Abstract
Selenocysteine (Sec) is translationally incorporated into proteins in response to the UGA codon. The tRNA specific to Sec (tRNASec) is first ligated with serine by seryl-tRNA synthetase (SerRS). In the present study, we determined the 3.1 Å crystal structure of the tRNASec from the bacterium Aquifex aeolicus, in complex with the heterologous SerRS from the archaeon Methanopyrus kandleri. The bacterial tRNASec assumes the L-shaped structure, from which the long extra arm protrudes. Although the D-arm conformation and the extra-arm orientation are similar to those of eukaryal/archaeal tRNASecs, A. aeolicus tRNASec has unique base triples, G14:C21:U8 and C15:G20a:G48, which occupy the positions corresponding to the U8:A14 and R15:Y48 tertiary base pairs of canonical tRNAs. Methanopyrus kandleri SerRS exhibited serine ligation activity toward A. aeolicus tRNASec in vitro. The SerRS N-terminal domain interacts with the extra-arm stem and the outer corner of tRNASec. Similar interactions exist in the reported tRNASer and SerRS complex structure from the bacterium Thermus thermophilus. Although the catalytic C-terminal domain of M. kandleri SerRS lacks interactions with A. aeolicus tRNASec in the present complex structure, the conformational flexibility of SerRS is likely to allow the CCA terminal region of tRNASec to enter the SerRS catalytic site.
INTRODUCTION
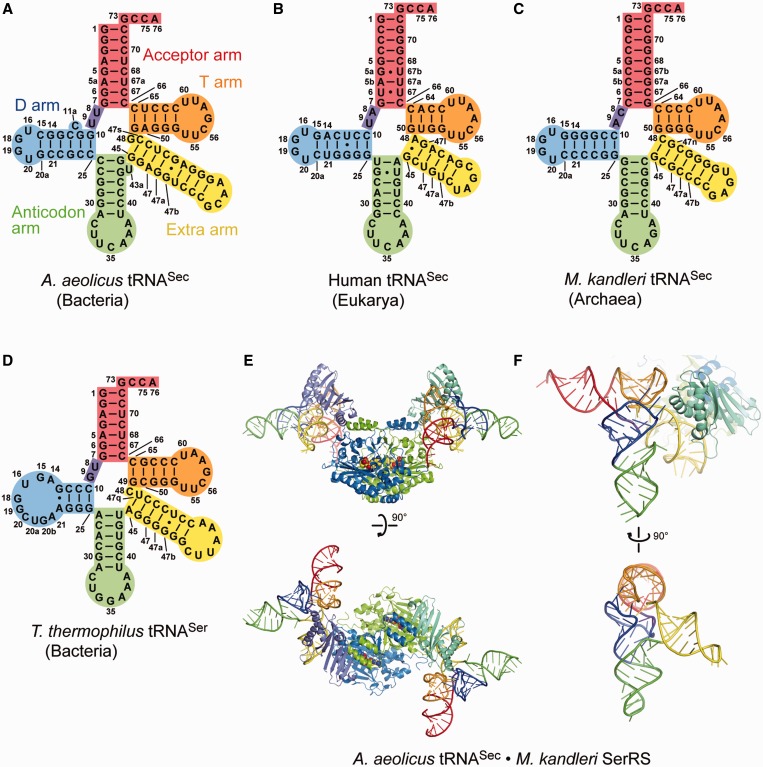
The twenty-first amino acid, selenocysteine (Sec), contains selenium and is translationally incorporated into proteins in the three domains of life, Bacteria, Eukarya and Archaea (1). The Sec-specific tRNA (tRNASec) is the longest tRNA, and its anticodon is complementary to the stop codon UGA (2). The secondary structures of the tRNASec species from Bacteria and Eukarya/Archaea are different from the canonical structure, and even from each other (3–5). The sequence-based cloverleaf model of the bacterial tRNASec secondary structure revealed that the acceptor and T arms have 8- and 5-bp stems, respectively. This ‘8 + 5’ secondary structure differs not only from the ‘7 + 5’ secondary structure of the canonical tRNAs, but also from the ‘9 + 4’ secondary structure of eukaryal/archaeal tRNASecs (Figure 1A–D). The D arm of tRNASec has a 6-bp stem and a four-nucleotide loop, in contrast to the 3–4-bp D stem and 7–12-nucleotide D loop of the canonical tRNA. In addition, both the bacterial and eukaryal/archaeal tRNASecs have a remarkably long extra arm, which is even longer than those of the type-2 tRNAs, such as tRNASer and tRNALeu. The extra arms of the eukaryal/archaeal and bacterial tRNASers have 4–5- and 5–7-bp stems, respectively, while those of the tRNASecs have 6–7- and 6–9-bp stems, respectively (4). In each organism, the extra arm of tRNASec is always longer than that of tRNASer.
Figure 1.
Overall structures. (A–D) The structure-based cloverleaf models of A. aeolicus tRNASec (A), human tRNASec (B), M. kandleri tRNASec (C) and T. thermophilus tRNASer (D). (E and F) Overall structures of A. aeolicus tRNASec in complex with M. kandleri SerRS. M. kandleri SerRS and its ligand Ser-SA are shown as a ribbon diagram and a sphere model, respectively. The acceptor arm, AD linker, D arm, anticodon arm, extra arm and T arm are colored red, blue-violet, blue, lime, yellow and orange, respectively. The SerRS structure is hidden in the bottom panel of (F).
Sec is synthesized on tRNASec through multiple steps. First, tRNASec is ligated with serine by seryl-tRNA synthetase (SerRS) (2). In Eukarya/Archaea, the Ser moiety of Ser-tRNASec is phosphorylated by O-phosphoseryl-tRNASec kinase (PSTK) and is then converted to Sec by the selenocysteine synthase SepSecS (6,7). By contrast, the bacterial selenocysteine synthase SelA directly converts the Ser moiety of Ser-tRNASec to Sec (8). SerRS is naturally responsible for serine ligation to tRNASer, which has the canonical ‘7 + 5’ secondary structure and the characteristic extra arm. The crystal structure of the SerRS•tRNASer complex from the bacterium Thermus thermophilus revealed that the N-terminal helical domain of SerRS recognizes the extra arm of tRNASer (9). In addition, the long extra arm of tRNASec is reportedly important for serine ligation by SerRS.
