Summary
Background
Actin-based cellular motility requires spatially and temporally coordinated remodeling of a network of branched actin filaments. This study investigates how cofilin and Arp2/3 complex, two main players in the dendritic nucle-ation model, interact to produce sharp spatial transitions between densely branched filaments and long, unbranched filaments.
Results
We found that cofilin binding reduces both the affinity of actin filaments for Arp2/3 complex and the stability of branches. We used fluorescence spectroscopy to measure the kinetics of cofilin association with filaments and the resulting dissociation of Arp2/3 complex and TIRF microscopy to visualize filament severing and the loss of actin filament branches. Cofilin severs filaments optimally when few actin subunits are occupied but dissociates branches rapidly only at higher occupancies. Effective debranching is nevertheless achieved, as a result of cooperative binding and reduced affinity of Arp2/3 complex for the filament, at cofilin concentrations below those required for direct competition.
Conclusions
Cofilin rapidly dissociates Arp2/3 complex and branches by direct competition for binding sites on the actin filament and by propagation of structural changes in the actin filament that reduce affinity for Arp2/3 complex.
Introduction
Cellular motility requires precise spatial and temporal control over the assembly and disassembly of actin filaments on a time scale of seconds. Actin can self-assemble, but the polymers are quite stable compared with actin filaments in cells. Multiple lines of evidence have implicated ADF/cofilin proteins in promoting the assembly and turnover of actin filaments in yeast actin patches [1] and actin filament comet tails of Listeria [2, 3]. Paradoxically, cofilins nucleate filaments at high concentrations [4–7] and accelerate release of the γ-phosphate from ADP-Pi-actin filaments at intermediate concentrations [8], but sever ADP-actin filaments at low concentrations [6, 9–11]. Cofilin also promotes dissociation of actin filament branches mediated by Arp2/3 complex [12]. This activity was attributed to the ability of cofilin to dissociate phosphate from ADP-Pi-actin filaments.
Here we show that simple binding of cofilins to actin filaments rapidly dissociates actin filament branches. This debranching reaction is so fast that it can account for the turnover of actin filament branches on a time scale of a few seconds at the leading edge of motile cells. We provide a mechanistic explanation for how cofilin uses differential occupancy of filaments to exert its various activities. These insights strengthen the case for cofilin exerting differential effects on the actin system in accordance with its local concentration in the cell [6].
Results
Binding of S. pombe Cofilin to ADP and ADP-Pi Actin Filaments
To characterize the kinetics of fission yeast cofilin binding to actin filaments under the conditions that we used to study the effects of cofilin on Arp2/3 complex, we measured the time course of quenching of the fluorescence of pyrenyl-actin [3, 9] at five concentrations of ADP-actin (0.5–5 µM) and three concentrations of ADP-Pi-actin as a function of cofilin concentration (1–5 µM). Fluorescence decreased rapidly in milliseconds to seconds as cofilin bound to filaments composed of ADP or ADP-Pi subunits (Figure 1A–1D). We interpret this fluorescence change as the binding of cofilin to polymerized actin. Under these conditions, binding was much faster than when cofilin concentrations were limiting [6, 8].
Figure 1. Binding of Cofilin to Pyrenyl ADP and ADP-Pi Filaments.
Time courses of normalized fluorescence change upon mixing cofilin with pyrenyl-actin filaments. Actin filaments were mixed with cofilin at time zero to give either (A) 1 µM actin or (B) 5 µM actin with the following concentrations of cofilin: circles, 1 µM; triangles, 2 µM; squares, 3 µM; long dash, 4 µM; short dash, 5 µM cofilin.
(A and B) Conditions for ADP-actin filaments: 1 or 5 µM pyrenyl-ATP-actin was polymerized at 22°C for 1 hr in 10 mM imidazole (pH 7.0), 50 mM KCl, 2 mM Tris-HCl, 1 mM MgCl2, 1 mM EGTA, 0.2 mM ATP, 0.5 mM DTT, 1 mM NaN3.
(C and D) Conditions for ADP-Pi actin filaments: 1 µM (C) or 5 µM (D) pyrenyl-ATP-actin was polymerized at 22°C for 1 hras in (A) and (B) but with 15.4 mM H2KPO4, 9.6 mM HK2PO4, and 20 mM KCl rather than 50 mM KCl.
At given concentrations of actin and cofilin, the fluorescence change was about ten times faster with ADP-actin filaments than ADP-Pi-actin filaments in the presence of 25 mM phosphate. The time courses were identical in 20% and 100% pyrenyl-actin, all following double exponentials (Figure 1) with a rapid, large-amplitude component and a slow, small-amplitude component. The small component was more prominent at substoichiometric ratios of cofilin to actin and decreased to less than 6% of the total change at high cofilin concentrations. The observed rate constants of the small amplitude component were, however, independent of the concentrations of actin and cofilin and always two or more magnitudes smaller than the rate constant of the fast component.
The observed rate constants of the large-amplitude, fast exponential components increased linearly with cofilin concentrations. These experiments were conducted with similar concentrations of cofilin and actin, so both reactants were depleted during the reactions. Because the reactions followed exponential time courses, the rate-enhancing effects of cooperativity [13–16] must have offset the slowing of the reactions resulting from depletion of both reactants.
Cofilin Dissociates Arp2/3 Complex as It Binds to Actin Filaments
We used S. pombe Arp2/3 complex labeled with pyrene on Cys167 of the ARPC2 subunit (pyrenyl-Arp2/3 complex) to measure association of the complex with actin filaments, because its fluorescence is 6-fold higher when bound to actin filaments [17]. This assay showed that Arp2/3 complex binds to (k+ = 1.5 × 10−4 µM−1 s−1) and dissociates from (k- = 1.0 × 10−3 s−1) actin filaments very slowly [17].
Addition of cofilin to ADP-actin filaments decorated with pyrenyl-Arp2/3 complex resulted in the rapid decrease in fluorescence (Figure 2A). The most likely explanation for the decline in fluorescence is dissociation of pyrenyl-Arp2/3 complex from actin filaments, but the discussion considers an alternative mechanism. The rate of this fluorescence change was orders of magnitude faster than the spontaneous dissociation of Arp2/3 complex from ADP-actin filaments (control plot in Figure 2A and [17]). A given cofilin concentration caused the fluorescence of pyrenyl-Arp2/3 complex and the fluorescence of pyrenyl-ADP-actin filaments to decline with similar time courses (Figure 2A). Plots of the observed rate constants for the fast phases of the two reactions versus cofilin concentration had similar slopes of 2.8 µM−1s−1for pyr-enyl ADP-actin filaments and 2.4 µM−1s−1 for pyrenyl-Arp2/3 complex bound to ADP-actin filaments (Figure 2C).
Figure 2. Dissociation of Pyrenyl-Arp2/3 Complex from Actin Filaments by Cofilin.
Time courses of normalized fluorescence changes upon mixing reactants
(A) Comparison of fluorescence changes of actin bound pyrenyl-Arp2/3 complex ± cofilin and pyrenyl-actin filaments + cofilin: gray circles, 5 µM cofilin mixed with 5 µM pyrenyl actin filaments; black circles, 600 nM pyrenyl-Arp2/3 complex bound to 5 µM unlabeled actin filaments mixed with 5 mM cofilin; black line, 600 nM pyrenyl-Arp2/3 complex bound to 5 µM unlabeled actin filaments mixed with buffer. Conditions: Pyrene or unlabeled actin monomers were incubated in KMEI-F buffer to generate ADP-actin filaments. Pyrenyl-Arp2/3 complex in 10 mM imidazole (pH 7.0), 50 mM KCl, 1 mM MgCl2, 1 mM EGTA, 0.2 mM ATP (1 × KMEI) was incubated overnight with black ADP-actin filaments at 4°C.
