Abstract
Corpus callosum (CC) area abnormalities have been reported in magnetic resonance imaging (MRI) studies of adults and youths with bipolar disorder (BPD), suggesting interhemispheric communication may be abnormal in BPD and may be present early in the course of illness and affect normal neuromaturation of this structure throughout the lifecycle. Neuroimaging scans from 44 youths with DSM-IV BPD and 22 healthy controls (HC) were analyzed using cross-sectional area measurements and a novel method of volumetric parcellation. Univariate analyses of variance were conducted on CC subregions using both volume and traditional area measurements. Youths with BPD had smaller middle and posterior callosal regions, and reduced typical age-related increases in CC size. The cross-sectional area and novel volumetric methodologies resulted in similar findings. Future longitudinal assessments of CC development would track the evolution of callosal abnormalities in youths with BPD and allow exploration of the functional significance of these findings.
Keywords: Bipolar disorder, Corpus callosum, Neuroimaging, Youths, MRI
Introduction
The corpus callosum (CC) is composed of approximately 180 million fibers and is the largest white matter fiber tract in the brain (Tomasch 1954). The CC is crucial for normal inter-hemispheric transfer of sensory, motor and higher-order cerebral information (Gazzaniga 2000; Tomasch 1954). Topographically, anterior callosal regions, such as the genu, contain fibers from the prefrontal cortex, while the body (proceeding from front to back) contains fibers from the premotor, motor, somatosensory, parietal, and temporal cortices, with the most posterior section containing fibers from the occipital cortex (de Lacoste et al. 1985; Pandya and Seltzer 1986; Witelson 1989).
From a neurodevelopmental perspective, the CC forms approximately 8 weeks after conception, with myelination beginning shortly after birth (Barkovich 1990; Paus et al. 2001). The CC is a spatially extended set of white matter fibers that cross the inter-hemispheric midline to confer connectivity between the cerebral hemispheres and hence is a volumetric (space filling) structure. The “size” of the CC has been traditionally represented by the cross sectional area of its decussation (midline crossing). Using such measures, the CC has been shown to increase in size throughout childhood and adolescence and up through early adulthood due to normative ongoing myelination (Giedd et al. 1996; Keshavan et al. 2002; Pujol et al. 1993). After early childhood, significant increases are limited to middle and posterior callosal regions such as the body, isthmus, and splenium, with the latter being most dramatic (Giedd et al. 1999). Later in adulthood, the callosum begins a slow and possibly region specific decline, particularly within the genu (Burke and Yeo 1994; Woodruff et al. 1997; Bastin et al. 2008; Pagani et al. 2008).
Aberrant communication between specific regions of the cerebral hemispheres, particularly the prefrontal, parietal and temporal lobes, may be important in the pathophysiology of several neuropsychiatric disorders including bipolar disorder (BPD) (Soares and Mann 1997). In fact, the results of one study described CC signal intensity abnormalities in adults with BPD, but not in those with major depression, suggesting that problems with interhemispheric communication may be more specific to BPD (Brambilla et al. 2004). Few MRI investigations have examined the CC area in adults with BPD, and the results are mixed. Compared to healthy controls (HC), adults with BPD showed smaller total callosal area (Brambilla et al. 2003; Coffman et al. 1990) and smaller subregion areas in the genu, posterior body and isthmus (Brambilla et al. 2003). In contrast, two studies did not find differences in area measurements of the CC (Hauser et al. 1989; Walterfang et al. 2009) although Walterfang and colleagues did find overall callosal thinning, most prominently in the splenium. In a more recent study, first episode, medication-naïve adults with BPD had significantly smaller total CC, anterior body, posterior body and isthmus areas compared with HC (Atmaca et al. 2007b). Furthermore, Brambilla and colleagues found an absence of typical age-related changes (decline) in total callosal, genu, anterior body, and isthmus in adults with BPD (Brambilla et al. 2003). Brambilla postulated that the absence of a normal age-related decline in CC size might reflect abnormal callosal maturation possibly due to aberrant myelination and/or pruning. Diffusion tensor imaging (DT-MRI) studies in adults with BPD have also found abnormalities in regions of the CC such as reduced fractional anisotrophy (FA) in anterior and middle CC regions (Wang et al. 2008) and increased (Yurgelun-Todd et al. 2007) and decreased (Chaddock et al. 2009) FA in the genu compared to HC.
While diffusion, signal intensity and area measurements of the CC indicate that abnormalities may exist in adults with BPD, no studies have evaluated the CC volumetrically. Furthermore, there is only one published MRI investigation of the CC area, but not volume, in youths with BPD (Yasar et al. 2006). These authors reported a shape difference marked by reduced splenium circularity in youths with BPD compared to HC, but did not find differences between groups in the length or area of the CC. A recent study in adolescents evaluated the white matter signal intensity in adolescents with BPD and found a reduction in the genu, anterior body, posterior body, isthmus and splenium in BPD youths as compared to HC (Caetano et al. 2008). There have been two DT-MRI studies, one in adolescents and another in children with BPD, that found reduced FA in the genu, body and splenium in adolescents at genetic-risk for BPD (Barnea-Goraly et al. 2009) and in the body of the CC in children with BPD (Frazier et al. 2007).
The present study represents the first application of a comprehensive set of measures that permit the comparison of total CC and callosal subregion areas and volumes in youths with BPD and HC. To our knowledge, this is the first study to evaluate the CC using a comprehensive system of sub-regional volumetric analysis in youths with BPD and directly comparing these findings to those of the traditional cross-sectional area methodology (Witelson 1989). The extension of the traditional cross-sectional area measures with a volumetric representation of the corpus callosum that is more representative of the spatial extent of the structure provides both an opportunity to corroborate the cross-sectional measures and examine the extent to which the interpretation of such changes are consistent with a spatially distributed set of changes in the fiber system.
We hypothesized that (1) posterior callosal volumes would be reduced in youths with BPD given that these structures are rapidly maturing during childhood and adolescence and as a result may be more vulnerable during this time of significant brain myelination and pruning, particularly around the time of illness onset; (2) consistent with the hypothesized role of the prefrontal cortex in mood dysregulation and cognitive abnormalities in BPD (Soares and Mann 1997; Wilder-Willis et al. 2001), genual volume was expected to be abnormal (i.e., smaller) in youths with BPD; and (3) consistent with Brambilla and colleagues’ findings, there would be an absence of typical age-related changes in specific callosal volumes in youths with BPD (Brambilla et al. 2003). Specifically, youths with BPD were hypothesized to have reduced callosal growth in posterior regions.
Methods
This paper reports a novel application of callosal subregion volumetric analysis on a library of MRI scans that has been used in prior studies of gray matter volumes (Frazier et al. 2005a, b, 2008). This group of investigators has not previously reported on any structural white matter analyses. The full details of the diagnostic and scanning methods have been reported elsewhere (Frazier et al. 2005a, b) and will be briefly described here.
Participants
The Institutional Review Boards at McLean Hospital and Cambridge Health Alliance approved this study. Inclusion criteria for all participants in this analysis were: age 6–16 years old and right-handedness. Individuals with BPD were diagnosed using DSM-IV criteria in semi-structured and clinical interviews; HC participants had no DSM-IV Axis I diagnoses or a family history of mood or psychotic disorders in first-degree relatives, based on parental interview. Exclusion criteria for all participants included: major sensorimotor handicaps; full scale IQ <70, learning disability, history of claustrophobia, head trauma, loss of consciousness, autism, schizophrenia, anorexia or bulimia nervosa, alcohol or drug dependence/abuse based on DSM-IV criteria (during 2 months prior to scan, or total past history of ≥12 months), electroconvulsive therapy; active medical or neurological disease; metal fragments or implants; and current pregnancy or lactation.
