Abstract
The activation, differentiation and subsequent effector functions of CD4 T cells depend on interactions with a multitude of MHCII-expressing antigen presenting cells (APCs). To evaluate the individual contribution of various APCs to CD4 T cell function, we have designed a new murine tool for selective in vivo expression of MHCII in subsets of APCs. Conditional expression of MHCII in B cells was achieved using a cre-loxP approach. After intravenous or subcutaneous priming, partial proliferation and activation of CD4 T cells was observed in mice expressing MHCII only by B cells. Restricting MHCII expression to B cells constrained secondary CD4 T cell responses in vivo, as demonstrated in a CD4 T cell-dependent model of autoimmunity, EAE. These results highlight the limitations of B cell antigen presentation during initiation and propagation of CD4 T cell function in vivo using a novel system to study individual APCs by the conditional expression of MHCII.
INTRODUCTION
Multiple antigen presenting cells (APCs) expressing MHCII can engage in cognate interactions that are critical to the development, differentiation and effector functions of CD4 T cells. While DCs are recognized as potent initiators of CD4 T cell responses (1), antigen-specific B cells are actually more adept at acquiring and presenting soluble cognate antigens in vivo compared to DCs (2). Contributing to the complexity involved in MHCII-dependent responses in vivo is the substantial reliance to date on indirect experimental models that have limited the ability to discern the degree to which individual APC subsets orchestrate CD4 T cell function.
Traditionally, B cells have been considered accessory APCs to DCs (3). However, accumulating evidence suggests that B cells regulate antigen-specific CD4 T cell immune responses, such as priming and memory responses (4, 5). Potent regulatory and tolerogenic properties have also been attributed to B cells (6, 7). Further, a role for B cell antigen presentation has been implicated during disease, as anti-CD20-mediated B cell depletion is an effective therapy for human autoimmune diseases such as multiple sclerosis (MS), apparently independent of effects on antibody levels (8). Whether B cells alone are capable of directing cognate CD4 T cell behavior during autoimmunity has not been directly tested.
To examine the individual contribution of various APC subsets to CD4 T cell function, we have established a new in vivo system to conditionally express MHCII. Herein, we demonstrate that MHCII expression can be restricted to cell lineages using a cre-loxP system. Examining B cell antigen presentation, we found in vivo priming of CD4 T cells by B cells alone does occur, but is limited. Moreover, secondary responses coordinated by B cells were also restricted, and B cell antigen presentation was not sufficient to support CD4 T cell-dependent autoimmune encephalomyelitis. These results demonstrate the limited sufficiency of B cell antigen presentation to direct CD4 T cell responses while providing evidence of the utility of this system for the study of individual APC contributions in vivo.
MATERIALS AND METHODS
Mice
C57Bl/6, B6.PL, 2D2, CMVCre and CD19Cre mice were purchased from Jackson laboratory (Bar Harbor, ME). MHCII−/− mice were used as described (9). A polyadenylation stop sequence flanked by loxP sites (10) was targeted to the first intron of the IAβ locus, making use of a retrieval vector PL253 and BAC recombineering (11). No endogenous sequences were deleted by the insertion. Mice bred for homozygosity of this construct are termed IAβbstopf/f. The final targeting vector was verified by sequencing of all essential elements, linearized and electroporated into the LK1 C57BL6 ES cell line (12). Southern blot analysis confirmed appropriate targeting (Supplemental Figure 1).
Antibodies and flow cytometry
Antibodies were purchased from BD Biosciences (San Jose, CA) and eBioscience (San Diego, CA). Samples were acquired on FACSCalibur™ or LSRII™ flow cytometers (BD, San Jose, CA) or Beckman Coulter Gallios™ (Brea, CA). Gating for % CFSE dilution was relative to the undivided peak in each individual experiment.
Cell purification, lines and culture
CD4 T and B cells were positively selected using mouse L3T4 and CD19 microbeads (Miltenyi Biotec, Auburn, CA). RNA purification and RT-PCR was performed as described (13). 1–5×106 B16 cells (14) were injected SQ into mice to purify bone marrow-derived DCs (BMDCs) that were cultured in RPMI medium with 10% FBS and 10ng/mL GM-CSF (R&D Systems). Peritoneal macrophages were isolated by peritoneal lavage from mice 3–5 days after i.p. injection of 40ug of ConA (Sigma, St. Louis, MO). T cell hybridomas were generated as previously described (15).
Proteins and peptides
MOG protein expression and purification was performed as reported (9). MOG35-55 was commercially synthesized (CSBio, Menlo Park, CA). Listeriolysin O peptide fragment 190–201 (LLO190-201) was synthesized at Washington University in St. Louis (16).
