Abstract
In this editorial, we integrate improved understanding of functional and structural brain stem anatomy with lessons learned from other disciplines on brainstem function to provide an alternative interpretation to the data used to support the brainstem migraine generator theory.
Keywords: Dorsal pons, PAG, headache, trigeminovascular, spinal trigeminal nucleus
Introduction
A large body of scientific evidence support the view that migraine pathophysiology involves inherited abnormal brain functions (1), that the initiation of the headache phase of a migraine attack depends on flow of nociceptive signals that originate in pain-sensitive cranial organs conveyed through peripheral nociceptors to central trigeminovascular neurons (2), and that the transition from episodic to chronic migraine and perhaps the duration of an attack depend on dysfunctional brainstem nuclei that modulate neuronal excitability and pain (3). In contrast, the enticing and provocative hypothesis that inherently dysfunctional brainstem nuclei constitute a so-called migraine generator lacks convincing evidence. Through this review we attempt to convince the reader that there is currently little or no evidence to support the brainstem migraine generator theory, that brainstem activation is not specific to migraine headache (4), and that the periaqueductal gray (PAG) is not activated during migraine. Through careful examination of upto-date understanding of the brainstem, we propose to replace this somewhat simplistic view with the more sophisticated concept that activation in nearby nuclei in the dorsolateral pons (DLP) reflects a potential role for the DLP in facial and pericranial muscle tenderness, abnormal tactile sensation, transmission of nociceptive signals to the hypothalamus, amygdala and basal forebrain, motion sickness and nausea, altered auditory perception and, perhaps most importantly, modulation of pain.
The ongoing debate over the validity of the brainstem migraine generator theory
One of the leading theories on the origin of migraine headache suggests that the cause of this malady lies in abnormal functioning of the brainstem PAG. Theoretically, defected PAG functioning can either enhance activity of neurons that facilitate pain transmission in the dorsal horn more than usual, or suppress activity of neurons that inhibit pain transmission in the dorsal horn less than usual (5). This theory received a big boost when the first imaging studies on headache became available. Following the publication of ‘Brain stem activation in spontaneous human migraine attacks’ in 1995 (6), the notion that the PAG is involved in migraine has become widespread even though the PAG was not defined as a region showing activation in that report.
Proponents of this theory, which was first conceived in a clinical study in which implementation of stimulating electrodes in the PAG of 175 intractable pain patients resulted in short-lasting (12 patients) or long-lasting (three patients) post-operative migraine-like headache (7), contend its validity based on (a) imaging studies in which activation was noted in the dorsal rostral brainstem during spontaneous migraine attacks (6,8) and (b) three case reports of patients whose episodic headache was believed to be associated with pontine cavernous angioma that extended over the dorsal rostral pons and the PAG (9–11). Scientifically, there is much logic and evidence to propose that diminished modulation by the PAG can weaken the inhibitory signals that help determine the magnitude and duration of activity in nociceptive trigeminovascular neurons. This is not the case, however, when proposing that the PAG itself is a migraine generator. To advance the brainstem migraine generator theory from the opinion phase to being evidence based, answers should be provided to questions such as: (a) How would increased activation in the PAG drive or produce migraine? (b) Are there any connections with the PAG that can de-novo activate pain pathways? (c) Given that the PAG is positioned to modulate pain processing at all spinal cord segments through indirect projections to laminae I–II, V and X (12), how and why mechanistically might dysfunctional PAG generate headache but not pain in other bodily areas?
In the absence of answers to these questions, opponents of this theory assert (a) that in the Raskin et al. study (7), electrical stimulation of the PAG did not trigger a headache in 174/175 patients (in fact they produced varying levels of pain relief in 160 patients), that persistent post-operative headache lasting 3 months or longer is routinely observed in 9–38% of patients undergoing craniotomy for a wide variety of procedures with or without electrode placement (13–17); (b) that dorsal pons/PAG activation was caused by the nociceptive input it receives from activated trigeminovascular neurons during migraine; and (c) that migraine-like headache seen in patients diagnosed with brainstem cavernous angioma, which may result in brainstem damage, is not different from the migraine-like headache seen in patients diagnosed with cavernous angiomas anywhere else in the brain (18).
In the absence of convincing scientific evidence, the ongoing debate over the validity of the brainstem migraine generator theory continues to be a hot topic of debate. This debate should continue to press researchers to try to find data to support or refute the notion of a brainstem migraine generator. Direct evidence for the brainstem migraine generator theory should include, for example the following: (i) repeated and consistent initiation of migraine-like headache within minutes or seconds of stimulating candidate brainstem nuclei in non-migraineurs; (ii) repeated and consistent initiation of migraine-like headache within minutes or seconds of stimulating candidate brainstem nuclei in migraineurs; (iii) imaging of abnormal/altered brainstem activity before onset of headache that is followed by increased activity in brain areas that contain central trigeminovascular neurons immediately after the onset of headache and absence of any altered/enhanced activity in the trigeminal ganglion and the dorsal root ganglia of C2 and C3; (iv) initiation of migraine in individuals in whom the trigeminal ganglion and the dorsal root ganglia of C2 and C3 were densely blocked during the aura or prodrome phase; (v) long-lasting activation of completely quiescent trigeminovascular neurons by stimulating candidate brainstem nuclei; or (vi) post-mortem histopathology showing potential anatomical and/or molecular abnormalities in candidate brainstem nuclei as compared between migraineurs and non-migraineurs.
A mistaken identity: migraine-associated dorsal pons activation does not include the PAG
In 1995 Weiller and colleagues (6) published a paper in which they imaged brain activity in nine individuals during spontaneous migraine attacks. Using positron emission tomography, they described enhanced activity in several brain regions including the DLP (Figure 1). Given the authors’ scientific interest in the brainstem, they legitimately suggested that the ‘the pathogenesis of migraine is related to an imbalance in activity between brainstem nuclei regulating antinociception’ (such as the PAG). They did not actually note PAG activation. Throughout the 15 years that followed the publication of this paper, it was considered the strongest evidence for the validity of the PAG ‘brainstem migraine generator theory’ (8,19–25).
