Abstract
NADPH oxidases of the NADPH oxidase (NOX) family are dedicated reactive oxygen species-generating enzymes that broadly and specifically regulate redox-sensitive signalling pathways that are involved in cancer development and progression. They act at specific cellular membranes and microdomains through the activation of oncogenes and the inactivation of tumour suppressor proteins. In this Review, we discuss primary targets and redox-linked signalling systems that are influenced by NOX-derived ROS, and the biological role of NOX oxidases in the aetiology of cancer.
Cellular transformation is a multistep process that requires an as yet undetermined sequence of genetic alterations and changes in intracellular signalling1. The hallmarks of cancer, first reviewed by Hanahan and Weinberg in 2000, and updated in 2011, indicate aberrant events that mediate cellular transformation2,3. An underlying event not discussed in these reviews is the role of oxidative stress in these processes. The role of oxidative stress in cellular transformation was first described in 1981 by Oberley and colleagues4. This seminal paper described the generation of intracellular hydrogen peroxide, stimulated by insulin, as a second messenger that induces cellular proliferation. Additionally, the authors suggested that increased super-oxide production leads to cellular immortality. This work became known as ‘the free radical theory of cancer’ (REF. 4). Similar to the importance of free radicals, was the discovery of enzymes that are involved in scavenging these radicals, which are referred to as antioxidants5. Oxidative stress is caused by an imbalance between the production of reactive oxygen species (ROS) and the ability of cells to neutralize their reactive intermediates. As early as 1981, when oxidative stress had only been hypothesized to have a role in cellular transformation and the enzymatic sources of ROS were still unknown, it was suggested that enzymes biochemically similar to the NADPH oxidase (NOX) NOX2 (also known as gp91phox) of the human neutrophil were present in nonphagocytic cells4,6. A decade later, other investigators detected high levels of super-oxide and hydrogen peroxide in various cancer cells7,8 and confirmed that ROS production could be reduced by diphenyleneiodonium (DPI), a chemical inhibitor of flavoprotein-containing enzymes such as NOX oxidases.
Currently, several sources of ROS in cells and tissues have been identified: the mitochondrial electron transfer chain and NADPH oxidases of the NOX family are the two major sources implicated in cancer. ROS derived from these two major sources are not mutually exclusive, and recent evidence suggests crosstalk exists between these major producers, which is highlighted in this Review. The role of mitochondrial-derived ROS in cancer, produced as a byproduct of mitochondrial respiration, has previously been reviewed9–11. In large part, the biological roles of NOX oxidases in cancer have been identified based on the knowledge gained during examination of the physiological and pathophysiological roles of NOX oxidases in diabetic kidney disease, cardiovascular disease, Alzheimer’s disease, fibrosis and atherosclerosis12–14. Although primarily based on in vitro data, owing to the early status of determining the function of NOX in cancer, this Review highlights the roles of the NOX complexes in cellular transformation and the maintenance of the malignant phenotype.
The NOX family as a source of ROS in cancer
Since 1999, the discovery and characterization of NOX2 homologues in various cells and tissues has facilitated a better understanding of the molecular mechanisms underlying oxidative stress in cancer. Seven membrane-bound NOX catalytic isoforms, referred to as NOX1 to NOX5, dual oxidase 1 (DUOX1) and DUOX2 have been identified, each of which displays similar but distinct structural, biochemical and subcellular localization characteristics13,15–23. The NOX catalytic subunits that have been implicated in cancer include NOX1, NOX2, NOX4 and NOX5. The roles of DUOX1, DUOX2 and NOX3 in carcinogenesis are not well established and so are not further discussed here. However, it is noteworthy that the DUOX promoters are highly methylated in lung cancer, but the biological importance of this posttranslational modification remains unclear24–26.
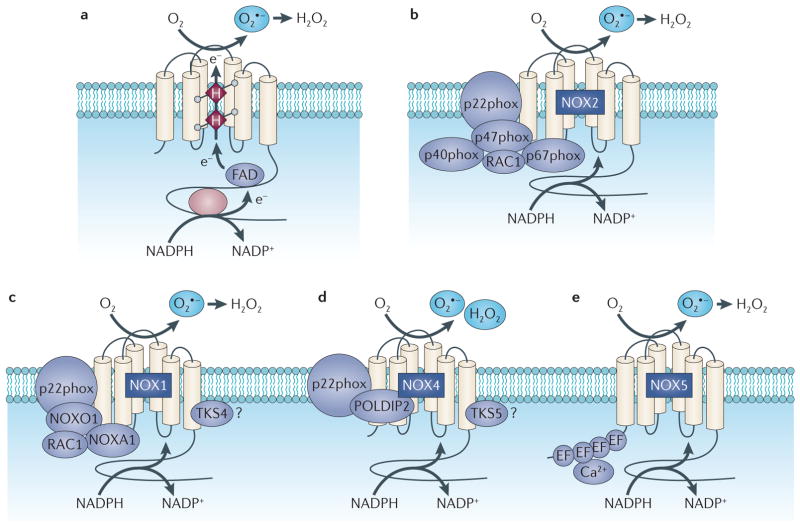
NOX-dependent redox signalling occurs at cellular membranes and intracellular structures where the NOX catalytic and regulatory subunits are localized. The NOX catalytic subunits NOX2, NOX1, NOX4 and NOX5 have been detected at the plasma membrane27–31. NOX4 has additionally been detected in the endoplasmic reticulum32, mitochondrial27,33,34 and nuclear membranes35. NOX subunits also reside at specific subcellular microdomains such as NOX4 at focal adhesions35, NOX1 at caveoli and lipid rafts35,36, and NOX1 and NOX4 at invadopodia37. With some exceptions, the NOX catalytic subunits have six amino-terminal transmembrane-spanning segments, conserved histidine residues that bind two haems, and carboxy-terminal binding sites for FAD and NADPH. NOX catalytic subunits transfer electrons from NADPH across biological membranes to molecular oxygen. With the exception of NOX5, the facilitation of electron transfer requires the small membrane-bound protein p22phox and cytosolic NOX-regulatory subunits. Most NOX catalytic isoforms use different regulatory subunits. For example, activation of NOX2 oxidase requires p22phox and the recruitment of cytosolic regulatory subunits p47phox, p67phox, p40phox and the GTPase RAC1. On cell stimulation, NOX2 complex formation and activation are mediated by initial phosphorylation and conformational change of the p47phox subunit38,39. p47phox organizes the translocation of the NOX activator subunit p67phox and p40phox to the membrane and docks through its SRC homology domains to the proline-rich region of the p22phox subunit with subsequent recruitment of RAC1. Once the complex is formed, electrons are donated from NADPH and transferred to FAD, reducing it to FADH2, which is mediated by the activation domain of p67phox. to the A single electron is then transferred from FADH2 first iron centre of the NOX catalytic subunit, which can only accept one electron. Oxygen is bound to the second haem centre and receives the electron from the inner first haem. One-electron reduction of molecular oxygen (O2) generates superoxide anion (O2·), which is then dismutated to hydrogen peroxide (H2O2), either spontaneously at an acid pH or catalytically by superoxide dismutase (SOD). For NOX4, hydrogen peroxide rather than superoxide is the predominant species detected by standard molecular reagents. As there is no thermo-dynamically viable mechanism for a haemcatalysed two-electron reduction of oxygen, the predominant detection of hydrogen peroxide generation is probably the result of the rapid kinetics of dismutation and/or due to a conserved histidine residue located in the extended E-loop of the NOX4 molecule, which may serve as a source of protons or as a trapping mechanism for the spontaneous dismutation of superoxide at this site13,30,40,41.
Reconstitution assays and the characterization of NOX isoforms show that p47phox and p67phox do not robustly activate other NOX isoforms in non-phagocytic cells. This led to the identification and cloning of human homologues of p47phox (also known as NOXO1) and p67phox (also known as NOXA1)15,42. Although the mechanisms of activation for NOX1 and NOX3 are similar to those of NOX2, NOX1 interacts with NOXO1 and NOXA1 in addition to tyrosine kinase substrate 4 (TKS4; also known as SH3PXD2B) and RAC1 on activation13,19,21,37,42–46. By contrast, the isoforms NOX4 and NOX5 do not require any of the classic cytosolic subunits13. In some circumstances, NOX4 interacts with proteins that enhance its activity, namely DNA polymerase-δ-interacting protein 2 (POLDIP2) and tyrosine kinase substrate 5 (TKS5; also known as SH3PXD2A)37,47,48. NOX4 seems to be a constitutively active enzyme that is primarily regulated at the level of its expression in response to various stimuli13, and NOX5 has N-terminal cytoplasmic EF-hand calcium-binding motifs that bind calcium for activation. A summary of the NOX catalytic subunits with their regulatory subunits (NOX complexes) is shown in FIG. 1. The structure and function of individual NOX oxidase subunits have been comprehensively reviewed13,19,21.
Figure 1. Structure and molecular organization of the NADPH oxidases of the NOX family.
Part a shows the topology and the enzymatic reaction catalysed by the NADPH oxidase (NOX) enzymes. Parts b–e represent the molecular structure of NOX2, NOX1, NOX4 and NOX5, which are predominantly expressed in carcinoma cells. All NOX proteins can form a complex with p22phox, but the cytosolic subunits differ between the NOX oxidase isoforms. e, electron; FAD, flavin, superoxide; POLDIP2, DNA polymerase-δ-interacting adenine dinucleotide; H, Haem; H2O2, hydrogen peroxide; O2 protein 2; TKS, tyrosine kinase substrate.
NOX catalytic and regulatory subunits have been implicated in one or more of the most common cancer types. Increased mRNA and/or protein expression of NOX1, NOX2, NOX4 and NOX5 or their regulatory components has been detected at higher levels in various cultured cancer cell lines34,49–61 or human tumours34,55,58,59,61–75 compared with normal controls at early and late stages of tumorigenesis, suggesting that ROS derived from NADPH oxidases may be important in both the initiation and the maintenance phases of tumour development (TABLE 1).
Table 1.
