendocrine control of the inflammatory response in rats. J Neurosci. 1995;15:4678–4686. doi: 10.1523/JNEUROSCI.15-06-04678.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green PG, Janig W, Levine JD. Negative feedback neuroendocrine control of inflammatory response in the rat is dependent on the sympathetic postganglionic neuron. J Neurosci. 1997;17:3234–3238. doi: 10.1523/JNEUROSCI.17-09-03234.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green PG, Strausbaugh HJ, Levine JD. Annexin I is a local mediator in neural-endocrine feedback control of inflammation. J Neurophysiol. 1998;80:3120–3126. doi: 10.1152/jn.1998.80.6.3120. [DOI] [PubMed] [Google Scholar]
- Gudas JM, Klein RC, Oka M, Cowan KH. Posttranscriptional regulation of the c-myb proto-oncogene in estrogen receptor-positive breast cancer cells. Clin Cancer Res. 1995;1:235–243. [PubMed] [Google Scholar]
- Guillot X, Semerano L, Decker P, Falgarone G, Boissier MC. Pain and immunity. Joint Bone Spine. 2012;79:228–236. doi: 10.1016/j.jbspin.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Harukuni I, Takahashi H, Traystman RJ, Bhardwaj A, Kirsch JR. Oxotremorine-induced cerebral hyperemia does not predict infarction volume in spontaneously hypertensive or stroke-prone rats. Crit Care Med. 2000;28:190–195. doi: 10.1097/00003246-200001000-00031. [DOI] [PubMed] [Google Scholar]
- Hennig T, Mogensen C, Kirsch J, Pohl U, Gloe T. Shear stress induces the release of an endothelial elastase: role in integrin alpha(v)beta(3)-mediated FGF-2 release. J Vasc Res. 2011;48:453–464. doi: 10.1159/000327009. [DOI] [PubMed] [Google Scholar]
- Hester TO, Jones RO, Archer SM, Haydon RC. Prophylactic antibiotic drops after tympanostomy tube placement. Arch Otolaryngol Head Neck Surg. 1995;121:445–448. doi: 10.1001/archotol.1995.01890040069011. [DOI] [PubMed] [Google Scholar]
- Inoue A, Yanagisawa M, Kimura S, Kasuya Y, Miyauchi T, Goto K, Masaki T. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc Natl Acad Sci U S A. 1989;86:2863–2867. doi: 10.1073/pnas.86.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata M, Suzuki S, Asai Y, Inoue T, Takagi K. Involvement of nitric oxide in a rat model of carrageenin-induced pleurisy. Mediators Inflamm. 2010;2010:682879. doi: 10.1155/2010/682879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal A, Bendeck M, Langille BL. Structural changes and recovery of function after arterial injury. Arterioscler Thromb. 1992;12:307–317. doi: 10.1161/01.atv.12.3.307. [DOI] [PubMed] [Google Scholar]
- Jang SN, Her SH, Do KR, Kim JS, Yoon HJ, Lee JM, Jin SW. A case of congenital bilateral coronary-to-right ventricle fistula coexisting with variant angina. Korean J Intern Med. 2008;23:216–218. doi: 10.3904/kjim.2008.23.4.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski MP, Koerber HR. Neurotrophic factors and nociceptor sensitization. In: Kruger L, Light AR, editors. Translational pain research: from mouse to man. Boca Raton, FL: CRC; 2010. Chap 2. [Google Scholar]