(B) ADP-Pi filaments were generated as in Figures 1C and 1D. Pyrenyl-Arp2/3 complex in 10 mM imidazole pH 7.0, 1 mM MgCl2, 1mM EGTA, 15.4 mM H2KPO4, 9.6 mM HK2PO4, 20 mM KCl, and 0.2 mM ATP (1 × MEI-phosphate buffer) was incubated overnight with unlabeled ADP-Pi filaments at 4°C.
(C) The dependence of observed rate constant for binding of cofilin (filled symbols) to and dissociation of pyrenyl-Arp2/3 complex (open symbols) from 5 µM ADP-actin (circles) and 5 µM ADP-Pi filaments (triangles) on cofilin concentrations.
Cofilin brought about a change in the fluorescence of pyrenyl-ADP-Pi-actin filaments faster than the change in the fluorescence of pyrenyl-Arp2/3 complex fluorescence bound to ADP-Pi-actin filaments (Figure 2B). The observed rate constant for the fast phase of the pyrenyl-Arp2/3 complex fluorescence change showed a linear dependence of 0.13 µM−1s−1 on cofilin concentrations, whereas the observed rate constants showed a dependence of 0.32 µM−1 s−1 on cofilin concentration at the same concentrations of pyrenyl-ADP-actin filaments (Figure 2C).
Effect of Cofilin on Equilibrium Binding of Arp2/3 Complex to Actin Filaments
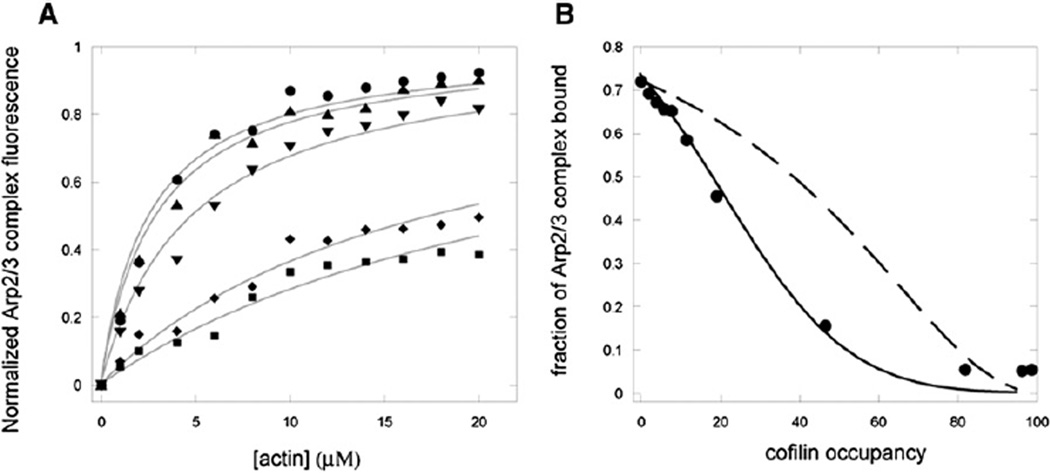
To learn more about the basis for the rapid change in the fluorescence of pyrenyl-Arp2/3 complex upon addition of cofilin, we measured the effect of cofilin on equilibrium binding of 600 nM pyrenyl-Arp2/3 complex to ADP-actin filaments. In control experiments without cofilin, the fluorescence of pyrenyl-Arp2/3 complex increased with the concentration of actin filaments and saturated at about 20µM polymerized actin (Figure 3A). We interpret this as pyrenyl-Arp2/3 complex binding to the filaments. The hyperbolic dependence of fractional saturation of the fluorescence change on polymerized actin concentration gave a Kd of 2 µM, or a KDR (affinity for binding sites composed of three subunits) of 0.55 µM without cofilin.
Figure 3. Cofilin Reduces the Affinity of Arp2/3 Complex for ADP-Actin Filaments.
(A) Dependence of equilibrium fluorescence of 600 nM pyrenyl-Arp2/3 complex on the concentration of ADP-actin filaments and on saturation of the filaments with cofilin: circles, no cofilin; triangles, 10% saturation; inverted triangles, 20% saturation; rhombuses, 30% saturation; squares, 45% saturation. Conditions: 600 nM Arp2/3 complex, 10 mM imidazole (pH 7), 2 mM Tris-HCl, 1 mM MgCl2, 1 mM EGTA, 0.2 mM ATP, 0.5 mM DTT, 1 mM NaN3, 50 mM KCl incubated over night at 4°C with a range of concentrations of ADP-actin filaments and cofilin. Fluorescence was measured at room temperature.
(B) Dependence of the normalized equilibrium fluorescence of pyrenyl-Arp2/3 complex with 5 µM ADP-actin filaments on the fractional saturation of the filaments with cofilin. Filled circles: Experimental observations with 600 nM pyrenyl Arp2/3 complex, 5 µM ADP-actin filaments, and a range of cofilin concentrations incubated in KEMI-F buffer for 24 hr at 4°C. The fraction of actin-bound Arp2/3 complex was calculated from the increase in fluorescence relative to the initial value. The dashed line is a theoretical calculation of fraction bound Arp2/3 complex assuming direct competition between Arp2/3 complex and cofilin for actin filaments. Here cofilin competes solely by limiting the number of 3-subunit binding sites for Arp2/3 complex by a factor of (1 – c)1.94 as described in Equation 5 and Figure S1. Arp2/3 complex has a constant affinity (KDR = 0.67 µM) for filaments whereas cofilin associates with increasing affinity (Kd = 8.2 µM to 0.11 µM), as calculated from the fractional occupancy and free cofilin concentration in Equation 7. The solid line is another theoretical calculation of fraction bound Arp2/3 complex assuming both competition for binding sites (Equation 5) and a cofilin-induced structural propagation in the filament that increases the affinity of cofilin (Kd = 8.2 µM to 0.11 µM) and decreases the affinity of Arp2/3 complex (KDR = 0.67 µM to 23 µM, calculated from Equation 6) for actin filaments as filaments change from zero to full cofilin occupancy.
We then measured binding of pyrenyl-Arp2/3 complex to actin filaments with four levels of cofilin occupancy (10%, 20%, 30%, and 45%) over the range of actin concentrations. Arp2/3 complex binds to three consecutive actin subunits, so we adjusted the actin concentration to reflect the number of 3-monomer sites available for binding as cofilin occupies 10%–45% of the filament (Equation 3). Filaments 10% saturated with cofilin bound Arp2/3 complex similarly to bare actin filaments (Figure 3A) with a KDR of 0.66 µM. When cofilin occupied more than 20% of the subunits, the fluorescence change of pyrenyl-Arp2/3 complex was less at each actin concentration than with bare actin filaments (Figure 3A) and the fluorescence change did not saturate at 20 µM actin 20%–45% occupied by cofilin. Assuming that these curves saturate at the same fluorescence as the control, fits of the data to hyperbolas gave KDRs of 0.87 µM with 20% cofilin saturation, 1.5 µM with 30% cofilin saturation, and 2.5 µM with 45% cofilin saturation (Figure 3A).
