Abstract
Limbal stem cell deficiency (LSCD) leads to severe ocular surface abnormalities that can result in the loss of vision. The most successful therapy currently being used is transplantation of limbal epithelial cell sheets cultivated from a limbal biopsy obtained from the patient´s healthy, contralateral eye or cadaveric tissue. In this study, we investigated the therapeutic potential of murine vibrissae hair follicle bulge-derived stem cells (HFSC) as an autologous SC source for ocular surface reconstruction in patients bilaterally affected by LSCD.
This study is an expansion of our previously published work showing transdifferentiation of HFSC into cells of a corneal epithelial phenotype in an in vitro system. In this study we used a transgenic mouse model, K12rtTA/rtTA/tetO-cre/ROSAmTmG, which allows for HFSC to change color, from red to green, once differentiation to corneal epithelial cells occurs and Krt12, the corneal epithelial-specific differentiation marker, is expressed. HFSC were isolated from transgenic mice, amplified by clonal expansion on a 3T3 feeder layer and transplanted on a fibrin carrier to the eye of LSCD wild type mice (n=31). The HFSC transplant was able to reconstruct the ocular surface in 80% of the transplanted animals; differentiating into cells with a corneal epithelial phenotype, expressing Krt12, repopulating the corneal SC pool while suppressing vascularization and conjunctival ingrowth. These data highlight the therapeutic properties of using HFSC to treat LSCD in a mouse model while demonstrating a strong translational potential and points to the niche as a key factor for determining stem cell differentiation.
Introduction
Over the past couple of years tissue engineering has become a rapidly growing field of research that is anticipated to reveal its translational potential through application of adult stem cells (SC) in clinical practice. It is believed that SC with their inherent plasticity could be utilized to regenerate the natural complexity present in native tissue, while providing factors required for lineage maintenance and differentiation. Tissue engineering consists of a cell-supporting scaffold or matrix, cells and cell signalling molecules each of which contribute to the therapeutic success of the construct. Recently many research groups have turned their attention to examining the various adult SC types already known, in an effort to identify the most suitable source for tissue reconstruction [1].
Homeostasis of the corneal epithelium is crucial for corneal transparency and unimpaired vision and is provided by a population of adult SC. These SC are localized in the basal epithelium of the limbus within protected epithelial ridges, the palisades of Vogt [2]. Their maintenance and function are controlled by various intrinsic and extrinsic factors provided by a unique local microenvironment or niche [3–5]. Since limbal SC reside within small clusters in the basal epithelium in a very close spatial relationship with specific basement membrane and matrix components [6] as well as with stromal fibroblasts and blood vessels, they are set in a very unique environment and are provided with specific growth and survival factors that have not been completely identified yet [3].
Dysfunction or loss of the limbal SC population either congenital or acquired by varying pathologies e.g., in cases of chemical burns may result in partial or total limbal stem cell deficiency (LSCD), which untreated often leads to functional blindness. One of the therapies currently being used to treat this disorder is transplantation of cultivated autologous epithelial cell sheets. This approach requires taking a limbal biopsy from the patient´s healthy, contralateral eye in order to isolate stem and progenitor cells and expand them on a transplantable carrier [7, 8]. For this reason this therapy is only applicable to patients suffering from unilateral LSCD who still possess a healthy eye allowing for a biopsy to be taken. In cases of bilateral LSCD an allogenic limbal biopsy is taken either from an immunologically related living donor or cadaveric tissue. Due to donor number limitations and the requirement for a prolonged systemic immunosuppressive therapy, the success rate of this therapeutic approach is drastically decreased. For this reason the focus of current research in this field has turned to the search for alternative autologous SC sources appropriate for transplantation and ocular surface restoration.
Several research groups have focused on treating LSCD through grafting of cultivated oral mucosa epithelial cells; however, this treatment has not provided satisfactory long-term results. It should also be mentioned that oral mucosal epithelial cell grafts seldom transdifferentiate to a corneal epithelium phenotype, e.g., expression of keratin 12 (Krt12) [9]. Therefore there is still a need for an alternative autologous SC source to improve the treatment of patients with bilateral LSCD. Recently some research groups have focused on utilizing the hair follicle (HF) as a source of multiple populations of adult SC [10] for regenerative medicine. The HF harbours mesenchymal SC in the dermal papilla and connective tissue sheath, which possess the potential to differentiate into several cell lineages including hematopoietic, adipogenic, osteogenic, chondrogenic, myogenic, and neurogenic under the influence of appropriate conditions in vitro and in vivo [11–13]. In addition, the HF comprises a population of adult SC of epithelial origin, which resides in the bulge region of the outer root sheath and can differentiate either under normal physiological conditions into HF and sebaceous glands or after injury into epidermis [10, 14, 15].
This bulge-derived keratinocyte population is characterized by expression of a wide spectrum of cytokeratins, i.e., Krt5, Krt15, Krt17, and Krt19 [16–18]. Krt15 has been reported to be a specific SC and progenitor cell marker and has been successfully utilized for purification and enrichment of SC by means of clonal growth on a 3T3 feeder cell layer [19].
As we showed in our previous study, HFSC have a high degree of plasticity and can cross cell lineage boundaries and differentiate into a different cell phenotype when given appropriate stimuli in vitro. Utilizing conditioned media harvested from corneal and limbal stromal fibroblasts, we demonstrated that HFSC were capable of being reprogrammed ex vivo into cells with a corneal epithelial phenotype [19]. In order to expand on our preliminary in vitro findings, we performed in vivo experiments using a transgenic mouse model that allows for HFSC to change color, from red to green, once differentiation to corneal epithelial cells occurs and Krt12, the corneal epithelial-specific differentiation marker, is expressed. Given the low success rate of the current treatment for patients with bilateral LSCD as well as the unsatisfactory long-term results of using cultivated oral mucosa epithelial cells there is a need for an alternative SC source. The data presented here demonstrate the positive impact that HFSC will have on treatment of LSCD. Therapeutically it is important that the source of autologous SCs is readily accessible and that the cells are easily isolated. It is also of utmost importance that the SCs persist and, once differentiated, express cornea epithelial specific proteins such as Krt12 and Pax6. Here we show that HFSC are cable of doing just this in a mouse model demonstrating the translational potential of using these cells in the treatment of LSCD.
Materials and Methods
Triple Transgenic Mice
Animal care and use conformed to the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research. All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Cincinnati. Genetically modified mouse lines such as Krt12rtTA [20], tetO-Cre [21] and Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J(ROSAmTmG) mice [22] have been previously described. Compound transgenic mice were generated by breeding each individual mouse line to create a triple transgenic mouse (K12rtTA/rtTA/tetO-cre/ROSAmTmG). Genotypes were identified by polymerase chain reaction (PCR) using oligonucleotide primers specific for each transgene.
Culture media
Basic culture medium for murine hair follicle (HF) epithelial cells was prepared containing three parts of Dulbecco´s modified Eagle´s medium (DMEM) without calcium and magnesium and one part of Ham´s F12 medium (HyClone, U.S.) supplemented with 10% fetal calf serum (FCS) (Invitrogen, Germany), 10 ng/ml hEGF (Chemicon, U.S.), 500 mg/l L-glutamin (Invitrogen, U.S.), and human corneal growth supplement (HCGS) consisting of 0.2% bovine pituitary extract, 0.18 µg/ml hydrocortisone, 5 µg/ml insulin and 5 µg/ml transferrin (Tebu-Bio, France) as well as 10,000 units/ml penicillin, 10,000 µg/ml streptomycin, 25 µg/ml amphotericin B (Invitrogen, U.S.). A final calcium concentration of 0.4 mM was achieved using a 0.2 M CaCl2 solution (Invitrogen, U.S.).
