Abstract
Objectives
We assessed the impact of tocilizumab (TCZ), a humanised monoclonal anti-interleukin-6 receptor antibody, on antibody response following administration of the 23-valent pneumococcal polysaccharide vaccine (PPV23).
Methods
A total of 190 patients with rheumatoid arthritis (RA) received PPV23. Patients were classified into TCZ (n=50), TCZ + methotrexate (MTX) (n=54), MTX (n=62) and RA control (n=24) groups. We measured serotype-specific IgG concentrations of pneumococcal serotypes 6B and 23F using ELISA and functional antibody activity using a multiplexed opsonophagocytic killing assay, reported as the opsonisation indices (OIs), before and 4–6 weeks after vaccination. Positive antibody response was defined as a 2-fold or more increase in the IgG concentration or as a ≥10-fold or more increase in the OI.
Results
IgG concentrations and OIs were significantly increased in all treatment groups in response to vaccination. The TCZ group antibody response rates were comparable with those of the RA control group for each serotype. MTX had a negative impact on vaccine efficacy. Multivariate logistic analysis confirmed that TCZ is not associated with an inadequate antibody response to either serotype. No severe adverse effect was observed in any treatment group.
Conclusions
TCZ does not impair PPV23 immunogenicity in RA patients, whereas antibody responses may be reduced when TCZ is used as a combination therapy with MTX.
Keywords: Methotrexate, Rheumatoid Arthritis, Vaccination, Infections
Introduction
Streptococcus pneumoniae (pneumococcus) infection is responsible for substantial mortality and morbidity among adults aged ≥65 years or those with underlying chronic or immunosuppressive conditions. The CDC Advisory Committee on Immunization Practice has recommended the use of the 23-valent pneumococcal polysaccharide vaccine (PPV23) for prevention of invasive pneumococcal disease in at-risk populations.1 Patients with rheumatoid arthritis (RA) are at an increased risk of contracting infectious diseases because of immunological changes that are intrinsic to RA and that result from immunosuppressive agents, and thus it is likely that pneumococcal vaccination can benefit this patient population.
Tocilizumab (TCZ), a humanised monoclonal antibody against the interleukin-6 (IL-6) receptor, is effective and generally well tolerated when administered either as monotherapy or in combination with methotrexate (MTX) in patients with moderate to severe RA. IL-6 was originally identified as a factor essential for B cell differentiation into antibody-producing plasma cells,2 and IL-6-deficient mice had reduced antigen-specific IgG following immunisation with a T-cell-dependent antigen.3 PPV23 induces serotype-specific IgG in a T-cell-independent polysaccharide antigen pathway, which can enhance pneumococcal opsonisation, phagocytosis and killing by phagocytic cells.4 PPV23 immunogenicity is often impaired in certain groups of immunocompromised patients,1 but evidence of PPV23 efficacy and safety is lacking in RA patients receiving TCZ.
The objective of the present study was to evaluate the influence of TCZ therapy on antibody response to PPV23 in RA patients. We determined the serum concentrations of serotype-specific IgG using ELISAs and the functional antibody activity using multiplexed opsonophagocytic killing assays (OPAs) in RA patients being treated with TCZ, MTX or TCZ and MTX, and in control RA patients who received neither drug.
Methods
Patients
RA patients who were receiving TCZ therapy (at least the first dose of an intravenous infusion of 8 mg/kg every 4 weeks) and/or MTX (4–18 mg per week) for ≥12 weeks at our rheumatology outpatient clinics were invited to participate in this open-label study. RA patients who had been treated with bucillamine or salazosulfapyridine were also included as RA controls. All participants fulfilled the 1987 American College of Rheumatology criteria for RA diagnosis. Exclusion criteria were current prednisolone use (≥10 mg/day), current use of immunosuppressive antirheumatic drugs other than MTX (such as tacrolimus, cyclosporine, leflunomide, cyclophosphamide and azathioprine), a recent history (within 6 months) of pneumococcal infection and a history of pneumococcal vaccination. Patients who had changed treatments during the follow-up period or those who had received biological agents other than TCZ were also excluded from this study.
