Abstract
The hippocampus is classically involved in memory consolidation, spatial navigation and is involved in the stress response. Migraine is an episodic disorder characterized by intermittent attacks with a number of physiological and emotional stressors associated with or provoking each attack. Given that migraine attacks can be viewed as repeated stressors, alterations in hippocampal function and structure may play an important role in migraine pathophysiology. Using high-resolution magnetic resonance imaging, hippocampal morphometric and functional differences (in response to noxious heat stimulation) were compared in age and gender-matched acute episodic migraineurs with high (HF) versus low (LF) frequency of migraine attacks. Morphometric results were compared with age and gender-matched healthy control (HC) cohort. Significant larger bilateral hippocampal volume was found in LF group relative to the HF and HC groups suggestive of an initial adaptive plasticity that may then become dysfunctional with increased frequency. Functional correlates of greater deactivation (LF > HF) in the same hippocampal regions in response to noxious stimulation was also accompanied by overall reduction in functional connectivity of the hippocampus with other brain regions involved in pain processing in the HF group. The results implicate involvement of hippocampus in the pathophysiology of the migraine.
Keywords: Headache, Pain, Migraine, fMRI, Functional connectivity, Morphometry
Introduction
The hippocampus is classically known to be involved in memory and learned behavior (Eichenbaum et al. 1999; van der Flier and Scheltens 2009) and seizure activity (Schwartzkroin 1994). It is a key brain region providing inhibitory feedback to the hypothalamic-pituitary-adrenal axis (HPA axis) that controls reactions to stress. Hippocampus is intimately affected by stress and glucocorticoids, working in concert with excitatory amino acids and other intracellular and extracellular mediators (McEwen 1999,2001; McEwen and Gianaros 2010b; Rodrigues et al.2009); and these mediators, as a consequence of their increased levels and activity under prolonged stress, may alter hippocampus both structurally (regeneration of neurons, loss of synapses) and functionally (abnormal level of neurotransmitters, impaired inhibition) (Rothman and Mattson 2010) and cause damage. The initial changes influenced by these mediators however may be completely adaptive to maintain homeostasis in the face of new challenges or stressors (McEwen 1999).
There is growing support for the concept of hippocampus being involved in pain processing (Khanna and Sinclair 1989; Liu and Chen 2009; McKenna and Melzack 1992; Prado and Roberts 1985; Rains 2009; Sauro and Becker 2009; Yeung et al. 1977). More recent imaging studies of pain in humans report hippocampal activation in a variety of conditions (e.g., Becerra et al. 2001; Ploghaus et al.2003) and such activation may be associated with processing related to anxiety (Bingel et al. 2011; Ploghaus et al. 2001). One notion based on such studies is that the hippocampal formation amplifies aversive effects as a protective mechanism to define appropriate behavioral responses.
Migraine is an episodic pain disorder characterized by intermittent attacks. A number of physiological and emotional stressors are associated with or provoke each migraine attack which may result in deleterious effects on the hippocampus through repeated glucocorticoid release (Ising and Braun 2000) as well as increased excitatory amino acid activity (McEwen 1999). Given that migraine is a complex behavior that includes prodromal phase with underlying behavioral changes (e.g., tiredness, yawning) and also changes in brain systems (viz., cortical spreading depression) (Eikermann-Haerter and Ayata 2010; Dreier 2011) differences in the appraisal of pain and sensitivity may differ across patients as a function of the attack frequency and such changes may alter neural networks that are involved in perceptual, autonomic and other components of pain (Piche et al. 2010). Such changes may also be maladaptive in the long term (allostatic load) (McEwen 1998; McEwen 2001, 2008) by potentially causing damage with repeated stressful challenges and/or by failure to shut off the response after the challenge is past. The role of the hippocampus in migraine pathology however has not been characterized. In our prior studies in migraine patients, we reported hippocampal and temporal lobe dysfunction when compared with healthy controls (Moulton et al. 2010) suggesting that hippocampal–temporal regions are altered by migraine.
In this study, we hypothesized that the increased frequency of migraine attacks which leads to increased repeated exposure to the stressors in migraine would involve differences in hippocampus as a function of the frequency of migraine attacks. Hippocampal morphometric and functional differences were compared in acute episodic migraineurs for whom the migraine attacks had progressed and had high frequency of migraine attacks to those who had low frequency migraine attacks. By evaluating multimodal aspects of hippocampal function and structure, a pattern of significant alteration in this region in migraine patients is presented.
Methods
Subjects
The study met the criteria of the Helsinki accord for experimentation of pain in human subjects (http://www.history.nih.gov/research/downloads/helsinki.pdf) and approved informed consent forms were obtained from all subjects. Migraine subjects included in the study were chosen from screening a group of 60 patients, out of which 10 subjects were selected for each group, matched for age, age of onset, and medication type. The subjects (1) met the criteria for episodic migraine as classified as per the International Classification for Headache (ICHD-2; http://www.i-h-s.org/upload/ct_clas/ihc_II), (2) had Beck Depression Inventory II (BDI-II) scores ≤25, (3) suffered from episodic migraine for three years or longer, (4) had no migraine 72 h prior to the scan and no symptoms of developing one during or 24 h after the scan, (5) LF sufferers (3M, 7F, age 40.2 ± 3.6) had 1–2 and HF sufferers (3M, 7F, age 43.9 ± 3.4) had 8–14 headache days per month; and (6) stable frequency levels were present for at least a year prior to the scan and (7) all subjects were right handed. For comparison, morphometric data of age- and gender-matched healthy controls (3M, 7F, age 39.1 ± 3.2) with no history of migraine were acquired and analyzed. None of the participants reported the use of either opioids or barbiturates (Bigal et al. 2008).
Quantitative sensory testing
Quantitative sensory testing (QST) was performed using a 1.6 cm × 1.6 cm contact thermode (TSA-II, Medoc Advanced Medical Systems, Ramat Yishai, Israel) prior to the MR session to determine the pain threshold for each subject. The temperature increased from a 32 °C baseline temperature at the 1 °C/s rate until stopped by the subject at the first onset of pain. This test was repeated three times and the thresholds were averaged and the corresponding temperature was recorded as the pain threshold (THR). QST was performed on the dorsum of the hand on the predominant side of migraine attacks. If there was no predominant side, the QST was performed on the left side. 1/20 patients had only left side and 2/20 had only right-sided attacks with the rest experiencing migraine attacks on both sides with either equal attacks on both sides (5/20), or predominant left-sided (5/20) or predominantly right-sided attacks (7/20).