We previously reported the crystal structures of human tRNASec and the archaeal tRNASec•PSTK complex (10,11), and those of the human tRNASec•SepSecS complex (12), mouse tRNASec (13) and another archaeal tRNASec•PSTK complex have also been solved (14). These studies confirmed the ‘9 + 4’ secondary structure, and revealed the characteristic features of their tertiary cores. In contrast, the bacterial tRNASec structure, which putatively adopts the ‘8 + 5’ secondary structure, has not been elucidated.
In this study, we solved the crystal structure of the bacterial tRNASec from Aquifex aeolicus, in complex with the heterologous SerRS from the archaeon Methanopyrus kandleri, and confirmed the ‘8 + 5’ secondary structure of bacterial tRNASec. Moreover, the tertiary core structure of the bacterial tRNASec is distinctly different from those of its eukaryal and archaeal counterparts. On the other hand, the long extra arm of tRNASec is recognized by M. kandleri SerRS in a similar manner to that of T. thermophilus tRNASer by its homologous SerRS, even though the N-terminal domain of M. kandleri SerRS adopts a fold characteristic of the SerRSs from methanogenic archaea, and thus is distinct from those of the canonical SerRSs (15,16).
MATERIALS AND METHODS
Protein and tRNA preparation
Aquifex aeolicus tRNASec, M. kandleri tRNASec and Archaeoglobus fulgidus tRNACys were prepared by in vitro transcription, as described (11,17,18). The M. kandleri SerRS gene was cloned into the pET26b vector (Novagen). The M. kandleri SerRS protein with 11 mutations, R55Y-E58Y-E62Y-R116Y-D118Y-E189R-D193R-E379Y-E383R-E497Y-E499Y, which targeted the protein surface to reinforce crystal packing, was used for crystallization. The native and selenomethionine (SeMet)-substituted M. kandleri SerRS mutant proteins were overexpressed and purified, as described (17). Furthermore, lysine methylation of SerRS was also performed to improve the crystallization efficiency (17).
Crystal structure determination
The complex of M. kandleri SerRS and A. aeolicus tRNASec was prepared by mixing the components at final concentrations of 80 and 120 μM, respectively, in 20 mM Tris–HCl buffer (pH 7.5), containing 400 mM NaCl, 10 mM MgCl2, 3 mM 2-ME and 500 μM 5′-O-[N-(l-seryl)sulfamoyl]adenosine (Ser-SA, a non-hydrolyzable analog of seryl-adenylate). Crystallization was performed at 20°C by the sitting-drop vapor diffusion method, and the phase was determined by a combination of the molecular replacement and multi-wavelength anomalous dispersion methods, using a platinum-labeled crystal (17). Labeling was performed by soaking for 10–15 h in harvest buffer containing 20 mM K2Pt(CN)4 (17). In the first phase determination step, we obtained the preliminary phase by molecular replacement, using the C-terminal core domain of the reported structure of Methanosarcina barkeri SerRS [PDB ID: 2CJ9 (19)]. The phase information was used for detecting the heavy atom sites for subsequent experimental phasing. In the later step, we placed the N-terminal domain of M. barkeri SerRS, based on the FO map generated by the experimental phasing. Model building began with the modification of the placed N- and C-terminal domains (17). The final data used for structure refinement were collected from the platinum-labeled crystal, using the SerRS with 11 mutations, Lys methylation and SeMet substitution (Table 1), while the data used for phase determination were collected from a different platinum-labeled crystal without SeMet substitution (17). The structures were refined against the diffraction data with the CNS (21) and Phenix (22) programs, with iterative cycles of positional and temperature-factor refinements (Table 1). The Coot (23) and CueMol programs were used for manual fitting of the models to the electron density map.
Table 1.
Data collection and refinement statistics
| Data collection | |
| Beam line | Photon Factory BL5A |
| Wavelength (Å) | 0.97848 |
| Space group | I432 |
| Cell parameters | a = b = c = 272.8 Å |
| Resolution (Å) | 50.0–3.10 (3.21–3.10)a |
| Unique reflections | 31 714 (3126)a |
| Completeness (%) | 99.9 (100.0)a |
| Redundancy | 15.0 (14.6)a |
| Rsymb | 0.094 (0.847)a |
| I/σ(I) | 28.4 (3.49)a |
| Structure refinement | |
| Working-set reflections | 29 484 |
| Test-set reflections | 1556 |
| Resolution (Å) | 50.0–3.10 |
| Number of SerRS subunits | 1 (one half of dimer) |
| Number of RNA molecules | 1 |
| Number of protein atoms | 4363 |
| Number of RNA atoms | 2090 |
| Number of ligands | 1 (Ser-SA) |
| Number of ions | 1 (Zn2+), 8 (sulfate), 15 (Pt2+) |
| Number of solvent molecules | 0 |
| Rwork/Rfreec | 0.204/0.260 |
| Average B factor (Å2) | |
| Overall | 128.4 |
| Protein | 112.0 |
| RNA | 163.0 |
| Ligand | 71.4 |
| Ion | 152.2 |
| RMSD bond lengths (Å) | 0.009 |
| RMSD bond angles (°) | 1.25 |
| Ramachandran-plot analysisd | |
| Most favored regions (%) | 95.2 |
| Additional allowed regions (%) | 4.8 |
| Generously allowed regions (%) | 0.0 |
| Disallowed regions (%) | 0.0 |
aThe statistics in the highest-resolution shell are given in parentheses.
bRsym = ∑hkl∑i[|Ii(hkl) − <I(hkl) > |]/∑hkl∑i[Ii(hkl)], where Ii(hkl) is the intensity of the ith measurement of hkl and <I(hkl)> is the average value of Ii(hkl) for all ith measurements.
cRwork, free = ∑hkl(||Fobs|−k|Fcalc||)/∑hkl(|Fobs|), where Rwork and Rfree are calculated using the working-set and test-set reflections (5% of the total reflections), respectively.
dProcheck (20) was used for Ramachandran-plot analysis.
Aminoacylation assay
The serine-ligation activities of the A. aeolicus and M. kandleri SerRSs were measured, using [14C]-L-serine (Moravek). The reaction was performed at 60°C, in 50 mM Hepes-NaOH buffer (pH 7.5), containing 100 mM KCl, 20 mM MgCl2, 2.0 mM dithiothreitol, 4.0 mM ATP, 0.1 mg/ml bovine serum albumin and 100 μM [14C]-l-serine (5.96 GBq/mol). The time course of the reaction was monitored by removing aliquots at 1, 2 and 4 min, while the initial reaction rates to calculate the KM and kcat values were measured by removing aliquots at 30 s. The reaction was quenched by transferring each aliquot to a filter paper pre-equilibrated with 5% trichloroacetic acid (TCA), and the radioactivities of the acid-insoluble fractions were quantified by liquid scintillation counting, after the filters were washed three times with 5% ice-cold TCA and twice with 100% ethanol.
