Abstract
Background/Purpose
A phase II randomized controlled trial of recombinant human relaxin suggested that 25 ug/kg/day was safe and clinically effective in improving skin disease and functional disability in scleroderma. We report the results of a large randomized, double-blind, placebo-controlled clinical trial comparing placebo with recombinant human relaxin, 10 ug/kg of body weight per day and 25 ug/kg per day, given for 24 weeks in patients with stable, diffuse, moderate to severe scleroderma (SSc).
Methods
Men and women 18 to 70 years of age with diffuse SSc, disease duration ≤ 5 years since the onset of the first non-Raynaud sign or symptom, a baseline modified Rodnan skin score (MRSS) of 20 or greater, or at least 16 if truncal involvement was present. Recombinant human relaxin (10 or 25 ug/kg/day), or placebo was administered for 24 weeks as a continuous subcutaneous infusion and there was a follow-up safety visit at week 28.
Results
The primary outcome measure, the MRSS, was similar between the 3 groups at baseline and at weeks 4, 12, and 24 (P=NS). Secondary outcomes such as functional disability were similar in all 3 groups and the forced vital capacity significantly decreased in the relaxin groups (p< 0.04). The discontinuation of relaxin (both doses) at week 24 led to statistically significant declines in creatinine clearance and serious renal adverse events (defined as either doubling of baseline serum creatinine, renal crisis, or grade 3 or 4 hypertension) in 7 patients who had received relaxin therapy but in none who had received placebo (p=0.04).
Conclusion
Recombinant relaxin was not significantly better than placebo in improving total skin score, pulmonary function, or functional disability in patients with diffuse SSc. In addition, relaxin was associated with serious renal adverse events, the majority of which occurred after stopping the infusion. If relaxin is used therapeutically for any conditions other than scleroderma, close monitoring of blood pressure and renal function must be performed.
Introduction
Relaxin is a naturally occurring protein, structurally related to the insulin family of peptides which is produced primarily by the ovary and/or placenta in pregnancy and by the prostate of mammals. Relaxin has been implicated in a number of pregnancy-related functions including relaxation of the cervix and vagina at delivery.
Relaxin has anti-fibrotic properties: it downregulates collagen production and increases collagen degradation (1). In vitro studies show that relaxin acts directly on TGF-beta 1-stimulated fibroblasts to decrease myofibroblast differentiation and collagen secretion while increasing expression of the metalloproteinases— enzymes responsible, in part, for collagen degradation (2;3). Relaxin acts in synergy with interferon-gamma to reduce collagen over-expression by fibroblasts isolated from patients with scleroderma(3). In addition, recombinant human relaxin prevents the development of bleomycin-induced pulmonary fibrosis in rodents (4).
Phase I and II studies of recombinant human relaxin in patients with diffuse scleroderma demonstrated that steady-state serum concentrations of relaxin up to 60 times higher than those seen in normal pregnancy could be safely achieved with continuous subcutaneous infusion (5;6). In addition, a previous phase II randomized controlled trial suggested that 25 ug/kg/day was safe and well tolerated, and was likely to be clinically effective in improving skin disease and functional disability(5).
We here report the results of a phase III randomized, double blind, controlled trial comparing placebo with recombinant human relaxin, 10 ug/kg of body weight per day and 25 ug/kg per day, given for 24 weeks in patients with stable, diffuse, moderate to severe scleroderma. The dosage of 25 ug/kg/day was selected on the basis of pharmacokinetics and clinical efficacy results from earlier studies (5;6). On the basis of preclinical and earlier clinical studies, we hypothesized that this serum concentration would have antifibrotic effects. In order to explore further dose-response relationships, a lower dose of 10 ug/kg/day was included. The 25 mg/kg/day dose was used to replicate the Phase II study (5).
Methods
Patients
Before screening, all patients gave full informed voluntary written consent according to the principles of the Declaration of Helsinki and in compliance with United States Food and Drug Administration requirements. Patients with scleroderma meeting the American College of Rheumatology criteria (7) were recruited through United States member institutions of the Scleroderma Clinical Trials Consortium (SCTC). The criteria for entry and study design were purposely kept nearly identical to the previous study, which compared relaxin in doses of 25 ug/kg/day and 100 ug/kg/day to placebo. Men and women 18 to 70 years of age were eligible if they had a history of systemic sclerosis with diffuse scleroderma (defined as skin thickening proximal, as well as distal, to the elbows or knees, with or without involvement of the face and neck), for ≤ 5 years since the onset of the first sign or symptom of SSc other than Raynaud’s phenomenon. A baseline modified Rodnan skin score (MRSS) of at least 20, or at least 16 if truncal involvement was present, was required for entry into the treatment phase of the study. Patients were excluded if their MRSS varied by more than 5 units from screening to the first treatment day.
We also excluded patients who had limited cutaneous scleroderma (skin thickening distal, but not proximal, to the knees and elbows, with or without facial involvement); eosinophilic fasciitis; eosinophilic myalgia syndrome; or scleroderma in conjunction with any other definable connective tissue disease, such as rheumatoid arthritis, systemic lupus erythematosus, or polymyositis/ dermatomyositis. We excluded patients with a substantial history of environmental exposure to “tainted” rapeseed oil, vinyl chloride, trichlorethylene, or silica dust. Also excluded were patients with onset of renal crisis in the previous 2 months prior to enrollment, or chronic renal failure (serum creatinine ≥ 2.0 mg/dL), and patients with severe pulmonary disease (forced vital capacity < 50% predicted and diffusion capacity of carbon monoxide < 40% predicted); severe cardiovascular disease (uncontrolled hypertension, symptomatic coronary artery disease, second or third AV nodal block and/ or bifascicular block, congestive heart failure, cor pulmonale, or symptomatic pericardial effusion), gastrointestinal (gastrointestinal bleeding requiring blood transfusion or surgical intervention within last 6 months or weight < 70% of ideal body weight) or hematological disease (hemoglobin ≤ 8 mg/dl, platelets ≤ 100/ul, white cell count ≤ 3,000/ul or polymorphonuclear cells ≤ 1,000 ul) were excluded. Other exclusion criteria included pregnancy and currently breast feeding.
Patients were required to discontinue putative “disease-modifying” treatments for scleroderma (including immunosuppressives, potassium aminobenzoate, photopheresis, colchicine, or any other experimental therapy) at least 4 weeks before the study. Patients were excluded if they were receiving more than 10 mg of prednisone (or equivalent) per day.