Noxious thermal stimulation
Similar to QST, thermal stimulation side was the migraine dominant side. If there was no predominant side, the stimulation was delivered to the left hand. For stimulation during functional imaging, three blocks of stimulation (30 s baseline/15 s stimulation @THR + 1) were delivered from a baseline temperature of 32 °C. The rate of temperature change was 4 °C/s. The 15 s pain stimulation period did not include the ramp-up and ramp-down periods of the thermode from the baseline temperature. The ramps were modeled in defining the explanatory variables (EVs) for fMRI data analysis. During the stimulations and fMRI data collection, subjects rated their pain using a visual analog scale (VAS) system.
Imaging
All data were collected on a 3 Tesla Siemens Trio scanner with an 8-channel phased array head coil (Erlangen, Germany). For structural data, high-resolution T1-weighted datasets were collected from each patient using a 3D MPRAGE pulse sequence (TR/TE/TI = 2,100/2.74/1,100 ms, FA = 12, 128 sagittal slices, res = 1.33 × 1.0 × 1.0 mm3). For acquiring functional data, a gradient-echo (GE) echo planar imaging (EPI) sequence (TE/TR = 30/2,500, res = 3.5 × 3.5 × 3.5 mm3, matrix = 64 × 64, 74 volumes, 41 slices) was used.
Data analysis
Structural analysis
Segmentation was performed with the automatic parcellation tools of the Freesurfer image analysis software (http://www.surfer.nmr.mgh.harvard.edu/). These tools enable labeling subcortical and cortical tissue classes using an atlas-based Bayesian segmentation procedure (Fischl et al. 2002). The processing steps included motion correction of the volumetric T1-weighted MPRAGE images, followed by removal of non-brain tissue (Segonne et al. 2004), automated Talairach transformation, and segmentation of the subcortical white matter and deep gray matter volumetric structures (Fischl et al. 2002, 2004) including the left and right hippocampus in each subject. Using IBM SPSS 19.0 statistics package, analysis of variance (ANOVA) was used to compare hippocampal volume in the three cohorts for both left and right hippocampus separately. Post hoc pairwise group comparisons were explored with Tukey’s honestly significant difference. Pearson correlation values were calculated for the left/right hippocampal volumes and an estimate of the total number of migraine attacks experienced throughout life for each patient. One subject from the HF cohort and two subjects from the LF cohort were not able to specify the exact age of their migraine onset instead they remembered the age range (ex. started during high school years). For these subjects, number of migraine attacks was estimated using the lower end range, mid range, and higher end range values. The estimated linear correlation between the hippocampal size and the number of migraine attacks was still significant for all of these scenarios. These subjects were not included in the final correlation analysis results reported due to the lack of information on the exact age of migraine onset for them. As a separate control, anatomical data of a separate cohort of 22 migraine patients (11 women and 11 men) were also analyzed and the correlation of the total number of attacks (frequency × duration) experienced throughout life (ranging from 140 to 4,100 with no significant difference between men and women) with the hippocampal volume was measured.
Functional analysis
fMRI analysis was carried out using FMRIB Software Library (FSL) (http://www.fmrib.ax.ac.uk/fsl), version 4.1.3. The initial two volumes were removed from each of the functional scans to allow for signal equilibration. The pre-statistical processing for each subject consisted of skull stripping using a brain extraction tool (BET) with bias field correction and neck removal and motion correction. The volumes were spatially smoothed with a 5-mm full-width at half-maximum filter, and a 60-s high-pass temporal filter was applied. First-level fMRI analysis of single subject data was performed using FMRI Expert Analysis Tool (FEAT) Version 5.98. Patients with right-sided migraines had their images flipped along the y axis to correspond with the majority of the patients with left-sided migraine. The recorded temperature traces for each subject during the MRI scan were rescaled from 0 to 1 and were used as explanatory variables (EVs) along with their temporal derivatives to achieve a better fit to the data. EVs were convolved with a gamma function (phase shift = 0 s, half width = 3 s, and a mean lag = 6 s) as the hemodynamic response function. The ramp ups and ramp downs were modeled in defining the explanatory variables (EVs) for fMRI data analysis. Subjects’ brains were spatially normalized to the MNI152 brain for group analysis. Normalization was performed using FLIRT (FMRIB’s Linear Image Registration Tool) following a two-stage process: first a low-resolution image of the whole brain (that was acquired with the similar imaging parameters as to the FMRI acquisition separately) was linearly registered to the high-resolution structural MPRAGE image. Then, the MPRAGE image was linearly (affine) registered to the standard image (MNI 152 average brain). These two transformations were combined, to transform the low-resolution FMRI images (and the statistic images derived from the first-level analyses) straight into standard space, when applied later, during group analysis.
Group activation maps were generated by fMRI expert analysis tool (FEAT) fMRIB’s Local Analysis of Mixed Effects (FLAME1). Statistical parametric maps were thresholded using a Gaussian Mixture Model (GMM) technique (Pendse et al. 2009). In this approach, the overall z statistic distribution is modeled by combination of Gaussian distributions representing “deactivation”, “null” and “activation” distributions. Various causes, such as unmodeled structured noise, signal inhomogeneities, variance in vascular flow or BOLD response could cause a deviation in the theoretical N(0,1) “null” distribution. Hence, instead of basing inference on a fixed parametric form (such as N(0,1)), the “null” distribution is adaptively estimated from the data. Alternative hypothesis testing is then performed based on the “activation” and “deactivation” maps thresholded at posterior probability P > 0.5.
Functional connectivity (Fc) analysis
The strength of correlation of fMRI response across voxels in the brain with anatomical hippocampus ROI was measured using a seed correlation-based approach (Fox et al. 2005; Zhang et al. 2008) for each subject and the two cohorts were compared using mixed effects group analysis (FLAME1). The ROIs for the Fc analysis were extracted by automatic segmentation of the T1-weighted anatomical volumes for each subject individually using Freesurfer (http://www.surfer.nmr.mgh.harvard.edu/). Preprocessing steps were similar to the steps described for functional analysis above. For each subject, the WM and CSF masks were created in anatomical space using Freesurfer tools (http://www.surfer.nmr.mgh.harvard.edu/). All time courses in the brain were orthogonalized with respect to the eigen time courses of WM and CSF masks that were computed by singular value decomposition (SVD) (Golub and Loan 1996). fMRI time courses from each seed ROI were also extracted using SVD. The time courses were normalized for general linear model (GLM) analysis. The resulting GLM analysis parameter estimates (correlation coefficients) were transformed into normally distributed quantities using a Fisher z transform, registered to MNI space and entered into a mixed effects group analysis (FLAME1). The group statistical parametric maps were threshold using a GMM technique (see above).
