Abstract
Background
Mutations in the JARID1C (Jumonji AT-rich interactive domain 1C) gene were recently associated with X-linked mental retardation (XLMR). Mutations in this gene are reported to be one of the relatively more common causes of XLMR with a frequency of approximately 3% in males with proven or probable XLMR. The JARID1C protein functions as a histone 3 lysine 4 (H3K4) demethylase and is involved in the demethylation of H3K4me3 and H3K4me2.
Methods
Mutation analysis of the JARID1C gene was conducted in the following cohorts: probands from 23 XLMR families linked to Xp11.2, 92 males with mental retardation and short stature, and 172 probands from small XLMR families with no linkage information.
Results
Four novel mutations consisting of two missense mutations, p.A77T and p.V504M, and two frame shift mutations, p.E468fsX2 and p.R1481fsX9, were identified in males with mental retardation. Two of the mutations, p.V504M and p.E468fsX2, are located in the JmjC domain of the JARID1C gene where no previous mutations have been reported. Additional studies showed that the missense mutation, p.V504M, was a de novo event on the grandpaternal X chromosome of the family. Clinical findings of the nine affected males from the four different families included mental retardation (100%), short stature (55%), hyperreflexia (78%), seizures (33%) and aggressive behaviour (44%). The degree of mental retardation consisted of mild (25%), moderate (12%) and severe (63%).
Conclusion
Based on the clinical observations, male patients with mental retardation, short stature and hyperreflexia should be considered candidates for mutations in the JARID1C gene.
Mental retardation (MR) is characterised by limitation in both intellectual functioning (IQ <70) and adaptive behaviour as exhibited in conceptual, social and practical skills.1 The estimated prevalence of MR is 1–3% in the general population.2,3 However, even with thorough clinical and laboratory evaluations, a specific aetiology can be assigned in only about 50% of the patients.4 The excess of males in the MR population has been attributed, in part, to genes located on the X chromosome.5 X-linked mental retardation (XLMR) may affect 2–3 males per 1000 and X-linked genes appear to cause MR in 15–25% of males with MR.5 Over 80 genes associated with XLMR have now been identified.4,6–10
The JARID1C gene encodes a protein of 1560 amino acids that belongs to a family of histone demethylases and is mainly involved in demethylation of tri- and dimethylated H3K4 (histone H3 lysine 4).11,12 The JARID1C protein contains several conserved DNA motifs, such as a JmjN domain, an ARID/BRIGHT domain, a JmjC domain, a C5HC2 zinc finger domain and PHD zinc finger domains.13 Mutations in the JARID1C gene have been found to cause XLMR.13–15 Thirteen different mutations have been reported in three studies involving 378 European families with non-syndromic XLMR giving an estimated gene frequency of approximately 3% in individuals with suspected XLMR.13–15 Recently, one missense mutation was reported in a patient with autism spectrum disorder (ASD).16 The reported mutations have been located throughout the JARID1C gene.
As part of our XLMR candidate gene testing, we screened probands from 23 XLMR families linked to the Xp11.2 region for mutations in the JARID1C gene. We also screened two cohorts consisting of 92 probands with MR plus short stature and 172 probands from small XLMR families with no linkage information. We identified four novel mutations in JARID1C: p.A77T, p.V504M, p.E468GfsX2 and p.R1481GfsX9.
PATIENTS AND METHODS
Patients and controls
One hundred and ninety-five unrelated individuals from families with XLMR (23 probands linked to the Xp11.2 region and 172 probands from small XLMR families with no linkage information) were included in this study. Since short stature was one of the common clinical findings in patients previously reported with JARID1C mutations, an additional cohort of 92 males with MR and short stature was included in the mutation screening. Thus a total of 287 unrelated males with MR were screened for JARID1C mutations. Clinical findings for the four families with a JARID1C mutation are summarised below and in table 1.
Table 1.
Clinical features in affected males with mutations in the JARID1C gene
| Clinical features/family number | K8545 | K8835 | K8140 | K9374 | Total |
|---|---|---|---|---|---|
| Age range of individuals evaluated | 13–46 years | 16–38 years | 26–28 years | 43 years | |
| Number of affected males evaluated | 3 | 3 | 2 | 1 | 9 |
| Mental retardation: | |||||
| Severe | 2 | – | 2 | 1 | 5/8 (63%) |
| Moderate | – | 1 | – | – | 1/8 (12%) |
| Mild | – | 2 | – | – | 2/8 (25%) |
| Head circumference <3rd centile | 1/3 | 0/3 | 0/2 | 1/1 | 2/9 (22%) |
| Head circumference >97th centile | 0/3 | 0/3 | 2/2 | 0/1 | 2/9 (22%) |
| Height <3rd centile | 2/3 | 0/3 | 2/2 | 1/1 | 5/9 (55%) |
| Deep set eyes | 2/3 | 0/3 | 0/2 | 0/1 | 2/9 (22%) |
| Strabismus | 2/3 | 0/3 | 0/2 | 0/1 | 2/9 (22%) |
| Prominent ears | 1/3 | 0/3 | 0/2 | 0/1 | 1/9 (11%) |
| High narrow palate | 0/3 | 2/3 | 1/2 | 0/1 | 3/9 (33%) |
| Small testes | 1/3 | 0/3 | 1/2 | 0/1 | 2/9 (22%) |
| Broad hand/tapering fingers | 2/3 | 0/3 | 0/2 | 0/1 | 2/9 (22%) |
| Camptodactyly and clinodactyly | 0/3 | 1/3 | 0/2 | 0/1 | 1/9 (11%) |
| Brachydactyly | 1/3 | 0/3 | 0/2 | 1/1 | 2/9 (22%) |
| Club feet | 1/3 | 0/3 | 0/2 | 0/1 | 1/9 (11%) |
| Café au lait spot | 0/3 | 0/3 | 1/2 | 0/1 | 1/9 (11%) |
| Muscle hypotrophy | 0/3 | 0/3 | 0/2 | 0/1 | 0/9 (0%) |
| Facial hypotonia | 0/3 | 0/3 | 0/2 | 0/1 | 0/9 (0%) |
| Hyperreflexia/spasticity | 1/3 | 3/3 | 2/2 | 1/1 | 7/9 (78%) |
| Seizures | 1/3 | 0/3 | 2/2 | 0/1 | 3/9 (33%) |
| Aggressive behaviour | 3/3 | 1/3 | 0/2 | 0/1 | 4/9 (44%) |
A control panel consisting of 364 males and 274 females with normal intelligence was used for polymorphism studies. The ethnicity of the individuals in the control panel consisted of Caucasians and African Americans. Informed consent was obtained from all patients, relatives and control individuals to participate in the study.
