Abstract
Objective
To assess relationships of acetabular volume (AV), femoral head volume (FV), and portion of the femoral head within in the acetabulum (FVIA) with each other and with degrees of hip joint laxity and degenerative joint disease from youth to maturity in dogs predisposed to developing hip joint osteoarthritis (OA).
Animals
46 mixed-breed half- or full-sibling hound-type dogs.
Procedures
The distraction index (DI), AV, FV, FVIA, and degree of osteoarthritis (OA score) were quantified in 1 hip joint at 16, 32, and 104 weeks of age. Relationships among variables were evaluated within and between ages. Ratios corresponding to OA scores were compared within ages. Differences among 16-week ratios corresponding to 32-week OA scores and among 16- and 32-week ratios corresponding to 104-week OA scores were evaluated.
Results
Significant positive relationships existed between FV and AV across ages as well as between FVIA/FV and FVIA/AV and between DI and OA score across and within most ages. Such relationships also existed within these variables across most ages. Negative relationships of DI and OA scores with FVIA/FV and FVIA/AV within and among all ages were significant. Sixteen-week AVs, FVs, and FVIAs were greater and FV/AVs and OA scores were less than 32- and 104-week values. The 32-week FVIA/FV was less than 16- and 104-week values, and the 32-week FVIA/AV was less than the 104-week value. The FVIA/FV and FVIA/AV were lower and the DI was higher with higher OA scores within and among most ages.
Conclusions and Clinical Relevance
Structural volumes in lax canine hip joints changed predictably relative to each other during growth, despite degenerative changes. Measures developed in this study may augment current diagnosis and treatment strategies for hip dysplasia in dogs.
Hip dysplasia is a multifaceted disease with variable degrees of degenerative hip joint changes.1 The pathophysiology of hip dysplasia in dogs and humans has been the subject of intense investigation.1–4 Several diagnostic techniques including palpation,5 radiography,5–8 CT,6,7,9,10 and MRI9 are used in both species for assessment of hip joints and diagnosis of hip dysplasia. Radiography is the criterion reference standard for hip joint imaging,11 but it is limited by a lack of cartilaginous and bony detail. Standard radiographic measures of coxofemoral joint articulation have variable sensitivity.5,12 Magnetic resonance imaging is a sensitive way to assess articular structures,9 but CT provides greater bone detail9,13 and allows multiplanar assessment and evaluation of articular surface characteristics.13,14 Three-dimensional joint models for evaluation and quantification of joint changes are routinely constructed from 2-D CT images.6,10,13,15–20
Characteristic changes in the femoral head and acetabulum develop in dogs and humans with hip dysplasia.1,21 The acetabulum becomes shallower and decreases in concavity.1,22 Osteophytes often form at the junction of the femoral head and neck, but the femoral head itself becomes progressively smaller.1 The purpose of the study reported here was to evaluate relationships between articular structure volume ratios in hip joints of a homogeneous group of dogs with joint laxity characteristic of hip dysplasia and to assess the relationships of articular structure volume ratios with each other and with the degree of joint laxity and degenerative disease (radiographic OA scores) at intervals from youth to maturity. Given that the acetabulum and femoral head undergo progressive structural changes in dysplastic hips,1 the first hypothesis was that the FV/AV would remain unchanged with increasing age and disease progression because of comparable decreases in FV and AV. Similarly, femoral head loss and acetabular flattening are characteristic of hip dysplasia.1 On the basis of this knowledge, the second hypothesis tested was that FVIA/FV and FVIA/AV would decrease with increasing degree of joint disease and would therefore have a negative relationship with the DI and radiographic OA score. The final 2-part hypothesis tested was that the FVIA/FV and FVIA/AV would differ among dogs with various degrees of degenerative disease as assessed radiographically within ages, and that there would be significant differences between 16-week ratios corresponding to 32-week OA scores and between 16- and 32-week ratios corresponding to 104-week OA scores.
Materials and Methods
Animals
One hip joint in 46 purpose-bred, mixed-breed, hound-type dogs with moderate to severe hip joint laxity was selected for evaluation by means of a randomized block design. Dogs were the progeny of 1 sire and 6 dams. The DI at 16 weeks of age was required to be ≥0.39 for inclusion in the study. Dogs were housed in temperature-regulated, 1.2 × 2.4-m runs, with daily 1-hour free play sessions for the duration of the study. They were fed commercially available puppy fooda up to 18 months of age, after which the dogs received free-choice adult food.b Water was provided ad libitum. The study was performed in accordance with Institutional and National Institutes of Health regulations governing the treatment of vertebrate animals. It was initiated after approval by the Institutional Animal Use and Care Committee of Louisiana State University.
Radiography
When dogs were 16, 32, and 104 weeks of age, radiography was performed with the dogs anesthetized.8 Radiographs were submitted to a commercial establishmentc for quantification of the DI, which is an accepted metric to quantify passive hip joint laxity.23 Results were provided on a standardized form in which degenerative joint disease could be classified as none, mild, moderate, or severe. The degenerative joint disease designation was converted to a numeric score (none = 0, mild = 1, moderate = 2, and severe = 3) for purposes of statistical analysis. It is reported as OA score for this investigation.
3-D CT evaluation
Pelvic CT scans with the hip joint extended were performed while dogs were anesthetized for radiography. Three-dimensional reconstructions of hip joints were computer generatedd from 2-D CT slices (Figure 1). Threshold limits were set at 537 to 3,056 Hounsfield units for all computer reconstructions. Virtual joint models were disarticulated, and the AV and FV were quantified on individual acetabulae and femoral heads, respectively. The FVIA was quantified on articulated 3-D hip images. Measurements were performed on images from each data collection point independently by 2 investigators. The mean of each measurement was used for all statistical analyses.
Figure 1.
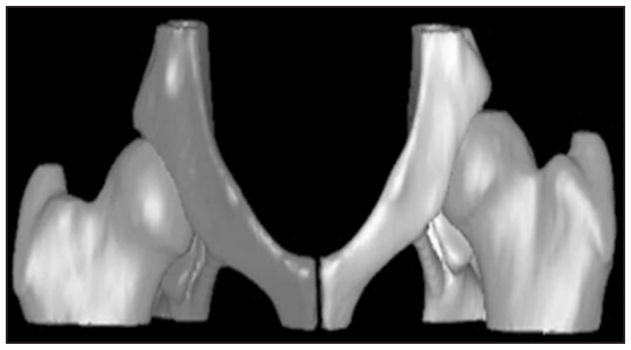
Three-dimensional computer reconstruction of canine hip joints, as generated by use of 2-D CT data.
