Abstract
For most cutaneous basal cell and squamous cell carcinomas (nonmelanoma skin cancers [NMSC]) data are insufficient to permit evidence-based choices among treatments. To compare tumor recurrence after treatments, we conducted a prospective cohort study of consecutive patients with primary NMSC treated with the most common treatments in two practices in 1999–2000. Recurrence was determined from medical records by observers blinded to treatment type. 24.3% of tumors (N=361) were treated with destruction with electrodessication / curettage, 38.3% (N=571) with excision, and 37.4% (N=556) with histologically-guided serial excision (Mohs surgery). Follow-up was available for 1174 patients with 1488 tumors (93.8%) at median 7.4 years; overall 5-year tumor recurrence rate [95% Confidence Interval] was 3.3% [2.3, 4.4]. Unadjusted recurrence rates did not differ after treatments: 4.9% [2.3, 7.4] after destruction, 3.5% [1.8, 5.2] after excision, and 2.1% [0.6, 3.5] after Mohs surgery (P=0.26), and no difference was seen after adjustment for risk factors. In tumors treated only with excision or Mohs surgery, the hazard of recurrence was not significantly different, even after adjustment for propensity for treatment with Mohs surgery. These data indicate that common treatments for NMSC were at least 95% effective, and further studies are needed to guide therapeutic choices for different clinical subgroups.
INTRODUCTION
Cutaneous basal cell carcinomas and squamous cell carcinomas—also called nonmelanoma skin cancer (NMSC)--are the most common malignancy.(Rogers et al., 2010) Typically, NMSCs affect quality of life but not survival, and the primary goal of treatment is the prevention of recurrence, since recurrent tumors are more difficult to treat. The most common treatments for primary NMSCs are tumor destruction with electrodessication and curettage, simple excision, and histologically guided serial excision, called Mohs surgery. For primary tumors existing data are insufficient to permit evidence-based choices among therapies,(Bath-Hextall et al., 2004) which vary in cost.(Essers et al., 2006; Otley, 2006; Wilson et al., 2011) Substantial unexplained variations exist in treatment choices,(Chren, 2004) and the dearth of data has resulted in “competing camps” for different treatments.(Williford and Feldman, 2004)
A randomized controlled trial of treatments for facial basal cell carcinomas (BCC) determined that 5-year recurrence rates after excision (4.1%) and Mohs surgery (2.5%) were similar (P=0.40).(Mosterd et al., 2008). These results have been controversial, however.(Feldman et al., 2008; Otley, 2005; Williford and Feldman, 2004) We found similar recurrence rates in 495 patients treated at a Veterans Affairs Medical Center (VA),(Chren et al., 2011), who were part of a larger prospective cohort study of patients treated at two hospitals. These results were reported first because follow-up from the electronic medical records at VA was complete sooner than at the other site. The sample size was too small to permit comparisons of effectiveness among treatments, however.
We now report complete follow-up on the entire cohort from the two hospitals. Our goal was to compare the effectiveness of the most common treatments for preventing recurrence of NMSC.
RESULTS
Patient, Tumor, and Care Characteristics
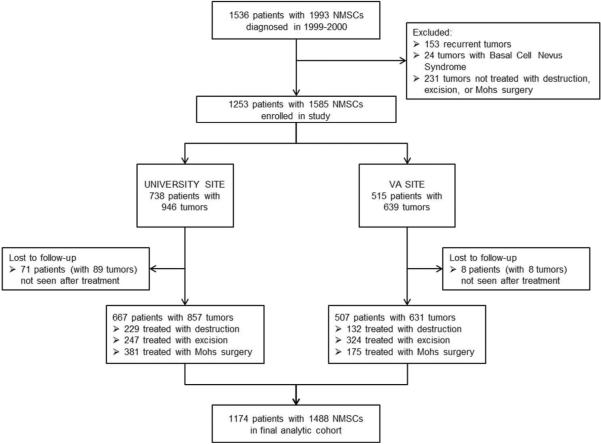
The study enrolled 1253 eligible patients with 1585primary NMSCs treated with destruction, excision, or Mohs surgery. Follow-up information was available for 1174 patients (93.7%) with 1488 tumors (93.8%) (Figure 1). Patients lost to follow-up were similar to those with follow-up in most features but were more likely to be female (38% vs. 26%), to have worse mental health status (median SF-12 Mental Component Score 41.2 vs 51.5), and to have BCC rather than SCC (89% vs 75%).
Figure 1.
Flow Diagram. Derivation of analytic cohort from consecutive patients diagnosed with NMSC during 1999–2000.
Patients, tumors, and care differed in the treatment groups (Table 1). For example, tumors treated with destruction were much less likely to be located in the H-zone of the face, and much less likely to have histological risk factors for recurrence(NCCN, 2011). Tumors treated with Mohs surgery were smaller, and much more likely to be located in the H-zone of the face.
Table 1.