We also tested equilibrium binding of600 nMpyrenyl-Arp2/3 complex to 5 µM ADP-actin filaments occupied with a range of cofilin densities (Figure 3B). Without cofilin, the fluorescence indicated that 70% of Arp2/3 complex bound to bare actin filaments. The fluorescence declined hyperbolically with cofilin occupancy to a level corresponding to only 5% of Arp2/3 complex bound to filaments more than 80% saturated with cofilin. Judging from the fluorescence of pyrenyl-Arp2/3 complex, about 5% of Arp2/3 complex remained bound to actin filaments in cofilin concentrations as high as 60 mM, where cofilin nearly fully occupied the filaments.
Cofilin Induces Rapid Loss of Actin Filament Branches
We used TIRF microscopy to visualize the effect of cofilin on branched filaments in real time (Figure 4A and 4B; Movie S1–Movie S4 available online). We grew branched actin filaments from 2 µM 40% Oregon green-labeled ATP-actin with 40 nM Arp2/3 complex and 120 nM of its activator Wsp1p-VCA. Filaments elongated at a steady rate of 10 subunits/s, and growing branches began to appear after about 45 s. When a sufficient number of branched filaments were present, we washed out actin, Arp2/3 complex, and Wsp1p-VCA with solutions containing a range of concentrations of cofilin and observed the consequences.
Figure 4. Real-Time Observations of Cofilin Severing Actin Filaments and Dissociating Branches by Fluorescence Microscopy.
(A and B) Time series of total internal reflection fluorescence micrographs showing the formation of actin filament branches and dissociation of branches by cofilin. Conditions: 10 mM imidazole (pH 7.0), 50 mM KCl, 1 mM MgCl2, 1 mM EGTA, 100 mM DTT, 0.2 mM ATP, 50 mM CaCl2, 15 mM glucose, 20 mg/ml catalase, 100 mg/ml glucose oxidase, and 0.5% methylcellulose (15 cP at 2%).
(A) Time course of actin filament branching and elongation by 2 mM Mg-ATP-actin monomers (40% Oregon green), 20 nM Arp2/3 complex, and 200 nM VCA imaged every 10 s.
(B) Time course after addition of 1 µM cofilin imaged every second.
(C) Time course of the loss of branches after adding a range of concentrations of cofilin.
(D) Dependence of the severing and debranching rates on the cofilin occupancy of the actin filaments during the first 30 s after adding cofilin. Rates are the total number of observed severing and debranching events divided by the time of observation (30 s).
All concentrations of cofilin tested (150 pM-10 mM) severed actin filaments, with the rate of severing and the time course of severing events depending on the cofilin concentration [6, 18]. We observed a single severing event with 150 pM cofilin (Movie S1). Above 1 nM cofilin the rate of severing increased to a maximum in the range of 10–50 nM cofilin (Movie S2; Figure 4D). At these low cofilin concentrations, severing persisted throughout observations at a steady rate of 0.6–1.3 × 10−3 severing events/mm actin/s until the filament became very short. As previously observed when images were acquired every 10 s [6], cofilin concentrations greater than 100 nM did not sever filaments persistently over time. However, we observed a new feature by acquiring images every second. During the first few seconds after flowing high concentrations of cofilin through the observation chamber, we observed severing by cofilin concentrations as high as 5 µM (Movie S4). At 100 nM cofilin, 33% of all severing events occurred within 4 s of cofilin addition, whereas at 5 µM 50% of severing happened within 2 s (Movie S4). With 50 nM cofilin, an average filament was severed 6–7 times within 30 s after introduction of cofilin (Movie S2), producing short filaments of only a few hundred subunits. With 75 nM cofilin, a typical filament severed 4–5 times and cofilin greater than 100 nM severed only 2–3 times (Movie S3), leaving them as longer filaments of variable lengths. Our results agree with previous studies [6,18] where severing is stronger at low rather than high densities of cofilin on the filaments.
Actin filament branches disappeared rapidly in the presence of cofilin. We identified branch points by their “y” shape and scored their loss when a protruding arm disappeared from one frame to the next. The rate and extent of branch point destruction increased with cofilin concentration in a hyperbolic fashion (Figure 4D), quite distinct from the peak of severing activity at low cofilin concentrations. The fraction of branch points surviving for 30 s decreased with cofilin concentration (Figure 4C). In 200 nM cofilin, the fraction of surviving branches declined from more than 40% of branches at 10 s to only 10% in 30 s. Thus, high nanomolar concentrations are sufficient for debranching. At micromolar concentrations, branches disappear on a second time scale, with 80% of branches lost within 5 s in 5 µM cofilin, similar to the rapid loss of pyrenyl-Arp2/3 complex fluorescence in these concentrations of cofilin (Figure 2A). Cofilin concentrations above 5µM did not increase the rate of branch dissociation or reduce the fraction of surviving branch points below 10%.
Discussion
Our key finding is that cofilin binding to actin filaments strongly promotes dissociation of Arp2/3 complex and actin-filament branches mediated by Arp2/3 complex. We propose that cofilin induces dissociation by decreasing the affinity of Arp2/3 complex for actin filaments by at least 5-fold as cofilin binds and stabilizes the 162° short-pitch helix conformation of the filament. The γ-phosphate of ATP is an intrinsic inhibitor of cofilin binding to actin filaments, so its dissociation is a key event facilitating the process. Here we consider how the occupancy of cofilin binding sites on actin filaments influences the properties of the filament, binding of Arp2/3 complex, and the stability of branches.
Mechanism of Cooperative Binding of Cofilin to Actin Filaments
Cofilins bind both actin monomers and filaments, with higher affinities for ADP-actin than ATP- or ADP-Pi-actin [8, 19]. It decreases the distance between fluorescent probes attached to Gln 41 (in subdomain 2) and Cys374 (in subdomain 1) in monomeric actin [20]. In an ATP-actin-twinfilin cocrystal, the C-terminal ADF-H domain interacts with the hydrophobic groove between subdomains 1 and 3 of actin through an α-helix containing many residues conserved among ADF/cofilins [21]. Cofilin bound to this site in an actin filament would displace the DNase I binding loop of the adjacent subunit along the long-pitch actin helix, favoring a more tightly twisted 162° conformation observed by electron microscopy [13, 22, 23] and weaken contacts along the long-pitch helix [24–26]. Radiolytic protein foot printing [27] and competitive crosslinking studies [28] support this conclusion. Binding to this site also allosterically closes the nucleotide binding cleft between subdomains 2 and 4 of actin [24].
Cofilin binding to actin filaments strongly favors a conformation with a 162° short-pitch helix [13] and vice versa. The twist of actin filaments is intrinsically variable [29, 30], but the shortpitch helical twist of bare actin filaments is about 167° except for rare segments with a 162° conformation [31]. The paucity of these 162° segments was proposed to explain the very slow rate of cofilin binding to actin filaments [8]. Cofilin binding to rare subunits in the 162° state shifts the equilibrium of an unknown number of neighboring subunits toward the 162° state [31] and increases the microsecond torsional flexibility and intersubunit angular disorder of actin filaments [32, 33]. Local propagation of the 162° state around sites of cofilin binding and the higher affinity of cofilin for subunits in the 162° state explain cooperative binding, as observed in equilibrium binding experiments [5,13,15], micrographs of filaments having bare and cofilin-decorated segments [13] and in time course of cofilin binding (Figure 1) [16]. We estimate that cofilin binding to the 162° state is favored by 2–3 orders of magnitude over the 167° state, consistent with other estimates of the ability of cofilin to shift filaments from a small fraction of subunits in the 162° state to a majority of subunits in the 162° state [31].