AV measurements
To measure AV, the acetabular joint space was isolated on 2-D CT images with a series of lines that extended from the dorsal to ventral bony margins of the acetabulum (Figure 2). The acetabulum was separated from the reconstructed pelvic model (Figure 3). A 3-D model was then created of the acetabulum with the joint space filled. The bony acetabulum was subsequently subtracted from the model to give a 3-D impression of the acetabulum, and the volume of the impression was quantified with the software volume tool.d
Figure 2.
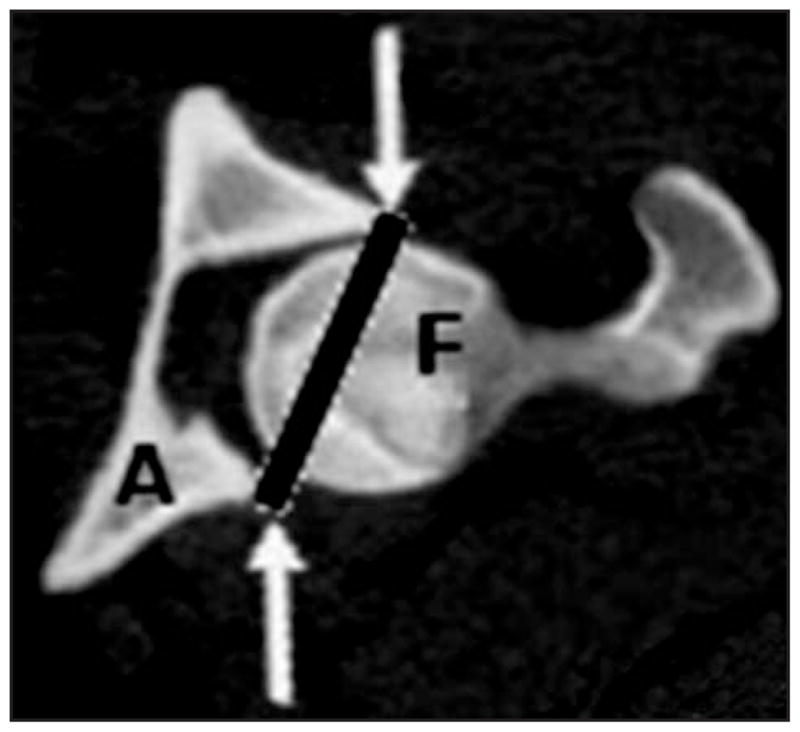
Two-dimensional CT image showing identification of the surface plane of the acetabulum (A) in canine hip joints. Lines from the ventral to the dorsal aspect of each acetabulum (arrows) extending across the femoral head (F) on each slice permitted generation of a plane over the lateral surface of the acetabulum in the 3-D model.
Figure 3.
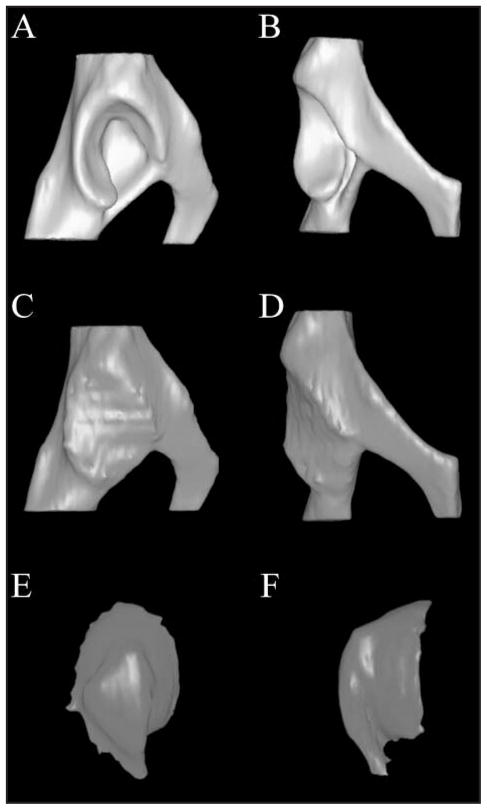
Lateral (A) and ventrodorsal (B) views of a 3-D CT model of a canine acetabulum. The acetabular cavity was filled to the level of the surface plane as shown in lateral (C) and ventrodorsal (D) views. A so-called 3-D cast of the acetabulum was created by subtracting the bony acetabulum from the filled joint space as shown in medial (E) and dorsoventral (F) views. The casts have been rotated approximately 180° from their position in the acetabulum.
FV measurements
For purposes of this study, FV was measured medial to the capital physis. Briefly, the dorsal and ventral limits of the physis or physeal scar were identified on 2-D images, and the capital epiphysis was isolated from the femur at the level of the physis on 3-D images (Figure 4). The FV was subsequently quantified as described for AV measurements.
Figure 4.
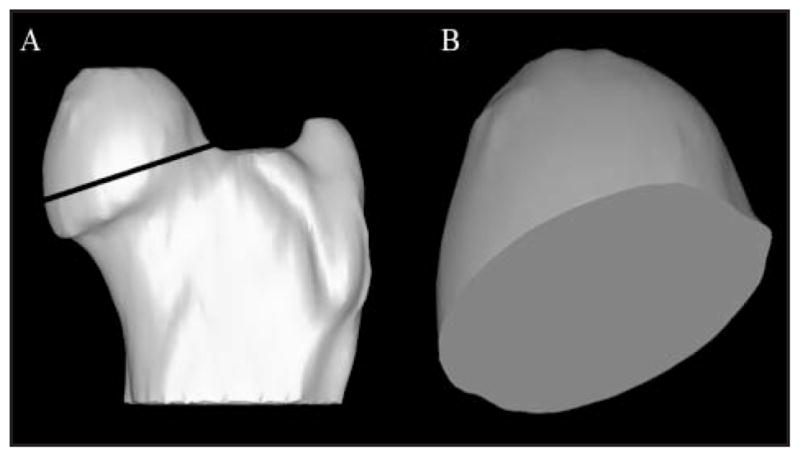
Three-dimensional CT model of the proximal aspect of a canine femur illustrating isolation of the capital epiphysis (A) and craniolateral to caudomedial view (B) of the isolated femoral head.