NOX oxidase subunit expression in common cancer types
| Cancer type | NADPH oxidase subunit | Refs |
|---|---|---|
| Bladder | p67phox | 53 |
| NOX4 | 59 | |
| NOX1 | 73 | |
| NOX4 | 34 | |
| Breast | NOX1 | 52,65 |
| NOX5 | 55 | |
| RAC1 | 66,68 | |
| TKS5 | 58 | |
| Colon and rectal | NOX4 | 63 |
| NOX1 | 55,67 | |
| NOXO1 and NOXA1 | 55 | |
| RAC1 | 66,68 | |
| Kidney | NOX4 | 49,64 |
| NOX1 | 64 | |
| p22phox | 49,64 | |
| Leukaemia | NOX2 and NOX4 | 55,57 |
| NOX5 | 70 | |
| Lung | RAC1 | 68 |
| Melanoma | NOX4 | 55,61 |
| NOX2, p22phox, p47phox and p67phox | 51 | |
| NOX5 | 55 | |
| Non-Hodgkin’s lymphoma | p22phox | 69,71 |
| Pancreatic | NOX4 and p22phox | 60 |
| Prostatic | NOX4 | 56 |
| NOX1 | 62,72 | |
| NOX2 | 56 | |
| NOX5 | 50,54 | |
| p22phox | 56 | |
| Thyroid | NOX4 and p22phox | 74 |
| Ovarian | NOX4 | 55,75 |
| NOX1 | 55,65 |
Biological roles of NOX-derived ROS in cancer
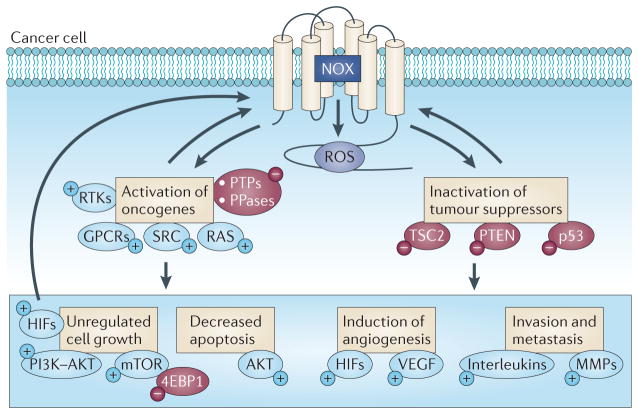
There are two biological outcomes in response to the exposure of cells to ROS. ROS are chemically reactive and can directly damage biomolecules such as nucleic acids, resulting in genomic instability. ROS can also directly or indirectly activate or inactivate redox-sensitive signalling pathways, damage lipids and modify proteins to alter cellular functions, promoting autonomous cell growth and immortality. Therefore, a fundamental question is whether NOX-derived ROS have a role in genomic instability or a biochemical role in the initiation and maintenence of cellular transformation? We address this question by examining the biological and molecular bases of NOX-derived ROS actions in the principal hallmarks of cancer (FIG. 2).
Figure 2. Integration of NOX oxidase-derived ROS with the hallmarks of cancer.
This figure shows the potential signalling pathways that are activated (+) or inactivated (−) by NADPH oxidase (NOX) oxidases and how they relate to some of the suggested hallmarks of cancer cells, such as deregulated cell growth and angiogenesis. GPCRs, G protein-coupled receptors; HIFs, hypoxia-inducible factors; MMPs, matrix metalloproteinases; PPase, protein phosphatase; PTPs, protein tyrosine phosphatases; ROS, reactive oxygen species; RTK, receptor tyrosine kinase; TSC2, tuberous sclerosis 2; VEGF, vascular endothelial growth factor.
NOX-derived ROS and genomic instability
Defining direct roles for NOX-derived ROS in mutagenesis and genomic instability is complex when considering putative interactions between other intracellular reactive species. In general, superoxide is converted into hydrogen peroxide enzymatically by the cytosolic antioxidant superoxide dismutase 1 (SOD1) or the mitochondrial SOD2 and is then converted to water by glutathione peroxidase or catalase. However, this conversion is not 100% efficient and in some conditions SOD expression and activity can be altered. SOD1 activity is reduced in human renal cancer tissue76, and SOD1 and SOD2 expression are reduced in prostate cancer tissue77, whereas SOD1 expression is increased in breast cancer tissue78. Alterations of SOD expression can be a ‘double-edged sword’. For example, overexpression of SOD1, as is the case in breast cancer, will theoretically generate high levels of hydrogen peroxide, capable of diffusing across biological membranes and constitutively activating redox-signalling pathways. Low expression of SOD1 promotes interactions of superoxide, which cannot cross biological membranes79, with other cellular reactive species such as nitric oxide (NO) to produce peroxynitrite (OONO−) or hydrogen peroxide (H2O2) to form a hydroxyl radical (•OH), which are both highly reactive intermediates that can quickly damage surrounding macromolecules such as nucleic acids, lipids and proteins. Oxidation of nucleic acids results in the formation of adducts with deoxyguanidine (8-OH-dG), which if not repaired can potentially generate mutations80. Mitochondrial DNA is highly susceptible to damage because it is not protected by histones and is therefore directly exposed to ROS. In addition, DNA repair capacity is less efficient in the mitochondria10. The recent finding that NOX4 localizes to the mitochondria27,34 suggests that NOX oxidases might mediate mitochondrial DNA damage. Moreover, there is a growing body of evidence linking mitochondrial metabolism to carcinogenesis. Notably, NOX4-derived ROS can directly oxidize mitochondrial complex subunits, leading to mitochondrial dysfunction33,81. The identification of a superoxide-generating NOX4 in the nucleus raises the possibility of direct alteration of nuclear DNA and proteins by the oxi-dase82. Overexpression of HRAS V12 in normal thyroid cells induces NOX4-derived ROS, DNA replication rate and DNA damage, leading to cellular senescence. Silencing of NOX4 or the treatment of cells with the antioxidant N-acetyl-cysteine (NAC) both reduce RAS-mediated DNA lesions, DNA foci formation and cell senescence83. Although the mechanisms have not been elucidated, NOX4-mediated DNA lesions in cells expressing oncogenic HRAS may be a result of increased DNA replication rate. As RAS signalling is activated in a number of cancers, which do not undergo cellular senescence, NOX4 may have a subsequent role in nuclear genomic hits and instability in these cancers.
Taken together, the subcellular localization where reactive species are generated, interactions between reactive species, expression of antioxidants present in the milieu and functional DNA repair pathways all have roles and can mediate outcomes of mutagenesis and genomic instability.
Role of NOX oxidases in autonomous cell growth and survival
In general, the roles and mechanisms by which NOX oxidases mediate autonomous cell growth and cell survival remain unclear and require further investigation. However, there are some initial studies that have provided insight into the function of NOX oxidases in these processes. Before the cloning of NOX isoforms and their identification in somatic cells, it was suggested that superoxide and hydrogen peroxide function as mitogenic stimuli through biochemical processes that are common to growth factors. The addition of low concentrations of superoxide or hydrogen peroxide to cultured cells, including fibroblasts, epidermal cells, and leukaemic and osteoblastic cells, stimulates growth and induces the expression of immediate early genes, such as FOS and JUN84–86. Conversely, antioxidants that neutralize hydrogen peroxide inhibit tyrosine kinase-dependent signalling87–89.
NOX1-, NOX2- and NOX4-derived ROS mediate cell proliferation of normal cultured cells in response to various growth stimuli that activate receptor tyrosine kinases (RTKs), such as epidermal growth factor receptor (EGFR) and platelet-derived growth factor receptor (PDGFR)90–93, suggesting that the mitogenic effects of NOX-mediated ROS in cancer cells with aberrant growth factor signalling may be a universal occurrence. NOX-derived ROS promote growth factor-induced tyrosine autophosphorylation predominantly by inactivating protein tyrosine phosphatases (PTPs), resulting in hyperphosphorylation and activation of the RTK and downstream signalling molecules88,94,95. Nucleophilic amino acids, including the sulphur-containing cysteine residues, are highly susceptible to redox modification96. The catalytic domains of PTPs contain redox-sensitive cysteine residues that undergo oxidative modification, which is predominantly mediated by hydrogen peroxide, resulting in impaired catalytic activity97–100. The modification of nucleophile amino acid residues is determined and surrounding amino by a characteristiclly low pKa acids, as well as its location in the tertiary structure of a protein making it susceptible or not to redox modification96,101. The oxidation of cysteine residues can be mild and reversible (by intracellular-reducing agents) or severe and irreversible. In-gel phosphatase activity assays suggest that NOX-derived ROS selectively target intracellular PTPs rather than nonspecifically oxidize PTPs, with the specificity of PTP oxidation probably attributed to the proximity of the NOX-derived ROS to the phosphatase101–104. To date, phosphatases that are known to be regulated by NOX oxidases include the tyrosine phosphatases PTP1B, PTEN, SRC homology region 2 domain-containing phosphatase 1 (SHP1), SHP2, PTP-PEST, CD45, low-molecular-weight protein tyrosine phosphatase (LMW-PTP) and the serine/threonine phosphatase PP1 (REFS 105,106). This list is likely to grow, as protein phosphatases are an abundant group of proteins in mammalian cells107. Constitutive NOX-derived ROS that are produced in hepatoma and epidermoid carcinoma cells potentiate autonomous cell growth through the inactivation of PTP1B101. Alternatively, in liver tumour cells, NOX1 regulates autocrine cell growth by regulating mRNA expression of EGFR and one of its ligands, transforming growth factor-α (TGFα), through redox-dependent activation of p38MAPK and AKT signalling pathways108.
Inactivation of phosphatases by NOX-derived ROS is linked to cell survival, in large part through the regulation of conventional survival pathways such as Janus kinase (JAK)–signal transducer and activator of transcription (STAT), AKT, nuclear factor-κB (NF-κB) and p53 signalling. In pancreatic cancer cells, insulin-like growth factor 1 (IGF1) or serum can activate the anti-apoptotic JAK2–STAT pathway and AKT, through NOX4-mediated inhibition of LMW-PTP and PP1 phosphatases, respectively98,109. Whereas inducible expression of BCR–ABL in chronic myelogenous leukaemia cells enhances NOX4-dependent cell survival through inactivation of PP1 and upregulation of the PI3K–AKT survival pathway110. In other studies, the activation of the G protein-coupled receptor (GPCR) leukotriene receptor BLT2 leads to cell survival through the induction of NOX1- and NOX4-derived ROS production through an ERK- and AKT-dependent mechanism in cultured bladder and breast cancer cell lines (REFS 52,111). NOX1- and NOX4-derived ROS promote cell survival in colon cancer cells and melanoma cells, respectively, through a putative NF-κB mechanism51,67, and inhibition of AKT by NOX4-derived ROS promotes cell survival in cultured pancreatic cancer cells60,112.
More recently, a novel link between NOX activation and inactivation of the pro-apoptotic tumour suppressor protein p53 has been described in cancer cells. NOX1 mediates cell survival through sirtuin 1 (SIRT1) activation, which causes deacetylation and inactivation of p53, ultimately impairing p53-mediated transcriptional induction of pro-apoptotic genes113–115. The putative link between NOX and SIRT1 activity could be important for many other biological outcomes, as SIRT deacetylates a broad range of protein substrates and has been implicated in chemotherapy resistance116 and in the regulation of FOXO-dependent expression of glucogenesis-promoting genes in cancer117. The link between NOX and deacetylation needs further exploration.