Cofilin binds slower to filaments of ADP-Pi-actin than ADP-actin. The presence of phosphate in the nucleotide cleft may directly slow cofilin binding or propagation of the structural change. Cofilin accelerates release of the γ-phosphate from ADP-Pi actin filaments [8], but the actual rate constant is not known. Thus, phosphate dissociation may limit the rate of cofilin binding to ADP-Pi filaments and propagation of the conformational change along the filament. Alternatively, the equilibrium between 162° state and 167° state may be less favorable for cofilin binding to ADP-Pi filaments than ADP actin filaments.
Mechanism of Arp2/3 Complex Dissociation by Cofilin
Mixing cofilin with actin filaments decorated with pyrenyl-Arp2/3 complex causes the fluorescence to decline orders of magnitude faster than when Arp2/3 complex spontaneously dissociates. Our interpretation is that dissociation of pyrenyl-Arp2/3 complex from the actin filament causes the fluorescence change, because the magnitude of the change is comparable to the fluorescence change when Arp2/3 complex binds to filaments. Alternatively, cofilin might induce conformational changes in the mother filament that reduce the quantum yield of bound pyrenyl-Arp2/3 complex, but the pyrene on C167 of ARPC2 is deeply buried in the interface between Arp2/3 complex and the mother filament in 3D reconstructions of branch junctions from electron tomography [34]. It is unlikely that a conformational change in the actin filament could decrease the quantum yield 6-fold to the level of unbound Arp2/3 complex without influencing the affinity of the filament for Arp2/3 complex, so we performed a titration experiment to investigate how cofilin displaces Arp2/3 complex.
We considered two potentially complementary mechanisms for cofilin displacing Arp2/3 complex from actin filaments, because our equilibrium analysis shows that cofilin not only occludes binding sites, but also decreases the affinity Arp2/3 complex has for unoccupied sites. The first mechanism assumes that cofilin competes directly with Arp2/3 complex for polymerized actin subunits. The second is that cofilin binding propagates a structural change along the actin filament that reduces the affinity of the filament for Arp2/3 complex. The following analysis concludes that both mechanisms contribute to the effect of cofilin on equilibrium binding (Figure 3) of Arp2/3 complex to actin filaments. However, Arp2/3 complex naturally dissociates so slowly from actin filaments (k- = 1.0 × 10−3 s−1 [17]) that direct competition cannot be involved when cofilin rapidly dissociates Arp2/3 complex from filaments in our kinetics experiments (Figure 2).
Model for Direct Competition
The methods section derives the Equation 3–Equation 6 describing direct competition between cofilin and Arp2/3 complex for binding actin filaments. In these equations, c is the fractional occupancy of actin subunits by cofilin. The model takes into account the fact that Arp2/3 complex binds to three successive actin subunits along the long pitch strand of a filament [34]. Therefore, direct competition with cofilin bound to a filament reduces the number of binding sites of Arp2/3 complex on the filament in proportion to (1 – c)3, whereas stochastic simulations show that the binding capacity decreases by approximately (1 – c)1.94 (Figure S1). The model also takes into account cooperative binding of cofilin to actin filaments.
Solution of these equations shows that at equilibrium direct competition with cooperative binding of cofilin to filaments reduces binding sites for Arp2/3 complex with a convex dependence on cofilin occupancy (Figure 3B, dashed line), quite different from the observed concave dependence of pyrenyl-Arp2/3 complex fluorescence on cofilin occupancy. Dissociation of Arp2/3 complex by direct competition for binding sites requires higher cofilin concentrations (Figure 3B, dashed line) than observed (Figure 3B, open symbols) even with cooperative binding of cofilin to actin filaments, because cofilin and Arp2/3 complex have similar affinities for ADP-actin filaments (0.11–8 µM and 2 µM).
Model for a Propagated Conformational Change
Since subsaturating densities of cofilin dissociate the majority of bound Arp2/3 complex from ADP-actin filaments in equilibrium experiments (Figure 3B), and since cofilin displaces Arp2/3 complexmuchfaster than its natural rate of dissociation in transient kinetic experiments, we considered a second mechanism whereby cofilin binding propagates a conformational change allosterically along filaments to reducethe affinity for Arp2/3 complex. We assumed that cofilin binding to a filament reduces the effective binding capacity by (1 – c)1.94 and also exponentially attenuates the affinity for Arp2/3 complex for unoccupied actin subunits. We assumed that the KDR increases from 0.55µM for bare actin filaments to 2.5µm for filaments 45% occupied with cofilin, as observed (Figure 3A), and to 20 mM when fully occupied as predicted by the exponential change in affinity in the equilibrium experiment (Equation 6).
Solution of these equations gives a theoretical curve that agrees with the observed reduction in pyrenyl-Arp2/3 complex fluorescence as a function of cofilin occupancy in equilibrium experiments (Figure 3B, solid line). This mechanism also explains how cofilin can dissociate Arp2/3 complex from filaments much faster than Arp2/3 complex equilibrates on and off of filaments. Insufficient time is available for cofilin to compete for binding sites, but a conformational change can propagate rapidly to Arp2/3 complex from adjacent cofilin binding sites.
The model of the branch junction based on EM tomography [34] offers a physical mechanism for cofilin dissociating Arp2/3 complex. Three subunits of the mother actin filament are locally untwisted to form a complex interface with Arp2/3 complex. Cofilin binding to adjacent sites may twist the mother filament in the opposite direction to decrease the affinity of the filament for Arp2/3 complex by 5-fold at half saturation and ~30-fold when fully saturated, above and beyond any direct competition. The dependence of the affinity of Arp2/3 complex for actin filaments on cofilin occupancy balances the free energy change needed for cooperative binding of cofilin to actin. Dissociation of counter ions provides the free energy for cofilin to bind cooperatively to ADP-actin filaments [16]. A decrease in affinity of Arp2/3 complex for sites consisting of three actin subunits from 0.55 µM to 20 µM gives rise to a ΔG of roughly −12 kJ/mol, balancing the positive ΔG needed for cooperative cofilin binding.
Cofilin binding to ADP-actin filaments and dissociation of pyrenyl-Arp2/3 complex follow similar time courses and dependence on the concentration of cofilin (Figure 2B), so the effect of cofilin binding is transmitted rapidly to Arp2/3 complex. In contrast, cofilin dissociates Arp2/3 complex 20-fold slower from ADP-Pi-actin filaments than from ADP filaments, whereas it binds ADP-Pi-actin filaments only 4-fold slower than ADP filaments. The lag between cofilin binding and dissociation could be due to a rate-limiting intermediate step, possibly Pi release, which follows structural propagation but precedes conformational change in subunit that diminishes Arp2/3 complex affinity. Cofilin binding might also affect the rate of ATP hydrolysis and Pi release from Arp2, which is a prerequisite in dissociation of the complex [35].