FVIA volume measurements
The FVIA was quantified on articulated 3-D hip images. The portion of the femoral head within the dorsal and ventral margins of the acetabulum was isolated with a reference line on 2-D images (Figure 5). The reference line was then used to isolate the portion of the femoral head within the acetabular cavity on the 3-D image (Figure 6). The FVIA was quantified as described for AV measurements.
Figure 5.
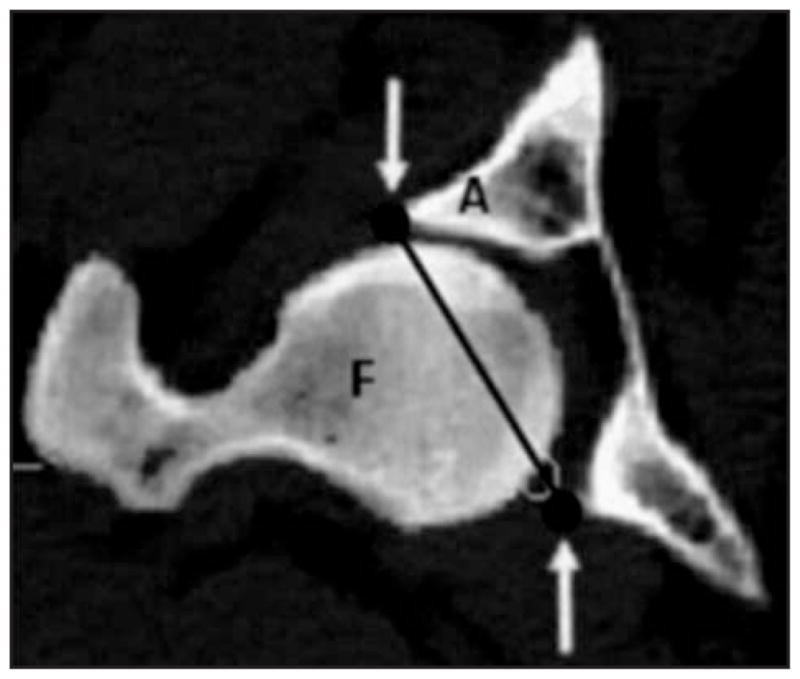
Two-dimensional CT image showing the reference line (arrows) created to isolate the femoral head (F) within the acetabulum (A) of a canine hip joint.
Figure 6.
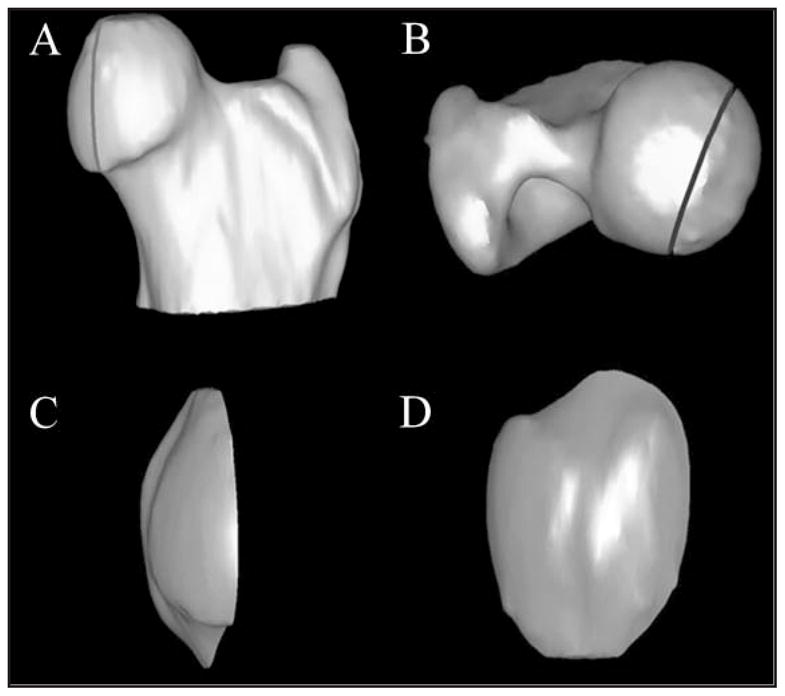
Craniocaudal (A) and dorsoventral (B) views of a 3-D CT model showing measurement of the FVIA in a canine hip joint by use of data collected in Figure 5. The reference line from Figure 5 was used to isolate the portion of the femoral head within the acetabular cavity on the 3-D image. Craniocaudal (C) and mediolateral (D) views of the same model have been rotated approximately 45° and 90°, respectively, from their position relative to the proximal aspect of the femur.
Repeatability and reproducibility
Reproducibility (IeCC) and repeatability (IaCC) were assessed for all measures. The measurement techniques were tested by 3 people performing a complete set of measurements 3 times on 8 randomly selected hip joints. One of the individuals was well versed in the radiographic anatomy and measuring techniques, and 2 of the individuals had no previous experience with the anatomy or the technique.24 They were trained on the day of testing by the experienced individual. Measurements were made in random order, and repetitions were not performed until a complete data set was obtained.
Statistical analysis
Mean ± SD values for FV, AV, FVIA, FV/AV, FVIA/FV, FVIA/AV, DI, and OA score at each data collection point were calculated. The relationship between AV and FV was assessed at specific ages (16, 32, and 104 weeks) and across all ages with the Pearson product moment correlation (r). Relationships of volume ratios with one another and with DI and OA scores within and between age groups were assessed similarly. Repeated-measures ANOVAs were performed to evaluate differences in volumes and volume ratios across ages. Differences between ratio values corresponding to OA scores at 32 and 104 weeks of age, 16-week ratios corresponding to 32-and 104-week OA scores, and 32-week ratios corresponding to 104-week OA scores were also evaluated with ANOVA models. Significant differences were evaluated with the Tukey post hoc test. Repeatability and reproducibility were assessed by calculation of IaCCs and IeCCs, respectively. An IeCC or IaCC > 0.75 was considered excellent,6,24 0.50 to 0.74 was considered good, 0.25 to 0.49 was considered fair, and < 0.24 was considered poor. Analyses were performed with commercially available software programs.e–g Significance was set at a value of P < 0.05.