RESULTS AND DISCUSSION
Structure determination
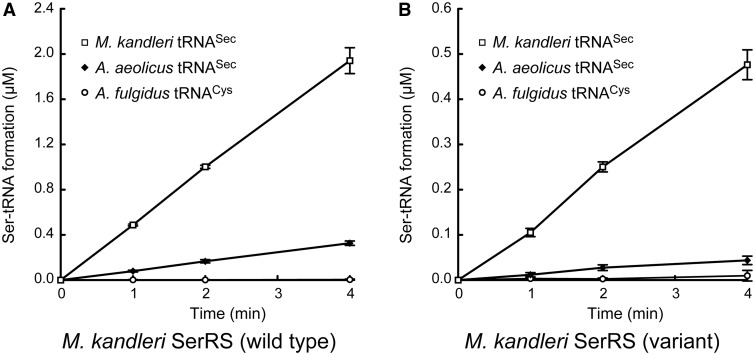
To elucidate the tertiary structure of bacterial tRNASec, for comparison to the eukaryal and archaeal tRNASec species, we attempted to crystallize various bacterial tRNASec species, and obtained crystals of A.aeolicus tRNASec. However, these crystals diffracted X-rays to only 5.5 Å resolution. Because many tRNA structures have been determined in the protein-bound forms, we tested the complex of A. aeolicus tRNASec with A. aeolicus SerRS, but the crystals still only diffracted to 8 Å resolution. On the other hand, we successfully determined the 3.1 Å resolution crystal structure of A. aeolicus tRNASec in its heterologous complex with M. kandleri SerRS (Figure 1E and F). In the process of resolution improvement, we introduced 11 mutations, R55Y-E58Y-E62Y-R116Y-D118Y-E189R-D193R-E379Y-E383R-E497Y-E499Y, methylated the lysine residues and substituted SeMet for Met, as only 5.7 Å resolution data were obtained using the wild-type SerRS without Lys methylation or SeMet substitution (17). The crystals containing the wild-type and variant SerRSs are isomorphic (17), indicating that the overall structures of tRNASec and SerRS and their contact surfaces in the crystals are the same. The mutated sites are located within non-conserved regions, but not in the tRNASec contact surface.Furthermore, the variant SerRS retained the serine-ligation activity toward the M. kandleri and A. aeolicus tRNASec s (Figure 2). The following structure descriptions are exclusively about the crystal structure using the variant SerRS, unless otherwise noted. The asymmetric unit contains one tRNASec molecule and one subunit of the SerRS homodimer. Aquifex aeolicus tRNASec consists 99 nucleotides (nucleotides 1–76 in Figure 1A). G73 and the 3′-CCA region in the acceptor arm are partially disordered, and the final model lacks the 3′-terminal A76.
Figure 2.
Seine-ligation activity. Activities of 100 nM wild-type (A) and variant (B) M. kandleri SerRSs toward 10 μM tRNASec (M. kandleri or A.aeolicus). The variant was the SerRS with the 11 mutations, Lys methylation and SeMet subutitution, used for crystallization. A.fulgidus tRNACys (10 μM) was used for estimating the background serine-ligation level. Each experiment was performed in quadruplicate, and the values were averaged. The error bars show standard deviations.
Overall structure
The tertiary structure of A. aeolicus tRNASec confirmed the putative ‘8 + 5’ secondary structure. Aquifex aeolicus tRNASec forms an L shape with the acceptor, T, D and anticodon arms, from which the long extra arm protrudes (Figure 3A and E). The acceptor stem consists of 8 bp, 1:72, 2:71, 3:70, 4:69, 5:68, 5a:67a, 6:67 and 7:66. The T stem includes 5 bp between nucleotides 49–53 and 61–65. The D stem is composed of 6 bp formed between nucleotides 10–15 and 25–20a, with the bulge of C11a, while the D loop has four nucleotides, 16, 18, 19 and 20 (nucleotide 17 is missing). As C26 and G44 are paired, the anticodon stem has 6 bp, from 26:44 to 31:39, with the bulge of U43a. The extra arm (nucleotides 45–47, 47a–47s and 48) of A. aeolicus tRNASec possesses a 9-bp stem and the linker G48. The length of the extra-arm stem (or the extra stem) varies from 6 to 9 bp among the bacterial tRNASecs (4). The extra-arm orientation is similar to those of the eukaryal/archaeal tRNASecs and even to that of tRNASer (Figure 3). In addition, tRNASec has U8 and U9 as the linker connecting the acceptor stem and the D stem (‘AD linker’). The bulged nucleotides, C11a and U43a, are completely flipped out from the D and anticodon stems, respectively. In this context, bulged nucleotides are frequently observed at or near the two positions in the cloverleaf models of bacterial tRNASecs (4), and they might fine-tune the twist angles of the D and anticodon stems.
Figure 3.
