Abstract
CHARGE (coloboma, heart defects, atresia of choanae, retardation of growth and development, genital hypoplasia, and ear abnormalities) and 22q11.2 deletion syndromes are variable, congenital malformation syndromes that show considerable phenotypic overlap. We further explored this clinical overlap and proposed recommendations for the genetic diagnosis of both syndromes. We described 2 patients clinically diagnosed with CHARGE syndrome, who were found to carry a 22q11.2 deletion, and searched the literature for more cases. In addition, we screened our cohort of CHD7 mutation carriers (n = 802) for typical 22q11.2 deletion features and studied CHD7 in 20 patients with phenotypically 22q11.2 deletion syndrome but without haploinsufficiency of TBX1. In total, we identified 5 patients with a clinical diagnosis of CHARGE syndrome and a proven 22q11.2 deletion. Typical 22q11.2 deletion features were found in 30 patients (30/802, 3.7%) of our CHD7 mutation-positive cohort. We found truncating CHD7 mutations in 5/20 patients with phenotypically 22q11.2 deletion syndrome. Differentiating between CHARGE and 22q11.2 deletion syndromes can be challenging. CHD7 and TBX1 probably share a molecular pathway or have common target genes in affected organs. We strongly recommend performing CHD7 analysis in patients with a 22q11.2 deletion phenotype without TBX1 haploinsufficiency and conversely, performing a genome-wide array in CHARGE syndrome patients without a CHD7 mutation.
Key Words : CHARGE syndrome, CHD7, 22q11.2 deletion syndrome, TBX1
CHARGE syndrome (OMIM 214800, coloboma, heart defects, atresia of choanae, retardation of growth and development, genital hypoplasia, and ear abnormalities) is a highly variable, multiple congenital malformation syndrome that shows considerable clinical overlap with other syndromes like Kallmann syndrome [Kim et al., 2008; Jongmans et al., 2009], VACTERL association (vertebral anomalies, anal atresia, cardiac defects, tracheo-oesophageal fistula, oesophageal atresia, renal anomalies, and limb defects) [Källén et al., 2004; Solomon, 2011], Goldenhar syndrome (oculo-auriculo-vertebral spectrum) [Van Meter and Weaver, 1996; Källén et al., 2004], and SOX2 anophthalmia syndrome [Engelen et al., 2011]. The most striking similarity of clinical features, however, is seen with 22q11.2 deletion syndrome as illustrated by Jyonouchi et al. [2009]. Here, we further explored the clinical similarities between CHARGE syndrome and 22q11.2 deletion syndrome.
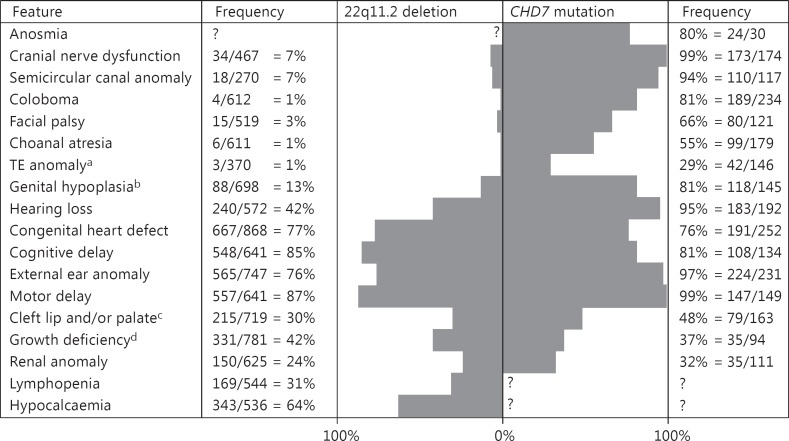
CHARGE syndrome has an estimated birth incidence of 5.8-6.7 per 100,000 live births [Janssen et al., 2012]. A patient is currently diagnosed with CHARGE syndrome if the clinical diagnostic criteria of Blake et al. [1998] or of Verloes [2005] are fulfilled. The major clinical features include choanal atresia, coloboma of the eye, hypoplastic semicircular canals, external ear anomalies, and cranial nerve dysfunction (as summarised in fig. 1) [Bergman et al., 2011b]. CHARGE syndrome is inherited in an autosomal dominant fashion, but most cases are sporadic due to de novo mutations in the CHD7 gene [Zentner et al., 2010b; Bergman et al., 2011b]. CHD7 codes for a chromodomain helicase DNA-binding protein that has a cell type-specific and embryonic stage-dependent function in regulating the expression of other developmental genes [Vissers et al., 2004; Schnetz et al., 2009; Zentner et al., 2010a]. Heterozygous CHD7 mutations are found in more than 90% of the patients, who fulfil the clinical criteria of CHARGE syndrome, but can also be detected in patients with an atypical phenotype [Jongmans et al., 2006; Bergman et al., 2011b].
Fig. 1.
Frequency of the most common clinical features seen in patients with a 22q11.2 deletion and patients with a CHD7 mutation. Frequency is depicted as number of patients with feature/number of patients investigated; data is based on 943 patients from Children's Hospital of Philadelphia Database for 22q11.2 deletion group and on 280 patients indentified at the Radboud University Nijmegen Medical Centre (Nijmegen, The Netherlands) with a CHD7 mutation as described in Bergman et al. [2011b]. TE anomaly = Tracheo-oesophageal anomaly. a In 22q11.2 deletion group only tracheo-oesophageal fistula. b Including 28 patients with hypospadias in 22q11.2 deletion group. c 148 patients in 22q11.2 deletion group had a submucosal cleft. d Height <2.5 SD in CHD7 cohort or below 5th percentile in 22q11.2 deletion cohort.
Chromosome 22q11.2 microdeletions have an estimated birth incidence between 10 and 26 per 100,000 live births and cause a highly variable clinical phenotype including velocardiofacial syndrome (OMIM 192430) which is the combination of velopharyngeal incompetence and other palate abnormalities, congenital heart defects, and dysmorphic facial features. DiGeorge syndrome (OMIM 188400) is another associated phenotype that includes features of congenital heart defects of the outflow tract, hypocalcaemia and immunodeficiency [McDonald-McGinn et al., 1993; Schwinger et al., 2010; McDonald-McGinn and Sullivan, 2011]. Since velocardiofacial syndrome and DiGeorge syndrome describe variable clinical expressions of the same entity, the term 22q11.2 deletion syndrome is now more commonly used (features summarised in fig. 1) [Jyonouchi et al., 2009; McDonald-McGinn and Sullivan, 2011]. This syndrome is inherited in an autosomal dominant manner from a parent in 10% of new cases but mostly occurs de novo [McDonald-McGinn and Sullivan, 2011]. The majority of patients with velocardiofacial syndrome and DiGeorge syndrome have a 3.0- (90%) or 1.5-Mb (8%) hemizygous deletion of chromosome 22q11.2 that can be identified using fluorescence in situ hybridization (FISH), multiplex ligation-dependent probe amplification (MLPA) or genome-wide array analysis [Schwinger et al., 2010; Gennery, 2012]. Mutations in the TBX1 gene located in the commonly deleted region cause a similar phenotype, and thus TBX1 haploinsufficiency appears to significantly contribute to the features of 22q11.2 deletion syndrome [Merscher et al., 2001].