Results
Animals
The hound-type dogs used in the study included 19 males and 27 females with a mean ± SD body weight of 5.4 ± 1.0 kg at 16 weeks of age, 21.7 ± 3.6 kg at 32 weeks of age, and 25.8 ± 3.7 kg at 104 weeks of age.
Reproducibility and repeatability
All measures had fair to excellent reproducibility (IeCCs: AV, 0.86 ± 0.05; FV, 0.47 ± 0.13; and FVIA, 0.93 ± 0.01) and good to excellent repeatability (IaCCs: AV, 0.93 ± 0.05; FV, 0.64 ± 0.18; and FVIA, 0.97 ± 0.01). The 16-week AV, FV, and FVIA were significantly greater than the corresponding volumes at 32 and 104 weeks of age (Table 1).
Table 1.
Mean ± SD values for AV, FV, FVIA in 1 hip joint from each of 46 mixed-breed hound-type dogs with hip joint osteoarthritis as measured at 16, 32, and 104 weeks of age.
| Variable | 16 weeks | 32 weeks | 104 weeks |
|---|---|---|---|
| AV (mm3) | 1.65 × 104± 0.49 × 104a | 1.07 × 104± 0.16 × 104b | 1.03 × 104± 0.22 × 104b |
| FV (mm3) | 1.39 × 104± 0.39 × 104a | 1.11 × 104± 0.22 × 104b | 1.02 × 104± 0.25 × 104b |
| FVIA (mm3) | 0.48 × 104± 0.23 × 104a | 0.30 × 104± 0.86 × 104b | 0.35 × 104± 0.15 × 104b |
Values with different superscript letters within rows are significantly (P < 0.05) different from each other.
The relationship between AV and FV was significant with all time points combined (r = 0.81) and at 16 (r = 0.91), 32 (r = 0.53), and 104 (r = 0.52) weeks of age. The 16-week FV/AVs were significantly less than those at 32 and 104 weeks (Table 2). The 16- and 104-week FVIA/FVs were significantly greater than the 32-week values. The FVIA/AVs at 32 weeks were significantly less than those at 104 weeks. None of the dogs had radiographic evidence of OA at 16 weeks of age, and the DI did not differ significantly among data collection points. Several relationships between volume ratios were also significant, as were several relationships between volume ratios and the DI and between the DI and OA score (Table 3). Various measures of hip joint volume were significantly correlated with OA score as well (Table 4).
Table 2.
Mean ± SD values for FV/AV, FVIA/FV, FVIA/AV, OA score,* and DI in 1 hip joint from each of 46 mixed-breed hound-type dogs with hip joint OA as measured at 16, 32, and 104 weeks of age.
| Variable | 16 weeks | 32 weeks | 104 weeks |
|---|---|---|---|
| FV/AV | 0.85 ± 0.12a | 1.04 ± 0.18b | 1.01 ± 0.26b |
| FVIA/FV | 0.34 ± 0.13b | 0.26 ± 0.16a | 0.35 ± 0.14b |
| FVIA/AV | 0.29 ± 0.10a,b | 0.28 ± 0.17a | 0.34 ± 0.11b |
| OA score | 0.00 ± 0.00a | 0.65 ± 1.12b | 0.93 ± 1.24b |
| DI | 0.65 ±± 0.15 | 0.74 ± 0.19 | 0.70 ± 0.22 |
Radiographs of hip joints were assigned OA scores for degree of degenerative joint disease as follows: none = 0, mild = 1, moderate = 2, and severe = 3.
See Table 1 for remainder of key.
Table 3.
Pearson product moment correlations (P value) for hip joint volume ratios, OA scores, and DI values in 1 hip joint from each of 46 mixed-breed hound-type dogs with hip joint OA as measured at 16, 32, and 104 weeks of age.
| Variable | 16 weeks
|
32 weeks
|
104 weeks
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FV/AV | FVIA/FV | FVIA/AV | DI | FV/AV | FVIA/FV | FVIA/AV | OA | DI | FV/AV | FVIA/FV | FVIA/AV | OA | |
| 16 wk | |||||||||||||
| FVIA/FV | −0.17 (0.27) | — | — | — | — | — | — | — | — | — | — | — | — |
| FVIA/AV | 0.11 (0.46) | 0.92 (< 0.001) | — | — | — | — | — | — | — | — | — | — | — |
| DI | 0.09 (0.55) | −0.59 (< 0.001) | −0.59 (< 0.001) | — | — | — | — | — | — | — | — | — | — |
| 32 wk | |||||||||||||
| FV/AV | 0.16 (0.30) | −0.08 (0.61) | −0.01 (0.95) | −0.13 (0.40) | — | — | — | — | — | — | — | — | — |
| FVIA/FV | −0.14 (0.34) | 0.63 (< 0.001) | 0.58 (< 0.001) | −0.64 (< 0.001) | 0.14 (0.36) | — | — | — | — | — | — | — | — |
| FVIA/AV | −0.10 (0.53) | 0.58 (< 0.001) | 0.55 (< 0.001) | −0.63 (< 0.001) | 0.34 (0.02) | 0.96 (< 0.001) | — | — | — | — | — | — | — |
| OA | −0.06 (0.71) | −0.51 (< 0.001) | −0.49 (< 0.001) | 0.52 (< 0.001) | −0.17 (0.27) | −0.64 (< 0.001) | −0.65 (< 0.001) | — | — | — | — | — | — |
| DI | 0.05 (0.74) | −0.43 (0.002) | −0.39 (0.007) | 0.68 (< 0.001) | −0.27 (0.07) | −0.71 (< 0.001) | −0.77 (< 0.001) | 0.67 (< 0.001) | — | — | — | — | — |
| 104 wk | |||||||||||||
| FV/AV | 0.23 (0.13) | −0.20 (0.18) | −0.09 (0.55) | 0.06 (0.71) | 0.17 (0.27) | −0.22 (0.14) | −0.13 (0.40) | 0.11 (0.48) | 0.04 (0.80) | — | — | — | — |
| FVIA/FV | −0.31 (0.03) | 0.43 (0.003) | 0.28 (0.06) | −0.37 (0.01) | −0.11 (0.47) | 0.55 (< 0.001) | 0.51 (< 0.001) | −0.33 (0.03) | −0.50 (< 0.001) | −0.58 (< 0.001) | — | — | — |
| FVIA/AV | −0.22 (0.15) | 0.36 (0.01) | 0.24 (0.10) | −0.39 (0.008) | 0.04 (0.79) | 0.58 (< 0.001) | 0.60 (< 0.001) | −0.36 (0.01) | −0.61 (< 0.001) | −0.17 (0.26) | 0.88 (< 0.001) | — | — |
| OA | −0.02 (0.87) | −0.47 (< 0.001) | −0.49 (< 0.001) | 0.60 (< 0.001) | −0.20 (0.19) | −0.67 (< 0.001) | −0.73 (< 0.001) | 0.87 (< 0.001) | 0.74 (< 0.001) | 0.07 (0.65) | −0.39 (0.007) | −0.46 (0.001) | — |
| DI | 0.04 (0.78) | −0.53 (< 0.001) | −0.51 (< 0.001) | 0.73 (< 0.001) | −0.28 (0.06) | −0.78 (< 0.001) | −0.83 (< 0.001) | 0.70 (< 0.001) | 0.94 (< 0.001) | 0.02 (0.90) | −0.48 (< 0.001) | −0.60 (< 0.001) | 0.76 (< 0.001) |
— = Not applicable.