In vitro and in vivo proliferation experiments
Spleens were processed for B cell depletion by AutoMACS. Single cell suspensions were irradiated with 2000 Rads, washed and then combined with antigen. 1×105 APCs were cultured with 5×104 hybridomas and antigen overnight at 37° C. Proliferation of CTLL-2 was measured after addition of supernatant by 3H-thymidine incorporation (16). CD4 T cells were isolated from 2D2 mice and labeled with CFSE (Invitrogen, Grand Island, NY). For priming studies, 2×106 CFSE-labeled congenic (CD45.1) CD4 T cells were transferred i.v. one day prior to immunization.
ELISPOT assays
IFN-γ and IL-2 ELISPOT assays were performed as described (16) using 5×105 splenocytes/well in triplicate with 1×105 2D2 CD4 T cells and stimulated with no antigen or varying doses of MOG35-55/ml.
Immunizations and Induction of EAE
Thymic grafting was performed prior to the induction of active EAE as reported (9). Immunization i.v. with 50 μg MOG35-55 or 100 μg rMOG was done with 50 μg CpG (IDT, Coralville, IA). Active SQ immunization with rMOG or MOG35-55 was performed as reported (9). Passive EAE was induced as described with 1×107 MOG-specific, Thy1.1+ encephalitogenic cells that are almost exclusively Th1 (13).
Statistics
Statistical analysis was performed using two-tailed Student’s t-tests.
RESULTS AND DISCUSSION
Conditional inactivation of IAβb in vivo results in abrogation of MHCII expression
To test the sufficiency of antigen presentation by specific lineages of APCs, we designed a mouse system in which MHCII is conditionally expressed. We successfully targeted a stop sequence flanked by loxP sites (10) to the IAβ locus (Supplemental Figure 1). Southern blot analysis confirmed germ-line transmission of the construct in several founder mice. We examined mice homozygous for the insert, termed IAβbstopf/f mice, for expression of MHCII. In peripheral blood, spleen, and BM compartments, MHCII expression was abolished (Supplemental Figure 1D). Consistent with the lack of MHCII and positive selection in the thymus, CD4 T cells were absent. By flow cytometry, splenic and BM DCs from mice treated with Flt3-ligand expressed no discernable MHCII (Supplemental Figure 1E). Thus, we have successfully generated mice in which a removable knock-in stop construct in the IAβ chain locus eliminates MHCII expression as detected by FACS.
Conditional in vivo gene repair of IAβ in B cells
Our targeted insert results in elimination of MHCII expression, but retains the capacity to re-express MHCII in a cell lineage-specific manner. To explore the contribution of B cell antigen presentation to CD4 T cell responses, we reconstituted MHCII expression using the CD19Cre mouse (17). CD19Cre and IAβbstopf/f mice bred to homozygosity for the IAβ allele, termed CD19Cre/IAβbstopf/f, were examined for MHCII expression in lymphoid organs. Expression of MHCII in B cells was identical in both CD19Cre/IAβbstopf/f and WT BM and spleen by FACS (Fig. 1A and Supplemental Figure 2A). The development and functionality of B cells was not altered (Supplemental Figure 2). In contrast, CD11c+CD19− cells were devoid of MHCII expression in CD19Cre/IAβbstopf/f mice. Of note, small fractions of CD19+ cells also express CD11c and/or CD11b (18), and CD19+CD11c+ cells from CD19Cre/IAβbstopf/f mice were observed to be MHCII+. However, all CD11c+ cells from CD19Cre/IAβbstopf/f mice expressing MHCII were CD19+ (Supplemental Figure 2E). We also examined the expression of MHCII in other subsets of APCs. WT BMDC and peritoneal macrophages exhibited clear expression of MHCII. However, both types of APCs from IAβbstopf/f mice had no MHCII expression detectable by FACS, and only APCs expressing CD19 were MHCII+ in CD19Cre/IAβbstopf/f mice (Supplemental Figure 2F).
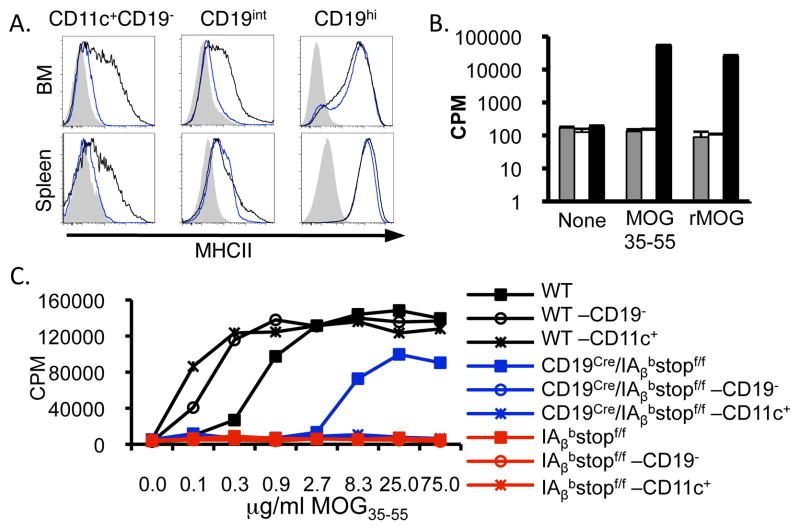
Figure 1.