Intervention
We administered recombinant human relaxin (10 ug/kg/day or 25 ug/kg/day) or placebo for 24 weeks by continuous subcutaneous infusion, using microinfusion pumps (Panomat T-series, Disetronic Medical Systems, Inc., Minneapolis, MN). Recombinant human relaxin was produced by Connetics Corporation (Palo Alto, CA) in Escherichia coli. The placebo was a sterile acetate buffer solution that was identical in composition to the buffer used for relaxin.
Patients were randomly assigned to receive placebo or recombinant human relaxin (10 ug/kg/day or 25 ug/kg/day) in a 2:1:2 ratio. Randomization was performed at a centralized data management organization (Pacific Research Associates, Los Altos, CA). “Biased coin” randomization was used to stratify patients on the basis of disease duration (≤2.5 years or >2.5 to ≤ 5 years) and use of D-penicillamine in the previous 6 months. The same randomization procedure was used to replace patients who withdrew before completing 4 weeks of treatment. The patients were recruited between 1999-2000.
Patient prescription for the study medication were forwarded to a centralized pharmacy for preparation of blinded supplies of the study drug. Each patient’s dose was based on screening body weight. The dose was adjusted only if body weight changed by 10% or more during the study. Treatment was administered 24 hours per day for 24 weeks. Continuous subcutaneous infusion was chosen as the mode of administration to eliminate the need for six daily subcutaneous injections, to conserve drug supply, and to mimic the constancy of relaxin concentrations usually seen during pregnancy. The infusion site and needle were changed at least every 72 hours.
Assessments
The primary measure of efficacy was the modified Rodnan skin score (MRSS), a clinical evaluation of skin thickness in 17 body surface areas (face, chest, and abdomen, and right and left fingers, hands, forearms, upper arms, thighs, lower legs and feet)(8). Each area was assessed for thickness on a 0 to 3 scale (0 = normal, 1 = mild but definite thickening, 2 = moderate skin thickening and 3 = severe skin thickening). The total score (the sum of scores from all 17 body areas) ranged from 0 to 51. The MRSS is both accurate and reproducible (with an interobserver standard deviation of ± 4.6 units and an intraobserver standard deviation of ± 2.5 units)(8). Before the study began, investigators underwent (re)training and standardization training.
Secondary measures of efficacy included: blood pressure measurement, maximal oral aperture (lip to lip); maximal hand extension (distance between the tip of the thumb and tip of the fifth finger in maximum hand opening); tenderness and swelling of the metacarpophalangeal joints (as a unit), wrists, elbows, and knees (eight joints); number of skin ulcers; Medical Outcomes Short From -36 version 1 (SF-36) (9); Health Assessment Questionnaire-Disability Index (HAQ-DI) (10;11); global disease visual analogue assessments by patients and investigator (0 to 100 mm line); and pulmonary function tests, including forced vital capacity (FVC as percent predicted) and diffusing capacity for carbon monoxide (DLCO as percent predicted), corrected for hemoglobin. We have previously reported the SF-36 and HAQ-DI data in this clinical trial (12;13) and those results will not be reported here; this manuscript presents the results of the original randomized clinical trial.
Renal crisis was determined to be present when the patient’s physician-investigator detected renal insufficiency (serum creatinine ≥2.0 mg/day, or a doubling of serum creatinine above the value at baseline, in the absence of another defined cause) and/or malignant hypertension (systolic blood pressure [BP] ≥160 mm Hg or diastolic BP ≥110 mm Hg on at least 2 occasions, a minimum of 12 hours apart), accompanied by persistent urine abnormalities(i.e., proteinuria) or evidence of microangiopathic hemolytic anemia (MAHA). The following equation may help to explain the definition of renal crisis. Renal crisis = (↑ serum creatinine) and/or (↑ BP + abnormal urinalysis or MAHA).
Statistical Analysis
Patients who completed at least 4 weeks of relaxin or placebo were considered evaluable for efficacy in a last-observation-carried-forward analysis. Baseline demographic data were evaluated for comparability of the three treatment groups by 1- way analysis of variance (ANOVA) for continuous variables and Fisher exact test for categorical variables. All efficacy and laboratory variables were evaluated by analysis of covariance (ANCOVA), adjusting for values at week 0. Frequencies of adverse events in the treatment groups were compared using the Fisher exact test. A two-tailed P value of 0.05 was considered significant for all comparisons. Continuous data are presented as mean ± standard error (SE) and dichotomous data as number (percent).
The prospective primary efficacy hypothesis was that relaxin therapy would reduce the MRSS by > 4 units after 24 weeks of treatment. This reduction was considered clinically meaningful based on consensus by SSc experts (14) and has been confirmed in a data driven analysis of another early diffuse SSc clinical trial (15).
Based on the phase II study, it was hypothesized that 25 ug/kg/day dose of relaxin will have at least 4 units greater improvement in MRSS compared to placebo at the end of the study(5). The sample size was selected to provide confirmation of the efficacy, safety, and dose-response effects of the 25 ug/kg/day dose compared to placebo with a power of 0.80 with α2 of 0.05.
Role of the Funding Source
The funding source provided the study drug, underwrote the costs of the trial and participated fully with the investigators in protocol design, analysis, and interpretation, but did not influence the decision to submit this manuscript, nor did they in any way contribute or influence the content of the manuscript.
Results
Baseline Characteristics
The study recruited 239 patients with diffuse SSc of which 8 did not complete the baseline visit. In 231 patients with baseline visit, the mean (SE) disease duration (from first non-Raynaud’s symptom) was 2.2 (0.1) years; 147 (64%) patients had disease duration of ≤ 2.5 years. The majority of patients were female (86%) and Caucasian (71%) with mean (SE) age of 46.2 (0.7) years. There were no statistical differences in the immunosuppressives used in the past and current use of oral vasodilators (Table 1). The mean baseline MRSS was 27.1 (0.6) units and mean baseline HAQ-DI was 2.21 (0.7) units indicating moderate-to-severe disease(11). There were no significant differences in the baseline characteristics between placebo, 10ug/kg/day, and 25 ug/kg/day groups (Table 1).
Table 1.