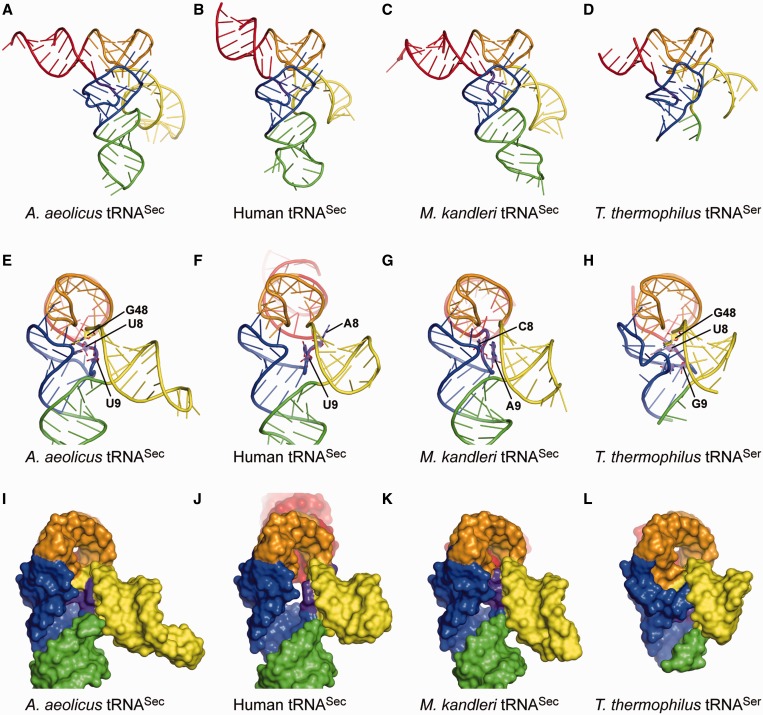
Structure comparisons. (A–D) Overall views of the present A. aeolicus tRNASec (A), human tRNASec (B) [PDB ID: 3A3A (10)], M. kandleri tRNASec (C) [PDB ID: 3ADB (11)] and T. thermophilus tRNASer (D) [PDB ID: 1SER (9)] structures. The structures of M. kandleri tRNASec and T. thermophilus tRNASer are those in their complexes with PSTK and SerRS, respectively. The ribose-phosphate backbones and the bases are shown as tubes and ladders, respectively. Each arm is indicated in the same color as in Figure 1. G73 and the terminal CCA are disordered in the human tRNASec structure, while the distal portion of the acceptor arm, most of the anticodon arm and the extra-arm loop are disordered in the T. thermophilus tRNASer structure. (E–L) Side views of the ladder and surface models of A. aeolicus tRNASec (E and G), human tRNASec (F and J), M. kandleri tRNASec (G and K) and T. thermophilus tRNASer (H and L). The linker nucleotides at positions 8, 9 and 48 are shown as plate-stick models in (E–H). The eukaryal/archaeal tRNASecs lack the linker nucleotide corresponding to G48 in bacterial tRNASec. The orientation of the extra arm is similar among tRNASecs and tRNASer.
Tertiary interactions
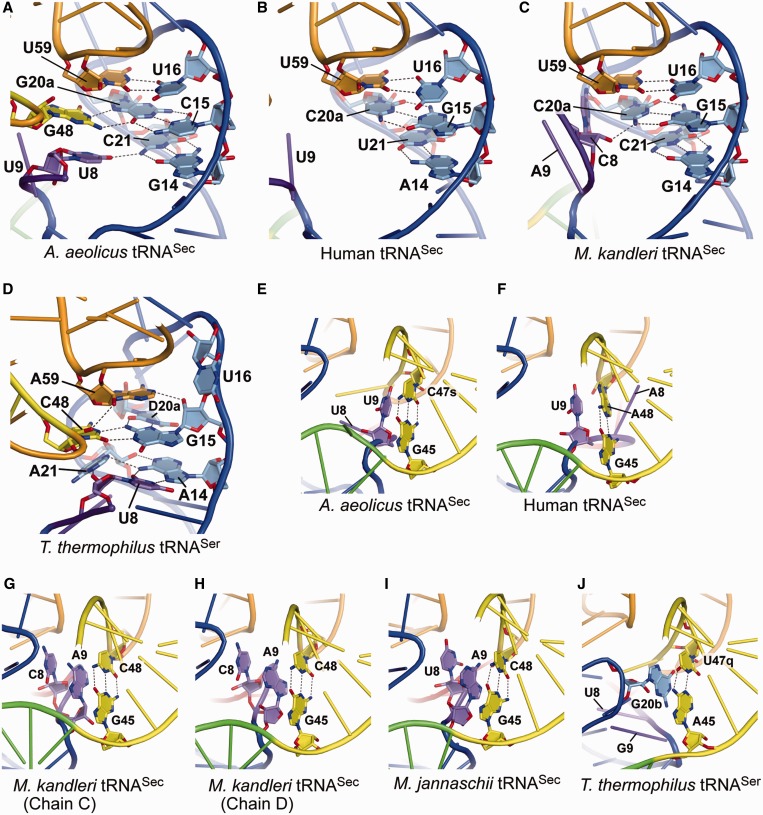
The D-loop•T-loop interaction includes the universal G18:U55 bp, as well as the tRNASec-specific U16:U59 bp (Figure 4A) and the U20:G19:C56 base triple (Figure 6B). The interaction of U8 with C21 in the D stem forms the G14:C21:U8 base triple (Figure 4A). In the eukaryal/archaeal tRNASecs, however, the nucleotide at position 8 is not involved in the tertiary base-pairing interaction (Figure 4B and C). G48, the linker between the extra stem and the T stem, is stacked on U8, and interacts with G20a to form the unique C15:G20a:G48 base triple (Figure 4A). C15, G20a, and G48 are completely conserved in the bacterial tRNASecs (4). The eukaryal/archaeal tRNASecs lack this base triple, as the extra stem is directly connected to the T stem, and there are no nucleotides corresponding to those at positions 48 and 49 (Figure 1B and C).
Figure 4.
Tertiary interactions. (A–D) Close-up views of the tertiary interactions with the AD linker and the D arm. The linker nucleotides U8 and G48 in A.aeolicus tRNASec interact with G14:C21 and C15:G20a in the D stem, respectively, to form unique base triples (A). In contrast, the D stem of human tRNASec lacks the tertiary interaction (B), while C8 in M. kandleri tRNASec interacts with the sixth D-stem base pair, G15:C21 (C). The N4 and O3′ atoms of C8 hydrogen bond with the O5′ and N4 atoms of C21, respectively. The nucleotides A14–A21 in T. thermophilus tRNASer are included in the D loop, and form the canonical tertiary pairs U8:A14 and G15:C48 (D). Furthermore, U8:A14 interacts with A21, and G15:C48 interacts with D20a and A59, thus building a tertiary core. (E–I) The tertiary stacking of the linker nucleotide at position 9 on the first extra-arm stem for the A. aeolicus (E), human (F), M. kandleri (G and H) and M. jannaschii (I) tRNASecs. Two distinct A9 conformations are observed in the two M. kandleri tRNASec molecules in the co-crystal with PSTK [PDB ID: 3ADD (11)]. The asymmetric unit contains two tRNASec molecules (chains C and D) and one PSTK dimer, and the A9s in chains C and D adopt different ribose puckering modes, C3′-endo and C2′-endo, respectively (G and H). The C2′-endo puckering is assumed by the U9s of the A. aeolicus (E) and human (F) tRNASecs. On the other hand, the asymmetric unit of the M. jannaschii tRNASec•PSTK co-crystal contains one tRNASec molecule and one-half of a PSTK dimer [PDB ID: 3AM1 (14)], and the A9 ribose puckering is C2′-endo (I). C8 and U8 of the M. kandleri and M. jannaschii tRNASecs, respectively, are further stacked on A9. (J) The tertiary stacking in T. thermophilus tRNASer. The D-loop nucleotide G20b is stacked on the first base pair of the extra-arm stem.