MHCII expression is functionally abrogated in IAβbstopf/f mice and restricted to B cells in CD19Cre/IAβbstopf/f mice. MHCII expression was analyzed after crossing CD19Cre mice (17) to IAβbstopf/f mice. (A) BM (top) and spleen (bottom) expression of MHCII in IAβbstopf/f (shaded), CD19Cre/IAβbstopf/f (blue line) and WT (black line) mice. Histograms are from singlet cells gated on CD11c+CD19− cells (left), and CD19int (middle) and CD19hi populations. Data representative of six or more mice per group analyzed in three experiments. (B) BMDC generated from IAβbstopf/f (gray), CD19Cre/IAβbstopf/f (white) and WT (black) mice were pulsed with MOG35-55 peptide or rMOG protein then co-cultured with the MOG-specific T cell hybridoma, MOG.15. IL-2 production was assayed by CTLL-2 proliferation assay using 3H-thymidine incorporation (16). (C) MOG35-55 was added to spleens from WT (black), IAβbstopf/f (red), and CD19Cre/IAβbstopf/f (blue) mice before (square) and after (circle) depletion of B cells. Further enrichment for DCs was also performed (X). CTLL-2 were incubated with supernatants from MOG.15 and splenic APC cultures with varying doses of MOG35-55. Data are representative of triplicate samples in two separate experiments.
To verify the functional degree to which MHCII expression is restricted to B cells in CD19Cre/IAβbstopf/f mice, we tested the ability of several APC subsets to present antigen to CD4 T cells. BMDC from WT mice elicited a robust response to the immuno-dominant CD4 T cell peptide of MOG (MOG35-55) by an IAb-restricted CD4 T cell hybridoma, MOG.15. In contrast, BMDC from either IAβbstopf/f or CD19Cre/IAβbstopf/f mice failed to generate antigen specific responses (Fig. 1B). Thus, DCs from IAβbstopf/f and CD19Cre/IAβbstopf/f mice cannot generate MHCII-dependent CD4 T cell proliferation. To determine the level of functional splenic MHCII expression, irradiated splenocytes from WT, IAβbstopf/f and CD19Cre/IAβbstopf/f mice were incubated with MOG35-55. The MOG-specific hybridoma, MOG.15 responded in a dose-dependent fashion to antigen with WT or CD19Cre/IAβbstopf/f splenocytes, but not to antigen with splenocytes from IAβbstopf/f mice (Fig. 1C). Importantly, removal of CD19+ cells from the spleen prior to incubation with MOG35-55 abrogated antigen presentation by CD19Cre/IAβbstopf/f, but not WT, splenocytes (Fig. 1C). These results confirm the absence of functionally relevant levels of MHCII expression in IAβbstopf/f mice and demonstrate the expression of MHCII in CD19Cre/IAβbstopf/f mice is restricted to B cells. Additional enrichment for CD11c+ cells did not result in any detectable antigen-specific response by either IAβbstopf/f or CD19Cre/IAβbstopf/f splenocytes (Fig. 1C). Similar results were obtained using the non-self antigen, LLO190-201 (16) (Supplemental Figure 2G).
B cells are capable of limited CD4 T cell priming
CD19Cre/IAβbstopf/f mice provide the optimal system in which to examine the in vivo capacity for B cells alone to drive peripheral CD4 T cell responses de novo. We assessed the ability of B cells to stimulate proliferation of MOG-specific 2D2 TCR transgenic CD4 T cells. MOG35-55 together with CpG was delivered i.v. to mice that had received 2D2 CD4 T cells labeled with CFSE. Virtually all 2D2 cells isolated from the spleen of WT mice exhibited some degree of proliferation after i.v. immunization (92.5±10.9%). In contrast, no CD4 T cell proliferation was observed in the absence of MHCII (4.5±2.7% in IAβbstopf/f mice, p<0.01 vs. WT) as expected due to dependence by rapid homeostatic proliferation on TCR-MHCII interactions. 2D2 CD4 T cells in CD19Cre/IAβbstopf/f mice proliferated after i.v. immunization with MOG35-55, but only 55.3±7.1% diluted CFSE (p<0.01 vs. WT and vs. IAβbstopf/f; Fig. 2A&C). Similar results were observed following protein administration (Fig. 2B&D). Examination of activation and differentiation markers revealed partial down-regulation of CD62L on 2D2 cells in CD19Cre/IAβbstopf/f, but not IAβbstopf/f mice, compared with WT mice after peptide, but not protein, immunization (Fig. 2G). No difference in other markers, including CD69, CD25, FoxP3, PD-1, or BTLA, was observed (data not shown).