Characteristics of 1174 patients with 1488 nonmelanoma skin cancers treated with destruction, excision, or Mohs surgery1,2
| Characteristic | Treatment Group | |||||||
|---|---|---|---|---|---|---|---|---|
| University Site | VA Site | |||||||
| Destruction | Excision | Mohs Surgery | P | Destruction | Excision | Mohs Surgery | P | |
| PATIENT CHARACTERISTICS | n=144 | n=203 | n=320 | n=92 | n=268 | n=147 | ||
| Age, years, median (IQR) | 60 (49–72) | 61 (51–75) | 68 (52–77) | 0.003 | 75 (64–80) | 75 (65–80) | 72 (63–78) | 0.14 |
| Gender male | 66% | 56% | 53% | 0.04 | 100% | 96% | 97% | 0.12 |
| Skin type I or II (Fitzpatrick, 1988) (never tans or tans a little) | 45% | 49% | 43% | 0.63 | 35% | 30% | 40% | 0.20 |
| Health status (SF-12)(Ware et al., 1996) | ||||||||
| Physical Component Score, median (IQR) | 52.6 (42.0–56.5) | 52.6 (46.6–55.9) | 53.1 (43.5–56.5) | 0.97 | 43.4 (30.2–52.8) | 42.7 (32.1–53.1) | 40.3 (34.3–51.2) | 0.93 |
| Mental Component Score, median (IQR) | 52.5 (45.8–57.8) | 52.4 (42.9–57.0) | 51.6 (41.5–57.2) | 0.68 | 49.2 (40.4–57.9) | 51.5 (37.9–57.7) | 50.4 (41.2–58.0) | 0.75 |
| Comorbidity (Charlson index),(Katz et al.. 1996) median (IQR) | 0.0 (0.0–2.5) | 1.0 (0.0–2.5) | 1.0 (0.0–2.0) | 0.92 | 2.0 (1.0–4.0) | 1.0 (0.8–3.0) | 1.0 (0.0–6.0) | 0.78 |
| History of prior NMSC | 57% | 43% | 48% | 0.04 | 55% | 58% | 48% | 0.17 |
| History of organ transplantation | 1% | 4% | 4% | 0.27 | 1% | 2% | 3% | 0.43 |
| History of HIV | 8% | 4% | 2% | 0.04 | 1% | 1% | 1% | 0.96 |
| Number of NMSCs at presentation, mean ± Standard Deviation | 1.4 ± 0.8 | 1.2 ± 0.6 | 1.4 ± 0.9 | 0.01 | 1.2 ± 0.5 | 1.2 ± 0.6 | 1.4 ± 1.0 | 0.41 |
| TUMOR CHARACTERISTICS | n=229 | n=247 | n=381 | n=132 | n=324 | n=175 | ||
| Histological type, basal cell carcinoma | 81% | 74% | 78% | <0.001 | 86% | 65% | 75% | <0.001 |
| Histopathological risk factor for recurrence(NCCN, 2011) | 7% | 27% | 32% | <0.001 | 2% | 21% | 18% | <0.001 |
| Tumor diameter, mm, median (IQR) | 9.0 (6.0–12.0) | 8.0 (5.2–12.0) | 8.0 (5.0–11.0) | 0.04 | 8.0 (5.0–14.2) | 10.0 (6.0–15.0) | 7.0 (5.0–10.5) | <0.001 |
| Tumor present in the `H-zone' of the face | 6% | 14% | 58% | <0.001 | 18% | 35% | 79% | <0.001 |
| CARE CHARACTERISTICS | ||||||||
| Training level of treating clinician | ||||||||
| Attending physician | 91% | 90% | 98% | <0.001 | 4% | 9% | 97% | <0.001 |
| Resident physician | 9% | 10% | 2% | 75% | 90% | 3% | ||
| Nurse practitioner | NA | NA | NA | 20% | 1% | 0% | ||
| Annual visits to dermatology over follow-up period, median (IQR) | 1.5 (0.5–2.4) | 0.9 (0.3–2.2) | 1.1 (0.4–2.6) | 0.02 | 1.9 (1–3.1) | 2.1 (1.1–3.3) | 2.0 (0.8–3.0) | 0.62 |
Data were missing at the university site/VA site for the following number of patients or tumors: skin type 300/158, health status 319/187, comorbidity 297/147, tumor diameter 110/103, H-zone 0/1, training level of clinician 11/25.
P value refers to comparison among the treatment groups.
Size of excisional margins was available for 289 tumors (50.6% of excised tumors); the median margin size was 3.0 mm (IQR 3.0 –.0). Tumor remained at the margins of the excised specimen for 29 tumors (5.1%). Of these tumors, 2 were re-treated with destruction, 11 with additional excisions, 7 with Mohs surgery, and 9 tumors were not re-treated.
Follow-up
The overall median follow-up time after treatment was 7.4 years (3.0–8.8); follow-up duration did not differ (P=0.16) among treatment groups. 652 patients were alive in December 2011 [median follow-up time, 8.5 (7.3, 9.2) years] and 522 had died [median follow-up time, 3.9 (1.6, 7.0) years].
328 patients (41.6% of those alive) with 396 tumors (40.4%) consented to examinations by the study dermatologist. Examined patients and tumors were similar (all P-values>0.1) to those not examined in almost all features, but examined patients had better mental health status (median Mental Component Scores 53.7 vs. 50.0, P=0.002]. Recurrence was suspected in 35 (9%) examined tumor locations. Based on subsequent medical record review, 24 were determined not to be recurrent, 8 were verified to be recurrent, none was probably recurrent, and for 3 tumors, recurrence was uncertain (for these tumors, the study dermatologist had judged the likelihood of recurrence as <20% for two tumors, and 21–40% for one tumor).
Tumor Recurrence
Fifty tumors recurred, and three tumors probably recurred. Overall, the unadjusted 5-year recurrence rate was 3.3% [95% CI 2.3, 4.4]. Unadjusted 5-year recurrence rates did not differ significantly (P=0.26) among treatments: 4.9% [2.3, 7.4]after destruction, 3.5% [1.8, 5.2] after excision, and 2.1% [0.6, 3.5], after Mohs surgery.