The overlap between CHARGE syndrome and 22q11.2 deletion syndrome has long been recognised [Emanuel et al., 1992; de Lonlay-Debeney et al., 1997; Digilio et al., 1997; Devriendt et al., 1998; Herman and Siegel, 1998]. The overlapping clinical features include congenital conotruncal heart defects, cleft palate, ear abnormalities, hearing loss, growth deficiency, developmental delay, renal abnormalities, hypocalcaemia, and immune deficiency [Jyonouchi et al., 2009; Bergman et al., 2011b]. Clinical features like coloboma, choanal atresia, facial nerve palsy, tracheo-oesophageal fistula, hypoplastic semicircular canals, micropenis, or hypogonadotropic hypogonadism generally occur more often in patients with CHARGE syndrome than in those with 22q11.2 deletion syndrome, although not exclusively (see fig. 1). Because of the clinical resemblance between the 2 syndromes, in our recent review, we recommended that CHD7 is a good candidate gene to analyse in patients with clinical features of 22q11.2 deletion syndrome, but who do not have a deletion or mutation of TBX1. We further recommended that a whole-genome array should be performed in patients suspected of CHARGE syndrome but without a CHD7 mutation or deletion [Bergman et al., 2011b].
The overlap between CHARGE and 22q11.2 deletion syndrome and the variable expression of both syndromes can hamper clinical diagnosis but also provides interesting clues to the aetiology and pathogenesis of both syndromes. We provided further details of the overlap between the 2 syndromes by describing case reports of patients diagnosed with CHARGE syndrome but carrying a 22q11.2 deletion, by reporting the typical 22q11.2 deletion features present in a CHD7-positive cohort and by describing the results of CHD7 sequencing in a cohort of patients with features of 22q11.2 deletion syndrome but without a deletion or mutation of TBX1. The molecular pathways underlying this clinical resemblance and the implications for genetic diagnostic work were discussed.
Methods
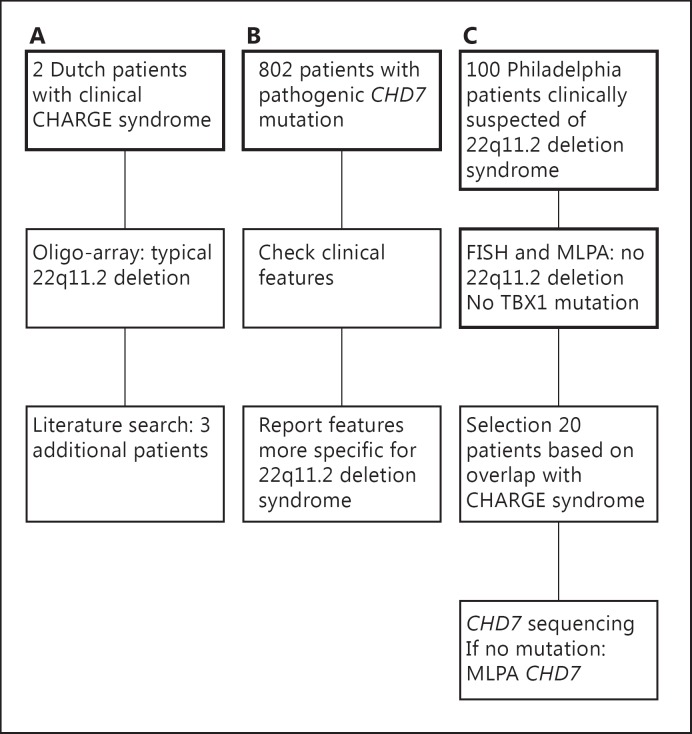
A schematic overview of our study design is shown in figure 2.
Fig. 2.
Overview of our study design. This flow diagram illustrates the 3 different parts of our study. The data in thickened boxes was available at the start of the study. A Clinical CHARGE patients with 22q11.2 deletions; B patients with a CHD7 mutation and features of 22q11.2 deletion syndrome; C CHD7 analysis in patients clinically presenting as 22q11.2 deletion syndrome.
Patients
We described the clinical findings of 2 Dutch patients, who were diagnosed with CHARGE syndrome according to the clinical criteria of Blake et al. [1998] and/or Verloes [2005], but who appeared to have a 22q11.2 deletion. We further summarised the available clinical data of 3 patients described in the literature with clinically typical CHARGE syndrome and a 22q11.2 deletion.
We screened our database of 802 patients with a pathogenic CHD7 mutation for clinical features more specific for 22q11.2 deletion syndrome, like hypocalcaemia, thymus anomalies and immunological problems, or who were reported to have a DiGeorge or 22q11.2 deletion phenotype.
In addition, we analysed CHD7 in a cohort of 20 patients from the Department of Paediatrics, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, Pa., USA. These patients were selected from a cohort of 100 cases, who were clinically suspected of 22q11.2 deletion syndrome, but in whom FISH did not detect a deletion of chromosome 22q11.2 and MLPA did not find any atypical deletions of the 22q11.22 region. In the patients selected for CHD7 analysis, point mutations in the TBX1 gene had also been excluded. The 20 patients were selected, because their clinical features overlapped with features seen in CHARGE syndrome, including the presence of congenital heart defects, coloboma, immune defects, ear anomalies, renal malformations, and hearing loss.
CHD7 Analysis
Blood samples from all patients were drawn after informed consent. DNA was isolated according to standard procedures. The 37 coding exons of CHD7 (exons 2-38, RefSeq NM_017780.02) and their flanking intronic sequences were amplified by PCR and sequenced as described earlier [Jongmans et al., 2006]. If no mutations were identified, CHD7 was screened for whole-exon deletions and duplications by MLPA using a commercially available set of probes: the SALSA P201 kit (MRC-Holland, Amsterdam, The Netherlands; http://www.mrc-holland.com) [Bergman et al., 2008].
Analysis of 22q11.2 Deletions and TBX1 Sequencing
Patients gave informed consent, and blood samples were taken. 22q11.2 deletions were detected or excluded in the Dutch patients by array comparative genomic hybridisation (CGH) using an Agilent 180 K oligonucleotide array (custom design no. 023363, Agilent Technologies Incorporation, Santa Clara, Calif., USA) and/or FISH using probe RP11-481H20 and RP11-590C5. The array CGH procedures were carried out according to the manufacturer's protocols. Normal male or female reference DNA was used as a control, and DNA analytics version 4.0.81 (Agilent Technologies Incorporation) was used to analyse the results.
The 20 Philadelphia cases were studied by FISH using the commercially available probe N25 and then analysed by an MLPA assay specific for the chromosome 22q11.22 region (MRC Holland; SALSA P250 kit) to identify deletions, whose proximal endpoints are distal to the standard FISH probes and would not have been identified in the original cohort [Jalali et al., 2008]. Point mutations in the TBX1 gene were excluded by capillary sequencing following PCR amplification as previously reported [Gong et al., 2001].
Results
Clinical CHARGE Patients with 22q11.2 Deletions
Table 1 lists all the patients with a CHARGE syndrome phenotype known to carry a 22q11.2 deletion including the 2 described below.
Table 1.