Baseline Measures in 231 patients with scleroderma
| Outcome Measure | Placebo (n = 94) | Relaxin 10 ug/kg/day (n = 42) | Relaxin 25 ug/kg/day (n = 95) | p-value* |
|---|---|---|---|---|
| Age‡ | 46.2 (0.7) | 46.1 (1.0) | 46.4 (1.6) | 0.994 |
| Female† | 79 (84%) | 38 (90%) | 81 (85%) | 0.983 |
| Ethnicity† | ||||
| Caucasian | 62 (66%) | 33 (79%) | 70 (74%) | 0.271 |
| African-American | 19 (20%) | 5 (12%) | 8 (8%) | |
| Hispanic | 10 (11%) | 3 (7%) | 14 (15%) | |
| Asian | 2 (2%) | 0 (0) | 0 (0) | |
| Others | 1 (1%) | 1 (1%) | 3 (3%) | |
| Disease duration (years) ‡ | 2.3 (0.1) | 1.9 (0.2) | 2.2 (0.1) | 0.189 |
| Disease duration <= 2.5 years† | 55 (59%) | 32 (76%) | 60 (63%) | 0.141 |
| Disease duration > 2.5 years† | 39 (41%0 | 10 (24%) | 35 (37%) | |
| Use of D-Penicillamine within last 6 months† | 88 (94%) | 40 (95%) | 84 (88%) | 0.189 |
| Immunosuppressives used in the past | ||||
| Methotrexate | 33 (35%) | 14 (33%) | 32 (34%) | 0.97 |
| Oral prednisone | 18 (19%) | 15 (36%) | 19 (20%) | 0.08 |
| Minocycline | 16 (17%) | 8 (19%) | 15 (16%) | 0.90 |
| Hydroxchloroquinine | 3 (3%) | 5 (12%) | 6 (6%) | 0.14 |
| Cyclophosphamide | 3 (3%) | 3 (7%) | 5 (5%) | 0.60 |
| Concomitant therapies | ||||
| ACE inhibitors or ARB’s | 18 (19%) | 12 (31%) | 20 (21%) | 0.29 |
| Calcium channel blockers | 39 (41%) | 14 (33%) | 35 (37%) | 0.63 |
| Modified Rodnan skin score‡ | 27.1 ± 0.6 | 28.6 ± 1.1 | 28 ± 0.8 | 0.465 |
| Maximum oral aperture (mm) ‡ | 43.6 ± 0.9 | 43.8 ± 1.5 | 43.2 ± 1.0 | 0.921 |
| Right hand extension (mm) ‡ | 166.6 ± 3.4 | 155.6 ± 4.3 | 162.2 ± 3.5 | 0.185 |
| Left hand extension (mm) ‡ | 169.3 ± 3.5 | 163.2 ± 4.0 | 169.1 ± 3.3 | 0.544 |
| Total musculoskeletal assessment (synovitis) score‡ | 2.8 ± 0.4 | 3.4 ± 0.6 | 2.7 ± 0.4 | 0.603 |
| Physician global assessment (mm) ‡ | 49.5 ± 2.2 | 49.8 ± 3.3 | 51.6 ± 2.1 | 0.773 |
| Patient global assessment (mm) ‡ | ||||
| Diffusing capacity (% of corrected value) ‡ | 69.6 ± 2.1 | 71.4 ± 3.5 | 67.2 ± 2.4 | 0.545 |
| Forced vital capacity (% of predicted) ‡ | 85.8 ± 1.7 | 87.1 ± 2.0 | 81.8 ± 1.6 | 0.095 |
| Total number of cutaneous ulcers‡ | 0.9 ± 0.2 | 0.8 ± 0.3 | 0.5 ± 0.1 | 0.309 |
| HAQ-DI‡ | 2.21± 0.07 | 2.37 ± 0.11 | 2.21 ± 0.08 | 0.464 |
| SF-36 PCS‡ | 33.9 ± 10.3 | 30.8 ± 9.1 | 33.3 ± 11.6 | 0.277 |
| SF-36 MCS‡ | 50.6 ± 9.0 | 47.4 ± 8.2 | 48.8 ± 11.3 | 0.175 |
| Creatinine clearance (ml/min) ‡ | 128.0 ± 4.1 | 115.5 ± 5.9 | 127.1 ± 4.9 | 0.26 |
| Systolic blood pressure (mm Hg) ‡ | 116.5 ± 1.9 | 115.6 ± 2.5 | 120.4 ± 1.9 | 0.224 |
| Diastolic blood pressure (mm Hg) ‡ | 71.3 ± 1.0 | 68.8 ± 1.6 | 71.1 ± 0.9 | 0.332 |
| Hemoglobin (gm/dl) ‡ | 12.6 ± 0.1 | 12.9 ± 0.2 | 12.8 ± 0.2 | 0.596 |
p value using 1-way ANOVA for continuous variables and Fisher’s exact test for categorical variables.
Results presented as mean ± standard error (SE) for continuous variables
Results presented as Number (%) for dichotomous variables
Course of Study
All 231 SSc patients completed the 4-week follow-up visit and were included in the efficacy and safety analyses. One hundred ninety-five patients completed the 24-week study, 21 dropped out due to subjects’ request or non-compliance, 14 due to adverse events, and 1 person died (Appendix Figure).
Primary Outcome Measure
The MRSS, was similar among the 3 groups at weeks 4, 12, and 24 (Table 2, Figure 1). The average MRSS declined over the course of the 24 weeks. Subanalysis of groups by disease duration (≤ 2.5 years and > 2.5 years) showed similar patterns of improvement in MRSS (-4.6 [0.8] in the placebo arm vs. −4.4 [1.0] in the 25 ug/kg/day arm; p=0.57 in the ≤ 2.5 years of disease duration, and -5.4 [1.2] in placebo arm vs. −6.6 [0.9] in 25 ug/kg/day arm; p=0.29 in the >2.5 years of disease duration).
Table 2.