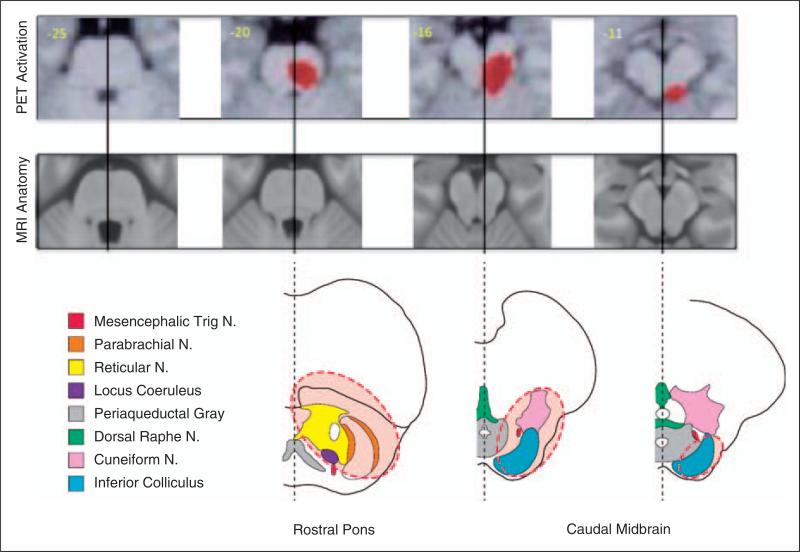
Figure 1.
Anatomical correlation with dorsal pons activation. To re-examine the dorsal pons activation images from (6) we superimposed them on MRI anatomical and brainstem atlas slices [adapted from Duvernoy's Atlas of the Human Brainstem and Cerebellum (34)] of the same region. The area highlighted by dashed lines (in red) corresponds to the bold signal in the fMRI images on top. It can be seen that there is minimal, if any, activation in the PAG. Instead, activation is seen in the cuneiform nucleus, rostral trigeminal nuclear complex, the locus coeruleus and the inferior colliculus. N., nucleus.
Imaging studies provide overwhelming evidence against PAG involvement in the brainstem migraine generator
Three sets of evidence have led us to question the theory that the PAG can ‘generate’ migraine: lack of PAG activation in migraine, lack of specificity to migraine, and lack of specificity to pain.
Dorsal pons activation in migraine
A careful review of the pattern of activation seen in the DLP of migraine patients is summarized in Table 1. As noted, the PAG area is not reported or included as an area activated.
Table 1.
Studies reporting brainstem activation in migraine patients.
| Study type | Stimulus | DLP activation | PAG activation | References |
|---|---|---|---|---|
| fMRI | Odor in migraine patients | + | – | (65) |
| fMRI | Heat in migraine patients | – Cuneiform nucleus | – | (3) |
| PET | Migraine patients | + Persisted with sumatriptan | – | (67) |
| PET | Migraine patients | + | – | (68) |
| PET | Migraine patients | + Persisted with sumatriptan | – | (6) |
| PET | Migraine patients | + | – | (8) |
fMRI: functional magnetic resonance imaging, PET: positron emission tomography.
Activation in DLP is not specific to migraine
Examination of non-migraine pain studies suggest that DLP activation is not specific to migraine (26–28). In fact, it is commonly seen in patients diagnosed with neuropathic pain and in individuals in whom mild somatic (midline lower abdomen skin) and visceral (rectum) pain is induced experimentally by electrical stimuli (Table 2 and Figure 2).
Table 2.
Examples of studies reporting brainstem activation in pain.
| Study type | Stimulus | DLP activation | PAG activation | References |
|---|---|---|---|---|
| fMRI | Somatic and visceral | + | – | (26) |
| fMRI | Cold in neuropathic pain | + | + | (27) |
| fMRI | Pain expectation | + Cuneiform nucleus | – | (59) |
| PET | Placebo effects on μ opioid activity | + Cuneiform nucleus | + | (56) |
fMRI: functional magnetic resonance imaging, PET: positron emission tomography.
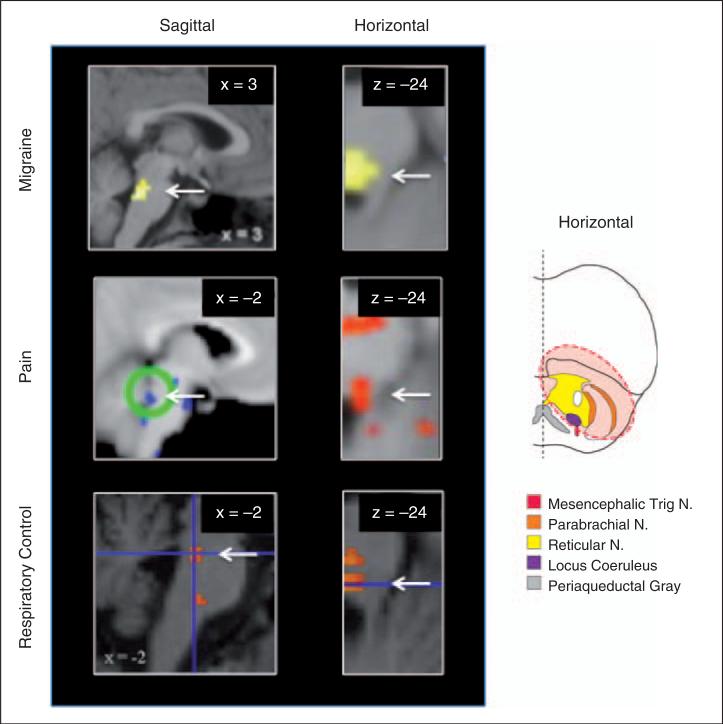
Figure 2.