The small GTPase RAS has a key role in the establishment of growth autonomy. In human colorectal tumours, a positive correlation of NOX1 expression with KRAS mutations has been described118 but this is not the case in prostate cancer in which NOX1 expression is high and KRAS mutations are absent or very infrequent72. Approximately 30% of all human cancers harbour oncogenic mutations in one of the RAS genes, giving rise to constitutively activated RAS signalling. Independent of RAS mutations, RAS can be directly activated by oxidation on susceptible cysteine residues within the molecule; however, a direct role for NOX-dependent activation of RAS has not been studied in cancer100,119,120. Activation of RAS promotes NOX-dependent oxidative stress in cancer cells. Overexpression of KRAS in several cell types increases the transcription of NOX1 through RAF–MEK–ERK-dependent phosphorylation of the transcription factor GATA6 with subsequent binding to the NOX1 promoter121,122. NOX1-dependent ROS generation is required for RAS-induced anchorage- independent cell growth and tumour formation in athymic mice122. In a subset of RAS-transformed cells, NOX1 may potentiate cell proliferation and anchorage-independent cell growth through the cooperation of RAS–MEK and canonical WNT–β-catenin-dependent signalling123,124. Binding of WNT–β-catenin to its receptor dissociates the axin-binding protein Dishevelled (DVL) from nucleoredoxin (NRX), resulting in DVL-dependent stabilization of β-catenin. Stabilized β-catenin migrates into the nuclei and associates with transcription factor T cell factor (TCF)/LEF to activate cell cycle and proliferation-controlling genes such as cyclin D1 and MYC. Overexpression of NRX inhibits WNT-induced cell proliferation in RAS-transformed cells, which is reversed by co-expression of NOX1 (REF. 123). NOX1-dependent activation of WNT–β-catenin-mediated cell growth occurs through redox targeting of NRX with the disruption of NRX–DVL complexes123. More recently, Du and colleagues demonstrated that superoxide, produced by NOX2, mediates pancreatic cancer cell growth in KRAS-transformed pancreatic ductal epithelial cell lines, through as yet unidentified mechanisms125.
Maintaining cell growth is necessary for cancer cell proliferation and survival. The PI3K–AKT–mTOR signalling pathway induces many responses in cells, including the maintenance of mRNA translation, cell survival and metabolism, and is activated in the majority of cancers126–131. The tumour suppressor PTEN, a dual lipid and tyrosine phosphatase, inhibits PI3K signalling and is mutated or post-translationally inactivated in cancer. As with most other PTPs, PTEN is susceptible to inhibition by ROS-mediated cysteine oxidation132. In normal cells that are stimulated with growth factors, PTEN has been identified as a primary target of NOX1-derived hydrogen peroxide; however, a role for NOX4-derived ROS has also been implicated in PTEN inactivation133–136. Although these studies were conducted in normal cells that were stimulated with growth factors, the overall observation is highly relevant for cancer, as inactivation of PTEN results in increased PI3K-dependent production of phosphati-dylinositol-3,4,5-trisphosphate and activation of AKT by phosphoinositide-dependent 1 (PDK1) at Thr308 and the mTORC2 complex at Ser473 (REFS 133,137–139). p22phox-based NOX-derived ROS production is enhanced in von Hippel–Lindau (VHL)-deficient renal carcinoma cells (RCC). Gene silencing of p22phox-based NOX oxidases in VHL-deficient cells inhibits phosphorylation of AKT on its mTORC2-dependent site, Ser473, suggesting a novel role of NOX oxidases in the regulation of mTORC2, through as yet unidentified mechanisms27,49. In addition, p22phox, probably through its interaction with NOX4 and NOX1, indirectly activates mTORC1 signalling downstream of PTEN through redox inactivation of tuberin in RCC cells. The tuberin–harmatin hetero-dimer is a key negative upstream regulator of mTORC1 signalling. When tuberin is phosphorylated by AKT on Thr1462 this results in its dissociation from hamartin with subsequent ubiquitin-mediated degradation140. Treatment of VHL-deficient cells with DPI or downregulation of p22phox results in decreased tuberin phosphorylation on Thr1462 and stabilizes tuberin protein levels27. Inactivation of tuberin by p22phox-based NOX oxidases results in mTORC1-dependent phosphorylation of initiation factor 4E-binding protein 1 (4EBP1) and ribosomal protein S6 kinase 1 (S6K), which are required for the stimulation of cap-dependent translation of key target genes that are involved in tumorigenesis27,49,141.
Glucose uptake is crucial for cancer cell growth and survival. Glucose transporters are upregulated in cancer cells and enhance glucose uptake. There are various mechanisms by which NOX oxidases facilitate glucose uptake, most of which have been studied in renal cancer cells. The VHL tumour suppressor protein is inactivated in ~70–80% of clear cell RCC cells. VHL is the substrate recognition subunit of a multimeric E3 ubiquitin ligase complex, which under normal oxygen conditions targets the α-subunits of the hypoxia-inducible factors (HIFα) for regulated protein degradation142–144. Post-translational hydroxylation of the HIFα subunits, by proline hydroxylases, is necessary for binding of the VHL–E3 complex145. In the absence of VHL, HIFα subunits are stabilized. However, in VHL-deficient RCC cells, maintaining HIF2α expression requires ongoing mRNA translation, as treatment of VHL-deficient cells with cyclohexamide, a general translation inhibitor, reduces HIFα protein expression49. NOX1- and NOX4-derived ROS levels are increased in VHL-deficient cells and maintain HIF2α expression through an AKT-dependent mRNA translational mechanism27,49,146. Importantly, HIF2α is a master transcriptional regulator of genes that are involved in cell growth, survival and angiogenesis, suggesting an indirect role for NOX oxidases in the aformentioned biological processes. For example, HIF2α is the transcriptional regulator of the renal mitogen TGFα, suggesting an indirect role of NOX-derived ROS in RCC autonomous cell growth. Consistent with this, treatment of VHL-deficient RCC cells with the flavoprotein inhibitor DPI reduces HIF2α expression and cell growth49. Similarly, HIF2α regulates expression of glucose transporter 1 in RCC cells, suggesting a role for p22phox-based NOX oxidases in glucose uptake in VHL-deficient cells. Conversely, stabilization of HIFα-dependent glucose uptake in cancer cells that are VHL competent relies on mechanisms that inhibit proline hydroxylation. For example, fumarate hydratase (FH)-deficient renal cancer cells proliferate rapidly and require HIF1α-dependent glucose uptake for survival147,148. HIF1α is stabilized in FH-deficient cells, in spite of VHL expression, through p47phox-dependent NOX oxidase- and fumerate-dependent inhibition of proline hydroxylation148.
In leukaemic cells, however, NOX2- and NOX4- derived ROS promote glucose uptake, cell survival and proliferation through putative mechanisms involving SRC activation and translocation of GLUT1-containing vesicles, which is independent of HIF57,149.
Role of NOX-derived ROS in angiogenesis
Tumour angio-genesis is necessary for vascularizing solid tumours in order to provide nutrients and oxygen for growth past a small diameter of ~1–2 mm. New blood vessel formation, arising from the existing vasculature, requires angiogenic growth factors (such as vascular endothelial growth factor (VEGF), which activates matrix metal-loproteinases (MMPs)), the proliferation of endothelial cells and the formation of blood vessel tubules. VEGF is a target for therapeutic angiogenesis in many cancers, and evidence suggests that compartmentalized NOX-derived ROS coordinate the angiogenic switch by enabling increased VEGF production by tumour cells. This propagates VEGF autocrine (for survival and migration) and paracrine (for tumour angiogenesis) signalling106,150. These processes are initiated when a tumour experiences hypoxia. Human NOX4 and NOX2 promoters harbour putative hypoxia-responsive elements (HREs) to which HIFs bind under hypoxic conditions and which thus may be characterized as oxygen sensors that are activated when solid tumours experience low oxygen tension. Indeed, NOX4 and NOX2 mRNAs are upregulated in vitro and in vivo in response to stabilization of HIFs, whereas NOX1-based oxidase complexes are post-translationally activated under hypoxic conditions151–155. NOX1- and NOX4-generated ROS increase VEGF and VEGFR mRNA levels and other markers of the angiogenic switch, thereby promoting vascularization and rapid expansion of malignant melanomas, prostate cancer and ovarian cancer cells in chicken chorioallantoic membranes or in vivo xenograft mouse models72,75,156,157. In ovarian cancer cells, NOX4-derived ROS and mitochondrial-derived ROS were found to regulate VEGF levels through HIF1α expression, and both sources of ROS were found to be required for tumour-induced angiogenesis and tumour growth75. NOX-dependent upregulation of VEGF mRNA expression can also be independent of HIFα. In RAS-transformed colon cancer cells, silencing of NOX1 using small inhibitory RNAs, inhibits ERK-dependent phosphorylation of the transcription factor SP1, thereby inhibiting SP1-dependent upregulation of VEGF mRNA and neovascularization in nude mice158. The subcutaneous injection of melanoma cells or lung carcinoma cells in NOX1-deficient mice or in wild-type mice treated with GKT136901 (an inhibitor of NOX1 and NOX4) reduces tumour angiogenesis through AKT-dependent suppression of the anti-inflammatory nuclear hormone receptor peroxisome-proliferator activated receptor-α (PPARα), a negative regulator of nuclear factor-κB (NF-κB) signalling159. siRNA-mediated downregulation of NOX1 in cultured melanoma or lung carcinoma cells inhibits NF-κB activation of VEGF and MMPs159.
Paracrine activation of VEGFR2 in endothelial cells by VEGF that is secreted by tumour cells is initiated at caveoli and lipid rafts by NOX2-dependent complexes. In addition, biological events, such as proliferation, migration and tube formation, possibly involve the generation of ROS by NOX1- and NOX4-dependent complexes at sites of focal adhesions and membrane ruffles in endothelial cells106.
A role for NOX oxidases in cell invasion and metastasis
Tumour metastasis is a multistep process that requires extracellular remodelling and intracellular changes such as epithelial–mesenchymal transition (EMT), reduced cell adhesion and increased migration, as well as degradation of the extracellular matrix. The tumour environment is complex, with cancer cells surrounded by various other cell types, including stromal cells, endothelial cells and inflammatory cells, which secrete high levels of growth factors, such as TGFβ, and cytokines, such as interleukins, into the extracellular fluid. NOX oxidases are responsive to soluble mediators that are present in the extracellular fluid, which influence the stimulation of cell migration and invasion. Stromal cells secrete TGFβ when co-cultured with breast cancer epithelial cells, stimulating NOX4-dependent migration of epithelial cells160. However, tumour-associated macrophages (TAMs) secrete interleukin-1β (IL-1β) when co-cultured with breast cancer epithelial cells, stimulating NOX-derived ROS with subsequent activation of the SRC–MAPK–AP1 pathway in breast cancer cells, leading to the transcriptional upregulation of cyclooxygenase 2 (COX2) levels, which is associated with increased proliferation and the metastasis of breast cancer cells161. A more recent finding suggests that NOX4 has a role in cytokine production in renal cancer cells that are exposed to hypoxia162. Renal cancer cells, but not normal kidney cells, exposed to hypoxia secrete IL-6 and IL-8 in a NOX4-dependent manner. In turn, the exposure of renal cancer cells to IL-6 and IL-8 induces migration and invasion162.