Results
Psychophysical results
There was no significant difference in the patients’ headache intensity [LF: 7.7 ± 2.4 (mean ± SD), HF: 7.2 ± 1.8 (mean ± SD)] on a 0–10 subjective scale. However, the headache unpleasantness rating was significantly different between the two groups, 8.5 ± 1.8 (mean ± SD) and 6.7 ± 1.4 (mean ± SD) in the LF and HF group, respectively (P < 0.028). There were no significant differences between the QST thresholds (LF: 46.06 ± 4.26 °C and HF: 45.89 ± 2.77 °C). Beck depression index (BDI) ratings for subjects were all <10, and not significantly different of (LF: 2.1 ± 2.5 and HF: 1.9 ± 2.4). HF group had suffered from an average of 9.3 ± 2.6 (mean ± SD) attacks per month, whereas the LF group had an average of 1.7 ± 0.5 attacks per month. HF migraineurs on average had suffered from an average of 2,474 ± 1210 (mean ± SD) migraines throughout their lives compared with an average of 185 ± 75 (mean ± SD) attacks for LF migraineurs.
Structural differences in hippocampus
Significant hippocampal volumetric differences were observed among the three cohorts (left: F = 3.3, P = 0.047; and right: F = 5.061, P = 0.011). Tukey’s test results were as follows (left: HC vs. LF: P < 0.040; LF vs. HF: P < 0.050) and (right: HC vs. LF: P < 0.019; LF vs. HF: P < 0.020). Figure 1 shows the volumetric comparisons for both the (1) raw and (2) normalized hippocampal volumes [normalized to an estimate of the total intracranial volume (eTIV) (Buckner et al. 2004)]. A paired t test was also performed to determine if there were any differences between left and right hippocampus across all of the subjects. The difference between the left and right hippocampus was not significant (P = 0.186), while the correlation between the left and right hippocampal volumes was significantly high (r = 0.703, P = 0.0003). Moreover, smaller hippocampal volume was observed in LF [left r = −0.62 (P < 0.05), right r = −0.64 (P < 0.043)] and HF [left r = −0.63 (P < 0.033), right r = −0.58 (P < 0.049)] cohorts with an increase in the estimate of the total number of migraine attacks that the patients had experienced throughout their lives (Fig. 2). There was a strong correlation between the total number of attacks and the hippocampus volume as for left hemisphere r = −0.55 (P < 0.009) and for right hemisphere r = −0.468 (P < 0.032) in a separate control cohort of 22 episodic migraineurs (Online Resource 1).
Fig. 1.
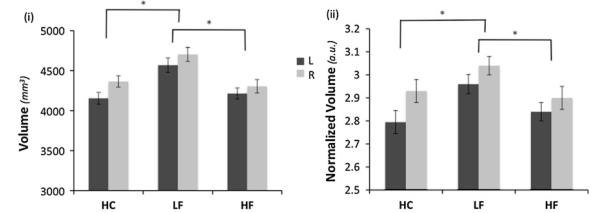
Differences in hippocampal volume. The bar plots show the hippocampus volume comparisons for low frequency (LF), high frequency (HF) and healthy controls (HC). LF migraineurs had a significantly larger hippocampal volume. (i) Raw volume comparisons—for the statistical analysis the left and right hippocampal volumes of the cohorts were compared while using the total intracranial volume and age as covariates. (ii) The same comparison for normalized volumes (normalized to the total intracranial volume) volumes. Bar heights represent the mean value for each volumetric measurement. Error bars represent the 95 % confidence interval of the mean. Asterisk denotes a significance level of the corresponding P value reported (see text)
Fig. 2.
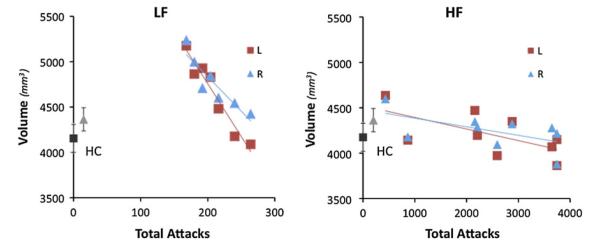
Migraine attacks and hippocampal volumetric differences. The plots represent the correlation between the left and right hippocampal volumes and estimate of the total number of migraine attacks in low frequency (LF left panel) and high frequency (HF right panel) migraine patients. Average hippocampal volume in healthy control (HC) subjects are also shown in gray scale colors in each panel for comparison
Differences in response to painful stimulation
Contrast analysis of the HF versus LF migraine group in response to the “pain threshold +1 °C” stimuli, Fig. 3, revealed significantly higher deactivation response in bilateral hippocampus (posterior probability >0.5, corrected for multiple comparisons using GMM) in the LF patients relative to HF patients, Table 1. It should be noted here that the analysis that was performed was a whole brain analysis and differences in activation response to noxious stimulation were observed in other cortical and subcortical regions of the brain that are not the focus of this paper and will be discussed elsewhere.
Fig. 3.
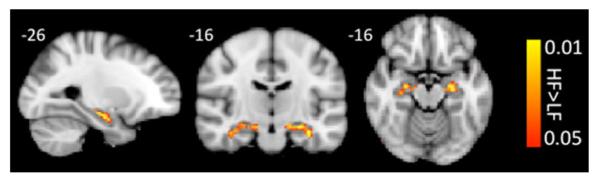
Response to pain. Contrast analysis of the HF versus LF migraine group in response to the “pain threshold +1 °C” stimuli revealed significantly higher deactivation in bilateral hippocampus in the LF patients
Table 1.