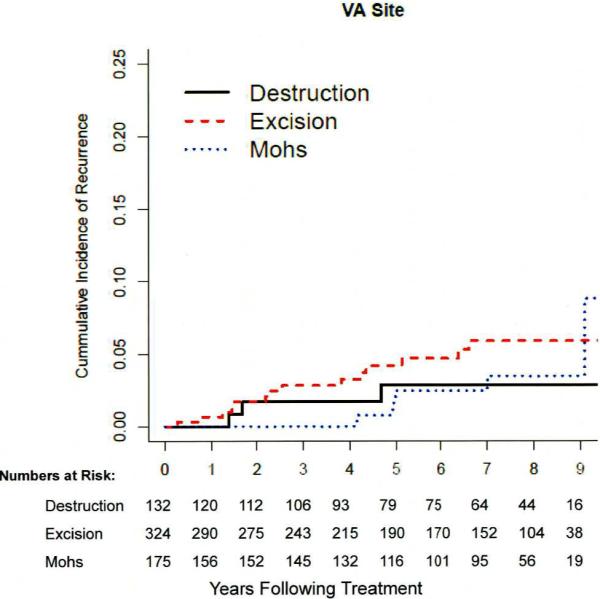
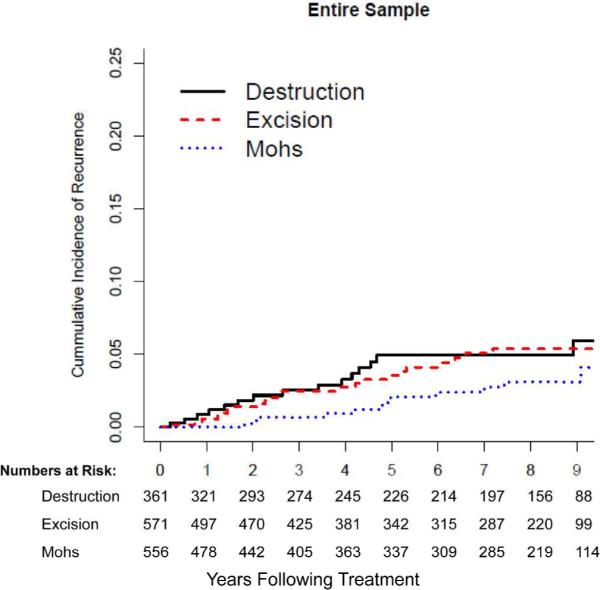
The median time of detection of recurrence was 3.9 years (1.8–5.3), and did not differ (P>0.11) at the two clinical sites or among treatment groups. Figure 2 depicts the cumulative incidence of recurrence in the treatment groups.
Figure 2a, b, c.
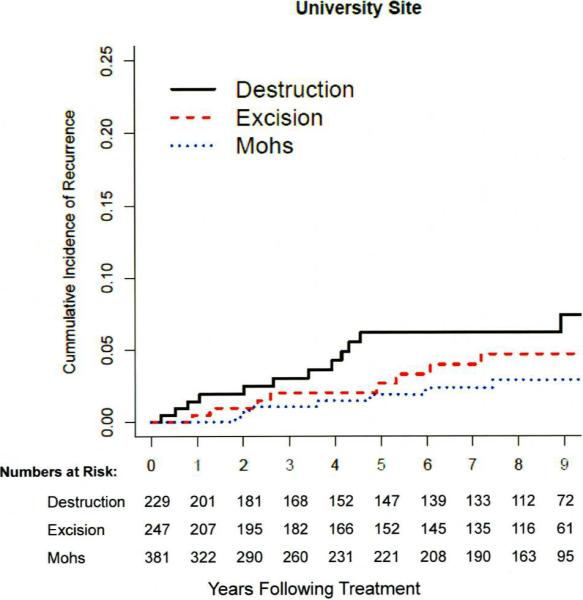
a). Cumulative incidence of recurrence among 667 patients with857 NMSCs at the university site. No statistically significant difference (P=0.09) detected in tumors treated with destruction, excision, or Mohs surgery.
b). Cumulative incidence of recurrence among 507 patients with 631 NMSCs at the VA site. No statistically significant difference (P=0.56) detected in tumors treated with destruction, excision, or Mohs surgery.
c). Cumulative incidence of recurrence among 1174 patients with 1488 NMSCs in the entire sample. No statistically significant difference (P=0.26) detected in tumors treated with destruction, excision, or Mohs surgery.
The median surgical margin size for excised tumors that recurred was 3.0 mm (3.0-3.0) and for excised tumors that did not recur was 3.0 mm (IQR 3.0–4.0). One of the recurrences occurred after an incomplete excision; this tumor had been subsequently treated with a second excision.
Table 2 reports 5-year recurrence rates in clinical subgroups. Few characteristics were significantly related to tumor recurrence, including characteristics conventionally considered high-risk.(NCCN, 2011) Overall, tumors in patients with a history of HIV, in those with multiple NMSCs at presentation, and in those who visited dermatology more often were more likely (P<0.01) to recur.
Table 2.
