Abstract
Purpose of Review
The process of reverse cholesterol transport (RCT) is critical for disposal of excess cholesterol from the body. Although it is generally accepted that RCT requires biliary secretion, recent studies show that RCT persists in genetic or surgical models of biliary insufficiency. Discovery of this non-biliary pathway has opened new possibilities of targeting the intestine as an inducible cholesterol excretory organ. In this review we highlight the relative contribution and therapeutic potential for both biliary and non-biliary components of reverse cholesterol transport (RCT).
Recent Findings
Recently, the proximal small intestine has gained attention for its underappreciated ability to secrete cholesterol in a process called transintestinal cholesterol efflux (TICE). Although this intestinal pathway for RCT is quantitatively smaller than the biliary route under normal physiological conditions, the TICE pathway is highly inducible, providing a novel therapeutic opportunity for treatment of atherosclerotic cardiovascular disease (ASCVD). In fact, recent studies show that intestine-specific activation of RCT protects against ASCVD in mice.
Summary
It is well known that the small intestine plays a gatekeeper role in the maintenance of cholesterol balance. Through integrated regulation of cholesterol absorption and TICE, the small intestine is a key target for new therapies against ASCVD.
Keywords: cholesterol, lipoprotein, bile, reverse cholesterol transport
Introduction
Cholesterol is a key component of membranes in all vertebrates, and plays important roles in membrane dynamics, signal transduction, bile acid synthesis, and steroid hormone production [1,2]. However, excessive cholesterol accumulation in the body can promote the development of several chronic diseases including ASCVD. Although statin drugs have modestly improved mortality due to ASCVD, one of every six deaths in the United States is still attributable to this cholesterol-driven disease [3]. Given this unmet need for effective therapies, there is increasing interest in targeting new pathways that either reduce low-density lipoprotein (LDL) cholesterol or increase high-density lipoprotein (HDL)-driven RCT. The purpose of this review is to discuss recent insights into a novel intestinal pathway for RCT that has the potential to address this unmet therapeutic need. Here we discuss the current state of knowledge surrounding RCT, with particular emphasis on the relative contribution and therapeutic potential for both biliary and non-biliary components of RCT.
A New Model for Reverse Cholesterol Transport
The process of RCT was originally proposed in the 1970s as a means for the body to rid itself of excess cholesterol [4], and experimental evidence has supported active RCT in all mammals studied [2,4,5,6]. Importantly the efflux of cholesterol from macrophages in the artery wall, and subsequent disposal of this cholesterol through the RCT pathway is highly predictive of ASCVD burden in humans and rodents [7,8]. Classically, RCT was thought to involve the movement of cholesterol from peripheral tissues to the liver for excretion into bile and subsequent loss through the feces [2,4–6]. In this classic model of RCT biliary secretion was obligatory for fecal disposal [4–6]. However, emerging evidence supports an unexpected role for the small intestine in actively excreting plasma-derived cholesterol in a process known as TICE [9–12]. Given that several comprehensive reviews have been recently written surrounding the historical evidence for TICE [9–12], this will not be discussed in detail here. Instead, we will highlight the most recent advances in our understanding of the TICE pathway. In agreement with previous studies in humans [13,14], dogs [15,16], rats [17–19], and mice [20–29] we recently established that TICE exists in novel genetic and surgical mouse models [29]. In these studies we demonstrated that the specific process of macrophage RCT as well as mass fecal neutral sterol loss persist in both genetic and surgical models of biliary cholesterol insufficiency [29]. Indeed, both biliary and non-biliary pathways are operative in delivering cholesterol to the lumen of the small intestine for fecal excretion [9–29]. However, our understanding surrounding the relative contributions of the biliary and non-biliary components to RCT in animal models is still in its infancy.