Clinical features in patients with clinical CHARGE syndrome and a 22q11.2 deletion
| Features | Patients |
||||
|---|---|---|---|---|---|
| Emanuel et al., 1992 | Digilio et al., 1997 | Devriendt et al., 1998 | our patient 1 | our patient 2 | |
| Coloboma | u | + | + | + | − |
| Heart defect | u | + | + | + | + |
| Choanal atresia* | u | + | + | + | + |
| Growth retardation | u | u | + | u | u |
| Developmental delay | u | u | + | + | + |
| Genital hypoplasia | u | + | − | − | − |
| Ear anomaly | u | + | + | − | + |
| SCC hypoplasia | u | u | u | + | − |
| Cleft lip and/or palate | u | − | − | − | − |
| Hearing loss | u | u | + | + | − |
| Feeding difficulties** | u | u | u | + | + |
| Facial palsy | u | u | u | − | − |
| TE anomaly | u | − | u | − | − |
| Hypocalcaemia | u | + | − | − | − |
| Immunological abn. | u | + | u | − | − |
| Thymus abn. | u | − | − | u | − |
| Other | CHARGE association, no further information | unilateral absent radius, hypoplastic ulna | preauricular tags, pit in left cheek | bronchotracheomalacia, GERD | pharyngomalacia |
Features more commonly seen in CHARGE syndrome than in 22q11.2 deletion syndrome are highlighted in grey.
abn. = Abnormalities; GERD = gastro-oesophageal reflux disease; SCC = semicircular canal; TE = tracheo-oesophageal anomaly; u = unexamined/unknown; + = present; − = not present.
Atresia or stenosis of choanae.
Only if necessitating tube feeding.
Patient 1. This girl was briefly described by Bergman et al. [2011b]. She was born by caesarean section because of foetal distress at 33 + 3 weeks of gestation. The pregnancy was complicated by gestational diabetes mellitus and polyhydramnios. Her birth weight was 2,830 g (97.7th percentile), and her Apgar scores were 9 and 9 after 1 and 5 min, respectively. During the first 4 weeks of life, she had feeding problems necessitating nasogastric tube feeding. She was diagnosed with bronchotracheomalacia and gastro-oesophageal reflux disease. Several congenital anomalies were noticed: coloboma of the left iris and both retinas, unilateral choanal stenosis, mild pulmonic stenosis, and bilateral mixed hearing loss. A CT scan of the mastoid showed abnormal semicircular canals as well as an abnormal vestibulum and an abnormal basal convolution of the cochlea. The girl had a developmental motor delay; she started walking at the age of 2 years and 3 months. At her last examination, at the age of 3 years, she had a normal language comprehension (quotient score of 91, comprehension scales of the Dutch Reynell Developmental Language Scales). Physical examination showed simple ears, an anteriorly placed anus and a hockey-stick line crease on both palms. The diagnosis CHARGE syndrome was made based on the clinical diagnostic criteria of both Blake et al. [1998] and Verloes [2005]. Analysis of CHD7 did not show a mutation or deletion. Subsequently, array CGH showed a de novo 2.6-Mb loss of 22q11.2 (proximal breakpoint 17,210,818-17,270,293; distal breakpoint 19,891,492-19,870,318). The deletion was confirmed by FISH analysis.
Patient 2. This female infant was born prematurely at 33 + 4 weeks with a birth weight of 1,565 g (10th-20th percentile). The pregnancy was complicated by polyhydramnios. Postnatally, she experienced respiratory distress and was suspected of having a partial choanal atresia. She had a congenital heart defect consisting of arterial septal defect, ventricular septal defect and patent ductus arteriosis. Morphologic evaluation at the age of 2 days revealed microcephaly (28 cm; −5.6 SD below mean), short palpebral fissures, dysmorphic ears with broad superior crus of the antihelices, overfolded helices, and a bulbous nasal tip. CHARGE syndrome or a chromosomal abnormality was suspected, and array CGH, fundoscopy, renal ultrasound, and brain imaging with special attention for the semicircular canals were suggested. Array CGH showed a 2.9-3.0 Mb deletion of 22q11.21 (proximal breakpoint 17,210,818-17,270,293; distal breakpoint 20,142,009-20,247,225) that was confirmed by FISH analysis. The other investigations had normal results, except for subtle abnormalities of the brain MRI scan with slightly delayed myelinisation and mildly enlarged ventricles.
At the age of 2 months, the ventricular septal defect was surgically corrected. From age 4-22 months, she required a tracheostomy because of the combination of very narrow choanae and pharyngomalacia. At the age of 10 months, a gastrostoma with feeding tube were placed. At her last examination at the age of 2 years and 4 months, her height was 86 cm (-1 SD). Her head circumference was not measured, but she was normocephalic before (at the age of 22 months, her head circumference was 47.7 cm (-0.7 SD)). She was still being fed through her feeding tube and had just started to use some spoken words in addition to sign language after the removal of her tracheotomy.
CHD7 Mutations in Phenotypic 22q11.2 Deletion Syndrome
Patients with a CHD7 Mutation and Features of 22q11.2 Deletion Syndrome. Table 2 summarises the 30 patients out of our international database of 802 patients, in which typical features of 22q11.2 deletion syndrome were described [Janssen et al., 2012]. We also included 3 additional patients from the literature, in whom the precise CHD7 mutation was not mentioned [Chopra et al., 2009]. All 33 patients had features that are more commonly seen in deletion 22q11.2 syndrome than in CHARGE syndrome. A 22q11.2 deletion was excluded in 25 of the 33 patients (76%). In one patient, a paternally inherited 2.5-Mb 22q11.23 deletion located distal to the 22q11.2 deletion syndrome region was identified in addition to the CHD7 mutation [Kaliakatsos et al., 2010]. At least, 16 of the 33 patients died in infancy.
Table 2.