Family K8545
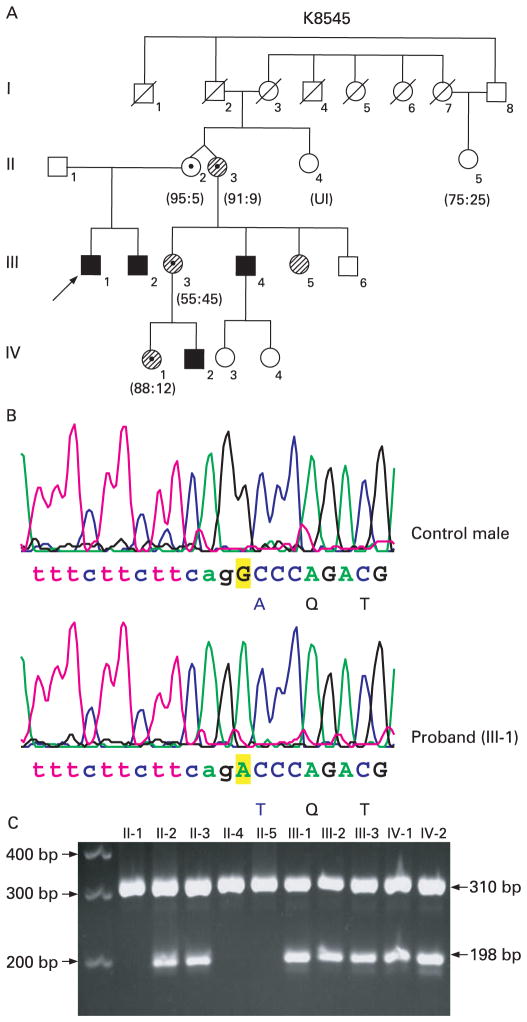
Family K8545 consists of four affected individuals (III-1, III-2, III-4 and IV-2) in two generations (fig 1A). The presence of slightly broad hands with brachydactyly and tapering fingers in the proband prompted the mutation screening of the Coffin–Lowry gene (RPS6KA3) which was found to be negative. After finding the JARID1C mutation in the proband (III-1), the family was revisited for a complete clinical evaluation. The three affected individuals (III-1, III-2 and IV-2) who were clinically evaluated had MR with delayed speech. Two of the affected individuals (III-2 and IV-2) had short stature (<3rd centile) and one had hyperreflexia (IV-2). The head circumference for individuals III-1 and III-2 was in the 10th–25th centile and for individual IV-2 it was below the 5th centile. Other clinical findings were deep set eyes, prominent nasal bridge, prominent ears, club feet and aggressive behaviour (table 1). It is of interest to note that the three carrier females (II-3, III-3 and IV-1) who were examined had mild MR (fig 1A). Individual II-3 is the monozygotic twin of II-2. Unlike her sister, she has mild learning disabilities.
Figure 1.
Mutation analysis of family K8545. (A) Partial pedigree of K8545. The arrow indicates the proband (III-1). Affected males are indicated by a black box and obligate carriers with a dotted circle. The circles with hatched lines indicate that these individuals have mental retardation and learning disability. The numbers below the female symbols indicate the X-inactivation status. (B) Sequence analysis of exon 3 of the JARID1C gene from a control individual and the proband (III-1) showing the G>A substitution (c.229G>A) which is highlighted in yellow. The amino acid change (p.A77T) is shown in blue. Intronic sequences are shown in lower case. (C) A 2% agarose gel electrophoresis of the allele specific polymerase chain reaction (AS-PCR) showing the segregation of the c.229G>A alteration. The 198 bp band was generated by primer sets (Ex3-MSPF/R) specific for the mutation. This band is associated with the mutation. The 310 bp band is generated by a primer set for a control gene (KIAA1111 Ex5F/R).