Growth factors and cytokines that are present in the tumour milieu can alternatively negatively influence the antitumour ability of immune effector cells, such as T cells, in part through the expansion of myeloid-derived suppressor cells (MDSCs). MDSCs are immature myeloid progenitor cells that suppress T cell function in cancer. Interestingly, NOX2 is upregulated in a STAT3-dependent manner and hyperactivated in MDSCs in a variety of tumour models and human MDSCs163. MDSCs that were isolated from the spleens of EL-4 tumour-bearing NOX2-deficient mice failed to suppress T cell function and resulted in differentiated MDSC cells; however, the lack of NOX2 did not result in tumour rejection163. Taken together, and owing to the fact that NOX2 is highly expressed in infiltrating immune cells, these data suggest novel roles for NOX2 in cancer immunology, which should be further explored.
Agonists that enhance metastasis induce structural intracellular changes in tumour cells, such as the formation of invasive microdomains called invadopo-dia164. NOX1 and NOX4 are present in invadopodia, and NOX4 also localizes to focal adhesions35,106,164–166. The subcellular localization of NOX1 and NOX4 to these membrane domains allows the formation of active redox signalling platforms that concentrate NOX-derived ROS in a small area, allowing them to coordinate cell adhesion, migration and invasive matrix degradation35,106,165. SRC and the SRC family of kinases are also located in invadopodia or in focal adhesions and are crucial for the invasiveness and metastatic potential characteristic of some cancer cells164,166–170. SRC proteins can function as both upstream modulators and downstream effectors of NOX oxidases108,123,171. SRC contributes to the activation of NOX2 and NOX1 through phosphorylation of p47phox and NOXA1, respectively, or through the activation of the small GTPase RAC1, leading to the formation of an active NOX enzymatic complex123,171–173. Moreover, TKS4 and TKS5 are phosphorylated by SRC during NOX activation, which is necessary for invadopodia formation and NOX1- and NOX4-derived ROS generation at this microdomain37,58,164,174,175. Note that p22phox also seems to interact with TKS proteins in the NOX complex37. SRC is also reported to be a downstream target of NOX1-, NOX2- and NOX4-derived ROS108,176,177. NOX-dependent SRC regulation is predominantly mediated through the control of its tyrosine phosphorylation, which is regulated by PTP–PEST that is also present in invadopodia165,168.
Other factors, such as MMPs, are present at sites of invadopodia. The activation of MMPs by hydrogen peroxide has been demonstrated in several cancers and may be partly dependent on an RTK–PI3K–NF-κB signalling pathway178–180. Proteolytic degradation of the extracellular matrix has been shown to involve NOX-dependent upregulation of MMP expression and activity181. Binding of leukotriene B4 to BLT2 elicits NOX4-dependent ROS generation that mediates STAT3- and MMP2-dependent invasiveness and metastasis of ovarian cancer cells182. In RAS-transformed cells, the BLT2–NOX1–NF-κB–MMP9 cascade was also reported to be important for tumour cell invasion and metastasis183–185. Additionally, NOX1-derived ROS, NF-κB and MMP9 are required for the motility of KRAS-transformed normal kidney cells and for the EGF-mediated migration of colon cancer cells through NOX1-dependent inhibition of LMW-PTP, thus blocking RHO activity, a key regulator of cytoskeletal contractility186,187.
Taken together, NOX oxidases coordinate a number of biological responses that are involved in cellular invasion and metastasis through intimate redox regulation of SRC and NF-κB signalling at discrete subcellular sites, such as invadopodia, and diffusely through moderating communication in the tumour milieu.
Perspectives
NADPH oxidase complexes regulate broad, but specific, redox-signalling pathways in various cell types through the discrete localization of NOX-derived ROS to the plasma membrane, mitochondria, endosomes, focal adhesions, invadopodia, caveolae, endoplasmic reticulum and nuclei, which together coordinate the activation of oncogenes or the inactivation of tumour suppressor proteins that are involved in tumour cell growth and survival, angiogenesis and cell invasion. A summary of oncogenes activated by, and tumour suppressor proteins inactivated by, NOX-derived ROS in these processes is depicted in FIG. 2.
It should be emphasized that this area of cancer research is still in its infancy and the biochemical roles for specific NOX complexes in cancer that are relevant to DNA repair, cellular metabolism and the Warburg effect, microRNA regulation, cancer immunology and cancer stem cell biology remain unexplored. Much like the discovery of kinases and phosphatases, NOX-dependent redox post-translational modifications may represent the next major advances in cell and cancer biology. Identification of NOX target proteins should move the field forwards. The lack of available NOX knockout and transgenic animal models has hindered and will continue to hinder our understanding of the role of NOX oxidases in cancer. It is noteworthy that the gene encoding NOX5 is absent in the mouse genome.
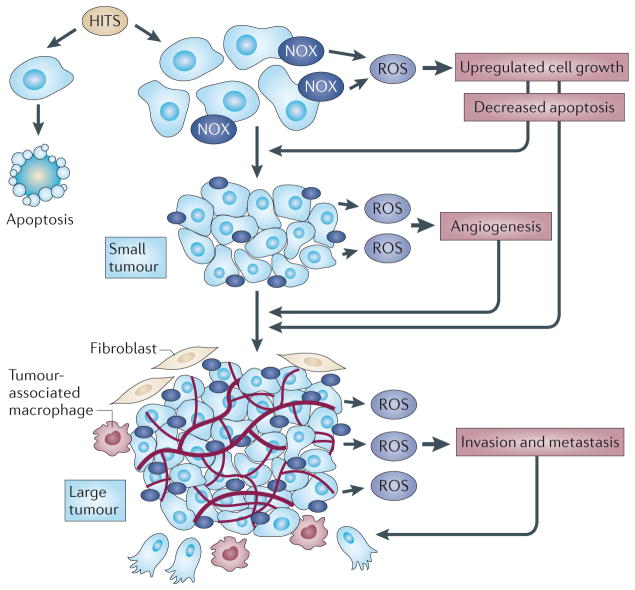
In summary, most of the studies discussed above are predominantly based on in vitro work, and so fail to address the biological complexity that exists in tumours, such as intratumour heterogeneity, microenvironmental changes and the systemic influence of factors, including growth factor levels, inflammation and nutrition. In these changing environments, some cells will gain function while others that have intact apoptotic pathways will undergo necrosis or apoptosis. As outlined in FIG. 3, we hypothesize that, owing to the pleio-tropic nature of NOX-derived ROS, tumour cells that upregulate NOX oxidases and NOX-derived ROS will progress and those that do not will necrose, apoptose or exhibit a biochemically benign phenotype. As the tumour progresses, NOX-derived ROS will maintain activated growth pathways, evade cell death and initiate angiogenesis and metastasis.
Figure 3. A hypothesis for the function of NOX-derived ROS in the progression of carcinogenesis.
Multiple genetic and epigenetic alterations (HITS) are required for cellular transformation. The cells that upregulate NADPH oxidase (NOX) oxidases and NOX-derived reactive oxygen species (ROS) will maintain unregulated cell growth, evade cell death and progress towards the development of a clinically relevant tumour. Cells that do not activate NOX-derived ROS and that have intact apoptotic pathways will undergo apoptosis or necrosis. In tumours of approximately ~1–2 mm in diameter, NOX oxidases sense hypoxia and can help to mediate activation of the angiogenic switch while maintaining unregulated cell growth and evasion of cell death. As the tumour continues to grow, a complex tumour environment, including stromal cells, endothelial cells and tumour-associated macrophages (TAMs), secrete various metastatic agonists. NOX oxidases are responsive to the extracellular fluid surrounding the tumour environment and facilitate invasion and metastasis.
At a glance.
As early as 1981, it was suggested that enzymes biochemically similar to the NADPH oxidase (NOX) NOX2 were present in all cell types, not just in neutrophils. A decade later, high levels of superoxide and hydrogen peroxide were found in various cancer cells, and these levels could be reduced by diphenyleneiodonium (DPI), a chemical inhibitor of flavoprotein-containing enzymes, such as NOX oxidases.
The mitochondrial electron transfer chain and NADPH oxidases of the NOX family are the two major sources of reactive oxygen species (ROS) that are implicated in cancer.
Seven membrane-bound NOX catalytic isoforms (NOX1–5) and dual oxidase 1 (DUOX1) and DUOX2 have been identified, each of which displays similar but distinct structural, biochemical and subcellular localization characteristics.
NOX-dependent redox signalling occurs at cellular membranes and intracellular structures where the NOX catalytic and regulatory subunits are localized. The NOX catalytic subunits NOX2, NOX1, NOX4 and NOX5 have been detected in the plasma membrane. NOX4 has additionally been detected in the endoplasmic reticulum, mitochondrial and nuclear membranes. NOX subunits also reside at specific subcellular microdomains, such as NOX4 at focal adhesions, NOX1 at caveoli and lipid rafts, and NOX1 and NOX4 at invadopodia.
NOX catalytic and regulatory subunits have been implicated in one or more of the most common cancer types. Increased mRNA and/or protein expression of NOX1, NOX2, NOX4 and NOX5 or their regulatory components have been detected at higher levels in various cultured cancer cell lines or human tumours compared with normal controls at early and late stages of tumorigenesis, suggesting that ROS derived from NADPH oxidases may be important in both the initiation and the maintenance phases of tumour development.
Initial studies indicate that NOX oxidases can effect several of the hallmarks of cancer, including genomic instability, autonomous growth and survival, angiogenesis, invasion and metastasis.
Acknowledgments
The authors acknowledge H. E. Abboud, R. A. Clark and R. Li for critical reading of the Review and F. Wauquier for unconditional assistance. Supported by Veterans Administration Career Development Award, NIH R01 NCI CA131272 (K.B.).
Glossary
- Focal adhesions
Specific types of large macromolecular assemblies through which both mechanical force and regulatory signals are transmitted
- Invadopodia
Protrusions of the plasma membrane that contain adhesive proteins and proteolytic enzymes
- Dismutation
A specific type of redox reaction in which a species is simultaneously reduced and oxidized to form two different products, in this case molecular oxygen and hydrogen peroxide
Footnotes
Competing interests statement
The authors declare no competing financial interests.