Vaccine
We used commercially available PPV23 (Pneumovax NP, Merck Sharp & Dohme Corp., Tokyo, Japan) containing 25 μg each of 23 capsular polysaccharide types. From October 2011 to March 2012, each patient received a single dose of vaccine (0.5 ml) subcutaneously in the upper arm. For RA patients receiving TCZ, the vaccination was performed on the same day as the TCZ infusion.
ELISAs for serotype-specific IgG and multiplexed OPAs
Sera were collected immediately before and 4–6 weeks after vaccination and stored at −30°C until tested. To measure serotype-specific IgG concentrations and functional antibody activity against pneumococcus serotypes 6B and 23F, we performed ELISAs and multiplexed OPAs, respectively. For detailed protocols, see online supplementary text.
Antibody response
Fold increases relative to pre-vaccination values (post-vaccination value to pre-vaccination value ratios) were determined. Positive antibody response was defined as a 2-fold or more increase in IgG concentrations or as a 10-fold or more increase in opsonisation indices (OIs).5
Monitoring adverse effects
Adverse events that occurred during a follow-up period of 4–6 weeks after vaccination were recorded. Systemic adverse effects included fever, headache, myalgia, asthenia and fatigue. Local adverse events included pain/tenderness, swelling/induration and erythema at the injection sites.
Statistical analysis
To access the PPV23 immunogenicity in patients in each treatment group, IgG concentrations and OIs before and after vaccination were transformed into logarithmic values. IgG geometric mean concentrations (GMCs) and geometric mean OIs (GM-OIs) were calculated as the exponential of an arithmetic mean of log-transformed values. For details regarding statistical analysis, see online supplementary text.
Results
Clinical and demographic characteristics
A total of 190 RA patients were divided into four groups according to their ongoing anti-RA therapy. There was one group of 50 patients treated with TCZ as monotherapy (TCZ group), 62 patients treated with MTX alone (MTX group), 54 patients who received a combination therapy consisting of TCZ and MTX (TCZ+MTX group) and 24 patients who did not receive either drug (RA control group). Prior to participating in this study, no patients had received a pneumococcal vaccination. Patients’ clinical and demographic characteristics are shown in table 1.
Table 1.
Clinical and demographic characteristics of RA patients prior to pneumococcal vaccination
| MTX group (n=62) | TCZ+MTX group (n=54) | TCZ group (n=50) | RA control (n=24) | p Values between treatment groups | |
|---|---|---|---|---|---|
| Male/female | 11/51 | 4/50 | 7/43 | 5/19 | NS |
| Age, mean (95% CI) (years) | 68.3 (66.6 to 70.1) | 65.1 (63.1 to 67.0) | 68.3 (65.8 to 70.8) | 69.2 (65.3 to 73.1) | NS |
| RA duration, mean (95% CI) (years) | 10.0 (7.8 to 12.1) | 9.1 (7.3 to 10.8) | 12.5 (9.6 to 15.3) | 11.3 (6.0 to 16.6) | NS |
| MTX dose, median (IQR) (mg/week) | 8 (6 to 8) | 8 (6 to 8) | – | – | NS |
| MTX duration, median (IQR) (months) | 48 (14.3 to 86.3) | 48.5 (26 to 81) | – | – | NS |
| TCZ duration, median (IQR) (weeks) | – | 56 (16 to 95) | 58 (15 to 98) | – | NS |
| Use of prednisolone, number of patients (%) | 17 (27.4) | 14 (25.9) | 12 (24) | 1 (4.2) | 0.018 (M vs C) |
| 0.029 (T/M vs C) | |||||
| 0.049 (T vs C) | |||||
| Prednisolone dose, median (IQR) (mg/day) | 0 (0 to 2) | 0 (0 to 1) | 0 (0 to 1) | 0 (0 to 1) | NS |
| Positive RF, number of patients (%) | 35 (56.5) | 39 (72.2) | 31 (62) | 8 (33.3) | 0.001 (T/M vs C) |
| 0.021 (T vs C) | |||||
| Positive anti-CCP Abs, number of patients (%) | 44 (71.0) | 46 (85.2) | 41 (82) | 11 (45.8) | 0.029 (M vs C) |
| 0.0003 (T/M vs C) | |||||
| 0.001 (T vs C) | |||||
| Lymphocytes, mean (95% CI) (/μl) | 1374 (1230 to 1517) | 1651 (1420 to 1881) | 1717 (1545 to 1890) | 1600 (1358 to 1842) | NS |
| Serum IgG, mean (95% CI) (mg/dl) | 1286 (1194 to 1377) | 1172 (1075 to 1269) | 1196 (1121 to 1271) | 1394 (1258 to 1530) | NS |
Data were obtained immediately before pneumococcal vaccination. p Values between treatment groups were determined using the Mann–Whitney U test, ANOVA (analysis of variance) with a Tukey's HSD (honesty significant difference) post hoc test, the Kruskal–Wallis test with a Scheffe post hoc test, the χ² test or Fisher's exact probability test.