Hippocampal clusters resulting from contrast analysis of low versus high frequency (LF>HF) migraine patients in response to noxious heat (threshold +1 °C)
| Region | Laterality | z stat | X (mm) | Y (mm) | Z (mm) | Vol (cm3) |
|---|---|---|---|---|---|---|
| Hippocampus | L | −2.8757 | −34 | −14 | −22 | 0.512 |
| Hippocampus | R | −2.1564 | 24 | −12 | −16 | 0.872 |
Differences in the functional connectivity of hippocampus
Significant differences in functional connectivity of the hippocampus were observed in the high frequency versus the low frequency migraine patients during intermittent heat stimuli (pain threshold +1 °C on hand). Functional connectivity contrast maps (HF–LF) are presented in Fig. 4. Significantly reduced functional connectivity with hippocampus was observed in high frequency versus low frequency migraine patients in contralateral supra marginal gyrus, bilateral temporal pole, contralateral fronto-orbital, bilateral NAc, bilateral anterior insula, bilateral middle frontal and contralateral paracingulate.
Fig. 4.
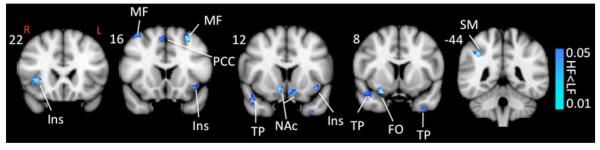
Functional connectivity contrast maps. Functional connectivity contrast map of the hippocampus during intermittent heat stimuli (pain threshold +1 °C on hand) in high versus low frequency migraine patients. PCC posterior cingulate cortex, PCin paracingulate, SM supramarginal, SF superior frontal, Ins (ant) anterior insula, TP temporal pole, NAc nucleus accumbens, FO frontal orbital, MF middle frontal, PAG periaqeductal gray
Discussion
The results of this study suggest significant functional and morphological differences in the hippocampus in migraine patients: (1) significant larger bilateral hippocampal volume was found in LF group relative to the HF and HC groups suggestive of an initial adaptive plasticity in the LF group that may then become dysfunctional with increased frequency, (2) the evidence for hippocampal dysfunction related to functional correlates of greater deactivation in the hippocampus (LF > HF) was also accompanied by overall reduction in functional connectivity of the hippocampus with other brain regions involved in pain processing in the HF group, (3) although no differences were observed for pain intensity between the two groups, significant differences in pain unpleasantness (LF > HF), suggestive of alterations in brain systems involved in affective processing that includes the hippocampus (see below).
Pain unpleasantness and migraine frequency
We found no difference in response to pain intensity (neither for pain threshold) between the two groups. We did expect that there may have been differences in pain intensity based on the data from the field that has suggested, but not defined an increased frequency in allodynia in patients with increased migraine frequency, particularly in chronic daily headache when compared with healthy controls (Schwedt et al. 2011). The reason for this could be that both groups may have had allodynia in their sensitized state, but differences were not observed because of the sensitivity of the quantitative sensory testing. In support of this, as noted in the data from Schwedt et al., no differences were observed between migraine groups (episodic and chronic). However, we did find a significant difference to pain unpleasantness. Although coupled with stimulus intensity, unpleasantness is a term that has been used in the field to describe the subjective experience of the emotional component of pain (i.e., experience of aversion associated with pain) that may include suffering. Howard Fields (1999) has construed pain unpleasantness as (1) primary or stimulus bound unpleasantness; or (2) secondary unpleasantness, a higher level process that has a highly variable relationship to stimulus intensity and is largely determined by memories and contextual features. Thus, our data may reflect a contribution of hippocampal function in emotional processing of pain that may become more severe with increased attacks.
Alterations in hippocampal volume
We are unaware of other reports in the literature on the concurrent hippocampal changes with the migraine disease. The results show a larger volume in LF migraineurs compared with healthy controls. The hippocampal volume in the HF group is comparable to the HC group. The LF migraineurs had ≤2 attacks per month, but significantly the volume trend was to the normative levels with increased number of total attacks (see Fig. 2). We interpret these results as either (1) initial adaptive plasticity of the hippocampus (see Glasper et al. 2011) or (2) a larger hippocampus (i.e., a pre-existing condition) in migraineurs that then decreases with increased attack frequency. Evidence for the first interpretation has come from animal models on neurogenesis in the structure that is known to persist into adulthood. Stress may mediate adaptive structural plasticity through remodeling of dendrites and synapses (McEwen 2010). With repeated stress involved in migraine attacks including pain (Milde-Busch et al. 2011; Peterlin et al.2009), glucocorticoids, cortical spreading depression, gonadal hormones (Craft et al. 2004), elevated and prolonged levels of excitatory amino acids may be released. What begins as a structural and protective response ends up as potential damage to the structure (McEwen 2001). While the HF group has a smaller average hippocampal volume than the LF group the negative correlation with the total number of attacks trend is suggestive of continued morphological rearrangements that may result in abnormal function. Indeed, our findings in the HF group of altered function (increased activity in the hippocampus with a noxious stressor), altered functional connectivity with other brain structures, and alterations in pain unpleasantness in this group are supportive of this notion. Certainly in animals chronic stress can damage the hippocampus, human conditions such as post-traumatic stress disorder. It does not seem that migraineurs have a persisting/genetic contribution relating to having a larger hippocampus since our preliminary studies in pre-adolescent children with and without migraine do not seem to differ. The issue is salient since hippocampal volume has been shown to predict vulnerability to stress in post-traumatic stress disorder (Gilbertson et al. 2002). To clarify these observations, studies in larger cohorts and comparisons between HF and chronic daily headache will be necessary.
The plasticity of the hippocampal volume relates to mechanisms that enhance or diminish dendritric complexity, synapse number, dentate gyrus neuronal number and even novel neural connections (Bourne and Harris 2008; Leuner and Gould 2010; McEwen 2001; McEwen and Gianaros 2010a, b). Plasticity of hippocampal volume is also observed across a number of conditions including: (1) decreased volume in Alzheimer’s disease (Devanand et al. 2007); major depression (Sheline 2003); Cushing’s disease (Starkman et al. 1999); PTSD (Woon et al. 2010); schizophrenia (Koolschijn et al. 2010); a 20-year history of chronic perceived stress (Gianaros et al. 2007); chronic inflammation (Marsland et al. 2008); jet lag (Cho 2001); and acute, or as noted above, in chronic pain (Zimmerman et al. 2009); (2) increased volume is observed after electroconvulsive therapy (Nordanskog et al. 2010) and sustained, moderate exercise (Erickson et al. 2011); and (3) vary in volume in the rostrocaudal extent of the structure with acquisition of special skills (Hufner et al. 2010). Thus, our observation of altered volume in the hippocampus, while not new in central nervous system (CNS) diseases and after chronic stress conditions, is new in the context of migraine and in the context of migraine frequency.