The Relative Contribution of Biliary and Non-Biliary Pathways for RCT
To design both safe and effective therapeutic strategies for targeting RCT, additional work is needed to quantify the relative contributions of the biliary and non-biliary pathways to RCT. Clearly targeting the non-biliary TICE pathway is a much safer option, given that increasing biliary cholesterol secretion promotes cholesterol gallstone disease [30–32], which is a major healthcare burden already affecting more than 20 million people in the United States [33,34]. To selectively target TICE we must attempt to understand the flux through each arm of the RCT pathway. In chow-fed C57BL/6J mice the non-biliary pathway accounts for approximately 33% of total fecal neutral sterol loss [23] and roughly 20% of total fecal sterol loss in FVB mice [20]. Although these TICE estimations in chow-fed mice are quite underwhelming, it is important to point out that TICE is a highly dynamic pathway that can be upregulated under certain conditions. In support of this, in three independent mouse models (ABCG5/G8−/−, Mdr2−/−, and NPC1L1−LiverTg) with severely diminished biliary cholesterol secretion, fecal neutral sterol loss is only modestly decreased [35] or not altered at all [20,21,29]. Furthermore, in dogs and rats with complete biliary diversion, fecal neutral, but not acidic, sterol los is actually increased 2–7 fold [15–19]. This unequivocally indicates that the non-biliary TICE pathway can be stimulated under conditions of biliary insufficiency, and has the capacity to maintain [20,21,29] or even increase [15–19] fecal cholesterol loss. Non-biliary RCT is also quite sensitive to pharmacological intervention [20,23,24,27,29]. In fact, activation of the liver × receptor (L×R) can dramatically augment non-biliary macrophage RCT [29] and increase mass fecal neutral sterol loss [20,23,29]. The dynamic nature of TICE is best illustrated with LXR activation, where the contribution of TICE to fecal cholesterol loss increases from 33% in vehicle treated mice to 63% in LXR agonist-treated mice [23]. Furthermore, small molecule activation of the peroxisome proliferator activated receptor δ also stimulates TICE specifically without affecting biliary cholesterol levels [24]. Most recently it was shown that the cholesterol absorption inhibitor ezetimibe specifically activates TICE [27]. The active search for novel pharmacologic activators of TICE is ongoing, but before testing such compounds in clinical trials we must first understand the capacity of TICE in humans.
Although there are studies in humans showing that the gut can contribute to the pool of cholesterol in the intestinal lumen [13,14], the relative contribution of the biliary and non-biliary pathways to RCT has not been firmly established. A data set that hints at the contribution of the TICE pathway comes from a study of three patients with complete obstruction of the common bile duct due to carcinoma of the head of the pancreas [14]. Because this illness totally removed the input of cholesterol from the biliary pathway, the amount of intestinal cholesterol secretion could be measured. In these patients it was determined the intestine secreted on average 350 mg cholesterol/day. If the average input of cholesterol into the gut from bile is 800–1,300 mg/day [36,37], then 20–30% of the endogenous cholesterol in the lumen of the human intestine could originate from the TICE pathway. Collectively, studies in both animal models and man suggest that TICE accounts for approximately 20–30% of fecal neutral sterols under normal conditions. However, TICE can be potently stimulated by either biliary insufficiency or by drug treatment, providing strong evidence that the TICE pathway is highly dynamic and amenable to pharmacologic intervention.
Targeting the TICE Pathway for Prevention of ASCVD
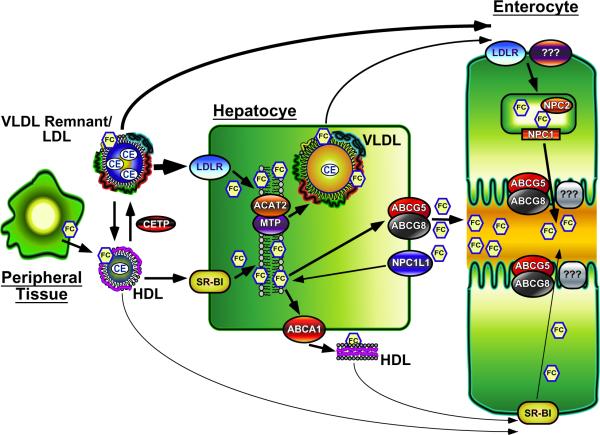
Within the last year there has been exciting new evidence that specifically targeting intestinal RCT can have profound effects on ASCVD progression [38,39]. These important studies have demonstrated that intestine-specific activation of LXR signaling can promote macrophage RCT as well as protect against ASCVD in mice [38,39]. Although providing evidence that selective pharmacological manipulation of the small intestine can protect against atherosclerosis, much additional work is needed to design novel therapeutic strategies specifically targeting TICE. The question now becomes how do we target non-biliary RCT without increasing biliary RCT and consequently the risk of gallstone formation? The answer will come from ongoing studies characterizing the following aspects of non-biliary RCT (Figure 1): (1) Plasma lipoproteins targeted to the intestine, (2) Intravascular metabolism of TICE-competent lipoproteins, (3) Intestinal lipoprotein receptors/transporters on the basolateral membrane, (4) Proteins involved in cholesterol trafficking within enterocytes, (5) Cholesterol transporter(s) on the brush border membrane, and (6) Acceptors of cholesterol in the intestinal lumen.