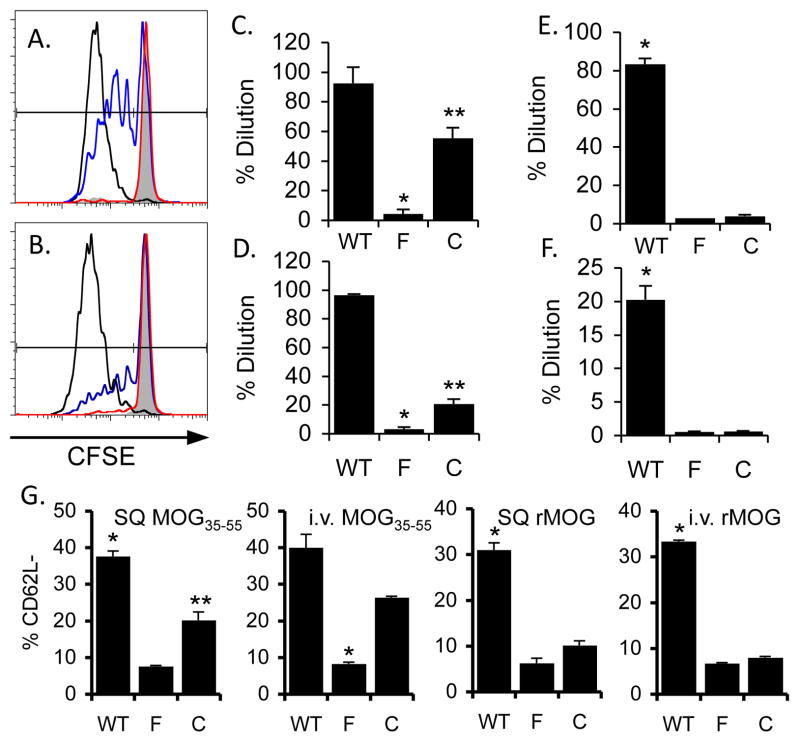
Figure 2.
B cells have a limited capacity to prime CD4 T cells in vivo. (A–F) CFSE labeled 2D2 T cells were adoptively transferred into IAβbstopf/f (“F”; red), CD19Cre/IAβbstopf/f (“C”; blue) and WT (black) mice one day prior to immunization with 50 μg MOG35-55 i.v. (A) or 150 μg rMOG i.v. (B) and 50 μg CpG. Shown are representative FACS plots from at least two different experiments with three mice per group three days after immunization. Percent of dividing 2D2 cells are shown for i.v. peptide (C) or protein (D), or SQ MOG35-55 (E) or rMOG SQ (E) in CFA. (G) Percent donor 2D2 cells isolated from mice immunized with SQ or i.v. MOG35-55, or SQ or i.v. rMOG, with CD62Llow expression. Each graph represents at least three mice per group. *p <0.001 (C–F) or p<0.01 (G) in comparison to either other group; **p <0.01 in comparison to WT (C&D) or IAβbstopf/f (G).
After SQ immunization with MOG35-55, 83.3±3.0% of 2D2 cells in the draining LN of WT mice exhibited CFSE dilution. In contrast, over 90% of 2D2 cells remained undiluted in both IAβbstopf/f and CD19Cre/IAβbstopf/f mice (Fig. 2E). Minimal proliferation of 2D2 cells was induced in IAβbstopf/f or CD19Cre/IAβbstopf/f mice following protein immunization as well (Fig. 2F), demonstrating that MHCII expression by B cells does not contribute to antigen-specific CD4 T cells proliferation after SQ immunization. However, 2D2 cells still exhibited a significant reduction in CD62L expression in CD19Cre/IAβbstopf/f compared with IAβbstopf/f mice after SQ peptide immunization (20.2±2.3% vs. 7.6±0.3%, respectively, p<0.05; Fig. 2G).