Culture medium for 3T3 murine fibroblasts contained DMEM (high glucose 4500g/l), supplemented with 10% FCS (Invitrogen, U.S.) and 10,000 units/ml penicillin, 10,000 µg/ml streptomycin, 25 µg/ml amphotericin B (Invitrogen, U.S.).
Isolation of the vibrissae hair follicle-derived stem cells
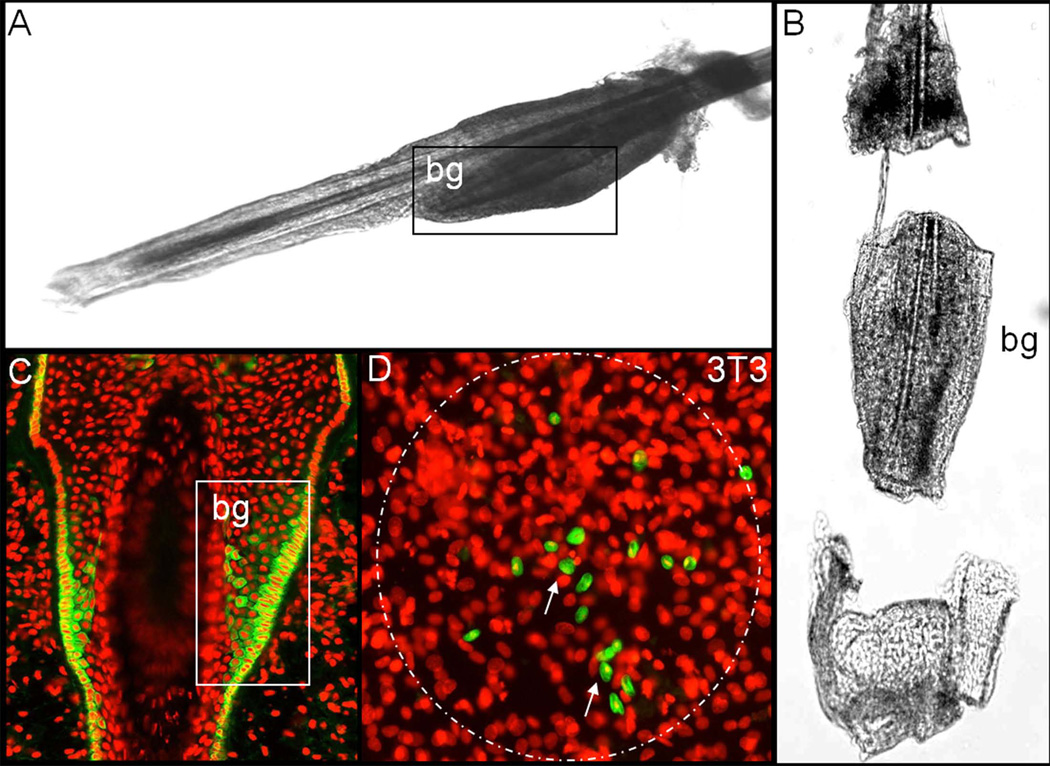
We proceeded according to the method already established by our research group on C57BL/6 wild type mice [19]. Upper lips containing the pads with the vibrissae were dissected from 3–5 week old triple transgenic mice and their inner surface exposed. The subcutaneous fat and the connective tissue were carefully removed and the vibrissae pulled away from the pad. The collagen capsule was cut open and almost completely removed by loosening it from the epithelial core and pulling it down along the hair shaft. The whole preparative procedure was performed under a dissecting microscope using very fine forceps and scissors. The isolated epithelial cores were cut into three portions (Fig. 1) and the middle part harboring the HF bulge and its SC were transferred into a 35 mm plastic dish containing 2 ml of collagenase (2 mg/ml) (Roche, Switzerland) and incubated for approximately 1 hour at 37°C to completely remove the mesenchymal remains of the capsule. Afterwards the HF were transferred to a fresh dish containing 5 ml dispase/trypsin (2.4 U/0.05%) (Roche/Invitrogen) solution and digested for another 1.5 hours, in order to obtain a single cell suspension of the epithelial cell population.
Figure 1.
Stem cell isolation and expansion (A, B) Murine vibrissae hair follicles were isolated under a dissecting microscope, in order to access the bulge region harbouring the epithelial stem cells (SC), which express keratin 15 (Krt15) (C), the mesenchymal capsule was completely removed. (D) Following the enzymatic digestion of the epithelial core´s central portion, the cells were seeded on a 3T3 feeder cell layer to purify and enrich the SC by means of clonal expansion. The presence of the putative SC marker Krt15 (green) was confined to several small cell clusters (arrow) within each holoclone. bg: bulge; nuclear staining: propidium iodide (red). A,B,C: reprinted with permission [19].
Clonal growth assay
To select and enrich stem and progenitor cells from the epithelial cell suspension, a clonal growth assay was performed. Epithelial cells were seeded at a density of 1×103 cells/cm2 onto a mitomycin C-treated, cell cycle inactivated [19], NIH 3T3 (ATCC, U.S.) feeder cell layer in 6-well culture plates. Cells were cultivated for 14–21 days at 37°C, 5% CO2 and in a humidified atmosphere prior to transfer of the holoclones to a fibrin carrier. The culture medium (see above) was changed every second day.
Cell transfer and cultivation on a fibrin carrier
Once the holoclones reached an appropriate size they were transferred to a fibrin carrier according to our standard subcultivation method [19]. Briefly, the 3T3 feeder layer was removed using Versene (Invitrogen, U.S.) for 60 sec. at room temperature followed by washing with PBS several times. The remaining, still adherent holoclones were trypsinized (0.25%Trypsin-EDTA) (Invitrogen, U.S.) for 15 min at 37°C, centrifuged and counted. Clonal cells were seeded at a density of 1×105 cells/cm2 onto a fibrin carrier and cultivated for 3 days, under the same conditions, to obtain a confluent epithelial cell layer ready for transplantation.
The fibrin carrier was prepared by dissolving fibrinogen and thrombin stock solutions (Tissucol; Baxter, Germany) in 1.1% NaCl and 1 mM CaCl2 to a final concentration of 10 mg/ml fibrinogen and 3 IU/ml thrombin [23].
Limbal stem cell deficiency and HFSC transplantation
Total limbal stem cell deficiency was performed on 6–8 week old C57BL/6 (Jackson Labs, U.S.) mice using an Algerbrush II corneal rust ring remover with a 0.5 mm burr. Mice were anesthetized with ketamine (87 mg/kg) and xylazine (13 mg/kg) followed by the removal of the entire limbal and corneal epithelium. Following limbal stem cell deficiency, HFSC on the fibrin carrier were sutured onto the eye. After surgical recovery, mice were treated with anti-inflammatory drops (Inflanefran® Forte, Allergan, U.S.) for the first 5 days and then with Avastin® (bevacizumab) (Genentech, Inc, U.S.) eye drops both of which were administered daily to both control and experimental animals. To monitor for conversion into corneal epithelial cells, mice were fed chow containing doxycycline (1 g/kg; Custom Animal Diets, U.S.) until samples were collected. Control mice were fed regular chow. Samples were collected at 3 d (n=3), 1 w (n=5), 2 w (n=8), 3 w (n=7), 5 w (n=5), and 7 w (n=3). Assessment of the success rate of ocular surface reconstruction using HFSC was made based on data collected throughout a five week period. The mouse cornea epithelium has a turnover rate of approximately 2 weeks; specimens collected at 5 weeks will have turned over approximately 2.5 times and was therefore chosen as the endpoint to assess the therapeutic potential of the HFSC transplant. The preliminary therapeutic outcome at 7 weeks has also been added to Table 1 but note that overall percentages were based on the 5 week time point.