All participants provided written assent, and their parents (or legal guardians) provided written informed consent for their child's participation. All children, including HCs, underwent diagnostic semi-structured interviews (Kiddie Schedule for Affective and Schizophrenic Disorders: Epidemiologic Version-KSADS-E) (Orvaschel and Puig-Antich 1987) to confirm the diagnosis of BPD after undergoing a clinical interview by board-certified child psychiatrists. Parents were also administered a KSADS-E regarding their children (see (Frazier et al. 2005b) for further details).
Handedness was assessed using the Edinburgh Handedness Questionnaire (Oldfield 1971). Measures of current psychopathology were obtained using the Mania Rating Scale (MRS) (Young et al. 1978) and Global Assessment of Functioning scale (GAF (American Psychiatric Association 1994)).
MRI protocol
Structural imaging was performed at the McLean Hospital Brain Imaging Center on a 1.5 Tesla Scanner (Signa; GE Medical Systems, Milwaukee, WI). The acquisitions included a 3-D inversion recovery-prepped spoiled gradient recalled echo coronal series, which was used for structural analysis (124 slices, prep=300 msec, TE=1 min, flip angle=25°, FOV= 24 cm2, slice thickness 1.5 mm, acquisition matrix 256×192, number of excitations=2). All scans were clinically reviewed by a neuroradiologist to rule out gross pathology.
Image analysis
Structural scans were transferred to the MGH Center for Morphometric Analysis (CMA) and coded and catalogued for blind analysis. Image analysis was done on Sun Microsystems, Inc. (Mountainview, CA) workstations using Cardviews software (Caviness et al. 1996). In short, a comprehensive segmentation of the cerebrum into gray and white matter structures was performed, followed by volumetric and cross-sectional (2D) analysis of the corpus callosum within the context of the cerebral white matter.
Gray and white matter segmentation
Brain images were positionally normalized to overcome variations in head position by using a standard 3-dimensional coordinate system on each scan that used the midpoints of the decussations of the anterior and posterior commissure (AC and PC, respectively) and the midsagittal plane at the level of the posterior commissure as points of reference for rotation and translation (Frazier et al. 2005a). The datasets were then segmented into gray, white, and CSF tissue classes using a semi-automated intensity contour algorithm for external border definition and signal intensity histogram distributions for delineation of gray-white borders. This technique, described in detail elsewhere (Filipek et al. 1989; Seidman et al. 1997), yields separate components of neocortex, subcortical gray nuclei, white matter (WM), and ventricular system subdivisions that correspond to the natural tissue boundaries distinguished by signal intensities on T1-weighted images. Total cerebral volume (TCV) was defined as all gray and white matter in the cerebrum and did not include CSF, cerebellum or brainstem.
Volumetric and cross-sectional analysis of the corpus callosum division
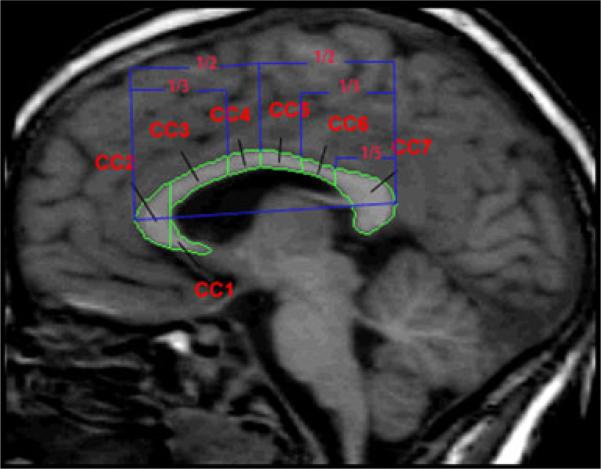
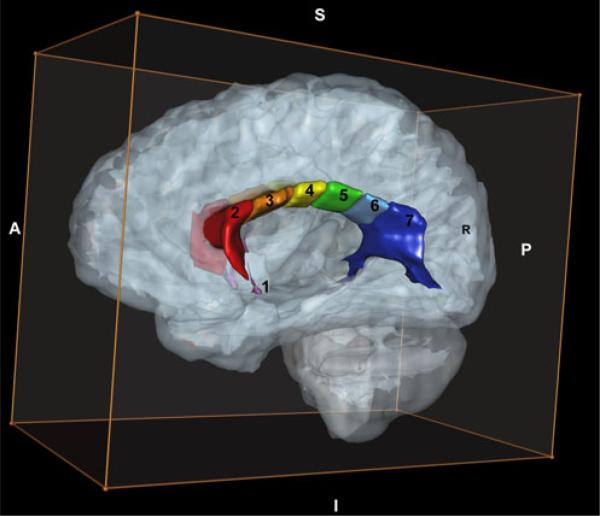
The positional normalization procedure facilitates generation of a ‘true’ midsaggital view of the brain as the image plane that includes the AC, PC and midsaggital plane points identified above. In this image, cross-sectional area measurements were obtained for total CC and the seven subregions based on the subdivisions described by Witelson (5) (see Fig. 1). Specifically, the CC is divided into seven subregions which include: rostrum (CC1), genu (CC2), anterior body (CC3), midbody (CC4), posterior body (CC5), isthmus (CC6) and splenium (CC7) using anterior and posterior definitions as described in Witelson (5) (see Fig. 1). For volumetric measures of the CC, we utilized a comprehensive white matter parcellation method to subdivide the cerebral WM into peripheral and deep divisions based upon a set of topographic relationships and geometric constraints related to cortical and subcortical structures as guided by known generalized white matter organizational principles (Makris et al. 1999; Meyer et al. 1999). Within the deep WM divisions, regions related to the major association projections and commissural systems are delineated. Volu-metric assessment of the CC is provided as a distinct subset of regions within this WM parcellation system. In this method, the volumetric CC is bounded superiorly by a spline that undercuts the cingulate cortex, inferiorally by the lateral ventricle, and laterally by a vertical line segment at the most superior extent of the lateral ventricle. The resultant volumetric CC is also subdivided anteriorally-posteriorally into several regions (CC1-CC7) following the identical proportional definitions of Witelson, above (See Fig. 2).
Fig. 1.
Mid-sagittal MRI of the corpus callosum subdivisions based on callosal subdivisions by Witelson (in blue). Key: CC1 = Rostrum, CC2 = Genu, CC3 = Anterior body, CC4 = Midbody, CC5 = Posterior body, CC6 = Isthmus, CC7 = Splenium
Fig. 2.
Three-dimensional MRI of the corpus callosum subdivisions. Key: 1 = Rostrum, 2 = Genu, 3 = Anterior body, 4 = Midbody, 5 = Posterior body, 6 = Isthmus, 7 = Splenium
Data analyses
SPSS 15.0 for Windows (SPSS, Inc., Chicago, IL) was used for statistical analysis. All statistical tests were two-tailed with α=0.05. Equality of groups on demographic and clinical variables was evaluated by t-tests for continuous variables and chi-square tests for categorical variables. Pearson's correlations were performed with the BPD group on clinical variables and psychoactive medications for those CC subregions that differed significantly between BPD youths and HC.
A series of Analyses of Variance (ANOVA) were performed on CC1 through CC7 and total CC as dependent variables with sex, age, and TCV as covariates to compare CC volumes and CC midsagittal areas between youths with BPD and HC to determine if there were group differences. For callosal regions where age was noted to be a significant variable in the original model, additional ANOVA's were performed, in which each diagnostic group was subdivided into two age groups (<12 and ≥12 years), and then an age group-by-diagnosis interaction term was introduced into the ANOVA to assess for possible different developmental trajectories with age. An age of <12 was used in order to approximate prepubertal youths and to distribute both gender and age between diagnostic groups as evenly as possible. Planned orthogonal contrasts were used to explore diagnostic effects within each gender (i.e. male BPD vs male HC; female BPD vs female HC). A series of post-hoc exploratory analyses were performed to evaluate the effects of full scale IQ (FSIQ), ethnicity, and ADHD on callosal regions.