Activation in the DLP across different conditions as measured by functional magnetic resonance imaging (fMRI). Examples of DLP activation in migraine (top, reproduced from (65) with permission), neuropathic pain in response to cold (middle left image, reproduced from (27) with permission), and experimentally induced pain (middle right image, reproduced from (26) with permission) and in breath holding (bottom, reproduced from (32) with permission). Note that the horizontal slice for breath holding has been flipped vertically from the original to align with the other figure. The x and y coordinates are provided in the top left hand corner for each slice. The diagram on the right provides a reference point to the level of the 3 horizontal sections. White arrows indicate location of DLP. N, nucleus.
Activation in DLP is not specific to pain
Furthermore, examination of non-pain functional imaging studies suggest that DLP activation is also not specific to pain (Figure 2, bottom images; Table 3). It is activated in response to bladder distention (29), empathy-related recognition and expression of emotions (30), changes in heart rate, heart rate variability, and plasma catecholamines during rectal distention (31), visceral stimulation in patients diagnosed with irritable bowel syndrome (26,28), breath holding (32) and sympathetic-nerve-related activity (33).
Table 3.
Examples of studies reporting brainstem activation in non-pain conditions.
| Study type | Stimulus | DLP activation | PAG activation | References |
|---|---|---|---|---|
| fMRI | Bladder fullness | + | + | (29) |
| fMRI | Alexithymia/empathy | + | – | (30) |
| fMRI | Autonomic changes | + | + | (31) |
| fMRI | Autonomic changes (respiratory control) | + | + | (69) |
| fMRI | Blue light | + (Locus coeruleus) | – | (70) |
| fMRI | Tinnitus | + (Inferior colliculus) | – | (71) |
| fMRI | Breath holding | + (Inferior colliculus) | – | (32) |
fMRI: functional magnetic resonance imaging.
If not a migraine generator, what is the putative role of the DLP region in migraine?
To identify the putative role of the DLP region in migraine, we outline below all the anatomical nuclei within the ‘dorsolateral pons’ and then attempt to assign to them potential functions in migraine.
Anatomical identification
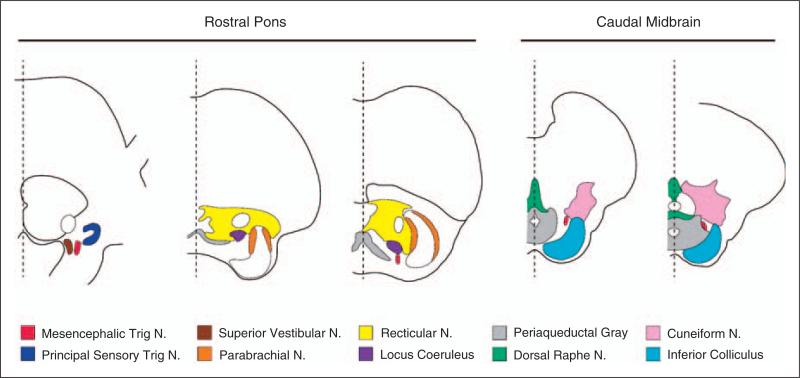
As summarized in Figure 3, anatomical sections of the rostral DLP and caudal midbrain (34) include the mesencephalic and principal sensory trigeminal nuclei, the dorsolateral pontine reticular nucleus, locus coeruleus (LC), the parabrachial nuclei, the cuneiform nucleus, the vestibular nuclei, and the inferior colliculus.
Figure 3.
Detailed anatomy of the rostral dorsolateral pons and caudal midbrain. Identification of nuclei follow reference (34). N, nucleus.
Functional considerations
Mesencephalic trigeminal nucleus
This nucleus contains primary sensory neurons that innervate facial and cranial muscles (35). These primary afferent neurons participate in regulating facial motor activity, such as jaw opening and proprioception of extraocular eye muscles. They are also involved in periodontal sensation (36,37). A role in migraine is unknown.
Principal sensory trigeminal nucleus
This region of the brainstem trigeminal nuclear complex receives afferent information relating to light touch of the face and proprioceptive information from the jaw (38). In human imaging studies it is activated mainly by innocuous stimulation (39,40), and although there is some anatomical evidence for activation by noxious stimulation (41) experiments in monkeys suggest that the principal sensory nucleus is not essential for facial pain sensation (42). Although a role in migraine is unknown, it may be involved in the altered tactile sensation that develops in the scalp and facial skin during migraine.
Reticular nucleus
The dorsolateral pontine reticular nucleus contains cholinergic neurons (43) and, although its role is poorly understood, it is thought to be involved in the integration of information that is passed from the cerebral to the cerebellar cortex. A role in migraine is unknown.
Locus coeruleus
The LC is the largest noradrenergic nucleus in the brain. Through heavy innervation of several forebrain regions, it is thought to have a role in a number of vital functions, including wakefulness, responses to stress, regulation of emotion, opioid therapy rapid behavioral adaptation to changing environmental imperatives (44–47). Although a specific role in migraine is unknown, LC involvement in the inhibition of nociceptive reflexes (48) and firing of thalamic and prefrontal cortex neurons in response to noxious stimuli (49) raise the possibility that it is involved in normal (and perhaps abnormal) pain modulation during migraine.
Parabrachial nucleus
The parabrachial nuclei constitute one of the better-understood relay brainstem nuclei for several modalities of sensory perception, including pain. They receive selective input from lamina I neurons at all spinal cord segments and the medullary dorsal horn and project to a variety of brain areas (e.g., basal ganglia, amygdala, hypothalamus) involved in homeostasis and regulation of affective, autonomic and endocrine responses (50–54). Together with evidence for selective activation of parabrachial neurons by dural stimulation (55), it is reasonable to speculate that the parabrachial nuclei are activated during migraine and that such activity may be involved in altered autonomic functions and amygdala-mediated emotions, such as anger and fear.