Patients with a CHD7 mutation, who show clinical features of 22q11.2 deletion syndrome
| ID | Type of mutation | Features |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| coloboma | heart defect | choanal atresia* | growth retardation | developm. delay | genital hypoplasia | ear anomaly | SCC hypoplasia | cleft lip and/orpalate | hearing loss | feeding difficulties** | facial palsy | TE anomaly | hypocalcaemia | immunological abn. | thymus abn. | other | ||
| 0d | del | + | + | − | − | u | + | + | − | − | u | u | + | u | + | + | + | |
| 0n | del wg | − | + | − | u | + | u | + | u | − | u | u | u | u | u | u | u | 22q11 phenotype, long slender fingers |
| P99 | fs | + | + | − | u | + | u | + | u | u | + | + | u | + | u | + | + | |
| P129d | fs | + | + | + | + | + | + | + | + | − | + | u | + | u | + | + | u | PTH def |
| P137 | fs | + | + | − | + | + | u | + | + | u | + | + | u | − | u | u | + | bronchomalacia, torti-collis, GERD, absent thumbs |
| P147 | fs | + | + | − | + | u | + | + | u | u | + | u | u | − | u | u | + | fissure upper lobe right long, hypothyroidism |
| P197e | fs | + | + | − | u | u | + | + | + | u | u | u | + | u | + | + | + | laryngomalacia, erythroderma, total alopecia |
| P238 | fs | u | u | u | u | u | u | u | u | u | u | u | u | u | u | + | u | |
| P245a | fs | + | + | u | u | + | u | + | u | u | u | u | u | u | + | u | + | |
| P293m | fs | + | + | u | u | u | u | u | u | u | + | u | u | + | + | + | + | tracheomalacia, PTH def, ectopia of one kidney |
| P304 | fs | + | u | + | u | u | + | + | − | u | + | u | u | u | + | + | u | |
| P767 | fs | − | + | u | − | + | + | + | + | − | + | + | + | − | + | u | u | PTH def, OSAS |
| P800i | fs | + | + | − | − | + | + | + | + | − | + | + | + | + | u | − | + | obstructive apneus |
| P866 | fs | u | u | u | u | u | + | + | u | u | u | u | u | u | + | u | u | |
| P875 | fs | − | − | + | + | + | + | + | + | + | + | + | + | − | + | + | + | laryngeomalcia, hypothyreoidy, vermis dysplasia |
| P987h | fs | + | + | u | u | u | + | + | u | u | + | u | u | u | + | u | u | PTH def, limb abnormality |
| P29a | non | + | + | u | u | u | + | + | u | + | u | u | u | u | u | u | + | abnormal limbs |
| P40 | non | + | + | + | + | u | u | + | u | u | u | u | u | u | u | + | u | |
| P37b | non | − | + | + | u | u | + | + | + | u | + | u | − | − | + | u | + | PTH def |
| P44c | non | + | + | + | u | u | − | + | u | u | u | u | + | u | + | + | + | PTH def, laryngomalacia, abnormal limbs |
| P122a | non | + | + | + | u | u | + | u | u | u | u | u | u | + | + | + | u | |
| P189b | non | u | + | − | u | u | + | + | u | u | − | u | u | + | + | + | u | hypothyroidism, partial agenesis corpus callosum, horseshoe kidney |
| P256fg | non | u | + | u | u | u | + | u | u | + | u | u | u | u | u | u | + | terminated pregnancy, left isomerism, hypoplastic optic nerves cortical brain atrophy |
| P279ikl | non | − | + | + | + | + | + | + | + | − | + | + | u | − | u | − | + | |
| P780ik | non | − | + | + | + | + | + | + | + | − | + | − | − | + | + | − | − | NEC, hydrocephalus, GERD |
| P834i | non | + | + | u | + | + | + | + | + | + | + | u | + | u | u | u | + | laryngomalacia, PTH def, corpus callosum hypoplasia, horseshoe kidney |
| P898 | non | + | + | u | u | u | u | + | u | + | + | u | u | u | u | + | u | hydrocephaly glottic web, laryngomalacia, vertical talus preauricular tags |
| P1003j | non | − | + | + | u | u | − | + | + | u | u | u | u | u | + | + | + | |
| 0a | non | u | + | u | u | u | + | + | u | u | u | u | u | u | + | u | + | |
| P616 | Sp1 | + | + | − | + | u | + | + | + | u | u | u | + | − | + | u | + | scalp cutis aplasia, PTH def |
| 0o | u | + | + | u | u | u | u | + | u | − | + | u | u | + | u | + | + | |
| 0o | u | + | + | + | + | + | u | u | u | − | + | u | + | u | u | + | u | seizures |
| 0o | u | + | + | u | u | u | u | u | u | − | + | u | − | u | u | + | u | bulbar palsy |
Features more commonly seen in 22q11.2 deletion syndrome than in CHARGE syndrome are highlighted in grey.
u = Unexamined/unknown; + = present; − = not present; abn. = abnormalities; del = deletion exon 4; fs = frameshift; developm. = developmental; GERD = gastro-oesophageal reflux disease; ID = patient ID in online database of CHD7 mutations (www.chd7.org); non = nonsense; NEC = necrotising enterocolitis; OSAS = obstructive sleep apnea syndrome; PTH def = low parathyroid hormone or hypoparathyroidism; SCC = semicircular canal; spl = splice site; TE = tracheo-oesophageal; U = mutations unknown (all patients described in Chopra et al. [2008]); wg = whole gene; 0 = not mentioned in the online database.
Chopra et al., 2008.
Atresia or stenosis of choanae.
Only if necessitating tube feeding.
CHD7 Analysis in Patients Clinically Presenting as 22q11.2 Deletion Syndrome. We identified 5 pathogenic CHD7 mutations in our group of 20 patients that were clinically suspected of 22q11.2 deletion syndrome.
Table 3 summarises the mutations and known clinical features of the 5 patients clinically presenting as deletion 22q11.2 syndrome, but in whom a CHD7 mutation was identified. Remarkably, all 5 patients carried a truncating mutation in the CHD7 gene. In addition, we identified 2 silent CHD7 mutations (c.4014C>T, p.Gly1338Gly and c.6216C>G, p.Pro2072Pro) in 2 other patients. Both silent mutations had been identified in the 1,000 Genomes project (DbSNP rs199828744, rs188679907).
Table 3.
Features of 5 patients with clinical 22q11.2 deletion syndrome but without TBX1 haploinsufficiency, in whom CHD7 mutations were found
| Patient ID | CHD7 | CHD7 | Clinical features* |
|---|---|---|---|
| CH95-172 | c.493_496delinsGG | p.Pro165fs | suspected of having 22q11.2 deletion syndrome, no further information available |
| CH95-218 | c.2440C>T | p.Gln814X | ADHD, speech therapy, sensorineural/conductive hearing loss, scoliosis, myopia, retinal coloboma, dysmorphic features |
| CH94-143 | c.3024T>G | p.Tyr1008X | thymic hypoplasia, CLP, hypocalcemia, normal cardiac ultrasound, auricular dysplasia |
| CH96-184 | c.4357C>T | p.Gln1453X | low PTH, low Ca, low T cells, small thymus, dysmorphia, micrognathia, low-set malformed ears, choanal atresia, velopharyngeal incompetence, laryngotracheomalacia, ASD |
| CH99-214F | c.4424del | p.Glu1475fs | suspected of having 22q11.2 deletion syndrome, no further information available |
Typical CHARGE features are indicated in bold.
Discussion
We highlighted the clinical overlap between 22q11.2 deletion and CHARGE syndrome. Although some of the clinical features are present far more often in one of the syndromes (fig. 1), none are seen exclusively in either 22q11.2 deletion or CHARGE syndrome. For example, the presence of choanal atresia or semicircular canal hypoplasia does not exclude a 22q11.2 deletion, and severe T-cell dysfunction also occurs in CHARGE syndrome.
Remarkably, almost exclusively, we found truncating CHD7 mutations in both groups of patients suspected of having TBX1 haploinsufficiency (tables 2, 3). This is in line with our previous observation that CHD7 missense mutations result in a milder phenotype. Features also seen in 22q11.2 syndrome, like congenital heart defects and cleft palate, occur more often in patients with a truncating mutation than in those with a missense mutation [Bergman et al., 2012b]. Thus, the clinical overlap between these syndromes is predominantly seen in the more severely affected patients with a 22q11.2 deletion or CHD7 mutation.