Secondary CD4 T cell responses, including those critical for inducing autoimmune encephalomyelitis, are limited in CD19Cre/IAβbstopf/f mice
B cells participate in cognate interactions with CD4 T cells that appear essential to immune activity, autoimmunity, and tolerance (19, 20). To evaluate the ability of B cells alone to promote CD4 T cell function after the priming phase, we co-cultured previously primed CD4 T cells with antigen and splenocytes from IAβbstopf/f, CD19Cre/IAβbstopf/f, or WT mice. ELISPOT analysis revealed an increase in antigen-specific IFNγ production by 2D2 CD4 T cells cultured with splenocytes from WT mice compared with IAβbstopf/f splenocytes (p<0.01) (Fig. 3A). Cognate interactions between previously primed 2D2 CD4 T cells and splenocytes from CD19Cre/IAβbstopf/f mice also produced greater IFNγ as compared with IAβbstopf/f (p<0.01), but reduced in comparison to WT (p<0.05) (Fig. 3A). In contrast, 2D2 production of IL-2 and IL-17 that was observed to result from cognate interactions with WT splenocytes was not elicited by splenocytes from IAβbstopf/f or CD19Cre/IAβbstopf/f mice. Conversely, antigen-specific production of GM-CSF was equally elicited by splenocytes from CD19Cre/IAβbstopf/f and WT mice, but not IAβbstopf/f mice (Fig. 3A).
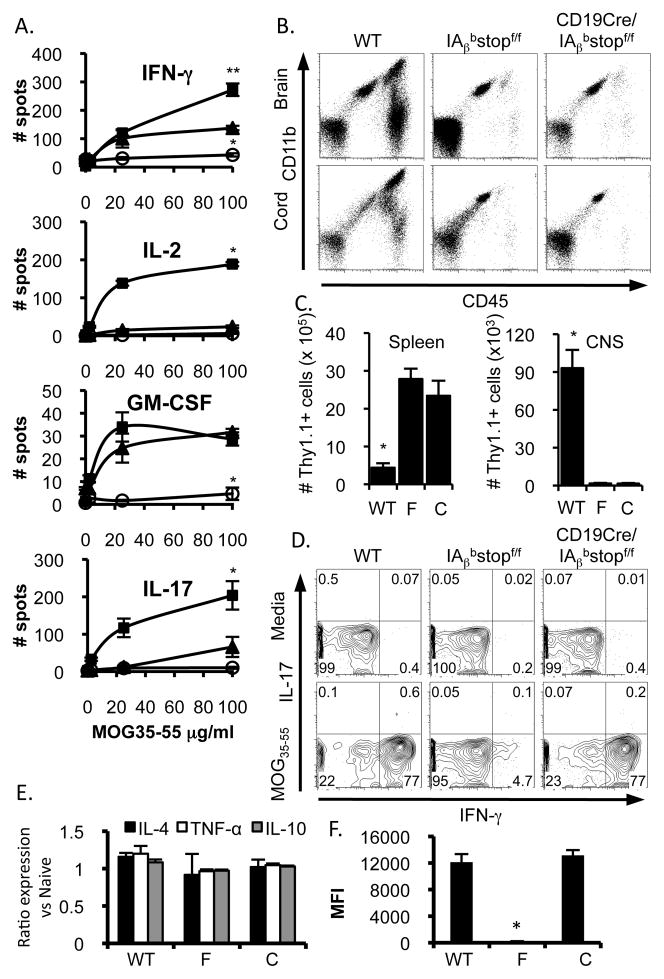
Figure 3.
Secondary CD4 T cell responses in vivo are limited when cognate interactions are restricted to B cells. (A) Ten days after SQ immunization with MOG35-55, 2D2 CD4 T cells were re-stimulated with splenocytes and varying doses of MOG35-55 from IAβbstopf/f (circles), CD19Cre/IAβbstopf/f (triangles) and WT (squares) mice primed four days prior with i.v. CpG and MOG35-55. Cytokine production was assayed by ELISPOT. Data represent three mice per group in four experiments. For the upper panel, *p<0.01 (IAβbstopf/f compared with WT or CD19Cre/IAβbstopf/f), **p<0.05 (WT compared with CD19Cre/IAβbstopf/f); all other panels, *p<0.01 compared to either other group. (B) Ten days following EAE induction, mononuclear cells from the brain and spinal cord were assessed by CD11b and CD45 expression. Data representative of three mice per group from two experiments. (C) The number of donor cells was quantified in the spleen and CNS; *p<0.05 when comparing WT to either IAβbstopf/f (“F”) or CD19Cre/IAβbstopf/f (“C”). Data representative of three mice per group in two experiments. (D) 30 days after passive EAE induction, splenocytes were isolated and cultured overnight with media (top) or MOG35-55 (bottom). IFN-γ and IL-17 production was assayed by intracellular staining. Plots are gated on CD4+Thy1.1+ cells. Data representative of at least two mice per group in two separate experiments. (E) Seven days after transfer of encephalitogenic cells, B cell mRNA levels for IL-4, TNF-α and IL-10 was compared to splenic B cells from naïve mice. Data representative of three mice per group. (F) B cell expression of MHCII was assessed by flow cytometry ten days following transfer of encephalitogenic CD4 T cells. *p<0.05 when comparing IAβbstopf/f (“F”) to either WT or CD19Cre/IAβbstopf/f (“C”). Data representative of three mice per group.