Cuneiform nucleus
The cuneiform nucleus is seen as one component of a functional circuit that forms the basis for descending modulation of pain by the brainstem (26,56–58). Given the notion that defective modulatory pain pathways contribute significantly to the patho-physiology of migraine, it is reasonable to propose a role along these lines. Given that the cuneiform nucleus is activated when patients merely expect the pain to come (59), it may not be surprising that it is abnormally active during the interictal state (3).
Vestibular nucleus
The vestibular nucleus is believed to mediate motion sickness symptoms such as nausea and dizziness, which are some of the hallmarks of migraine headache (60,61). Activation of vestibular nuclei during migraine may thus be associated with the nausea and dizziness.
Inferior colliculus
The inferior colliculus receives auditory signals from the cochlear nuclei, establishes reciprocal connections with the auditory cortex, and as such has an important role in auditory perception (62,63). Given the large number of patients who complain about abnormal intolerance to noise during migraine, it may be that at least a part of this hypersensitivity is mediated by the inferior colliculus and that the activation of this area during migraine reflects abnormal processing of auditory signals.
Brainstem involvement in migraine relates to oscillatory state of allostatic load: a proposal
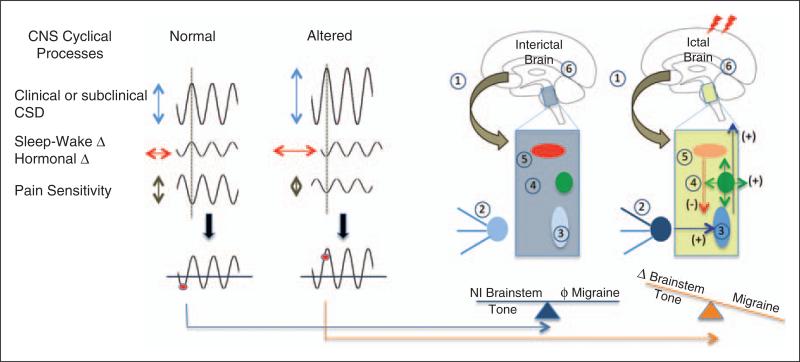
As migraine depends on environmental conditions, genetic predisposition and physiological functions, the susceptibility of an actual migraine attack may be determined by allostatic load (defined in this context as the wear and tear of brain functions as a result of too much stress or ineffcient management of stress) (64). Figure 4 illustrates a model in which initiation is determined by elements such as cortical spreading depression, oscillatory susceptibility, insuffciency modulation of nociceptive inputs by the PAG, or vascular drivers. Specifically, we propose that in the susceptible person, the onset of migraine must coincide with a the cyclic rhythmicity of brainstem activity that is regulated by the effort to maintain homeostasis (Figure 5).
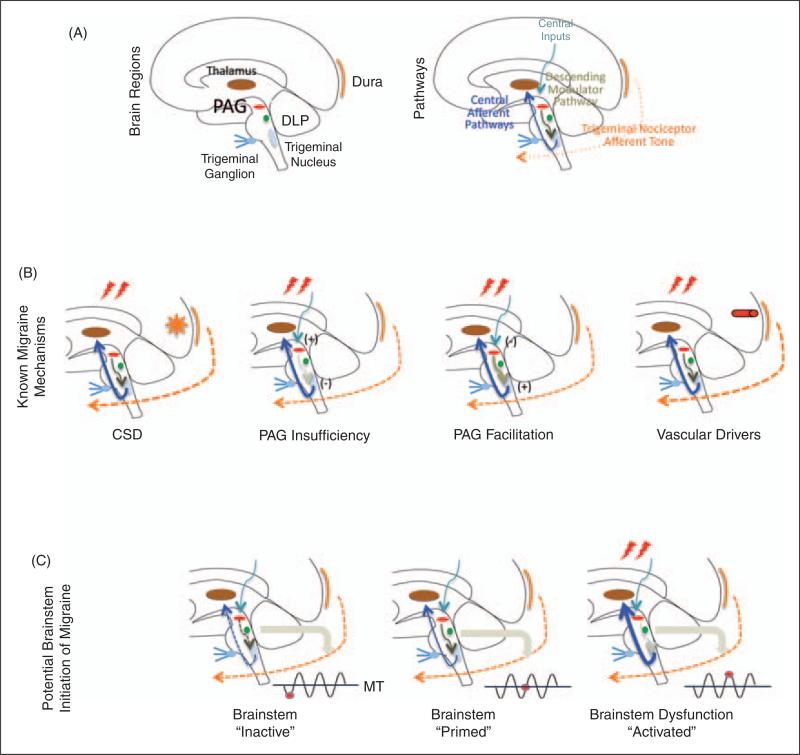
Figure 4.