Mean change in outcome measures from baseline to week 24 in 195 patients who completed the study *
| Outcome Measure | Placebo (n = 94) | Relaxin 10 ug/kg/day (n = 42) | p-value (Relaxin 10 m/kg/d vs plac) | Relaxin 25 ug/kg/day (n = 95) | p-value (Relaxin 25 m/kg/d vs plac) |
|---|---|---|---|---|---|
| Modified Rodnan skin score | -4.9 ± 0.7 | -4.3 ± 1.3 | 0.72 | -5.2 ± 0.7 | 0.365 |
| Maximum oral aperture (mm) | 0.1 ± 0.6 | 0.7 ± 1.2 | 0.629 | 1.3 ± 0.8 | 0.258 |
| Right hand extension (mm) | -3.3 ± 1.3 | -0.1 ± 2.1 | 0.358 | -0.6 ± 2.5 | 0.52 |
| Left hand extension (mm) | -1.9 ± 1.9 | -3.8 ± 2.3 | 0.334 | -3.0 ± 2.4 | 0.69 |
| Total musculoskeletal assessment (synovitis) score | -0.3 ± 0.4 | -0.7 ± 0.5 | 0.848 | -1.1 ± 0.3 | 0.17 |
| Physician global assessment (mm) | -7.2 ± 2.2 | -3.4 ± 3.4 | 0.182 | -8.0 ± 2.3 | 0.879 |
| Diffusing capacity (% predicted of corrected value) | 0.2 ± 1.5 | 2.3 ± 2.1 | 0.361 | 0.3 ± 1.1 | 0.93 |
| Forced vital capacity (% predicted) | -0.6 ± 0.9 | -4.3 ± 1.3 | 0.033 | -2.3 ± 1.0 | 0.016 |
| Total cutaneous ulcers (n) | -0.1 ± 0.2 | 1.1 ± 0.6 | 0.017 | 0.1 ± 0.1 | 0.758 |
| Score on HAQ-DI | -0.01 ± 0.05 | 0.08 ± 0.06 | 0.19 | 0.07 ± 0.04 | 0.225 |
| Predicted creatinine clearance (ml/min) | -5.6 ± 3.1 | 8.2 ± 6.3 | 0.046 | 6.1 ± 3.4 | 0.015 |
| Systolic blood pressure (mm Hg) | -1.0 ± 1.7 | 2.1 ± 2.6 | 0.483 | -2.7 ± 1.9 | 0.688 |
| Diastolic blood pressure (mm Hg) | 0.0 ± 1.1 | 0.8 ± 1.8 | 0.455 | -2.9 ± 1.3 | 0.018 |
| Hemoglobin (gm/dl) | -0.4 ± 0.1 | -1.24 ± 0.2 | 0.001 | -1.41 ± 0.1 | <0.001 |
Results presented as mean ± standard error = mean (SE)
Figure 1.
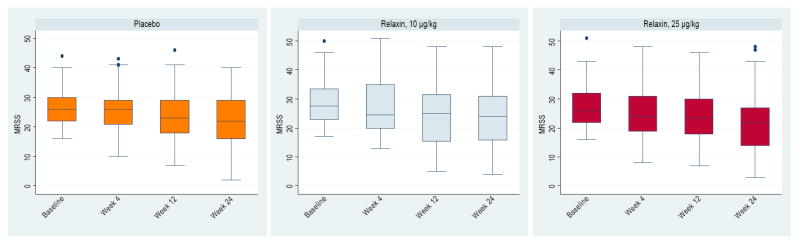
Change in modified Rodnan skin score over 24 weeks. Data are presented as box plots, where the boxes represent the 25th to 75th percentiles, the lines within the boxes represent the median, and the lines outside the boxes represent the minimum to 25th percentile and 75th percentile to maximum values. Despite individual variability at each time point, the MRSS decreased over 24 weeks and there were no statistical differences among the 3 groups during the trial.
Secondary Outcome Measures
Other efficacy measures, including change in DLCO% predicted, HAQ-DI, and physician global assessment were similar among the 3 groups (Table 2). The decline in FVC% predicted was significantly greater in the 10 and 25 ug/kg/day doses (mean of -4.3% and -2.3%, respectively) compared to the placebo group (mean of -0.6%; p=0.03 vs. 10 ug/kg/day and p= 0.02 vs. 25 ug/kg/day). The number of patients with baseline FVC% predicted ≤70% were similar in the 3 groups (p=0.13) and the declines in their FVC% predicted were similar at 24 week (p=0.27) Patients were not screened for active interstitial lung disease either by high-resolution computer tomography or bronchoalveolar lavage.
Safety
Both doses of relaxin were associated with an increase in creatinine clearance from baseline (mean change = 8.2 ml/min with 10 ug/kg/day dose and 6.1 ml/min with 25 ug/kg/day dose) compared to a decline in placebo group (mean change = -5.6 ml/min; p< 0.05 vs. both relaxin doses). In addition, the 25 ug/kg/day dose was associated with a statistically significant decline in diastolic blood pressure (mean change of -2.9 mmHg vs. mean change of 0.0 mmHg in placebo; p=0.02) but no significant impact on systolic blood pressure (p=0.67; Table 3). There was no significant difference between placebo and the 10 ug/kg/day relaxin dose regarding change in either systolic or diastolic blood pressure (p> 0.05).
Table 3.
Renal adverse events during the trial and 4 week safety period.
| Renal Adverse Events | Placebo group (n = 94) | Relaxin (10 ug/kg/day) (n = 42) | P value* | Relaxin (25 ug/kg/day) (n = 95) | P value† | All Relaxin Therapy | P value‡ |
|---|---|---|---|---|---|---|---|
| During Treatment | |||||||
| Renal Crisis | 1 | 0 | 1.00 | 0 | 1.00 | 1 | 1.00 |
| Doubling of serum creatinine | 1 | 0 | 1.00 | 1 | 1.00 | 1 | 1.00 |
| Grade 3 or 4 HTN | 0 | 0 | - | 0 | - | 0 | - |
| Any Renal event | 1 | 0 | 1.00 | 1 | 1.00 | 1 | 1.00 |
| After Treatment | |||||||
| Renal Crisis | 0 | 2 | 0.09 | 2 | 0.50 | 4 | 0.15 |
| Doubling of serum creatinine | 0 | 0 | - | 2 | 0.50 | 2 | 0.52 |
| Grade 3 or 4 HTN | 0 | 0 | - | 1 | 1.00 | 1 | 1.00 |
| Any Renal event | 0 | 2 | 1.00 | 5 | 0.06 | 7 | 0.04 |
Grade 3 hypertension was defined as requiring one or more antihypertensive medications and grade 4 hypertension was defined as life-threatening consequences due to hypertension (e.g., hypertensive crisis)
Relaxin 10 ug/kg/day vs. placebo
Relaxin 25 ug/kg/day vs. placebo
Combined 10 and 25 ug/kg/day vs. placebo
Discontinuation of relaxin at week 24 was associated with a further decline in creatinine clearance compared to placebo (Figure 2). At the post study visit (week 28), the mean decline in creatinine clearance in the relaxin 10 ug/kg/day and in 25 ug/kg/day groups were -8.4 ml/min and -9.8 ml/min, respectively, compared to a decline of -0.9 ml/min in the placebo group (p=0.07 for comparison with each relaxin group and 0.03 for comparison with the combined relaxin group). This decline in creatinine clearance from baseline values reached statistical significance for 25 ug/kg/day group (p=0.02) and showed a trend (p=0.09) for the10 ug/kg/day group. In addition, all relaxin patients had combined elevations of the mean (SE) systolic blood pressure of 3.0 [1.7] mm Hg compared to the placebo group (-0.7 [1.9]; p=0.053) and non-significant elevations of their mean (SE) diastolic blood pressure of 2.5 (1.1) mm Hg compared to the placebo group (0.6 [1.3]); p=0.27).