How can we explain the remarkable clinical overlap between these 2 syndromes? It is possible that both syndromes could be present simultaneously, but this must be extremely rare, as no cases have been reported yet. However, it cannot be excluded, since not all mutations in CHD7 and TBX1 are detectable by current techniques. It is well known that no CHD7 mutation can be detected in 5-10% of the patients with a clinical diagnosis of CHARGE, suggesting either undetectable CHD7 mutations (e.g. in the promoter region) or the existence of a second gene that can cause CHARGE syndrome when mutated. Nonetheless, both syndromes should be included in a common differential diagnosis as discussed in our recent reviews [McDonald-McGinn et al., 1993; Bergman et al., 2011b; McDonald-McGinn and Sullivan, 2011].
The most likely explanation for the phenotypic overlap between both syndromes is that the causative genes, CHD7 and TBX1, function in the same embryonic pathway or in pathways with a common target. The CHD7 gene is expressed ubiquitously during human embryonic development with a high expression in the foetal inner ear, eye, central nervous system, and in the neural crest of the pharyngeal arches [Sanlaville et al., 2006]. The CHD7 protein belongs to the chromodomain helicase DNA-binding (CHD) family [Woodage et al., 1997] and is thought to regulate gene transcription by ATP-dependent chromatin modification during embryogenesis [Schnetz et al., 2009]. CHD7 cooperates with, amongst others, PBAF (polybromo- and BRG1-associated factor containing complex) in controlling neural crest gene expression and cell migration [Bajpai et al., 2010].
The TBX1 gene codes for the T-box transcription factor TBX1 that regulates the expression of downstream growth and transcription factors that are involved in the development of the heart, thymus, parathyroid, and palate. TBX1 physically interferes with SMAD1, influencing its binding to SMAD4 and thus signal transduction [Fulcoli et al., 2009]. Interestingly, CHD7 was found to colocalise with SMAD1 (OMIM 601595) and other transcription factors at enhancer elements near genes that are repressed [Schnetz et al., 2010]. Thus, both TBX1 and CHD7 regulate gene transcription and might well regulate the transcription of the same genes.
Mice with heterozygous Chd7 mutations show semicircular canal defects, septal heart defects, cleft palate, choanal atresia, hyposmia, olfactory bulb anomalies, testes hypoplasia, hearing loss, and low body weight [Bosman et al., 2005; Adams et al., 2007; Hurd et al., 2007, 2011; Layman et al., 2009; Bergman et al., 2010]. Other CHARGE features, e.g. coloboma, external ear anomalies and tracheo-oesophageal defects, have not been reported in Chd7-deficient mice. This discrepancy in phenotype between man and mice might be caused by species-specific differences in CHD7 requirements or differences in genetic background [Zentner et al., 2010b].
Mice with haploinsufficiency for Tbx1 show the full range of malformations that can be present in the 22q11.2 deletion syndrome [Merscher et al., 2001]. Tissue-specific conditional mutagenesis of Tbx1 has shown its role in the secondary heart field [Xu et al., 2004], pharyngeal mesoderm [Zhang et al., 2006], pharyngeal endoderm [Arnold et al., 2006], pharyngeal epithelia [Zhang et al., 2005], and otic epithelium [Xu et al., 2007].
Surprisingly, mice with a double heterozygous mutation of Chd7 and Tbx1 show a severe cardiovascular phenotype and severely reduced postnatal viability compared to mice with a heterozygous mutation of Chd7 or Tbx1 [Randall et al., 2009]. The synergistic haploinsufficiency of both genes resulted in an enhanced effect on the fourth pharyngeal arch morphogenesis, abnormal thymus development and malformations of the semicircular canals. These observations in mice together with our observations in the patients presented in this paper suggest that both genes act in the same developmental pathway. Randall et al. [2009] hypothesised that Chd7 might modulate Tbx1 expression but were unable to prove that the expression of either gene changed in mouse embryos mutated at the other locus. Hurd et al. [2011] showed that Tbx1 expression was expanded more ventrally in the developing inner ear of a Chd7 null mouse conditional mutant (Chd7Gt/Gt) compared to the wild-type mouse embryo. This effect was not seen in heterozygously mutated mice (Chd7Gt/+), suggesting that there is a dose-dependent inhibiting effect of Chd7 on Tbx1 in the inner ear which might be essential for inner ear neurogenesis [Hurd et al., 2011].
As an alternative theory, a shared convergent pathway via fibroblast growth factor 8 (FGF8, OMIM 600483) has been suggested, but has not been proven [Randall et al., 2009]. It was shown that reduced Chd7 dosage in the olfactory placode, pituitary and hypothalamus in mice reduced the expression of the FGF8 receptor Fgfr1 (OMIM 136350) [Layman et al., 2011]. FGF8 and its receptor FGFR1 are interesting linking factors, since both are also involved in the pathogenesis of other organs frequently affected in CHARGE syndrome, like the combination of hypogonadotropic hypogonadism and anosmia [Pallais et al., 1993; Bergman et al., 2011a, 2012a]. The fact that this combination is seldom seen in patients with 22q11.2 deletion could be explained by the more ubiquitous expression of CHD7 compared to TBX1.
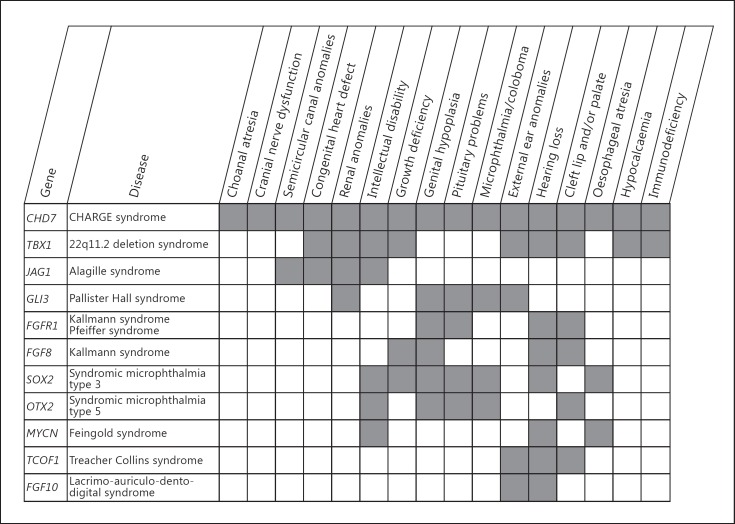
The tight relationship between 22q11.2 deletion and CHARGE syndrome is not an isolated observation. Both syndromes share common features with other syndromes that may reveal further clues for interaction of their causative genes and underlying embryonic pathways. For instance, SOX2 mutations (OMIM 184429) result in a phenotype characterised by anophthalmia, tracheo-oesophageal abnormalities, pituitary defects, and genital abnormalities. Like CHD7, SOX2 is assumed to play a role in neural stem cells, and Engelen et al. [2011] showed that CHD7 is a SOX2 transcriptional cofactor with their common target genes being JAG1, GLI3 and MYCN (Notch and Shh signalling pathways). Shh signalling regulates the expression of TBX1 in the pharyngeal arch probably through transcription factors of the FOX family [Yamagishi et al., 2003]. TBX1 has also been described as an upstream regulator of the Notch-signalling effector HES1 in the pharyngeal arch and a downstream target of JAG2 in tooth morphogenesis [Mitsiadis et al., 2010; van Bueren et al., 2010]. CHD7 and TBX1 have been described to interact with proteins known from other overlapping syndromes (fig. 3). Thus, CHD7 and TBX1 may also interact indirectly through different pathways, like the Notch and Shh signalling pathways.