Pearson's correlations and coefficient of variation (defined as the standard deviation divided by the mean) analyses were performed between traditional cross-sectional area and volumetric measurements for total CC and subregions in order to compare the two methodologies. Cohen's d effect sizes were calculated for total CC and each of the seven subregions for both methodologies.
Results
Data from 66 participants (44 children with DSM-IV BPD, age 10.6±3.0 years (mean ± SD) and 22 HC, age 10.5±3.1 years (mean ± SD) are included in this report (see Table 1 for demographic information). Thirty-one (70%) youths with BPD and 14 (64%) healthy controls were below age 12. The youths with BPD had a mean MRS score of 20.8±9.5 (range 0–38). For the BPD group, seven were manic, 28 mixed, four depressed and five were euthymic at the time of scanning. Twelve youths with BPD (27%) had histories of psychosis (five girls and seven boys). Clinical and treatment characteristics of the BPD group are shown in Table 2. At the time of assessment, 33 (75%) of the youths with BPD were on atypical antipsychotics; 10 (22.7%) were taking stimulants; 8 (18.2%) were taking lithium; 18 (40.9%) were on a mood stabilizer other than lithium (i.e. divalproic acid); 13 (29.5%) were on antidepressants; 8 (18.2%) were on beta-adrenergic agents; and 1 (2.3%) was taking a benzodiazepine (clonazepam).
Table 1.
Characteristics of youths with bipolar disorder (BPD) and healthy controls (HC)
| Characteristic | BPD (n=44) | HC (n=22) | Statistical significance of group difference |
|---|---|---|---|
| Age, years (mean ± SD) | 10.6±3.0 | 10.5±3.1 | ns |
| Edinburgh Laterality Quotient (mean ± SD) | 77.1±31.8 | 71.8±27.5 | ns |
| # Females (%) | 19 (43.2) | 7 (31.8) | ns |
| # Caucasian (%) | 43 (97.7) | 19 (86.3) | 0.02 |
| Height (cm) (mean ± SD) | 54.1±1.6 | 54.4±2.3 | ns |
| Weight (kg) (mean ± SD) | 104.6±38.6 | 91.6±41.3 | ns |
| Head circumference (cm) (mean ± SD) | 54.1±1.6 | 54.4±2.3 | ns |
| # Prepubertal (%) | 26 (59.0) | 12 (54.5) | ns |
| # Hollingshead low (III–V) Socioeconomic status (%) | 6 (13.6) | 4 (18.2) | ns |
| Full Scale Intelligence Quotient (mean ± SD) | 101.0 (13.1) | 114.06 (2.8) | 0.001 |
Table 2.
Clinical and treatment characteristics of youths with bipolar disorder (n=44)
| Characteristic | Mean ± SDa |
|---|---|
| Current global assessment of functioning | 50.0±6.3 |
| Mania Rating Score (MRS) | 20.8±9.5 |
| Psychosis Scaled Score (MRS) | 2.73 (3.2) |
| Age at onset of BPD (years) | 6.3±3.7 |
| Duration of BPD (years) | 2.5±3.1 |
| History of hospitalizations (n,%) | 14.0 (31.8) |
| Chlorpromazine equivalents at time of study (n=39) | 117.0±108.9 |
| Number of psychoactive medications at time of studyb | 2.3±1.2 |
| Presence of attention deficit hyperactivity disorder (n,%) | 21 (47.7) |
Unless otherwise indicated
Includes atypical antipsychotics, antidepressants, sedatives, mood stabilizers, and stimulants
Anatomic measurements
Table 3 displays the volumetric and area means and standard deviations for the callosal subregions, respectively. UNIANOVAs were performed on the CC1-CC7 and total CC volumes and areas using sex, age, FSIQ and TCV as covariates. To evaluate the effect of sex on callosal regions, a sex-by-diagnosis interaction term was reintroduced into the original models. After thorough analysis, sex, ethnicity and FSIQ were not found to be significant covariates for either cross-sectional or volumetric analyses and were removed from the initial overall models.
Table 3.
Comparison of volume (cc) and area (mm) measurements for corpus callosum regions; Unadjusted means are reported
| Region | Volumetric measurements (cc) |
Area measurements (mm) |
Area and volume correlations, r (p value) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BPD (n=44) | HC (n=22) | Significant findings (p value) | Effect sizeb | CVa | BPD (n=44) | HC | Significant findings (p value) | Effect sizeb | CVa | ||
| CC1 (Rostrum) | 0.26±0.19 | 0.29±0.16 | ns | – | 0.68 | 23.15±11.09 | 24.53±7.91 | Age (0.025) | – | 0.43 | 0.59 (≤0.01) |
| CC2 (Genu) | 3.66±0.76 | 3.97±0.79 | TCV (≤0.01) | – | 0.20 | 113.59±21.98 | 122.78±18.25 | TCV (0.03) | – | 0.18 | 0.35 (≤0.01) |
| CC3 (Anterior body) | 1.67±0.40 | 1.77±0.50 | TCV (≤0.01) | – | 0.25 | 86.19±17.23 | 88.27±21.13 | – | – | 0.21 | 0.64 (≤0.01) |
| CC4 (Anterior midbody) | 1.45±0.24 | 1.52±0.25 | TCV (0.002) AgeGrpaDX (≤0.01) |
0.70 | 0.17 | 68.64±10.66 | 70.31±12.33 | TCV (0.04) Age (0.02) |
– | 0.16 | 0.43 (≤0.01) |
| CC5 (Posterior body) | 1.17±0.20 | 1.38±0.23 | TCV (≤0.01) AgeGrpaDX (≤0.01) |
0.75 | 0.17 | 55.06±8.97 | 61.74±11.99 | AgeGrpaDX (0.008) | 0.60 | 0.17 | 0.64 (≤0.01) |
| CC6 (Isthmus) | 0.98±0.26 | 1.12±0.34 | AgeGrp (0.04) | – | 0.28 | 49.55±10.84 | 55.18±15.52 | AgeGrpaDX (≤0.01) | 0.67 | 0.24 | 0.74 (≤0.01) |
| CC7 (Splenium) | 4.23±1.10 | 4.33±1.25 | TCV (0.04) AgeGrpaDX (0.01) |
0.59 | 0.27 | 154.33±24.93 | 161.55±29.33 | TCV (0.01) AgeGrpaDX (0.03) |
0.52 | 0.17 | 0.77 (≤0.01) |
| Total CC | 13.42±2.06 | 14.38±2.45 | TCV (<0.0001) AgeGrpaDX (0.01) |
0.57 | 0.16 | 550.51±77.96 | 584.36±93.83 | TCV (0.03) AgeGrpaDX (0.05) |
0.47 | 0.15 | 0.74 (≤0.01) |
CV coefficient of variation
Effect sizes were only calculated for diagnostic differences
Total CC
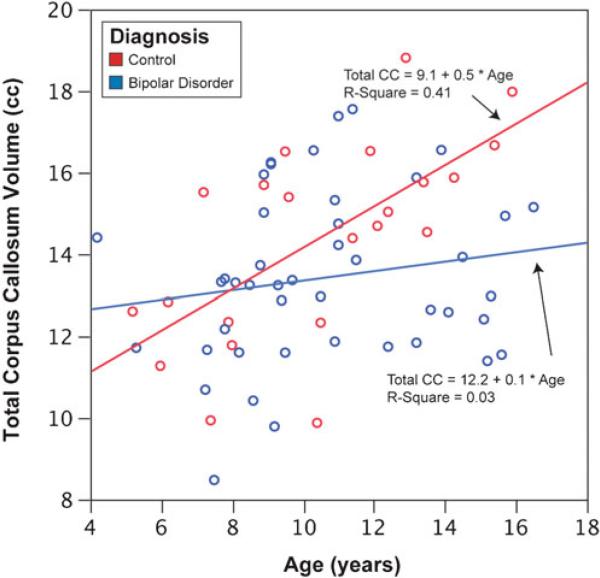
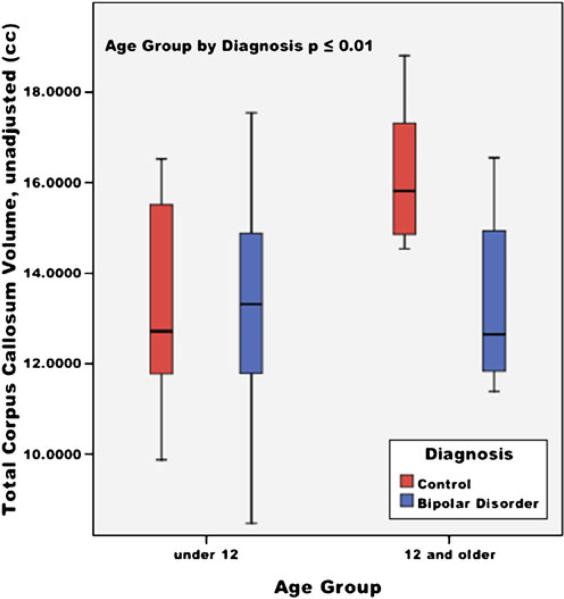
Significant effects of TCV (F=19.4, p<0.01) and for age group-by-diagnosis interaction term (F=4.60, p=0.01) for the volumetric measurements of total CC were found. For the area measurement of the total CC, significant effects were also found for TCV (F=5.15, p=0.03) and age group-by-diagnosis interaction term (F=3.08, p=0.05). Volumetric and area measurements found that the older HC (15.5 cc) had significantly larger total CC than the younger HC group (13.1 cc), whereas there was not a significant difference among the BPD age groups (13.6 and 13.7 cc). There was no significant difference between the younger BPD the younger HC. Linear regression of total CC by age and a box plot of the age group-by-diagnosis interaction are provided in Figs. 3 and 4.
