Abstract
Purpose
Over the last thirty years, prenatal care utilization, both the proportion of women receiving the recommended number of visits and the average number of visits, has increased substantially. Although infant mortality has fallen, preterm birth has increased. We hypothesized that prenatal care may lead to lower infant mortality in part by increasing the detection of obstetrical problems for which the clinical response may be to medically induce preterm birth.
Methods
We examine whether medically induced preterm birth mediates the association between prenatal care and infant mortality using newly developed methods for mediation analysis. Data are the cohort version of the national linked birth certificate and infant death data for 2003 births. Analyses adjust for maternal sociodemographic, geographic and health characteristics.
Results
Receiving more prenatal care visits than recommended was associated with medically induced preterm birth (OR=2.44 (95% CI: 2.40,2.49) compared with fewer visits than recommended). Medically induced preterm birth was itself associated with higher infant mortality (OR=5.08, 95% CI: 4.61,5.60)), but that association was weaker among women receiving extra prenatal care visits (OR=3.08 95% CI: 2.88,3.30)) compared to women receiving the recommended number of visits or fewer.
Conclusions
These analyses suggest that some of the benefit of prenatal care in terms of infant mortality may be mediated by medically induced preterm birth. If so, using preterm birth rates as a metric for tracking birth policy and outcomes could be misleading.
Introduction
Relatively poor birth outcomes in the U.S. have been a concern for decades. In 1985 the Institute of Medicine (IOM) concluded a report by recommending increased support for prenatal care: “Every additional dollar spent for prenatal care would save $3.38 in the total cost of caring for LBW infants”.2 Prenatal care was thought of as a preventive intervention for preterm birth, a view supported by numerous studies that showed that women who initiated prenatal care early in pregnancy had fewer preterm and low birthweight babies.1,2 Since that time funding and utilization of prenatal care has increased considerably.3,4 However, preterm birth rates and low birth weight rates have increased rather than declined, and some quantitative studies suggest that prenatal care is ineffective at reducing these rates.5–7 Revisiting the problem of preterm birth in a 2006 report, the IOM acknowledged that the problem of preterm birth was more complex than the IOM and others had once thought, and that solutions are not obvious.1
Perhaps surprisingly, infant mortality has nonetheless steadily declined over this period.8 This, too, was not what scientists or policy makers would have predicted, since preterm birth is the leading cause of infant mortality and has been used as a primary birth outcome in part for that reason. Clearly, the relationship between prenatal care, preterm birth, and infant mortality are more complicated than what has been implicitly assumed.
This paper examines empirical evidence to assess a different understanding of how prenatal care might affect birth outcomes. While it may be a preventive intervention for some women (as traditionally thought), for others it may function primarily as an opportunity for early detection of obstetrical complications. Many of the problems so detected are treated by early delivery through labor induction or caesarian section (or both). To the extent that prenatal care functions in this way, it may lead to an increase in preterm birth, yet also be associated with a decrease in both fetal and infant mortality. By this view, the effect of prenatal care on infant mortality may, in part, come about by (appropriately) increasing the rate of medically induced preterm deliveries.
We evaluate the associations between prenatal care, medically induced preterm birth and infant mortality and the extent to which medically induced preterm birth acts as a mediator for the effect of prenatal care on infant mortality. One of the key challenges in assessing the overall effect of prenatal care is dealing with the problems of confounding. Prenatal care may simply serve as a marker for higher socioeconomic status, more conscientious health behaviors, or other factors that truly predict lower risk of preterm birth. Problems of confounding are aggravated further when questions of mediation are in view.9–11 Confounding of the intermediate-outcome relationship (e.g. common causes of medically induced preterm birth and infant mortality) can bias analyses of direct and indirect effects. To address these questions we will employ recent methodological developments in causal mediation analysis12,13 along with sensitivity analysis techniques14 to attempt to assess and potentially correct for problems of unmeasured confounding.