- Joseph EK, Levine JD. Mitochondrial electron transport in models of neuropathic and inflammatory pain. Pain. 2006;121:105–114. doi: 10.1016/j.pain.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Joseph EK, Levine JD. Multiple PKCepsilon-dependent mechanisms mediating mechanical hyperalgesia. Pain. 2010;150:17–21. doi: 10.1016/j.pain.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph EK, Gear RW, Levine JD. Mechanical stimulation enhances endothelin-1 hyperalgesia. Neuroscience. 2011;178:189–195. doi: 10.1016/j.neuroscience.2011.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano Y, Sampei K, Matsudo H. Time course of capillary structure changes in rat skeletal muscle following strenuous eccentric exercise. Acta Physiol Scand, 2004;180:291–299. doi: 10.1111/j.0001-6772.2003.01250.x. [DOI] [PubMed] [Google Scholar]
- Khasar SG, Lin YH, Martin A, Dadgar J, McMahon T, Wang D, Hundle B, Aley KO, Isenberg W, McCarter G, Green PG, Hodge CW, Levine JD, Messing RO. A novel nociceptor signaling pathway revealed in protein kinase C epsilon mutant mice. Neuron. 1999;24:253–260. doi: 10.1016/s0896-6273(00)80837-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasar SG, Burkham J, Dina OA, Brown AS, Bogen O, Alessandri-Haber N, Green PG, Reichling DB, Levine JD. Stress induces a switch of intracellular signaling in sensory neurons in a model of generalized pain. J Neurosci. 2008;28:5721–5730. doi: 10.1523/JNEUROSCI.0256-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodorova A, Navarro B, Jouaville LS, Murphy JE, Rice FL, Mazurkiewicz JE, Long-Woodward D, Stoffel M, Strichartz GR, Yukhananov R, Davar G. Endothelin-B receptor activation triggers an endogenous analgesic cascade at sites of peripheral injury. Nat Med. 2003;9:1055–1061. doi: 10.1038/nm885. [DOI] [PubMed] [Google Scholar]
- Khodorova A, Montmayeur JP, Strichartz G. Endothelin receptors and pain. J Pain. 2009a;10:4–28. doi: 10.1016/j.jpain.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodorova A, Zou S, Ren K, Dubner R, Davar G, Strichartz G. Dual roles for endothelin-B receptors in modulating adjuvant-induced inflammatory hyperalgesia in rats. Open Pain J. 2009b;2:30–40. doi: 10.2174/1876386300902010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkup AJ, Booth CE, Chessell IP, Humphrey PP, Grundy D. Excitatory effect of P2X receptor activation on mesenteric afferent nerves in the anaesthetised rat. J Physiol. 1999;520:551–563. doi: 10.1111/j.1469-7793.1999.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori K, Suzuki H. Electrical responses of smooth muscle cells during cholinergic vasodilation in the rabbit saphenous artery. Circ Res. 1987;61:586–593. doi: 10.1161/01.res.61.4.586. [DOI] [PubMed] [Google Scholar]
- Laskin DL, Chen L, Hankey PA, Laskin JD. Role of STK in mouse liver macrophage and endothelial cell responsiveness during acute endotoxemia. J Leukoc Biol. 2010;88:373–382. doi: 10.1189/jlb.0210113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laziz I, Larbi A, Grebert D, Sautel M, Congar P, Lacroix MC, Salesse R, Meunier N. Endothelin as a neuroprotective factor in the olfactory epithelium. Neuroscience. 2011;172:20–29. doi: 10.1016/j.neuroscience.2010.10.063. [DOI] [PubMed] [Google Scholar]
- Lo EJ, Green PG, Miao FJ, Relchling DB, Levine JD. Bradykinin-induced neurogenic migration of neutrophils into the rat knee joint. Neuroreport. 1999;10:3821–3824. doi: 10.1097/00001756-199912160-00018. [DOI] [PubMed] [Google Scholar]
- Lundborg G, Dahlin LB, Hansson HA, Kanje M, Necking LE. Vibration exposure and peripheral nerve fiber damage. J Hand Surg Am. 1990;15:346–351. doi: 10.1016/0363-5023(90)90121-7. [DOI] [PubMed] [Google Scholar]