Fig. 3.
Linear regression of the total corpus callosum volumes (cc) and age (years) in youths with bipolar disorder and healthy controls
Fig. 4.
Total corpus callosum volumes by age group (under 12 and 12 and older) in youths with bipolar disorder and healthy controls; unadjusted mean volumes (cc), ±95% confidence interval
CC1 (Rostrum), CC2 (Genu) and CC3 (Anterior body)
For CC1, area measurement found age (F=5.28, p=0.03) to be a significant covariate. For CC2, significant effects were found for TCV in CC2 volume (F=12.64, p<0.01) and area (F=5.18, p=0.03) measurements, respectively. Significant effects for TCV in CC3 volume (F=7.75, p<0.01) were also found.
CC4 (Midbody)
Significant effects for TCV (F=18.1, p<0.01) and for age group-by-diagnosis interaction term (F=6.97, p<0.01) for the CC4 volumetric measurements were found. Healthy controls had smaller CC4 volumes in the younger than 12 age group and larger volumes in the 12 and older age group compared to youths with BPD. Area measurements for the CC4 region noted significant effects for TCV (F=4.38, p=0.04) and age (F=6.08, p=0.02).
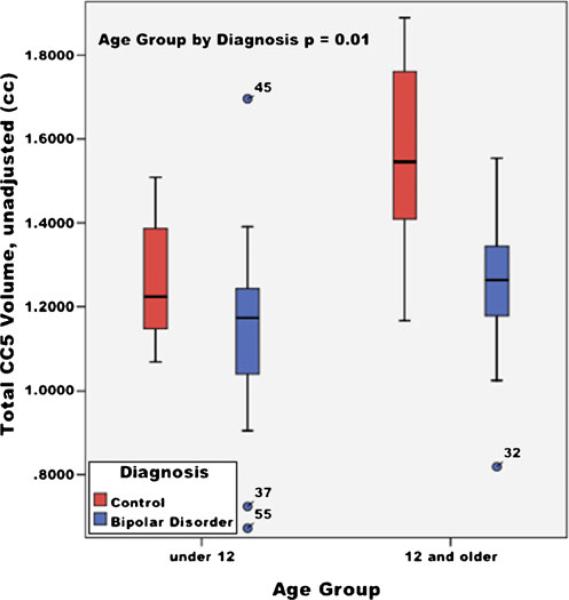
CC5 (Posterior body)
Significant effects for TCV (F=8.05, p<0.01) and age group-by-diagnosis interaction term (F=7.45, p<0.01) for the volumetric measurements of CC5 were found (see Fig. 5). A significant age group-by-diagnosis interaction term (F=5.18, p<0.01) for the area measurements of CC5 was also found. For both area and volumetric measurements, youths with BPD had smaller CC5 volumes in both age groups compared to HC.
Fig. 5.
Total CC5 volumes by age group (under 12 and 12 and older) in youths with bipolar disorder and healthy controls; unadjusted mean volumes (cc), ±95% confidence interval
CC6 (Isthmus)
A significant effect for age group (F=4.25, p=0.04) for the volumetric measurements of CC6 and age group-by-diagnosis (F=6.33, p<0.01) for the area measurement of CC6 were found, with youths with BPD having smaller CC6 in both age groups compared to HC.
CC7 (Splenium)
Significant effects for TCV (F=4.34, p=0.04 and F=6.73, p=0.01) and for age group-by-diagnosis interaction term (F=5.02, p=0.01 and F=3.88, p=0.03) were found for the volumetric and area measurements of CC7, respectively. Volumetric and area measurements in the 12 and under group found that youths with BPD had larger CC7 than HC, while in the 12 and older group, the HC's had larger total CC7 than the BPD youths.
In summary, BPD youths had smaller midbody (volumetric measurement only), posterior body, isthmus (cross-sectional measurement only), splenium and total CC relative to HC. Each of these regions also showed an age-by-diagnosis interaction, with HC aged 12 and older having larger volumes than similarly aged youths with BPD. Furthermore, TCV (means: BPD=1146.9±101.5; HC=1203.8±54.6) was found to have significant sex (F=16.61, p=<0.01) and diagnostic effects (F=6.85, p=0.01) with BPD females having the smallest TCV.
Post-hoc subgroup analyses
BPD without ADHD compared to HC
Similar UNIANOVAs were again performed on the CC1-CC7 and total CC volumes and areas as described above on participant subgroups including BPD youths without ADHD (n=23) compared to HC (n=22) and for youths with BPD with ADHD (BPD+ADHD) (n=21) compared to youths with BPD only (n=23). For BPD only compared to HC, subregion volume measurements for CC1-CC3 regions remained the same, i.e., nonsignificant between groups. Significant age group-by-diagnosis interactions were again found for the CC4 (p≤0.01), CC7 (p=0.01) and total CC volume (p=0.03). CC5 had significant age group (p=0.005) and diagnostic effects (p=0.002) and CC6 had significant diagnostic effects (p=0.04), which were not found before. For area measurements CC5 had diagnostic effects (0.03), and CC6 and CC7 had age group by diagnosis interactions, (p=0.04 and p=0.05, respectively). Total CC area only trended toward significance (p=0.07) for age group by diagnosis interaction.
BPD versus BPD+ADHD
Significant age group-by-diagnosis differences interactions were found between the BPD only group and BPD+ADHD for area measurements in the CC5 (p=0.05) and CC6 (p=0.05) regions, with individuals with BPD only, having reduced area measurements in both age groups compared to participants with BPD+ADHD. For volume measurements, similar results were found for CC6 [Age group (p=0.05); and diagnosis (p=0.02)] and CC5 but only at a trend level [Age group (p=0.02); diagnosis (p=0.09)].