Table 1.
Assessment of the therapeutic potential of HFSC to treat a mouse model of LSCD
| Time Post-transplantation |
Number | Goblet Cells Peripheral Cornea |
Vascularization | Ocular Surface Reconstruction |
|---|---|---|---|---|
| 3d | 3 | 0/3 (0%) | 0/3 (0%) | 3/3 (100%) |
| 1 wk | 5 | 1/5 (20%) | 0/5 (0%) | 4/5 (80%) |
| 2 wk | 8 | 1/8 (12.5%) | 1/8 (12.5%) | 7/8 (87.5%) |
| 3 wk | 7 | 2/7 (29%) | 1/7 (14%) | 5/7 (71%) |
| 5 wk | 5 | 1/5 (20%) | 1/5 (20%) | 4/5 (80%) |
| 7 wk | 3 | 1/3 (33%) | 1/3 (33%) | 2/3 (67%) |
Assessment of Barrier Function
The integrity of the corneal epithelium was assessed via a fluorescein uptake assay. Briefly, fluorescein stain was added to the eyes and allowed to permeate for 30 sec. Subsequently the eyes were washed extensively with PBS and examined using an epi-fluorescent stereomicroscope (Stemi SVII, Carl Zeiss).
Histology and Immunofluorescence
For all staining procedures, eyes were enucleated and fixed in 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4 at 4°C overnight. Following fixation, the eyes were washed extensively in PBS before proceeding with the staining.
Whole Mount Imaging
Corneas were treated with 0.2% sodium borohydride for 30 min at room temperature followed by washing in 0.1 M phosphate buffer, pH 7.4. Corneas were then stained with 4’,6-diamidino-2-phenylindole (DAPI) and visualized using a Zeiss Observer Z1 microscope with an apotome attachment.
Immunofluorescence
Ten micrometer cryosections were washed twice in PBS containing 0.15% glycine and 0.5% BSA (bovine serum albumin) for 10 min followed by three 10 min washes in PBS. Sections were blocked with 2% BSA at room temperature followed by incubation with the primary antibody in PBST (PBS, 0.03% Tween20) containing 0.5% BSA overnight at 4°C. The primary antibodies used were as follows: Krt12 (1:200, Kao lab [24]), Krt10 (1:500, Covance), Krt4 (1:150, Novus Biologicals), Krt15 (1:500, Covance), Pax6 (1:300, Covance). Proteins were visualized by indirect immunofluorescence with appropriate secondary antibody conjugates containing AlexaFluor 647 (Invitrogen, U.S.). All specimens were counterstained with DAPI for nuclei and observed using a Zeiss Observer Z1 microscope. In order to stain cultured SC clones we proceeded according to our previously established protocol [19].
Results
HFSC isolation: Phenotypical characterization of SC clones (holoclones)
To isolate the HFSC we proceeded according to the protocol previously established by our research group on C57BL/6 wild type mice [19], which has been slightly modified. Murine vibrissae HF were isolated under a dissecting microscope using very fine surgical instruments. In order to access the bulge region harboring the SC, the mesenchymal capsule surrounding the epithelial core was carefully removed and the remaining epithelial core cut into three portions (Fig. 1A, B). Subsequently the middle portion underwent a multi-step enzymatic digestion to finally obtain a single cell suspension. The cells were seeded on a 3T3 feeder cell layer to purify and expand stem and progenitor cells by means of clonal growth as well as to enrich them for further purposes. The clones were characterized phenotypically to determine the grade of differentiation using immunohistochemistry. The presence of the putative SC marker Krt15, previously reported to be expressed abundantly in murine bulge cells [19, 25, 26] (Fig. 1C), was confined to several small cell clusters within each SC clone (holoclone) [27] (Fig. 1D). This observation confirmed previous reports, that Krt15 positive cells are clonogenic and possess a high proliferative capacity and ability to give rise to holoclones [28]. Some of the central superficial cells of the holoclones already revealed a very low and scarce expression of Krt10, an epidermal differentiation marker, thus marking the beginning of epidermal differentiation after 2 weeks of culture. The corneal epithelial differentiation marker Krt12 was not detected within the colonies as determined by immunohistochemistry (data not shown). Our culture method for murine SC by means of clonal growth on a 3T3 feeder cell layer is a successful tool to select and enrich HFSC.
Clonal growth, subcultivation on a fibrin carrier and transplantation
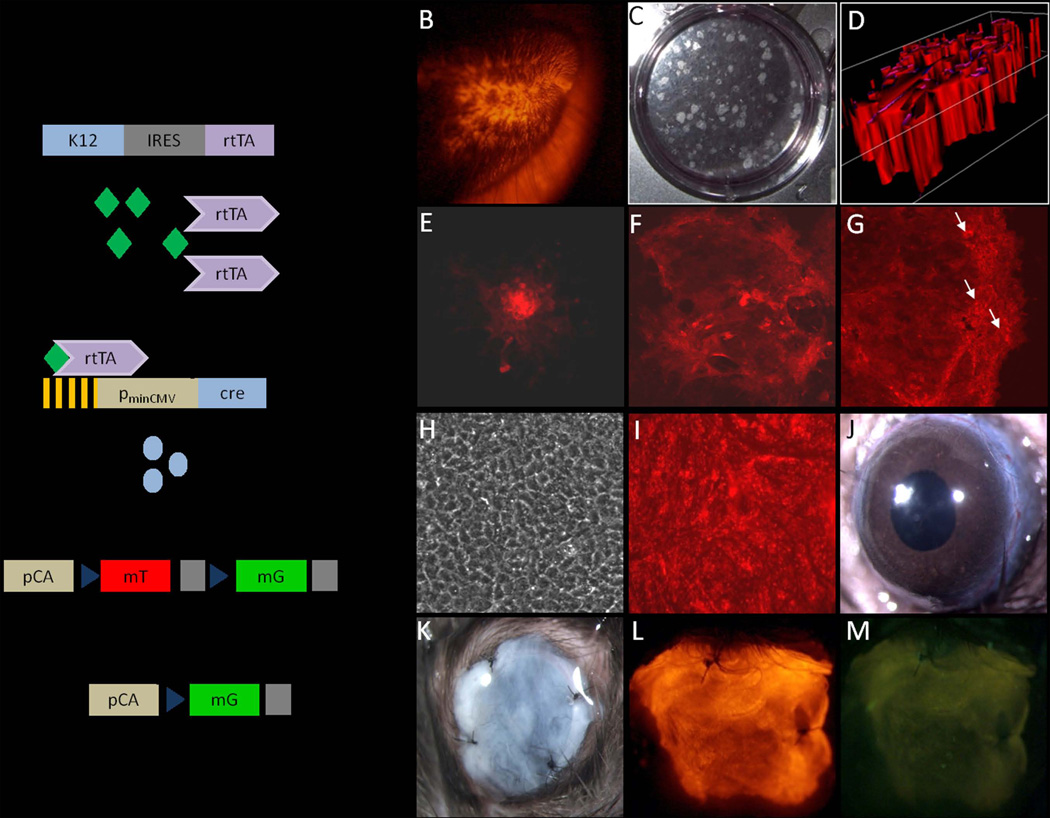
In order to expand on our previous work in vitro [19] and to show the potential of HFSC to differentiate into corneal epithelial cells in vivo, an inducible, tissue specific triple transgenic mouse model (K12rtTA/rtTA/TetO-Cre/ ROSAmTmG) was utilized in this study. Due to the monoallelic nature of Krt12, mice homozygous for rtTA were generated to ensure all cells expressing Krt12 also expressed rtTA [29]. In this transgenic mouse line, only those cells expressing Krt12 will be positive for EGFP (enhanced green fluorescence protein), when the mouse has been given doxycycline. In the absence of doxycycline, all cells express the membrane-bound tdtomato red fluorescent protein (mT) (Fig. 2A). Vibrissae HF were dissected from the lips of whole body red mice (Fig. 2B) and the enzymatically derived epithelial cell suspension was seeded onto a 3T3 feeder layer to obtain holoclones (Fig. 2C, D) as described above.