We utilized our in vivo mechanism for conditional expression of MHCII to test whether B cells alone are capable of coordinating CD4 T cell-mediated autoimmune neuro-inflammation during passive EAE. I.v. transfer of 1×107 previously primed, encephalitogenic Th1 CD4 T cells (13) to WT mice resulted in 100% incidence of EAE, while IAβbstopf/f recipient mice lacking MHCII remained entirely free of disease. Similarly, CD19Cre/IAβbstopf/f recipient mice were fully resistant to clinical disease, demonstrating an insufficiency of antigen presentation by B cells to support CD4 T cell-mediated EAE (Table I).
Table I.
Clinical features of EAE in IAβbstopf/f and CD19Cre/IAβbstopf/f mice.
| Mouse group | Model* | Incidence | Day of Onset (mean ± SEM) | Maximum Disease (mean ± SEM)** |
|---|---|---|---|---|
| WT (n= 16) | Passive | 100% | 6.1 ± 0.2 | 3.4 ± 0.2 |
| IAβbstopf/f (n=14) | Passive | 0 | N/A | 0 |
| CD19Cre/IAβbstopf/f (n=20) | Passive | 0 | N/A | 0 |
| CD19Cre/Cre/IAβbstopf/f (n=19) | Passive | 0 | N/A | 0 |
| WT (n=9) | Active | 100% | 9.3 ± 0.5 | 4.0 ± 0.0 |
| IAβbstopf/f (n=13) | Active | 0 | N/A | 0 |
| CD19Cre/IAβbstopf/f (n=15) | Active | 0 | N/A | 0 |
As described in Materials and Methods, passive disease was induced by transfer of 1×107 encephalitogenic CD4 T cells as described in Materials and Methods, while active EAE was induced by immunization with MOG35-55 or rMOG.
Maximal disease score of each mouse during the course of 30 days following receipt of encephalitogenic T cells or immunization.
To examine the extent of inflammation within the CNS after passive transfer of encephalitogenic CD4 T cells, CNS mononuclear cells were isolated at the peak of disease in WT mice from spinal cord and brain tissue of each mouse group. In contrast to WT CNS tissue, minimal evidence of microglial activation or leukocyte infiltration was observed in IAβbstopf/f mice (as expected (21)) or CD19Cre/IAβbstopf/f mice (Fig. 3B). At 10 days post transfer, 19.3% of mononuclear cells isolated from the CNS of WT mice were donor cells. In contrast, both IAβbstopf/f and CD19Cre/IAβbstopf/f mice had minimal accumulation (<2.5%) of donor lymphocytes within the CNS, prohibiting further characterization. Absolute numbers of donor cells paralleled this disparity (Fig. 3C). Donor CD4 T cells accumulated within the spleen of IAβbstopf/f and CD19Cre/IAβbstopf/f mice, however (Fig. 3C), and ex vivo antigen-specific recall production of IFN-γ was identical in CD19Cre/IAβbstopf/f and WT mice (Fig. 3D), serving as further evidence that B cell antigen presentation alone can elicit cognate secondary CD4 T cell responses. The cytokine profile and MHCII expression of B cells following induction of EAE did not differ between mouse groups (Figs. 3F&G). Thus, B cells can support antigen-specific secondary CD4 T cell responses, but have a limited capacity to propagate EAE.
CD19+CD11c+ splenocytes are one unique niche of APCs that may exert functionally distinct influences on CD4 T cells (18). The regulatory influences of these indoleamine-producing cells, mediated via CD19, are unlikely to be responsible for the lack of disease following transfer of encephalitogenic T cells, as CD19Cre/IAβbstopf/f mice homozygous for Cre expression, which eliminates CD19 expression and the indoleamine-mediated suppressive effects and is associated with heightened EAE disease severity (22), retain full resistance to EAE (Table 1).
The functional relevance of B cell antigen presentation during autoimmune responses was further investigated using the frequently employed active EAE model system. To avoid constraints related to homeostatic proliferation and variability associated with transfer of CD4 T cells prior to immunization, we reconstituted the CD4 T cell compartment of IAβbstopf/f and CD19Cre/IAβbstopf/f mice by WT thymus grafting (Supplemental Figure 2H) (9). The results of our active EAE experiments parallel those of passive EAE, as both IAβbstopf/f and CD19Cre/IAβbstopf/f mice immunized with peptide or protein were entirely resistant to disease (Table 1). To verify the ability for our system to generate sufficient levels of MHCII to support passive EAE, we used CMVCre mice - in which ubiquitous Cre expression is under the control of a human CMV minimal promoter - to rescue expression of MHCII. CMVCre/IAβbstopf/f mice have identical expression of MHCII in lymphoid tissue compared with WT mice (Supplemental Figure 2I) and are fully susceptible to passive EAE (onset at 6.25 ± 0.25 days with maximal severity of 2.25 ± 0.72; n = 4).