Family K8835
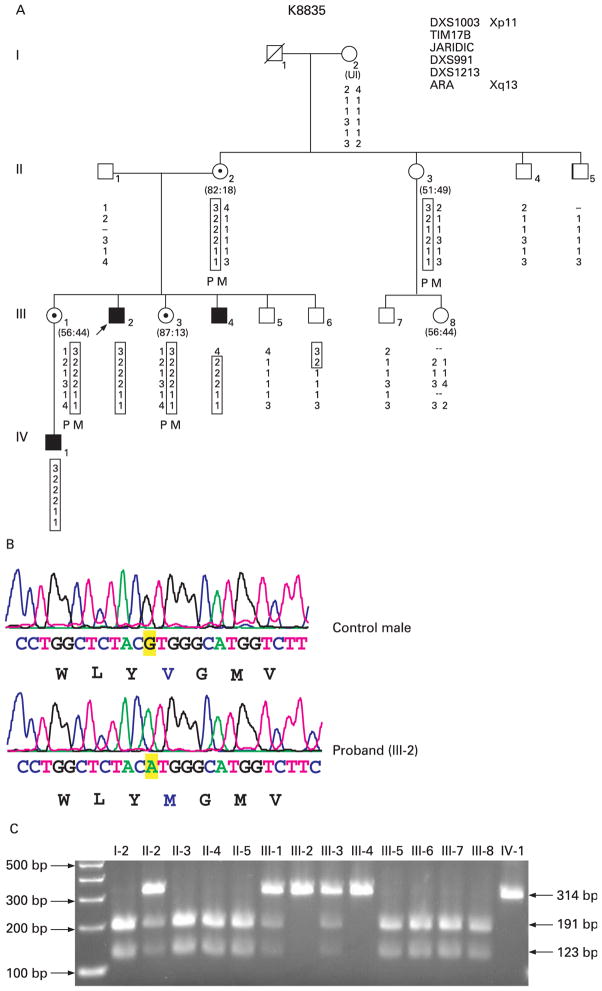
Family K8835 consists of three affected individuals (III-2, III-4 and IV-1) in two generations (fig 2A). All affected individuals had significant developmental and speech delay. The height ranged from the 5th–10th centile. Head circumference ranged from the 75th–95th centile for individuals III-2 and III-4 and in the 25th–50th centile for individual IV-1. Other clinical features included high narrow palate, camptodactyly and clinodactyly of the fifth fingers bilaterally (table 1).
Figure 2.
Mutation analysis of family K8835. (A) Haplotype analysis of family K8835 with microsatellite markers flanking the JARID1C gene. The boxed numbers indicate the haplotype shared among the individuals. The letters “P” and “M” indicate the paternal and the maternal allele, respectively. The haplotype analysis shows that the c.1510G>A mutation is a de novo event arising in the grandpaternal allele of individual II-2. The arrow indicates the proband. Affected males are indicated by a black box and obligate carriers with a dotted circle. The numbers in parentheses under the females indicate the X-inactivation status. (B) Sequence analysis of exon 11 of the JARID1C gene from a control individual and the proband (III-2) showing the G>A substitution (c.1510G>A). The G to A change is highlighted in yellow and the amino acid change (p.V540M) is shown in blue. (C) A 2% agarose gel electrophoresis of the BsaA1 restriction endonuclease digestion of amplified DNA fragments showing the segregation of the c.1510G>A alteration. The normal males have bands of 123 bp and 191 bp, whereas the affected males (III-2, III-4, and IV-1) have a single 314 bp band.
Individual IV-1 has an unusual gait, frequent mood swings and repetitive behaviours including stomping and hand flapping. Two-point linkage in this family had been done previously with 28 polymorphic markers spread along the X chromosome. Linkage was established between DXS1003 at Xp11.3 and DXS990 at Xq21.33 with a maximum LOD score of 1.97 (unpublished data).
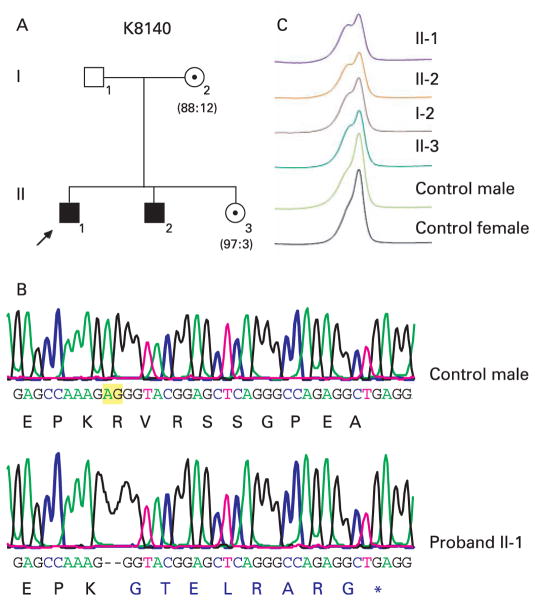
Family K8140
Family K8140 consists of two affected males with significant developmental delay, speech delay, short stature (<3rd–5th centile), macrocephaly (>97th centile), seizures and hyper-reflexia (fig 3A). The proband (II-1) has a history of seizures (table 1). He had developmental delay, spastic diplegia, and pronounced speech delay. He also had a 4×2.2 cm café au lait spot on the left thigh and multiple freckles. Other clinical findings were broad forehead, broad nasal tip and high palate. Individual II-3, the sister who carries the mutation, had normal development except for mild speech delay and increased deep tendon reflexes. Haplotype analysis utilising microsatellite markers on the X chromosome in this family indicated possible linkage to two regions on the X chromosome, DXS996-DXS992 (Xp22.3-Xp21.2) and DXS8015-DXS8017 (Xp11.4-Xq22) (unpublished data).