(A) The migraine circuit, showing peripheral and central structures involved in nociceptive drive (dura, trigeminal afferents, trigeminal ganglion and trigeminal nucleus), modulation of nociceptive inputs (DLP, PAG) and cortical processing (thalamus, primary somatosensory cortex). (B) Principles that govern brainstem modulation of nociceptive drive during migraine. Activation of nociceptors, for example by cortical spreading depression (CSD), mild head trauma in migraine patients or inflammation, triggers activity in central trigeminovascular neurons. The magnitude of activation is then enhanced by insufficient synaptic inhibition due to PAG deficiency or due to abnormally enhanced synaptic strength caused by overactive pain facilitatory neurons in the PAG. (C) Brainstem ‘state of tone’ can limit afferent nociceptive drive in migraine-susceptible individuals. Brainstem activity, like that in other parts of the brain, fluctuates over time and such fluctuations may correlate with functional processing (66) that may be adaptive or not. This applies to modulation of nociceptive signals. The effectiveness or gating of these signals (whether they exceed brainstem tone and are therefore inhibited) depends on the threshold of neural networks that modify these afferent signals. Thus, the robustness of the ‘gate’ that allows nociceptive signals to drive central trigeminovascular neurons (and thus headache) is dictated by brainstem tone. Since tone may vary over time (diurnal, stress, hormonal, sympathetic drive, cortical and subcortical influences, etc.), the threshold to limit afferent signals will vary: when the brainstem tone is high (red dot below line of migraine threshold (MT), nociceptive signals are inhibited or limited; and when the brainstem tone is low (red dot above MT), afferent signals are not effectively blocked. Three functional brainstem states are defined in this model: 1) normal state: when cyclical phase brainstem activity is high (less sensitive to stimuli), the potency of pain facilitation (enhanced synaptic strength in the dorsal horn) is too high to allow normal nociceptive signals from the periphery to ‘drive’ the central neurons into the active state (left); 2) threshold state: At threshold, the system has reached a primed state that could tip into a functional state that would allow for nociceptive drive from the dura to activate the central trigeminovascular neurons (middle); 3) migraine state: When cyclical brainstem activity is high (more sensitive to stimuli), nociceptive signals from the periphery ‘drive’ the central neurons into the active state (right). In migraineurs, the state of brainstem tone may be unstable or less robust than in healthy individuals by prophylactic and thus they may be susceptible to activation of the cascade of networks that trigger the migraine attack by normal afferent nociceptive signals, which would be inhibited in healthy individuals or prophylactic treatment.
Figure 5.
CNS cyclical process and brainstem thresholds. Genetic, physiological, pharmacological, social and other interactions define migraineurs’ susceptibility. When processes are in synchrony (a harmonic or repetitive frequency), the model suggests that the migraine potential is sub-threshold (red circle); however, when these are altered either in magnitude, phase or duration, the system becomes unstable and the migraine threshold is exceeded. These components affect the brainstem tone as shown on the right of the figure for the interictal state: 1) cortical processes affect subcortical processing; 2) afferent input through the trigeminal ganglion is normal; 3) trigeminal nucleus function is normal/unchallenged; 4) DLP function is normal or not activated; 5) PAG functioning is not challenged; 6) brain systems acting on the brainstem are in a balanced tone. During migraine, however, we postulate the following cascade of events: 1) normal cortical inhibition of brainstem pathways is altered; 2) there is an afferent barrage of nociceptive input from trigeminal ganglion neurons to 3) the spinal trigeminal nucleus; 4) there is increased activation of the DLP; 5) there is diminished brainstem inhibition of nociceptive signals resulting from altered modulatory/inhibitory tone by the PAG; and 6) all this results in increased afferent inputs to higher brain centers that themselves may be sensitized or affected by brainstem nuclei.
Conclusions
Careful synthesis of imaging data that show activation of the DLP during migraine suggests that previous attempts to assign to this region a specific role had unintentionally narrowed the scope of our thinking. Rather than limiting the academic debate to the brainstem migraine generator theory, we should broaden our scientific assessment of the DLP to include potential involvement in many other processes; facial and pericranial muscle tenderness (mesencephalic trigeminal nucleus); abnormal tactile sensation (principal sensory trigeminal nucleus); transmission of nociceptive signals to the hypothalamus, amygdala and basal forebrain, areas that are involved in regulation of endocrine (parabrachial nucleus); motion sickness and nausea (vestibular nucleus); altered auditory perception (inferior colliculus); and modulation of pain (LC and cuneiform nucleus). Existing evidence clearly supports the notion that the brainstem has an important role in the complex pathophysiology of migraine headache. The topic debated in this commentary is not whether it is involved in migraine pathophysiology but what specific role the brainstem has in migraine headache.
Acknowledgements
We thank Eric Moulton, Center for Pain and the Brain, Harvard Medical School for the brain cartoon. We thank M Jakubowski for his helpful comments on the manuscript and his help with the design of the figures.
Funding
This work has been supported by NIH grants to DB (K24 NS064050; R01 NS056195; R01 NS073997) and RB (R01 NS069847; R01 NS051484; P01 NS035611).