Methods
The National Center for Health Statistics (NCHS) links birth certificates to death certificates through the following year, but these “cohort” linked birth-infant death files are released several years after the “period” linked birth year certificate files, which include the death certificates for the same calendar year only. We used the 2003 NCHS linked birth-infant death cohort files, which was the most recent year available at the time of the analysis. The files include data on all births in the US in 2003, followed through 2004 to assess infant survival status (n=3,918,541). The research was approved by the Institutional Review Board at the University of Chicago and the Harvard School of Public Health.
Adequacy of prenatal care is categorized using a modification of the Adequacy of Prenatal Care Utilization index (APNCU, called the “Kotelchuck index”), which we have previously described.15 The APNCU combines two care dimensions: the month prenatal care began, divided into two-month intervals, and the ratio of actual visits to the expected number based on the recommended visit schedule of the American College of Obstetricians and Gynecologists (the recommended schedule has not changed in 20 years: one visit per month for the first six months, two per month in the 7th and 8th months and weekly visits from the 9th month onward). Based on these two pieces of information, prenatal care is categorized as: missing, inadequate, intermediate, adequate, and adequate-plus. The APNCU defines “adequate plus” as a ratio of reported to recommended visits greater than 1.1. Koroukian and Rimm16 criticized the “adequate plus” category because only one extra visit boosts the ratio to 1.1 when the recommended number of visits is fewer than 10, which is the case for most preterm births. Thus preterm deliveries are more easily categorized as “adequate plus.” One extra visit does not result in “adequate plus” care if gestational age is greater than 36 weeks. We thus instead use a modification of the APNCU that does not categorize a course of prenatal care as “adequate plus” unless it includes at least two more visits than are expected.17 We refer to this modified “adequate plus” category as “superadequate.” The modification also collapses the relatively infrequent “intermediate” and “inadequate” categories into a “not adequate” category.17 Prenatal care is treated as a categorical, not ordinal, variable in the analysis.
Preterm birth (<37 weeks gestation) was categorized according to the gestational age variable in the NCHS data derived from the last menstrual period. Medically induced preterm birth is defined, as in prior literature,18–21 as a cesarean or labor induction at <37 weeks. It is difficult to distinguish from the NCHS data preterm cesarean with prior spontaneous labor, versus without prior spontaneous labor. To attempt to partially address this we exclude from the “medically induced preterm birth” category pregnancies with preterm cesarean accompanied by precipitous labor or tocolysis. Moreover, for preterm induction, we use data only on labor induction, not labor augmentation, to define “medically induced.” It is not possible to entirely distinguish using the NCHS data preterm births that are genuinely “medically indicated” from those that are merely “medically induced.”
The birth certificate forms included a number of demographic variables that were used to control for confounding. The variables included in the analysis were plurality; maternal age, race, Hispanic origin, place of birth, education, marital status, gravidity, smoking, alcohol consumption, prior low-weight birth, prior 4000+ grams birth and geographic divisions of the country. We combined maternal race and ethnicity to form the following categories: White non-Hispanic, Black non-Hispanic, Hispanic, Asian/Pacific Islander, and Native American. Maternal age was categorized as less than 15, six consecutive age categories of five years each, and a final category of 45 years or more; years of maternal education was categorized as 0–8, 9–11, 12, 13–15 and 16 or more; maternal place of birth as U.S., Mexico, Canada and the rest of the world, and gravidity as 1, 2 and 3 or more. These covariates were included in the models below and were selected a priori to attempt to make confounding assumptions plausible; no automated covariate selection methods were used. Missing data were handled by complete case analysis and an indicator for missing prenatal care. All analyses employed over 90% of the observations (and over 93% of observations for all demographic summaries) so resulting biases due to missing data are unlikely to be severe.