Figure 2.
Subcultivation on a fibrin carrier and transplantation. (A) Diagram of the triple transgenic mouse model showing the inducible, tissue specific nature of this system. In this system, only in corneal epithelial cells can rtTA (reverse tetracycline transcriptional activator) be expressed. In the presence of doxycycline (dox), the rtTA/dox complex binds to the tetracycline operator elements (Tet-O) to drive the expression of Cre recombinase. Cre is then able to excise the tdTomato transgene encoding red fluorescent protein allowing for expression of EGFP. Following this recombination event EGFP expression is under the control of the chicken actin (pCA) promoter. Vibrissae hair follicles (HFs) were dissected from lips of whole body red triple transgenic mice. (B, C) The enzymatically isolated epithelial cells were seeded onto a 3T3 feeder cell layer to grow holoclones; white light (C); red light, 3-D imaging of a holoclone (D). (E–G) The clonal growth was monitored over the 2 week culture period and showed progressively growing red fluorescent holoclones, whereas EGFP expression was completely absent. The clones were photographed on culture day 3 (E), 7 (F), and 14 (G). (H, I) After two weeks of culture, the clones were transferred onto a biodegradable fibrin carrier and cultured for several days to form a confluent cell sheet appropriate for transplantation; white light (H), red and green light merged (I). (J) Prior to transplantation limbal stem cell deficiency was generated on murine eyes using an Algerbrush II corneal rust ring remover with a 0.5 mm burr. (K) The transplants were anchored to the conjunctiva with four sutures with the cells facing the intact recipient´s corneal epithelium basement membrane. (L, M) Examination of the sutured eye revealed that the HFSC were still intact and expressing the red fluorescent tdtomato protein, but as expected lacked EGFP. Magnification: B – 8X, E-I– 100X, J-M – 20X
The clonal growth was monitored over the 2 week culture period and revealed progressively growing holoclones (Fig. 2E–G) that expressed mT and showed no EGFP expression when observed under UV light. The clones were comprised of mostly small, compact epithelial cells. Several large, flat cells, indicative of the early stages of differentiation, were found only within the center, most superficial layer of the holoclone. After approximately 2 weeks of culture the holoclones were transferred to a biodegradable fibrin carrier and cultivated for several days until they formed a confluent cell sheet. Immunofluorescent staining revealed that the HFSC on the fibrin carrier expressed Krt15 as well as low levels of Krt10 but were negative for Pax6 and Krt12 indicating that the cells had not started to express the corneal epithelial differentiation markers (data not shown). Examination prior to transplantation under white and UV light (Fig. 2H, I) revealed a dense multilayered cell sheet of red fluorescent cells. The fibrin carriers with the cells facing the intact recipient´s corneal epithelium basement membrane were transplanted onto the ocular surface of C57BL/6 wild type LSCD mice (Fig. 2J). The transplants were anchored to the conjunctiva with four sutures (Fig. 2K). Examination of the sutured eye under UV light revealed that the HFSC were still intact and expressing mT, but as expected lacked EGFP expression (Fig. 2L, M).
Reconstruction of the corneal epithelium
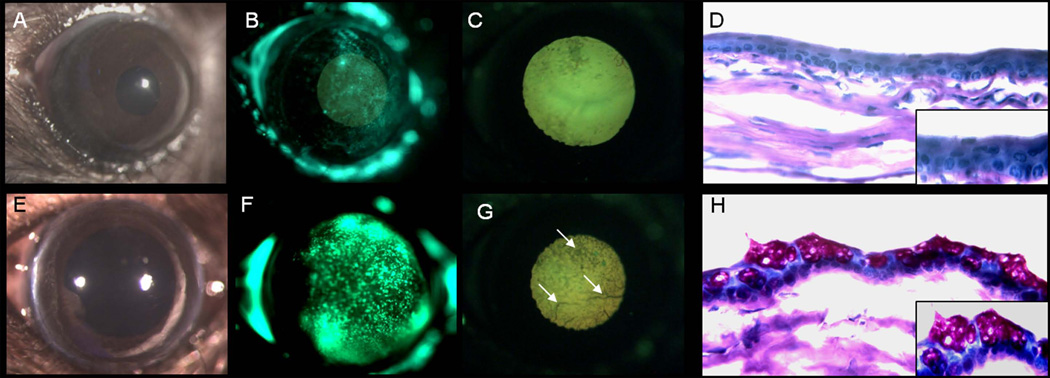
The cornea not only provides the major refractive power for producing a focused image on the retina, but also serves as a barrier to environmental factors that could damage the eye. The barrier function of the cornea is provided by the continuously renewed corneal epithelium that stratifies and forms intercellular tight junctions. To determine the success of the HFSC transplant in resurfacing the corneal epithelium, the mice, prior to euthanasia, were subjected to a fluorescein uptake assay to demonstrate the proper reconstruction of the intercellular tight junctions. Four weeks after receiving the HFSC graft mice had significantly less fluorescein stain present in the cornea when compared to control mice not receiving the transplant (Fig. 3B, F). This suggests that the HFSC are able to resurface the cornea; reforming intercellular tight junctions.
Figure 3.