References
- 1.Terwindt GM, Ophoff RA, Haan J, Sandkuijl LA, Frants RR, Ferrari MD. Migraine, ataxia and epilepsy: a challenging spectrum of genetically determined calcium channelopathies. Dutch Migraine Genetics Research Group. Eur J Hum Genet. 1998;6:297–307. doi: 10.1038/sj.ejhg.5200206. [DOI] [PubMed] [Google Scholar]
- 2.Olesen J, Burstein R, Ashina M, Tfelt-Hansen P. Origin of pain in migraine: evidence for peripheral sensitisation. Lancet Neurol. 2009;8:679–690. doi: 10.1016/S1474-4422(09)70090-0. [DOI] [PubMed] [Google Scholar]
- 3.Moulton EA, Burstein R, Tully S, Hargreaves R, Becerra L, Borsook D. Interictal dysfunction of a brainstem descending modulatory center in migraine patients. PLoS One. 2008;3:e3799. doi: 10.1371/journal.pone.0003799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linnman C, Moulton EA, Barmettler G, Becerra L, Borsook D. Neuroimaging of the periaqueductal gray: State of the field. Neuroimage. 2011;60:505–522. doi: 10.1016/j.neuroimage.2011.11.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25:319–325. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- 6.Weiller C, May A, Limmroth V, Juptner M, Kaube H, Schayck RV, et al. Brain stem activation in spontaneous human migraine attacks. Nat Med. 1995;1:658–660. doi: 10.1038/nm0795-658. [DOI] [PubMed] [Google Scholar]
- 7.Raskin NH, Hosobuchi Y, Lamb S. Headache may arise from perturbation of brain. Headache. 1987;27:416–420. doi: 10.1111/j.1526-4610.1987.hed2708416.x. [DOI] [PubMed] [Google Scholar]
- 8.Bahra A, Matharu MS, Buchel C, Frackowiak RS, Goadsby PJ. Brainstem activation specific to migraine headache. Lancet. 2001;357:1016–1017. doi: 10.1016/s0140-6736(00)04250-1. [DOI] [PubMed] [Google Scholar]
- 9.Katsarava Z, Egelhof T, Kaube H, Diener HC, Limmroth V. Symptomatic migraine and sensitization of trigeminal nociception associated with contralateral pontine cavernoma. Pain. 2003;105:381–384. doi: 10.1016/s0304-3959(03)00203-3. [DOI] [PubMed] [Google Scholar]
- 10.Afridi S, Goadsby PJ. New onset migraine with a brain stem cavernous angioma. J Neurol Neurosurg Psychiatry. 2003;74:680–682. doi: 10.1136/jnnp.74.5.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goadsby PJ. Neurovascular headache and a midbrain vascular malformation: evidence for a role of the brainstem in chronic migraine. Cephalalgia. 2002;22:107–111. doi: 10.1046/j.1468-2982.2002.00323.x. [DOI] [PubMed] [Google Scholar]
- 12.Fields HL, Malick A, Burstein R. Dorsal horn projection targets of ON and OFF cells in the rostral ventromedial medulla. J Neurophysiol. 1995;74:1742–1759. doi: 10.1152/jn.1995.74.4.1742. [DOI] [PubMed] [Google Scholar]
- 13.Schaller B, Baumann A. Headache after removal of vestibular schwannoma via the retrosigmoid approach: a long-term follow-up-study. Otolaryngol Head Neck Surg. 2003;128:387–395. doi: 10.1067/mhn.2003.104. [DOI] [PubMed] [Google Scholar]
- 14.Harner SG, Beatty CW, Ebersold MJ. Headache after acoustic neuroma excision. Am J Otol. 1993;14:552–555. [PubMed] [Google Scholar]
- 15.Gokalp HZ, Arasil E, Erdogan A, Egemen N, Deda H, Cerci A. Tentorial meningiomas. Neurosurgery. 1995;36:46–51. doi: 10.1227/00006123-199501000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Kaur A, Selwa L, Fromes G, Ross DA. Persistent headache after supratentorial craniotomy. Neurosurgery. 2000;47:633–636. doi: 10.1097/00006123-200009000-00020. [DOI] [PubMed] [Google Scholar]
- 17.Gee JR, Ishaq Y, Vijayan N. Postcraniotomy headache. Headache. 2003;43:276–278. doi: 10.1046/j.1526-4610.2003.03053.x. [DOI] [PubMed] [Google Scholar]
- 18.Sakakibara Y, Taguchi Y, Uchida K. [A case of cavernous angioma presenting as migrainous attack]. No Shinkei Geka. 2010;38:287–291. [PubMed] [Google Scholar]
- 19.Diener HC, May A. New aspects of migraine pathophysiology: lessons learned from positron emission tomography. Curr Opin Neurol. 1996;9:199–201. doi: 10.1097/00019052-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Welch KM, Nagesh V, Aurora SK, Gelman N. Periaqueductal gray matter dysfunction in migraine: cause or the burden of illness? Headache. 2001;41:629–637. doi: 10.1046/j.1526-4610.2001.041007629.x. [DOI] [PubMed] [Google Scholar]
- 21.Tajti J, Uddman R, Edvinsson L. Neuropeptide localization in the “migraine generator” region of the human brainstem. Cephalalgia. 2001;21:96–101. doi: 10.1046/j.1468-2982.2001.00140.x. [DOI] [PubMed] [Google Scholar]
- 22.Ebersberger A. [Pathophysiology of migraine: models to explain the generation of migraine headache]. Anaesthesist. 2002;51:661–667. doi: 10.1007/s00101-002-0342-5. [DOI] [PubMed] [Google Scholar]
- 23.Tfelt-Hansen PC, Koehler PJ. One hundred years of migraine research: major clinical and scientific observations from 1910 to 2010. Headache. 2011;51:752–778. doi: 10.1111/j.1526-4610.2011.01892.x. [DOI] [PubMed] [Google Scholar]
- 24.Lambert GA, Zagami AS. The mode of action of migraine triggers: a hypothesis. Headache. 2009;49:253–275. doi: 10.1111/j.1526-4610.2008.01230.x. [DOI] [PubMed] [Google Scholar]
- 25.May A. [The window into headache research: what have we learned from functional and structural neuroimaging]. Schmerz. 2010;24:130–136. doi: 10.1007/s00482-010-0898-y. [DOI] [PubMed] [Google Scholar]