For direct and indirect effects control needs to be made not only for confounders of the exposure-outcome relationship but also of the intermediate-outcome relationship and of the exposure-intermediate relationship.10,12,14 In addition, there should be no intermediate-outcome confounders that are themselves affected by the exposure.12,13 Because of the need to control for intermediate-outcome confounding, adjustment was also made for chronic hypertension, pregnancy associated hypertension, and eclampsia/pre-eclampsia which might be common causes of medically induced preterm birth and infant mortality. See Figure 1.
Figure 1.
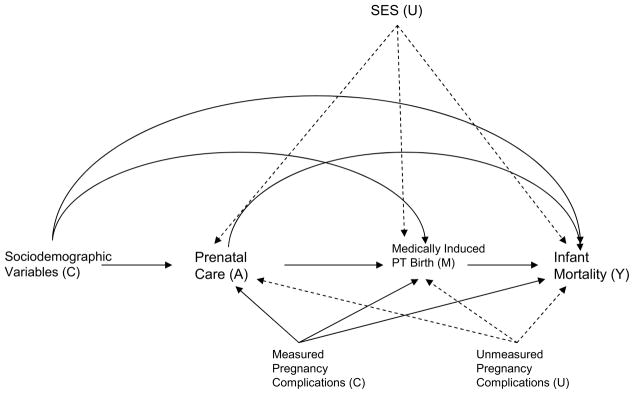
Relationships between prenatal care (A), medically induced preterm birth (M), and infant mortality (Y) with measured confounders (C) and unmeasured confounders (U); dashed arrows indicates sources of unmeasured confounding
The effect of prenatal care on medically induced preterm birth and also the effect of prenatal care and medically induced preterm birth on infant mortality were assessed using logistic regression models. We fit a logistic regression model for medically induced preterm birth (M) conditional on prenatal care categories (A) and the set of confounders (C):
Robust standard errors were used in this and all subsequent models to account for potential clustering in multiple births. Odds ratios are reported; because the outcome is rare these can be interpreted as risk ratios. We fit a logistic regression model for infant mortality (Y) on prenatal care categories (A), medically induced preterm birth (M), an interaction between prenatal care and medically induced preterm birth and the confounders (C):
The logistic regression for infant mortality was also fit without including medically induced preterm birth and its interaction with prenatal care so as to also assess total effects of prenatal care.
Logistic regression models for medically induced preterm birth and for infant mortality were combined to obtain direct and indirect effects using odds ratios for mediation analysis for dichotomous outcomes using SAS macros.12,13 For this mediation analysis we assessed the effect of “superadequate” versus “not adequate” prenatal care as mediated by medically induced preterm birth. The natural indirect effect odds ratio compares the odds of infant mortality of those with superadequate care if medically induced preterm birth were set to what it would have been with superadequate versus with not adequate prenatal care. The natural direct effect odds ratio compares the odds of infant mortality with superadequate versus not adequate prenatal care if, in both cases, medically induced preterm birth were set to what it would have been with not adequate care.
We used sensitivity analysis for direct and indirect effects to assess and adjust for violations in the confounding assumptions.14 Socioeconomic status might confound the relationships between prenatal care and either medically induced preterm birth or infant mortality. Common causes of medically induced preterm birth and infant mortality, such as thrombophilia, premature rupture of membrane, lupus, other immune diseases, etc. for which data is either unavailable or incomplete in the NCHS files, might confound the mediator-outcome (medically-induced preterm birth-infant mortality) relationship, but are all relatively uncommon.
All statistical analyses were performed using SAS (SAS Institute, Cary, NC), version 9.2.
Results
Pregnancy and maternal demographic characteristics of the 2003 birth cohort are presented in Table 1. Table 2 reports the odds ratios from a logistic regression model for medically induced preterm birth conditional on prenatal care categories, with not adequate care as the reference group, along with the set of measured confounders. The odds of medically induced preterm birth were 0.52 (95% CI: 0.51, 0.53) times lower for mothers with adequate versus not adequate care. The odds of medically induced preterm birth were 2.44 (95% CI: 2.40, 2.49) times higher for mothers with super-adequate versus inadequate care. Estimates are not adjusted for unmeasured confounders such as thrombophilia, premature rupture of membrane, lupus and other immune diseases and thus reflect associations due to prenatal care and to confounding by pregnancy complications or residual confounding of SES. The impact of unmeasured confounding is assessed through sensitivity analysis below.