Figure 6.
Interactions between tRNASec and SerRS. (A and B) Close-up views of the interaction between A. aeolicus tRNASec and M. kandleri SerRS. The SerRS residues that interact with tRNASec are represented by stick models. Possible hydrogen bonds are depicted as dashed lines. (C) An overall view of the T. thermophilus tRNASer•SerRS complex. In the absence of the tRNASer interaction, the coiled-coil is disordered.
U9, in the AD linker, is stacked on the first base pair of the extra stem (G45:C47s) (Figure 4E). Similar base stacking of the nucleotide at position 9 is also observed in the eukaryal/archaeal tRNASecs (Figure 4F–I) (10,11,14). U9 of human tRNASec is only stacked on the A48 base of the G45:A48 pair, while A9 of the archaeal tRNASecs is stacked on the interbase hydrogen-bonding region of the base pair G45:C48. Interestingly, two distinct A9 conformations are observed in the M. kandleri tRNASec molecules (chains C and D) in the complex with the PSTK dimer [PDB ID: 3ADD (11)] (Figure 4G and H). A9 in chain D has the same C2′-endo ribose puckering and base orientation as those of the U9s in the A. aeolicus and human tRNASecs, while the A9 in chain C has the C3′-endo puckering and a different base orientation. On the other hand, both A9s exhibit the C2′-endo puckering in the two molecules of tRNASec from another archaeon, Methanocaldococcus jannaschii in complex with the PSTK dimer [PDB ID: 3AM1 (14)] (Figure 4I). The corresponding base stacking is also observed in tRNASer. The D-loop nucleotide G20b is stacked on the first extra-stem base pair of T. thermophilus tRNASer (Figure 4J), which results in essentially the same extra-arm orientation between tRNASec and tRNASer.
Regardless of these interior differences in the secondary and tertiary structures, the overall exterior structure of the bacterial tRNASec is similar to those of the eukaryal/archaeal tRNASecs (Figure 3A–C and E–G). This is due to the conservation of the D-arm conformation, the D-loop•T-loop interaction, the total length of the acceptor-T arm and the orientation of the long extra arm. It is surprising that the distinct 8 + 5 and 9 + 4 secondary structures result in the same exterior structure, while the total number of base pairs in the acceptor-T arm is the same (13 bp). The difference in the boundary between the acceptor and T arms is compensated by the linker nucleotides, U8 and G48, which are involved in the unique base triples, G14:C21:U8 and C15:G20a:G48, in the bacterial tRNASec.
These two unique base triples in the bacterial tRNASec occupy the positions corresponding to the U8:A14 and R15:Y48 tertiary base pairs, conserved in the canonical tRNAs (Figure 4D). Here, R and Y denote G/A and C/U, respectively. In the canonical tRNAs, these tertiary base pairs form the tight ‘tertiary core’, with additional non-conserved base triples in the D stem, such as 45:10:25, 12:23:9 and 13:22:46 (24,25), or 12:23:9 in the case of a long extra arm (9). In contrast, the eukaryal tRNASec completely lacks the corresponding interaction, and has a cavity instead of the tertiary core (10) (Figure 3J). On the other hand, the ribose and base moieties of C8 in the archaeal tRNASec interact with the base and phosphate moieties of C20a, respectively (11) (Figure 4C), thus generating an incomplete core (Figure 3K). Along with the shift of the acceptor-T boundary from the 9 + 4 to 8 + 5 secondary structure, the bacterial tRNASec generates the tertiary core, without any alterations in the overall exterior structure. The tertiary core reinforces the bacterial tRNASec conformation, although its contribution is relatively weak, as compared with those observed in the canonical tRNAs.
Structure of M. kandleri SerRS
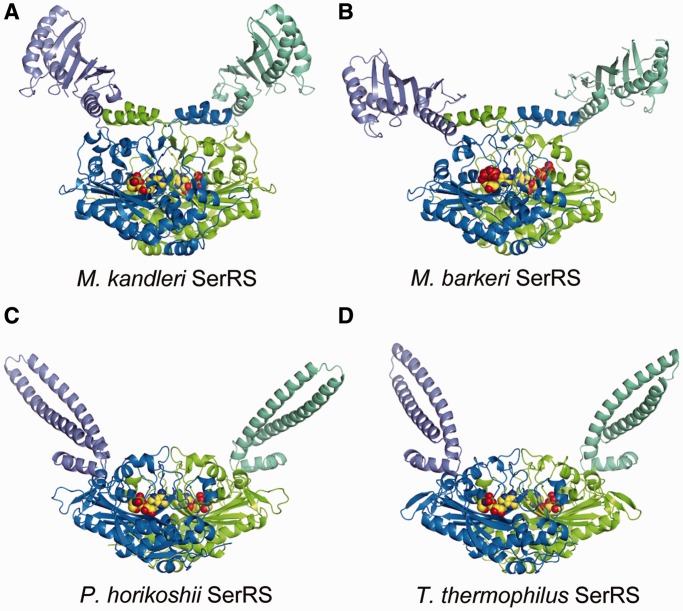
The M. kandleri SerRS, used for co-crystallization with A. aeolicus tRNASec, is an atypical SerRS unique to methanogenic archaea. The aminoacyl-tRNA synthetases are divided into two classes, classes I and II, based on the two structurally unrelated catalytic core domains (26), and both the typical and atypical SerRSs belong to class II. Similar to the reported M. barkeri SerRS structure (19), M. kandleri SerRS consists of an N-terminal domain (residues 1–167) and a C-terminal catalytic domain (residues 168–527) (Figure 5A and B). The globular N-terminal domain is composed of a six-stranded β sheet and four α helices, in contrast to the coiled-coil N-terminal domain of the typical SerRSs (15,16,27) (Figure 5C and D). The SerRS homodimer is formed by inter-subunit interactions between the C-terminal catalytic domains.
Figure 5.