- 26.Dunckley P, Wise RG, Fairhurst M, Hobden P, Aziz Q, Chang L, Tracey I. A comparison of visceral and somatic pain processing in the human brainstem using functional magnetic resonance imaging. J Neurosci. 2005;25:7333–7341. doi: 10.1523/JNEUROSCI.1100-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Becerra L, Morris S, Bazes S, Gostic R, Sherman S, Gostic J, et al. Trigeminal neuropathic pain alters responses in CNS circuits to mechanical (brush) and thermal (cold and heat) stimuli. J Neurosci. 2006;26:10646–10657. doi: 10.1523/JNEUROSCI.2305-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naliboff BD, Berman S, Chang L, Derbyshire SW, Suyenobu B, Vogt BA, et al. Sex-related differences in IBS patients: central processing of visceral stimuli. Gastroenterology. 2003;124:1738–1747. doi: 10.1016/s0016-5085(03)00400-1. [DOI] [PubMed] [Google Scholar]
- 29.Xiang B, Biji S, Liu JX, Chu WC, Yeung DK, Yeung CK. Functional brainstem changes in response to bladder function alteration elicited by surgical reduction in bladder capacity: a functional magnetic resonance imaging study. J Urol. 2010;184:2186–2191. doi: 10.1016/j.juro.2010.06.095. [DOI] [PubMed] [Google Scholar]
- 30.Moriguchi Y, Decety J, Ohnishi T, Maeda M, Mori T, Nemoto K, et al. Empathy and judging other's pain: an fMRI study of alexithymia. Cereb Cortex. 2007;17:2223–2234. doi: 10.1093/cercor/bhl130. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki H, Watanabe S, Hamaguchi T, Mine H, Terui T, Kanazawa M, et al. Brain activation associated with changes in heart rate, heart rate variability, and plasma catecholamines during rectal distention. Psychosom Med. 2009;71:619–626. doi: 10.1097/PSY.0b013e31819b69ca. [DOI] [PubMed] [Google Scholar]
- 32.McKay LC, Adams L, Frackowiak RS, Corfield DR. A bilateral cortico-bulbar network associated with breath holding in humans, determined by functional magnetic resonance imaging. Neuroimage. 2008;40:1824–1832. doi: 10.1016/j.neuroimage.2008.01.058. [DOI] [PubMed] [Google Scholar]
- 33.Barman SM, Gebber GL, Kitchens H. Rostral dorsolateral pontine neurons with sympathetic nerve-related activity. Am J Physiol. 1999;276:H401–H412. doi: 10.1152/ajpheart.1999.276.2.H401. [DOI] [PubMed] [Google Scholar]
- 34.Naidich TP, Duvernoy HM, Delman BN, Sorensen AG, Kollias SS, Haacke EM. Duvernoy's Atlas of the Human Brain Stem and Cerebellum. Springer; Wien: 2009. [Google Scholar]
- 35.Lazarov NE. Comparative analysis of the chemical neuroanatomy of the mammalian trigeminal ganglion and mesencephalic trigeminal nucleus. Prog Neurobiol. 2002;66:19–59. doi: 10.1016/s0301-0082(01)00021-1. [DOI] [PubMed] [Google Scholar]
- 36.Nomura S, Mizuno N. Differential distribution of cell bodies and central axons of mesencephalic trigeminal nucleus neurons supplying the jaw-closing muscles and periodontal tissue: a transganglionic tracer study in the cat. Brain Res. 1985;359:311–319. doi: 10.1016/0006-8993(85)91442-8. [DOI] [PubMed] [Google Scholar]
- 37.Jerge CR. Organization and function of the trigeminal mensencephalic nucleus. J Neurophysiol. 1963;26:379–392. doi: 10.1152/jn.1963.26.3.379. [DOI] [PubMed] [Google Scholar]
- 38.Luo P, Moritani M, Dessem D. Jaw-muscle spindle afferent pathways to the trigeminal motor nucleus in the rat. J Comp Neurol. 2001;435:341–353. doi: 10.1002/cne.1034. [DOI] [PubMed] [Google Scholar]
- 39.Borsook D, Burstein R, Becerra L. Functional imaging of the human trigeminal system: opportunities for new insights into pain processing in health and disease. J Neurobiol. 2004;61:107–125. doi: 10.1002/neu.20085. [DOI] [PubMed] [Google Scholar]
- 40.Nash PG, Macefield VG, Klineberg IJ, Murray GM, Henderson LA. Differential activation of the human trigeminal nuclear complex by noxious and non-noxious orofacial stimulation. Hum Brain Mapp. 2009;30:3772–3782. doi: 10.1002/hbm.20805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kiknadze GI, Dzamoeva EI, Lazriev IL. [Ultrastructure of the trigeminal principal sensory nucleus in the norm and during pain threshold stimulation]. Morfologiia. 2000;118:31–35. [PubMed] [Google Scholar]
- 42.Lisney SJ. Observations on facial nociception in a monkey after destruction of the rostral part of the trigeminal sensory nuclear complex. Pain. 1985;21:129–135. doi: 10.1016/0304-3959(85)90282-9. [DOI] [PubMed] [Google Scholar]
- 43.Reiner PB, Vincent SR. Topographic relations of cholinergic and noradrenergic neurons in the feline pontomesencephalic tegmentum: an immunohistochemical study. Brain Res Bull. 1987;19:705–714. doi: 10.1016/0361-9230(87)90058-x. [DOI] [PubMed] [Google Scholar]
- 44.Nistico G, Nappi G. Locus coeruleus, an integrative station involved in the control of several vital functions. Funct Neurol. 1993;8:5–25. [PubMed] [Google Scholar]
- 45.Aston-Jones G, Rajkowski J, Kubiak P, Valentino RJ, Shipley MT. Role of the locus coeruleus in emotional activation. Prog Brain Res. 1996;107:379–402. doi: 10.1016/s0079-6123(08)61877-4. [DOI] [PubMed] [Google Scholar]
- 46.Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- 47.Bouret S, Sara SJ. Network reset: a simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci. 2005;28:574–582. doi: 10.1016/j.tins.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 48.Jones SL. Descending noradrenergic influences on pain. Prog Brain Res. 1991;88:381–394. doi: 10.1016/s0079-6123(08)63824-8. [DOI] [PubMed] [Google Scholar]