Table 1.
Maternal and pregnancy characteristics for all live births in the United States (2003), from the National Center for Health Statistics Linked Birth Certificate Infant Mortality Files (n=3,918,541).
| Percentage | |
|---|---|
| Maternal Race/Ethnicity | |
| White Non Hispanic | 57.6 |
| Black Non-Hispanic | 14.2 |
| Hispanic | 22.0 |
| Asian & Pacific Islander | 5.2 |
| Native American | 1.0 |
| Maternal Age in Years | |
| < 15 | 0.2 |
| 15–19 | 10.0 |
| 20–24 | 25.0 |
| 25–29 | 26.6 |
| 30–34 | 24.1 |
| 35–39 | 11.6 |
| 40–45 | 2.5 |
| 45–49 | 0.1 |
| Maternal Years of School | |
| 0–8 | 6.0 |
| 9–11 | 15.2 |
| 12 | 30.4 |
| 13–15 | 21.4 |
| 16+ | 27.0 |
| Maternal Marital Status | |
| Married | 65.8 |
| Unmarried | 34.2 |
| Pregnancy Plurality | |
| Singleton | 96.6 |
| Twin | 3.2 |
| Triplet+ | 0.2 |
| Maternal Gravidity | |
| Gravid 1 | 40.0 |
| Gravid 2 | 32.5 |
| Gravid 3+ | 27.5 |
Table 2.
Adjusted odds ratios and 95% confidence intervals for medically induced preterm birth, from the National Center for Health Statistics Natality Files 2003*
| Estimate (CI) | |
|---|---|
| Prenatal Care (Reference Not Adequate) | |
| Adequate | 0.52 (0.51,0.53) |
| Superadequate | 2.44 (2.40,2.49) |
| Missing | 1.77 (1.70,1.84) |
| Maternal Race/Ethnicity (Reference White Non-Hispanic) | |
| Black Non-Hispanic | 1.44 (1.41,1.47) |
| Hispanic | 1.16 (1.13,1.19) |
| Asian & Pacific Islander | 1.09 (1.05,1.13) |
| Native American | 1.20 (1.13,1.28) |
| Maternal Age in Years (Reference 25–29) | |
| < 15 | 1.02 (0.88,1.18) |
| 15–19 | 0.79 (0.77,0.81) |
| 20–24 | 0.87 (0.85,0.89) |
| 30–34 | 1.19 (1.17,1.21) |
| 35–39 | 1.46 (1.43,1.50) |
| 40–45 | 1.81 (1.74,1.88) |
| 45–49 | 2.10 (1.86,2.35) |
| Pregnancy Plurality (%)(Reference Singleton) | |
| Twin | 9.31 (9.14,9.48) |
| Triplet+ | 38.5 (35.7,41.5) |
Adjusted also for geographic division, marital status, maternal place of birth, education, gravidity, smoking and drinking during pregnancy, chronic hypertension, pregnancy associated hypertension, and eclampsia/pre-eclampsia
Table 3 reports the odds ratios from a logistic regression model for infant mortality on prenatal care categories (with not adequate care as the reference), and the measured confounders. The odds of infant mortality were 0.62 (95% CI: 0.58, 0.66) times lower for mothers with adequate versus not adequate care. The odds of infant mortality were 1.04 (95% CI: 0.99,1.09) times higher for mothers with super-adequate versus inadequate care.
Table 3.