anti-CCP Abs, anti-cyclic citrullinated peptide antibodies; M, MTX group; MTX, methotrexate; NS, not significant; RA, rheumatoid arthritis; RF, rheumatoid factor; T, TCZ group; T/M, TCZ+MTX group; C, RA control; TCZ, tocilizumab.
Serotype-specific IgG concentrations
After vaccination, serotype-specific IgG GMCs to pneumococcal serotypes 6B and 23F in all four groups were increased significantly (p<0.0005; table 2). For serotype 6B, a significantly higher post-GMC was obtained in the TCZ group compared with that in the TCZ+MTX group (p=0.004). The TCZ group also showed a significantly greater fold increase than did the TCZ+MTX group (p=0.036). For serotype 23F, the TCZ group also showed a significantly higher post-GMC than did the MTX group (p=0.027). Increases were twofold or more in all treatment groups, and there were no statistically significant differences.
Table 2.
Concentrations of pneumococcal polysaccharide antigen serotype-specific IgG antibodies and opsonisation indices in the RA treatment groups before and after 23-valent pneumococcal polysaccharide vaccination
| Serotype | MTX group (n=62) | TCZ+MTX group (n=54) | TCZ group (n=50) | RA control group (n=24) | p Values between treatment groups |
|---|---|---|---|---|---|
| IgG GMCs (μg/ml) | |||||
| 6B | |||||
| Before | 1.2 (1.0 to 1.5) | 1.1 (0.9 to 1.3) | 1.3 (1.0 to 1.7) | 1.1 (0.8 to 1.6) | NS |
| After | 2.2 (1.7 to 2.7)* | 1.7 (1.3 to 2.3)* | 6.1 (2.6 to 4.9)* | 2.5 (1.5 to 4.4)* | 0.004 (T/M vs T) |
| Fold increase | 1.5 (1.1 to 3.0) | 1.6 (1.2 to 1.9) | 2.8 (1.4 to 4.4) | 1.8 (1.3 to 3.7) | 0.036 (T/M vs T) |
| 23F | |||||
| Before | 1.0 (0.8 to 1.2) | 0.9 (0.7 to 1.2) | 1.3 (1.0 to 1.7) | 1.0 (0.6 to 1.5) | NS |
| After | 2.4 (1.8 to 3.3)* | 2.5 (1.8 to 3.5)* | 4.6 (3.4 to 6.4)* | 3.6 (1.8 to 5.7)* | 0.027 (M vs T) |
| Fold increase | 2.6 (1.4 to 4.1) | 2.9 (1.0 to 6.9) | 3.4 (1.5 to 6.8) | 3.5 (1.7 to 5.6) | NS |
| GM-OIs | |||||
| 6B | |||||
| Before | 18.8 (18.7 to 32.1) | 24.5 (14.7 to 42.1) | 43.8 (22.4 to 85.6) | 20.70 (7.0 to 61.0) | NS |
| After | 115.6 (64.1 to 206.4)* | 232.8 (124.0 to 437.0)* | 692.3 (265.1 to 1366)* | 262.4 (74.4 to 916.0)* | 0.001 (M vs T) |
| Fold increase | 4.5 (1 to 12.5) | 6.8 (1.7 to 35.5) | 12 (3.5 to 62.4) | 8.5 (2.2 to 52.0) | NS |
| 23F | |||||
| Before | 10.1 (6.6 to 15.3) | 15.5 (10.3 to 23.6) | 27.9 (15.2 to 51.4) | 17.6 (7.5 to 42.1) | 0.018 (M vs T) |
| After | 72.2 (39.3 to 133.0)* | 124.0 (62.2 to 244.7)* | 437.0 (221.4 to 862.6)* | 219.2 (82.3 to 578.2)* | 0.001 (M vs T) |
| 0.042 (M/T vs T) | |||||
| Fold increase | 7 .0 (2.7 to 15.8) | 5.0 (1 to 40) | 18.8 (2.7 to 75.1) | 11.0 (3.1 to 30.6) | NS |
IgG GMCs and GM-OIs are expressed as the mean (95% CI). Fold increases are expressed as the median (IQR). Differences between pre- and post-vaccination GMCs of serotype-specific IgG and those between pre- and post-vaccination GM-OIs were assessed using a paired-sample t test. The four treatment groups were compared using ANOVA (analysis of variance) with a Tukey's HSD (honestly significant difference) post hoc test or the Kruskal–Wallis test with a Scheffe post hoc test.