Using our newly designed murine system in which subsets of APCs are capable of conditionally expressing MHCII in vivo, we have addressed the hypothesis that individual APCs contribute to CD4 T cell-mediated autoimmunity in unique ways by examining the contribution of B cells to CD4 T cell responses. Our findings are based on a knock-in model of MHCII expression rather than a transgenic approach that has been utilized in the past which suggested that CD4 T cell responses are not driven only by the quantity of MHCII in vivo per se, but rather the cellular source of MHCII (23). Not surprisingly, i.v. delivery of antigen, rather than SQ, was found to be optimal for stimulating initial CD4 T cell proliferation (Fig. 2), and CD19Cre/IAβbstopf/f mice are resistant to active EAE induced by SQ immunization (Table 1). Nonetheless, B cells may be relevant for SQ antigen delivery in combination with DCs. For example, while DCs alone are sufficient to mediate peptide-induced active EAE, generation of disease following SQ immunization with protein may require B cells (9).
Contrary to several lines of evidence suggesting an APC role for B cells during primary and secondary responses, including disease states such as EAE and MS, our data indicate that antigen presentation solely by B cells is not sufficient for full CD4 T cell function. However, one manner in which B cells may participate in the propagation of CD4 T cell auto-reactivity is by the uptake and presentation of cognate antigen that is limited in availability (24). This may be relevant early in disease when intact myelin does not provide excess available antigen and only antigen-specific uptake of target will efficiently result in sufficient CD4 T cell activation. Another possibility involves the potential for B cells to drive unique CD4 T cell cytokine production upon re-stimulation after priming. Differential CD4 T cell production of GM-CSF, but not IL-17 and partial production of IFN-γ resulting from antigen-specific interactions with B cells may engender a restricted set of effector responses. Alternatively, B cells are capable of down-modulating CD4 T cell function (20, 25), and B cell-mediated tolerance may be mediated in part by MHCII. The cognate basis for the tolerogenic nature of B cells warrants further investigation, one which will undoubtedly be more feasible given the flexibility of our new system for conditional murine MHCII expression. Overall, comparison of CD4 T cell responses in mice expressing MHCII in specific APC lineages using this system with other cell-specific Cre mice will address the exclusive contributions by other APCs to CD4 T and greatly enhance our understanding of the pathways by which immune and autoimmune processes are generated and propagated.
Supplementary Material
Footnotes
Funded by the NINDS (5K08NS062138) and the Barnes-Jewish Foundation. We thank the Alvin J. Siteman Cancer Center (supported in part by an NCI Cancer Center Support Grant #P30 CA91842) and Barnes-Jewish Hospital for the use of the Embryonic Stem Cell Core, which provided all ES cell services.
References
- 1.Reis e Sousa C. Dendritic cells in a mature age. Nat Rev Immunol. 2006;6:476–483. doi: 10.1038/nri1845. [DOI] [PubMed] [Google Scholar]
- 2.Pape KA, Catron DM, Itano AA, Jenkins MK. The humoral immune response is initiated in lymph nodes by B cells that acquire soluble antigen directly in the follicles. Immunity. 2007;26:491–502. doi: 10.1016/j.immuni.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 4.Whitmire JK, Asano MS, Kaech SM, Sarkar S, Hannum LG, Shlomchik MJ, Ahmed R. Requirement of B cells for generating CD4+ T cell memory. J Immunol. 2009;182:1868–1876. doi: 10.4049/jimmunol.0802501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Constant SL. B lymphocytes as antigen-presenting cells for CD4+ T cell priming in vivo. J Immunol. 1999;162:5695–5703. [PubMed] [Google Scholar]
- 6.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 7.Frommer F, Heinen TJ, Wunderlich FT, Yogev N, Buch T, Roers A, Bettelli E, Muller W, Anderton SM, Waisman A. Tolerance without clonal expansion: self-antigen-expressing B cells program self-reactive T cells for future deletion. J Immunol. 2008;181:5748–5759. doi: 10.4049/jimmunol.181.8.5748. [DOI] [PubMed] [Google Scholar]
- 8.Bar-Or A, Fawaz L, Fan B, Darlington PJ, Rieger A, Ghorayeb C, Calabresi PA, Waubant E, Hauser SL, Zhang J, Smith CH. Abnormal B-cell cytokine responses a trigger of T-cell-mediated disease in MS? Ann Neurol. 2010;67:452–461. doi: 10.1002/ana.21939. [DOI] [PubMed] [Google Scholar]