Adjusted odds ratios and 95% confidence intervals for infant mortality, from the National Center for Health Statistics Natality Files 2003*
| Estimate (CI) | |
|---|---|
| Prenatal Care (Reference Not Adequate) | |
| Adequate | 0.62 (0.58,0.66) |
| Superadequate | 1.04 (0.99,1.09) |
| Missing | 1.96 (1.79,2.13) |
| Maternal Race/Ethnicity (Reference White Non-Hispanic) | |
| Black Non-Hispanic | 1.86 (1.76,1.96) |
| Hispanic | 1.02 (0.95,1.10) |
| Asian & Pacific Islander | 1.17 (1.04,1.31) |
| Native American | 1.29 (1.09,1.52) |
| Maternal Age in Years (Reference 25–29) | |
| < 15 | 1.23 (0.89,1.69) |
| 15–19 | 1.07 (1.00,1.16) |
| 20–24 | 1.00 (0.95,1.05) |
| 30–34 | 1.05 (0.99,1.10) |
| 35–39 | 1.09 (1.01,1.17) |
| 40–45 | 1.38 (1.22,1.55) |
| 45–49 | 1.55 (1.06,2.21) |
| Pregnancy Plurality (%)(Reference Singleton) | |
| Twin | 4.67 (4.42,4.94) |
| Triplet+ | 13.4 (11.7,15.5) |
Adjusted also for geographic division, marital status, maternal place of birth, education, gravidity, smoking and drinking during pregnancy, chronic hypertension, preganancy associated hypertension, and eclampsia/pre-eclampsia
Table 4 reports the odds ratios from a logistic regression model for infant mortality on prenatal care categories (with not adequate care as the reference), medically induced preterm birth, an interaction between prenatal care and medically induced preterm birth and the measured confounders. There is negative interaction on the odds ratio scale with interaction ratio of 0.57 (P<0.001) between superadequate care and medically induced preterm birth so that although medically induced preterm birth increases the odds of infant mortality by a factor of 5.08 (95% CI: 4.61,5.60) for those with not adequate care, it increases the odds of infant mortality by a factor of only 3.08 (95% CI: 2.88,3.30) for those with superadequate care. The odds of infant mortality for adequate care and medically induced preterm birth is also higher than for superadequate care and medically induced preterm birth.
Table 4.
Adjusted odds ratios and 95% confidence intervals for infant mortality by prenatal care and medically induced preterm birth status, from the National Center for Health Statistics Natality Files 2003*
| Not Medically Induced Preterm Birth | Medically Induced Preterm Birth | |
|---|---|---|
| Prenatal Care (Reference Not Adequate) | (REFERENCE) | 5.08 (4.61,5.60) |
| Adequate | 0.60 (0.56,0.64) | 5.71 (5.24,6.22) |
| Superadequate | 1.07 (1.01,1.14) | 3.08 (2.88,3.30) |
| Missing | 1.98 (1.80,2.19) | 6.87 (5.94,7.95) |
Adjusted also for geographic division, marital status, plurality, maternal race, age, place of birth, education, gravidity, smoking and drinking during pregnancy, chronic hypertension, pregnancy-induced
Estimates from logistic regressions for medically induced preterm birth and infant mortality were used with sensitivity analysis techniques for unmeasured confounding to yield estimates of the effect of superadequate care on infant mortality mediated through medically induced preterm birth. Ignoring possible unmeasured confounding gave a direct effect odds ratio of 0.98 (95% CI: 0.90,1.04) and an indirect effect odds ratio of 1.11 (95% CI: 1.10,1.12). These estimates are subject to unmeasured confounding by common causes of medically induced preterm birth and infant mortality.
Using sensitivity analysis, we can calculate the effect of unmeasured confounding. If an unmeasured confounder increases the risk of infant mortality by 5 times with a prevalence amongst those with superadequate care of 50% for those with medically induced preterm birth and 5% for those without medically induced preterm birth, and is 3 times as likely amongst those with superadequate care as it is amongst those with inadequate care, this would give a direct effect odds ratio of 0.87 and an indirect effect odds ratio of 1; more extreme sensitivity analysis parameters (e.g. larger effect sizes of the unmeasured confounder or larger prevalence differences of the unmeasured confounder) would give an indirect effect odds ratio less than 1. Although the mediated effect is protective for sensitivity analysis parameters corresponding to substantial unmeasured confounding it seems plausible the effect could be in either direction.