Fig. 3.
Multiple congenital anomaly syndromes having clinical overlap with CHARGE syndrome and 22q11.2 microdeletion syndrome. In figure 3, we show the overlapping clinical features of CHARGE and 22q11.2 deletion syndrome with known genetic syndromes. All the genes mentioned in this figure or their proteins have been associated with either CHD7 or TBX1. The expression of FGFR1, OTX2 and TBX1 depends on CHD7 in some tissues; TBX1 and FGF8 are in epistasis in ectodermal development; binding of the protein treacle which is encoded by TCOF1 partly depends on the presence of CHD7, and SOX2 and CHD7 are cofactors that regulate the expression of JAG1, MYCN and GLI3, amongst others [Randall et al., 2009; Hurd et al., 2010; Zentner et al., 2010a; Engelen et al., 2011; Layman et al., 2011]. Figure 3 shows that the linked molecular pathways are reflected by the shared clinical features of the syndromes.
In conclusion, the clinical diagnosis of 2 highly variable syndromes, CHARGE and 22q11.2 deletion syndrome, can prove challenging. The syndromes should therefore be included in a common differential diagnosis, and we strongly recommend performing CHD7 analysis in any patients with a 22q11.2 deletion phenotype but without TBX1 haploinsufficiency and performing a genome-wide array for 22q11.2 deletions in clinical CHARGE patients without a CHD7 mutation. We have shown that there is strong clinical evidence that both molecular pathways are linked, although the precise nature of this link needs further exploration.
Acknowledgements
We thank Jackie Senior for editing the manuscript and the Nuts Ohra Fund for financial support (project 0901-80 to N.C.-J.). These studies were also partly supported by grants from the National Institutes of Health, USA (HL084410, MH087636 and HD070454).
References
- Adams ME, Hurd EA, Beyer LA, Swiderski DL, Raphael Y, Martin DM. Defects in vestibular sensory epithelia and innervation in mice with loss of Chd7 function: implications for human CHARGE syndrome. J Comp Neurol. 2007;504:519–532. doi: 10.1002/cne.21460. [DOI] [PubMed] [Google Scholar]
- Arnold JS, Werling U, Braunstein EM, Liao J, Nowotschin S, et al. Inactivation of Tbx1 in the pharyngeal endoderm results in 22q11DS malformations. Development. 2006;133:977–987. doi: 10.1242/dev.02264. [DOI] [PubMed] [Google Scholar]
- Bajpai R, Chen DA, Rada-Iglesias A, Zhang J, Xiong Y, et al. CHD7 cooperates with PBAF to control multipotent neural crest formation. Nature. 2010;463:958–962. doi: 10.1038/nature08733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman JE, de Wijs I, Jongmans MC, Admiraal RJ, Hoefsloot LH, van Ravenswaaij-Arts CM. Exon copy number alterations of the CHD7 gene are not a major cause of CHARGE and CHARGE-like syndrome. Eur J Med Genet. 2008;51:417–425. doi: 10.1016/j.ejmg.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Bergman JE, Bosman EA, van Ravenswaaij-Arts CM, Steel KP. Study of smell and reproductive organs in a mouse model for CHARGE syndrome. Eur J Hum Genet. 2010;18:171–177. doi: 10.1038/ejhg.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman JE, Bocca G, Hoefsloot LH, Meiners LC, van Ravenswaaij-Arts CM. Anosmia predicts hypogonadotropic hypogonadism in CHARGE syndrome. J Pediatr. 2011a;158:474–479. doi: 10.1016/j.jpeds.2010.08.032. [DOI] [PubMed] [Google Scholar]
- Bergman JE, Janssen N, Hoefsloot LH, Jongmans MC, Hofstra RM, van Ravenswaaij-Arts CM. CHD7 mutations and CHARGE syndrome: the clinical implications of an expanding phenotype. J Med Genet. 2011b;48:334–342. doi: 10.1136/jmg.2010.087106. [DOI] [PubMed] [Google Scholar]
- Bergman JE, de Ronde W, Jongmans MC, Wolffenbuttel BH, Drop SL, et al. The results of CHD7 analysis in clinically well-characterized patients with Kallmann syndrome. J Clin Endocrinol Metab. 2012a;97:E858–E862. doi: 10.1210/jc.2011-2652. [DOI] [PubMed] [Google Scholar]
- Bergman JE, Janssen N, van der Sloot AM, de Walle HE, Schoots J, et al. A novel classification system to predict the pathogenic effects of CHD7 missense variants in CHARGE syndrome. Hum Mutat. 2012b;33:1251–1260. doi: 10.1002/humu.22106. [DOI] [PubMed] [Google Scholar]
- Blake KD, Davenport SL, Hall BD, Hefner MA, Pagon RA, et al. CHARGE association: an update and review for the primary pediatrician. Clin Pediatr (Phila) 1998;37:159–173. doi: 10.1177/000992289803700302. [DOI] [PubMed] [Google Scholar]
- Bosman EA, Penn AC, Ambrose JC, Kettleborough R, Stemple DL, Steel KP. Multiple mutations in mouse Chd7 provide models for CHARGE syndrome. Hum Mol Genet. 2005;14:3463–3476. doi: 10.1093/hmg/ddi375. [DOI] [PubMed] [Google Scholar]
- Chopra C, Baretto R, Duddridge M, Browning MJ. T-cell immunodeficiency in CHARGE syndrome. Acta Paediatr. 2009;98:408–412. doi: 10.1111/j.1651-2227.2008.01077.x. [DOI] [PubMed] [Google Scholar]
- de Lonlay-Debeney P, Cormier-Daire V, Amiel J, Abadie V, Odent S, et al. Features of DiGeorge syndrome and CHARGE association in five patients. J Med Genet. 1997;34:986–989. doi: 10.1136/jmg.34.12.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devriendt K, Swillen A, Fryns JP. Deletion in chromosome region 22q11 in a child with CHARGE association. Clin Genet. 1998;53:408–410. doi: 10.1111/j.1399-0004.1998.tb02755.x. [DOI] [PubMed] [Google Scholar]
- Digilio MC, Giannotti A, Marino B, Guadagni AM, Orzalesi M, Dallapiccola B. Radial aplasia and chromosome 22q11 deletion. J Med Genet. 1997;34:942–944. doi: 10.1136/jmg.34.11.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuel BS, Budarf ML, Sellinger B, Goldmuntz E, Driscoll DA. Detection of microdeletions of 22q11.2 with fluorescence in situ hybrydization (FISH): diagnosis of DiGeorge syndrome (DGS), velo-cardio-facial (VCF) syndrome, CHARGE association and conotruncal cardiac malformations. Am J Hum Genet. 1992;51:A3. [Google Scholar]
- Engelen E, Akinci U, Bryne JC, Hou J, Gontan C, et al. Sox2 cooperates with Chd7 to regulate genes that are mutated in human syndromes. Nat Genet. 2011;43:607–611. doi: 10.1038/ng.825. [DOI] [PubMed] [Google Scholar]