- 9.Wu GF, Shindler KS, Allenspach EJ, Stephen TL, Thomas HL, Mikesell RJ, Cross AH, Laufer TM. Limited sufficiency of antigen presentation by dendritic cells in models of central nervous system autoimmunity. J Autoimmun. 2011;36:56–64. doi: 10.1016/j.jaut.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dragatsis I, Zeitlin S. A method for the generation of conditional gene repair mutations in mice. Nucleic Acids Res. 2001;29:E10. doi: 10.1093/nar/29.3.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu P, Jenkins NA, Copeland NG. A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res. 2003;13:476–484. doi: 10.1101/gr.749203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keskintepe L, Norris K, Pacholczyk G, Dederscheck SM, Eroglu A. Derivation and comparison of C57BL/6 embryonic stem cells to a widely used 129 embryonic stem cell line. Transgenic Res. 2007;16:751–758. doi: 10.1007/s11248-007-9125-8. [DOI] [PubMed] [Google Scholar]
- 13.Archambault AS, Sim J, McCandless EE, Klein RS, Russell JH. Region-specific regulation of inflammation and pathogenesis in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2006;181:122–132. doi: 10.1016/j.jneuroim.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Mach N, Gillessen S, Wilson SB, Sheehan C, Mihm M, Dranoff G. Differences in dendritic cells stimulated in vivo by tumors engineered to secrete granulocyte-macrophage colony-stimulating factor or Flt3-ligand. Cancer Res. 2000;60:3239–3246. [PubMed] [Google Scholar]
- 15.Velazquez C, Vidavsky I, van der Drift K, Gross ML, Unanue ER. Chemical identification of a low abundance lysozyme peptide family bound to I-Ak histocompatibility molecules. J Biol Chem. 2002;277:42514–42522. doi: 10.1074/jbc.M202316200. [DOI] [PubMed] [Google Scholar]
- 16.Carrero JA, Vivanco-Cid H, Unanue ER. Listeriolysin o is strongly immunogenic independently of its cytotoxic activity. PLoS One. 2012;7:e32310. doi: 10.1371/journal.pone.0032310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rickert RC, Roes J, Rajewsky K. B lymphocyte-specific, Cre-mediated mutagenesis in mice. Nucleic Acids Res. 1997;25:1317–1318. doi: 10.1093/nar/25.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson BA, 3rd, Kahler DJ, Baban B, Chandler PR, Kang B, Shimoda M, Koni PA, Pihkala J, Vilagos B, Busslinger M, Munn DH, Mellor AL. B-lymphoid cells with attributes of dendritic cells regulate T cells via indoleamine 2,3-dioxygenase. Proc Natl Acad Sci U S A. 2010;107:10644–10648. doi: 10.1073/pnas.0914347107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan J, Harvey BP, Gee RJ, Shlomchik MJ, Mamula MJ. B cells drive early T cell autoimmunity in vivo prior to dendritic cell-mediated autoantigen presentation. J Immunol. 2006;177:4481–4487. doi: 10.4049/jimmunol.177.7.4481. [DOI] [PubMed] [Google Scholar]
- 20.Ray A, Basu S, Williams CB, Salzman NH, Dittel BN. A Novel IL-10-Independent Regulatory Role for B Cells in Suppressing Autoimmunity by Maintenance of Regulatory T Cells via GITR Ligand. J Immunol. 2012;188:3188–3198. doi: 10.4049/jimmunol.1103354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slavin AJ, Soos JM, Stuve O, Patarroyo JC, Weiner HL, Fontana A, Bikoff EK, Zamvil SS. Requirement for endocytic antigen processing and influence of invariant chain and H-2M deficiencies in CNS autoimmunity. J Clin Invest. 2001;108:1133–1139. doi: 10.1172/JCI13360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsushita T, Fujimoto M, Hasegawa M, Komura K, Takehara K, Tedder TF, Sato S. Inhibitory role of CD19 in the progression of experimental autoimmune encephalomyelitis by regulating cytokine response. Am J Pathol. 2006;168:812–821. doi: 10.2353/ajpath.2006.050923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lemos MP, Fan L, Lo D, Laufer TM. CD8alpha+ and CD11b+ dendritic cell-restricted MHC class II controls Th1 CD4+ T cell immunity. J Immunol. 2003;171:5077–5084. doi: 10.4049/jimmunol.171.10.5077. [DOI] [PubMed] [Google Scholar]
- 24.Krishnamoorthy G, Lassmann H, Wekerle H, Holz A. Spontaneous opticospinal encephalomyelitis in a double-transgenic mouse model of autoimmune T cell/B cell cooperation. J Clin Invest. 2006;116:2385–2392. doi: 10.1172/JCI28330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest. 2008 doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.