A value of P < 0.05 was considered significant.
Table 4.
Mean ± SD hip joint volume ratios and DI values corresponding to each radiographic OA score in 1 hip joint from each of 46 mixed-breed hound-type dogs with hip joint OA as measured at 16, 32, and 104 weeks of age.
| Variable | 0 | 1 | 2 | 3 |
|---|---|---|---|---|
| Volume ratios at 16 wk by OA score at 16 wk | ||||
| FV/AV | 0.85 ± 0.12 | — | — | — |
| FVIA/FV | 0.34 ± 0.13 | — | — | — |
| FVIA/AV | 0.29 ± 0.09 | — | — | — |
| DI | 0.65 ± 0.15 | — | — | — |
| Volume ratios at 32 wk by OA score at 32 wk | ||||
| FV/AV | 1.06 ± 0.19 | 1.02 ± 0.08 | 0.97 ± 0.10 | 0.98 ± 0.24 |
| FVIA/FV | 0.34 ± 0.14a | 0.17 ± 0.09b | 0.06 ± 0.07b | 0.07 ± 0.09b |
| FVIA/AV | 0.36 ± 0.14a | 0.18 ± 0.10b | 0.05 ± 0.06b | 0.08 ± 0.11b |
| DI | 0.67 ± 0.16a | 0.78 ± 0.09a,b | 0.92 ± 0.06a,b | 1 ± 0b |
| Volume ratios at 104 wk by OA score at 104 wk | ||||
| FV/AV | 0.99 ± 0.20 | 10.88 ± 0.11 | 0.99 ± 0.03 | 1.13 ± 0.43 |
| FVIA/FV | 0.40 ± 0.14 | 0.34 ± 0.15 | 0.27 ± 0.02 | 0.28 ± 0.13 |
| FVIA/AV | 0.38 ± 0.10a | 0.29 ± 0.11a,b | 0.27 ± 0.01a,b | 0.28 ± 0.08b |
| DI | 0.57 ± 0.18a | 0.72 ± 0.13a | 0.76 ± 0.04a,b | 1.00 ± 0.00b |
The 16-week FVIA/FVs and FVIA/AVs were significantly greater in hip joints with a 32-week OA score of 0 than in hip joints with a 32-week OA score of 3 (Table 5). The 16-week DIs in hip joints that had a 32-week OA score of 0 were significantly lower than in hip joints with a 32-week OA score of 2 or 3. The 16-week FVIA/FVs and FVIA/AVs were significantly greater and the 16-week DIs were significantly lower in hip joints with a 104-week OA score of 0 or 1 than those in hip joints with a 104-week OA score of 3. The 32-week FVIA/FVs and FVIA/AVs in hip joints with an OA score of 0 or 1 at 104 weeks were significantly greater than those in hip joints with a 104-week OA score of 3. Additionally, the 32-week DIs in hip joints with an OA score of 0 at 104 weeks were significantly lower than in hip joints with a 104-week OA score of 1 or 3.
Table 5.
Mean ± SD hip joint volume ratios and DIs in 1 hip joint from each of 46 mixed-breed hound-type dogs with hip joint osteoarthritis at 16 and 32 weeks of age corresponding to each radiographic OA score as measured at 32 and 104 weeks of age.
| Variable | 0 | 1 | 2 | 3 |
|---|---|---|---|---|
| Volume ratios at 16 wk by OA score at 32 wk | ||||
| FV/AV | 0.85 ± 0.12 | 0.86 ± 0.11 | 0.87 ± 0.09 | 0.84 ± 0.16 |
| FVIA/FV | 0.38 ± 0.11a | 0.32 ± 0.08a,b | 0.27 ± 0.19a,b | 0.20 ± 0.10b |
| FVIA/AV | 0.32 ± 0.09a | 0.27 ± 0.06a,b | 0.24 ± 0.19a,b | 0.17 ± 0.08b |
| DI | 0.59 ± 0.12a | 0.65 ± 0.07a,b | 0.85 ± 0.15b | 0.83 ± 0.17b |
| Volume ratios at 16 wk by OA score at 104 wk | ||||
| FV/AV | 0.86 ± 0.11 | 0.81 ± 0.13 | 0.87 ± 0.12 | 0.86 ± 0.14 |
| FVIA/FV | 0.38 ± 0.11a | 0.42 ± 0.10a | 0.26 ± 0.06a,b | 0.22 ± 0.11b |
| FVIA/AV | 0.32 ± 0.09a | 0.33 ± 0.07a | 0.23 ± 0.05a,b | 0.19 ± 0.10b |
| DI | 0.57 ± 0.11a | 0.65 ± 0.07a | 0.71 ± 0.08a,b | 0.82 ± 0.15b |
| Volume ratios at 32 wk by OA score at 104 wk | ||||
| FV/AV | 1.07 ± 0.18 | 1.01 ± 0.22 | 1.07 ± 0.11 | 0.98 ± 0.20 |
| FVIA/FV | 0.35 ± 0.13a | 0.27 ± 0.11a | 0.19 ± 0.04a,b | 0.06 ± 0.08b |
| FVIA/AV | 0.37 ± 0.14a | 0.26 ± 0.09a | 0.20 ± 0.03a,b | 0.07 ± 0.09b |
| DI | 0.64 ± 0.16a | 0.81 ± 0.14b | 0.81 ± 0.07a,b | 0.96 ± 0.07b |
Discussion
Characterization of joint structure volumes before, during, and after onset and progression of phenotypic alterations attributable to hip dysplasia in dogs is a unique approach to study the disease. Our hypothesis that the FV/AV would remain unchanged with increasing age and disease progression because of comparable decreases in the FV and AV was accepted in part because FV and AV decreased with time in a predictable fashion relative to each other, despite disease and age progression. However, the 16-week FV/AV was significantly lower than the 32-and 104-week values. The FVIA/FV and FVIA/AV both had negative relationships with the DI and radiographic OA score within and between all age groups, supporting the hypothesis that FVIA/FV and FVIA/AV would decrease with increasing degree of joint disease and would therefore have a negative relationship with the DI and radiographic OA scores. The final hypothesis was that the FVIA/FV and FVIA/AV would differ among dogs with different OA scores within ages and that there would be significant differences between 16-week ratios corresponding to different 32-week OA scores and between 16-and 32-week ratios corresponding to different 104-week OA scores. This hypothesis was accepted because FVIA/FV and FVIA/AV were significantly different among OA scores within and between ages. The information obtained from the present study suggested that comparable decreases in AV and FV develop in lax canine hips, irrespective of the presence of OA. In addition, the FVIA/FV and FVIA/AV had significant relationships with laxity and OA scores, and the FVIA/FV and FVIA/AV corresponded to hip joint OA severity prior to and after onset of the disease.