Mechanism of Debranching
We hypothesize that debranching results from cofilin-induced dissociation of Arp2/3 complex from the mother filament. Low occupancy of a filament by cofilin promotes some dissociation of Arp2/3 complex and branches, so concentrations of cofilin far below the Kd dissociate branches (Figure 4). Even low nanomolar concentrations of cofilin dissociate some branches at 15% the maximal rate, destroying 5% of branch points per second. The rate of debranching increased hyperbolically with cofilin concentration into the micromolar range, where cofilin binds ADP-actin filaments and dissociates Arp2/3 complex and branches on a subsecond time scale.
When cofilin occupies filaments at the same density, Arp2/3 complex and branches dissociate at similar rates. For example, at 40% cofilin occupancy, pyrenyl-Arp2/3 complex fluorescence decreased with an observed rate constant of 7 s−1 and the majority of branch points were destroyed in 3 s, which, at an average of 25 branch points per sample, corresponds to a dissociation rate constant of 7.5 s−1 This rate of branch dissociation with cofilin is about 35-fold faster than the natural rate of dissociation branches originating from ADP-actin filaments (0.2 s−1) in the absence of cofilin [36].
In addition to these effects on the mother filament, actions of cofilin on daughter filaments may also influence branch stability [12]. Weak binding of cofilin to ADP-Pi subunits in daughter filaments promotes dissociation of Pi and conversion to ADP-actin. This may promote dissociation of daughter filaments from Arp2/3 complex at branch junctions, as indicated by the fact that the affinity of Arp2/3 complex for the pointed ends of ADP-actin daughter filaments is 25-fold lower than for ATP-actin filaments [12].
Mechanism of Severing
In agreement with previous work [6, 18, 25], we observed that concentrations of about 10 nM S. pombe cofilin sever filaments at the highest rate. Prolonged severing is optimal at low concentrations of cofilin where low occupancy of the filaments creates boundaries between different twist states where severing is favored. We also observed a transient phase of severing at cofilin concentrations into the micromolar range, corresponding to the time when low densities of cofilin occupy the filaments. With time, the gaps between cofilins are filled in and the filament is stable in the 162° twist state.
Kinetic and Biochemical Pathways to Sequential Effects of Cofilin at the Leading Edge of Cells
The effects of cofilin binding on the rates of phosphate dissociation from ADP-Pi-actin [8], branch dissociation, and severing help to explain the spatial and temporal transition from branched to unbranched filaments about 1 µm behind the leading edge of motile cells [37]. Newly formed ATP-actin filament branches inevitably age by the following reactions. (1) Polymerized actin hydrolyzes bound ATP at 0.3 s−1 [8]. (2) Cofilin binds cooperatively but weakly to ADP-Pi actin filaments (0.45 µM−1 s−1) and (3) slowly dissociates Arp2/3 complex (0.13 µM−1 s−1) as the 162° state propagates locally. (3) Cofilin increases the rate of spontaneous dissociation of γ-phosphate from 0.002 s−1 [38] to at least 0.035 s−1 [8] allowing (4) cofilin to bind faster (1.8 µM−1 s−1), with 10- to 15-fold higher affinity [8] to ADP-actin (~0.5 µM) on a subsecond time scale. Cofilin’s induction and propagation of the 162° twist state allows small numbers of bound cofilins to promote dissociation of Arp2/3 complex and branches.
In addition to these kinetic effects largely intrinsic to actin and cofilin, other actin-binding proteins such as Aip1 [39] surely contribute to the spatial regulation of the actin filament network to produce a narrow zone of branches that mature into long unbranched filaments. The formation of long unbranched filaments is the most mysterious aspect of the process. Depending on concentrations of broken ends, capping protein, profilin and tropomyosin, rapid annealing of severed fragments of filaments [40] may contribute. Annealed filaments may resist severing because of association with tropomyosin [41–43] and possibly because local cofilin concentrations are too high for effective severing.
A biochemical gradient of active cofilin that is highest near the leading edge may augment these kinetic effects. The combined activity of inhibitory phosphoinositides in the plasma membrane [44] and localized activation by dephos-phorylation of cofilin [45–47] may create such a gradient. PIP2 can sequester cofilin from actin. LIM-kinase spread throughout the cytoplasm [48] phosphorylates a serine near the N terminus [49, 50] and blocks cofilin from binding either actin monomers or filaments [12]. Slingshot phosphatase concentrated near the leading edge [51, 52] may dephosphor-ylate and activate cofilin [47]. Farther from the leading edge, phosphorylation may reduce the concentration of active cofilin, enabling effective severing and recycling of filaments into monomers.
Experimental Procedures
Protein Purification and Labeling
Actin was purified from acetone powder of chicken skeletal muscle, gel filtered through an S-300 column in G-buffer (0.2 mM ATP, 0.5 mM DTT, 0.1 mM CaCl2,1 mM NaN3,2 mM Tris-HCl [pH 8.0]) [53] and labeled with pyrene [54] or Oregon green iodoacetemide [55]. RecombinantS. pombe cofilin was expressed from a pMW172 plasmid in E. coli BL21(DE3)pLysS cells [8, 56] and purified by size-exclusion and ion-exchange chromatography. GST-WASp-VCA was expressed from a pGEX6T plasmid in E. coli BL21(DE3)pLysS cells and purified by affinity chromatography followed by Source Q ion-exchange chromatography [17]. Arp2/3 complex was purified from S. pombe double mutant strain ARPC2 A317C- ARPC4 C167S and labeled with pyrene [17].
Chemicals
KCl, MgCl2, EGTA, imidazole, CaCl2, ATP, DTT, NaN3, Tris, methylcellulose, catalase, and glucose oxidase were purchased from Sigma. Pyrene iodoacetamide and Oregon green iodoacetamide were purchased from Invitrogen.
Fluorescence Assays
Experiments were performed with an Alphascan fluorimeter (Photon Technology International) equipped with a MiniMixer stopped-flow mixer (KinTek), with excitation and emission wavelengths of 365 nm and 390 nm, at 100 counts per second. Pyrenyl-actin monomers were polymerized in 10 mM imidazole (pH 7.0), 2 mM Tris-HCl, 1 mM MgCl2,1 mM EGTA, 0.2 mM ATP, 0.5 mM DTT, 1 mM NaN3, and 50 mM KCl (KMEI-F buffer) or 19.9 mM KCl with 9.63 mM H2KPO4 and 15.37 mM HK2PO4 (MEI-F buffer) for 1 hr at 22°C to generate ADP and ADP-Pi filaments. Unlabelled actin filaments for measuring dissociation of Arp2/3 complex were polymerized in the presence of 600 nM pyrenyl-Arp2/3 complex in similar conditions. In both assays, time courses of fluorescence emission upon rapid mixing with cofilin were fit to double exponentials to give the observed rate constants.