- Fulcoli FG, Huynh T, Scambler PJ, Baldini A. Tbx1 regulates the BMP-Smad1 pathway in a transcription independent manner. PLoS One. 2009;4:e6049. doi: 10.1371/journal.pone.0006049. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gennery AR, Slatter MA, Rice J, Hoefsloot LH, Barge D, et al. Mutations in CHD7 in patients with CHARGE syndrome cause T-B + natural killer cell + severe combined immune deficiency and may cause Omenn-like syndrome. Clin Exp Immunol. 2008;153:75–80. doi: 10.1111/j.1365-2249.2008.03681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennery AR. Immunological aspects of 22q11.2 deletion syndrome. Cell Mol Life Sci. 2012;69:17–27. doi: 10.1007/s00018-011-0842-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong W, Gottlieb S, Collins J, Blescia A, Dietz H, et al. Mutation analysis of TBX1 in non-deleted patients with features of DGS/VCFS or isolated cardiovascular defects. J Med Genet. 2001;38:E45. doi: 10.1136/jmg.38.12.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman TE, Siegel MJ. Special imaging casebook. CHARGE association-DiGeorge syndrome with congenital short esophagus and single kidney. J Perinatol. 1998;18:322–324. [PubMed] [Google Scholar]
- Hoover-Fong J, Savage WJ, Lisi E, Winkelstein J, Thomas GH, et al. Congenital T cell deficiency in a patient with CHARGE syndrome. J Pediatr. 2009;154:140–142. doi: 10.1016/j.jpeds.2008.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd EA, Capers PL, Blauwkamp MN, Adams ME, Raphael Y, et al. Loss of Chd7 function in gene-trapped reporter mice is embryonic lethal and associated with severe defects in multiple developing tissues. Mamm Genome. 2007;18:94–104. doi: 10.1007/s00335-006-0107-6. [DOI] [PubMed] [Google Scholar]
- Hurd EA, Poucher HK, Cheng K, Raphael Y, Martin DM. The ATP-dependent chromatin remodeling enzyme CHD7 regulates pro-neural gene expression and neurogenesis in the inner ear. Development. 2010;137:3139–3150. doi: 10.1242/dev.047894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd EA, Adams ME, Layman WS, Swiderski DL, Beyer LA, et al. Mature middle and inner ears express Chd7 and exhibit distinctive pathologies in a mouse model of CHARGE syndrome. Hear Res. 2011;282:184–195. doi: 10.1016/j.heares.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H, Takada H, Kusuda T, Goto T, Ochiai M, et al. Successful cord blood transplantation for a CHARGE syndrome with CHD7 mutation showing DiGeorge sequence including hypoparathyroidism. Eur J Pediatr. 2010;169:839–844. doi: 10.1007/s00431-009-1126-6. [DOI] [PubMed] [Google Scholar]
- Jalali GR, Vorstman JA, Errami A, Vijzelaar R, Biegel J, et al. Detailed analysis of 22q11.2 with a high density MLPA probe set. Hum Mutat. 2008;29:433–440. doi: 10.1002/humu.20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen N, Bergman JE, Swertz MA, Tranebjaerg L, Lodahl M, et al. Mutation update on the CHD7 gene involved in CHARGE syndrome. Hum Mutat. 2012;33:1149–1160. doi: 10.1002/humu.22086. [DOI] [PubMed] [Google Scholar]
- Jongmans MC, Admiraal RJ, van der Donk KP, Vissers LE, Baas AF, et al. CHARGE syndrome: the phenotypic spectrum of mutations in the CHD7 gene. J Med Genet. 2006;43:306–314. doi: 10.1136/jmg.2005.036061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongmans MC, van Ravenswaaij-Arts CM, Pitteloud N, Ogata T, Sato N, et al. CHD7 mutations in patients initially diagnosed with Kallmann syndrome – the clinical overlap with CHARGE syndrome. Clin Genet. 2009;75:65–71. doi: 10.1111/j.1399-0004.2008.01107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyonouchi S, McDonald-McGinn DM, Bale S, Zackai EH, Sullivan KE. CHARGE (coloboma, heart defect, atresia choanae, retarded growth and development, genital hypoplasia, ear anomalies/deafness) syndrome and chromosome 22q11.2 deletion syndrome: a comparison of immunologic and nonimmunologic phenotypic features. Pediatrics. 2009;123:e871–e877. doi: 10.1542/peds.2008-3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaliakatsos M, Giannakopoulos A, Fryssira H, Kanariou M, Skiathitou AV, et al. Combined microdeletions and CHD7 mutation causing severe CHARGE/DiGeorge syndrome: clinical presentation and molecular investigation by array-CGH. J Hum Genet. 2010;55:761–763. doi: 10.1038/jhg.2010.95. [DOI] [PubMed] [Google Scholar]
- Källén K, Robert E, Castilla EE, Mastroiacovo P, Källén B. Relation between oculo-auriculo-vertebral (OAV) dysplasia and three other non-random associations of malformations (VATER, CHARGE, and OEIS) Am J Med Genet A. 2004;127A:26–34. doi: 10.1002/ajmg.a.20643. [DOI] [PubMed] [Google Scholar]
- Kim HG, Kurth I, Lan F, Meliciani I, Wenzel W, et al. Mutations in CHD7, encoding a chromatin-remodeling protein, cause idiopathic hypogonadotropic hypogonadism and Kallmann syndrome. Am J Hum Genet. 2008;83:511–519. doi: 10.1016/j.ajhg.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layman WS, McEwen DP, Beyer LA, Lalani SR, Fernbach SD, et al. Defects in neural stem cell proliferation and olfaction in Chd7 deficient mice indicate a mechanism for hyposmia in human CHARGE syndrome. Hum Mol Genet. 2009;18:1909–1923. doi: 10.1093/hmg/ddp112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layman WS, Hurd EA, Martin DM. Reproductive dysfunction and decreased GnRH neurogenesis in a mouse model of CHARGE syndrome. Hum Mol Genet. 2011;20:3138–3150. doi: 10.1093/hmg/ddr216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald-McGinn DM, Sullivan KE. Chromosome 22q11.2 deletion syndrome (DiGeorge syndrome/velocardiofacial syndrome) Medicine (Baltimore) 2011;90:1–18. doi: 10.1097/MD.0b013e3182060469. [DOI] [PubMed] [Google Scholar]
- McDonald-McGinn DM, Emanuel BS, Zackai EH. 22q11.2 deletion syndrome. In: Pagon RA, Bird TD, Dolan CR, Stephens K, Adam MP, editors. Gene Reviews. University of Washington: Seattle; 1993. http://www.ncbi.nlm.nih.gov/books/NBK1116/?partid=1282. [Google Scholar]
- Merscher S, Funke B, Epstein JA, Heyer J, Puech A, et al. TBX1 is responsible for cardiovascular defects in velo-cardio-facial/DiGeorge syndrome. Cell. 2001;104:619–629. doi: 10.1016/s0092-8674(01)00247-1. [DOI] [PubMed] [Google Scholar]