Structures of SerRSs. Ribbon models of SerRS (dimer), colored as in Figure 1E. The structures of the SerRSs from the methanogenic archaea M. kandleri (A) and M. barkeri (B) [PDB ID: 2CJA (19)] are atypical, while those of the SerRSs from the archaeon Pyrococcus horikoshii (C) [PDB ID: 2DQ0 (16)] and the bacterium T. thermophilus (D) [PDB ID: 1SET (27)] are universal. The present M. kandleri SerRS structure (A) is that in the SerRS•tRNASec complex, while the others are the tRNA-free structures (B–C). The sphere models represent the seryl-adenylate intermediate analog Ser-SA (A, C and D) and ATP (B). In the tRNA-free structure of M. barkeri SerRS (B), the N-terminal domains are partially disordered, and the N-terminal-domain orientation differs significantly from that of the present SerRS•tRNASec complex structure (A). The N-terminal domains of the universal SerRSs have a long coiled-coil, which is responsible for tRNA binding (C and D).
Interaction of M. kandleri SerRS with A. aeolicus tRNASec
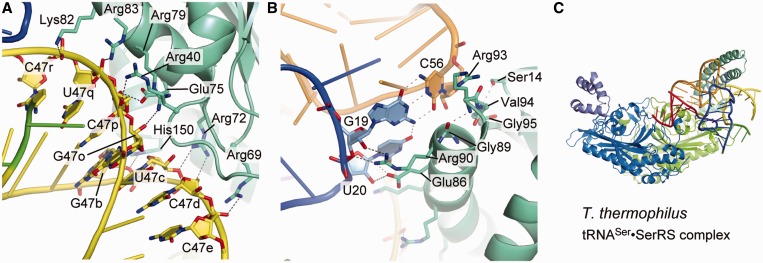
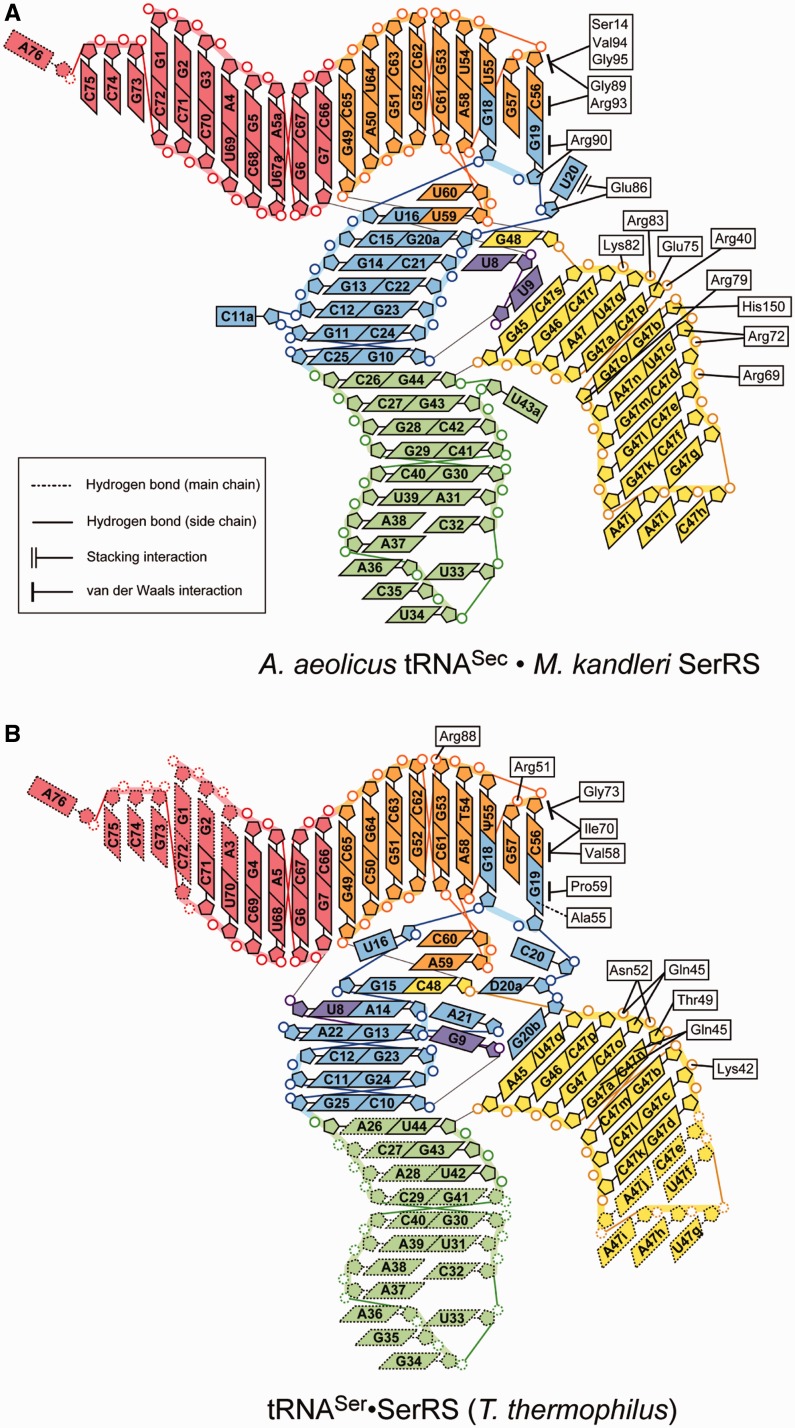
Methanopyrus kandleri SerRS exhibited serine-ligation activity toward A. aeolicus tRNASec in vitro, although the activity was lower than that toward M. kandleri tRNASec (Figure 2 and Table 2). One SerRS homodimer binds two tRNASec molecules. The N-terminal domain of SerRS interacts with the extra stem and the D and T loops of tRNASec (Figures 6A and B, and 7A). The acceptor and anticodon arms do not interact with SerRS. The backbone of the second to eighth base pairs of the extra stem forms polar interactions with the SerRS residues (Figure 6A). The side chains of Arg40, Arg69, Arg72, Lys82 and Arg83 interact with the phosphate groups of C47p, C47e, C47d, C47r and U47q, respectively. The side chains of Arg72, Glu75 and His150 interact with the ribose moieties of C47d, C47p and G47b, respectively. The Arg76 side chain interacts with the ribose moieties of two nucleotides, C47o and C47p. The U20:G19:C56 base triple in the D-loop•T-loop interface interacts with SerRS (Figure 6B). The side chains of Glu86 and Arg90 interact with the ribose moieties of U20 and G19, respectively. Ser14, Gly89, Arg93, Val94 and Gly95 form van-der-Waals interactions with the base and ribose moieties of C56.