Figure 1.
The Role of Lipoproteins in TICE
Non-biliary RCT almost certainly relies on plasma lipoproteins to move cholesterol from either peripheral tissues and/or the liver to the small intestine for secretion. Importantly, we believe that the liver plays a central role in producing lipoproteins necessary for TICE, and support for this concept has been recently reviewed [9]. Although historically HDL has been implicated in promoting RCT [4,5,7,8], there are several studies indicating that HDL is not rate limiting in biliary or non-biliary RCT. A key observation supporting this is that ABCA1−/− mice, which have virtually no circulating HDL cholesterol, have normal bilary and fecal cholesterol levels [40,41], and display similar LXR-induced increases in RCT as wild type mice [42]. Likewise, mice deficient in apolipoprotein A-I have normal biliary and fecal cholesterol levels [43]. Furthermore, intestinal uptake of HDL-cholesteryl ester (CE) was similar in vehicle-treated control and MDR2−/− mice, and was reduced to the same extent in these two genotypes following LXR agonist treatment [44]. In several mouse models with augmented TICE [21,45,46], apolipoprotein E (apoE)-rich HDL is significantly increased implicating this lipoprotein class in TICE. However we have recently eliminated apoE expression in a mouse model where TICE predominates (NPC1L1−liverTg mice), and found no effect on biliary or non-biliary fecal cholesterol excretion (Temel RE, Brown JM, unpublished data).
In contrast to HDL, hepatic apoB-containing lipoproteins such as VLDL appear to play an important role in TICE. For instance, the concentration of VLDL cholesterol was significantly elevated in LXR agonist-treated ABCA1−/− mice, while the level of HDL cholesterol was unchanged [42]. Furthermore, we recently reported that antisense oligonucleotide (ASO)-mediated knockdown of acyl-CoA:cholesterol acyltransferase 2 (ACAT2) promotes TICE due to the apparent ability of nascent VLDL to deliver 2–3 fold more cholesterol to the proximal small intestine [22]. We have also recently discovered that knockdown of hepatic MTP, a protein essential for the lipidation and production of VLDL [47], caused a greater than 50% reduction in TICE (Temel RE, Brown JM, unpublished data). Based upon this experimental evidence, we believe that following lipolysis of the core TG, VLDL remnants or further catabolic products of VLDL (IDL, LDL) can deliver cholesterol to the small intestine for secretion. However, much additional work is needed to determine the mechanism by which apoB-containing lipoproteins can be specifically targeted to the proximal small intestine to promote TICE.
Receptors Involved in Intestinal Lipoprotein Cholesterol Uptake
Under the assumption that HDL is involved in delivering cholesterol to the small intestine, several studies have analyzed the role of the HDL receptor, SR-BI, in TICE. Using an intestinal perfusion system, TICE was found to be paradoxically increased, not decreased, in SR-BI deficient (SR-BI−/−) mice [25]. When treated with vehicle or LXR agonist, control and SR-BI−/− mice showed the same amount of intestinal uptake of HDL CE [44]. In addition, we (Temel RE, Brown JM, unpublished data) recently found that non-biliary RCT is unaffected in mice with intestine-specific overexpression of SR-BI [48]. These results strongly indicate that intestinal SR-BI is not involved in TICE and further support the conclusion that HDL does not participate in delivering cholesterol to the gut for secretion through the TICE pathway.