Figure 3.
Mutation analysis of family K8140: (A) Pedigree of K8140 showing two affected brothers. The arrow indicates the proband (II-1). Obligate carriers are indicated with a dotted circle. The numbers below the females indicate the X-inactivation status. (B) Sequence analysis of exon 26 showing a two bp deletion (c.4441_4442delAG) which is highlighted in yellow. The amino acids in blue indicate five of the novel amino acids which result from the deletion before termination (p.R1418GfsX9). (C) DHPLC chromatogram showing the segregation of the deletion in the family.
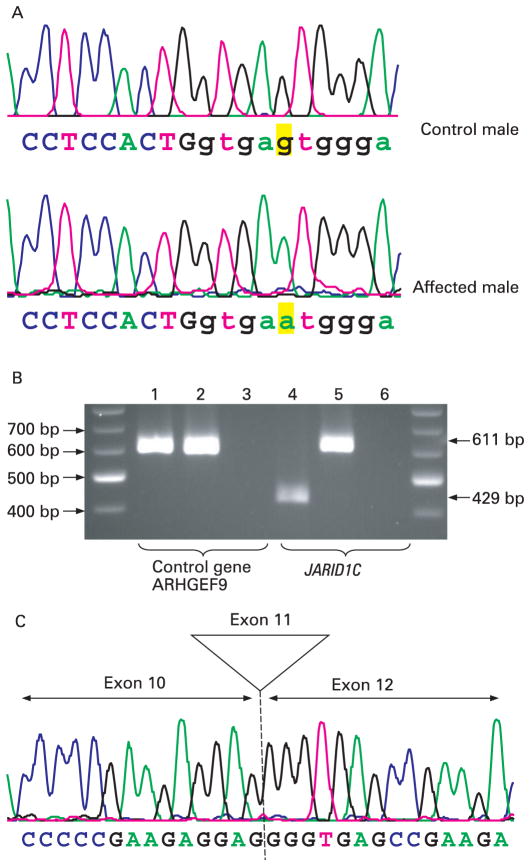
Family K9374
The proband was initially seen at the age 24 years for severe MR. He was adopted soon after birth and details of his childhood and family history were unavailable. The last physical examination at the age of 43 years showed a height of 133.3 cm (<3rd centile), and head circumference of 51.2 cm (<3rd centile) (table 1). The patient was edentulous and had mild pectus excavatum. The hands had mild brachydactyly with shortening of the nails. There was mild asymmetry of the arms with the left forearm slightly longer than the right and a similar discrepancy in the legs. The right foot appeared smaller than the left. He had hypertonia with brisk patellar reflexes, but no clonus.
Mutation screening
Mutation analysis of the 26 coding exons of the JARID1C gene was done by denaturing high performance liquid chromatography (DHPLC) using the WAVE apparatus (Transgenomic equipped with a DNA Sep column (Transgenomic, Omaha, Nebraska, USA) at two to three different melting temperatures. Exon 26 was large and required three sets of overlapping primers to give complete coverage. The sequence of the primers, amplicon size and the DHPLC conditions are available upon request.
Genomic DNA (40–100 ng) from affected individuals was used as a template in a polymerase chain reaction (PCR) to generate amplicons for each exon of the JARID1C gene. To ensure the proper formation of duplex DNA, equal volumes of the PCR product obtained from four patients were mixed together before heteroduplex analysis. The samples were denatured at 95°C for 12 min and allowed to re-nature over 45 min by decreasing the temperature from 95°C to 25°C before DHPLC analysis.
Sequence analysis
DNA fragments exhibiting an abnormal elution profile by DHPLC were sequenced in both directions on the MegaBACE 1000 (GE Healthcare, Piscataway, New Jersey, USA) using the DYEnamic dye terminator kit (GE Healthcare) according to the manufacturer’s protocol. The alignment and analysis of the sequence was done by the DNASTAR program (Lasergene).
X-inactivation studies
X-inactivation was determined by methylation analysis of the androgen receptor (AR) locus as described elsewhere.17
Mutation analysis
For the alteration, c.229G>A in family K8545, an allele specific PCR (AS-PCR) was designed to test for segregation in the family and to screen the normal control samples as described previously.18 The primer pairs used were as follows: Ex3-MSPF: 5′-ctttgagccatttcttcttccTgA- 3′ and MSPR: 5′-tcaaggatattggggtttgg-3′. A 198 bp amplification product was seen only in the patient and not in the control individual. To ensure that DNA from normal individuals could be amplified, the above primers were multiplexed with a primer set from a control gene (KIAA1111 Ex5F/R) which gave a PCR product of 310 bp.
The c.1510G>A alteration in K8835 results in a loss of a BsaA1 restriction site in the abnormal sequence. Therefore, upon digestion with BsaA1 (New England Biolabs, Beverly, Massachusetts, USA), the altered sequence yields an undigested fragment of 314 bp, whereas the normal sequence yields two fragments of 123 bp and 191 bp.