Method comparison
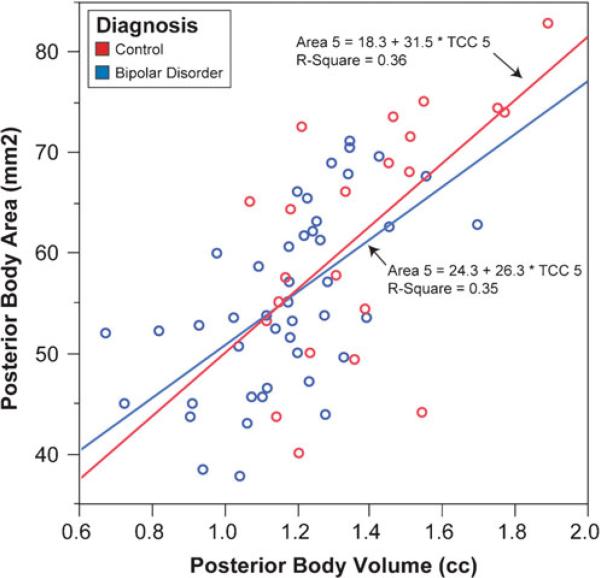
Correlations were performed between area and volume for total CC as well as subsections CC1 through CC7 for all participants, then by each group with a comparison of slope coefficients between the diagnostic groups. Overall, the two methods exhibited correlation coefficients that ranged from 0.35 (CC2) to 0.77 (CC7) with corresponding p-values ranging from 0.004 to <0.0001 based on the data of all 66 participants. The average correlation coefficient was 0.61. Subsection CC5 exemplifies this average and is depicted in Fig. 6 with fit lines for both healthy controls (r(22)=0.60, p=0.003) and youths with Bipolar Disorder (r(44)=0.59, p<0.0001). Similar results were found when correlations between areas and volumes were calculated within each group, although there was a modest difference for CC2: the correlation between CC2 area and CC2 volume was 0.46 (p=0.002) in youths with Bipolar Disorder, but 0.02 (p>0.9) in healthy controls (Fisher's Z-transform=–1.7, p=0.09). Comparisons of the remaining coefficients yielded Fisher's Z-transforms ranging from 0.04 (CC5) to 1.2 (CC6) with corresponding p-values 0.9–0.2. The strength of each correlation between CC area and volume was unrelated to the coefficient of variation for the corresponding region (Spearman rho(eight regions)=–0.05, p>0.9).
Fig. 6.
Linear regression of the correlation between volume and area measurements for CC5 in youths with bipolar disorder and healthy controls
Clinical variables
Pearson's correlations were performed on the continuous clinical variables listed in Table 2 with the volumetric measurements for the CC4 (volume only), CC5, CC6 (area only), CC7 and total CC and there were no significant findings or trends found.
Discussion
To our knowledge, this is the first study to evaluate the CC using a comprehensive system of sub-regional volumetric analysis in youths with BPD and directly comparing these findings to those of the traditional cross-sectional area methodology (Witelson 1989). Consistent with emerging neurodevelopmental models of psychiatric disorders, this study documents CC volume and growth abnormalities in youth with BPD and expands conceptualizations of the pathophysiology of BPD to include disturbances in inter-hemispheric connectivity in middle and posterior callosal regions. The comparison of the traditional cross-sectional area measures with volume measurements of the corpus callosum that is more representative of the entirety of the structure was performed to corroborate the cross-sectional measurement technique and examine whether or not volumetric examination provides more robust and reliable findings. Overall, our results suggest that the methods provide very similar results.
Our findings are consistent with prior studies of adults with BPD that found smaller posterior and total callosal areas (Brambilla et al. 2003; Coffman et al. 1990). However, unlike adult studies, anterior callosal regions were not found to be different in youths with BPD compared to HC, which may indicate that this finding occurs later in life secondary to abnormal brain maturation or illness progression. Furthermore, Brambilla and colleagues found an absence of the typical age-related decline in total callosal, genu, anterior body, and isthmus, in adults with BPD (Woodruff et al. 1997). This current study in youths with BPD found a reduction in regions of the CC that typically increase with age, including the mid-body (volumetric measurement only), posterior body, isthmus (cross-sectional area measurement only), and the splenium as compared to HC. Overall, youths with BPD had smaller callosal volumes in middle and posterior regions in the 12 and older group as compared to similarly aged HC. These findings may reflect abnormal callosal maturation or growth trajectories, which could be due to aberrant myelination and/or pruning. Topographically, these middle and posterior callosal regions interhemispherically connect motor, somatosensory, parietal, temporal and occipital cortices (de Lacoste et al. 1985; Pandya and Seltzer 1986; Witelson 1989), which are involved in primary, secondary and heteromodal (area 7b) somatosensory functions. Therefore, these findings suggest problems with interhemispheric connectivity between these key regions of the brain may begin early in the course of BPD illness and affect normal neuromaturation (both progressive and regressive) of this structure throughout the lifecycle.
Our findings are supported by structural and functional MRI studies, which have noted abnormalities in youths with BPD in cortical regions thought to be interhemisperically connected by regions of the middle and posterior callosal regions such as the parietal and temporal cortices. For example, Frazier and colleagues (Frazier et al. 2005a) compared HC to youths with BPD and found reduced bilateral parietal lobe and left temporal lobe in the BPD group. On further analysis of the parietal lobe and temporal lobe, smaller volumes were noted in the bilateral post-central gyri, left superior temporal gyrus (STG), and left fusiform gyrus in the BPD group. In a longitudinal voxel based morphometric (VBM) study of an older group of adolescents and young adults, Farrow and associates (Farrow et al. 2005) looked at 25 youths with schizophrenia and eight participants with BPD, first-episode, who had MRI scans at first presentation and 2 years later and 22 HC who had one set of MRI scans. Youths with BPD compared with HC had decreased gray matter volumes in the temporal lobe (bilateral inferior temporal gyrus/uncus, left insula, left posterior inferior/middle temporal gyrus), and parietal lobe (left posterior cingulate gyrus). In addition, the BPD group also had reduced WM in bilateral posterior parietotemporal junctions. Furthermore, reductions in WM were found in the follow-up scans relative to the time one scan in the BPD group in regions of the right posterior frontal/parietal cortex, left temporoparietal junction, right parietooccipital junction, left parietal lobe, and right cerebellum (Farrow et al. 2005). Cognitive-based fMRI studies in youths with BPD have also noted abnormalities in the primary motor cortex, parietal lobe (precuneus, posterior parietal cortex) and temporal lobe (middle temporal gyrus, left STG/insula) regions in early-onset BPD (Chang et al. 2004; Nelson et al. 2007; Adler et al. 2005). Emotion-based fMRI studies in youths with BPD report abnormalities in regions of the temporal (parahippocampal gyrus, left superior/middle temporal gyrus, right insula) and parietal (right posterior parietal cortex, paracentral lobule, and precuneus) lobe (Dickstein et al. 2007; Pavuluri et al. 2007; Rich et al. 2006, 2008). Deficits in specific parietal and temporal lobe functions have been found in visuospatial ability and verbal memory in both adults and youths with BPD (Cahill et al. 2007; Joseph et al. 2008). In summary, these findings suggest that mid and posterior callosal regions are reduced and show atypical development in early-onset BPD. These callosal abnormalities in interhemispheric connectivity may be associated with the corresponding cortical gray matter (GM) deficits and neurocognitive and emotional disturbances reported in youths with BPD.