As has been shown in the rodent models of neuropathic pain hippocampus volume loss may be a result of mechanisms that could include dendritic pruning in medial prefrontal cortex (Metz et al. 2009) and as such may correlate with abnormal function. Dendritic spines are the targets of excitatory inputs in the CNS and these spines are modulated by inhibitory GABAergic synapses. Glutamatergic input from cortical pyramidal cells and subcortical sources transmits activity generated through sensory inputs as well as spontaneous activity of the nervous system while dendritic GABAergic inputs regulate these dendritic computations of pyramidal cells (Klausberger 2009). Based on these insights on potential dendritic changes that may alter volume, the repeated (presumably excitatory) inputs to the hippocampus with repeated migraine attacks may drive the changes (McEwen 1999). A similar trend has been observed in subgenual prefrontal cortex in major depression disease (Drevets et al. 1997) where smaller volume and increased activity are reported, which is consistent with an excitatory amino acid induced dendritic shrinkage. Elevated levels of glucocorticoids that occur during stress may play an important contributing role in the process of neuronal cell death in other conditions such as major depression (Sapolsky 2000). Support for abnormal glutamatergic effects on the hippocampus associated with pain have come from both animal and human studies (Niddam et al. 2011; Zimmerman et al. 2009).
Finally, it should be noted that the accuracy of the hippocampal segmentation using Freesurfer has been verified by comparison to the manual tracing with a correlation of r = 0.82 with the manual segmentation (Morey et al. 2009). Moreover, our estimated hippocampal volume in healthy subjects was in agreement with the reported measurements values (4,190 ± 526.7 mm3) of the hippocampal volume using Freesurfer in a healthy cohort (35.4 ± 11 years) (Morey et al. 2009).
Alterations in hippocampal function
We observed significant differences in hippocampal function between the two groups. In addition, these changes were observed to be in the same regions that volumetric alterations are reported here. Similar functional–structural alterations have been reported in the literature for other brain structures (DaSilva et al. 2008) but not for the hippocampus. The hippocampus is one of a number of brain regions involved in stress. Termination of the stress response under the acute stress is through a negative feedback to the hypothalamic–pituitary axis. However, ongoing stress may impair the feedback mechanisms and result in prolonged responses (McEwen 2001). Diminished connectivity was observed with HF < LF for the hippocampus. Our connectivity analysis shows decreased connectivity between regions that included the temporal lobe, insula and nucleus accumbens (see Fig. 4), suggestive of diminished functional outputs. Altered function in the hippocampus may have significant consequences on other systems as has been reported for the hippocampal-accumbens system (see Nason et al. 2011). Such alterations in outputs may also have other effects, for example, hippocampal spreading depression which activates the caudal trigeminal nucleus in rodents (Kunkler and Kraig 2003). In our prior report, painful heat produces hyperexcitability in temporal pole in episodic migraine vs. controls, that also shows an increased connectivity with the hippocampus and the trigeminal nucleus (Moulton et al. 2010). The decreased functional connectivity observed here for HF versus LF may reflect diminished interactions with brain regions involved in cognitive, associative and measures of interoception (insula) as they may contribute to the “migraine” experience. Alternatively, the decreased connectivity may also be interpreted as increased connectivity in the LF group which may be explained in the context of the initial changes of the brain in the form of larger hippocampal volume in the LF cohort that would facilitate increased functional connectivity of the hippocampus in the LF cohort with the rest of the brain specially the areas in pain processing.
Caveats
The number of subjects studied in this study is relatively small in favor of having two cohorts of patients at the two extremes of the disease while controlling for multiple variables, such as age, duration of the disease and etc. Since aside from the frequency, the patients were highly matched, we believe that the despite the small sample size the other sources of variability were well accounted for. Our voxel-based comparisons show significance after correction for multiple comparisons utilizing robust adaptive techniques, such as, mixture modeling to determine statistical significance minimizing false positive as well as false negatives.
Although the subjects were matched, a mixed male/female sample was used in this study. Recent studies have suggested sex-related differences in migraine both in structure and function (Liu et al. 2011; Maleki et al. 2012). Given the sample size and the number of male participants we were not able to determine the influence of sex on the observed differences in the hippocampal volume as a function of migraine attack frequency. However, given our findings on the sex-related structural differences in migraine in (Maleki et al. 2012), where there were no sex-related differences in hippocampus volume in migraineurs, but in parahippocampal gyrus, we believe that it is acceptable to assume that the frequency of attacks will have the same effect on the hippocampal volume in both male and female migraineurs.
In the patient cohorts, the only difference between the cohorts was related to triptan use. This is a potential confounder of the data in that the drug may itself produce changes in brain structure and function, albeit intermittent use with the use with migraine attacks. While the direct effects of triptans are still a matter of debate (Tfelt-Hansen 2010), and longitudinal studies will be needed to clarify their impact on the central nervous system central nervous system central nervous system, there are studies supporting direct effects of triptans on CNS (Boshuisen and den Boer 2000; Cupini and Calabresi 2005; Dodick and Martin 2004).
Conclusions
Our results support a role of the hippocampus in migraine where structural and functional changes may be the result of repeated stress and, as a consequence, that, in turn, alter biological responses (including the stress response) over time, as a negative cascade adding to the disease burden through allostatic overload. These responses would appear to be maladaptive, and lead to allostatic load over time, and have significant implications for disease progression.
Acknowledgments
The work was supported in by grants from NIH (K24 NS064050 (NINDS) and R01 NS056195 (NINDS) and R01-NS073997 (NINDS) to DB) and an Investigator Initiated Grant from Merck and Co.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00429-012-0437-y) contains supplementary material, which is available to authorized users.
Contributor Information
Nasim Maleki, Departments of Anesthesia and Radiology, Center for Pain and the Brain, MCL, MGH and CHB, Children’s Hospital Boston and Harvard Medical School, Boston, MA, USA.
Lino Becerra, Departments of Anesthesia and Radiology, Center for Pain and the Brain, MCL, MGH and CHB, Children’s Hospital Boston and Harvard Medical School, Boston, MA, USA; Department of Psychiatry, P.A.I.N. Group, McLean Hospital and Harvard Medical School, Belmont, MA, USA; Departments of Psychiatry and Radiology, Massachusetts General Hospital, Harvard Medical School, Charlestown, MA, USA.