HFSC enhanced barrier function and suppressed resurfacing by the conjunctiva following LSCD. (A, B, E, F) Transplant of the HFSC to the LSCD eye (A–D) resulted in reestablishment of tight junctions revealed by a decrease in fluorescein uptake in the ocular surface when compared to controls; white light (A, E), green light (B, F). (C, G) Stereomicroscopic examination with UV light also revealed a reduction in vascularization in eyes receiving the HFSC transplant (C) when compared to the control (G). (D, H) Periodic acid Schiff staining demonstrated the ability of the SC transplant to suppress resurfacing by the conjunctiva with little to no goblet cells present (D); however, without treatment the ocular surface is entirely covered by goblet cells (H). All images are from specimens 4 weeks after LSCD in the presence of the HFSC transplant (A–D) or absence (E–H). Magnification: A-C, E-G – 20X; D, H – 400X; inset 630X
It is known that, if left untreated, an eye with LSCD will be resurfaced by conjunctiva resulting in the presence of goblet cells and blood vessels on the ocular surface. In order to examine the level of conjunctiva overgrowth as well as to assess the general morphology of the corneal surface, a periodic acid Schiff (PAS) stain was performed. In the absence of the HFSC transplant, the morphology of the corneal surface was severely compromised as can be seen by the presence of goblet cells and blood vessels (Fig. 3 E–H). In contrast, the eyes receiving the HFSC transplant had a corneal surface closely resembling a normal corneal epithelium; with an average thickness of 3–5 cell layers, little to no goblet cells (Fig. 3D), and minimal vascularization (Fig. 3A, C). Inflammation was minimal in both the control and treated eyes due in part to the use of anti-inflammatory eye drops and Avastin® (Fig. 3D, H). The HFSC graft was able to reconstruct the ocular surface following LSCD in approximately 80% of the cases. The remaining 20% of the eyes contained a minor degree of conjunctiva ingrowth within the peripheral aspects of the cornea (Table 1).
Differentiation into corneal epithelial cells
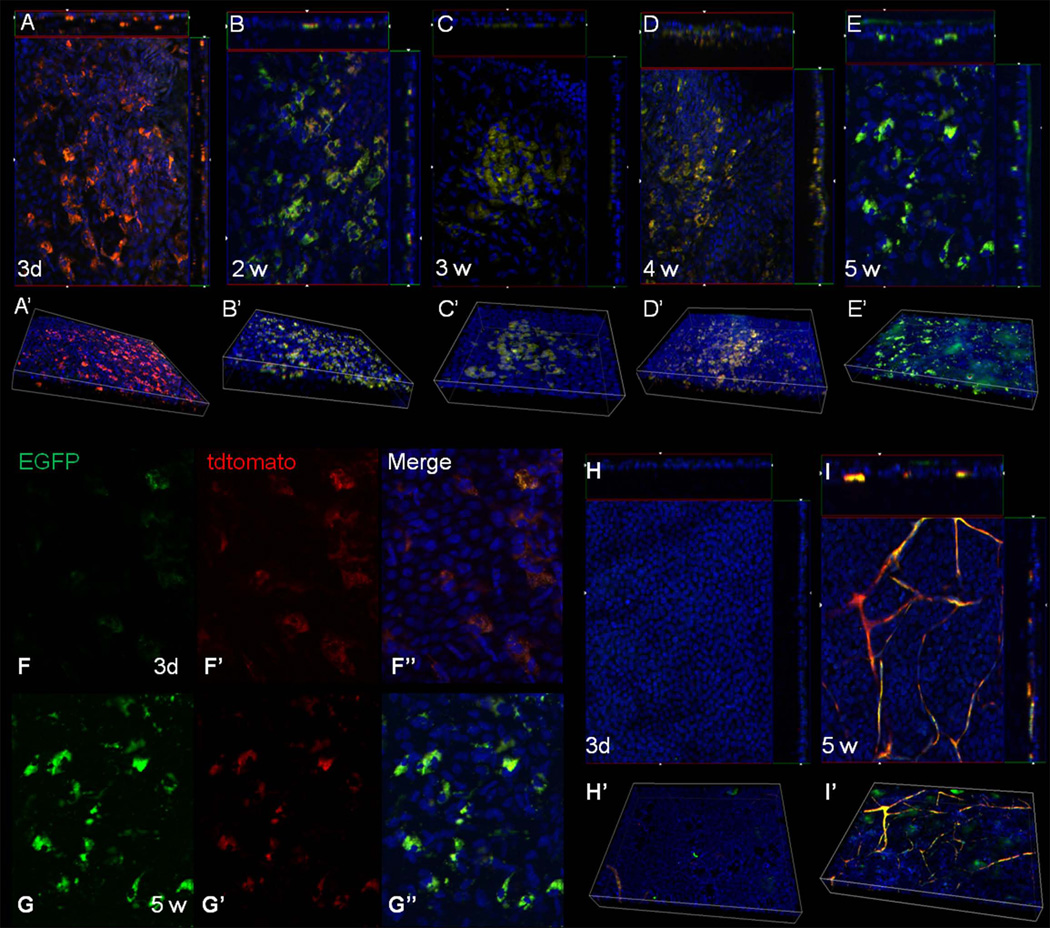
The data presented thus far suggest that HFSC have the potential to properly resurface the cornea in a LSCD mouse model. In order to show conclusively, that the SC transplant was capable of differentiating into cells with a corneal epithelial phenotype the presence of EGFP was examined. Since the HFSC were isolated from the triple transgenic mouse and subsequently transplanted onto a LSCD C57BL/6 mouse eye, the presence of green or red fluorescent cells can only come from the donor cells. As stated earlier, the conversion of red to green fluorescence only occurs when the cells express Krt12. Based on this unique feature of the system, we were able to show that the HFSC transplant was maintained for at least five weeks seemingly not undergoing host graft rejection. In addition, the transplant gave rise to EGFP expressing cells, which were localized primarily to the basal layer of the epithelium (Fig. 4A–E). EGFP expression was first detected in a few cells three days post-transplantation, while the majority of the HFSC still expressed mT (tdtomato red fluorescent protein), indicating that these cells had not yet undergone differentiation (Fig. 4A, F). By 14 days post-transplantation the number of EGFP expressing cells increased and was maintained through the length of the experiment (4B–E). Aside from autofluorescence from the vast network of blood vessels, the control eye did not contain any EGFP expressing cells (Fig. 4H, I). During the examination we noticed that some cells co-expressed both mT and EGFP (Fig. 4F, G), this is due to the half-life of the mT protein following excision of the mT gene in differentiated cells. One must keep in mind that the turn-over rate of the mouse corneal epithelium is approximately 14 days; therefore, cells that started expressing Krt12 in the early days (for example 3 days) following transplantation will be lost by day 20 due to desquamation of terminally differentiated epithelial cells. The fact that EGFP expressing cells are seen 21–39 days post-transplantation indicate that differentiation into Krt12 expressing cells does not occur all at once, but rather that these SC may have re-settled into their niche giving rise to new cells as needed.
Figure 4.