Figure 2.
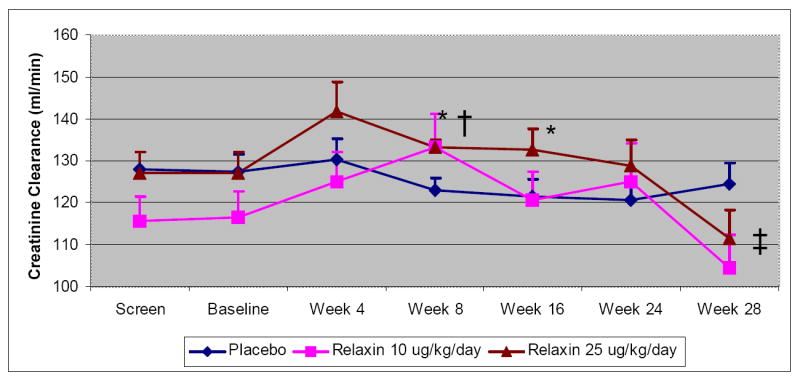
Change in creatinine clearance over 24 weeks. Therapy with relaxin was associated with an increase in creatinine clearance during the trial and an abrupt decline after the therapy was discontinued. Data is presented as mean change with bars representing the standard error.
*p< 0.05 for comparison of relaxin 25 ug/kg/day vs. placebo, adjusted for baseline value
† p< 0.05 for comparison of relaxin 10 ug/kg/day vs. placebo, adjusted for baseline value
‡ p= 0.07 for comparison of relaxin 10 ug/kg/day and 25 ug/kg/day vs. placebo, adjusted for baseline value
This change in renal function was accompanied by serious renal adverse events (defined as either doubling of serum creatinine, renal crisis, or grade 3 or 4 essential hypertension) in the relaxin group. Grade 3 hypertension was defined as requiring one or more antihypertensive medications and grade 4 hypertension was defined as life-threatening consequences due to hypertension (e.g., hypertensive crisis). After relaxin was stopped, 2 patients in each of the relaxin groups had new-onset renal crisis, 2 patients in the 25 ug/kg/day group doubled their serum creatinine, and 1 patient in the 25 ug/kg/day group had grade 3 or 4 hypertension (Table 3). In comparison, during the trial, 1 placebo patient and 1 relaxin 25 ug/kg/day patient had doubling of their serum creatinine and 1 in the placebo group developed new-onset renal crisis. This unusual number of serious renal adverse events (N=6 of 169, 3.6%) 1-23 days after the relaxin infusion was stopped led the investigators to recommend daily blood pressure monitoring and gradual discontinuation of relaxin therapy. Subsequent to implementation of this recommendation, only 1 serious renal adverse event (of 61 patients, 1.6%) was noted in the relaxin arm after drug discontinuation.
Another adverse event that was statistically different among the 3 groups was a decline in the mean (SD) serum hemoglobin (-0.41 [0.13] g/dl) in the placebo arm vs.-1.36 [0.09] g/dl in the relaxin groups; p<0.001) at week 24). The mean (SD) decrease in hemoglobin was found predominantly in the female patients receiving relaxin (-1.33 [1.02] g/dl in the female relaxin group vs. -0.32 [0.94] g/dl in the female placebo group, p<0.001) and not in the male patients (-1.04 [1.10] g/dl vs. -0.99 [2.03] g/dl, p=0.9). This difference was no longer significant at the off-drug week 28 follow-up visit(-0.52[0.12] mg/dl in placebo vs. −0.70 [0.11] mg/dl in the combined relaxin arms; p=0.43). Menorrhagia was reported in 27.3% and 30.4% of female patients in the relaxin 10 ug/kg/day and 25 ug/kg/day groups compared to 6.3% in the placebo group (p=0.004 for 10 ug/kg/day and <0.0001 for 25 ug/kg/day). Other adverse events were similar among the 3 groups (Table 4).
Table 4.
Adverse events during the 24- week trial presented as number (percentage).