Jennifer Brawn, Departments of Anesthesia and Radiology, Center for Pain and the Brain, MCL, MGH and CHB, Children’s Hospital Boston and Harvard Medical School, Boston, MA, USA.
Bruce McEwen, Laboratory of Neuroendocrinology, The Rockefeller University, New York, USA.
Rami Burstein, Department of Anesthesia, Beth Israel Deaconess Hospital, Harvard Medical School, Boston, MA, USA.
David Borsook, Departments of Anesthesia and Radiology, Center for Pain and the Brain, MCL, MGH and CHB, Children’s Hospital Boston and Harvard Medical School, Boston, MA, USA; Department of Psychiatry, P.A.I.N. Group, McLean Hospital and Harvard Medical School, Belmont, MA, USA; Departments of Psychiatry and Radiology, Massachusetts General Hospital, Harvard Medical School, Charlestown, MA, USA.
References
- Becerra L, Breiter HC, Wise R, Gonzalez RG, Borsook D. Reward circuitry activation by noxious thermal stimuli. Neuron. 2001;32(5):927–946. doi: 10.1016/s0896-6273(01)00533-5. doi:S0896-6273(01)00533-5. [DOI] [PubMed] [Google Scholar]
- Bigal ME, Serrano D, Buse D, Scher A, Stewart WF, Lipton RB. Acute migraine medications and evolution from episodic to chronic migraine: a longitudinal population-based study. Headache. 2008;48(8):1157–1168. doi: 10.1111/j.1526-4610.2008.01217.x. doi:HED121710.1111/j.1526-4610.2008.01217.x. [DOI] [PubMed] [Google Scholar]
- Bingel U, Wanigasekera V, Wiech K, Ni Mhuircheartaigh R, Lee MC, Ploner M, Tracey I. The effect of treatment expectation on drug efficacy: imaging the analgesic benefit of the opioid remifentanil. Sci Transl Med. 2011;3(70):70ra14. doi: 10.1126/scitranslmed.3001244. doi:10.1126/scitranslmed.3001244. [DOI] [PubMed] [Google Scholar]
- Boshuisen ML, den Boer JA. Zolmitriptan (a 5-HT1B/1D receptor agonist with central action) does not increase symptoms in obsessive compulsive disorder. Psychopharmacology (Berl) 2000;152(1):74–79. doi: 10.1007/s002130000529. [DOI] [PubMed] [Google Scholar]
- Bourne JN, Harris KM. Balancing structure and function at hippocampal dendritic spines. Annu Rev Neurosci. 2008;31:47–67. doi: 10.1146/annurev.neuro.31.060407.125646. doi:10.1146/annurev.neuro.31.060407.125646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23(2):724–738. doi: 10.1016/j.neuroimage.2004.06.018. doi:10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Cho K. Chronic ‘jet lag’ produces temporal lobe atrophy and spatial cognitive deficits. Nat Neurosci. 2001;4:567–568. doi: 10.1038/88384. [DOI] [PubMed] [Google Scholar]
- Craft RM, Mogil JS, Aloisi AM. Sex differences in pain and analgesia: the role of gonadal hormones. Eur J Pain. 2004;8(5):397–411. doi: 10.1016/j.ejpain.2004.01.003. doi:10.1016/j.ejpain.2004.01.003S1090380104000175. [DOI] [PubMed] [Google Scholar]
- Cupini LM, Calabresi P. Medication-overuse headache: pathophysiological insights. J Headache Pain. 2005;6(4):199–202. doi: 10.1007/s10194-005-0184-z. doi:10.1007/s10194-005-0184-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DaSilva AF, Becerra L, Pendse G, Chizh B, Tully S, Borsook D. Colocalized structural and functional changes in the cortex of patients with trigeminal neuropathic pain. PLoS One. 2008;3(10):e3396. doi: 10.1371/journal.pone.0003396. doi:10.1371/journal.pone.0003396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devanand DP, Pradhaban G, Liu X, Khandji A, De Santi S, Segal S, Rusinek H, Pelton GH, Honig LS, Mayeux R, Stern Y, Tabert MH, de Leon MJ. Hippocampal and entorhinal atrophy in mild cognitive impairment: prediction of Alzheimer disease. Neurology. 2007;68(11):828–836. doi: 10.1212/01.wnl.0000256697.20968.d7. doi:10.1212/01.wnl.0000256697.20968.d7. [DOI] [PubMed] [Google Scholar]
- Dodick DW, Martin V. Triptans and CNS side-effects: pharmacokinetic and metabolic mechanisms. Cephalalgia. 2004;24(6):417–424. doi: 10.1111/j.1468-2982.2004.00694.x. doi:10.1111/j.1468-2982.2004.00694.xCHA694. [DOI] [PubMed] [Google Scholar]
- Dreier JP. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat Med. 2011;17(4):439–447. doi: 10.1038/nm.2333. doi:10.1038/nm.2333. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386(6627):824–827. doi: 10.1038/386824a0. doi:10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Dudchenko P, Wood E, Shapiro M, Tanila H. The hippocampus, memory, and place cells: is it spatial memory or a memory space? Neuron. 1999;23(2):209–226. doi: 10.1016/s0896-6273(00)80773-4. doi:S0896-6273(00)80773-4. [DOI] [PubMed] [Google Scholar]
- Eikermann-Haerter K, Ayata C. Cortical spreading depression and migraine. Curr Neurol Neurosci Rep. 2010;10(3):167–173. doi: 10.1007/s11910-010-0099-1. doi:10.1007/s11910-010-0099-1. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108(7):3017–3022. doi: 10.1073/pnas.1015950108. doi:101595010810.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields HL. Pain: an unpleasant topic. Pain Suppl. 1999;6:S61–S69. doi: 10.1016/S0304-3959(99)00139-6. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. doi:S089662730200569X. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, Dale AM. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23(Suppl 1):S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. doi:10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. doi:10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Jennings JR, Sheu LK, Greer PJ, Kuller LH, Matthews KA. Prospective reports of chronic life stress predict decreased grey matter volume in the hippocampus. Neuroimage. 2007;35:795–803. doi: 10.1016/j.neuroimage.2006.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5(11):1242–1247. doi: 10.1038/nn958. doi:10.1038/nn958nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasper ER, Schoenfeld TJ, Gould E. Adult neurogenesis: optimizing hippocampal function to suit the environment. Behav Brain Res. 2011 doi: 10.1016/j.bbr.2011.05.013. doi:10.1016/j.bbr.2011.05.013. [DOI] [PubMed] [Google Scholar]