Table 2.
The steady-state kinetics of aminoacylation
| SerRS | tRNASec | KM (μM) | kcat (s−1) | kcat/KM (s−1μM−1) |
|---|---|---|---|---|
| M. kandleri | M. kandleri | 0.57 ± 0.20 | 0.30 ± 0.10 | 0.77 ± 0.34 |
| M. kandleri | A. aeolicus | 109 ± 15 | 0.43 ± 0.05 | 0.0040 ± 0.00004 |
| A. aeolicus | M. kandleri | 0.52 ± 0.18 | 0.053 ± 0.009 | 0.19 ± 0.09 |
| A. aeolicus | A. aeolicus | 3.6 ± 0.5 | 0.42 ± 0.02 | 0.13 ± 0.02 |
The KM and kcat values of the wild-type M.kandleri and A.aeolicus SerRSs were measured.
Each experiment was performed 3–6 times, and the values were averaged.
The SEM values are shown as ‘±’.
Figure 7.
Comparison with tRNASer and SerRS interaction. A diagram representing the tRNASec•SerRS interactions observed in the present complex structure (A), and that representing the interactions between tRNASer and the SerRS N-terminal domain in the T. thermophilus tRNASer•SerRS complex [PDB ID: 1SER (9)] (B). In both of the complexes, the SerRS N-terminal domain interacts with the extra-arm stem and the outer corner of the tRNA.
The KM value of M. kandleri SerRS for A. aeolicus tRNASec is much higher than that for M. kandleri tRNASec (Table 2). Interestingly, the KM value of A. aeolicus SerRS for A. aeolicus tRNASec is also higher than that for M. kandleri tRNASec (Table 2), suggesting that the affinity of the bacterial tRNASec for SerRS is lower, as compared with that of the archaeal tRNASec.
In the crystal structure, SerRS interacts with the extra stem and the outer corner of tRNASec via its N-terminal domain, while the acceptor arm lacks interactions with SerRS. Therefore, this complex might be assumed to represent the pre-binding state of SerRS and tRNASec, where only the N-terminal domain binds to tRNASec. These tRNA–SerRS interactions are homologous to those between the N-terminal domain of the common type of SerRS and tRNASer observed in the T. thermophilus SerRS•tRNASer complex structure (9) (Figures 6C and 7B).
The unique secondary and tertiary structures of tRNASec are responsible for its specific interactions with PSTK, SepSecS, SelA and EF-Sec. However, tRNASec must also interact with SerRS to receive serine, and it has a long extra arm to mimic tRNASer. The long extra arm of tRNASer and tRNASec is the determinant element for the SerRS interaction (9,28). Furthermore, the extra arm orientation of tRNASec is adjusted to resemble that of tRNASer, by the stacking interaction on the first extra-stem base pair by the nucleotides at positions 9 and 20b of tRNASec and tRNASer, respectively. On the other hand, the length of the acceptor stem is not important for SerRS (29,30), owing to the flexibility between its N- and C-terminal domains. The present complex structure indicated that tRNASec is recognized by SerRS in the same manner as tRNASer, and is thereby distinguished from the other tRNAs. The conformational flexibility between the N- and C-terminal domains may allow tRNASec to retain the specific interaction between its extra arm and the SerRS N-terminal domain, when its 3′-terminal CCA region enters the SerRS catalytic site.
ACCESSION NUMBERS
The atomic coordinates and structure factors have been deposited in the Protein Data Bank (ID: 3W3S).
FUNDING
Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for Scientific Research (A) [20247008 to S.Y.]; JSPS Grants-in-Aid for Scientific Research (C) [20570148 to S.S.]; JSPS Research Fellowships (to Y.I.); the JSPS Global Centers of Excellence Program (Integrative Life Science Based on the Study of Biosignaling Mechanisms); the Targeted Proteins Research Program of the Ministry of Education, Culture, Sports, Science and Technology. Funding for open access charge: RIKEN; The source of funding is the Targeted Proteins Research Program of the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Conflict of interest statement. None declared.
ACKNOWLEDGEMENTS
The authors thank the staffs of the Photon Factory beam lines (Tsukuba, Japan) and SPring-8 BL41XU (Harima, Japan) for assistance with our X-ray diffraction experiments. The authors also thank A. Ishii and T. Nakayama for assistance in the manuscript preparation.
REFERENCES
- 1.Böck A, Forchhammer K, Heider J, Leinfelder W, Sawers G, Veprek B, Zinoni F. Selenocysteine: the 21st amino acid. Mol. Microbiol. 1991;5:515–520. doi: 10.1111/j.1365-2958.1991.tb00722.x. [DOI] [PubMed] [Google Scholar]
- 2.Leinfelder W, Zehelein E, Mandrand-Berthelot MA, Böck A. Gene for a novel tRNA species that accepts L-serine and cotranslationally inserts selenocysteine. Nature. 1988;331:723–725. doi: 10.1038/331723a0. [DOI] [PubMed] [Google Scholar]
- 3.Baron C, Sturchler C, Wu XQ, Gross HJ, Krol A, Böck A. Eukaryotic selenocysteine inserting tRNA species support selenoprotein synthesis in Escherichia coli. Nucleic Acids Res. 1994;22:2228–2233. doi: 10.1093/nar/22.12.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abe T, Ikemura T, Sugahara J, Kanai A, Ohara Y, Uehara H, Kinouchi M, Kanaya S, Yamada Y, Muto A, et al. tRNADB-CE 2011: tRNA gene database curated manually by experts. Nucleic Acids Res. 2011;39:D210–D213. doi: 10.1093/nar/gkq1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sturchler C, Westhof E, Carbon P, Krol A. Unique secondary and tertiary structural features of the eucaryotic selenocysteine tRNASec. Nucleic Acids Res. 1993;21:1073–1079. doi: 10.1093/nar/21.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuan J, Palioura S, Salazar JC, Su D, O'Donoghue P, Hohn MJ, Cardoso AM, Whitman WB, Söll D. RNA-dependent conversion of phosphoserine forms selenocysteine in eukaryotes and archaea. Proc. Natl Acad. Sci. USA. 2006;103:18923–18927. doi: 10.1073/pnas.0609703104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu XM, Carlson BA, Mix H, Zhang Y, Saira K, Glass RS, Berry MJ, Gladyshev VN, Hatfield DL. Biosynthesis of selenocysteine on its tRNA in eukaryotes. PLoS Biol. 2007;5:e4. doi: 10.1371/journal.pbio.0050004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forchhammer K, Böck A. Selenocysteine synthase from Escherichia coli. Analysis of the reaction sequence. J. Biol. Chem. 1991;266:6324–6328. [PubMed] [Google Scholar]