Measurements performed were for the most part reproducible and repeatable; however, the FV values were less reproducible than the AV or FVIA values. This may have indicated that identification of anatomic landmarks required for the measures, such as boundaries of the capital epiphysis and physeal scar, is subjective and difficult as previously reported.25 More experience with anatomic landmark identification may increase the reproducibility of the FV measurements.
Two-dimensional CT image measurements are routinely performed in human medicine10,26,27 and have been applied in dogs for research purposes.6,7,28–33 The sensitivity of 3-D CT to detect and characterize minor hip joint abnormalities is superior to that of 2-D radiography.10,26 Also, CT is an important imaging modality for assessment of hip joint structures in children with minor symptoms of hip dysplasia,34 classification of human dysplastic hip joints,20 surgical planning,15–17,19,26,34 and postoperative assessment.10,35 Two-dimensional measures on 3-D canine hip CT images reported by others6 differ from the measures developed in the present study because our measures included volume quantification on 3-D images. Such volume measurements allowed assessment of the acetabulum and femoral head in multiple dimensions. Ratios were used to account for growth and in anticipation of breed differences. Because volumetric hip joint measures are relatively new in canine versus human medicine,34,36 outcomes were compared with established radiographic measures: DI and OA scores. The measures described here provided novel information about the relationships of articulating canine hip joint structures over the progression of hip joint changes characteristic of hip dysplasia in dogs.
Given the lack of significant changes in AV or FV after dogs were 32 weeks of age, the most significant loss of volume in the hip joints developed prior to that age. It has been proposed that acetabular changes may be an initiating1,2,4 factor in the development of hip dysplasia in dogs. Our results suggested that the acetabulum undergoes a more rapid but consistent decrease in volume, compared with the femoral head, because an increase was detected in the FV/AVs with no comparable decrease in the FVIA/AVs between 16 and 32 weeks of age. Furthermore, the FVIA/AV was most closely related to the DI and OA score at and after 32 weeks of age, whereas the FVIA/FV was most strongly related to the DI and OA before 32 weeks. Finally, the FVIA/FV at 104 weeks did not differ significantly among OA scores at the same age, whereas the FVIA/AV did. All of these findings suggested that the earliest joint changes associated with hip dysplasia develop in the acetabulum.1 It is possible that the measures developed in our study will be useful to further define the chronology and potential contributions of hip structures to pathological changes. A key finding was that the relative volumetric relationship of the hip joint articular structures remained consistent despite age and OA in dogs with lax hip joints characteristic of hip dysplasia as well as the apparently more rapid change in AV. This was supported by the strong relationships between FV and AV at all ages evaluated as well as the finding that the FV/AV was similar between OA severities across ages. The FVIA/FVs and FVIA/AVs were also significantly aligned from youth to adulthood in the study dogs, suggesting that despite changes in the shape of the FV and AV, the relative position of the femoral head in the acetabulum remained fairly consistent when normalized to their volumes. A slight increase in FVIA between 32 and 104 weeks of age may have been attributable to joint capsular thickening1 and reflected in the significant increase in FVIA/FV and FVIA/AV at the same time.
The FVIA/FV, FVIA/AV, and DI all involve evaluations of the relationship between the acetabulum and femoral head. The ratios, however, incorporate 3-D volume measures, whereas the DI is a 2-D assessment of the femoral head–acetabulum relationship normalized to the femoral head radius. As expected, the FVIA/FV and FVIA/AV decreased and the DI increased with increasing OA score. A potential predictive value of the volume ratios to identify dogs that will develop severe OA versus those that will develop no or mild OA was suggested by 16- and 32-week FVIA/FVs and FVIA/AVs, compared with OA scores at 104 weeks of age. Of interest is that the relationships between the FVIA/FV and FVIA/AV at younger ages corresponding to OA scores at older ages have the same relationships at 16 and 32 weeks while DI relationships changed between ages. The relationships of FVIA/FV and FVIA/AV with OA score were the strongest at 32 weeks of age and subsequently decreased. On the other hand, the relationship between DI and OA increased in strength. The 32-week FVIA/FVs, FVIA/AVs, and DIs had stronger relationships with 104-week OA scores than with the 16-week values. Despite this, the 16-week values had measurable relationships with the OA scores at 104 weeks of age, suggesting some conformational attributes associated with OA. Whether intervention prior to 32 weeks of age will inhibit disease progression is an area for future investigation. The findings in the present study reinforce the concept that in addition to laxity, predisposing and potentially programmed conditions such as abnormal composition, structural alterations, or failure of homeostasis contribute to initiation and progression of hip dysplasia in dogs.