- Golub G, Cv Loan. Matrix computations. 3rd edn The Johns Hopkins University Press; London: 1996. [Google Scholar]
- Hufner K, Binetti C, Hamilton DA, Stephan T, Flanagin VL, Linn J, Labudda K, Markowitsch H, Glasauer S, Jahn K, Strupp M, Brandt T. Structural and functional plasticity of the hippocampal formation in professional dancers and slackliners. Hippocampus. 2010 doi: 10.1002/hipo.20801. doi:10.1002/hipo.20801. [DOI] [PubMed] [Google Scholar]
- Ising H, Braun C. Acute and chronic endocrine effects of noise: review of the research conducted at the institute for water, soil and air hygiene. Noise Health. 2000;2(7):7–24. [PubMed] [Google Scholar]
- Khanna S, Sinclair JG. Noxious stimuli produce prolonged changes in the CA1 region of the rat hippocampus. Pain. 1989;39(3):337–343. doi: 10.1016/0304-3959(89)90047-X. [DOI] [PubMed] [Google Scholar]
- Klausberger T. GABAergic interneurons targeting dendrites of pyramidal cells in the CA1 area of the hippocampus. Eur J Neurosci. 2009;30(6):947–957. doi: 10.1111/j.1460-9568.2009.06913.x. doi:10.1111/j.1460-9568.2009.06913.x. [DOI] [PubMed] [Google Scholar]
- Koolschijn PC, van Haren NE, Cahn W, Schnack HG, Janssen J, Klumpers F, Hulsho ffPol HE, Kahn RS. Hippocampal volume change in schizophrenia. J Clin Psychiatry. 2010;71(6):737–744. doi: 10.4088/JCP.08m04574yel. doi:10.4088/JCP.08m04574yel. [DOI] [PubMed] [Google Scholar]
- Kunkler PE, Kraig RP. Hippocampal spreading depression bilaterally activates the caudal trigeminal nucleus in rodents. Hippocampus. 2003;13(7):835–844. doi: 10.1002/hipo.10139. doi:10.1002/hipo.10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Gould E. Structural plasticity and hippocampal function. Annu Rev Psychol. 2010;61(111–140):C111–C113. doi: 10.1146/annurev.psych.093008.100359. doi:10.1146/annurev.psych.093008.100359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Qin W, Nan J, Li J, Yuan K, Zhao L, Zeng F, Sun J, Yu D, Dong M, Liu P, von Deneen KM, Gong Q, Liang F, Tian J. Gender-related differences in the dysfunctional resting networks of migraine suffers. PLoS One. 2011;6(11):e27049. doi: 10.1371/journal.pone.0027049. doi:10.1371/journal.pone.0027049PONE-D-11-10682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu MG, Chen J. Roles of the hippocampal formation in pain information processing. Neurosci Bull. 2009;25(5):237–266. doi: 10.1007/s12264-009-0905-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleki N, Linnman C, Brawn J, Burstein R, Becerra L, Borsook D. Her vs. his migraine: multiple sex differences in brain function and structure brain. 2012. in press. [DOI] [PMC free article] [PubMed]
- Marsland AL, Gianaros PJ, Abramowitch SM, Manuck SB, Hariri AR. Interleukin-6 covaries inversely with hippocampal grey matter volume in middle-aged adults. Biol Psychiatry. 2008;64:484–490. doi: 10.1016/j.biopsych.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. New Engl J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann N Y Acad Sci. 2001;933:265–277. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008;583(2–3):174–185. doi: 10.1016/j.ejphar.2007.11.071. doi:10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stress, sex, and neural adaptation to a changing environment: mechanisms of neuronal remodeling. Ann N Y Acad Sci. 2010;1204(Suppl):E38–E59. doi: 10.1111/j.1749-6632.2010.05568.x. doi:10.1111/j.1749-6632.2010.05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gianaros P. Stress- and allostasis-induced brain plasticity. Annu Rev Med. 2010a doi: 10.1146/annurev-med-052209-100430. doi:10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann NY Acad Sci. 2010b;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna JE, Melzack R. Analgesia produced by lidocaine microinjection into the dentate gyrus. Pain. 1992;49(1):105–112. doi: 10.1016/0304-3959(92)90195-H. [DOI] [PubMed] [Google Scholar]
- Metz AE, Yau HJ, Centeno MV, Apkarian AV, Martina M. Morphological and functional reorganization of rat medial prefrontal cortex in neuropathic pain. Proc Natl Acad Sci USA. 2009;106(7):2423–2428. doi: 10.1073/pnas.0809897106. doi:10.1073/pnas.0809897106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milde-Busch A, Blaschek A, Heinen F, Borggrafe I, Koerte I, Straube A, Schankin C, von Kries R. Associations between stress and migraine and tension-type headache: results from a school-based study in adolescents from grammar schools in Germany. Cephalalgia. 2011;31(7):774–785. doi: 10.1177/0333102410390397. doi:10.1177/0333102410390397. [DOI] [PubMed] [Google Scholar]
- Morey RA, Petty CM, Xu Y, Hayes JP, Wagner HR, 2nd, Lewis DV, LaBar KS, Styner M, McCarthy G. A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. Neuroimage. 2009;45(3):855–866. doi: 10.1016/j.neuroimage.2008.12.033. doi:10.1016/j.neuroimage.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton EA, Becerra L, Maleki N, Pendse G, Tully S, Hargreaves R, Burstein R, Borsook D. Painful heat reveals hyperexcit-ability of the temporal pole in interictal and ictal migraine states. Cereb Cortex. 2010 doi: 10.1093/cercor/bhq109. doi:10.1093/cercor/bhq109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nason MW, Jr, Adhikari A, Bozinoski M, Gordon JA, Role LW. Disrupted activity in the hippocampal-accumbens circuit of type III neuregulin 1 mutant mice. Neuropsychopharmacology. 2011;36(2):488–496. doi: 10.1038/npp.2010.180. doi:10.1038/npp.2010.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niddam DM, Tsai SY, Lu CL, Ko CW, Hsieh JC. Reduced Hippocampal glutamate–glutamine levels in irritable bowel syndrome: preliminary findings using magnetic resonance spectroscopy. Am J Gastroenterol. 2011 doi: 10.1038/ajg.2011.120. doi:10.1038/ajg.2011.120. [DOI] [PubMed] [Google Scholar]