It would be logical to assume that the LDL receptor (LDLR) could be involved in TICE since apoB-containing lipoproteins appear to be the primary shuttle for moving cholesterol to the gut. However, the small intestine of LDLR deficient mice was able to accumulate cholesterol that originated from VLDL-containing liver perfusate [22]. In addition, LXR agonist treatment significantly increases TICE [20,23,28] in spite of increased ubiquitination and degradation of intestinal LDLR protein [49]. Other members in the LDL receptor family such as LRP, VLDL receptor (VLDLR), and apoER2 are also expressed in the gut [50,51], and could conceivably act as primary or secondary receptors for apoB-containing lipoproteins. However, LXR-driven upregulation of the E3 ubiquitin ligase Idol targets not only the LDLR but also VLDLR and apoER2 for proteasomal degradation [49,52]. Thus, the uptake of apoB-containing lipoproteins by the small intestine could involve a currently unidentified receptor.
Trafficking of Cholesterol Across Enterocytes and Into the Intestinal Lumen
The factors involved in trafficking cholesterol through the enterocytes for the TICE pathway remain poorly understood. If apoB-containing lipoproteins are involved in delivering cholesterol to the small intestine then it is likely that these lipoproteins are endocytosed and ultimately degraded in lysosomes. Therefore, TICE would require NPC1 and NPC2 to move the cholesterol out of the lysosomal compartment [53]. Interestingly, compared to control mice, mice deficient in NPC1 have double the amount of cholesterol in the small intestine [54]. Since NPC1 and NPC2 deficient mice do not have a defect in cholesterol absorption [54], the intestinal accumulation of cholesterol in the absence of NPC1 [54] is almost certainly the result of LDL cholesterol being trapped in the lysosomes [53]. Rab9 and LIMP2 could also be involved in intracellular trafficking of cholesterol derived from TICE, given that their intestinal expression was significantly elevated in mice treated with PPARδ agonists [24]. However, much additional work is obviously required to fully understand the subcellular trafficking itinerary of TICE-derived cholesterol within the enterocyte.
After being moved from the basolateral to apical membrane of the enterocytes, cholesterol is presumably actively effluxed into the lumen of the gut. Because of their ability to pump cholesterol into the intestinal lumen, the heterodimeric sterol transporters ABCG5 and ABCG8 would logically play an essential role in TICE. However, in a study using stable isotopes to measure the contribution of several pathways to fecal cholesterol excretion, it was found that although reduced by 40%, TICE was not eliminated in mice lacking ABCG5 [23]. Moreover, no difference in intestinal cholesterol secretion was observed in control and ABCG8 deficient mice subjected to intestinal perfusion [28]. Importantly, ABCG5 and ABCG8 are necessary for LXR-stimulated biliary and non-biliary RCT [23,56,57]. These results indicate that although ABCG5 and ABCG8 do contribute to LXR-stimulated RCT [23,56,57], other apical transporters are likely involved in the secretion of cholesterol from enterocytes into the intestinal lumen under normal physiological conditions.
The secretion of cholesterol into the intestinal lumen would presumably require an acceptor. Intestinal perfusion of mice showed that a mixture of bile acids and phospholipid could drive TICE [28]. However, the amount of phospholipid in the perfusate appeared to determine the level of intestinal cholesterol secretion [28]. The dependence on phospholipid for TICE is similar to what is observed for the movement of cholesterol into the bile [20]. Moreover, the highest levels of TICE have been reported to occur in the proximal small intestine, a region of the gut that should contain a high concentration of bile-derived phospholipid. Studies examining the luminal acceptors of TICE hold great promise for therapeutic interventions that would not require systemic distribution.
Conclusion
The process of RCT is strongly negatively correlated with ASCVD burden in humans and rodents [7,8]. Although RCT was historically believed to rely solely on biliary secretion [2,4–6], recent evidence unequivocally demonstrates that a non-biliary pathway for RCT exists [9–29]. Unfortunately, to date very little progress has been made to identify the molecular mechanisms that define TICE. In order to effectively target this pathway, the following challenges will need to be addressed: (1) establishing quantitative methods to measure nonbiliary fecal sterol loss in primates and man, (2) characterizing the hepatic and plasma lipoprotein metabolism that is requisite for nonbiliary fecal sterol loss, (3) identifying the intestinal transport proteins/receptors involved, (3) identifying the lumenal acceptor molecules promoting TICE, and (4) identifying bona fide drug targets to specifically modulate the intestinal component of RCT. Advancement in these areas has strong potential for novel anti-ASCVD therapies.