- Mitsiadis TA, Graf D, Luder H, Gridley T, Bluteau G. BMPs and FGFs target Notch signalling via jagged 2 to regulate tooth morphogenesis and cytodifferentiation. Development. 2010;137:3025–3035. doi: 10.1242/dev.049528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallais JC, Au M, Pitteloud N, Seminara S, Crowley WF. Kallmann syndrome. In: Pagon RA, Bird TD, Dolan CR, Stephens K, Adam MP, editors. Gene Reviews. University of Washington: Seattle; 1993. http://www.ncbi.nlm.nih.gov/books/NBK1116/?partid=1282. [Google Scholar]
- Randall V, McCue K, Roberts C, Kyriakopoulou V, Beddow S, et al. Great vessel development requires biallelic expression of Chd7 and Tbx1 in pharyngeal ectoderm in mice. J Clin Invest. 2009;119:3301–3310. doi: 10.1172/JCI37561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanka M, Tangsinmankong N, Loscalzo M, Sleasman JW, Dorsey MJ. Complete DiGeorge syndrome associated with CHD7 mutation. J Allergy Clin Immunol. 2007;120:952–954. doi: 10.1016/j.jaci.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Sanlaville D, Etchevers HC, Gonzales M, Martinovic J, Clément-Ziza M, et al. Phenotypic spectrum of CHARGE syndrome in fetuses with CHD7 truncating mutations correlates with expression during human development. J Med Genet. 2006;43:211–217. doi: 10.1136/jmg.2005.036160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnetz MP, Bartels CF, Shastri K, Balasubramanian D, Zentner GE, et al. Genomic distribution of CHD7 on chromatin tracks H3K4 methylation patterns. Genome Res. 2009;19:590–601. doi: 10.1101/gr.086983.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnetz MP, Handoko L, Akhtar-Zaidi B, Bartels CF, Pereira CF, et al. CHD7 targets active gene enhancer elements to modulate ES cell-specific gene expression. PLoS Genet. 2010;6:e1001023. doi: 10.1371/journal.pgen.1001023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwinger E, Devriendt K, Rauch A, Philip N. Clinical utility gene card for: DiGeorge syndrome, velocardiofacial syndrome, Shprintzen syndrome, chromosome 22q11.2 deletion syndrome (22q11.2, TBX1) Eur J Hum Genet. 2010;18 doi: 10.1038/ejhg.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon BD. VACTERL/VATER association. Orphanet J Rare Dis. 2011;6:56. doi: 10.1186/1750-1172-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bueren KL, Papangeli I, Rochais F, Pearce K, Roberts C, et al. Hes1 expression is reduced in Tbx1 null cells and is required for the development of structures affected in 22q11 deletion syndrome. Dev Biol. 2010;340:369–380. doi: 10.1016/j.ydbio.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Laar I, Dooijes D, Hoefsloot L, Simon M, Hoogeboom J, Devriendt K. Limb anomalies in patients with CHARGE syndrome: an expansion of the phenotype. Am J Med Genet A. 2007;143A:2712–2715. doi: 10.1002/ajmg.a.32008. [DOI] [PubMed] [Google Scholar]
- Van Meter TD, Weaver DD. Oculo-auriculo-vertebral spectrum and the CHARGE association: clinical evidence for a common pathogenetic mechanism. Clin Dysmorphol. 1996;5:187–196. [PubMed] [Google Scholar]
- Verloes A. Updated diagnostic criteria for CHARGE syndrome: a proposal. Am J Med Genet A. 2005;133A:306–308. doi: 10.1002/ajmg.a.30559. [DOI] [PubMed] [Google Scholar]
- Vissers LE, van Ravenswaaij CM, Admiraal R, Hurst JA, de Vries BB, et al. Mutations in a new member of the chromodomain gene family cause CHARGE syndrome. Nat Genet. 2004;36:955–957. doi: 10.1038/ng1407. [DOI] [PubMed] [Google Scholar]
- Vuorela P, Ala-Mello S, Saloranta C, Penttinen M, Pöyhönen M, et al. Molecular analysis of the CHD7 gene in CHARGE syndrome: identification of 22 novel mutations and evidence for a low contribution of large CHD7 deletions. Genet Med. 2007;9:690–694. doi: 10.1097/gim.0b013e318156e68e. [DOI] [PubMed] [Google Scholar]
- Vuorela PE, Penttinen MT, Hietala MH, Laine JO, Huoponen KA, Kääriäinen HA. A familial CHARGE syndrome with a CHD7 nonsense mutation and new clinical features. Clin Dysmorphol. 2008;17:249–253. doi: 10.1097/MCD.0b013e328306a704. [DOI] [PubMed] [Google Scholar]
- Wincent J, Holmberg E, Strömland K, Soller M, Mirzaei L, et al. CHD7 mutation spectrum in 28 Swedish patients diagnosed with CHARGE syndrome. Clin Genet. 2008;74:31–38. doi: 10.1111/j.1399-0004.2008.01014.x. [DOI] [PubMed] [Google Scholar]
- Woodage T, Basrai MA, Baxevanis AD, Hieter P, Collins FS. Characterization of the CHD family of proteins. Proc Natl Acad Sci USA. 1997;94:11472–11477. doi: 10.1073/pnas.94.21.11472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Writzl K, Cale CM, Pierce CM, Wilson LC, Hennekam RC. Immunological abnormalities in CHARGE syndrome. Eur J Med Genet. 2007;50:338–345. doi: 10.1016/j.ejmg.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Xu H, Morishima M, Wylie JN, Schwartz RJ, Bruneau BG, et al. Tbx1 has a dual role in the morphogenesis of the cardiac outflow tract. Development. 2004;131:3217–3227. doi: 10.1242/dev.01174. [DOI] [PubMed] [Google Scholar]
- Xu H, Viola A, Zhang Z, Gerken CP, Lindsay-Illingworth EA, Baldini A. Tbx1 regulates population, proliferation and cell fate determination of otic epithelial cells. Dev Biol. 2007;302:670–682. doi: 10.1016/j.ydbio.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi H, Maeda J, Hu T, McAnally J, Conway SJ, et al. Tbx1 is regulated by tissue-specific forkhead proteins through a common Sonic hedgehog-responsive enhancer. Genes Dev. 2003;17:269–281. doi: 10.1101/gad.1048903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentner GE, Hurd EA, Schnetz MP, Handoko L, Wang C, et al. CHD7 functions in the nucleolus as a positive regulator of ribosomal RNA biogenesis. Hum Mol Genet. 2010a;19:3491–3501. doi: 10.1093/hmg/ddq265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentner GE, Layman WS, Martin DM, Scacheri PC. Molecular and phenotypic aspects of CHD7 mutation in CHARGE syndrome. Am J Med Genet A. 2010b;152A:674–686. doi: 10.1002/ajmg.a.33323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Cerrato F, Xu H, Vitelli F, Morishima M, et al. Tbx1 expression in pharyngeal epithelia is necessary for pharyngeal arch artery development. Development. 2005;132:5307–5315. doi: 10.1242/dev.02086. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Huynh T, Baldini A. Mesodermal expression of Tbx1 is necessary and sufficient for pharyngeal arch and cardiac outflow tract development. Development. 2006;133:3587–3595. doi: 10.1242/dev.02539. [DOI] [PMC free article] [PubMed] [Google Scholar]