Important information about hip joint morphology, including acetabular height, surface area,34 volume,34,36 acetabular coverage,15,18 joint congruency,15 and position of the acetabular cup,10 is obtained from 3-D CT models in humans with hip dysplasia. The information is incorporated into finite element models to predict and model disease progression.21,37,38 The measures described herein may permit modeling of the disease process in dogs. The finding that significant relationships of both FVIA/FV and FVIA/AV with the radiographic OA score were maintained for up to 104 weeks of age, despite severe pathological changes, reflected the potential value of the measures for this purpose. Finite element models may contribute to greater understanding of the pathogenesis of hip dysplasia by permitting consideration of factors that contribute to the disease in dogs, such as joint forces, abnormal growth, and preprogrammed joint degeneration both together and independently.
The results of the study reported here supported the supposition that the pathogenesis of hip dysplasia is the result of multiple factors. In addition, the measures used suggested that the major alterations of hip dysplasia occur between 16 and 32 weeks of age in affected dogs and that there is a strong association between the measures and the severity of OA. Finite element modeling based on the measures developed in this study may be useful to distinguish between programmed and mechanical contributions to the changes to advance diagnosis, prevention, and treatment of hip dysplasia as well as to provide insight into the pathophysiology of the disease.
Acknowledgments
Supported in part by the NIH-NIAMS (grant No. K01 AR02174-01), the Arthritis Foundation, and the Collie Health Foundation.
AbbreviAtions
- AV
Acetabular volume
- CT
Computed tomography
- DI
Distraction index
- FV
Femoral head volume
- FV/AV
Ratio of femoral head volume to acetabular volume
- FVIA
Portion of femoral head within the acetabulum
- FVIA/AV
Ratio of the portion of femoral head within the acetabulum to acetabular volume
- FVIA/FV
Ratio of the portion of femoral head within the acetabulum to femoral head volume
- IaCC
Intraclass correlation coefficient
- IeCC
Interclass correlation coefficient
- MRI
Magnetic resonance imaging
- OA
Osteoarthritis
Footnotes
PMI Nutrition Prime Formula, PMI Nutrition, Henderson, Colo.
PMI Nutrition Adult Formula, PMI Nutrition, Henderson, Colo.
PennHIP Analysis Center, Malvern, Pa.
Mimics, version 11.1, Materialise, Leuven, Belgium.
Microsoft Excel 2007, Microsoft Corp, Redmond, Wash.
SAS, version 9.0, SAS Institute Inc, Cary, NC.
GraphPad Prism, version 4.0, GraphPad Software Inc, San Diego, Calif.
References
- 1.Riser WH. The dog as a model for the study of hip dysplasia. Growth, form, and development of the normal and dysplastic hip joint. Vet Pathol. 1975;12:234–334. doi: 10.1177/030098587501200401. [DOI] [PubMed] [Google Scholar]
- 2.Schnelle GB. The veterinary radiologist: regional radiography—the pelvic region—part I. North Am Vet J. 1937;18:53–56. [Google Scholar]
- 3.Schnelle GB. Bilateral congenital subluxation of the coxo-femoral joints in a dog. University of Pennsylvania Bulletin. 1937;37:15–16. [Google Scholar]
- 4.Macys JR, Bullough PG, Wilson PD., Jr Coxarthrosis: a study of the natural history based on a correlation of clinical, radiographic, and pathologic findings. Semin Arthritis Rheum. 1980;10:66–80. doi: 10.1016/0049-0172(80)90015-3. [DOI] [PubMed] [Google Scholar]
- 5.Adams WM, Dueland RT, Meinen J, et al. Early detection of canine hip dysplasia: comparison of two palpation and five radiographic methods. J Am Anim Hosp Assoc. 1998;34:339–347. doi: 10.5326/15473317-34-4-339. [DOI] [PubMed] [Google Scholar]
- 6.Lopez MJ, Lewis BP, Swaab ME, et al. Relationships among measurements obtained by use of computed tomography and radiography and scores of cartilage microdamage in hip joints with moderate to severe joint laxity of adult dogs. Am J Vet Res. 2008;69:362–370. doi: 10.2460/ajvr.69.3.362. [DOI] [PubMed] [Google Scholar]
- 7.Farese JP, Todhunter RJ, Lust G, et al. Dorsolateral subluxation of hip joints in dogs measured in a weight-bearing position with radiography and computed tomography. Vet Surg. 1998;27:393–405. doi: 10.1111/j.1532-950x.1998.tb00146.x. [DOI] [PubMed] [Google Scholar]
- 8.Smith GK, Biery DN, Gregor TP. New concepts of coxofemoral joint stability and the development of a clinical stress-radiographic method for quantitating hip joint laxity in the dog. J Am Vet Med Assoc. 1990;196:59–70. [PubMed] [Google Scholar]
- 9.Preidler KW, Resnick D. Imaging of osteoarthritis. Radiol Clin North Am. 1996;34:259–271. [PubMed] [Google Scholar]
- 10.Kalteis T, Handel M, Herold T, et al. Position of the acetabular cup—accuracy of radiographic calculation compared to CT-based measurement. Eur J Radiol. 2006;58:294–300. doi: 10.1016/j.ejrad.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Khan O, Goodfellow T, Cosgrove DO, et al. Non-invasive imaging techniques in surgery. Ann R Coll Surg Engl. 1984;66:127–134. [PMC free article] [PubMed] [Google Scholar]
- 12.Corley EA, Keller GG, Lattimer JC, et al. Reliability of early radiographic evaluations for canine hip dysplasia obtained from the standard ventrodorsal radiographic projection. J Am Vet Med Assoc. 1997;211:1142–1146. [PubMed] [Google Scholar]
- 13.Atar D, Lehman WB, Grant AD. 2-D and 3-D computed tomography and magnetic resonance imaging in developmental dysplasia of the hip. Orthop Rev. 1992;21:1189–1197. [PubMed] [Google Scholar]