*p<0.0005 compared with pre-vaccination IgG GMCs or GM-OIs.
GMC, geometric mean concentration; GM-OI, geometric mean opsonisation index; M, MTX group; MTX, methotrexate; NS, not significant; RA, rheumatoid arthritis; T, TCZ group; T/M, TCZ+MTX group; TCZ, tocilizumab.
Opsonophagocytic killing assays
After vaccination, GM-OIs for the 6B and the 23F serotypes were increased significantly in all four groups (p<0.0005; table 2). For serotype 6B, the post-vaccination GM-OI was significantly higher in the TCZ group compared with that in the MTX group (p=0.001). The TCZ group also showed a significantly higher post-vaccination GM-OI for serotype 23F compared with the MTX group (p=0.001) or with the TCZ+MTX group (p=0.042). For either serotype, there were no significant differences in fold increases among the four treatment groups.
There was a moderate correlation between IgG concentrations and OIs for the 6B and the 23F serotypes (serotype 6B: r=0.623, p<0.0005; serotype 23F: r=0.601, p<0.0005).
Antibody response rates (percentages of patients with positive antibody response)
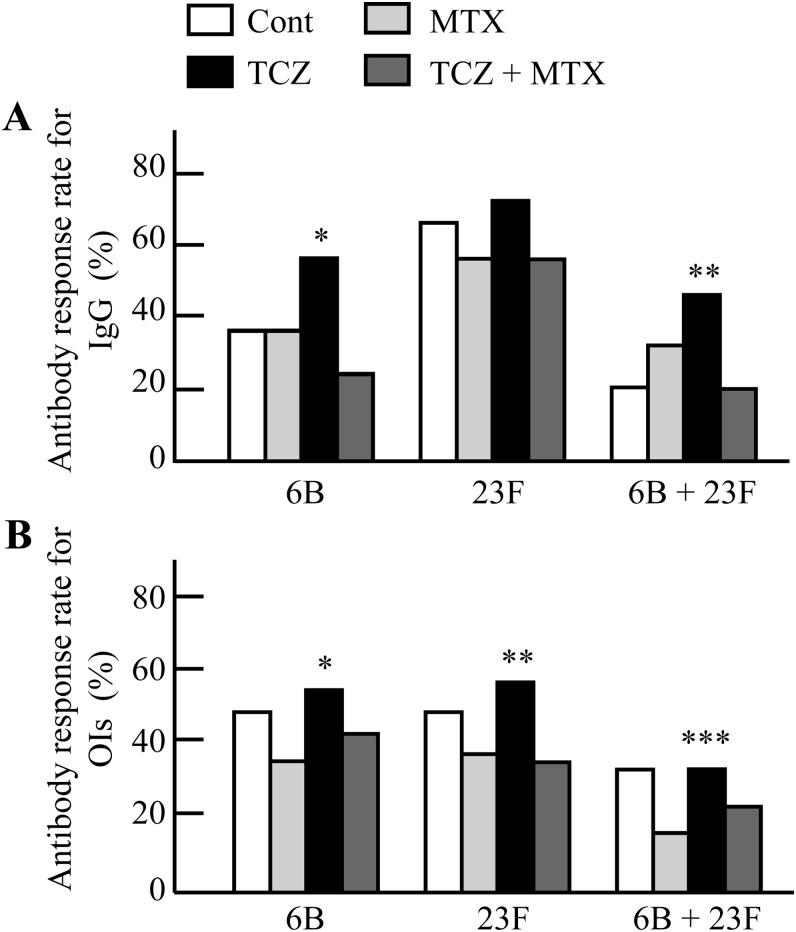
The TCZ group antibody response rates were comparable with those of the RA control group for serotypes 6B and 23F (figure 1).