Mean NMSC recurrence rates five years after treatment in clinical subgroups
| Characteristic | Recurrence rate [95% CI]1 | |||
|---|---|---|---|---|
| PATIENT CHARACTERISTICS | University Site n=857 | VA Site n=631 | Entire Sample n=1488 | |
| Age, years | < median of 69 years | 3.8% [1.9, 5.7] | 4.2% [1.3, 7.0] | 3.9% [2.3, 5.5] |
| ≥ median of 69 years | 2.4% [0.5, 4.4] | 2.8% [1.0, 4.7] | 2.7% [1.3, 4.0] | |
| Gender | Female | 3.2% [1.0, 5.3] | 0.0% [0.0, 20.6] | 3.0% [0.9, 5.0] |
| Male | 3.4 % [1.5, 5.2] | 3.5% [1.9, 5.2] | 3.5% [2.2, 4.7] | |
| Skin Type(Fitzpatrick, 1988) | Type I or II | 4.4% [1.2, 7.6] | 3.9% [0.5, 7.3] | 4.2% [1.8, 6.5] |
| Type III – VI | 3.5% [0.9, 6.0] | 3.5% [1.1, 5.9] | 3.5% [1.7, 5.2] | |
| Health Status (SF-12) | ||||
| Physical Component Score | < median of 48.7 | 5.3% [1.4, 9.2] | 2.3% [0.3, 4.4] | 3.5% [1.5, 5.4] |
| ≥ median of 48.7 | 2.8% [0.6, 5.0] | 4.4% [0.5, 8.1] | 3.4% [1.4, 5.3] | |
| Mental Component Score | < median of 51.5 | 3.4% [0.4, 6.2] | 5.3% [1.6, 8.9] | 4.3% [2.0, 6.6] |
| ≥ median of 51.5 | 4.2% [1.3, 7.0] | 1.1% [0, 2.6] | 2.7% [1.0, 4.4] | |
| Comorbidity (Charlson index) | < median of 1 | 2.4% [0.0, 4.7] | 6.1% [1.2, 10.7] | 3.8% [1.4, 6.0] |
| ≥ median of 1 | 5.0% [1.9, 8.1] | 2.5% [0.6, 4.4] | 3.6% [1.9, 5.2] | |
| History of prior NMSC | No history | 2.0% [0.4, 3.6] 3 | 2.6% [0.3, 4.8] | 2.3% [0.9, 3.6] 2 |
| History | 4.5% [2.2, 6.8] | 4.0% [1.7, 6.2] | 4.3% [2.7, 5.8] | |
| History of organ transplantation | No history | 3.4% [2.0, 4.9] | 3.5% [1.8, 5.2] | 3.5% [2.4, 4.6] |
| History | 0.0% [0.0, 12.8] | 0.0% [0.0, 23.2] | 0.0% [0.0, 8.6] | |
| History of HIV | No history | 2.5% [1.2, 3.8] 4 | 3.1% [1.5, 4.6] 4 | 2.8% [1.8, 3.7] 4 |
| History | 19.6%[4.0, 32.7] | 27.1%[0.0, 53.2] | 20.8%[6.6, 32.9] | |
| Number of NMSCs at presentation: | ≤ median of 1 | 1.9% [0.6, 3.3] 4 | 2.0% [0.5, 3.4] | 2.0% [1.0, 2.9] 4 |
| > median of 1 | 5.8% [2.7, 8.9] | 5.9% [2.2, 9.4] | 5.9% [3.5, 8.2] | |
| TUMOR CHARACTERISTICS | n=857 | n=631 | n=1488 | |
| Histological Type: | BCC | 3.3% [1.7,4.8] | 3.5% [1.6, 5.4] | 3.4% [2.1, 4.6] |
| SCC | 3.3% [0.4, 6.2] | 3.1% [0.0, 6.0] | 3.2% [1.1, 5.2] | |
| High-risk Histological Features(NCCN,2011): | No high-risk features | 3.9% [2.2, 5.7] | 3.6% [1.8, 5.5] | 3.8% [2.5, 5.0] |
| High-risk features | 1.2% [0.0, 2.9] | 2.3% [0.0, 5.4] | 1.6% [0.0, 3.1] | |
| Tumor Diameter, mm: | < median of 8 mm | 4.0% [1.5, 6.3] | 2.8% [0.3, 5.3] | 3.5% [1.7, 5.3] |
| ≥ median of 8 mm | 2.7% [0.8, 4.6] | 3.3% [1.0, 5.5] | 3.0% [1.5, 4.4] | |
| Tumor present in the `H-zone' of the face: | Not present | 3.7% [1.9, 5.4] | 3.0% [1.0, 5.0] | 3.4% [2.1, 4.8] |
| Present | 2.4% [0.3, 4.5] | 4.0% [1.2, 6.6] | 3.2% [1.5, 4.9] | |
| CARE CHARACTERISTICS | ||||
| Training Level of Treating Clinician: | Attending physician | 3.4% [1.9, 4.8] | 2.2% [0.0, 4.6] | 3.1% [1.8, 4.3] |
| Resident physician | 0.0% [0.0, 9.3] | 4.2% [1.9, 6.5] | 3.8% [1.7, 5.8] | |
| Nurse practitioner | Not applicable | 4.2% [0.0, 11.8] | Not applicable | |
| Treatment of NMSC: | Destruction | 6.2% [2.5, 9.6] 2 | 2.8% [0.0, 6.0] | 4.9% [2.3, 7.4] |
| Excision | 2.6% [0.3, 4.9] | 4.2% [1.7, 6.6] | 3.5% [1.8, 5.2] | |
| Mohs surgery | 1.9% 0.2, 3.5] | 2.4% [0.0, 5.1] | 2.1% [0.6, 3.5] | |
| Number of Annual Visits to Dermatology: | ≤ 2 visits | 2.0% [0.7, 3.3] 4 | 2.3% [0.3, 4.4] | 2.1 % [1.0, 3.2] 4 |
| > 2 visits | 6.6% [2.8, 10.2] | 4.3% [1.9, 6.7] | 5.2% [3.1, 7.2] | |
P values refer to comparisons of subgroups within each column.
0.05 ≤ P ≤ 0.10
0.01 ≤ P< 0.05
P< 0.01
Table 3 contains 5-year recurrence rates after each treatment, in subgroups conventionally considered high-risk.(NCCN, 2011) There were few differences in the recurrence rates; at the university site recurrence was less likely after Mohs surgery for tumors located in the H-zone of the face (P=0.05), and was somewhat more likely after destruction for invasive tumors (P=0.04).
Table 3.