Segregation analysis of the two base pair deletion (c.4441_4443delAG) in family K8140 was done by DHPLC and direct sequencing in both directions.
To test the c.1583+5G>A alteration in K9374, a reverse transcriptase (RT)-PCR experiment was done according to the procedure described previously.17 Primers were designed in exon 9 (5′-tacccgggaatacactctgc-3′) and in exon 13 (5′-ctcctgcacactggtttgtg-3′) in order to flank the alteration in the intron 11. To check the quality of the cDNA generated by the first strand synthesis, primers from the ARHGEF9 gene were used as a control.
Online computational analysis
To predict the effect of the mutations on the JARID1C protein both the PolyPhen (Polymorphism Phenotyping) program (http://genetics.bwh.harvard.edu/pph/) and the SIFT (Sorting Intolerant From Tolerant) program (http://blocks.fhcrc.org/sift/SIFT.html) were utilised.19,20 The donor and acceptor splice sequence was analysed by the splice site calculator (http://genes.mit.edu/burgelab/maxent/Xmaxentscan_scoreseq.html) which implements the scoring method described by Yeo et al.21
RESULTS
Mutation screening of the coding exons of the JARID1C gene using DHPLC identified abnormal patterns in exons 3, 11 and 26 in four probands. In family K8545, a G to A change was found at position 229 (c.229G>A) in exon 3, which resulted in an alanine to threonine change at amino acid 77 (p.A77T) (fig 1B). Alanine is a non-polar amino acid and threonine is an uncharged polar amino acid. PolyPhen analysis predicted that this missense mutation is possibly damaging and the SIFT analysis predicted that the change is not tolerated. This mutation is located in the ARID/BRIGHT domain of the JARID1C protein. Segregation analysis was done by AS-PCR and the mutation was found to segregate with MR in family K8545 (fig 1C). This alteration was not found in 782 X chromosomes (234 males and 274 females) from normal individuals. Although this alteration was found to be located at the boundary of intron 2/exon3 (fig 1B), the acceptor splice site calculations did not reveal any significant change in the information bits present in the normal (8.64) versus the altered sequence (8.12). This was also validated by RT-PCR experiments which revealed that the alteration did not affect the splicing of the JARID1C transcript (data not shown).
In family K8835, DHPLC analysis of exon 11 showed an abnormal pattern. Sequencing of exon 11 revealed a G to A substitution at position 1510 (c.1510G>A) (fig 2B). This alteration changed a conserved valine residue in the JmjC domain at position 504 to a methionine residue (p.V504M). The mutation segregates with the MR in this family (fig 2C). This variant was predicted to be damaging by PolyPhen and not tolerated by the SIFT program. As this family was linked to Xp11.4 the inclusion of the JARID1C mutation in the haplotype revealed that the mutation was a de novo mutation on the paternal allele of individual II-2 (CMS1179). Individual II-2 and her sister (II-3) have the same haplotype, but individual II-3 does not carry the mutation (fig 2A). This alteration was not found in 734 X chromosomes (364 males and 185 females) from normal individuals.
In the family K8140, sequencing of exon 26 revealed a 2 bp deletion (c.4441_4442delAG) which resulted in a frameshift at position 1481 (fig 3B). The frameshift added eight novel amino acids before ending in a stop codon (p.R1481GfsX9) (fig 3B). In addition to the eight novel amino acids, the truncated protein lacks 80 amino acids of the JARID1C protein at the C-terminal end where no conserved domains have been identified. The deletion was found to segregate in the family by both DHPLC and sequence analysis (fig 3C).
For K9374, sequencing of exon 11 revealed a base substitution of a G for an A in intron 11 at position +5 of the donor splice site (c.1583+5G>A) (fig 4A). The donor splice site calculation revealed that the maximum entropy (MaxENT) for the normal donor splice site (5′-CTGgtgagt-3′) in intron 11 was 10.10 which was significantly reduced to 3.53 for the altered sequence (5′-CTGgtgaat-3′). The ideal MaxENT score for a 5′ splice site is 11.81 and the greater the MaxENT value, the more efficient the splicing.21 This prompted us to analyse the effect of this alteration at the RNA level. RT-PCR analysis of K9374 revealed that only an abnormal transcript was present in the proband (fig 4B). Sequence analysis revealed that this abnormal transcript completely skipped exon 11, which caused a frame-shift mutation resulting in a stop codon (p.E468GfsX2) (fig 4C). Thus the truncated JARID1C protein lacks the JmjC domain, the C5HC2 domain and the PHD finger domain. Theoretically, a mutation in the 5′donor splice site should result in the inclusion of the intronic sequence, but skipping of the preceding exon has been observed in several cases.22,23
Figure 4.
Mutation analysis of family K9374. (A) Sequence analysis of exon 11 showing a G>A substitution (c.1583+5G>A) in intron 11 of the JARID1C gene. The G>A change is highlighted in yellow. The sequence from intron 11 is in lower case showing the exon/intron junction. (B) Reverse transcriptase-polymerase chain reaction (RT-PCR) analysis of K9374—lanes 1 and 4; affected male; lanes 2 and 5: control male; lanes 3 and 6: negative control (H2O). Lanes 1 and 2 show the amplification of a 600 bp band from a control gene (ARHGEF9). Lane 4 shows the 429 bp band from the affected male which is lacking the exon 11 of the JARID1C gene, whereas in lane 5 there is normal amplification of a 611 bp band from the JARID1C gene spanning exons 9 to 13. (C) Sequence chromatogram showing the sequence across the exon10/exon12 junction of the abnormal transcript.