- Nordanskog P, Dahlstrand U, Larsson MR, Larsson EM, Knutsson L, Johanson A. Increase in hippocampal volume after electroconvulsive therapy in patients with depression: a volumetric magnetic resonance imaging study. J ECT. 2010;26(1):62–67. doi: 10.1097/YCT.0b013e3181a95da8. doi:10.1097/YCT.0b013e3181a95da800124509-201003000-00017. [DOI] [PubMed] [Google Scholar]
- Pendse G, Borsook D, Becerra L. Enhanced false discovery rate using Gaussian mixture models for thresholding fMRI statistical maps. Neuroimage. 2009;47(1):231–261. doi: 10.1016/j.neuroimage.2009.02.035. doi:10.1016/j.neuroimage.2009.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterlin BL, Katsnelson MJ, Calhoun AH. The associations between migraine, unipolar psychiatric comorbidities, and stress-related disorders and the role of estrogen. Curr Pain Headache Rep. 2009;13(5):404–412. doi: 10.1007/s11916-009-0066-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piche M, Arsenault M, Rainville P. Dissection of perceptual, motor and autonomic components of brain activity evoked by noxious stimulation. Pain. 2010;149(3):453–462. doi: 10.1016/j.pain.2010.01.005. doi:10.1016/j.pain.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Ploghaus A, Becerra L, Borras C, Borsook D. Neural circuitry underlying pain modulation: expectation, hypnosis, placebo. Trends Cogn Sci. 2003;7(5):197–200. doi: 10.1016/s1364-6613(03)00061-5. [DOI] [PubMed] [Google Scholar]
- Ploghaus A, Narain C, Beckmann CF, Clare S, Bantick S, Wise R, Matthews PM, Rawlins JN, Tracey I. Exacerbation of pain by anxiety is associated with activity in a hippocampal network. J Neurosci. 2001;21(24):9896–9903. doi: 10.1523/JNEUROSCI.21-24-09896.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado WA, Roberts MH. An assessment of the antinociceptive and aversive effects of stimulating identified sites in the rat brain. Brain Res. 1985;340(2):219–228. doi: 10.1016/0006-8993(85)90917-5. [DOI] [PubMed] [Google Scholar]
- Rains JC. Epidemiology and neurobiology of stress and migraine. Headache. 2009;49(9):1391–1394. doi: 10.1111/j.1526-4610.2009.01477.x. doi:10.1111/j.1526-4610.2009.01477.x. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, LeDoux JE, Sapolsky RM. The influence of stress hormones on fear circuitry. Annu Rev Neurosci. 2009;32:289–313. doi: 10.1146/annurev.neuro.051508.135620. doi:10.1146/annurev.neuro.051508.135620. [DOI] [PubMed] [Google Scholar]
- Rothman SM, Mattson MP. Adverse stress, hippocampal networks, and Alzheimer’s disease. Neuromolecular Med. 2010;12(1):56–70. doi: 10.1007/s12017-009-8107-9. doi:10.1007/s12017-009-8107-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57(10):925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- Sauro KM, Becker WJ. The stress and migraine interaction. Headache. 2009;49(9):1378–1386. doi: 10.1111/j.1526-4610.2009.01486.x. doi:10.1111/j.1526-4610.2009.01486.x. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin PA. Role of the hippocampus in epilepsy. Hippocampus. 1994;4(3):239–242. doi: 10.1002/hipo.450040302. doi:10.1002/hipo.450040302. [DOI] [PubMed] [Google Scholar]
- Schwedt TJ, Krauss MJ, Frey K, Gereau RWT. Episodic and chronic migraineurs are hypersensitive to thermal stimuli between migraine attacks. Cephalalgia. 2011;31(1):6–12. doi: 10.1177/0333102410365108. doi:10.1177/0333102410365108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22(3):1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. doi:10.1016/j.neuroimage.2004.03.032S1053811904001880. [DOI] [PubMed] [Google Scholar]
- Sheline YI. Neuroimaging studies of mood disorder effects on the brain. Biol Psychiat. 2003;54:338–352. doi: 10.1016/s0006-3223(03)00347-0. [DOI] [PubMed] [Google Scholar]
- Starkman MN, Giordani B, Gebrski SS, Berent S, Schork MA, Schteingart DE. Decrease in cortisol reverses human hippocampal atrophy following treatment of Cushing’s disease. Biol Psychiatry. 1999;46:1595–1602. doi: 10.1016/s0006-3223(99)00203-6. [DOI] [PubMed] [Google Scholar]
- Tfelt-Hansen PC. Does sumatriptan cross the blood–brain barrier in animals and man? J Headache Pain. 2010;11(1):5–12. doi: 10.1007/s10194-009-0170-y. doi:10.1007/s10194-009-0170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Flier WM, Scheltens P. Alzheimer disease: hippocampal volume loss and Alzheimer disease progression. Nat Rev Neurol. 2009;5(7):361–362. doi: 10.1038/nrneurol.2009.94. doi:10.1038/nrneurol.2009.94. [DOI] [PubMed] [Google Scholar]
- Woon FL, Sood S, Hedges DW. Hippocampal volume deficits associated with exposure to psychological trauma and posttraumatic stress disorder in adults: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(7):1181–1188. doi: 10.1016/j.pnpbp.2010.06.016. doi:10.1016/j.pnpbp.2010.06.016. [DOI] [PubMed] [Google Scholar]
- Yeung JC, Yaksh TL, Rudy TA. Concurrent mapping of brain sites for sensitivity to the direct application of morphine and focal electrical stimulation in the production of antinociception in the rat. Pain. 1977;4(1):23–40. doi: 10.1016/0304-3959(77)90084-7. [DOI] [PubMed] [Google Scholar]
- Zhang D, Snyder AZ, Fox MD, Sansbury MW, Shimony JS, Raichle ME. Intrinsic functional relations between human cerebral cortex and thalamus. J Neurophysiol. 2008;100(4):1740–1748. doi: 10.1152/jn.90463.2008. doi:10.1152/jn.90463.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman ME, Pan JW, Hetherington HP, Lipton ML, Baigi K, Lipton RB. Hippocampal correlates of pain in healthy elderly adults: a pilot study. Neurology. 2009;73(19):1567–1570. doi: 10.1212/WNL.0b013e3181c0d454. doi:10.1212/WNL.0b013e3181c0d454. [DOI] [PMC free article] [PubMed] [Google Scholar]