Equilibrium Binding of Pyrenyl Arp2/3 Complex to Actin Filaments
ADP actin filaments generated as above were mixed with cofilin to achieve desired occupancy and incubated overnight at 4°C. Pyrenyl-Arp2/3 complex (600 nM) was included in overnight incubations for equilibrium assays or added just prior to measurements in kinetic assays. The concentration of cofilin added was calculated from the desired fractional occupancy (v) multiplied by the concentration of actin plus the concentration of free cofilin [L] at that occupancy. The free ligand concentration [L] was estimated from mathematical rearrangement of the Scatchard format equation for the McGhee-Von Hippel unlimited nearest neighbor cooperativity model, where v is the desired occupancy, Ka = 0.115 mM −1, and cooperativity factor ω = 8.5 [6, 15]. Alternatively, fractional occupancy (v) was calculated with the same equations if the total cofilin concentration was known.
| Equation 1 |
| Equation 2 |
The affinity of Arp2/3 complex for binding sites consisting of three actin subunits (KDR) at each of the cofilin occupancy (c) tested was fitted to the following equation, which reflects the decrease in the effective concentration of 3-subunit actin sites as cofilin occupies a filament as suggested by stochastic simulations (Figure S5). The coefficient of 3 arises from the theoretical concentration of 3-subunit binding sites as [actin]/3 at zero cofilin occupancy, whereas the factor of 0.83 accounts for the probabilistic reduction in effective binding capacity resulting from imperfect alignment of Arp2/3 complexes with solitary or consecutive unoccupied subunits in between.
| Equation 3 |
Analysis of Competitive Binding between Cofilin and Arp2/3 Complex
We estimated the concentration of actin-bound Arp2/3 complex (F) over a range of cofilin concentrations by comparing the end fluorescence values (Fend), arising from association of pyrenyl-Arp2/3 complex to actin filaments, with that of a sample with 20 µM actin and no cofilin (Fsat), because equilibrium binding suggests it to be a saturating condition where fluorescence increases 5-fold. Fo is the fluorescence value of Arp2/3 complex in the absence of actin.
| Equation 4 |
We modeled the concentration of Arp2/3 complex binding to ADP actin filaments with an equation that describes competitive binding
| Equation 5 |
where m = [actin], c = cofilin occupancy, and KDR being KdS of Arp2/3 complex for 3-monomer binding sites on actin filaments. Cofilin occupancy was estimated from Equation 1 and Equation 2. For the two theoretical curves, we either kept KDR constant at 0.55 µM, as predicted from equilibrium assays at zero cofilin occupancy, or varied KDR exponentially with cofilin occupancy as suggested by observed KDRs in equilibrium assays (Figure 3A).
| Equation 6 |
The affinity of cofilin for actin filaments changes with occupancy, so we estimated differential change in Kd with
| Equation 7 |
and experimental values for [L] (cofilin concentration) and q obtained in previous work [6]. The Kd of cofilin (K2) ranged from 8.22 µM for bare filaments to 0.11 µM for fully occupied filaments in the equilibrium and competitive binding assays.
Evanescent Wave Microscopy
Experiments were performed in flow cells made with Parafilm strips as spacers between cleaned slides and 24 × 50 coverslips [55, 57]. The proteins were in 1 × TIRF buffer: 50 mM KCl, 1 mM MgCl2, 1 mM EGTA, 10 mM imidazole (pH 7.0), 0.2 mM ATP, 100 mM DTT, 15 mM glucose 0.5% methyl cellulose, 20 µg/mL catalase, and 100 µg/mL glucose oxidase. Samples were excited by total internal reflection illumination at 488 nm and images were captured with an Orca ER C4742-95 CCD camera (Hamamatsu Corp., Bridgewater, NJ) controlled by Metamorph software (Molecular Devices, Union City, CA) on an Olympus IX70 microscope.
Debranching Assay
Flow cells were incubated with 2 volumes of 10 µl of 200 nM skeletal NEM-treated muscle myosin for 2 min, followed by a high salt TRIS-buffered saline wash (50 mM TRIS [pH 7.5], 600 mM NaCl) and 5 min incubation with Superblock (Pierce, Rockford, IL). Chambers were equilibrated with one volume of 1 × TIRF buffer prior to introduction of one volume of 200 nM VCA, 20 nM Arp2/3 complex, and 2 µM Mg-ATP-actin monomers (40% labeled with Oregon green on cys374) prepared by incubation of Ca-ATPactin for 5 min in Buffer G with 1/10 volume of 1 mM MgCl2, 10 mM EGTA. During polymerization of actin, images were acquired every 10 s until sufficient branches formed. Then we begin collecting images at one frame per second as we used capillary suction with a paper strip to replace the solution in the chamber with cofilin in 1 × TIRF buffer.
We numbered all visible branch points in the frame before the cofilin started to fill the chamber and designated the next frame as time zero for the debranching and severing reactions. We compared successive images to determine the times of severing and debranching, defined as loss a protruding arm from the “y” shaped branch points. We measured rates of severing by summing events along each filament over up to 60 frames and dividing by the observation time and the total filament length to give an averaged severing rate per µm of filament.
Stochastic Simulations
Simulations were performed in Matlab, where a matrix of n × 1 (n = 5,000, 10,000, 15,000, and 20,000) empty sites were successively filled randomly, one at a time with cofilin. The number of three consecutive empty sites, as well as the actual number of Arp2/3 complex that will fit in the matrix (binding sites), were surveyed after each successive fill. The number of site configurations and the number of binding sites were plotted against fractional occupancy (c), yielding a dependence of (1 – c)3 and (1 – c)1.94, respectively.
Supplementary Material
Acknowledgments
This work was supported by NIH research grant GM-026338. The authors thank Aditya Paul for stimulating discussions, Chad McCormick for guidance on image analysis, and Ernesto Andrianantoandro for help with the initial experiments on dissociation of pyrenyl-Arp2/3 complex from actin filaments with cofilin. We are particularly indebted to the anonymous reviewers whose constructive suggestions helped us analyze our data in a more rigorous fashion.
Footnotes
Supplemental Data
Supplemental Data include one figure and four movies and can be found with this article online at http://www.current-biology.com/supplemental/S0960-9822(09)00826-4.