Discussion
In this paper we have examined the relationships between prenatal care, medically induced preterm birth and infant mortality. We found that superadequate prenatal care was associated with higher risk of medically induced preterm birth even when compared with the group receiving inadequate care. Women receiving superadequate care were at about equal likelihood of infant mortality as those with inadequate care. Perhaps most striking, however, was that the infant mortality odds ratio for medically induced preterm birth was considerably lower among women who had received superadequate care compared to those who had received adequate or inadequate care. This suggests that superadequate care is leading to medically indicated preterm delivery, and that these medical inductions may be an appropriate response to a pregnancy complication. Our analyses of the direct and indirect effects of prenatal care on infant mortality were consistent with medically induced preterm birth being a mediator of the relationship between more prenatal care and lower infant mortality. The possibility that there may be unmeasured confounding, assessed via sensitivity analysis, however, made it difficult to draw a definitive conclusion. In addition it is possible that some covariates such as smoking, drinking, pregnancy associated hypertension, etc. that were controlled for as confounders of the medically-induced preterm birth and infant mortality relationship could themselves be affected by prenatal care. If this were the case, this would require use of alternative methods.10,12,18,19
The analysis is subject to some additional limitations. First, information is not available on the content of care (i.e. what is actually done during a prenatal care visit) limiting the types of conclusions that can be drawn. There are, moreover, important issues with regard to the accuracy of reporting on the birth certificate files20; risk behaviors such as drinking and smoking may be underreported in the birth certificate files. Delivery characteristics, such as induction, are likely also under-reported. 21 Those with superadequate care may have more accurate information than those who do not. Subsequent research could carry out similar analyses using other data sources with more accurate information, which in the U.S. would be possible only for much smaller and less representative study populations. More recent birth certificate files incorporating the newer birth certificate form may have more accurate information on medical indications. This analysis considered infant mortality but not fetal mortality; a fetuses-at-risk approach could potentially be used in future research.
Analytic studies have suggested that prenatal care is not particularly effective at lowering preterm birth and low birthweight rates.5–7 However, the differences in the intensity of prenatal care between groups compared were modest, and it is possible that studies may have reached different conclusions were they able to compare more substantially different prenatal care regimens. Nevertheless, given current evidence, it seems that one of three conclusions might be drawn, each with different policy implications: 1) that prenatal care as currently configured and delivered does not improve birth outcomes; 2) that traditional prenatal care might work as once thought – to improve birth outcomes by preventing preterm birth – but only in a subset of pregnant women; or 3) that prenatal care might improve birth outcomes in a different way than had originally been hypothesized, one that includes increasing preterm births – but only in a subset of pregnant women. There is some evidence that each conclusion may be operative for some women and that all may be part of the overall effect (or lack of effect) of prenatal care.
Our analysis here considered the possibility that the third scenario was valid, at least for some women: prenatal care improves birth outcomes in a different way than had originally been hypothesized. No prior analysis of prenatal care outcomes has taken into account crucial changes in the content or the purpose of prenatal care and distinguished between spontaneous and induced preterm births.22–25
It seems contrary to conventional wisdom that prenatal care might increase preterm birth and that preterm birth that is appropriately medically induced might in fact lower infant mortality. But it makes sense if one thinks of prenatal care not as a preventive intervention but, instead, as a complex screening test designed to detect problems during pregnancy for which the appropriate clinical response may be to deliver the baby early - either by induction or by c-section. This, of course, can lower infant mortality only in the context of a health system in which neonatal intensive care is available to care for the preterm infants thus delivered. Our result that the risk of infant mortality is lower for those with superadequate care who had a medically induced preterm birth than those with adequate or inadequate care who had a medically induced preterm birth may be evidence of this phenomenon. Indeed if prenatal care functions in this way, it would lead to precisely the changes that we see - increases in c-sections, increases in preterm and low birthweight deliveries, and decreased infant mortality. A recent study of women pregnant with twins showed precisely these results: those who received intensive prenatal care had an increased rate of c-sections and preterm birth and a decreased infant mortality rate.26 Our analysis here lends further support to this as a possible explanation. Further evidence for this mechanism comes from the temporal concurrence of increases in medically induced preterm birth rates and decreased infant and fetal mortality rates.27,28 All this evidence suggests that prenatal care may be serving this unrecognized function.