Key Points
TICE contributes ~30% of total RCT in normal conditions, but importantly TICE can be potently stimulated by either biliary insufficiency or by drug treatment, providing strong evidence that the TICE pathway is a highly dynamic pathway that is amenable to pharmacologic intervention.
The TICE pathway is a much more attractive drug target since increased biliary secretion promotes gallstone formation.
To effectively target TICE, we need to understand: hepatic cholesterol trafficking, plasma lipoprotein carriers and intravascular metabolism, basolateral transporters, trafficking pattern across the enterocyte, apical efflux mechanisms, and lumenal acceptors.
Acknowledgments
This work was supported by pathway to independence grants (K99/R00-HL088528 to R.E.T. and K99/R00-HL096166 to J.M.B.) from the National Heart, Lung, and Blood Institute. The authors report that they have no conflicts of interest.
Abbreviations Used
- ABCG5/G8
ATP-binding cassette transporters G5 and G8
- ACAT2
Acyl-CoA:cholesterol acyltransferase 2
- ASO
antisense oligonucleotide
- ASCVD
atherosclerotic cardiovascular disease
- HDL
high density lipoprotein
- LDL
low density lipoprotein
- LXR
liver × receptor
- MDR2
P-glycoprotein
- NPC1L1
Niemann Pick-C1 Like-1
- PPARδ
peroxisome proliferator-activated receptor delta
- RCT
reverse cholesterol transport
- SR-BI
scavenger receptor class B type I
- TICE
transintestinal cholesterol efflux
References
- 1.Ikonen E. Cellular cholesterol trafficking and compartmentalization. Nat Rev Mol Cell Biol. 2008;9:125–138. doi: 10.1038/nrm2336. [DOI] [PubMed] [Google Scholar]
- 2.Dietschy JM, Turley SD, Spady DK. Role of the liver in the maintenance of cholesterol and low density lipoprotein homeostasis in different animal species, including humans. J Lipid Res. 1993;34:1637–1659. [PubMed] [Google Scholar]
- 3.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics – 2011 update, a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glomset JA, Norum KR. The metabolic role of lecithin:cholesterol acyltransferase: perspectives from pathology. Adv Lipid Res. 1973;11:1–65. [PubMed] [Google Scholar]
- 5.Rader DJ. Regulation of reverse cholesterol transport and clinical implications. Am J Cardiol. 2003;92:42J–49J. doi: 10.1016/s0002-9149(03)00615-5. [DOI] [PubMed] [Google Scholar]
- 6.Dietshcy JM, Turley SD. Control of cholesterol turnover in the mouse. J Biol Chem. 2002;277:3801–3804. doi: 10.1074/jbc.R100057200. [DOI] [PubMed] [Google Scholar]
- 7.Khera AV, Cuchel M, de la Liera-Moya M, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This paper demonstrates that the measurement of cholesterol efflux capacity from macrophages, as a measure of HDL function, is inversely correlated with cardiovascular disease burden in humans.