Age-related abnormalities in the CC are not specific to BPD and have also been reported in schizophrenia (SZ) (Woodruff et al. 1997) and childhood-onset SZ (Jacobsen et al. 1997), suggesting that neurodevelopmental abnormalities of the CC may be important in the pathophysiology of other neuropsychiatric disorders as well. We did not find differences in anterior callosal regions including the rostrum, genu, or anterior body between diagnostic groups. Furthermore, age-related volume changes were not detected in these regions in youths with BPD compared to HC. In sharp contrast to Brambilla and colleagues, we did not find genual abnormalities, which may be a finding specific to adults with BPD (Woodruff et al. 1997). However, the lack of finding may also be due to insufficient power, the cross-sectional design of the study, or the normative individual variation in morphology of the CC, which could make detection of subtle developmental changes difficult (Giedd et al. 1996; Keshavan et al. 2002; Pujol et al. 1993). After early childhood, typically significant increases are limited to middle and posterior callosal regions such as the body, isthmus, and splenium, with the latter being most dramatic (Giedd et al. 1999). In addition, development (pruning and myelination) of the CC extends well into the second decade of life, and the children included in this study have not yet reached a developmental plateau in the neuromaturation of this complex brain structure. Finally, our lack of finding may also suggest that alternative metrics for evaluating the CC may be necessary at different developmental epochs. Metrics including thickness, shape, area, volume, length, diffusion and signal intensity may have different sensitivities in detecting microstructural callosal changes with age such as myelination and axonal size, number, composition and organization. Therefore, further studies of CC neuomaturation should include longitudinal designs with multimodal imaging and diverse analytic techniques.
Correlations with clinical variables were not found. There were no associations between any callosal subregion volumes or chlorpromazine equivalent dosages among those taking antipsychotics (n=27). Unfortunately the authors had limited dosage data on psychotropic medications used at the time of participant MRI scanning. The effects of psychotropic medications, such as mood stabilizers, on brain structures, particularly the CC, remain unknown. However, recent studies suggest that mood stabilizers are neuroprotective, particularly through their antiapoptotic effects (Atmaca et al. 2007a; Bielecka and Obuchowicz 2008). Further investigations of the impact of medications on specific brain regions in neuropsychiatric disorders are clearly warranted.
These findings should be interpreted with caution given the limitations of this study. For example, there were half as many HC participants as those with BPD. However, all structures showed good homogeneity of variance, which would attenuate the possible power-reducing effects of unequal group sizes, and the groups were well-matched on demographic characteristics. In addition, multiple comparisons were performed for each region. This study included both male and female participants and sex-structure interactions were assessed as they are thought to be important in CC regions, although the published literature is conflicting on this issue (Giedd et al. 1999). However, the impact of sex on the CC in this study was less of a concern as it was similarly distributed between diagnostic groups and could be adequately controlled for. Sex was used as a factor in all initial model analyses, and no sex or sex-by-diagnosis interactions were found. Another limitation of this study included the use of youths with comorbid ADHD. Callosal studies of ADHD youths have noted abnormalities in the splenium (Hutchinson et al. 2008; Valera et al. 2007), therefore, our findings of reduced splenial volumes may be secondary to the inclusion of youths with comorbid ADHD. Twenty-one participants with BPD (48%) in this study had comorbid ADHD. Our subgroup analysis indicated that the CC5 and CC6 regions were preferentially affected in youths with BPD only compared to BPD+ADHD youths. This finding was unexpected based on our prior study that suggested BPD+ADHD is a more severe subtype of BPD (Lopez-Larson et al. 2009). Ascertainment of a larger number of participants with BPD with and without comorbid ADHD is underway in order to assess the impact of ADHD status on this region. Finally, 27% of youths with BPD who participated in this study had histories of psychosis, which could have also impacted our findings.
This study directly compared volumetric callosum data to traditional cross-sectional area measurements for callosal subregions. Given that differences in callosal regions were detected using volumetric and cross-sectional area methodology suggests both methods are sensitive for evaluating callosal regions and detecting abnormalities. Overall, the methods were comparable with the exception of the difference in findings of the mid-body region, which was only significant in the volumetric analysis and the isthmus, for the cross-sectional area measurement. Interesting to note in the normative population, the area and volume measurements in the isthmus and CC4 regions were more correlated (CC4: r=.45, p=0.04; isthmus r=0.81, p<0.001) than in the BPD participants (CC4 r=0.41, p=0.01, and isthmus r=0.65, p<0.001), suggesting greater variability in the BPD group. Furthermore, methodological differences in the subdivision of the CC subregions could have arisen from the fact the single mid-sagittal area measurement was unable to pick up the possible shape or morphometric changes that may have occurred in the periphery of the structure. Medium to large effect sizes were seen for both methods. Further neuropsychiatric investigations of the CC should include both area and volumetric measurements to explore the differences in these techniques and to permit comparisons with prior studies, which have reported CC area.
By linking anatomical mal-development to specific cognitive and behavioral changes in children and adolescents, we may learn more about the functional organization of the brain, its development and its disruption in pediatric BPD and other early-onset mood and psychotic disorders. One important task of future research is to discern how these anatomical deviations might be related to neurodevelopment. Further cross-sectional and longitudinal studies of the CC are clearly warranted to understand more fully how neurodevelopmental changes in this important brain structure are mediated by interactions with symptom type and severity, comorbidity, psychosocial factors (e.g., stress), and medications.
Acknowledgements
This work was supported by several research grants from NIH: K08 MH01573 to JAF, U24 RR021382 to DNK, and K01 MH01798 to CM
Contributor Information
Melissa Lopez-Larson, The Brain Institute, University of Utah, 383 Colorow Drive, Salt Lake City, UT 84108, USA Melissa.Lopez-Larson@hsc.utah.edu; University of Utah Medical School, Salt Lake City, UT, USA.
Janis L. Breeze, Harvard Medical School, Boston, MA, USA Child and Adolescent Neuropsychiatric Research Program, Cambridge Health Alliance, Cambridge, MA, USA.
David N. Kennedy, Harvard Medical School, Boston, MA, USA Department of Neurology, Massachusetts General Hospital (MGH), Boston, MA, USA; Center for Morphometric Analysis, MGH, Charlestown, MA, USA; Child and Adolescent Neurodevelopment Initiative, University of Massachusetts Medical School, Worcester, MA, USA; Psychiatry Department, UMASS Memorial Medical Center, Worcester, MA, USA.
Steven M. Hodge, Center for Morphometric Analysis, MGH, Charlestown, MA, USA Child and Adolescent Neurodevelopment Initiative, University of Massachusetts Medical School, Worcester, MA, USA; Psychiatry Department, UMASS Memorial Medical Center, Worcester, MA, USA.
Lena Tang, Center for Morphometric Analysis, MGH, Charlestown, MA, USA.
Constance Moore, Harvard Medical School, Boston, MA, USA; Brain Imaging Center, McLean Hospital, Belmont, MA, USA.
Anthony J. Giuliano, Harvard Medical School, Boston, MA, USA
Nikos Makris, Harvard Medical School, Boston, MA, USA; Department of Neurology, Massachusetts General Hospital (MGH), Boston, MA, USA; Center for Morphometric Analysis, MGH, Charlestown, MA, USA.
Verne S. Caviness, Harvard Medical School, Boston, MA, USA Department of Neurology, Massachusetts General Hospital (MGH), Boston, MA, USA; Center for Morphometric Analysis, MGH, Charlestown, MA, USA.
Jean A. Frazier, Child and Adolescent Neurodevelopment Initiative, University of Massachusetts Medical School, Worcester, MA, USA Psychiatry Department, UMASS Memorial Medical Center, Worcester, MA, USA.