References
- 1.Knudson AG. Two genetic hits (more or less) to cancer. Nature Rev Cancer. 2001;1:157–162. doi: 10.1038/35101031. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Oberley LW, Oberley TD, Buettner GR. Cell division in normal and transformed cells: the possible role of superoxide and hydrogen peroxide. Med Hypotheses. 1981;7:21–42. doi: 10.1016/0306-9877(81)90018-9. The free radical theory of cancer is presented. [DOI] [PubMed] [Google Scholar]
- 5.McCord JM, Fridovich I. The utility of superoxide dismutase in studying free radical reactions. I. Radicals generated by the interaction of sulfite, dimethyl sulfoxide, and oxygen. J Biol Chem. 1969;244:6056–6063. This seminal paper describes the biochemical characterizaiton of superoxide dismutases involved in neutralizing ROS. [PubMed] [Google Scholar]
- 6.Dewald B, Baggiolini M, Curnutte JT, Babior BM. Subcellular localization of the superoxide-forming enzyme in human neutrophils. J Clin Invest. 1979;63:21–29. doi: 10.1172/JCI109273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bittinger F, et al. Production of superoxide by human malignant melanoma cells. Melanoma Res. 1998;8:381–387. doi: 10.1097/00008390-199810000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–798. This paper describes the detection of diphenyleneiodonium-inhibitable ROS in a large variety of cancer cells. [PubMed] [Google Scholar]
- 9.Ralph SJ, Rodriguez-Enriquez S, Neuzil J, Saavedra E, Moreno-Sanchez R. The causes of cancer revisited: “mitochondrial malignancy” and ROS-induced oncogenic transformation-why mitochondria are targets for cancer therapy. Mol Aspects Med. 2010;31:145–170. doi: 10.1016/j.mam.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Singh KK. Mitochondria damage checkpoint, aging, and cancer. Ann NY Acad Sci. 2006;1067:182–190. doi: 10.1196/annals.1354.022. [DOI] [PubMed] [Google Scholar]
- 11.Wallace DC. Mitochondria and cancer: Warburg addressed. Cold Spring Harb Symp Quant Biol. 2005;70:363–374. doi: 10.1101/sqb.2005.70.035. [DOI] [PubMed] [Google Scholar]
- 12.Barnes JL, Gorin Y. Myofibroblast differentiation during fibrosis: role of NAD(P)H oxidases. Kidney Int. 2011;79:944–956. doi: 10.1038/ki.2010.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. This is an extensive review of the structure and function of NOX oxidases. [DOI] [PubMed] [Google Scholar]
- 14.Drummond GR, Selemidis S, Griendling KK, Sobey CG. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nature Rev Drug Discov. 2011;10:453–471. doi: 10.1038/nrd3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banfi B, et al. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J Biol Chem. 2004;279:46065–46072. doi: 10.1074/jbc.M403046200. [DOI] [PubMed] [Google Scholar]
- 16.Banfi B, et al. A mammalian H+ channel generated through alternative splicing of the NADPH oxidase homolog NOH-1. Science. 2000;287:138–142. doi: 10.1126/science.287.5450.138. [DOI] [PubMed] [Google Scholar]
- 17.Cheng G, Cao Z, Xu X, van Meir EG, Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene. 2001;269:131–140. doi: 10.1016/s0378-1119(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 18.Dupuy C, et al. Purification of a novel flavoprotein involved in the thyroid NADPH oxidase. Cloning of the porcine and human cdnas. J Biol Chem. 1999;274:37265–37269. doi: 10.1074/jbc.274.52.37265. [DOI] [PubMed] [Google Scholar]
- 19.Geiszt M. NADPH oxidases: new kids on the block. Cardiovasc Res. 2006;71:289–299. doi: 10.1016/j.cardiores.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Geiszt M, Kopp JB, Varnai P, Leto TL. Identification of renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci USA. 2000;97:8010–8014. doi: 10.1073/pnas.130135897. This paper describes the cloning of the NOX isoform NOX4 and suggests that NOX4 is an oxygen sensor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lambeth JD, Kawahara T, Diebold B. Regulation of Nox and Duox enzymatic activity and expression. Free Radic Biol Med. 2007;43:319–331. doi: 10.1016/j.freeradbiomed.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shiose A, et al. A novel superoxide-producing NAD(P) H oxidase in kidney. J Biol Chem. 2001;276:1417–1423. doi: 10.1074/jbc.M007597200. [DOI] [PubMed] [Google Scholar]
- 23.Suh YA, et al. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 1999;401:79–82. doi: 10.1038/43459. This paper describes the cloning of the NOX isoform NOX1 and provides the first evidence that a NOX oxidase may mediate cellular transformation. [DOI] [PubMed] [Google Scholar]
- 24.Ginabreda MG, et al. Negative correlation between thyroperoxidase and dual oxidase H2O2-generating activities in thyroid nodular lesions. Eur J Endocrinol. 2008;158:223–227. doi: 10.1530/EJE-07-0602. [DOI] [PubMed] [Google Scholar]
- 25.Lacroix L, et al. Expression of nicotinamide adenine dinucleotide phosphate oxidase flavoprotein DUOX genes and proteins in human papillary and follicular thyroid carcinomas. Thyroid. 2001;11:1017–1023. doi: 10.1089/105072501753271699. [DOI] [PubMed] [Google Scholar]
- 26.Luxen S, Belinsky SA, Knaus UG. Silencing of DUOX NADPH oxidases by promoter hypermethylation in lung cancer. Cancer Res. 2008;68:1037–1045. doi: 10.1158/0008-5472.CAN-07-5782. [DOI] [PubMed] [Google Scholar]
- 27.Block K, Gorin Y, Abboud HE. Subcellular localization of Nox4 and regulation in diabetes. Proc Natl Acad Sci USA. 2009;106:14385–14390. doi: 10.1073/pnas.0906805106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borregaard N, Heiple JM, Simons ER, Clark RA. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J Cell Biol. 1983;97:52–61. doi: 10.1083/jcb.97.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Serrander L, et al. NOX5 is expressed at the plasma membrane and generates superoxide in response to protein kinase C activation. Biochimie. 2007;89:1159–1167. doi: 10.1016/j.biochi.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 30.Takac I, et al. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J Biol Chem. 2011;286:13304–13313. doi: 10.1074/jbc.M110.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Lohneysen K, Noack D, Jesaitis AJ, Dinauer MC, Knaus UG. Mutational analysis reveals distinct features of the Nox4-p22 phox complex. J Biol Chem. 2008;283:35273–35282. doi: 10.1074/jbc.M804200200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Buul JD, Fernandez-Borja M, Anthony EC, Hordijk PL. Expression and localization of NOX2 and NOX4 in primary human endothelial cells. Antioxid Redox Signal. 2005;7:308–317. doi: 10.1089/ars.2005.7.308. [DOI] [PubMed] [Google Scholar]
- 33.Ago T, et al. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res. 2010;106:1253–1264. doi: 10.1161/CIRCRESAHA.109.213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graham KA, et al. NADPH oxidase 4 is an oncoprotein localized to mitochondria. Cancer Biol Ther. 2010;10:223–231. doi: 10.4161/cbt.10.3.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24:677–683. doi: 10.1161/01.ATV.0000112024.13727.2c. [DOI] [PubMed] [Google Scholar]
- 36.Cheng G, Lambeth JD. NOXO1, regulation of lipid binding, localization, and activation of Nox1 by the Phox homology (PX) domain. J Biol Chem. 2004;279:4737–4742. doi: 10.1074/jbc.M305968200. [DOI] [PubMed] [Google Scholar]
- 37.Diaz B, et al. Tks5-dependent, nox-mediated generation of reactive oxygen species is necessary for invadopodia formation. Sci Signal. 2009;2:ra53. doi: 10.1126/scisignal.2000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burnham DN, Uhlinger DJ, Lambeth JD. Diradylglycerol synergizes with an anionic amphiphile to activate superoxide generation and phosphorylation of p47phox in a cell-free system from human neutrophils. J Biol Chem. 1990;265:17550–17559. [PubMed] [Google Scholar]
- 39.Nauseef WM, McCormick S, Renee J, Leidal KG, Clark RA. Functional domain in an arginine-rich carboxyl-terminal region of p47phox. J Biol Chem. 1993;268:23646–23651. [PubMed] [Google Scholar]
- 40.Leto TL, Morand S, Hurt D, Ueyama T. Targeting and regulation of reactive oxygen species generation by Nox family NADPH oxidases. Antioxid Redox Signal. 2009;11:2607–2619. doi: 10.1089/ars.2009.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 42.Banfi B, Clark RA, Steger K, Krause KH. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J Biol Chem. 2003;278:3510–3513. doi: 10.1074/jbc.C200613200. [DOI] [PubMed] [Google Scholar]
- 43.Brandes RP, Schroder K. Composition and functions of vascular nicotinamide adenine dinucleotide phosphate oxidases. Trends Cardiovasc Med. 2008;18:15–19. doi: 10.1016/j.tcm.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 44.Brandes RP, Weissmann N, Schroder K. NADPH oxidases in cardiovascular disease. Free Radic Biol Med. 2010;49:687–706. doi: 10.1016/j.freeradbiomed.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 45.Lassegue B, Griendling KK. NADPH oxidases: functions and pathologies in the vasculature. Arterioscler Thromb Vasc Biol. 2010;30:653–661. doi: 10.1161/ATVBAHA.108.181610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Selemidis S, Sobey CG, Wingler K, Schmidt HH, Drummond GR. NADPH oxidases in the vasculature: molecular features, roles in disease and pharmacological inhibition. Pharmacol Ther. 2008;120:254–291. doi: 10.1016/j.pharmthera.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 47.Lyle AN, et al. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ Res. 2009;105:249–259. doi: 10.1161/CIRCRESAHA.109.193722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morand S, et al. Duox maturation factors form cell surface complexes with Duox affecting the specificity of reactive oxygen species generation. FASEB J. 2009;23:1205–1218. doi: 10.1096/fj.08-120006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Block K, et al. NAD(P)H oxidases regulate HIF-2α protein expression. J Biol Chem. 2007;282:8019–8026. doi: 10.1074/jbc.M611569200. [DOI] [PubMed] [Google Scholar]
- 50.Brar SS, et al. NOX5 NAD(P)H oxidase regulates growth and apoptosis in DU 145 prostate cancer cells. Am J Physiol Cell Physiol. 2003;285:C353–C369. doi: 10.1152/ajpcell.00525.2002. [DOI] [PubMed] [Google Scholar]
- 51.Brar SS, et al. An NAD(P)H oxidase regulates growth and transcription in melanoma cells. Am J Physiol Cell Physiol. 2002;282:C1212–C1224. doi: 10.1152/ajpcell.00496.2001. [DOI] [PubMed] [Google Scholar]