- 8.Khera AV, Rader DJ. Future therapeutic directions in reverse cholesterol transport. Curr Atheroscler Rep. 2010;12:73–81. doi: 10.1007/s11883-009-0080-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Temel RE, Brown JM. A new framework for reverse cholesterol transport: non-biliary contributions to reverse cholesterol transport. World J Gastroenterol. 2010;16:5946–5952. doi: 10.3748/wjg.v16.i47.5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van der Velde AE, Brufau G, Groen AK. Transintestinal cholesterol efflux. Curr Opin Lipidol. 2010;21:167–171. doi: 10.1097/MOL.0b013e3283395e45. [DOI] [PubMed] [Google Scholar]
- 11.Brufau G, Groen AK, Kuipers F. Reverse cholesterol transport revisited: contribution of biliary versus intestinal cholesterol excretion. Arterioscler Thromb Vasc Biol. 2011;31:1726–1733. doi: 10.1161/ATVBAHA.108.181206. [DOI] [PubMed] [Google Scholar]
- 12.Vrins CL. From blood to gut: direct secretion of cholesterol via transintestinal cholesterol efflux. World J Gastroenterol. 2010;16:5953–5957. doi: 10.3748/wjg.v16.i47.5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simmonds WJ, Hofmann AF, Theodor E. Absorption of cholesterol from a micellular solution: intestinal perfusion studies in man. J Clin Invest. 1967;46:874–890. doi: 10.1172/JCI105587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng SH, Stanley MM. Secretion of cholesterol by intestinal mucosa in patients with complete common bile duct obstruction. Proc Soc Exp Biol Med. 1959;101:223–225. doi: 10.3181/00379727-101-24890. [DOI] [PubMed] [Google Scholar]
- 15.Sperry WM. Lipid Excretion IV. A study of the relationship of the bile to the fecal lipids with special reference to certain problems of sterol metabolism. J Biol Chem. 1927;71:351–378. [Google Scholar]
- 16.Pertsemlidis D, Kirchman EH, Ahrens EH., Jr. Regulation of cholesterol metabolism in the dog I. Effects of complete bile diversion and of cholesterol feeding on absorption, synthesis, accumulation, and excretion rates measured during life. J Clin Invest. 1973;52:2353–2367. doi: 10.1172/JCI107424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dietschy JM. The role of bile salts in controlling the rate of intestinal cholesterogenesis. J Clin Invest. 1968;47:286–300. doi: 10.1172/JCI105725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dietschy JM, Siperstein MD. Cholesterol synthesis by the gastrointestinal tract: localization and mechanisms of control. J Clin Invest. 1965;44:1311–1327. doi: 10.1172/JCI105237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bandsma RH, Stellard F, Vonk RJ, et al. Contribution of newly synthesized cholesterol to rat plasma and bile determined by mass isotopomer distribution analysis: bile-salt flux promotes secretion of newly synthesized cholesterol into bile. Biochem J. 1998;329:699–703. doi: 10.1042/bj3290699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kruit JK, Plosch T, Havinga R, et al. Increased fecal neutral sterol loss upon liver × receptor activation is independent of biliary sterol secretion in mice. Gastroenterology. 2005;128:147–156. doi: 10.1053/j.gastro.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 21.Temel RE, Tang W, Ma Y, et al. Hepatic Niemann-Pick C1-like 1 regulates biliary cholesterol concentration and is a target of ezetimibe. J Clin Invest. 2007;117:1968–1978. doi: 10.1172/JCI30060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown JM, Bell TA, III, Alger HM, et al. Targeted depletion of hepatic ACAT2-driven cholesterol esterification reveals a non-biliary route for fecal neutral sterol loss. J Biol Chem. 2008;283:10522–10534. doi: 10.1074/jbc.M707659200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Veen JN, van Dijk TH, Vrins CL, et al. Activation of liver × receptor stimulates trans-intestinal excretion of plasma cholesterol. J Biol Chem. 2009;284:19211–19219. doi: 10.1074/jbc.M109.014860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vrins CL, Van der Velde AE, Van den Oever K, et al. PPARδ activation leads to increased trans intestinal cholesterol efflux. J Lipid Res. 2009;50:2046–2054. doi: 10.1194/jlr.M800579-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Velde AE, Vrins CL, van den Oever K, et al. Regulation of direct transintestinal cholesterol excretion in mice. Am J Physiol Gastrointest Liver Physiol. 2008;295:G203–G208. doi: 10.1152/ajpgi.90231.2008. [DOI] [PubMed] [Google Scholar]
- 26.Brufau G, Kuipers F, Lin Y, et al. A reappraisal of the mechanism by which plant sterols promote neutral sterol loss in mice. PLoS One. 2011;6:e21576. doi: 10.1371/journal.pone.0021576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jakulj L, Vissers MN, van Roomen CP, et al. Ezetimibe stimulates faecal neutral sterol excretion depending on abcg8 function in mice. FEBS Lett. 2010;584:3625–3628. doi: 10.1016/j.febslet.2010.07.035. [DOI] [PubMed] [Google Scholar]
- 28.Van der Velde AE, Vrins CL, van den Oever K, et al. Direct intestinal cholesterol secretion contributes significantly to total fecal neutral sterol excretion in mice. Gastroenterology. 2007;133:967–975. doi: 10.1053/j.gastro.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 29.Temel RE, Sawyer JK, Yu L, et al. Biliary sterol secretion is not required for macrophage reverse cholesterol transport. Cell Metab. 2010;12:96–102. doi: 10.1016/j.cmet.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** First study to show that the specific process of macrophage RCT persists in mice genetically or surgically lacking the ability to secrete cholesterol into bile.