| Adverse Events | Placebo group (n = 94) | Relaxin (10 ug/kg/day) (n = 42) | P value* | Relaxin (25 ug/kg/day) (n = 95) | P value† |
|---|---|---|---|---|---|
| Body as a whole | 77 (81%) | 33 (78%) | 0.64 | 76 (80%) | 0.10 |
| Cardiovascular | 26 (28%) | 16 (38%) | 0.22 | 45 (47%) | 0.05 |
| Chest pain | 5 (5.3) | 4 (9.1) | 0.46 | 9 (9.8) | 0.28 |
| Peripheral vascular disease | 5 (5.3) | 0 (0.0) | 0.32 | 9 (9.8) | 0.28 |
| Tachycardia | 5 (5.3) | 1 (2.3) | 0.67 | 10 (10.9) | 0.19 |
| Digestive system | 47 (50%) | 27 (64%) | 0.12 | 56 (59%) | 0.21 |
| Gastrointestinal hemorrhage | 5 (5.3) | 1 (2.3) | 0.67 | 6 (6.5) | 0.77 |
| Melena | 5 (5.3) | 1 (2.3) | 0.67 | 6 (6.5) | 0.77 |
| Hemopoitic system | 20 (21%) | 15 (36%) | 0.08 | 34 (36%) | 0.03 |
| Anemia | 11 (11.6) | 11 (25) | 0.03 | 33 (35.9) | 0.0002 |
| Lymphadenopathy | 7 (7.4) | 1 (2.3) | 0.43 | 3 (3.3) | 0.21 |
| Metabolic | 1 (1%) | 1 (2%) | 0.56 | 3 (3%) | 0.32 |
| MSK | 42 (45%) | 20 (48%) | 0.12 | 37 (39%) | 0.42 |
| Respiratory system | 58 (62%) | 29 (69%) | 0.41 | 61 (64%) | 0.72 |
| Skin | 51 (54%) | 26 (62%) | 0.41 | 54 (57%) | 0.72 |
| Urogenital system | 32 (34%) | 24 (57%) | 0.01 | 44 (46%) | 0.08 |
| Menorrhagia | 6 (6.3) | 12 (27.3) | 0.0004 | 28 (30.4) | <.0001 |
| Metrorrhagia | 12 (12.6) | 12 (27.3) | 0.03 | 14 (15.2) | 0.69 |
* and † are P- values for relaxin 10 ug/kg/day compared to placebo and 25 ug/kg/day compared to placebo
In 14 patients, adverse events led to discontinuation of the study medication. In the placebo group, 2 patients discontinued due to anemia related to gastric antral vascular ectasia, 1 patient had anemia of unknown cause, and 1 patient had new-onset renal crisis (4 patients overall). In the 10 ug/kg/day group 1 patient discontinued due to anemia of unknown cause, 1 patient had congestive heart failure and pulmonary hypertension, 1 patient had fatigue and dizziness, and 1 patient had an allergic reaction to contrast dye with cardiac arrest (4 patients overall). In the 25 ug/kg/day group, 2 patients discontinued due to severe anemia of unknown cause, 1 patient each had menorrhagia, pericardial and pleural effusion, worsening lung function, and nausea/ vomiting (6 patients overall).
There were 2 deaths reported in the 25 ug/kg/day group. One patient died after treatment for 22 weeks; she developed uncontrolled congestive heart failure and died of cardiac arrest. Her condition was complicated by anemia, gastrointestinal bleeding, and hypertension. Another subject died of acute scleroderma renal failure, 3 weeks after stopping the relaxin therapy; this patient also had a history of pericarditis during her scleroderma renal crisis.
Discussion
We report the results of a phase III study of recombinant relaxin in diffuse scleroderma. This study was undertaken based on encouraging results of the phase II study and in vitro studies showing the antifibrotic potential of relaxin (5;6). The phase II study randomized patients with diffuse scleroderma into 3 groups— placebo, 25 ug/kg/day, and 100 ug/kg/day. The 25 ug/kg/day relaxin group had significantly improved total skin score and functional outcomes compared with the placebo and 100 ug/kg/day groups regarding (5).
Unlike the phase II study, the current phase III study did not find any difference among the placebo, 10 ug/kg/day, and 25 ug/kg/day relaxin-treated patients for change in skin score, diffusion capacity, or functional disability at 24 weeks. The primary outcome, the MRSS, declined statistically equally in all 3 groups over the course of the 24 week clinical trial. A decline in total skin score for all groups after entry into clinical trials has previously been reported in diffuse SSc (16;17). This change likely represents the natural history of diffuse SSc in patients entering SSc clinical trials.
The present trial incorporated patients with early diffuse SSc (16;18); subgroup analysis stratified by disease duration (≤2.5 years vs. > 2.5 years) showed no differences in the MRSS, diffusion capacity, or functional disability between the placebo and relaxin arms.(data not shown)
There were also statistically significant, but not clinically meaningful decreases in the FVC % predicted-favoring placebo (mean differences compared to placebo of -3.6% predicted (P=0.03) for the 10 mcg/kg/d relaxin group and - 1.7% predicted in the 25 mcg/kg/d relaxin group(p=0.02). These data support the data from the other secondary outcomes, indicating that relaxin had no positive effects in this clinical trial.
Changes in renal physiology associated with relaxin therapy were noted and were reminiscent of those seen in pregnancy: increase in creatinine clearance, lowering of systolic and diastolic blood pressures and decreases in hemoglobin (possibly related to dilutional effects of increased blood volume) (1;19). Relaxin causes renal vasodilatation and hyperfilteration in pregnancy by increasing nitric oxide production via stimulation of NO-synthase-II (1;19). In addition, relaxin acts on endothelin by binding to the endothelin B receptor (ETB), which is involved in renal vasodilatation, hyperfilteration, and reduced myogenic reactivity of small renal arteries. Therefore, it was not unexpected that effects of renal vasodilatation and hyperfilteration disappeared when relaxin was withdrawn.
Unexpected, however, were the abrupt appearance of severe hypertension and renal impairment in a disproportionate number of the patients who abruptly stopped active relaxin therapy since no such signal was seen in the phase II study. There are recognized abnormalities in SSc renal physiology which may have predisposed the SSc patients to such renal events. Many patients with SSc have reduced renal blood flow, higher plasma renin levels, both reclining or sitting at rest, after exposure to cold, or with sodium depletion (20). This suggests that the renovascular systems of patients with SSc are sensitive to changes in blood flow and other stimuli and may have contributed to the new-onset hypertension and in some cases full-blown renal crisis.
Although adverse renovasclar effects of relaxin have only been reported among patients with SSc, only a small number of people without scleroderma have received treatment with relaxin over any prolonged period of time(21). Until there has been significantly more experience in healthy controls or in patients with circulatory or renal abnormalities, we recommend that caution be employed in the use of relaxin. We suggest at a minimum that patients who receive relaxin therapy have their blood pressure monitored daily during and for several weeks following withdrawal of relaxin as a means of monitoring for new-onset hypertension or acute renal impairment. If hypertension appears, then serial measurement of serum creatinine and control of blood pressure are mandatory. Such monitoring seemed to have been successful in this study where only 1 additional serious renal adverse event was noted after relaxin withdrawal when the above precautions were taken.
There was a statistically significant decline in hemoglobin in the relaxin arms compared to placebo, a pattern of anemia also observed in previous studies of relaxin for SSc (5;6). In addition, menorrhagia was reported in 29% of patients in the relaxin groups compared to 6% in the placebo group (p < 0.01). Relaxin induces the expression of an angiogenic agent, vascular endothelial growth factor (VEGF) (22;23)and VEGF may cause menorrhagia by inducing neovascularization of the endometrial lining (22). In this study, a decline in hemoglobin may have been due to relaxin-induced menorrhagia or some other unknown factor (eg dilution). The drop in hemoglobin was no longer statistically different between placebo and relaxin arms at week 28 (4 weeks after relaxin was stopped). The effects of relaxin on creatinine clearance, blood pressure, and menorrhagia suggest that a biological effect was indeed achieved in this clinical trial.