References
- 1.Lappalainen P, Fedorov EV, Fedorov AA, Almo SC, Drubin DG. Essential functions and actin-binding surfaces of yeast cofilin revealed by systematic mutagenesis. EMBO J. 1997;16:5520–5530. doi: 10.1093/emboj/16.18.5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenblatt J, Agnew BJ, Abe H, Bamburg JR, Mitchison TJ. Xenopus actin depolymerizing factor/cofilin (XAC) is responsible for the turnover of actin filaments in Listeria monocytogenes tails. J. Cell Biol. 1997;136:1323–1332. doi: 10.1083/jcb.136.6.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carlier MF, Laurent V, Santolini J, Melki R, Didry D, Xia GX, Hong Y, Chua NH, Pantaloni D. Actin depolymerizing factor (ADF/cofilin) enhances the rate of filament turnover: Implication in actin based motility. J. Cell Biol. 1997;136:1307–1322. doi: 10.1083/jcb.136.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen H, Bernstein BW, Sneider JM, Boyle JA, Minamide LS, Bamburg JR. In vitro activity differences between proteins of the ADF/cofilin family define two distinct subgroups. Biochemistry. 2004;43:7127–7142. doi: 10.1021/bi049797n. [DOI] [PubMed] [Google Scholar]
- 5.Yeoh S, Pope B, Mannherz HG, Weeds A. Determining the differences in actin binding by human ADF and cofilin. J. Mol. Biol. 2002;315:911–925. doi: 10.1006/jmbi.2001.5280. [DOI] [PubMed] [Google Scholar]
- 6.Andrianantoandro E, Pollard TD. Mechanism of actin filament turnover by severing and nucleation at different concentrations of ADF/Cofilin. Mol. Cell. 2006;24:13–23. doi: 10.1016/j.molcel.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Kudryashov DS, Galkin VE, Orlova A, Phan M, Egelman EH, Reisler E. Cofilin cross-bridges adjacent actin protomers and replaces part of the longitudinal F-actin interface. J. Mol. Biol. 2006;358:785–797. doi: 10.1016/j.jmb.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 8.Blanchoin L, Pollard TD. Mechanism of interaction of Acanthamoeba actophorin (ADF/cofilin) with actin filaments. J. Biol. Chem. 1999;274:15538–15546. doi: 10.1074/jbc.274.22.15538. [DOI] [PubMed] [Google Scholar]
- 9.Nishida E, Maekawa S, Sakai H. Cofilin, a protein in porcine brain that binds to actin filaments and inhibits their interactions with myosin and tropomyosin. Biochemistry. 1984;23:5307–5311. doi: 10.1021/bi00317a032. [DOI] [PubMed] [Google Scholar]
- 10.Cooper JA, Blum JD, Williams RC, Jr., Pollard TD. Purification and characterization of actophorin, a new 15,000-dalton actin-binding protein from Acanthamoeba castellanii . J. Biol. Chem. 1986;261:477–485. [PubMed] [Google Scholar]
- 11.Ichetovkin I, Han J, Pang KM, Knecht DA, Condeelis JS. Actin filaments are severed by both native and recombinant Dictyostelium cofilin but to different extents. Cell Motil. Cytoskeleton. 2000;4:293–306. doi: 10.1002/(SICI)1097-0169(200004)45:4<293::AID-CM5>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 12.Blanchoin L, Pollard TD, Mullins RD. Interaction of ADF/ cofilin, Arp2/3 complex, capping protein and profilin in remodeling of branched actin filament networks. Curr. Biol. 2000;10:1273–1282. doi: 10.1016/s0960-9822(00)00749-1. [DOI] [PubMed] [Google Scholar]
- 13.McGough A, Pope B, Chiu W, Weeds A. Cofilin changes the twist of F-actin: Implications for actin filaments dynamics and cellular function. J. Cell Biol. 1997;138:771–781. doi: 10.1083/jcb.138.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ressad F, Didry D, Xia GX, Hong Y, Chua NH, Pantaloni D, Carlier MF. Kinetic analysis of the interaction of actin-depolymerizing factor (ADF)/cofilin with G- and F-actins. Comparison of plant and human ADFs and effect of phosphorylation. J. Biol. Chem. 1998;273:20894–20902. doi: 10.1074/jbc.273.33.20894. [DOI] [PubMed] [Google Scholar]
- 15.De La Cruz EM. Cofilin binding to muscle and non-muscle actin filaments: Isoform-dependent cooperative interactions. J. Mol. Biol. 2005;346:557–564. doi: 10.1016/j.jmb.2004.11.065. [DOI] [PubMed] [Google Scholar]
- 16.Cao W, Goodarzi JP, De La Cruz EM. Energetics and kinetics of cooperative cofilin-actin filament interactions. J. Mol. Biol. 2006;361:257–267. doi: 10.1016/j.jmb.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 17.Beltzner C, Pollard TD. Pathway of actin filament branch formation by Arp2/3 complex. J. Biol. Chem. 2007;283:7135–7144. doi: 10.1074/jbc.M705894200. [DOI] [PubMed] [Google Scholar]
- 18.Pavlov D, Muhlrad A, Cooper J, Wear M, Reisler E. Actin filament severing by cofilin. J. Mol. Biol. 2006;365:1350–1358. doi: 10.1016/j.jmb.2006.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blanchoin L, Pollard TD. Interaction of actin monomers with Acanthamoeba actophorin (ADF/cofilin) and profilin. J. Biol. Chem. 1998;273:25106–25111. doi: 10.1074/jbc.273.39.25106. [DOI] [PubMed] [Google Scholar]
- 20.Dedova IV, Dedov VN, Nosworthy NJ, Hambly BD, dos Remedios CG. Cofilin and DNase I affect the conformation of the small domain of actin. Biophys. J. 2002;82:3134–3143. doi: 10.1016/S0006-3495(02)75655-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paavilainen VO, Oksanen E, Goldman A, Lappalainen P. Structure of the actin-depolymerizing factor homology domain in complex with actin. J. Cell Biol. 2008;182:51–59. doi: 10.1083/jcb.200803100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galkin VE, Orlova A, VanLoock MS, Shvetsov A, Reisler E, Egelman EH. ADF/cofilin use an intrinsic mode of F-actin instability to disrupt actin filaments. J. Cell Biol. 2003;163:1057–1066. doi: 10.1083/jcb.200308144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamal JK, Benchaar SA, Takamoto K, Reisler E, Chance MR. Three-dimensional structure of cofilin bound to monomeric actin derived by structural mass spectrometry data. Proc. Natl. Acad. Sci. USA. 2007;104:7910–7915. doi: 10.1073/pnas.0611283104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bobkov AA, Muhlrad A, Kokabi K, Vorobiev S, Almo SC, Reisler E. Structural effects of cofilin on longitudinal contacts in F-actin. J. Mol. Biol. 2002;323:739–750. doi: 10.1016/s0022-2836(02)01008-2. [DOI] [PubMed] [Google Scholar]
- 25.Bobkov AA, Muhlrad A, Shvetsov A, Benchaar S, Scoville D, Almo SC, Reisler E. Cofilin/ADF affects lateral contacts in F-actin. J. Mol. Biol. 2004;337:93–104. doi: 10.1016/j.jmb.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 26.Bobkov AA, Muhlrad A, Pavlov DA, Kokabi K, Yilmaz A, Reisler E. Cooperative Effects of cofilin (ADF) on actin structure suggest allosteric mechanism of cofilin function. J. Mol. Biol. 2006;356:325–334. doi: 10.1016/j.jmb.2005.11.072. [DOI] [PubMed] [Google Scholar]
- 27.Guan JQ, Vorobiev S, Almo SC, Chance MR. Mapping the G-actin binding surface of cofilin using synchrotron protein footprinting. Biochemistry. 2002;7:5765–5775. doi: 10.1021/bi0121104. [DOI] [PubMed] [Google Scholar]
- 28.Mannherz HG, Ballweber E, Galla M, Villard S, Granier C, Steegborn C, Schmidtmann A, Jaquet K, Pope B, Weeds AG. Mapping the ADF/cofilin binding site on monomeric actin by competitive cross-linking and peptide array: evidence for a second binding site on monomeric actin. J. Mol. Biol. 2007;366:745–755. doi: 10.1016/j.jmb.2006.11.100. [DOI] [PubMed] [Google Scholar]