DISCUSSION
JARID1C belongs to a family of JARID1 proteins. It functions as a histone demethylase specific for di- and trimethyated histone 3 lysine 4 (H3K4) and may play an important role in development.11,24 Iwase et al11 found that four previously published JARID1C mutations (p.A388P, p.F642L, p.L731F and p.Y751L) reduced the JARID1C demethylase activity approximately 30–70% when compared to the wild type enzyme.
The two missense mutations, p.A77T and p.V504M, affect highly conserved alanine and valine residues, respectively, in the four members of the JARID1 family of proteins as well as in the orthologous proteins from different organisms (fig 5). The p.A77T mutation in K8545 is located in the ARID/BRIGHT domain. Proteins consisting of ARID domains bind to DNA by interacting with specific AT-rich sequences and are involved in chromatin remodelling.25,26 In the p.A77T mutation, a non-polar alanine is replaced by threonine, an uncharged polar amino acid. PolyPhen analysis of this variant predicted it to be possibly damaging. This mutation could affect the demethylase activity of the JARID1C protein.
Figure 5.
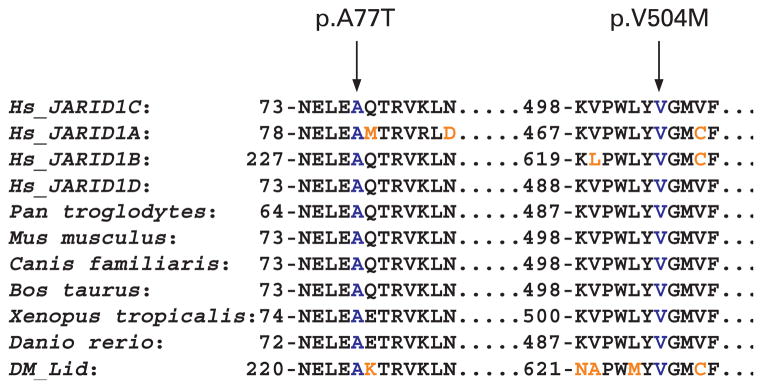
Partial amino acid sequence alignment of the JARID1C (ENSP00000364550) protein. Sequence alignment of the four members of JARID1 family of proteins (Hs_JARID1C, Hs_JARID1A, Hs_JARID1B and Hs_JARID1D) and the JARID1C orthologous proteins in Pan troglodytes (ENSPTRP00000038795), Mus musculus (ENSMUSP00000080814), Canis familiaris (ENSCAFP00000023835), Bos taurus (ENSBTAP00000019893), Xenopus tropicalis (ENSXETP00000015599), Danio rerio (ENSDARP00000040942), and Dm_lid (NP_723140). The missense mutations are indicated by the arrows and blue letters. Amino acids that differ from the JARID1C protein are shown in brown.
The p.V504M mutation found in the K8835 family falls in the JmjC domain of the JARID1C protein. Valine 504 is highly conserved (fig 5). At present, without any functional studies, it is not clear how the substitution of a valine with a methionine could affect the function of the JARID1C protein since both amino acids have hydrophobic side chains, although methionine does contain a sulfur group. However, the mutation is in the JmjC domain. In this domain, the amino acid histidine at position 514 is predicted to be critical for folding of the protein to form an enzymatically active pocket that binds to the cofactor Fe(II).27 A point mutation of this amino acid (p.H514Y) abolishes the demethylase activity of the JARID1C protein.11 Since the V504 residue is very close to this histidine, functional analyses utilising this mutation may provide insight into the demethylation activity of the JARID1C protein.
The two frameshift mutations, p.E468GfsX2 and p.R1481GfsX9, result in premature truncation of the JARID1C protein. The p.E468GfsX2 mutation is in the JmjC domain which results in a JARID1C protein that lacks the JmjC domain, C5HC2 domain and one PHD finger domain. The p.R1481GfsX9 mutation is in the C-terminal end of the protein and results in the deletion of 80 amino acids of the JARID1C protein.
In this study three different cohorts were screened for mutations in the JARID1C gene (table 2).
Table 2.
Breakdown of different cohorts screened for mutations in the JARID1C gene
| Cohorts | Number | Mutations found (%) |
|---|---|---|
| Probands from linked families | 23 | 2 (8.6) |
| Probands from small families with no linkage information | 172 | 1 (0.6) |
| Probands with short stature | 92 | 1 (1.6) |
We found two mutations in 23 linked families (9%), whereas Jensen et al13 reported two mutations in 31 linked families (6.4%). We also found one mutation in 172 probands without linkage information (0.6%), whereas Jensen et al13 reported five mutations in 179 families (2.8%) and Tzschach et al14 reported five mutations in 144 families (3.4%). This difference in the percentages may be attributed to the fact that a fraction of our 172 probands were males whose family history could not rule out autosomal inheritance. Finally, in our study, one mutation was found in a third cohort consisting of 92 probands (1%) with short stature (table 2).