- 30.Admirand WH, Small DM. The physiochemical basis of cholesterol gallstone formation in man. J Clin Invest. 1968;47:1043–1052. doi: 10.1172/JCI105794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Small DM, Bourges M, Dervichian DG. Ternary and quaternary aqueous systems containing bile salt, lecithin, and cholesterol. Nature. 1966;211:816–818. doi: 10.1038/211816a0. [DOI] [PubMed] [Google Scholar]
- 32.Grunhage F, Acalovschi M, Tirziu S, et al. Increased gallstone risk in humans conferred by common variant of hepatic ATP-binding cassette transporter for cholesterol. Hepatology. 2007;46:793–801. doi: 10.1002/hep.21847. [DOI] [PubMed] [Google Scholar]
- 33.Everhart JE, editor. The burden of digestive diseases in the United States. National Institute of Diabetes and Digestive and Kidney Diseases, U.S. Dept of Health and Human Services; Bethesda, MD: 2008. NIH Publication 09-6433. [Google Scholar]
- 34.Shaffer EA. Epidemiology of gallbladder stone disease. Best Practices and Research Clinical Gastroenterology. 2006;20:981–996. doi: 10.1016/j.bpg.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Yu L, Hammer RE, Li-Hawkins J, et al. Disruption of Abcg5 and Abcg8 in mice reveals their crucial role in biliary cholesterol secretion. Proc Natl Acad Sci USA. 2002;99:16237–16242. doi: 10.1073/pnas.252582399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grundy SM, Metzger AL. A physiological method for estimation of hepatic secretion of biliary lipids in man. Gastroenterology. 1972;62:1200–1217. [PubMed] [Google Scholar]
- 37.Mitchell JC, Stone BG, Logan GM, Duane WC. Role of cholesterol synthesis in regulation of bile acid synthesis and biliary cholesterol secretion in humans. J Lipid Res. 1991;32:1143–1149. [PubMed] [Google Scholar]
- 38.Yasuda T, Grillot D, Billheimer JT, et al. Tissue-specific liver × receptor activation promotes macrophage reverse cholesterol transport in vivo. Arterioscler Thromb Vasc Biol. 2010;30:781–786. doi: 10.1161/ATVBAHA.109.195693. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Study demonstrates that an intestine-specific small molecule LXR agonist increases macrophage RCT, supporting a prominent role of the small intestine in RCT.
- 39.Lo Sasso G, Murzilli S, Salvatore L, et al. Intestinal specific LXR activation stimulates reverse cholesterol transport and protects from atherosclerosis. Cell Metab. 2010;12:187–193. doi: 10.1016/j.cmet.2010.07.002. [DOI] [PubMed] [Google Scholar]; ** First study to show that mice expressing constituitively-active LXR exclusively in intestinal enterocytes have increased RCT rates and are protected against atherosclerosis.