NMSC recurrence rates five years after different treatments, in subgroups of tumors conventionally believed to be high-risk for recurrence
| Tumors at High Risk for Recurrence | 5 year recurrence rates [95% CI] | |||||||
|---|---|---|---|---|---|---|---|---|
| University site | VA site | |||||||
| No. | Destruction | Excision | Mohs surgery | No. | Destruction | Excision | Mohs surgery | |
| Squamous cell carcinoma | 189 | 4.9% [0, 11.4] | 6.1% [0, 12.5] | 0% [0, 4.0] | 177 | 0% [0, 8.5] | 3.1% [0, 6.5] | 4% [0, 11.4] |
| Diameter ≥ 20 mm | 211 | 15.4% [1.9, 27.0] | 0% [0, 7.4] | 1.6% [0, 4.6] | 198 | 0% [0, 16.1] | 3.7% [0, 7.3] | 0% [0, 10.3] |
| Invasive histologically | 577 | 5.1% [0, 10.5] | 1.4% [0, 3.2] | 2.2% [0.3, 4.1] 3 | 499 | 4.6% [0, 9.7] | 3.6% [1.1, 6.1] | 1.8% [0, 4.3] |
| Located in H-zone of face(Swanson etal., 1983) | 269 | 9.5% [0, 25.6] | 6.3% [0, 14.5] | 1.2% [0, 2.8] 2 | 275 | 0% [0.0, 19.5] | 5.8% [0.7, 10.7] | 3% [0, 6.3] |
| High-risk location and size(NCCN, 2011)' 1 | 185 | 0% [0.0, 41.0] | 7.0% [0, 15.9] | 0% [0.0, 3.4] | 175 | 0% [0, 36.9] | 4.8% [0, 10.0] | 3.2% [0, 7.5] |
Tumor located in H-zone of face or hand or genitals; size >=6 mm
P=0.05
P=0.04
The final Cox proportional hazard model adjusted for history of HIV infection, multiple NMSCs at presentation, tumor location in the H-zone of the face, histological subtype and invasiveness, and >2 annual dermatology visits during the follow-up period; for analyses of the entire sample, clinical site was also included. Adjusted 5-year recurrence rates were 2.8% [1.8, 3.8] overall, 3.8% [1.4, 6.1] after destruction, 3.3%[1.6, 4.9] after excision, and 1.7% [0.4, 3.0] after Mohs surgery (P=0.26) (Table S1).
Comparison of recurrence rates after excision or Mohs surgery
The difference in recurrence rates between excision and Mohs surgery was 1.6% [−0.1, 3.0]. There was no statistically significant difference in the hazard of tumor recurrence after Mohs surgery compared to excision in any of the adjusted models (by site, in the overall sample, and in propensity-matched pairs), which are described in Table 4.
Table 4.
Hazard of recurrence after treatment in NMSCs treated with Mohs surgery compared to excision
| Hazard Ratio [95% confidence interval] | ||||
|---|---|---|---|---|
| Sample of tumors treated with excision or Mohs surgery | Propensity-matched pairs3 | |||
| Unadjusted | Adjusted 1 | Propensity-adjusted2 | ||
| University site | 0.66 [0.24, 1.83] | 1.07 [0.37, 3.10] | 0.85 [0.30, 2.42] | 0.70 [0.24, 2.08] |
| VA site | 0.76 [0.30, 1.94] | 0.44 [0.17, 1.19] | 0.52 [0.21, 1.25] | 0.65 [0.24, 1.76] |
| Full sample | 0.65 [0.33, 1.27] | 0.65 [0.32, 1.33] | 0.62 [0.33, 1.20] | 0.61 [0.30, 1.24] |
All models adjusted for history of HIV, multiple NMSCs at presentation, tumor in H-zone of the face, tumor type (BCC vs. SCC), whether the tumor was histopathologically superficial or in situ,> 2 annual visits to dermatology, and in the entire sample, clinical site.
Adjusted by quintile of propensity score for performance of Mohs surgery for each tumor, > 2 annual visits to dermatology, and in the entire sample, clinical site.
Subsample of tumors matched on propensity score; 240 pairs at university site, 162 pairs at VA, 402 pairs at both sites. All models adjusted for > 2 annual visits to dermatology, and in the entire sample, clinical site.
DISCUSSION
In this large prospective cohort study of consecutive primary nonmelanoma skin cancers with excellent long-term follow-up, at least 95% of tumors were cured. Rates of tumor recurrence were similar after different treatments, even after adjustment for conventional risk factors in patients, tumors, or care. Destruction is recommended only for tumors at low risk for recurrence and those in cosmetically less important body locations,(NCCN, 2011) but, in the tumors treated with excision or Mohs surgery, the hazard of recurrence was not significantly different, even in analyses adjusted for propensity for treatment with Mohs surgery.
Comparison with Past Studies
Precise NMSC recurrence rates have not been known.(Bath-Hextall et al., 2004) Automated datasets usually have insufficient information, and comprehensive follow-up requires direct review of outpatient medical records (including progress notes, photographs, diagrams, and dermatopathology records) by experienced clinicians who are blinded to treatment type. Many previous studies have not followed patients for at least five years,(Smeets et al., 2004) nor adjusted for risk factors or differential follow-up using survival analysis techniques.
The recurrence rate after destruction (4.9%) was lower than expected, but the similarity of recurrence rates after excision and Mohs surgery is consistent with the findings of a randomized controlled trial of the treatments for facial BCCs.(Mosterd et al., 2008) Our results expand previous findings, since we prospectively enrolled a large consecutive sample at two sites, adjusted for risk factors and follow-up, and studied 5-year outcomes in practice.
We have previously described recurrence outcomes at only the VA site(Chren et al., 2011) because the electronic records there were more feasible to obtain. The sample size prevented comparisons among treatments, however. At both sites we found recurrence rates to be similarly low in many conventional `high risk' clinical subgroups(NCCN, 2011). Of particular note is the unexplained high recurrence rate in patients with HIV infection;(Chren et al., 2011) similar results were not found in patients who had received organ transplantations, a group at high risk for primary NMSCs.(Euvrard et al., 2003) Finally, recurrence was more common in patients who visited the dermatologist more often, which highlights the importance of adjustments for surveillance bias.