Figure 1.
(A) Percentages of patients with twofold or more increases in serotype-specific IgG concentrations for serotypes 6B and 23F in the rheumatoid arthritis (RA) treatment groups. *p=0.046 (TCZ vs MTX) and p=0.0009 (TCZ vs TCZ+MTX). **p=0.005 (TCZ vs TCZ+MTX) and p=0.044 (TCZ vs Cont). (B) Percentages of patients with 10-fold or more increases in OIs for serotypes 6B and 23F in the RA treatment groups. *p=0.019 (TCZ vs MTX). **p=0.027 (TCZ vs MTX) and p=0.020 (TCZ vs TCZ+MTX). ***p=0.028 (TCZ vs MTX). Data were compared using the χ2 test or Fisher's exact probability test. OIs, opsonisation indices; Cont, RA control group; MTX, methotrexate group; TCZ, tocilizumab group; TCZ+MTX, combination therapy group.
For the IgG concentration specific to serotype 6B, the antibody response rate was significantly higher in the TCZ group (56%) compared with that in the MTX group (37%) and the TCZ+MTX group (24%, p=0.046 and p=0.0009, respectively; figure 1A). For serotype 23F, there was no significant difference in the antibody response rate among the four treatment groups (Control: 67%; MTX: 57%; TCZ+MTX: 56%; TCZ: 72%). The percentage of patients with positive antibody response for both strains were significantly greater in the TCZ group (46%) compared with the TCZ+MTX group (20%, p=0.005) and the RA control group (21%, p=0.044).
For OIs specific to serotype 6B, the TCZ group showed a significantly higher antibody response rate than did the MTX group (56% vs 34%, p=0.019; figure 1B). For serotype 23F, the antibody response rates were significantly higher in the TCZ group (58%) compared with those in the MTX group (37%, p=0.027) and the TCZ+MTX group (35%, p=0.020). For both strains, a higher proportion of patients in the TCZ group responded to pneumococcal vaccination compared with the patients being treated with MTX alone (34% vs 16%, p=0.028).
Predictive factors for antibody response to PPV23
In a multivariate logistic regression analysis, TCZ use was not identified as the predictive factor for antibody response to pneumococcal vaccination for either IgG concentrations or OIs. The negative association of current MTX use with antibody response was confirmed for IgG concentrations specific to serotypes 6B and 23F (for serotype 6B: OR 0.45, 95% CI 0.25 to 0.82, p=0.009; for serotype 23F: OR 0.56, 95% CI 0.31 to 1.04, p=0.007) and OIs for serotype 23F (OR 0.54, 95% CI 0.29 to 0.99, p=0.046).
Vaccination safety
Two patients in the TCZ+MTX group had a fever. Local adverse events were observed in 12 patients (2 in the MTX group, 7 in the TCZ+MTX group and 3 in the TCZ group). All adverse effects were mild.
Discussion
Following immunisation with PPV23, IgG concentrations and OIs for the 6B and the 23F serotypes were significantly increased in all treatment groups. Antibody response rates in the TCZ group were comparable with those of the RA control group for each serotype. Ongoing use of MTX is likely to have affected the antibody response to PPV23.