- 52.Choi JA, et al. Pro-survival of estrogen receptor-negative breast cancer cells is regulated by a BLT2-reactive oxygen species-linked signaling pathway. Carcinogenesis. 2010;31:543–551. doi: 10.1093/carcin/bgp203. [DOI] [PubMed] [Google Scholar]
- 53.Huang HS, Liu ZM, Chen PC, Tseng HY, Yeh BW. TG-interacting factor-induced superoxide production from NADPH oxidase contributes to the migration/invasion of urothelial carcinoma. Free Radic Biol Med. doi: 10.1016/j.freeradbiomed.2012.06.014. (in the press) [DOI] [PubMed] [Google Scholar]
- 54.Huang WC, Li X, Liu J, Lin J, Chung LW. Activation of androgen receptor, lipogenesis, and oxidative stress converged by SREBP-1 is responsible for regulating growth and progression of prostate cancer cells. Mol Cancer Res. 2012;10:133–142. doi: 10.1158/1541-7786.MCR-11-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Juhasz A, et al. Expression of NADPH oxidase homologues and accessory genes in human cancer cell lines, tumours and adjacent normal tissues. Free Radic Res. 2009;43:523–532. doi: 10.1080/10715760902918683. mRNA expression levels of NOX catalytic and regulatory subunits are presented in a large variety of cultured cancer cell lines and tissues and are compared with normal controls. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kumar B, Koul S, Khandrika L, Meacham RB, Koul HK. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res. 2008;68:1777–1785. doi: 10.1158/0008-5472.CAN-07-5259. [DOI] [PubMed] [Google Scholar]
- 57.Prata C, et al. Nox-generated ROS modulate glucose uptake in a leukaemic cell line. Free Radic Res. 2008;42:405–414. doi: 10.1080/10715760802047344. [DOI] [PubMed] [Google Scholar]
- 58.Seals DF, et al. The adaptor protein Tks5/Fish is required for podosome formation and function, and for the protease-driven invasion of cancer cells. Cancer Cell. 2005;7:155–165. doi: 10.1016/j.ccr.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 59.Shimada K, Fujii T, Anai S, Fujimoto K, Konishi N. ROS generation via NOX4 and its utility in the cytological diagnosis of urothelial carcinoma of the urinary bladder. BMC Urol. 2011;11:22. doi: 10.1186/1471-2490-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vaquero EC, Edderkaoui M, Pandol SJ, Gukovsky I, Gukovskaya AS. Reactive oxygen species produced by NAD(P)H oxidase inhibit apoptosis in pancreatic cancer cells. J Biol Chem. 2004;279:34643–34654. doi: 10.1074/jbc.M400078200. This paper describes the first evidence that NOX4- derived ROS mediates cell survival in cancer cells. [DOI] [PubMed] [Google Scholar]
- 61.Yamaura M, et al. NADPH oxidase 4 contributes to transformation phenotype of melanoma cells by regulating G2-M cell cycle progression. Cancer Res. 2009;69:2647–2654. doi: 10.1158/0008-5472.CAN-08-3745. [DOI] [PubMed] [Google Scholar]
- 62.Arnold RS, et al. Nox1 expression determines cellular reactive oxygen and modulates c-fos-induced growth factor, interleukin-8, and Cav-1. Am J Pathol. 2007;171:2021–2032. doi: 10.2353/ajpath.2007.061144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bauer KM, Hummon AB, Buechler S. Right-side and left-side colon cancer follow different pathways to relapse. Mol Carcinog. 2012;51:411–421. doi: 10.1002/mc.20804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Block K, et al. The NADPH oxidase subunit p22phox inhibits the function of the tumor suppressor protein tuberin. Am J Pathol. 2010;176:2447–2455. doi: 10.2353/ajpath.2010.090606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Desouki MM, Kulawiec M, Bansal S, Das GM, Singh KK. Cross talk between mitochondria and superoxide generating NADPH oxidase in breast and ovarian tumors. Cancer Biol Ther. 2005;4:1367–1373. doi: 10.4161/cbt.4.12.2233. This paper demonstrates the existence of crosstalk between the mitochondria and NADPH oxidase in cancer. [DOI] [PubMed] [Google Scholar]
- 66.Fritz G, Just I, Kaina B. Rho GTPases are over-expressed in human tumors. Int J Cancer. 1999;81:682–687. doi: 10.1002/(sici)1097-0215(19990531)81:5<682::aid-ijc2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 67.Fukuyama M, et al. Overexpression of a novel superoxide-producing enzyme, NADPH oxidase 1, in adenoma and well differentiated adenocarcinoma of the human colon. Cancer Lett. 2005;221:97–104. doi: 10.1016/j.canlet.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 68.Gomez del Pulgar T, Benitah SA, Valeron PF, Espina C, Lacal JC. Rho GTPase expression in tumourigenesis: evidence for a significant link. Bioessays. 2005;27:602–613. doi: 10.1002/bies.20238. [DOI] [PubMed] [Google Scholar]
- 69.Hoffmann M, et al. A functional polymorphism in the NAD(P)H oxidase subunit CYBA is related to gene expression, enzyme activity, and outcome in non-Hodgkin lymphoma. Cancer Res. 2010;70:2328–2338. doi: 10.1158/0008-5472.CAN-09-2388. [DOI] [PubMed] [Google Scholar]
- 70.Kamiguti AS, et al. Expression and activity of NOX5 in the circulating malignant B cells of hairy cell leukemia. J Immunol. 2005;175:8424–8430. doi: 10.4049/jimmunol.175.12.8424. [DOI] [PubMed] [Google Scholar]
- 71.Lan Q, et al. Genetic polymorphisms in the oxidative stress pathway and susceptibility to non-Hodgkin lymphoma. Hum Genet. 2007;121:161–168. doi: 10.1007/s00439-006-0288-9. [DOI] [PubMed] [Google Scholar]
- 72.Lim SD, et al. Increased Nox1 and hydrogen peroxide in prostate cancer. Prostate. 2005;62:200–207. doi: 10.1002/pros.20137. [DOI] [PubMed] [Google Scholar]
- 73.Shimada K, et al. A novel human AlkB homologue, ALKBH8, contributes to human bladder cancer progression. Cancer Res. 2009;69:3157–3164. doi: 10.1158/0008-5472.CAN-08-3530. [DOI] [PubMed] [Google Scholar]
- 74.Weyemi U, et al. Intracellular expression of reactive oxygen species-generating NADPH oxidase NOX4 in normal and cancer thyroid tissues. Endocr Relat Cancer. 2010;17:27–37. doi: 10.1677/ERC-09-0175. [DOI] [PubMed] [Google Scholar]
- 75.Xia C, et al. Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res. 2007;67:10823–10830. doi: 10.1158/0008-5472.CAN-07-0783. [DOI] [PubMed] [Google Scholar]
- 76.Durak I, Canbolat O, Kavutcu M, Ozturk HS, Yurtarslani Z. Activities of total, cytoplasmic, and mitochondrial superoxide dismutase enzymes in sera and pleural fluids from patients with lung cancer. J Clin Lab Anal. 1996;10:17–20. doi: 10.1002/(SICI)1098-2825(1996)10:1<17::AID-JCLA4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 77.Bostwick DG, et al. Antioxidant enzyme expression and reactive oxygen species damage in prostatic intraepithelial neoplasia and cancer. Cancer. 2000;89:123–134. [PubMed] [Google Scholar]
- 78.Curtis CD, Thorngren DL, Nardulli AM. Immunohistochemical analysis of oxidative stress and DNA repair proteins in normal mammary and breast cancer tissues. BMC Cancer. 2010;10:9. doi: 10.1186/1471-2407-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Evans P, Halliwell B. Free radicals and hearing. Cause, consequence, and criteria. Ann NY Acad Sci. 1999;884:19–40. doi: 10.1111/j.1749-6632.1999.tb08633.x. [DOI] [PubMed] [Google Scholar]
- 80.Novo E, Parola M. Redox mechanisms in hepatic chronic wound healing and fibrogenesis. Fibrogenesis Tissue Repair. 2008;1:5. doi: 10.1186/1755-1536-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kartha GK, et al. Renal mitochondrial damage and protein modification in type-2 diabetes. Acta Diabetol. 2008;45:75–81. doi: 10.1007/s00592-008-0025-z. [DOI] [PubMed] [Google Scholar]
- 82.Spencer NY, et al. Control of hepatic nuclear superoxide production by glucose 6-phosphate dehydrogenase and NADPH oxidase-4. J Biol Chem. 2011;286:8977–8987. doi: 10.1074/jbc.M110.193821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Weyemi U, et al. ROS-generating NADPH oxidase NOX4 is a critical mediator in oncogenic H-Ras-induced DNA damage and subsequent senescence. Oncogene. 2012;31:1117–1129. doi: 10.1038/onc.2011.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Burdon RH. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radic Biol Med. 1995;18:775–794. doi: 10.1016/0891-5849(94)00198-s. [DOI] [PubMed] [Google Scholar]
- 85.Burdon RH, Gill V, Rice-Evans C. Oxidative stress and tumour cell proliferation. Free Radic Res Commun. 1990;11:65–76. doi: 10.3109/10715769009109669. [DOI] [PubMed] [Google Scholar]
- 86.Burdon RH, Rice-Evans C. Free radicals and the regulation of mammalian cell proliferation. Free Radic Res Commun. 1989;6:345–358. doi: 10.3109/10715768909087918. [DOI] [PubMed] [Google Scholar]
- 87.Bae YS, et al. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J Biol Chem. 1997;272:217–221. [PubMed] [Google Scholar]
- 88.Mahadev K, et al. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol Cell Biol. 2004;24:1844–1854. doi: 10.1128/MCB.24.5.1844-1854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 90.Chen K, Kirber MT, Xiao H, Yang Y, Keaney JF., Jr Regulation of ROS signal transduction by NADPH oxidase 4 localization. J Cell Biol. 2008;181:1129–1139. doi: 10.1083/jcb.200709049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lyle AN, Griendling KK. Modulation of vascular smooth muscle signaling by reactive oxygen species. Physiology. 2006;21:269–280. doi: 10.1152/physiol.00004.2006. [DOI] [PubMed] [Google Scholar]
- 92.Mesquita FS, et al. Reactive oxygen species mediate mitogenic growth factor signaling pathways in human leiomyoma smooth muscle cells. Biol Reprod. 2010;82:341–351. doi: 10.1095/biolreprod.108.075887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wagner B, et al. Mitogenic signaling via platelet-derived growth factor β in metanephric mesenchymal cells. J Am Soc Nephrol. 2007;18:2903–2911. doi: 10.1681/ASN.2006111229. [DOI] [PubMed] [Google Scholar]
- 94.Reddy MM, et al. NADPH oxidases regulate cell growth and migration in myeloid cells transformed by oncogenic tyrosine kinases. Leukemia. 2011;25:281–289. doi: 10.1038/leu.2010.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sastry SK, Elferink LA. Checks and balances: interplay of RTKs and PTPs in cancer progression. Biochem Pharmacol. 2011;82:435–440. doi: 10.1016/j.bcp.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 96.Rudolph TK, Freeman BA. Transduction of redox signaling by electrophile-protein reactions. Sci Signal. 2009;2:re7. doi: 10.1126/scisignal.290re7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chiarugi P, Cirri P. Redox regulation of protein tyrosine phosphatases during receptor tyrosine kinase signal transduction. Trends Biochem Sci. 2003;28:509–514. doi: 10.1016/S0968-0004(03)00174-9. [DOI] [PubMed] [Google Scholar]