References
- Adler CM, Delbello MP, Mills NP, Schmithorst V, Holland S, Strakowski SM. Comorbid ADHD is associated with altered patterns of neuronal activation in adolescents with bipolar disorder performing a simple attention task. Bipolar Disorders. 2005;7(6):577–588. doi: 10.1111/j.1399-5618.2005.00257.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. American Psychiatric Association; Washington: 1994. [Google Scholar]
- Atmaca M, Ozdemir H, Cetinkaya S, Parmaksiz S, Belli H, Poyraz AK, et al. Cingulate gyrus volumetry in drug free bipolar patients and patients treated with valproate or valproate and quetiapine. Journal of Psychiatric Research. 2007a;41(10):821–827. doi: 10.1016/j.jpsychires.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Atmaca M, Ozdemir H, Yildirim H. Corpus callosum areas in first-episode patients with bipolar disorder. Psychological Medicine. 2007b;37(5):699–704. doi: 10.1017/S0033291706009743. [DOI] [PubMed] [Google Scholar]
- Barkovich AJ. Normal development of the neonatal and infant brain. In: Barkovich AJ, editor. Pediatric neuroimaging. Raven Press; New York: 1990. pp. 5–34. [Google Scholar]
- Barnea-Goraly N, Chang KD, Karchemskiy A, Howe ME, Reiss AL. Limbic and corpus callosum aberrations in adolescents with bipolar disorder: a tract-based spatial statistics analysis. Biological Psychiatry. 2009;66(3):238–244. doi: 10.1016/j.biopsych.2009.02.025. [DOI] [PubMed] [Google Scholar]
- Bastin ME, Piatkowski JP, Storkey AJ, Brown LJ, Maclullich AM, Clayden JD. Tract shape modelling provides evidence of topological change in corpus callosum genu during normal ageing. Neuroimage. 2008;43(1):20–28. doi: 10.1016/j.neuroimage.2008.06.047. [DOI] [PubMed] [Google Scholar]
- Bielecka AM, Obuchowicz E. Antiapoptotic action of lithium and valproate. Pharmacological Reports. 2008;60(6):771–782. [PubMed] [Google Scholar]
- Brambilla P, Nicoletti MA, Sassi RB, Mallinger AG, Frank E, Kupfer DJ, et al. Magnetic resonance imaging study of corpus callosum abnormalities in patients with bipolar disorder. Biological Psychiatry. 2003;54(11):1294–1297. doi: 10.1016/s0006-3223(03)00070-2. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Nicoletti M, Sassi RB, Mallinger AG, Frank E, Keshavan MS, et al. Corpus callosum signal intensity in patients with bipolar and unipolar disorder. Journal of Neurology, Neurosurgery and Psychiatry. 2004;75(2):221–225. [PMC free article] [PubMed] [Google Scholar]
- Burke HL, Yeo RA. Systematic variations in callosal morphology: the effects of age, gender, hand preference, and anatomic asymmetry. Neuropsychology. 1994;8(4):563–571. [Google Scholar]
- Caetano SC, Silveira CM, Kaur S, Nicoletti M, Hatch JP, Brambilla P, et al. Abnormal corpus callosum myelination in pediatric bipolar patients. Journal of Affective Disorders. 2008;108(3):297–301. doi: 10.1016/j.jad.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill CM, Green MJ, Jairam R, Malhi GS. Do cognitive deficits in juvenile bipolar disorder persist into adulthood? The Journal of Nervous and Mental Disease. 2007;195(11):891–896. doi: 10.1097/NMD.0b013e318159288b. [DOI] [PubMed] [Google Scholar]
- Caviness VS, Makris N, Meyer DA, Kennedy DN. MRI-based topographic parcellation of human neocortex: an anatomically specified method with estimate of reliability. Journal of Cognitive Neuroscience. 1996;8(6):566–588. doi: 10.1162/jocn.1996.8.6.566. [DOI] [PubMed] [Google Scholar]
- Chaddock CA, Barker GJ, Marshall N, Schulze K, Hall MH, Fern A, et al. White matter microstructural impairments and genetic liability to familial bipolar I disorder. The British Journal of Psychiatry. 2009;194(6):527–534. doi: 10.1192/bjp.bp.107.047498. [DOI] [PubMed] [Google Scholar]
- Chang K, Adleman NE, Dienes K, Simeonova DI, Menon V, Reiss A. Anomalous prefrontal-subcortical activation in familial pediatric bipolar disorder. Archives of General Psychiatry. 2004;61:781–792. doi: 10.1001/archpsyc.61.8.781. [DOI] [PubMed] [Google Scholar]
- Coffman JA, Bornstein RA, Olson SC, Schwarzkopf SB, Nasrallah HA. Cognitive impairment and cerebral structure by MRI in bipolar disorder. Biological Psychiatry. 1990;27:1188–1196. doi: 10.1016/0006-3223(90)90416-y. [DOI] [PubMed] [Google Scholar]
- de Lacoste MC, Kirkpatrick JB, Ross ED. Topography of the human corpus callosum. Journal of Neuropathology and Experimental Neurology. 1985;44(6):578–591. doi: 10.1097/00005072-198511000-00004. [DOI] [PubMed] [Google Scholar]
- Dickstein DP, Rich BA, Roberson-Nay R, Berghorst L, Vinton D, Pine DS, et al. Neural activation during encoding of emotional faces in pediatric bipolar disorder. Bipolar Disorders. 2007;9(7):679–692. doi: 10.1111/j.1399-5618.2007.00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrow TF, Whitford TJ, Williams LM, Gomes L, Harris AW. Diagnosis-related regional gray matter loss over two years in first episode schizophrenia and bipolar disorder. Biological Psychiatry. 2005;58(9):713–723. doi: 10.1016/j.biopsych.2005.04.033. [DOI] [PubMed] [Google Scholar]
- Filipek PA, Kennedy DN, Caviness VS, Jr., Rossnick SL, Spraggins TA, Starewicz PM. Magnetic resonance imaging-based morphometry: development and applications to normal controls. Annals of Neurology. 1989;25(1):61–67. doi: 10.1002/ana.410250110. [DOI] [PubMed] [Google Scholar]
- Frazier JA, Breeze JL, Makris N, Giuliano AJ, Herbert MR, Seidman LJ, et al. Cortical gray matter differences identified by structural magnetic resonance imaging in pediatric bipolar disorder. Bipolar Disorders. 2005a;7:555–569. doi: 10.1111/j.1399-5618.2005.00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier JA, Chiu S, Breeze JL, Makris N, Lange N, Kennedy DN, et al. Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. The American Journal of Psychiatry. 2005b;162(7):1256–1265. doi: 10.1176/appi.ajp.162.7.1256. [DOI] [PubMed] [Google Scholar]
- Frazier JA, Breeze JL, Papadimitriou G, Kennedy DN, Hodge SM, Moore CM, et al. White matter abnormalities in children with and at risk for bipolar disorder. Bipolar Disorders. 2007;9(8):799–809. doi: 10.1111/j.1399-5618.2007.00482.x. [DOI] [PubMed] [Google Scholar]
- Frazier JA, Hodge SM, Breeze JL, Giuliano AJ, Terry JE, Moore CM, et al. Diagnostic and sex effects on limbic volumes in early-onset bipolar disorder and schizophrenia. Schizophrenia Bulletin. 2008;34(1):37–46. doi: 10.1093/schbul/sbm120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaniga MS. Cerebral specialization and interhemispheric communication: does the corpus callosum enable the human condition? Brain. 2000;123(Pt 7):1293–1326. doi: 10.1093/brain/123.7.1293. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Rumsey JM, Castellanos FX, Rajapakse JC, Kaysen D, Vaituzis AC, et al. A quantitative MRI study of the corpus callosum in children and adolescents. Brain Research. Developmental Brain Research. 1996;91(2):274–280. doi: 10.1016/0165-3806(95)00193-x. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Rajapakse JC, Vaituzis AC, Liu H, et al. Development of the human corpus callosum during childhood and adolescence: a longitudinal MRI study. Progress in Neuropsychopharmacology & Biological Psychiatry. 1999;23(4):571–588. doi: 10.1016/s0278-5846(99)00017-2. [DOI] [PubMed] [Google Scholar]