Results of the present study indicate that TCZ does not diminish T-cell-independent antibody production after PPV23 immunisation. In addition, we recently reported that RA patients receiving TCZ can produce an adequate antibody response to influenza vaccine, which are T-cell-dependent protein antigens.6 These findings suggest that both T-cell-dependent and T-cell-independent antibody response pathways are conserved in RA patients who are treated with TCZ. There is an increasing awareness of lethal synergism between influenza virus and pneumococcus; influenza virus contributes to secondary pneumococcal pneumonia and can subsequently increase mortality.7 8 In addition, a large-scale trial suggested that a significant proportion of viral pneumonia, including influenza, is attributable to bacterial co-infection and that this co-infection may be preventable by bacterial vaccination.9 Immunisation with both influenza and pneumococcal vaccines may, therefore, provide additive benefits for RA patients compared with a single vaccination, even if they are receiving TCZ therapy.
Previous studies have shown that MTX therapy reduced the antibody response to PPV23,10–13 which is in agreement with the data obtained in the present study. Although T-cell-dependent protein antigens may be more immunogenic than polysaccharide antigens in immunocompromised patients,14 MTX was also reported to be a strong predictive factor for an impaired antibody response to protein-conjugate pneumococcal vaccine.15 Offering PPV23 vaccination before introduction of MTX therapy may be considered in RA patients.11 16 In contrast, a study by Elkayam et al17 did not demonstrate a detrimental effect of immunosuppressive drugs such as MTX on PPV23 immunogenicity in RA patients. Coulson et al18 have also suggested that a single PPV23 administration offers up to 10 years of protection against the development of pneumococcal pneumonia in RA patients receiving MTX therapy. Determining serotype-specific IgG concentrations after PPV23 vaccination in patients receiving MTX therapy is recommended.19
In the present study, no patients were receiving high doses of prednisolone or antirheumatic agents with immunosuppressive effects other than MTX. In addition, there were no differences in the prednisolone dose among the four treatment groups, and the median dose of prednisolone was zero among all groups. The number of prednisolone users was significantly lower in the RA control group; however, there were no significant differences or trends in antibody response to each serotype compared with the other three groups. We can, therefore, say that the influence of such agents on PPV23-induced antibody response was minimal in the present study.
One limitation of this study is the relatively small number of patients in each group and the RA control group in particular. Since most RA patients had already received one or more immunosuppressive antirheumatic drugs, as recommended by the current therapeutic guidelines, it was difficult to recruit a sufficient number of patients who had never received such drugs. Another limitation is that we determined antibody response to only two pneumococcal serotypes. We chose serotypes 6B and 23F because these are the main causative serotypes of pneumococcal pneumonia in Japan and these are representative penicillin-resistant pneumococci.20 However, the immune response to PPV23 may not be consistent among the 23 serotypes. Lastly, unlike influenza vaccines, antibody levels that are protective against invasive pneumococcal disease in adults have not been clearly defined. We used a 2-fold increase in the IgG concentration or a 10-fold increase in the OI as a measure of positive antibody response to PPV23 in this study, which was also used in previous studies;5 however, how this threshold may best correlate with protection against invasive pneumococcal disease remains to be determined.
In conclusion, ongoing TCZ therapy does not preclude pneumococcal polysaccharide vaccination in RA patients; however, antibody responses may be reduced when TCZ is administered in combination with MTX.
Supplementary Material
Acknowledgments
The authors are grateful to Michiyo Hayakawa and Yumi Hattori for technical assistance in measuring serotype-specific IgG concentrations and OIs.
Footnotes
Contributors: All authors contributed to study conception and design, acquisition of data, analysis and interpretation of data, and drafting of the manuscript with regard to important intellectual content.
Funding: The study was supported by research grants from the Ministry of Health, Labour and Welfare of Japan and research funds from the National Hospital Organization (NHO), Japan.
Competing interests: TH has received lecture fees from Mitsubishi-Tanabe Pharmaceutical Co., Eisai Co. Ltd. and Abbott Japan Co. Ltd. The other authors have no financial relationships that could lead to a conflict of interest.
Patient consent: Obtained.