In this study, the range of mental retardation consisted of mild (25%), moderate (12%) and severe (63%) (table 1). Clinical evaluations did not reveal any consistent phenotype. Most of the affected individuals had short stature (55%) and hyper-reflexia (78%); a minority had seizures (33%) and aggressive behaviour (44%). Carrier females did not have any unusual features except three of four carrier individuals in family K8545 had mild MR. Based on the clinical findings listed in tables 1 and 3, males with MR, short stature, hyperreflexia and, to a lesser degree, seizures or aggressive behaviour should be considered for mutation screening in the JARID1C gene.
Table 3.
Summary of mutations and the clinical findings in the patients in this study
| Kindred | Nucleotide change* | Amino acid change | Domain | Clinical information |
|---|---|---|---|---|
| K8545 | c.229G>A | p.A77T | ARID/Bright | MR, short stature, strabismus, broad hand, hyperreflexia |
| K9374 | c.1583+5G>A | p.E468GfsX2 | JmjC | MR, short stature, microcephaly, maxillary hypoplasia, hyperreflexia |
| K8835 | c.1510G>A | p.V504M | JmjC | MR, short stature, high narrow palate, hyperreflexia, aggressive behaviour |
| K8140 | c.4441_4442delAG | p.R1481GfsX9 | MR, short stature, macrocephaly, hyperreflexia, seizures |
MR, mental retardation.
Mutation nomenclature is based on GenBank NM_004187.2, with nucleotide +1 as the A of the ATG initiation codon.
Acknowledgments
We would like to express our gratitude to the families for their participation in this study. We are also grateful to Linda M Wolf, MS, CGC, for providing family K8835. We would like to thank John Archie for helping with the splice site calculations and Cindy Skinner for sample coordination. We would like to acknowledge the technical assistance of Raewyn Lowe and Dana Schultz for DHPLC analysis and DNA sequencing respectively. This paper is dedicated to the memory of Ethan Francis Schwartz 1996–1998.
Funding: This work was supported, in part, by grants from NICHD (HD26202) to CES and the South Carolina Department of Disabilities and Special Needs (SCDDSN).
Footnotes
Competing interests: None declared.
Patient consent: Obtained.
Study contribution from CAM during employment at the Department of Medical and Molecular Genetics, Indiana University School of Medicine, Indianapolis, Indiana, USA.
References
- 1.Schalock RL, Luckasson RA, Shogren KA, Borthwick-Duffy S, Bradley V, Buntinx WH, Coulter DL, Craig EM, Gomez SC, Lachapelle Y, Reeve A, Snell ME, Spreat S, Tassé MJ, Thompson JR, Verdugo MA, Wehmeyer ML, Yeager MH. The renaming of mental retardation: understanding the change to the term intellectual disability. Intellect Dev Disabil. 2007;45:116–24. doi: 10.1352/1934-9556(2007)45[116:TROMRU]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 2.Larson SA, Lakin KC, Anderson L, Kwak Lee N, Anderson D. Prevalence of mental retardation and developmental disabilities: estimates from the 1994/1995 national health interview survey disability supplements. Am J Ment Retard. 2001;106:231–52. doi: 10.1352/0895-8017(2001)106<0231:POMRAD>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 3.Brosco JP, Mattingly M, Sanders LM. Impact of specific medical interventions on reducing the prevalence of mental retardation. Arch Pediatr Adolesc Med. 2006;160:302–9. doi: 10.1001/archpedi.160.3.302. [DOI] [PubMed] [Google Scholar]
- 4.Stevenson RE. Advances in X-linked mental retardation. Curr Opin Pediatr. 2005;17:720–4. doi: 10.1097/01.mop.0000184290.57525.fb. [DOI] [PubMed] [Google Scholar]
- 5.Stevenson RE, Schwartz CE, Schroer RJ. X-linked mental retardation. New York: Oxford University Press; 2000. [Google Scholar]
- 6.Renieri A, Pescucci C, Longo I, Ariani F, Mari F, Meloni I. Non-syndromic X-linked mental retardation: from a molecular to a clinical point of view. J Cell Physiol. 2005;204:8–20. doi: 10.1002/jcp.20296. [DOI] [PubMed] [Google Scholar]
- 7.Kleefstra T, Hamel BC. X-linked mental retardation: further lumping, splitting and emerging phenotypes. Clin Genet. 2005;67:451–67. doi: 10.1111/j.1399-0004.2005.00434.x. [DOI] [PubMed] [Google Scholar]
- 8.Raymond FL. X linked mental retardation: a clinical guide. J Med Genet. 2006;43:193–200. doi: 10.1136/jmg.2005.033043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ropers HH. X-linked mental retardation: many genes for a complex disorder. Curr Opin Genet Dev. 2006;16:260–9. doi: 10.1016/j.gde.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 10.Chiurazzi P, Schwartz CE, Gecz J, Neri G. XLMR genes: update 2007. Eur J Hum Genet. 2008;16:422–4. doi: 10.1038/sj.ejhg.5201994. [DOI] [PubMed] [Google Scholar]