- 29.Hanson J. Axial period of actin filaments. Nature. 1967;213:353–356. [Google Scholar]
- 30.Egelman EH, Francis N, DeRosier DJ. F-actin is a helix with a random variable twist. Nature. 1982;298:131–135. doi: 10.1038/298131a0. [DOI] [PubMed] [Google Scholar]
- 31.Galkin VE, Orlova A, Lukoyanova N, Wriggers W, Egelman EH. Actin depolymerizing factor stabilizes an existing state of F-actin and can change the tilt of F-actin subunits. J. Cell Biol. 2001;153:75–86. doi: 10.1083/jcb.153.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prochniewicz E, Janson N, Thomas DD, De la Cruz EM. Cofilin increases the torsional flexibility and dynamics of actin filaments. J. Mol. Biol. 2005;353:990–1000. doi: 10.1016/j.jmb.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 33.McCullough BR, Blanchoin L, Martiel J, De La Cruz EM. Cofilin increases the bending flexibility of actin filaments: Implications for severing and cell mechanics. J. Mol. Biol. 2008;381:550–558. doi: 10.1016/j.jmb.2008.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rouiller I, Xu X, Amann KJ, Egile C, Nickell S, Nicastro D, Li R, Pollard TD, Volkmann N, Hanein D. The structural basis of actin filament branching by the Arp2/3 complex. J. Cell Biol. 2008;180:887–895. doi: 10.1083/jcb.200709092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin AC, Welch MD, Drubin DG. Arp2/3 ATP hydrolysis-catalyzed branch dissociation is critical for endocytic force generation. Nat. Cell Biol. 2006;8:826–833. doi: 10.1038/ncb1443. [DOI] [PubMed] [Google Scholar]
- 36.Mahaffy RE, Pollard TD. Kinetics of the formation and dissociation of actin filament branches mediated by Arp2/3 complex. Biophys. J. 2006;91:3519–3528. doi: 10.1529/biophysj.106.080937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Svitkina TM, Verkhovsky AB, McQuade KM, Borisy GG. Keratocytes: Mechanism of cell body translocation. J. Cell Biol. 1997;139:397–415. doi: 10.1083/jcb.139.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Melki R, Fievez S, Carlier MF. Continuous monitoring of Pi release following nucleotide hydrolysis in actin or tubulin assembly using 2-amino-6-mercapto-7-metyhlpurine ribonucleoside and purinenucleoside phosphorylase as an enzyme-linked assay. Biochemistry. 1996;35:12038–12045. doi: 10.1021/bi961325o. [DOI] [PubMed] [Google Scholar]
- 39.Okada K, Obinata T, Abe H. XAIP1: A Xenopus homologue of yeast actin interacting protein 1 (AIP1), which induces disassembly of actin filaments cooperatively with ADF/cofilin family proteins. J. Cell Sci. 1999;112:1553–1565. doi: 10.1242/jcs.112.10.1553. [DOI] [PubMed] [Google Scholar]
- 40.Andrianantoandro E, Blanchoin L, Sept D, McCammon JA, Pollard TD. Kinetic mechanism of end-to-end anneal of actin filaments. J. Mol. Biol. 2001;312:721–730. doi: 10.1006/jmbi.2001.5005. [DOI] [PubMed] [Google Scholar]
- 41.Svitkina TM, Borisy GG. Arp2/3 complex and actin depolymerizing factor/cofilin in dendritic organization and treadmilling of actin filament array in lamellipodia. J. Cell Biol. 1999;145:1009–1026. doi: 10.1083/jcb.145.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DesMarais V, Ichetovkin I, Condeelis J, Hitchcock-DeGregori SE. Spatial regulation of actin dynamics:atropomyosin-free,actin-rich compartment at the leading edge. J. Cell Sci. 2002;115:4649–4660. doi: 10.1242/jcs.00147. [DOI] [PubMed] [Google Scholar]
- 43.Gupton SL, Anderson KL, Kole TP, Fischer RS, Ponti A, Hitchcock-DeGregori SE, Danuser G, Fowler VM, Wirtz D, Hanein D, Waterman-Storer CM. Cell migration without a lamellipodium: Translation of actin dynamics into cell movement mediated by tropomyosin. J. Cell Biol. 2005;168:619–631. doi: 10.1083/jcb.200406063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yonezawa N, Nishida E, Iida K, Yahara I, Sakai H. Inhibition of the interactions of cofilin, destrin, and deoxyribonuclease I with actin by phosphoinositides. J. Biol. Chem. 1990;265:8382–8386. [PubMed] [Google Scholar]
- 45.Agnew BJ, Minamide LS, Bamburg JR. Reactivation of phosphorylated actin depolymerizing factor and identification of the regulatory site. J. Biol. Chem. 1995;27:17582–17587. doi: 10.1074/jbc.270.29.17582. [DOI] [PubMed] [Google Scholar]
- 46.Moriyama K, Iida K, Yahara I. Phosphorylation of Ser-3 of cofilin regulates its essential function on actin. Genes Cells. 1996;1:73–86. doi: 10.1046/j.1365-2443.1996.05005.x. [DOI] [PubMed] [Google Scholar]
- 47.Niwa R, Nagata-Ohashi K, Takeichi M, Mizuno K, Uemura T. Control of actin reorganization by Slingshot,a family of phosphatases that dephosphorylate ADF/cofilin. Cell. 2002;108:233–246. doi: 10.1016/s0092-8674(01)00638-9. [DOI] [PubMed] [Google Scholar]
- 48.Foletta VC, Moussi N, Sarmiere PD, Bamburg JR, Bernard O. LIM kinase 1, a key regulator of actin dynamics, is widely expressed in embryonic and adult tissues. Exp. Cell Res. 2004;294:392–405. doi: 10.1016/j.yexcr.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 49.Arber S, Barbayannis FA, Hanser H, Schneider C, Stanyon CA, Bernard O, Caroni P. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature. 1998;393:805–809. doi: 10.1038/31729. [DOI] [PubMed] [Google Scholar]
- 50.Yang N, Higuchi O, Ohashi K, Nagata K, Wada A, Kangawa K, Nishida E, Mizuno K. Cofilin phosphorylation by LIMkinase 1 and its role in Rac-mediated actin reorganization. Nature. 1998;393:809–812. doi: 10.1038/31735. [DOI] [PubMed] [Google Scholar]
- 51.Nagata-Ohashi K, Ohta Y, Goto K, Chiba S, Mori R, Nishita M, Ohashi K, Kousaka K, Iwamatsu A, Niwa R. A pathway of neuregulin-induced activation of cofilin-phosphatase Slingshot and cofilin in lamellipodia. J. Cell Biol. 2004;165:465–471. doi: 10.1083/jcb.200401136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nishita M, Wang Y, Tomizawa C, Suzuki A, Niwa R, Uemura T, Mizuno K. Phosphoinositide 3-kinase-mediated activation of cofilin phosphatase Slingshot and its role for insulin-induced membrane protrusion. J. Biol. Chem. 2004;279:7193–7198. doi: 10.1074/jbc.M312591200. [DOI] [PubMed] [Google Scholar]
- 53.MacLean-Fletcher S, Pollard TD. Identification of a factor in conventional muscle actin preparations which inhibits actin filament self-association. Biochem. Biophys. Res. Commun. 1980;96:18–27. doi: 10.1016/0006-291x(80)91175-4. [DOI] [PubMed] [Google Scholar]
- 54.Cooper JA, Walker SB, Pollard TD. Pyrene actin: documentation of the validity of a sensitive assay for actin polymerization. J. Muscle Res. Cell Motil. 1983;4:253–262. doi: 10.1007/BF00712034. [DOI] [PubMed] [Google Scholar]
- 55.Kuhn JR, Pollard TD. Real-time measurements of actin filament polymerization by total internal reflection fluorescence microscopy. Biophys. J. 2005;88:1387–1402. doi: 10.1529/biophysj.104.047399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Quirk S, Maciver SK, Ampe C, Doberstein SK, Kaiser DA, VanDamme J, Vandekerckhove JS, Pollard TD. Primary structure of and studies on Acanthamoeba actophorin . Biochemistry. 1993;32:8525–8533. doi: 10.1021/bi00084a019. [DOI] [PubMed] [Google Scholar]
- 57.Paul A, Pollard TD. The role of the FH1 domain and profilin in formin-mediated actin-filament elongation and nucleation. Curr. Biol. 2008;18:9–19. doi: 10.1016/j.cub.2007.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.