- 9.Biou V, Yaremchuk A, Tukalo M, Cusack S. The 2.9 Å crystal structure of T. thermophilus seryl-tRNA synthetase complexed with tRNASer. Science. 1994;263:1404–1410. doi: 10.1126/science.8128220. [DOI] [PubMed] [Google Scholar]
- 10.Itoh Y, Chiba S, Sekine S, Yokoyama S. Crystal structure of human selenocysteine tRNA. Nucleic Acids Res. 2009;37:6259–6268. doi: 10.1093/nar/gkp648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiba S, Itoh Y, Sekine S, Yokoyama S. Structural basis for the major role of O-phosphoseryl-tRNA kinase in the UGA-specific encoding of selenocysteine. Mol. Cell. 2010;39:410–420. doi: 10.1016/j.molcel.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 12.Palioura S, Sherrer RL, Steitz TA, Söll D, Simonovic M. The human SepSecS-tRNASec complex reveals the mechanism of selenocysteine formation. Science. 2009;325:321–325. doi: 10.1126/science.1173755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ganichkin OM, Anedchenko EA, Wahl MC. Crystal structure analysis reveals functional flexibility in the selenocysteine-specific tRNA from mouse. PLoS One. 2011;6:e20032. doi: 10.1371/journal.pone.0020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sherrer RL, Araiso Y, Aldag C, Ishitani R, Ho JM, Söll D, Nureki O. C-terminal domain of archaeal O-phosphoseryl-tRNA kinase displays large-scale motion to bind the 7-bp D-stem of archaeal tRNASec. Nucleic Acids Res. 2011;39:1034–1041. doi: 10.1093/nar/gkq845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cusack S, Berthet-Colominas C, Hartlein M, Nassar N, Leberman R. A second class of synthetase structure revealed by X-ray analysis of Escherichia coli seryl-tRNA synthetase at 2.5 Å. Nature. 1990;347:249–255. doi: 10.1038/347249a0. [DOI] [PubMed] [Google Scholar]
- 16.Itoh Y, Sekine S, Kuroishi C, Terada T, Shirouzu M, Kuramitsu S, Yokoyama S. Crystallographic and mutational studies of seryl-tRNA synthetase from the archaeon Pyrococcus horikoshii. RNA Biol. 2008;5:169–177. doi: 10.4161/rna.5.3.6876. [DOI] [PubMed] [Google Scholar]
- 17.Itoh Y, Sekine S, Yokoyama S. Crystallization and preliminary X-ray crystallographic analysis of bacterial tRNASec in complex with seryl-tRNA synthetase. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2012;68:678–682. doi: 10.1107/S1744309112016004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukunaga R, Yokoyama S. Structural insights into the first step of RNA-dependent cysteine biosynthesis in archaea. Nat. Struct. Mol. Biol. 2007;14:272–279. doi: 10.1038/nsmb1219. [DOI] [PubMed] [Google Scholar]
- 19.Bilokapic S, Maier T, Ahel D, Gruic-Sovulj I, Söll D, Weygand-Durasevic I, Ban N. Structure of the unusual seryl-tRNA synthetase reveals a distinct zinc-dependent mode of substrate recognition. EMBO J. 2006;25:2498–2509. doi: 10.1038/sj.emboj.7601129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laskowski RA, MacArthur MW, Moss DS, Thornton JM. PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Cryst. 1993;26:283–291. [Google Scholar]
- 21.Adams PD, Pannu NS, Read RJ, Brunger AT. Cross-validated maximum likelihood enhances crystallographic simulated annealing refinement. Proc. Natl Acad. Sci. USA. 1997;94:5018–5023. doi: 10.1073/pnas.94.10.5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 24.Robertus JD, Ladner JE, Finch JT, Rhodes D, Brown RS, Clark BF, Klug A. Structure of yeast phenylalanine tRNA at 3 Å resolution. Nature. 1974;250:546–551. doi: 10.1038/250546a0. [DOI] [PubMed] [Google Scholar]
- 25.Kim SH, Suddath FL, Quigley GJ, McPherson A, Sussman JL, Wang AH, Seeman NC, Rich A. Three-dimensional tertiary structure of yeast phenylalanine transfer RNA. Science. 1974;185:435–440. doi: 10.1126/science.185.4149.435. [DOI] [PubMed] [Google Scholar]
- 26.Eriani G, Delarue M, Poch O, Gangloff J, Moras D. Partition of tRNA synthetases into two classes based on mutually exclusive sets of sequence motifs. Nature. 1990;347:203–206. doi: 10.1038/347203a0. [DOI] [PubMed] [Google Scholar]
- 27.Belrhali H, Yaremchuk A, Tukalo M, Larsen K, Berthet-Colominas C, Leberman R, Beijer B, Sproat B, Als-Nielsen J, Grübel G, et al. Crystal structures at 2.5 angstrom resolution of seryl-tRNA synthetase complexed with two analogs of seryl adenylate. Science. 1994;263:1432–1436. doi: 10.1126/science.8128224. [DOI] [PubMed] [Google Scholar]
- 28.Asahara H, Himeno H, Tamura K, Nameki N, Hasegawa T, Shimizu M. Escherichia coli seryl-tRNA synthetase recognizes tRNASer by its characteristic tertiary structure. J. Mol. Biol. 1994;236:738–748. doi: 10.1006/jmbi.1994.1186. [DOI] [PubMed] [Google Scholar]
- 29.Baron C, Böck A. The length of the aminoacyl-acceptor stem of the selenocysteine-specific tRNASec of Escherichia coli is the determinant for binding to elongation factors SELB or Tu. J. Biol. Chem. 1991;266:20375–20379. [PubMed] [Google Scholar]
- 30.Sherrer RL, Ho JM, Söll D. Divergence of selenocysteine tRNA recognition by archaeal and eukaryotic O-phosphoseryl-tRNASec kinase. Nucleic Acids Res. 2008;36:1871–1880. doi: 10.1093/nar/gkn036. [DOI] [PMC free article] [PubMed] [Google Scholar]