- 49.Condes-Lara M. Different direct pathways of locus coeruleus to medial prefrontal cortex and centrolateral thalamic nucleus: electrical stimulation effects on the evoked responses to nociceptive peripheral stimulation. Eur J Pain. 1998;2:15–23. doi: 10.1016/s1090-3801(98)90042-8. [DOI] [PubMed] [Google Scholar]
- 50.Gauriau C, Bernard JF. Pain pathways and parabrachial circuits in the rat. Exp Physiol. 2002;87:251–258. doi: 10.1113/eph8702357. [DOI] [PubMed] [Google Scholar]
- 51.Benarroch EE. Pain-autonomic interactions: a selective review. Clin Auton Res. 2001;11:343–349. doi: 10.1007/BF02292765. [DOI] [PubMed] [Google Scholar]
- 52.Bernard JF, Besson JM. The spino(trigemino)pontoamygdaloid pathway: electrophysiological evidence for an involvement in pain processes. J Neurophysiol. 1990;63:473–490. doi: 10.1152/jn.1990.63.3.473. [DOI] [PubMed] [Google Scholar]
- 53.Bernard JF, Huang GF, Besson JM. The parabrachial area: electrophysiological evidence for an involvement in visceral nociceptive processes. J Neurophysiol. 1994;71:1646–1660. doi: 10.1152/jn.1994.71.5.1646. [DOI] [PubMed] [Google Scholar]
- 54.Bernard JF, Bester H, Besson JM. Involvement of the spino-parabrachio -amygdaloid and -hypothalamic pathways in the autonomic and affective emotional aspects of pain. Prog Brain Res. 1996;107:243–255. doi: 10.1016/s0079-6123(08)61868-3. [DOI] [PubMed] [Google Scholar]
- 55.Malick A, Jakubowski M, Elmquist JK, Saper CB, Burstein R. A neurohistochemical blueprint for pain-induced loss of appetite. Proc Natl Acad Sci U S A. 2001;98:9930–9935. doi: 10.1073/pnas.171616898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wager TD, Scott DJ, Zubieta JK. Placebo effects on human mu-opioid activity during pain. Proc Natl Acad Sci U S A. 2007;104:11056–11061. doi: 10.1073/pnas.0702413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zemlan FP, Behbehani MM. Afferent projections to the nucleus cuneiformis in the rat. Neurosci Lett. 1984;52:103–109. doi: 10.1016/0304-3940(84)90358-6. [DOI] [PubMed] [Google Scholar]
- 58.Porro CA, Cavazzuti M, Galetti A, Sassatelli L. Functional activity mapping of the rat brainstem during formalin-induced noxious stimulation. Neuroscience. 1991;41:667–680. doi: 10.1016/0306-4522(91)90358-u. [DOI] [PubMed] [Google Scholar]
- 59.Keltner JR, Furst A, Fan C, Redfern R, Inglis B, Fields HL. Isolating the modulatory effect of expectation on pain transmission: a functional magnetic resonance imaging study. J Neurosci. 2006;26:4437–4443. doi: 10.1523/JNEUROSCI.4463-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cuomo-Granston A, Drummond PD. Migraine and motion sickness: what is the link? Prog Neurobiol. 2010;91:300–312. doi: 10.1016/j.pneurobio.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 61.Marcus DA, Furman JM, Balaban CD. Motion sickness in migraine sufferers. Expert Opin Pharmacother. 2005;6:2691–2697. doi: 10.1517/14656566.6.15.2691. [DOI] [PubMed] [Google Scholar]
- 62.Jones EG. Chemically defined parallel pathways in the monkey auditory system. Ann N Y Acad Sci. 2003;999:218–233. doi: 10.1196/annals.1284.033. [DOI] [PubMed] [Google Scholar]
- 63.Winer JA, Lee CC. The distributed auditory cortex. Hear Res. 2007;229:3–13. doi: 10.1016/j.heares.2007.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Borsook D, Maleki N, Becerra L, McEwen B. Understanding Migraine through the Lens of Maladaptive Stress Responses: A Model Disease of Allostatic Load. Neuron. 2012;73:219–234. doi: 10.1016/j.neuron.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 65.Stankewitz A, May A. Increased limbic and brainstem activity during migraine attacks following olfactory stimulation. Neurology. 2011;77:476–482. doi: 10.1212/WNL.0b013e318227e4a8. [DOI] [PubMed] [Google Scholar]
- 66.Janusonis S, Fite KV. Diurnal variation of c-Fos expression in subdivisions of the dorsal raphe nucleus of the Mongolian gerbil (Meriones unguiculatus). J Comp Neurol. 2001;440:31–42. doi: 10.1002/cne.1368. [DOI] [PubMed] [Google Scholar]
- 67.Afridi SK, Giffin NJ, Kaube H, Friston KJ, Ward NS, Frackowiak RS, Goadsby PJ. A positron emission tomographic study in spontaneous migraine. Arch Neurol. 2005;62:1270–1275. doi: 10.1001/archneur.62.8.1270. [DOI] [PubMed] [Google Scholar]
- 68.Aurora SK, Barrodale PM, Tipton RL, Khodavirdi A. Brainstem dysfunction in chronic migraine as evidenced by neurophysiological and positron emission tomography studies. Headache. 2007;47:996–1003. doi: 10.1111/j.1526-4610.2007.00853.x. (discussion 4–7) [DOI] [PubMed] [Google Scholar]
- 69.Pattinson KT, Mitsis GD, Harvey AK, Jbabdi S, Dirckx S, Mayhew SD, et al. Determination of the human brainstem respiratory control network and its cortical connections in vivo using functional and structural imaging. Neuroimage. 2009;44:295–305. doi: 10.1016/j.neuroimage.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 70.Vandewalle G, Schmidt C, Albouy G, Sterpenich V, Darsaud A, Rauchs G, et al. Brain responses to violet, blue, and green monochromatic light exposures in humans: prominent role of blue light and the brainstem. PLoS One. 2007;2:e1247. doi: 10.1371/journal.pone.0001247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smits M, Kovacs S, de Ridder D, Peeters RR, van Hecke P, Sunaert S. Lateralization of functional magnetic resonance imaging (fMRI) activation in the auditory pathway of patients with lateralized tinnitus. Neuroradiology. 2007;49:669–679. doi: 10.1007/s00234-007-0231-3. [DOI] [PubMed] [Google Scholar]