There is renewed interest in assessing the biological effects of relaxin in different experimental models since relaxin downregulates collagen production and increases collagen degradation (1). For example, relaxin deficient mutant mice show an age-related progression of dermal fibrosis and skin thickening along with internal organ fibrosis similar to scleroderma; treatment with recombinant human gene-2 relaxin reverses the dermal fibrosis in early disease(24). Recent in vitro and in vivo experiments have assessed the role of relaxin in modulating fibroblast function and collagen production in pulmonary, liver, kidney, and cardiac fibrosis (25-27). Recent studies characterizing relaxin receptors in tissue (LGR7, LGR8, GPCR135 and GPCR142) have emphasized the pleiotropic actions of this family of peptides on both tissue fibrosis, angiogenesis and vascular tone(28). These basic and translational research data have led to clinical trials in humans. In fact, clinicaltrials.gov (accessed on June 11th, 2008) lists 5 phase II clinical trials using relaxin for heart failure, pre eclampsia, or induction of labor. These ongoing investigations higlight the need to report this large clinical trial, especially as the adverse events seen in this study should inform the design of future clinical trials and should, in our view, require close follow-up after patients cease relaxin therapy.
In conclusion, recombinant human relaxin given by continuous subcutaneous infusion over a 24 week period of time was not significantly better than placebo in improving total skin score, pulmonary function, or function (as measured by HAQ-DI) in patients with diffuse cutaneous scleroderma. In addition, withdrawal of relaxin was associated with serious renal adverse events.
Acknowledgments
We wish to thank Vivien M. Hsu, MD and Deborah A. McCloskey, RN at UMDNJ and Mildred Sterz, RN at UCLA for their invaluable help during this study.
Dr. Khanna was supported by a National Institutes of Health Award (NIAMS K23 AR053858-01A1) and the Scleroderma Foundation (New Investigator Award).
Appendix figure
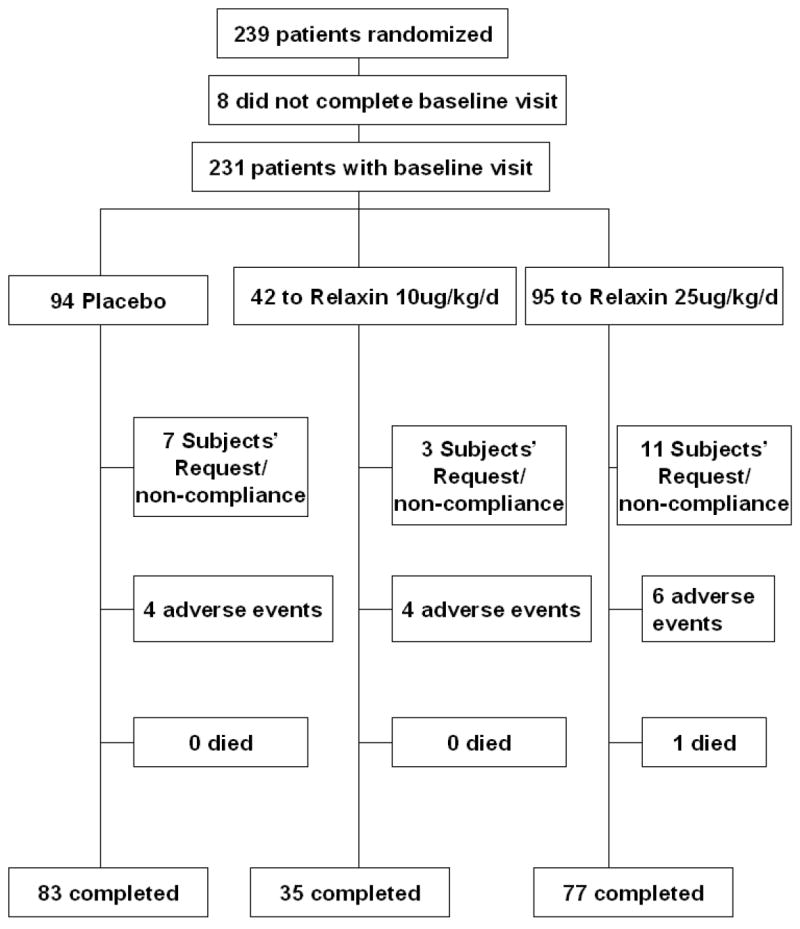
Trial profile
Reference List
- 1.Samuel CS, Hewitson TD. Relaxin in cardiovascular and renal disease. Kidney Int. 2006;69(9):1498–502. doi: 10.1038/sj.ki.5000264. [DOI] [PubMed] [Google Scholar]
- 2.Unemori EN, Amento EP. Relaxin modulates synthesis and secretion of procollagenase and collagen by human dermal fibroblasts. J Biol Chem. 1990;265(18):10681–5. [PubMed] [Google Scholar]
- 3.Unemori EN, Bauer EA, Amento EP. Relaxin alone and in conjunction with interferon-gamma decreases collagen synthesis by cultured human scleroderma fibroblasts. J Invest Dermatol. 1992;99(3):337–42. doi: 10.1111/1523-1747.ep12616665. [DOI] [PubMed] [Google Scholar]
- 4.Unemori EN, Beck LS, Lee WP, Xu Y, Siegel M, Keller G, et al. Human relaxin decreases collagen accumulation in vivo in two rodent models of fibrosis. J Invest Dermatol. 1993;101(3):280–5. doi: 10.1111/1523-1747.ep12365206. [DOI] [PubMed] [Google Scholar]
- 5.Seibold JR, Korn JH, Simms R, Clements PJ, Moreland LW, Mayes MD, et al. Recombinant human relaxin in the treatment of scleroderma. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2000;132(11):871–9. doi: 10.7326/0003-4819-132-11-200006060-00004. [DOI] [PubMed] [Google Scholar]
- 6.Seibold JR, Clements PJ, Furst DE, Mayes MD, McCloskey DA, Moreland LW, et al. Safety and pharmacokinetics of recombinant human relaxin in systemic sclerosis. J Rheumatol. 1998;25(2):302–7. [PubMed] [Google Scholar]
- 7.Preliminary criteria for the classification of systemic sclerosis (scleroderma) Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum. 1980;23(5):581–90. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 8.Clements PJ, Lachenbruch PA, Seibold JR, Zee B, Steen VD, Brennan P, et al. Skin thickness score in systemic sclerosis: an assessment of interobserver variability in 3 independent studies. J Rheumatol. 1993;20(11):1892–6. [PubMed] [Google Scholar]
- 9.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–83. [PubMed] [Google Scholar]
- 10.Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum. 1980;23(2):137–45. doi: 10.1002/art.1780230202. [DOI] [PubMed] [Google Scholar]
- 11.Khanna D, Clements PJ, Postlethwaite AE, Furst DE. Does Incorporation of Aids and Devices Make a Difference in the Score of the Health Assessment Questionnaire-Disability Index? Analysis from a Scleroderma Clinical Trial. J Rheumatol. 2008;35(3):466–8. [PubMed] [Google Scholar]