- 98.Lee JK, et al. NADPH oxidase promotes pancreatic cancer cell survival via inhibiting JAK2 dephosphorylation by tyrosine phosphatases. Gastroenterology. 2007;133:1637–1648. doi: 10.1053/j.gastro.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 99.Lee SR, Kwon KS, Kim SR, Rhee SG. Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor. J Biol Chem. 1998;273:15366–15372. doi: 10.1074/jbc.273.25.15366. [DOI] [PubMed] [Google Scholar]
- 100.Woo HA, et al. Reversing the inactivation of peroxiredoxins caused by cysteine sulfinic acid formation. Science. 2003;300:653–656. doi: 10.1126/science.1080273. [DOI] [PubMed] [Google Scholar]
- 101.Lou YW, et al. Redox regulation of the protein tyrosine phosphatase PTP1B in cancer cells. FEBS J. 2008;275:69–88. doi: 10.1111/j.1742-4658.2007.06173.x. [DOI] [PubMed] [Google Scholar]
- 102.Groen A, et al. Differential oxidation of protein-tyrosine phosphatases. J Biol Chem. 2005;280:10298–10304. doi: 10.1074/jbc.M412424200. [DOI] [PubMed] [Google Scholar]
- 103.Rhee SG, Chang TS, Bae YS, Lee SR, Kang SW. Cellular regulation by hydrogen peroxide. J Am Soc Nephrol. 2003;14:S211–S215. doi: 10.1097/01.asn.0000077404.45564.7e. [DOI] [PubMed] [Google Scholar]
- 104.Wright VP, Reiser PJ, Clanton TL. Redox modulation of global phosphatase activity and protein phosphorylation in intact skeletal muscle. J Physiol. 2009;587:5767–5781. doi: 10.1113/jphysiol.2009.178285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chan EC, Jiang F, Peshavariya HM, Dusting GJ. Regulation of cell proliferation by NADPH oxidase-mediated signaling: potential roles in tissue repair, regenerative medicine and tissue engineering. Pharmacol Ther. 2009;122:97–108. doi: 10.1016/j.pharmthera.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 106.Ushio-Fukai M. Localizing NADPH oxidase-derived ROS. Sci STKE. 2006;2006:re8. doi: 10.1126/stke.3492006re8. [DOI] [PubMed] [Google Scholar]
- 107.Almo SC, et al. Structural genomics of protein phosphatases. J Struct Funct Genomics. 2007;8:121–140. doi: 10.1007/s10969-007-9036-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sancho P, Fabregat I. NADPH oxidase NOX1 controls autocrine growth of liver tumor cells through up-regulation of the epidermal growth factor receptor pathway. J Biol Chem. 2010;285:24815–24824. doi: 10.1074/jbc.M110.114280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nitsche C, et al. The phosphatase PHLPP1 regulates Akt2, promotes pancreatic cancer cell death, and inhibits tumor formation. Gastroenterology. 2012;142:377–387.e5. doi: 10.1053/j.gastro.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Naughton R, Quiney C, Turner SD, Cotter TG. Bcr-Abl-mediated redox regulation of the PI3K/AKT pathway. Leukemia. 2009;23:1432–1440. doi: 10.1038/leu.2009.49. [DOI] [PubMed] [Google Scholar]
- 111.Seo JM, et al. Up-regulation of BLT2 is critical for the survival of bladder cancer cells. Exp Mol Med. 2011;43:129–137. doi: 10.3858/emm.2011.43.3.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mochizuki T, et al. Inhibition of NADPH oxidase 4 activates apoptosis via the AKT/apoptosis signal-regulating kinase 1 pathway in pancreatic cancer PANC-1 cells. Oncogene. 2006;25:3699–3707. doi: 10.1038/sj.onc.1209406. [DOI] [PubMed] [Google Scholar]
- 113.Luo J, et al. Negative control of p53 by Sir2α promotes cell survival under stress. Cell. 2001;107:137–148. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- 114.Puca R, et al. Nox1 is involved in p53 deacetylation and suppression of its transcriptional activity and apoptosis. Free Radic Biol Med. 2010;48:1338–1346. doi: 10.1016/j.freeradbiomed.2010.02.015. This is the first example that NOX1 activates SIRT1 deacetylase, which inhibits p53-dependent cell apoptosis. [DOI] [PubMed] [Google Scholar]
- 115.Vaziri H, et al. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–159. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- 116.Olmos Y, Brosens JJ, Lam EW. Interplay between SIRT proteins and tumour suppressor transcription factors in chemotherapeutic resistance of cancer. Drug Resist Updat. 2011;14:35–44. doi: 10.1016/j.drup.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 117.Frescas D, Valenti L, Accili D. Nuclear trapping of the forkhead transcription factor FoxO1 via Sirt-dependent deacetylation promotes expression of glucogenetic genes. J Biol Chem. 2005;280:20589–20595. doi: 10.1074/jbc.M412357200. [DOI] [PubMed] [Google Scholar]
- 118.Laurent E, et al. Nox1 is over-expressed in human colon cancers and correlates with activating mutations in K-Ras. Int J Cancer. 2008;123:100–107. doi: 10.1002/ijc.23423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Heo J. Redox control of GTPases: from molecular mechanisms to functional significance in health and disease. Antioxid Redox Signal. 2011;14:689–724. doi: 10.1089/ars.2009.2984. [DOI] [PubMed] [Google Scholar]
- 120.Lander HM, Ogiste JS, Teng KK, Novogrodsky A. p21ras as a common signaling target of reactive free radicals and cellular redox stress. J Biol Chem. 1995;270:21195–21198. doi: 10.1074/jbc.270.36.21195. [DOI] [PubMed] [Google Scholar]
- 121.Adachi Y, et al. Oncogenic Ras upregulates NADPH oxidase 1 gene expression through MEK-ERK-dependent phosphorylation of GATA-6. Oncogene. 2008;27:4921–4932. doi: 10.1038/onc.2008.133. [DOI] [PubMed] [Google Scholar]
- 122.Mitsushita J, Lambeth JD, Kamata T. The superoxide-generating oxidase Nox1 is functionally required for Ras oncogene transformation. Cancer Res. 2004;64:3580–3585. doi: 10.1158/0008-5472.CAN-03-3909. This paper describes the cooperation of RAS and NOX1 in cellular transformation. [DOI] [PubMed] [Google Scholar]
- 123.Kajla S, et al. A crucial role for Nox 1 in redox-dependent regulation of Wnt-β-catenin signaling. FASEB J. 2012;26:2049–2059. doi: 10.1096/fj.11-196360. [DOI] [PubMed] [Google Scholar]
- 124.Rimerman RA, Gellert-Randleman A, Diehl JA. Wnt1 and MEK1 cooperate to promote cyclin D1 accumulation and cellular transformation. J Biol Chem. 2000;275:14736–14742. doi: 10.1074/jbc.m910241199. [DOI] [PubMed] [Google Scholar]
- 125.Du J, et al. Regulation of pancreatic cancer growth by superoxide. Mol Carcinog. 2012 Mar 5; doi: 10.1002/mc.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 127.Luo J, Manning BD, Cantley LC. Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer Cell. 2003;4:257–262. doi: 10.1016/s1535-6108(03)00248-4. [DOI] [PubMed] [Google Scholar]
- 128.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pópulo H, Lopes JM, Soares P. The mTOR signalling pathway in human cancer. Int J Mol Sci. 2012;13:1886–1918. doi: 10.3390/ijms13021886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Porta C, Figlin RA. Phosphatidylinositol-3-kinase/Akt signaling pathway and kidney cancer, and the therapeutic potential of phosphatidylinositol-3-kinase/Akt inhibitors. J Urol. 2009;182:2569–2577. doi: 10.1016/j.juro.2009.08.085. [DOI] [PubMed] [Google Scholar]
- 131.Silvera D, Formenti SC, Schneider RJ. Translational control in cancer. Nature Rev Cancer. 2010;10:254–266. doi: 10.1038/nrc2824. [DOI] [PubMed] [Google Scholar]
- 132.Lee SR, et al. Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem. 2002;277:20336–20342. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]
- 133.Coant N, et al. NADPH oxidase 1 modulates WNT and NOTCH1 signaling to control the fate of proliferative progenitor cells in the colon. Mol Cell Biol. 2010;30:2636–2650. doi: 10.1128/MCB.01194-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cui W, et al. NOX1/nicotinamide adenine dinucleotide phosphate, reduced form (NADPH) oxidase promotes proliferation of stellate cells and aggravates liver fibrosis induced by bile duct ligation. Hepatology. 2011;54:949–958. doi: 10.1002/hep.24465. [DOI] [PubMed] [Google Scholar]
- 135.Kwon J, et al. Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc Natl Acad Sci USA. 2004;101:16419–16424. doi: 10.1073/pnas.0407396101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nisbet RE, et al. Rosiglitazone attenuates chronic hypoxia-induced pulmonary hypertension in a mouse model. Am J Respir Cell Mol Biol. 2010;42:482–490. doi: 10.1165/rcmb.2008-0132OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gan X, Wang J, Su B, Wu D. Evidence for direct activation of mTORC2 kinase activity by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 2011;286:10998–11002. doi: 10.1074/jbc.M110.195016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lamouille S, Connolly E, Smyth JW, Akhurst RJ, Derynck R. TGF-β-induced activation of mTOR complex 2 drives epithelial-mesenchymal transition and cell invasion. J Cell Sci. 2012;125:1259–1273. doi: 10.1242/jcs.095299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zinzalla V, Stracka D, Oppliger W, Hall MN. Activation of mTORC2 by association with the ribosome. Cell. 2011;144:757–768. doi: 10.1016/j.cell.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 140.Plas DR, Thompson CB. Akt activation promotes degradation of tuberin and FOXO3a via the proteasome. J Biol Chem. 2003;278:12361–12366. doi: 10.1074/jbc.M213069200. [DOI] [PubMed] [Google Scholar]
- 141.Sonenberg N. Translation factors as effectors of cell growth and tumorigenesis. Curr Opin Cell Biol. 1993;5:955–960. doi: 10.1016/0955-0674(93)90076-3. [DOI] [PubMed] [Google Scholar]
- 142.Clifford SC, et al. The pVHL-associated SCF ubiquitin ligase complex: molecular genetic analysis of elongin B and C, Rbx1 and HIF-1α in renal cell carcinoma. Oncogene. 2001;20:5067–5074. doi: 10.1038/sj.onc.1204602. [DOI] [PubMed] [Google Scholar]
- 143.Kaelin WG., Jr The von hippel-lindau tumor suppressor protein: an update. Methods Enzymol. 2007;435:371–383. doi: 10.1016/S0076-6879(07)35019-2. [DOI] [PubMed] [Google Scholar]
- 144.Pause A, et al. The von Hippel-Lindau tumor-suppressor gene product forms a stable complex with human CUL-2, a member of the Cdc53 family of proteins. Proc Natl Acad Sci USA. 1997;94:2156–2161. doi: 10.1073/pnas.94.6.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Min JH, et al. Structure of an HIF-1α-pVHL complex: hydroxyproline recognition in signaling. Science. 2002;296:1886–1889. doi: 10.1126/science.1073440. [DOI] [PubMed] [Google Scholar]