- McLeod JD, Piper PJ. Effect of removing the endothelium on the vascular responses induced by leukotrienes C4 and D4 in guinea-pig isolated heart. Eur J Pharmacol. 1992;212:67–72. doi: 10.1016/0014-2999(92)90073-d. [DOI] [PubMed] [Google Scholar]
- Medhora M, Narayanan J, Harder D. Dual regulation of the cerebral microvasculature by epoxyeicosatrienoic acids. Trends Cardiovasc Med. 2001;11:38–42. doi: 10.1016/s1050-1738(01)00082-2. [DOI] [PubMed] [Google Scholar]
- Miao FJ, Jänig W, Levine JD. Role of sympathetic postganglionic neurons in synovial plasma extravasation induced by bradykinin. J Neurophysiol. 1996;75:715–724. doi: 10.1152/jn.1996.75.2.715. [DOI] [PubMed] [Google Scholar]
- Milner P, Bodin P, Loesch A, Burnstock G. Rapid release of endothelin and ATP from isolated aortic endothelial cells exposed to increased flow. Biochem Biophys Res Commun. 1990;170:649–656. doi: 10.1016/0006-291x(90)92141-l. [DOI] [PubMed] [Google Scholar]
- Mirbod SM, Akbar-Khanzadeh F, Onozuka M, Jamali M, Watanabe K, Inaba R, Iwata H. A four-year follow-up study on subjective symptoms and functional capacities in workers using hand-held grinders. Ind Health. 1999;37:415–425. doi: 10.2486/indhealth.37.415. [DOI] [PubMed] [Google Scholar]
- Mironidou-Tzouveleki M, Tsartsalis S, Tomos C. Vascular endothelial growth factor (VEGF) in the pathogenesis of diabetic nephropathy of type 1 diabetes mellitus. Curr Drug Targets. 2011;12:107–114. doi: 10.2174/138945011793591581. [DOI] [PubMed] [Google Scholar]
- Mulder K, Colditz IG. Migratory responses of ovine neutrophils to inflammatory mediators in vitro and in vivo. J Leukoc Biol. 1993;53:273–278. doi: 10.1002/jlb.53.3.273. [DOI] [PubMed] [Google Scholar]
- Murase S, Terazawa E, Queme F, Ota H, Matsuda T, Hirate K, Kozaki Y, Katanosaka K, Taguchi T, Urai H, Mizumura K. Bradykinin and nerve growth factor play pivotal roles in muscular mechanical hyperalgesia after exercise (delayed-onset muscle soreness) J Neurosci. 2010;30:3752–3761. doi: 10.1523/JNEUROSCI.3803-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutafova-Yambolieva VN, Westfall DP. Endothelin-3 can both facilitate and inhibit transmitter release in the guinea-pig vas deferens. Eur J Pharmacol. 1995;285:213–216. doi: 10.1016/0014-2999(95)00501-b. [DOI] [PubMed] [Google Scholar]
- Nelson CP, Willets JM, Davies NW, Challiss RA, Standen NB. Visualizing the temporal effects of vasoconstrictors on PKC translocation and Ca2+ signaling in single resistance arterial smooth muscle cells. Am J Physiol Cell Physiol. 2008;295:C1590–C1601. doi: 10.1152/ajpcell.00365.2008. [DOI] [PubMed] [Google Scholar]
- Nowak G, Bakajsova D, Clifton GL. Protein kinase C-epsilon modulates mitochondrial function and active Na+ transport after oxidant injury in renal cells. Am J Physiol Renal Physiol. 2004;286:F307–F316. doi: 10.1152/ajprenal.00275.2003. [DOI] [PubMed] [Google Scholar]
- Ogasawara C, Sakakibara H, Kondo T, Miyao M, Yamada S, Toyoshima H. Longitudinal study on factors related to the course of vibration-induced white finger. Int Arch Occup Environ Health. 1997;69:180–184. doi: 10.1007/s004200050134. [DOI] [PubMed] [Google Scholar]