- 12.Khanna D, Furst DE, Clements PJ, Park GS, Hays RD, Yoon J, et al. Responsiveness of the SF-36 and the Health Assessment Questionnaire Disability Index in a systemic sclerosis clinical trial. J Rheumatol. 2005;32(5):832–40. [PubMed] [Google Scholar]
- 13.Khanna D, Park GS, Seibold J. SF-36 Scales in the Relaxin study. Rheumatology (Oxford) 2007;46(4):724. doi: 10.1093/rheumatology/kem009. [DOI] [PubMed] [Google Scholar]
- 14.Seibold JR, McCloskey DA. Skin involvement as a relevant outcome measure in clinical trials of systemic sclerosis. Curr Opin Rheumatol. 1997;9(6):571–5. doi: 10.1097/00002281-199711000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Khanna D, Furst DE, Hays RD, Park GS, Wong WK, Seibold JR, et al. Minimally Important Difference in Diffuse Systemic Sclerosis- Results from the D-Penicillamine Study. Ann Rheum Dis. 2006;65(10):1325–9. doi: 10.1136/ard.2005.050187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denton CP, Merkel PA, Furst DE, Khanna D, Emery P, Hsu VM, et al. Recombinant human anti-transforming growth factor beta1 antibody therapy in systemic sclerosis: A multicenter, randomized, placebo-controlled phase I/II trial of CAT-192. Arthritis Rheum. 2006;56(1):323–33. doi: 10.1002/art.22289. [DOI] [PubMed] [Google Scholar]
- 17.Merkel PA, Silliman N, Clements P, Denton CP, Furst DE, Mayes M, et al. Performance of the Modified Rodnan Skin Score in Clinical Trials. Arthritis Rheum. 2005;52:S283. [Google Scholar]
- 18.Clements PJ, Furst DE, Wong WK, Mayes M, White B, Wigley F, et al. High-dose versus low-dose D-penicillamine in early diffuse systemic sclerosis: analysis of a two-year, double-blind, randomized, controlled clinical trial. Arthritis Rheum. 1999;42(6):1194–203. doi: 10.1002/1529-0131(199906)42:6<1194::AID-ANR16>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 19.Conrad KP. Mechanisms of renal vasodilation and hyperfiltration during pregnancy. J Soc Gynecol Investig. 2004;11(7):438–48. doi: 10.1016/j.jsgi.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Clements PJ, Lachenbruch PA, Furst DE, Maxwell M, Danovitch G, Paulus HE. Abnormalities of renal physiology in systemic sclerosis. A prospective study with 10-year followup. Arthritis Rheum. 1994;37(1):67–74. doi: 10.1002/art.1780370110. [DOI] [PubMed] [Google Scholar]
- 21.Samuel CS, Hewitson TD, Unemori EN, Tang ML. Drugs of the future: the hormone relaxin. Cell Mol Life Sci. 2007;64(12):1539–57. doi: 10.1007/s00018-007-6543-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Unemori EN, Erikson ME, Rocco SE, Sutherland KM, Parsell DA, Mak J, et al. Relaxin stimulates expression of vascular endothelial growth factor in normal human endometrial cells in vitro and is associated with menometrorrhagia in women. Hum Reprod. 1999;14(3):800–6. doi: 10.1093/humrep/14.3.800. [DOI] [PubMed] [Google Scholar]
- 23.Palejwala S, Tseng L, Wojtczuk A, Weiss G, Goldsmith LT. Relaxin gene and protein expression and its regulation of procollagenase and vascular endothelial growth factor in human endometrial cells. Biol Reprod. 2002;66(6):1743–8. doi: 10.1095/biolreprod66.6.1743. [DOI] [PubMed] [Google Scholar]
- 24.Samuel CS, Zhao C, Yang Q, Wang H, Tian H, Tregear GW, et al. The relaxin gene knockout mouse: a model of progressive scleroderma. J Invest Dermatol. 2005;125(4):692–9. doi: 10.1111/j.0022-202X.2005.23880.x. [DOI] [PubMed] [Google Scholar]
- 25.Williams EJ, Benyon RC, Trim N, Hadwin R, Grove BH, Arthur MJ, et al. Relaxin inhibits effective collagen deposition by cultured hepatic stellate cells and decreases rat liver fibrosis in vivo. Gut. 2001;49(4):577–83. doi: 10.1136/gut.49.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heeg MH, Koziolek MJ, Vasko R, Schaefer L, Sharma K, Muller GA, et al. The antifibrotic effects of relaxin in human renal fibroblasts are mediated in part by inhibition of the Smad2 pathway. Kidney Int. 2005;68(1):96–109. doi: 10.1111/j.1523-1755.2005.00384.x. [DOI] [PubMed] [Google Scholar]
- 27.Mookerjee I, Unemori EN, Du XJ, Tregear GW, Samuel CS. Relaxin modulates fibroblast function, collagen production, and matrix metalloproteinase-2 expression by cardiac fibroblasts. Ann N Y Acad Sci. 2005;1041:190–3. doi: 10.1196/annals.1282.028. [DOI] [PubMed] [Google Scholar]
- 28.Park JI, Chang CL, Hsu SY. New Insights into biological roles of relaxin and relaxin-related peptides. Rev Endocr Metab Disord. 2005;6(4):291–6. doi: 10.1007/s11154-005-6187-x. [DOI] [PubMed] [Google Scholar]