Ethics approval: The ethics committees of participating hospitals approved the protocol for this study.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Advisory Committee on Immunization Practices Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 1997;46:1–24 [PubMed] [Google Scholar]
- 2.Muraguchi A, Hirano T, Tang B, et al. The essential role of B cell stimulatory factor 2 (BSF-2/IL-6) for the terminal differentiation of B cells. J Exp Med 1988;167:332–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kopf M, Baumann H, Freer G,et al. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature 1994;368:339–42 [DOI] [PubMed] [Google Scholar]
- 4.Mond JJ, Vos Q, Lees A,et al. T cell independent antigens. Curr Opin Immunol 1995;7:349–54 [DOI] [PubMed] [Google Scholar]
- 5.Dransfield MT, Nahm MH, Han MK,et al. Superior immune response to protein-conjugate versus free pneumococcal polysaccharide vaccine in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2009;180:499–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mori S, Ueki Y, Hirakata N,et al. Impact of tocilizumab therapy on antibody response to influenza vaccine in patients with rheumatoid arthritis. Ann Rheum Dis 2012;71:2006–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCullers JA, Rehg JE. Lethal synergism between influenza virus and Streptococcus pneumoniae: characterization of a mouse model and the role of platelet-activating factor receptor. J Infect Dis 2002;186:341–50 [DOI] [PubMed] [Google Scholar]
- 8.Peltola VT, Murti KG, McCullers JA. Influenza virus neuraminidase contributes to secondary bacterial pneumonia. J Infect Dis 2005;192:249–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madhi SA, Klugman KP. A role for Streptococcus pneumoniae in virus-associated pneumonia. Nat Med 2004;10:811–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mease PJ, Ritchlin CT, Martin RW,et al. Pneumococcal vaccine response in psoriatic arthritis patients during treatment with etanercept. J Rheumatol 2004;31:1356–61 [PubMed] [Google Scholar]
- 11.Kapetanovic MC, Saxne T, Sjoholm A,et al. Influence of methotrexate, TNF blockers and prednisolone on antibody responses to pneumococcal polysaccharide vaccine in patients with rheumatoid arthritis. Rheumatology (Oxford) 2006;45:106–11 [DOI] [PubMed] [Google Scholar]
- 12.Visvanathan S, Keenan GF, Baker DG,et al. Response to pneumococcal vaccine in patients with early rheumatoid arthritis receiving infliximab plus methotrexate or methotrexate alone. J Rheumatol 2007;34:952–7 [PubMed] [Google Scholar]
- 13.Gelinck LB, van der Bijl AE, Visser LG,et al. Synergistic immunosuppressive effect of anti-TNF combined with methotrexate on antibody responses to the 23 valent pneumococcal polysaccharide vaccine. Vaccine 2008;26:3528–33 [DOI] [PubMed] [Google Scholar]
- 14.Recommendations to assure the quality, safety and efficacy of pneumococcal conjugate vaccines. Replacement of: TRS 927, Annex 2. In: WHO Expert Committee on Biological Standardization. Geneva: World Health Organization, 2009: 1–57 [Google Scholar]
- 15.Kapetanovic MC, Roseman C, Jonsson G, et al. Antibody response is reduced following vaccination with 7-valent conjugate pneumococcal vaccine in adult methotrexate-treated patients with established arthritis, but not those treated with tumor necrosis factor inhibitors. Arthritis Rheum 2011;63:3723–32 [DOI] [PubMed] [Google Scholar]
- 16.Singh JA, Furst DE, Bharat A, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64:625–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elkayam O, Paran D, Caspi D,et al. Immunogenicity and safety of pneumococcal vaccination in patients with rheumatoid arthritis or systemic lupus erythematosus. Clin Infect Dis 2002;34:147–53 [DOI] [PubMed] [Google Scholar]
- 18.Coulson E, Saravanan V, Hamilton J,et al. Pneumococcal antibody levels after pneumovax in patients with rheumatoid arthritis on methotrexate. Ann Rheum Dis 2011;70:1289–91 [DOI] [PubMed] [Google Scholar]
- 19.Heijstek MW, Ott de Bruin LM, Bijl M, et al. EULAR recommendations for vaccination in paediatric patients with rheumatic diseases. Ann Rheum Dis 2011;70:1704–12 [DOI] [PubMed] [Google Scholar]
- 20.Oishi K, Yoshimine H, Watanabe H, et al. Drug-resistant genes and serotypes of pneumococcal strains of community-acquired pneumonia among adults in Japan. Respirology 2006;11:429–36 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.