- 40.Groen AK, Bloks VW, Bandsma RH, et al. Hepatobiliary cholesterol transport is not impaired in Abca1-null mice lacking HDL. J Clin Invest. 2001;108:843–850. doi: 10.1172/JCI12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xie C, Turley SD, Dietschy JM. ABCA1 plays no role in centripetal movement of cholesterol from peripheral tissues to the liver and intestine in the mouse. J Lipid Res. 2009;50:1316–1329. doi: 10.1194/jlr.M900024-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Plosch T, Kok T, Bloks VW, et al. Increased hepatobiliary and fecal cholesterol excretion upon activation of the liver × receptor is independent of ABCA1. J Biol Chem. 2002;277:33870–33877. doi: 10.1074/jbc.M206522200. [DOI] [PubMed] [Google Scholar]
- 43.Jolley CD, Woolett LA, Turley SD, Dietschy JM. Centripetal cholesterol flux to the liver is dictated by events in the peripheral organs and not by the plasma high density lipoprotein of apolipoprotein A-I concentrations. J Lipid Res. 1998;39:2143–2149. [PubMed] [Google Scholar]
- 44.Nijstad N, Gautier T, Briand F, et al. Biliary sterol secretion is required for functional in vivo reverse cholesterol transport in mice. Gastroenterology. 2011;140:1043–1051. doi: 10.1053/j.gastro.2010.11.055. [DOI] [PubMed] [Google Scholar]
- 45.Cao G, Beyer TP, Yang XP, et al. Phospholipid transfer protein is regulated by liver × receptors in vivo. J Biol Chem. 2002;277:39561–39565. doi: 10.1074/jbc.M207187200. [DOI] [PubMed] [Google Scholar]
- 46.Jiang XC, Beyer TP, Li Z, et al. Enlargement of high density lipoprotein in mice via liver × receptor activation requires apolipoprotein E and is abolished by cholesteryl ester transfer protein expression. J Biol Chem. 2003;278:49072–49078. doi: 10.1074/jbc.M304274200. [DOI] [PubMed] [Google Scholar]
- 47.Hussain MM, Rava P, Pan X, et al. Microsomal triglyceride transfer protein in plasma and cellular lipid metabolism. Curr Opin Lipidol. 2008;19:277–284. doi: 10.1097/MOL.0b013e3282feea85. [DOI] [PubMed] [Google Scholar]
- 48.Bietrix F, Yan D, Nauze M, et al. Accelerated lipid absorption in mice overexpressing intestinal SR-BI. J Biol Chem. 2006;281:7214–7219. doi: 10.1074/jbc.M508868200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zelcer N, Hong C, Boyadjian R, Tontonoz P. LXR regulates cholesterol uptake through Idol-dependent ubiquitination of the LDL receptor. Science. 2009;325:100–104. doi: 10.1126/science.1168974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Herz J, Hamann U, Rogne S, et al. Surface location and high affinity for calcium of a 500-kd liver membrane protein closely related to the LDL-receptor suggest a physiological role as lipoprotein receptor. EMBO J. 1988;7:4119–4127. doi: 10.1002/j.1460-2075.1988.tb03306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garcia-Miranda P, Peral MJ, Ilundain AA. Rat small intestine expresses the reelin-Disabled-1 signalling pathway. Exp Physiol. 2010;95:498–507. doi: 10.1113/expphysiol.2009.050682. [DOI] [PubMed] [Google Scholar]
- 52.Hong C, Duit S, Jalonen P, et al. The E3 ubiquitin ligase IDOL induces the degradation of the low density lipoprotein receptor family members VLDLR and ApoER2. J Biol Chem. 2010;285:19720–19726. doi: 10.1074/jbc.M110.123729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vance JE, Peake KB. Function of the Niemann-Pick type C proteins and their bypass by cyclodextrin. Curr Opin Lipidol. 2011;22:204–209. doi: 10.1097/MOL.0b013e3283453e69. [DOI] [PubMed] [Google Scholar]
- 54.Xie C, Turley SD, Pentchev PG, Dietschy JM. Cholesterol balance and metabolism in mice with loss of function of Niemann-Pick C protein. Am J Physiol. 1999;276:E336–E344. doi: 10.1152/ajpendo.1999.276.2.E336. [DOI] [PubMed] [Google Scholar]
- 55.Dixit SS, Sleat DE, Stock AM, Lobel P. Do mammalian NPC1 and NPC2 play a role in intestinal cholesterol absorption? Biochem J. 2007;408:1–5. doi: 10.1042/BJ20071167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu L, York J, von Bergmann K, et al. Stimulation of cholesterol excretion by the liver × receptor agonist requires ATP-binding cassette transporters G5 and G8. J Biol Chem. 2003;278:15565–15570. doi: 10.1074/jbc.M301311200. [DOI] [PubMed] [Google Scholar]
- 57.Calpe-Berdiel L, Rotllan N, Fievet C, et al. Liver × receptor-mediated activation of reverse cholesterol transport from macrophages to feces in vivo requires ABCG5/G8. J Lipid Res. 2008;49:1904–1911. doi: 10.1194/jlr.M700470-JLR200. [DOI] [PubMed] [Google Scholar]