- Hauser P, Dauphinais ID, Berrettini W, DeLisi LE, Gelernter J, Post RM. Corpus callosum dimensions measured by magnetic resonance imaging in bipolar affective disorder and schizophrenia. Biological Psychiatry. 1989;26(7):659–668. doi: 10.1016/0006-3223(89)90100-5. [DOI] [PubMed] [Google Scholar]
- Hutchinson AD, Mathias JL, Banich MT. Corpus callosum morphology in children and adolescents with attention deficit hyperactivity disorder: a meta-analytic review. Neuropsychology. 2008;22(3):341–349. doi: 10.1037/0894-4105.22.3.341. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Giedd JN, Rajapakse JC, Hamburger SD, Vaituzis AC, Frazier JA, et al. Quantitative magnetic resonance imaging of the corpus callosum in childhood onset schizophrenia. Psychiatry Research. 1997;68(2–3):77–86. doi: 10.1016/s0925-4927(96)03019-3. [DOI] [PubMed] [Google Scholar]
- Joseph MF, Frazier TW, Youngstrom EA, Soares JC. A quantitative and qualitative review of neurocognitive performance in pediatric bipolar disorder. Journal of Child and Adolescent Psychopharmacology. 2008;18(6):595–605. doi: 10.1089/cap.2008.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan MS, Diwadkar VA, DeBellis M, Dick E, Kotwal R, Rosenberg DR, et al. Development of the corpus callosum in childhood, adolescence and early adulthood. Life Sciences. 2002;70(16):1909–1922. doi: 10.1016/s0024-3205(02)01492-3. [DOI] [PubMed] [Google Scholar]
- Lopez-Larson MP, Michael ES, Terry JE, Breeze JL, Hodge SM, Tang L, et al. Subcortical differences among youths with ADHD compared to those with bipolar disorder with and without ADHD. Journal of Child and Adolescent Psychopharmacology. 2009;19(1) doi: 10.1089/cap.2008.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Meyer JW, Bates JF, Yeterian EH, Kennedy DN, Caviness VS. MRI-based topographic parcellation of human cerebral white matter and nuclei II. Rationale and applications with systematics of cerebral connectivity. Neuroimage. 1999;9(1):18–45. doi: 10.1006/nimg.1998.0384. [DOI] [PubMed] [Google Scholar]
- Meyer JW, Makris N, Bates JF, Caviness VS, Kennedy DN. MRI-based topographic parcellation of human cerebral white matter. Neuroimage. 1999;9(1):1–17. doi: 10.1006/nimg.1998.0383. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Vinton DT, Berghorst L, Towbin KE, Hommer RE, Dickstein DP, et al. Brain systems underlying response flexibility in healthy and bipolar adolescents: an event-related fMRI study. Bipolar Disorders. 2007;9(8):810–819. doi: 10.1111/j.1399-5618.2007.00419.x. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Orvaschel H, Puig-Antich J. Schedule for affective disorders and schizophrenia for school-age children: Epidemiologic 4th Version. Nova University, Center for Psychological Study; Ft. Lauderdale: 1987. [Google Scholar]
- Pagani E, Agosta F, Rocca MA, Caputo D, Filippi M. Voxel-based analysis derived from fractional anisotropy images of white matter volume changes with aging. Neuroimage. 2008;41(3):657–667. doi: 10.1016/j.neuroimage.2008.03.021. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Seltzer B. The topography of commissural fibers. In: Lepore F, Ptito M, Jasper HH, editors. Two hemispheres, one brain: Functions of the corpus callosum. Wiley; New York: 1986. pp. 47–73. [Google Scholar]
- Paus T, Collins DL, Evans AC, Leonard G, Pike B, Zijdenbos A. Maturation of white matter in the human brain: a review of magnetic resonance studies. Brain Research Bulletin. 2001;54(3):255–266. doi: 10.1016/s0361-9230(00)00434-2. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN, O'Connor MM, Harral E, Sweeney JA. Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biological Psychiatry. 2007;62(2):158–167. doi: 10.1016/j.biopsych.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Pujol J, Vendrell P, Junque C, Marti-Vilalta JL, Capdevila A. When does human brain development end? Evidence of corpus callosum growth up to adulthood. Annals of Neurology. 1993;34(1):71–75. doi: 10.1002/ana.410340113. [DOI] [PubMed] [Google Scholar]
- Rich BA, Vinton DT, Roberson-Nay R, Hommer RE, Berghorst LH, McClure EB, et al. Limbic hyper-activation during processing of neutral facial expressions in children with bipolar disorder. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(23):8900–8905. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich BA, Fromm SJ, Berghorst LH, Dickstein DP, Brotman MA, Pine DS, et al. Neural connectivity in children with bipolar disorder: impairment in the face emotion processing circuit. Journal of Child Psychology and Psychiatry. 2008;49(1):88–96. doi: 10.1111/j.1469-7610.2007.01819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman LJ, Faraone SV, Goldstein JM, Goodman JM, Kremen WS, Matsuda G, et al. Reduced subcortical brain volumes in nonpsychotic siblings of schizophrenic patients: a pilot magnetic resonance imaging study. American Journal of Medical Genetics. 1997;74(5):507–514. doi: 10.1002/(sici)1096-8628(19970919)74:5<507::aid-ajmg11>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Soares JC, Mann JJ. The anatomy of mood disorders: review of structural neuroimaging studies. Biological Psychiatry. 1997;41(1):86–106. doi: 10.1016/s0006-3223(96)00006-6. [DOI] [PubMed] [Google Scholar]
- Tomasch J. Size, distribution, and number of fibres in the human corpus callosum. The Anatomical Record. 1954;119(1):119–135. doi: 10.1002/ar.1091190109. [DOI] [PubMed] [Google Scholar]
- Valera EM, Faraone SV, Murray KE, Seidman LJ. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2007;61(12):1361–1369. doi: 10.1016/j.biopsych.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Walterfang M, Malhi GS, Wood AG, Reutens DC, Chen J, Barton S, et al. Corpus callosum size and shape in established bipolar affective disorder. Australian and New Zealand Journal of Psychiatry. 2009;43(9):838–845. doi: 10.1080/00048670903107534. [DOI] [PubMed] [Google Scholar]
- Wang F, Kalmar JH, Edmiston E, Chepenik LG, Bhagwagar Z, Spencer L, et al. Abnormal corpus callosum integrity in bipolar disorder: a diffusion tensor imaging study. Biological Psychiatry. 2008;64(8):730–733. doi: 10.1016/j.biopsych.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder-Willis KE, Sax KW, Rosenberg HL, Fleck DE, Shear PK, Strakowski SM. Persistent attentional dysfunction in remitted bipolar disorder. Bipolar Disorders. 2001;3(2):58–62. doi: 10.1034/j.1399-5618.2001.030202.x. [DOI] [PubMed] [Google Scholar]
- Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum: a postmortem morphological study. Brain. 1989;112(Pt 3):799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- Woodruff PW, Phillips ML, Rushe T, Wright IC, Murray RM, David AS. Corpus callosum size and interhemispheric function in schizophrenia. Schizophrenia Research. 1997;23(3):189–196. doi: 10.1016/s0920-9964(96)00103-x. [DOI] [PubMed] [Google Scholar]
- Yasar AS, Monkul ES, Sassi RB, Axelson D, Brambilla P, Nicoletti MA, et al. MRI study of corpus callosum in children and adolescents with bipolar disorder. Psychiatry Research. 2006;146(1):83–85. doi: 10.1016/j.pscychresns.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. The British Journal of Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd DA, Silveri MM, Gruber SA, Rohan ML, Pimentel PJ. White matter abnormalities observed in bipolar disorder: a diffusion tensor imaging study. Bipolar Disorders. 2007;9(5):504–512. doi: 10.1111/j.1399-5618.2007.00395.x. [DOI] [PubMed] [Google Scholar]