Recognition that prenatal care could be influencing birth outcomes in this way and further analysis of it will be important if we hope to more accurately assess the value of prenatal care and to design a system to provide such care in the most cost-effective and efficacious way. The continued use of preterm birth as a primary metric for tracking birth outcomes and policies would need to be reconsidered.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Behrman RE, Butler AS, editors. Preterm birth: causes, consequences, prevention. National Academies Press; Washington DC: 2006. [PubMed] [Google Scholar]
- 2.IOM. Committee to Study the Prevention of Low Birthweight. Washington DC: 1985. [Google Scholar]
- 3.Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S. Births: Final data for 2004. National vital statistics reports. 1. Vol. 55. Hyattsville, MD: National Center for Health Statistics; 2006. [PubMed] [Google Scholar]
- 4.Lauderdale DS, VanderWeele TJ, Siddique J, Lantos JD. Prenatal care utilization in excess of recommended levels: trends from 1985–2004. Medical Care Research and Reviews. 2010;67:609–622. doi: 10.1177/1077558709351530. [DOI] [PubMed] [Google Scholar]
- 5.Alexander GR, Korenbrot CC. The role of prenatal care in preventing low birthweight. Future Child. 1995;5:103–20. [PubMed] [Google Scholar]
- 6.Stevens-Simon C, Orleans M. Low-birthweight prevention programs: the enigma of failure. Birth. 1999 Sep;26(3):184–91. doi: 10.1046/j.1523-536x.1999.00184.x. [DOI] [PubMed] [Google Scholar]
- 7.Lu MC, Tache V, Alexander M, Kotelchuck M, Halfon N. Preventing low birthweight: is prenatal care the answer? J Matern Fetal Neonatal Med. 2003;13:162–80. doi: 10.1080/jmf.13.6.362.380. [DOI] [PubMed] [Google Scholar]
- 8.World Bank. [Last accessed: February 6, 2013.];World Development Indicators. Web Access: http://www.google.com/publicdata?ds=wb-wdi&met=sp_dyn_imrt_in&idim=country:USA&dl=en&hl=en&q=infant+mortality+us.
- 9.Judd CM, Kenny DA. Process analysis: estimating mediation in treatment evaluations. Evaluation Review. 1981;5:602–19. [Google Scholar]
- 10.Robins JM, Greenland S. Identifiability and exchangeability for direct and indirect effects. Epidemiology. 1992;3(2):143–55. doi: 10.1097/00001648-199203000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Valeri L, VanderWeele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychological Methods. doi: 10.1037/a0031034. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pearl J. Direct and indirect effects. Presented at In: Proceedings of the Seventeenth Conference on Uncertainty and Artificial Intelligence; San Francisco, CA. 2001. [Google Scholar]
- 13.VanderWeele TJ, Vansteelandt S. Odds ratios for mediation analysis with a dichotomous outcome. American Journal of Epidemiology. 2010;172:1339–1348. doi: 10.1093/aje/kwq332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.VanderWeele TJ. Bias formulas for sensitivity analysis for direct and indirect effects. Epidemiology. 2010;21(4):540–51. doi: 10.1097/EDE.0b013e3181df191c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kotelchuck M. An evaluation of the Kessner adequacy of prenatal care index and a proposed adequacy of prenatal care utilization index. Am J Public Health. 1994;84:1414–1420. doi: 10.2105/ajph.84.9.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koroukian SM, Rimm AA. The “adequacy of prenatal care utilization” (APNCU) index to study low birth weight: is the index biased? Journal of Clinical Epidemiology. 2002;55:296–305. doi: 10.1016/s0895-4356(01)00471-1. [DOI] [PubMed] [Google Scholar]