Induction of EGFP expression from the HFSC transplant. Corneas were harvested at various times following transplantation from mice fed doxycycline chow and examined for the expression of red and green fluorescent protein via whole mount imaging. A Z-stack through the entire thickness was obtained for each sample, with each slice having a thickness of 1.8 µM. (A–E) Images represent the cut-view of the Z-stack depicting the x-z axis along the top and the y-z axis along the right-hand side of the x-y image. A 3-D reconstruction of the Z-stack is shown below each cut-view image (A’–E’). Three days following transplant most of the HF bulge-derived SC expressed the red fluorescent protein (mT) indicating they had not yet differentiated (A). The presence of EGFP was observed two weeks post-transplantation (B) and was maintained throughout the length of the experiment; 3 wk (C), 4 wk (D), 5 wk (E). At all time points, EGFP was localized to the basal layer of the corneal epithelium. (F–G) Single color images taken from the Z-stack depicting the cellular localization of mT and EGFP at 3 d (F) and 5 wk (G). (H, I) Cut-view images and the corresponding 3-D images of corneas from mice not receiving the HFSC transplant 3 d (H, H’) and 5 w (I, I’) following LSCD. Magnification: 200X
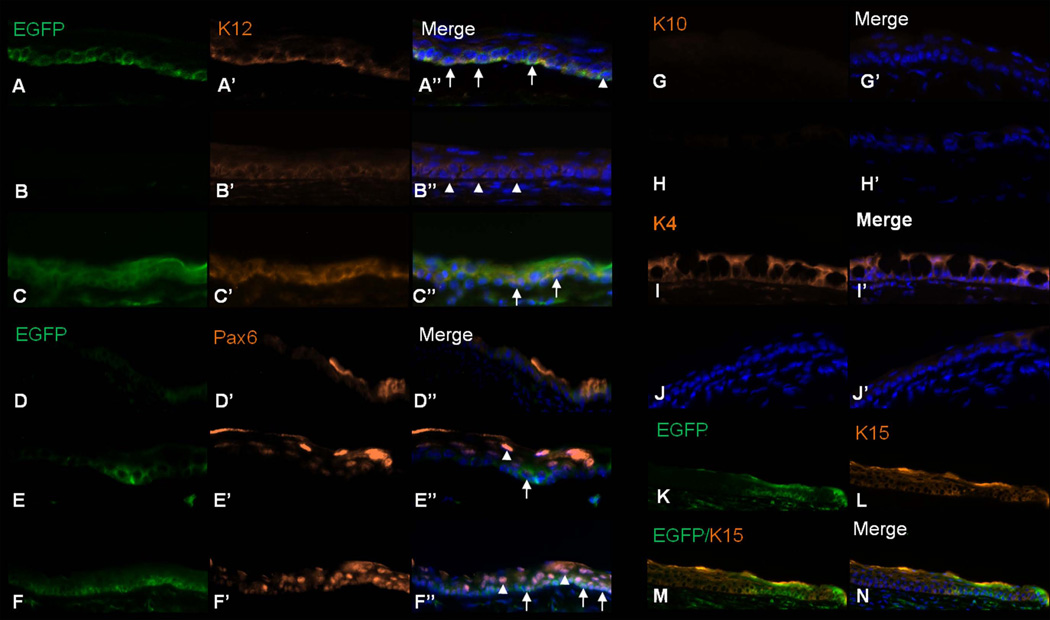
To confirm that the EGFP expressing cells were indeed of corneal phenotype, immunofluorescent staining using the corneal epithelial markers Krt12 and Pax6 was performed and revealed that these two populations were co-expressed (Fig. 5A–F). In several instances, Krt12 positive cells were seen in the absence of EGFP expression suggesting that these cells came from the host. In mice, unlike other species, the Krt3 gene is not present in the genome and thus Krt12 pairs with Krt5. As Krt5 is not specific to corneal epithelia we chose to examine the expression of only Krt12 [30].
Figure 5.
Differentiation of the HFSC transplant. (A–F) Expression of the corneal epithelial markers Krt12 and Pax6 were observed at 1 wk (A, D), 3 wk (B, E), and 5 wk (C–F) post-transplantation. Expression of Krt12 and Pax6 was co-localized with EGFP (arrows) and in a few instances expressed only in host cells (arrowheads). (G, H) Krt10 was not expressed in either the experimental (G) or control eyes (H) 3 wk s following LSCD. (I, J) 3 wk post-transplantation Krt4 was expressed only within eyes not receiving the HFSC graft (I), but not in the transplanted eyes (J). (K–N) 2 wk post-transplantation Krt15 expressing cells were present within the ocular surface and as expected did not co-localize with EGFP expression. Magnification: 200X
As SC have the potential to differentiate into various cell lineages and due to the resurfacing of the cornea with conjunctiva in a LSCD animal, we examined Krt10 (skin epidermal marker) [31] and Krt4 (conjunctiva marker) [32] expression. Krt10 was not expressed in either the control or SC-treated eyes (Fig. 5G, H); however, Krt4 was expressed highly in the control while minimally in the HFSC-treated eye (Fig. 5I, J). Despite the fact that a low level of Krt10 was present in the superficial layers of the holoclone, prior to transplant, these cells appear to have been lost in the ocular surface microenvironment. These data demonstrate that when placed in the appropriate microenvironment HFSC can differentiate into a cell type distinct from their lineage of origin in addition to suppressing conjunctival resurfacing; highlighting the promising therapeutic use of these SC.
A stem cell not only has the ability to differentiate but it also has the ability of self-renewal. To assess whether the HFSC transplant was able to re-establish the SC niche or if the transplant was exhausted of all SC, expression of Krt15, a putative stem cell marker, was examined. Krt15 was expressed in the ocular surface following HFSC transplant but only in those cells lacking EGFP expression and hence have not yet undergone differentiation (Fig. 5K–N). These data suggest that the HFSC were able to re-establish the SC niche.
Discussion
The identification of multiple sources of adult SC, has spurred research aimed at manipulating their high level of plasticity for the purpose of tissue engineering [33]. Currently, research in the area of SC-based tissue engineering is focusing on identification of an appropriate, easily accessible SC source, optimization of culture conditions and scaffold material, delivery method and a reliable method to control the reprogramming of the cells. A detailed molecular understanding of the mechanisms controlling the differentiation process along specific lineages would greatly facilitate the development of clinically applicable procedures.
Each distinct SC population will likely possess unique properties distinguishing it from the others, which will be beneficial for engineering tissue substitutes [34, 35]. Perfectly engineered tissue should not only mimic its physiological function but should also be easy to handle and apply to the wound site, readily adherent, sterile, non-toxic, and evoke a minimal inflammatory response from the recipient [36]. For these reasons, HFSC have drawn attention for their potential application in tissue engineering as has been seen in the development of skin substitutes [37, 38], which are so far the most highly developed and clinically utilized tissue equivalents in the field [36]. To further emphasize the common properties between the corneal epithelium and the hair follicle, reminiscent of their common embryological origin, Ferraris et al. showed that adult central corneal cells are able to respond to specific information and be reprogrammed into another cell phenotype. When recombined with embryonic mouse hair-forming dermis adult corneal epithelial cells retain the ability to be reprogrammed into an epidermis and to produce hair follicles with associated sebaceous glands [39]. Corneal epithelium differentiation into a hair-bearing epidermis provides evidence that transient amplifying cells are able to activate different genetic programs in response to a change in their fibroblast environment [40].
In our previous study, we show that transdifferentiation of HFSC into cells assuming a corneal epithelial phenotype is possible when the cells are cultured in limbal fibroblast-derived conditioned medium [19]. However, this in vitro reprogramming process cannot achieve the precision and complexity of a native in vivo system, which maintains an on-site pool of SC capable of initiating terminal differentiation when required; i.e., following injury or the physiological turnover of the corneal epithelium. Under the culture conditions we developed, SC start to lose their plasticity, if maintained in culture for an extended period of time, and differentiate into epidermal cells expressing Krt10 assuming the phenotype of their tissue of origin. This differentiation process is likely initiated by a wide spectrum of unidentified and non-physiological growth and survival factors present within the culture medium. Furthermore, our culture method is highly simplified when compared to the complex network of signals, stemming from soluble growth factors, secreted or matrix-embedded signalling molecules, cell-cell/cell-matrix interaction and physical forces; cells receive in vivo in order to maintain tissue homeostasis [41, 42].