- Okada M, Nishikibe M. BQ-788, a selective endothelin ET(B) receptor antagonist. Cardiovasc Drug Rev. 2002;20:53–66. doi: 10.1111/j.1527-3466.2002.tb00082.x. [DOI] [PubMed] [Google Scholar]
- Palmer RA, Collin J. Vibration white finger. Br J Surg. 1993;80:705–709. doi: 10.1002/bjs.1800800608. [DOI] [PubMed] [Google Scholar]
- Papir-Kricheli D, Frey J, Laufer R, Gilon C, Chorev M, Selinger Z, Devor M. Behavioural effects of receptor-specific substance P agonists. Pain. 1987;31:263–276. doi: 10.1016/0304-3959(87)90041-8. [DOI] [PubMed] [Google Scholar]
- Parada CA, Tambeli CH, Cunha FQ, Ferreira SH. The major role of peripheral release of histamine and 5-hydroxytryptamine in formalin-induced nociception. Neuroscience. 2001;102:937–944. doi: 10.1016/s0306-4522(00)00523-6. [DOI] [PubMed] [Google Scholar]
- Parada CA, Yeh JJ, Joseph EK, Levine JD. Tumor necrosis factor receptor type-1 in sensory neurons contributes to induction of chronic enhancement of inflammatory hyperalgesia in rat. Eur J Neurosci. 2003a;17:1847–1852. doi: 10.1046/j.1460-9568.2003.02626.x. [DOI] [PubMed] [Google Scholar]
- Parada CA, Yeh JJ, Reichling DB, Levine JD. Transient attenuation of protein kinase Cepsilon can terminate a chronic hyperalgesic state in the rat. Neuroscience. 2003b;120:219–226. doi: 10.1016/s0306-4522(03)00267-7. [DOI] [PubMed] [Google Scholar]
- Pate M, Damarla V, Chi DS, Negi S, Krishnaswamy G. Endothelial cell biology: role in the inflammatory response. Adv Clin Chem. 2010;52:109–130. [PubMed] [Google Scholar]
- Piechota A, Polánczyk A, Goraca A. Role of endothelin-1 receptor blockers on hemodynamic parameters and oxidative stress. Pharmacol Rep. 2010;62:28–34. doi: 10.1016/s1734-1140(10)70240-1. [DOI] [PubMed] [Google Scholar]
- Plant TD, Zöllner C, Kepura F, Mousa SS, Eichhorst J, Schaefer M, Furkert J, Stein C, Oksche A. Endothelin potentiates TRPV1 via ETA receptor-mediated activation of protein kinase C. Mol Pain. 2007;3:35. doi: 10.1186/1744-8069-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard MH, Pugh N, Wright I, Brownlee M. A vascular basis for repetitive strain injury. Rheumatology (Oxford) 1999;38:636–639. doi: 10.1093/rheumatology/38.7.636. [DOI] [PubMed] [Google Scholar]
- Radwin RG, Armstrong TJ, Vanbergeijk E. Vibration exposure for selected power hand tools used in automobile assembly. Am Ind Hyg Assoc J. 1990;51:510–518. doi: 10.1080/15298669091370013. [DOI] [PubMed] [Google Scholar]
- Rainbow RD, Norman RI, Everitt DE, Brignell JL, Davies NW, Standen NB. Endothelin-I and angiotensin II inhibit arterial voltage-gated K+ channels through different protein kinase C isoenzymes. Cardiovasc Res. 2009;83:493–500. doi: 10.1093/cvr/cvp143. [DOI] [PubMed] [Google Scholar]
- Randall MD, Thomas GR, Hiley CR. Effect of destruction of the vascular endothelium upon pressure/flow relations and endothelium-dependent vasodilatation in resistance beds of spontaneously hypertensive rats. Clin Sci (Lond) 1991;80:463–469. doi: 10.1042/cs0800463. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Pascual F, Busnadiego O, Lagares D, Lamas S. Role of endothelin in the cardiovascular system. Pharmacol Res. 2011;63:463–472. doi: 10.1016/j.phrs.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Russell FD, Davenport AP. Characterization of the binding of endothelin ETB selective ligands in human and rat heart. Br J Pharmacol. 1996;119:631–636. doi: 10.1111/j.1476-5381.1996.tb15720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez A, Recio P, Orensanz LM, Bustamante S, Navarro-Dorado J, Climent B, Benedito S, García-Sacristán A, Prieto D, Hernández M. Mechanisms involved in the effects of endothelin-1 in pig prostatic small arteries. Eur J Pharmacol. 2010;640:190–196. doi: 10.1016/j.ejphar.2010.04.059. [DOI] [PubMed] [Google Scholar]
- Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10:1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- Stoyneva Z, Lyapina M, Tzvetkov D, Vodenicharov E. Current pathophysiological views on vibration-induced Raynaud's phenomenon. Cardiovasc Res. 2003;57:615–624. doi: 10.1016/s0008-6363(02)00728-9. [DOI] [PubMed] [Google Scholar]
- Summer GJ, Romero-Sandoval EA, Bogen O, Dina OA, Khasar SG, Levine JD. Proinflammatory cytokines mediating burn-injury pain. Pain. 2008;135:98–107. doi: 10.1016/j.pain.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Sun ZW, Wang XD, Deng XM, Wallén R, Gefors L, Hallberg E, Andersson R. The influence of circulatory and gut luminal challenges on bidirectional intestinal barrier permeability in rats. Scand J Gastroenterol. 1997;32:995–1004. doi: 10.3109/00365529709011216. [DOI] [PubMed] [Google Scholar]
- Taguchi T, Matsuda T, Tamura R, Sato J, Mizumura K. Muscular mechanical hyperalgesia revealed by behavioural pain test and c-Fos expression in the spinal dorsal horn after eccentric contraction in rats. J Physiol. 2005;564:259–268. doi: 10.1113/jphysiol.2004.079483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toya SP, Malik AB. Role of endothelial injury in disease mechanisms and contribution of progenitor cells in mediating endothelial repair. Immunobiology. 2012;217:569–580. doi: 10.1016/j.imbio.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Triggle CR, Samuel SM, Ravishankar S, Marei I, Arunachalam G, Ding H. The endothelium: influencing vascular smooth muscle in many ways. Can J Physiol Pharmacol. 2012;90:713–738. doi: 10.1139/y2012-073. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Ninomiya H, Saotome M, Nomura A, Ohtsuka M, Yanagisawa M, Goto K, Masaki T, Hasegawa S. Endothelin, a novel vasoconstrictor peptide, as potent bronchoconstrictor. Eur J Pharmacol. 1988;154:227–228. doi: 10.1016/0014-2999(88)90106-9. [DOI] [PubMed] [Google Scholar]
- Van Langendonckt A, Donnez J, Defrère S, Dunselman GA, Groothuis PG. Antiangiogenic and vascular-disrupting agents in endometriosis: pitfalls and promises. Mol Hum Reprod. 2008;14:259–268. doi: 10.1093/molehr/gan019. [DOI] [PubMed] [Google Scholar]
- Vernon MW, Wilson EA. Studies on the surgical induction of endometriosis in the rat. Fertil Steril. 1985;44:684–694. [PubMed] [Google Scholar]
- Werner MF, Trevisani M, Campi B, André E, Geppetti P, Rae GA. Contribution of peripheral endothelin ETA and ETB receptors in neuropathic pain induced by spinal nerve ligation in rats. Eur J Pain. 2010;14:911–917. doi: 10.1016/j.ejpain.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Zhang J, Jin B, Li L, Block ER, Patel JM. Nitric oxide-induced persistent inhibition and nitrosylation of active site cysteine residues of mitochondrial cytochrome-c oxidase in lung endothelial cells. Am J Physiol Cell Physiol. 2005;288:C840–C849. doi: 10.1152/ajpcell.00325.2004. [DOI] [PubMed] [Google Scholar]