Potential limitations
Although we studied care at two hospitals, the clinicians were from a single dermatology department, which may limit generalizability.
Treatment groups differed substantially and although propensity adjusted analyses failed to detect a difference in recurrence rates, this study was not a randomized trial of treatments. Unmeasured characteristics may have affected the risk of recurrence.
Some patients may have received care at other sites, and, if so, actual recurrence rates may be higher. Over 80% of patients had had a skin examination at the study sites in the year before their final follow up date, however, indicating that the vast majority continued to receive follow up dermatologic care that we could review.
The minimum important difference in recurrence rates between therapies has not been established (Shuster, 1999), although some have acknowledged that a difference in recurrence rates of 4% might be considered clinically unimportant (McGovern and Leffell, 1999). We detected a difference between excision and Mohs surgery of 1.4% [−0.5, 3.5], and suggest that because the confidence limits exclude 4%, our results indicate that the treatments did not differ significantly in preventing recurrence in our practice settings. The overall sample may have been too small to permit detection of differences in select subgroups. For example, in unadjusted analyses at the university site, recurrence was lower after Mohs surgery than excision for tumors located in the H-zone of the face (1.2% vs 6.3%, P=0.05) (Table 3).
Treatment patterns may have changed since this cohort was assembled. Topical agents are now used to treat some histologically superficial NMSCs. Even when these tumors were eliminated, however, we found similar recurrence rates (Table 3). Also, the use of Mohs surgery doubled in the US from 2001 2006,(Viola et al., 2012) which suggests that the availability of and/or accepted indications for Mohs surgery are changing(Shuster, 1999). If Mohs surgery was used for subgroups of NMSCs other than those in which its efficacy was originally described, then our ability to detect its effectiveness in the overall cohort is lessened.
Outcomes other than recurrence are important, particularly for nonfatal tumors.(Feldman et al., 2008; Tinetti and Studenski, 2011) We have previously shown, however, that improvements in tumor-related quality of life (bother from the treatment site, including its appearance) were worse after destruction and similar after excision and Mohs surgery.(Chren et al., 2007) Thus, for both clinical and patient-reported outcomes, surgical excision and Mohs surgery led to similar outcomes.
Conclusions
Tumor recurrence was low after common treatments for nonmelanoma skin cancer. Recurrence rates were similar after excision or Mohs surgery, even after adjustment for conventional risk factors for recurrence. These results from routine clinical care demonstrate that future studies are needed to guide therapeutic choices for different clinical subgroups.
MATERIALS AND METHODS
Design, Setting, Patients, Baseline Data
We performed a prospective cohort study of all patients with NMSC diagnosed in 1999 and 2000 and treated in a university-based dermatology practice or the dermatology clinic of the nearby affiliated VA. This study was approved by the Institutional Review Boards, and when required, patients provided written informed consent. The investigation was conducted according to the Declaration of Helsinki principles.
Details about the study have been described.(Chren et al., 2004) (Chren et al., 2011) Eligible patients were all those with primary NMSC, defined histopathologically as BCC or squamous cell carcinoma (SCC) of the skin. We restricted the sample to tumors treated with the three most common therapies: destruction with electrodessication and curettage, excision, or Mohs surgery.
Most care at the university site was provided by attending dermatologists. Most care at the VA, except Mohs surgery, was provided by dermatology residents supervised by attending physicians. Dermatologic nurse practitioners provided a minority of care at the VA. Excisions and Mohs surgery were usually performed in specifically designated clinics. Mohs surgery at both sites was typically performed by a Mohs surgeon who was a member of the American College of Mohs Micrographic Surgery and Cutaneous Oncology.
For destruction, usually three cycles of electrodessication/curettage were performed. Most excisions were simple excisions, and margins of excised specimens were typically examined histologically in fixed specimens after closure.(Do, 2009)
Collection of Outcome Data
The primary source of data on recurrence was the medical record. At a median of 9.0 years after treatment trained dermatologic nurse practitioners who were blinded to treatment type reviewed all records using structured dataforms. To supplement this review, patients who consented were examined a median of 8.6 years after treatment by a dermatologist (MMC) blinded to treatment type. If any irregularity near the treatment site was noted (the examiner commented on the presence of scaling, papule, erythema, erosion, induration, or cyst like lesion), the patient was referred to his/her dermatologist for assessment.
For all tumors with evidence of recurrence, the entire record was reviewed again by an additional dermatologic clinician blinded to details from the original review, to validate the outcome.
Measures
Primary outcome
A tumor was defined as recurrent if the tumor type (BCC or SCC) and body location were identical to those of the primary tumor, and the lesion was described by the clinician as recurrent or previously treated. A tumor was defined as probably recurrent if the tumor type was the same as that of the primary tumor, and either (i) the body location of the suspected lesion was very close to that of the primary tumor (for example, “distal tip of left nasal ala”) or (ii) the body location of the suspected lesion may have been the same as that of the primary tumor (e.g., “right temple”) and the lesion was described by the clinician as recurrent or previously treated. A tumor was defined as of uncertain recurrence if a suspicious lesion was noted on examination by the study dermatologist, but there was no subsequent clinician evaluation.
The date of recurrence was the date of biopsy of the recurrent lesion.