- 17.VanderWeele TJ, Lantos JD, Siddique J, Lauderdale DS. A comparison of four prenatal care indices in birth outcome models: comparable results for predicting small-for-gestational-age outcome but different results for preterm birth or infant mortality. Journal of Clinical Epidemiology. 2009;62:438–445. doi: 10.1016/j.jclinepi.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 18.VanderWeele TJ. Marginal structural models for the estimation of direct and indirect effects. Epidemiology. 2009;20:18–26. doi: 10.1097/EDE.0b013e31818f69ce. [DOI] [PubMed] [Google Scholar]
- 19.VanderWeele TJ, Chiba Y. Sensitivity analysis for direct and indirect effects in the presence of exposure-induced mediator-outcome confounders. Epidemiology, Biostatistics, and Public Health. doi: 10.2427/9027. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Northam S, Knapp TR. The reliability and validity of birth certificates. J Obstet Gynecol Neonatal Nurs. 2006;35(1):3–12. doi: 10.1111/j.1552-6909.2006.00016.x. [DOI] [PubMed] [Google Scholar]
- 21.Lydon-Rochelle MT, Holt VL, Nelson JC, Cárdenas V, Gardella C, Easterling TR, Callaghan WM. Accuracy of reporting maternal in-hospital diagnoses and intrapartum procedures in Washington State linked birth records. Paediatr Perinat Epidemiol. 2005;19(6):460–71. doi: 10.1111/j.1365-3016.2005.00682.x. [DOI] [PubMed] [Google Scholar]
- 22.Pickett KE, Abrams B, Selvin S. Defining preterm delivery – the epidemiology of clinical presentation. Paediatr Perinat Epidemiol. 2000;14(4):305–308. doi: 10.1046/j.1365-3016.2000.00293.x. [DOI] [PubMed] [Google Scholar]
- 23.Moutquin JM. Classification and heterogeneity of preterm birth. Brit J Obstet Gynaecol. 2003;110(Suppl 20):30–33. doi: 10.1016/s1470-0328(03)00021-1. [DOI] [PubMed] [Google Scholar]
- 24.Savitz DA, Dole N, Herring AH, Kaczor D, Murphy J, Siega-Riz AM, Thorp JM, Jr, MacDonald TL. Should spontaneous and medically indicated preterm births be separated for studying aetiology? Paediatr Perinat Epidemiol. 2005;19(2):97–105. doi: 10.1111/j.1365-3016.2005.00637.x. [DOI] [PubMed] [Google Scholar]
- 25.Ananth CV, Vintzileos AM. Epidemiology of preterm birth and its clinical subtypes. J Matern Fetal Neonatal Med. 2006;19(12):773–782. doi: 10.1080/14767050600965882. [DOI] [PubMed] [Google Scholar]
- 26.Kogan MD, Alexander GR, Kotelchuck M, et al. Trends in twin births and prenatal care utilization, 1981–97. JAMA. 2000;284:335–41. doi: 10.1001/jama.284.3.335. [DOI] [PubMed] [Google Scholar]
- 27.Ananth CV, Joseph KS, Oyelese Y, et al. Trends in preterm birth and perinatal mortality among singletons: United States, 1989 through 2000. Obstetrics and Gynecology. 2005;105(5):1084–1091. doi: 10.1097/01.AOG.0000158124.96300.c7. [DOI] [PubMed] [Google Scholar]
- 28.Ananth CV, Vintzileos AM. Maternal-fetal conditions necessitating a medical intervention resulting in preterm birth. Am J Obstet Gynecol. 2006;195(6):1557–1563. doi: 10.1016/j.ajog.2006.05.021. [DOI] [PubMed] [Google Scholar]