The present study is the first to provide evidence demonstrating that HFSC can go beyond their lineage boundaries and terminally differentiate into a different epithelial cell phenotype in vivo when grafted into a specific niche microenvironment. Following generation of LSCD mice, HFSC grafts were transplanted onto the eyes and were shown to differentiate into cells with a corneal epithelial phenotype, expressing corneal markers Krt12 and Pax6, up to 5 weeks post transplantation. The presence of the epidermal differentiation marker Krt10 was suppressed and completely absent in the sutured eyes indicating a successful and complete reprogramming of the HFSC into a corneal phenotype. HFSC transplants were able regenerate the ocular surface and likely repopulate the SC pool. In the absence of the HFSC transplant, the morphology of the corneal surface following LSCD was severely compromised due to conjunctivalization as evidenced by the presence of goblet cells and blood vessels. In contrast, the eyes receiving the HFSC transplant had a corneal surface closely resembling a normal corneal epithelium; with an average thickness of 3–5 cell layers and little to no goblet cells present. Immunohistochemical examination demonstrated co-localization of EGFP and Krt12 expression in the same cells additionally confirming the transdifferentiation into a corneal cell phenotype. Additionally the presence of Pax6 was found co-localized with EGFP further confirming differentiation into corneal epithelial cells.
It is apparent that not all of the cells resurfacing the cornea are from the HFSC transplant, as can be seen by the presence of cells without green or red fluorescence. A likely reason for this may be that in the creation of limbal stem cell deficiency using the Algerbrush a few limbal SC or transient amplifying cells were left behind. These cells given the environment provided by the HFSC transplant were able to survive and contribute to the reconstruction of the corneal surface. Additionally the host cells contributing to the resurfacing of the cornea may come from the conjunctiva. Following removal of the limbal epithelium the conjunctival stem/progenitor cells begin to migrate to cover the denuded area. It is possible that when these cells migrate and reach the HFSC transplant environment that they are cued to differentiate into corneal epithelial cells. Whatever the reason may be, these observations highlight the ability of the HFSC transplant to provide an environment conducive to the survival and directed differentiation of stem/progenitor cells. Without the HFSC transplant, the ocular surface cannot be properly reconstituted but rather is replaced with conjunctiva which can lead to severe vision impairment. To fully reveal the translational implications of this research as well as the mechanism responsible for the therapeutic effect of the HFSC graft, future work including long-term monitoring of the HFSC transplant needs to be examined to assess the potential for stem cell depletion and/or graft rejection as well as the contribution of both host and donor cells to the reconstruction of the ocular surface. Taken together, these data suggest that the therapeutic potential of HFSC in treating LSCD may arise from two mechanisms; differentiation and survival.
Our results using the murine model demonstrate the promising, therapeutic potential of HFSC-engineered tissue to eventually treat patients with LSCD. Preliminary data from a pilot study, in cooperation with the Department of Neurosurgery in our clinic (data unpublished, ARVO, Fort Lauderdale, 2009), has shown that human skin biopsies (1 cm2) taken either from a retroauricular or temporal region of the human scalp allowed for HFSC isolation and enrichment by clonal expansion. Subsequent culture on a fibrin carrier in limbal fibroblast-derived conditioned medium resulted in their ex vivo differentiation into cells of a corneal phenotype expressing corneal epithelial markers such as Krt12 and Pax6. Preliminary in vivo engraftment experiments to LSCD New Zealand white rabbits revealed acceptance of the graft and an increasing number of transplanted cells differentiating into cells of a corneal epithelial phenotype post-operatively; indicating that like mouse HFSC, human HFSC also have a high degree of plasticity when transferred into a specific limbal-corneal environment. With a greater understanding of the corneal differentiation process we could optimize this therapeutic approach through standardization of the procedure thus making it more efficient. This improvement could be achieved prior to grafting through control of the culture microenvironment and the choice of scaffolding material with possible growth factor and signal molecule insertion to additionally direct and support the existing ex vivo transdifferentiation.
Acknowledgement
The study was in part supported by grants from NIH/NEI EY013755, Ohio Lions Eye Research Foundation and Research to Prevent Blindness to WWK.
Footnotes
Author contribution:
Ewa Anna Meyer-Blazejewska: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of the manuscript
Mindy K. Call: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of the manuscript
Osamu Yamanaka: collection and/or assembly of data
Hongshan Liu: collection and/or assembly of data
Ursula Schlötzer-Schrehardt: final approval of the manuscript
Friedrich E. Kruse: final approval of the manuscript
Winston W. Kao: conception and design, data analysis and interpretation, final approval of the manuscript
Disclosure of potential conflicts of interest
The authors indicate no potential conflicts of interest.
References
- 1.Polak JM, Bishop AE. Stem cells and tissue engineering: past, present, and future. Ann N Y Acad Sci. 2006;1068:352–366. doi: 10.1196/annals.1346.001. [DOI] [PubMed] [Google Scholar]
- 2.Cotsarelis G, Cheng SZ, Dong G, et al. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57(2):201–209. doi: 10.1016/0092-8674(89)90958-6. [DOI] [PubMed] [Google Scholar]
- 3.Li W, Hayashida Y, Chen YT, et al. Niche regulation of corneal epithelial stem cells at the limbus. Cell Res. 2007;17(1):26–36. doi: 10.1038/sj.cr.7310137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li DQ, Tseng SC. Three patterns of cytokine expression potentially involved in epithelial-fibroblast interactions of human ocular surface. J Cell Physiol. 1995;163(1):61–79. doi: 10.1002/jcp.1041630108. [DOI] [PubMed] [Google Scholar]
- 5.Ihanamaki T, Pelliniemi LJ, Vuorio E. Collagens and collagen-related matrix components in the human and mouse eye. Prog Retin Eye Res. 2004;23(4):403–434. doi: 10.1016/j.preteyeres.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Schlotzer-Schrehardt U, Dietrich T, Saito K, et al. Characterization of extracellular matrix components in the limbal epithelial stem cell compartment. Exp Eye Res. 2007;85(6):845–860. doi: 10.1016/j.exer.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 7.Pellegrini G, Traverso CE, Franzi AT, et al. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997;349(9057):990–993. doi: 10.1016/S0140-6736(96)11188-0. [DOI] [PubMed] [Google Scholar]