Additional variables
Health status was measured with the Short Form-12 instrument (SF-12),(Ware et al., 1996) comorbidity by an adapted Charlson instrument,(Charlson et al., 1987; Katz et al., 1996) and photosensitivity (skin type) by an item about sunburn and tanning.(Fitzpatrick, 1988) History of prior NMSC, organ transplantation, and infection with Human Immunodeficiency Virus (HIV) was obtained from the medical record. Tumors were classified according to the presence of histological risk factors for recurrence;(NCCN, 2011) facial tumors were categorized by location in the H-zone of the face, an area believed at higher risk for recurrence.(Swanson et al., 1983) We also counted each patient's visits to dermatology throughout follow-up.
For each tumor, follow-up ended at the last date when the patient received care. A patient was lost to follow up if there was no record of care after treatment.
Analytic Strategy
Statistical analyses were performed using R version 2.13. We defined recurrent tumors as those classified as recurrent or probably recurrent. Analyses were repeated with two alternative classifications: (i) recurrent tumors were only those classified as recurrent, and (ii) recurrent tumors were those classified as recurrent, probably recurrent, or uncertain. The conclusions were not substantively different with the alternative classifications, and are not reported. Because of differences in patients and tumors at the two clinical sites, we performed both site stratified and combined analyses. Where results are reported in the entire sample, the conclusions did not differ at the two clinical sites, except as noted.
We compared treatment groups using chi-square tests for categorical characteristics, and non parametric analysis of variance (Kruskal Wallis test) for continuous characteristics. The Huber-White method was used to adjust for patients with more than one tumor. Cumulative incidence of tumor recurrence over time was displayed using Kaplan-Meier plots. Data were right-censored at the last date of care. We determined unadjusted 5-year recurrence rates in the entire sample, in important clinical subgroups, and after each treatment in subgroups of tumors conventionally believed to be high-risk for recurrence, using the Kaplan-Meier method. The high-risk subgroups were tumors that were ≥ 20 millimeters in diameter, invasive histologically, located in the H-zone of the face,(Swanson et al., 1983) or considered high-risk by location and size.(NCCN, 2011) Exact binomial Confidence Intervals (CI) were used in cases where the recurrence rate was 0%.
We next performed a series of Cox proportional hazard models to calculate 5-year recurrence rates and hazard ratios, after adjustment for characteristics likely related to recurrence. The R function cox.zph was applied on all multivariate models to confirm that the proportionality assumption was met. Models were developed in the tumors treated with excision or Mohs surgery, because destruction is recommended only for low-risk tumors,(NCCN, 2011) and those treated with destruction were different in many respects from the other tumors. First we fit a large model with all potential predictors of recurrence. These variables included age, gender, history of prior NMSC, infection with HIV, multiple NMSCs (>1) at study presentation, tumor type (BCC vs. SCC), whether the tumor was histopathologically superficial or in situ, presence of histological risk factors for recurrence,(NCCN, 2011) tumor location in the H-zone of the face,(Swansonet al., 1983) tumor diameter > 10 mm, and > 2 annual dermatology visits. Because of the limited number of recurrences, we also obtained a more parsimonious set of independent variables by forcing treatment type and tumor type into the model with all the potential variables listed above and applying a forward stepwise selection based on AIC (Akaike Information Criterion). The results from both sets of models were similar, and only results from the parsimonious models are presented.
We also applied propensity score methods to adjust for measured differences between patients and tumors that might relate to choice of Mohs surgery vs. excision.(D'Agostino, 1998; Rubin, 1997) At each clinical site a propensity score for Mohs surgery was calculated for each tumor, using a logistic regression model for the performance of Mohs surgery. The model included age, gender, infection with HIV, history of prior NMSC, multiple NMSCs at presentation, tumor type, whether the tumor was histopathologically superficial or in situ, presence of histopathological risk factors for recurrence (NCCN, 2011), tumor location in the H-zone of the face,(Swanson et al., 1983) and whether the treatment choice was made by an attending physician, resident physician, or nurse practitioner. We then used quintiles of propensity as independent variables in Cox regression analyses of recurrence. We also modeled recurrence in subsamples of pairs of tumors matched on propensity score, using the R function match in the Matching package.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health (R01 AR 054983, K02 AR02203, and K24 AR052667), and by Investigator-Initiated Research Grants (IIRs 97010-2 and 04-043-3) and the Health Services Research Enhancement Award Program (REAP) of the Health Services Research and Development Service of the Department of Veterans Affairs. The funders had no role in the design and conduct of the study, in the collection, management, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the funders.
Dr. Chren had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Abbreviations used
- BCC
basal cell carcinoma
- CI
confidence interval
- HIV
Human Immunodeficiency Virus
- IQR
interquartile range
- NMSC
nonmelanoma skin cancer
- SCC
squamous cell carcinoma
- SF-12
Short Form-12 instrument of the Medical Outcomes Study
- US
United States
- VA
Veterans Affairs Medical Center
Footnotes
CONFLICT OF INTEREST Dr. Chren is a consultant for Genentech, Inc., about measurement of patient-reported outcomes.
Specific contributions of each author are: CHREN: conception and design, acquisition of data, analysis and interpretation of data, drafting of manuscript, critical revision of manuscript for important intellectual content, statistical analysis, obtaining funding, supervision.
LINOS: interpretation of data, critical revision of manuscript for important intellectual content
TORRES: acquisition of data, critical revision of manuscript for important intellectual content, administrative support, supervision
STUART: acquisition of data, critical revision of manuscript for important intellectual content, administrative support, supervision.
PARVATANENI: analysis and interpretation of data, drafting of manuscript, critical revision of manuscript for important intellectual content, statistical analysis, technical support.
BOSCARDIN: analysis and interpretation of data, critical revision of manuscript for important intellectual content, statistical analysis, supervision.