- 11.Iwase S, Lan F, de la Torre-Ubeita L, Huart M, Qi HH, Whetstine JR, Bonni A, Roberts TM, Shi Y. The X-linked mental retardation gene SMCX/JARID1C defines a family of histone H3 lysine 4 demethylases. Cell. 2007;128:1077–88. doi: 10.1016/j.cell.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 12.Tahiliani M, Mei P, Fang R, Leonor T, Rutenberg M, Shimizu F, Li J, Rao A, Shi Y. The histone H3K4 demethylase SMCX links REST target genes to X-linked mental retardation. Nature. 2007;447:601–5. doi: 10.1038/nature05823. [DOI] [PubMed] [Google Scholar]
- 13.Jensen LR, Amende M, Gurok U, Moser B, Gimmel V, Tzschach A, Janecke AR, Tariverdian G, Chelly J, Fryns JP, Van Esch H, Kleefstra T, Hamel B, Moraine C, Gecz J, Turner G, Reinhardt R, Kalscheuer VM, Ropers HH, Lenzner S. Mutations in the JARID1C gene, which is involved in transcriptional regulation and chromatin remodeling, cause X-linked mental retardation. Am J Hum Genet. 2005;76:227–36. doi: 10.1086/427563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tzschach A, Lenzner S, Moser B, Reinhardt R, Chelly J, Fryns JP, Kleefstra T, Raynaud M, Turner G, Ropers HH, Kuss A, Jensen LR. Novel JARID1C/SMCX mutations in patients with X-linked mental retardation. Hum Mutat. 2006;27:389. doi: 10.1002/humu.9420. [DOI] [PubMed] [Google Scholar]
- 15.Santos C, Rodriguez-Revenga L, Madrigal I, Badenas C, Pineda M, Mila M. A novel mutation in JARID1C gene associated with mental retardation. Eur J Hum Genet. 2006;14:583–6. doi: 10.1038/sj.ejhg.5201608. [DOI] [PubMed] [Google Scholar]
- 16.Adegbola A, Gao H, Sommer S, Browning M. A novel mutation in JARID1C/SMCX in a patient with autism spectrum disorder (ASD) Am J Med Genet A. 2008;146:505–11. doi: 10.1002/ajmg.a.32142. [DOI] [PubMed] [Google Scholar]
- 17.Abidi FE, Cardoso C, Lossi AM, Lowry RB, Depetris D, Mattei MG, Lubs HA, Stevenson RE, Fontes M, Chudley AE, Schwartz CE. Mutation in the 5′ alternatively spliced region of the XNP/ATR-X gene causes Chudley-Lowry syndrome. Eur J Hum Genet. 2005;13:176–83. doi: 10.1038/sj.ejhg.5201303. [DOI] [PubMed] [Google Scholar]
- 18.Abidi FE, Miano MG, Murray JC, Schwartz CE. A novel mutation in the PHF8 gene is associated with X-linked mental retardation with cleft lip/cleft palate. Clin Genet. 2007;72:19–22. doi: 10.1111/j.1399-0004.2007.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramensky V, Bork P, Sunyaev S. Human non-synonymous SNPs: server and survey. Nucleic Acids Res. 2002;30:3894–900. doi: 10.1093/nar/gkf493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng PC, Henikoff S. Predicting deleterious amino acid substitutions. Genome Res. 2001;11:863–74. doi: 10.1101/gr.176601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeo G, Burge CB. Maximum entropy modeling of short sequence motifs with applications to RNA splicing signals. J Comput Biol. 2004;11:377–94. doi: 10.1089/1066527041410418. [DOI] [PubMed] [Google Scholar]
- 22.Schneider S, Wildhardt G, Ludwig R, Royer-Pokora B. Exon skipping due to a mutation in a donor splice site in the WT-1 gene is associated with Wilms’ tumor and severe genital malformations. Hum Genet. 1993;91:599–604. doi: 10.1007/BF00205087. [DOI] [PubMed] [Google Scholar]
- 23.Sakai N, Santamarina-Fojo S, Yamashita S, Matsuzawa Y, Brewer HB., Jr Exon 10 skipping caused by intron 10 splice donor site mutation in cholesteryl ester transfer protein gene results in abnormal downstream splice site selection. J Lipid Res. 1996;37:2065–73. [PubMed] [Google Scholar]
- 24.Christensen J, Agger K, Cloos PAC, Pasini D, Rose S, Sennels L, Rappsilber J, Hansen KH, Salcini AE, Helin K. RBP2 belongs to a family of demethylases, specific for tri-and dimethylated lysine 4 on histone 3. Cell. 2007;128:1063–76. doi: 10.1016/j.cell.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Kortschak RD, Tucker PW, Saint R. ARID proteins come in from the desert. Trends Biochem Sci. 2000;25:294–9. doi: 10.1016/s0968-0004(00)01597-8. [DOI] [PubMed] [Google Scholar]
- 26.Kim S, Zhang Z, Upchurch S, Isern N, Chen Y. Structure and DNA-binding sites of the SWI1 AT-rich interaction domain (ARID) suggests determinants for sequence-specific DNA recognition. J Biol Chem. 2004;279:16670–6. doi: 10.1074/jbc.M312115200. [DOI] [PubMed] [Google Scholar]
- 27.Klose RJ, Kallin EM, Zhang Y. JmjC-domain-containing proteins and histone demethylation. Nat Rev Genet. 2006;7:715–27. doi: 10.1038/nrg1945. [DOI] [PubMed] [Google Scholar]