- 14.Bogner EA, Sofka CM. CT evaluation of total hip arthroplasty complication: dissociation of acetabular component. HSS J. 2007;3:112–114. doi: 10.1007/s11420-006-9026-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roach JW, Hobatho MC, Baker KJ, et al. Three-dimensional computer analysis of complex acetabular insufficiency. J Pediatr Orthop. 1997;17:158–164. doi: 10.1097/00004694-199703000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Brown GA, Firoozbakhsh K, Gehlert RJ. Three-dimensional CT modeling versus traditional radiology techniques in treatment of acetabular fractures. Iowa Orthop J. 2001;21:20–24. [PMC free article] [PubMed] [Google Scholar]
- 17.Jawish R, Khalife R, Ghorayeb J. Three-dimensional tomography analysis and anteversion study after periacetabular osteotomy of pelvis in children. J Child Orthop. 2007;1:357–363. doi: 10.1007/s11832-007-0063-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janzen DL, Aippersbach SE, Munk PL, et al. Three-dimensional CT measurement of adult acetabular dysplasia: technique, preliminary results in normal subjects, and potential applications. Skeletal Radiol. 1998;27:352–358. doi: 10.1007/s002560050397. [DOI] [PubMed] [Google Scholar]
- 19.Smet MH, Marchal GJ, Baert AL, et al. Three-dimensional imaging of acetabular dysplasia: diagnostic value and impact on surgical type classification. Eur J Radiol. 2000;34:26–31. doi: 10.1016/s0720-048x(00)00156-x. [DOI] [PubMed] [Google Scholar]
- 20.Ito H, Matsuno T, Hirayama T, et al. Three-dimensional computed tomography analysis of non-osteoarthritic adult acetabular dyplasia. Skeletal Radiol. 2009;38:131–139. doi: 10.1007/s00256-008-0601-x. [DOI] [PubMed] [Google Scholar]
- 21.Shefelbine SJ, Carter DR. Mechanobiological predictions of growth front morphology in developmental hip dysplasia. J Orthop Res. 2004;22:346–352. doi: 10.1016/j.orthres.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Buckley SL, Sponseller PD, Magid D. The acetabulum in congenital and neuromuscular hip instability. J Pediatr Orthop. 1991;11:498–501. doi: 10.1097/01241398-199107000-00015. [DOI] [PubMed] [Google Scholar]
- 23.Smith GK, Gregor TP, Rhodes WH, et al. Coxofemoral joint laxity from distraction radiography and its contemporaneous and prospective correlation with laxity, subjective score, and evidence of degenerative joint disease from conventional hip-extended radiography in dogs. Am J Vet Res. 1993;54:1021–1042. [PubMed] [Google Scholar]
- 24.Smith GK, Lafond E, Gregor TP, et al. Within-and between-examiner repeatability of distraction indices of the hip joints in dogs. Am J Vet Res. 1997;58:1076–1077. [PubMed] [Google Scholar]
- 25.Decking R, Brunner A, Decking J, et al. Reliability of the Crowe and Hartofilakidis classifications used in the assessment of the adult dysplastic hip. Skeletal Radiol. 2006;35:282–287. doi: 10.1007/s00256-005-0061-5. [DOI] [PubMed] [Google Scholar]
- 26.Tallroth K, Lepistö J. Computed tomography measurement of acetabular dimensions: normal values for correction of dysplasia. Acta Orthop. 2006;77:598–602. doi: 10.1080/17453670610012665. [DOI] [PubMed] [Google Scholar]
- 27.Jacobsen S, Romer L, Soballe K. Degeneration in dysplastic hips: a computed tomography study. Skeletal Radiol. 2005;34:778–784. doi: 10.1007/s00256-005-0019-7. [DOI] [PubMed] [Google Scholar]
- 28.Dueland RT, Adams WM, Fialkowski JP, et al. Effects of pubic symphysiodesis in dysplastic puppies. Vet Surg. 2001;30:201–217. doi: 10.1053/jvet.2001.23350. [DOI] [PubMed] [Google Scholar]
- 29.Patricelli AJ, Dueland RT, Adams WM, et al. Juvenile pubic symphysiodesis in dysplastic puppies at 15 and 20 weeks of age. Vet Surg. 2002;31:435–444. doi: 10.1053/jvet.2002.34766. [DOI] [PubMed] [Google Scholar]
- 30.Manley PA, Adams WM, Danielson KC, et al. Long-term outcome of juvenile pubic symphysiodesis and triple pelvic osteotomy in dogs with hip dysplasia. J Am Vet Med Assoc. 2007;230:206–210. doi: 10.2460/javma.230.2.206. [DOI] [PubMed] [Google Scholar]
- 31.Kishimoto M, Yamada K, Pae S, et al. Quantitative evaluation of hip joint laxity in 22 border collies using computed tomography. J Vet Med Sci. 2009;71:247–250. doi: 10.1292/jvms.71.247. [DOI] [PubMed] [Google Scholar]
- 32.Fujiki M, Kurima Y, Yamanokuchi K, et al. Computed tomographic evaluation of growth-related changes in the hip joints of young dogs. Am J Vet Res. 2007;68:730–734. doi: 10.2460/ajvr.68.7.730. [DOI] [PubMed] [Google Scholar]
- 33.Fujiki M, Misumi K, Sakamoto H. Laxity of canine hip joint in two positions with computed tomography. J Vet Med Sci. 2004;66:1003–1006. doi: 10.1292/jvms.66.1003. [DOI] [PubMed] [Google Scholar]
- 34.Lin CJ, Romanus B, Sutherland DH, et al. Three-dimensional characteristics of cartilaginous and bony components of dysplastic hips in children: three-dimensional computed tomography quantitative analysis. J Pediatr Orthop. 1997;17:152–157. doi: 10.1097/00004694-199703000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Pitto RP, Mueller LA, Reilly K, et al. Quantitative computer-assisted osteodensitometry in total hip arthroplasty. Int Orthop. 2007;31:431–438. doi: 10.1007/s00264-006-0257-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Egawa H, Powers CC, Beykirch SE. Can the volume of pelvic osteolysis be calculated without using computed tomography? Clin Orthop Relat Res. 2009;467:181–187. doi: 10.1007/s11999-008-0522-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Russell ME, Shivanna KH, Grosland NM, et al. Cartilage contact pressure elevations in dysplastic hips: a chronic overload model. J Orthop Surg Res. 2006;1:6. doi: 10.1186/1749-799X-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cilingir AC, Ucar V, Kazan R. Three-dimensional anatomic finite element modelling of hemi-arthroplasty of human hip joint. Trends Biomater Artif Organs. 2007;21:63–72. [Google Scholar]