References
- Bath-Hextall F, Bong J, Perkins W, et al. Interventions for basal cell carcinoma of the skin: systematic review. Bmj. 2004;329:705. doi: 10.1136/bmj.38219.515266.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlson ME, Pompei PP, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Chren MM, Sahay AP, Sands LP, et al. Variation in care for nonmelanoma skin cancer in a private practice and a Veterans Affairs clinic. Med Care. 2004;42:1019–26. doi: 10.1097/00005650-200410000-00011. [DOI] [PubMed] [Google Scholar]
- Chren MM, Sahay AP, Bertenthal DS, et al. Quality-of-life outcomes of treatments for cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2007;127:1351–7. doi: 10.1038/sj.jid.5700740. [DOI] [PubMed] [Google Scholar]
- Chren MM, Sahay AP, Sands LP, et al. Variation in care for nonmelanoma skin cancer in a private practice and a veterans affairs clinic. Med Care. 2004;42:1019–26. doi: 10.1097/00005650-200410000-00011. [DOI] [PubMed] [Google Scholar]
- Chren MM, Torres JS, Stuart SE, et al. Recurrence after treatment of nonmelanoma skin cancer: a prospective cohort study. Arch Dermatol. 2011;147:540–6. doi: 10.1001/archdermatol.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agostino RB., Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–81. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Do D. Mohs micrographic surgery for Basal cell carcinoma of the face. Arch Dermatol. 2009;145:1428–30. doi: 10.1001/archdermatol.2009.315. [DOI] [PubMed] [Google Scholar]
- Essers BA, Dirksen CD, Nieman FH, et al. Cost-effectiveness of Mohs Micrographic Surgery vs Surgical Excision for Basal Cell Carcinoma of the Face. Arch Dermatol. 2006;142:187–94. doi: 10.1001/archderm.142.2.187. [DOI] [PubMed] [Google Scholar]
- Euvrard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. N Engl J Med. 2003;348:1681–91. doi: 10.1056/NEJMra022137. [DOI] [PubMed] [Google Scholar]
- Feldman S, Pearce DJ, Williford PM. Surgical decision making for basal-cell carcinoma of the face. Lancet Oncol. 2008;9:1119–20. doi: 10.1016/S1470-2045(08)70292-4. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869–71. doi: 10.1001/archderm.124.6.869. [DOI] [PubMed] [Google Scholar]
- Katz JN, Chang LC, Sangha O, et al. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34:73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- McGovern TW, Leffell DJ. Mohs surgery: the informed view. Arch Dermatol. 1999;135:1255–9. doi: 10.1001/archderm.135.10.1255. [DOI] [PubMed] [Google Scholar]
- Mosterd K, Krekels GA, Nieman FH, et al. Surgical excision versus Mohs' micrographic surgery for primary and recurrent basal-cell carcinoma of the face: a prospective randomised controlled trial with 5-years' follow-up. Lancet Oncol. 2008;9:1149–56. doi: 10.1016/S1470-2045(08)70260-2. [DOI] [PubMed] [Google Scholar]
- NCCN . National Comprehensive Cancer Network. The complete library of NCCN linical Practice Guidelines in Oncology. version 1. 2011. Basal cell and squamous cell skin cancers. 2012. [Google Scholar]
- Otley CC. Mohs' micrographic surgery for basal-cell carcinoma of the face. Lancet. 2005;365:1226–7. doi: 10.1016/S0140-6736(05)74804-2. author reply 7. [DOI] [PubMed] [Google Scholar]
- Otley CC. Cost-effectiveness of Mohs micrographic surgery vs surgical excision for basal cell carcinoma of the face. Arch Dermatol. 2006;142:1235. doi: 10.1001/archderm.142.9.1235-a. author reply -6. [DOI] [PubMed] [Google Scholar]
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283–7. doi: 10.1001/archdermatol.2010.19. [DOI] [PubMed] [Google Scholar]
- Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127:757–63. doi: 10.7326/0003-4819-127-8_part_2-199710151-00064. [DOI] [PubMed] [Google Scholar]
- Shuster S. The case against micrographically controlled skin surgery. Acta Derm Venereol. 1999;79:2–3. [PubMed] [Google Scholar]
- Smeets NW, Kuijpers DI, Nelemans P, et al. Mohs' micrographic surgery for treatment of basal cell carcinoma of the face--results of a retrospective study and review of the literature. Br J Dermatol. 2004;151:141–7. doi: 10.1111/j.1365-2133.2004.06047.x. [DOI] [PubMed] [Google Scholar]
- Swanson NA, Grekin RC, Baker SR. Mohs surgery: techniques, indications, and applications in head and neck surgery. Head Neck Surg. 1983;6:683–92. doi: 10.1002/hed.2890060209. [DOI] [PubMed] [Google Scholar]
- Tinetti ME, Studenski SA. Comparative effectiveness research and patients with multiple chronic conditions. N Engl J Med. 2011;364:2478–81. doi: 10.1056/NEJMp1100535. [DOI] [PubMed] [Google Scholar]
- Viola KV, Jhaveri MB, Soulos PR, et al. Mohs Micrographic Surgery and Surgical Excision for Nonmelanoma Skin Cancer Treatment in the Medicare Population. Arch Dermatol. 2012 doi: 10.1001/archdermatol.2011.2456. in press. [DOI] [PubMed] [Google Scholar]
- Ware J, Jr., Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Williford PM, Feldman SR. Surgery for basal-cell carcinoma of the face. Lancet. 2004;364:1732–3. doi: 10.1016/S0140-6736(04)17411-4. [DOI] [PubMed] [Google Scholar]
- Wilson LS, Pregenzer M, Basu R, et al. Fee Comparisons of Treatments for Nonmelanoma Skin Cancer in a Private Practice Academic Setting. Dermatol Surg. 2011 doi: 10.1111/j.1524-4725.2011.02231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.