- 146.Maranchie JK, Zhan Y. Nox4 is critical for hypoxia-inducible factor 2-α transcriptional activity in von Hippel-Lindau-deficient renal cell carcinoma. Cancer Res. 2005;65:9190–9193. doi: 10.1158/0008-5472.CAN-05-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Isaacs JS, et al. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell. 2005;8:143–153. doi: 10.1016/j.ccr.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 148.Sudarshan S, et al. Fumarate hydratase deficiency in renal cancer induces glycolytic addiction and hypoxia-inducible transcription factor 1α stabilization by glucose-dependent generation of reactive oxygen species. Mol Cell Biol. 2009;29:4080–4090. doi: 10.1128/MCB.00483-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Maraldi T, et al. VEGF-induced ROS generation from NAD(P)H oxidases protects human leukemic cells from apoptosis. Int J Oncol. 2010;36:1581–1589. doi: 10.3892/ijo_00000645. [DOI] [PubMed] [Google Scholar]
- 150.Ushio-Fukai M, Nakamura Y. Reactive oxygen species and angiogenesis: NADPH oxidase as target for cancer therapy. Cancer Lett. 2008;266:37–52. doi: 10.1016/j.canlet.2008.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Diebold I, Petry A, Hess J, Gorlach A. The NADPH oxidase subunit NOX4 is a new target gene of the hypoxia-inducible factor-1. Mol Biol Cell. 2010;21:2087–2096. doi: 10.1091/mbc.E09-12-1003. This is the first paper to show that hypoxia-inducible factors regulate NOX4 gene expression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Diebold I, et al. The HIF1 target gene NOX2 promotes angiogenesis through urotensin-II. J Cell Sci. 2012;125:956–964. doi: 10.1242/jcs.094060. [DOI] [PubMed] [Google Scholar]
- 153.Goyal P, et al. Upregulation of NAD(P)H oxidase 1 in hypoxia activates hypoxia-inducible factor 1 via increase in reactive oxygen species. Free Radic Biol Med. 2004;36:1279–1288. doi: 10.1016/j.freeradbiomed.2004.02.071. [DOI] [PubMed] [Google Scholar]
- 154.Tojo T, et al. Role of gp91phox (Nox2)-containing NAD(P)H oxidase in angiogenesis in response to hindlimb ischemia. Circulation. 2005;111:2347–2355. doi: 10.1161/01.CIR.0000164261.62586.14. [DOI] [PubMed] [Google Scholar]
- 155.Vallet P, et al. Neuronal expression of the NADPH oxidase NOX4, and its regulation in mouse experimental brain ischemia. Neuroscience. 2005;132:233–238. doi: 10.1016/j.neuroscience.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 156.Arbiser JL, et al. Reactive oxygen generated by Nox1 triggers the angiogenic switch. Proc Natl Acad Sci USA. 2002;99:715–720. doi: 10.1073/pnas.022630199. This paper is the first evidence that NOX1 is involved in tumour angiogenesis in vitro and in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Govindarajan B, et al. Overexpression of Akt converts radial growth melanoma to vertical growth melanoma. J Clin Invest. 2007;117:719–729. doi: 10.1172/JCI30102. This paper describes the cooperation of AKT and NOX4 in invasive melanoma cell growth. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Komatsu D, Kato M, Nakayama J, Miyagawa S, Kamata T. NADPH oxidase 1 plays a critical mediating role in oncogenic Ras-induced vascular endothelial growth factor expression. Oncogene. 2008;27:4724–4732. doi: 10.1038/onc.2008.102. [DOI] [PubMed] [Google Scholar]
- 159.Garrido-Urbani S, et al. Targeting vascular NADPH oxidase 1 blocks tumor angiogenesis through a PPARα mediated mechanism. PLoS ONE. 2011;6:e14665. doi: 10.1371/journal.pone.0014665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tobar N, Guerrero J, Smith PC, Martinez J. NOX4-dependent ROS production by stromal mammary cells modulates epithelial MCF-7 cell migration. Br J Cancer. 2010;103:1040–1047. doi: 10.1038/sj.bjc.6605847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hou Z, Falcone DJ, Subbaramaiah K, Dannenberg AJ. Macrophages induce COX-2 expression in breast cancer cells: role of IL-1β autoamplification. Carcinogenesis. 2011;32:695–702. doi: 10.1093/carcin/bgr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fitzgerald JP, et al. Nox4 mediates renal cell carcinoma cell invasion through hypoxia-induced interleukin 6- and 8- production. PLoS ONE. 2012;7:e30712. doi: 10.1371/journal.pone.0030712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Corzo CA, et al. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J Immunol. 2009;182:5693–5701. doi: 10.4049/jimmunol.0900092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Courtneidge SA. Cell migration and invasion in human disease: the Tks adaptor proteins. Biochem Soc Trans. 2012;40:129–132. doi: 10.1042/BST20110685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Diaz B, Courtneidge SA. Redox signaling at invasive microdomains in cancer cells. Free Rad. Biol Med. 2012;52:247–256. doi: 10.1016/j.freeradbiomed.2011.09.016. This review outlines redox signalling events mediated by invadopodia NADPH oxidase complexes and their contribution to cancer cell invasion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wang Y, McNiven MA. Invasive matrix degradation at focal adhesions occurs via protease recruitment by a FAK-p130Cas complex. J Cell Biol. 2012;196:375–385. doi: 10.1083/jcb.201105153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Destaing O, Block MR, Planus E, Albiges-Rizo C. Invadosome regulation by adhesion signaling. Curr Opin Cell Biol. 2011;23:597–606. doi: 10.1016/j.ceb.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 168.Giannoni E, Taddei ML, Chiarugi P. Src redox regulation: again in the front line. Free Radic Biol Med. 2010;49:516–527. doi: 10.1016/j.freeradbiomed.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 169.Guarino M. Src signaling in cancer invasion. J Cell Physiol. 2010;223:14–26. doi: 10.1002/jcp.22011. [DOI] [PubMed] [Google Scholar]
- 170.Yeatman TJ. A renaissance for SRC. Nature Rev Cancer. 2004;4:470–480. doi: 10.1038/nrc1366. [DOI] [PubMed] [Google Scholar]
- 171.Gianni D, Taulet N, DerMardirossian C, Bokoch GM. c-Src-mediated phosphorylation of NoxA1 and Tks4 induces the reactive oxygen species (ROS)-dependent formation of functional invadopodia in human colon cancer cells. Mol Biol Cell. 2010;21:4287–4298. doi: 10.1091/mbc.E10-08-0685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Baumer AT, et al. Phosphatidylinositol 3-kinase-dependent membrane recruitment of Rac-1 and p47phox is critical for α-platelet-derived growth factor receptor-induced production of reactive oxygen species. J Biol Chem. 2008;283:7864–7876. doi: 10.1074/jbc.M704997200. [DOI] [PubMed] [Google Scholar]
- 173.Chowdhury AK, et al. Src-mediated tyrosine phosphorylation of p47phox in hyperoxia-induced activation of NADPH oxidase and generation of reactive oxygen species in lung endothelial cells. J Biol Chem. 2005;280:20700–20711. doi: 10.1074/jbc.M411722200. [DOI] [PubMed] [Google Scholar]
- 174.Gianni D, Bohl B, Courtneidge SA, Bokoch GM. The involvement of the tyrosine kinase c-Src in the regulation of reactive oxygen species generation mediated by NADPH oxidase-1. Mol Biol Cell. 2008;19:2984–2994. doi: 10.1091/mbc.E08-02-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gianni D, et al. Novel p47(phox)-related organizers regulate localized NADPH oxidase 1 (Nox1) activity. Sci Signal. 2009;2:ra54. doi: 10.1126/scisignal.2000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Block K, et al. Nox4 NAD(P)H oxidase mediates Src-dependent tyrosine phosphorylation of PDK-1 in response to angiotensin II: role in mesangial cell hypertrophy and fibronectin expression. J Biol Chem. 2008;283:24061–24076. doi: 10.1074/jbc.M803964200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Li Q, Zhang Y, Marden JJ, Banfi B, Engelhardt JF. Endosomal NADPH oxidase regulates c-Src activation following hypoxia/reoxygenation injury. Biochem J. 2008;411:531–541. doi: 10.1042/BJ20071534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Binker MG, Binker-Cosen AA, Richards D, Oliver B, Cosen-Binker LI. EGF promotes invasion by PANC-1 cells through Rac1/ROS-dependent secretion and activation of MMP-2. Biochem Biophys Res Commun. 2009;379:445–450. doi: 10.1016/j.bbrc.2008.12.080. [DOI] [PubMed] [Google Scholar]
- 179.Steinbrenner H, et al. Tumor promoter TPA stimulates MMP-9 secretion from human keratinocytes by activation of superoxide-producing NADPH oxidase. Free Radic Res. 2005;39:245–253. doi: 10.1080/10715760500053487. [DOI] [PubMed] [Google Scholar]
- 180.Yoon SO, Park SJ, Yoon SY, Yun CH, activates Chung AS. Sustained production of H2O2 pro-matrix metalloproteinase-2 through receptor tyrosine kinases/phosphatidylinositol 3-kinase/NF-κB pathway. J Biol Chem. 2002;277:30271–30282. doi: 10.1074/jbc.M202647200. [DOI] [PubMed] [Google Scholar]
- 181.Grote K, et al. Mechanical stretch enhances mRNA expression and proenzyme release of matrix metalloproteinase-2 (MMP-2) via NAD(P)H oxidase-derived reactive oxygen species. Circ Res. 2003;92:e80–e86. doi: 10.1161/01.RES.0000077044.60138.7C. [DOI] [PubMed] [Google Scholar]
- 182.Seo JM, Park S, Kim JH. Leukotriene B4 receptor-2 promotes invasiveness and metastasis of ovarian cancer cells through signal transducer and activator of transcription 3 (STAT3)-dependent up-regulation of matrix metalloproteinase 2. J Biol Chem. 2012;287:13840–13849. doi: 10.1074/jbc.M111.317131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Choi JA, Kim EY, Song H, Kim C, Kim JH. Reactive oxygen species are generated through a BLT2-linked cascade in Ras-transformed cells. Free Radic Biol Med. 2008;44:624–634. doi: 10.1016/j.freeradbiomed.2007.10.041. [DOI] [PubMed] [Google Scholar]
- 184.Kim EY, Seo JM, Cho KJ, Kim JH. Ras-induced invasion and metastasis are regulated by a leukotriene B4 receptor BLT2-linked pathway. Oncogene. 2010;29:1167–1178. doi: 10.1038/onc.2009.412. [DOI] [PubMed] [Google Scholar]
- 185.Kim EY, et al. BLT2 promotes the invasion and metastasis of aggressive bladder cancer cells through a reactive oxygen species-linked pathway. Free Radic Biol Med. 2010;49:1072–1081. doi: 10.1016/j.freeradbiomed.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 186.Shinohara M, et al. Reactive oxygen generated by NADPH oxidase 1 (Nox1) contributes to cell invasion by regulating matrix metalloprotease-9 production and cell migration. J Biol Chem. 2010;285:4481–4488. doi: 10.1074/jbc.M109.071779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Yang JQ, et al. v-Ha-RaS oncogene upregulates the 92-kDa type IV collagenase (MMP-9) gene by increasing cellular superoxide production and activating NF-κB. Free Radic Biol Med. 2001;31:520–529. doi: 10.1016/s0891-5849(01)00613-x. [DOI] [PubMed] [Google Scholar]