- 8.Shortt AJ, Secker GA, Notara MD, et al. Transplantation of ex vivo cultured limbal epithelial stem cells: a review of techniques and clinical results. Surv Ophthalmol. 2007;52(5):483–502. doi: 10.1016/j.survophthal.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 9.Inatomi T, Nakamura T, Koizumi N, et al. Midterm results on ocular surface reconstruction using cultivated autologous oral mucosal epithelial transplantation. Am J Ophthalmol. 2006;141(2):267–275. doi: 10.1016/j.ajo.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61(7):1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- 11.Jahoda CA, Whitehouse J, Reynolds AJ, et al. Hair follicle dermal cells differentiate into adipogenic and osteogenic lineages. Exp Dermatol. 2003;12(6):849–859. doi: 10.1111/j.0906-6705.2003.00161.x. [DOI] [PubMed] [Google Scholar]
- 12.Lako M, Armstrong L, Cairns PM, et al. Hair follicle dermal cells repopulate the mouse haematopoietic system. J Cell Sci. 2002;115(Pt 20):3967–3974. doi: 10.1242/jcs.00060. [DOI] [PubMed] [Google Scholar]
- 13.Richardson GD, Arnott EC, Whitehouse CJ, et al. Plasticity of rodent and human hair follicle dermal cells: implications for cell therapy and tissue engineering. J Investig Dermatol Symp Proc. 2005;10(3):180–183. doi: 10.1111/j.1087-0024.2005.10101.x. [DOI] [PubMed] [Google Scholar]
- 14.Cotsarelis G. Epithelial stem cells: a folliculocentric view. J Invest Dermatol. 2006;126(7):1459–1468. doi: 10.1038/sj.jid.5700376. [DOI] [PubMed] [Google Scholar]
- 15.Taylor G, Lehrer MS, Jensen PJ, et al. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell. 2000;102(4):451–461. doi: 10.1016/s0092-8674(00)00050-7. [DOI] [PubMed] [Google Scholar]
- 16.Kloepper JE, Tiede S, Brinckmann J, et al. Immunophenotyping of the human bulge region: the quest to define useful in situ markers for human epithelial hair follicle stem cells and their niche. Exp Dermatol. 2008;17(7):592–609. doi: 10.1111/j.1600-0625.2008.00720.x. [DOI] [PubMed] [Google Scholar]
- 17.Tiede S, Kloepper JE, Bodo E, et al. Hair follicle stem cells: walking the maze. Eur J Cell Biol. 2007;86(7):355–376. doi: 10.1016/j.ejcb.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Larouche D, Tong X, Fradette J, et al. Vibrissa hair bulge houses two populations of skin epithelial stem cells distinct by their keratin profile. Faseb J. 2008;22(5):1404–1415. doi: 10.1096/fj.07-8109com. [DOI] [PubMed] [Google Scholar]
- 19.Blazejewska EA, Schlotzer-Schrehardt U, Zenkel M, et al. Corneal limbal microenvironment can induce transdifferentiation of hair follicle stem cells into corneal epithelial-like cells. Stem Cells. 2009;27(3):642–652. doi: 10.1634/stemcells.2008-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chikama T, Hayashi Y, Liu CY, et al. Characterization of tetracycline-inducible bitransgenic Krt12rtTA/+/tet-O-LacZ mice. Invest Ophthalmol Vis Sci. 2005;46(6):1966–1972. doi: 10.1167/iovs.04-1464. [DOI] [PubMed] [Google Scholar]
- 21.Perl AK, Wert SE, Nagy A, et al. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc Natl Acad Sci U S A. 2002;99(16):10482–10487. doi: 10.1073/pnas.152238499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muzumdar MD, Tasic B, Miyamichi K, et al. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45(9):593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 23.Pellegrini G, Ranno R, Stracuzzi G, et al. The control of epidermal stem cells (holoclones) in the treatment of massive full-thickness burns with autologous keratinocytes cultured on fibrin. Transplantation. 1999;68(6):868–879. doi: 10.1097/00007890-199909270-00021. [DOI] [PubMed] [Google Scholar]
- 24.Bagutti C, Wobus AM, Fassler R, et al. Differentiation of embryonal stem cells into keratinocytes: comparison of wild-type and beta 1 integrin-deficient cells. Dev Biol. 1996;179(1):184–196. doi: 10.1006/dbio.1996.0250. [DOI] [PubMed] [Google Scholar]
- 25.Lavker RM, Sun TT. Epidermal stem cells: properties, markers, and location. Proc Natl Acad Sci U S A. 2000;97(25):13473–13475. doi: 10.1073/pnas.250380097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jensen UB, Yan X, Triel C, et al. A distinct population of clonogenic and multipotent murine follicular keratinocytes residing in the upper isthmus. J Cell Sci. 2008;121(Pt 5):609–617. doi: 10.1242/jcs.025502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barrandon Y, Green H. Three clonal types of keratinocyte with different capacities for multiplication. Proc Natl Acad Sci U S A. 1987;84(8):2302–2306. doi: 10.1073/pnas.84.8.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blanpain C, Lowry WE, Geoghegan A, et al. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118(5):635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 29.Hayashi Y, Call M, Liu CY, et al. Monoallelic Expression of Krt12 Gene during Corneal-type Epithelium Differentiation of Limbal Stem Cells. Invest Ophthalmol Vis Sci. 2010 doi: 10.1167/iovs.10-5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaloin-Dufau C, Pavitt I, Delorme P, et al. Identification of keratins 3 and 12 in corneal epithelium of vertebrates. Epithelial Cell Biol. 1993;2(3):120–125. [PubMed] [Google Scholar]
- 31.Leigh IM, Purkis PE, Whitehead P, et al. Monospecific monoclonal antibodies to keratin 1 carboxy terminal (synthetic peptide) and to keratin 10 as markers of epidermal differentiation. Br J Dermatol. 1993;129(2):110–119. doi: 10.1111/j.1365-2133.1993.tb03511.x. [DOI] [PubMed] [Google Scholar]
- 32.Ang LP, Tan DT, Beuerman RW, et al. Development of a conjunctival epithelial equivalent with improved proliferative properties using a multistep serum-free culture system. Invest Ophthalmol Vis Sci. 2004;45(6):1789–1795. doi: 10.1167/iovs.03-1361. [DOI] [PubMed] [Google Scholar]
- 33.Hodgkinson T, Yuan XF, Bayat A. Adult stem cells in tissue engineering. Expert Rev Med Devices. 2009;6(6):621–640. doi: 10.1586/erd.09.48. [DOI] [PubMed] [Google Scholar]
- 34.Sakaguchi Y, Sekiya I, Yagishita K, et al. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52(8):2521–2529. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- 35.Kern S, Eichler H, Stoeve J, et al. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24(5):1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 36.MacNeil S. Progress and opportunities for tissue-engineered skin. Nature. 2007;445(7130):874–880. doi: 10.1038/nature05664. [DOI] [PubMed] [Google Scholar]
- 37.Lenoir MC, Bernard BA, Pautrat G, et al. Outer root sheath cells of human hair follicle are able to regenerate a fully differentiated epidermis in vitro. Dev Biol. 1988;130(2):610–620. doi: 10.1016/0012-1606(88)90356-9. [DOI] [PubMed] [Google Scholar]
- 38.Gharzi A, Reynolds AJ, Jahoda CA. Plasticity of hair follicle dermal cells in wound healing and induction. Exp Dermatol. 2003;12(2):126–136. doi: 10.1034/j.1600-0625.2003.00106.x. [DOI] [PubMed] [Google Scholar]
- 39.Ferraris C, Chevalier G, Favier B, et al. Adult corneal epithelium basal cells possess the capacity to activate epidermal, pilosebaceous and sweat gland genetic programs in response to embryonic dermal stimuli. Development. 2000;127(24):5487–5495. doi: 10.1242/dev.127.24.5487. [DOI] [PubMed] [Google Scholar]
- 40.Pearton DJ, Ferraris C, Dhouailly D. Transdifferentiation of corneal epithelium: evidence for a linkage between the segregation of epidermal stem cells and the induction of hair follicles during embryogenesis. Int J Dev Biol. 2004;48(2–3):197–201. doi: 10.1387/ijdb.15272385. [DOI] [PubMed] [Google Scholar]
- 41.Guilak F, Cohen DM, Estes BT, et al. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell. 2009;5(1):17–26. doi: 10.1016/j.stem.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Estes BT, Gimble JM, Guilak F. Mechanical signals as regulators of stem cell fate. Curr Top Dev Biol. 2004;60:91–126. doi: 10.1016/S0070-2153(04)60004-4. [DOI] [PubMed] [Google Scholar]