Abstract
Prevention reduces tooth loss, but little evidence supports biannual preventive care for all adults. We used risk-based approaches to test tooth loss association with 1 vs. 2 annual preventive visits in high-risk (HiR) and low-risk (LoR) patients. Insurance claims for 16 years for 5,117 adults were evaluated retrospectively for tooth extraction events. Patients were classified as HiR for progressive periodontitis if they had ≥ 1 of the risk factors (RFs) smoking, diabetes, interleukin-1 genotype; or as LoR if no RFs. LoR event rates were 13.8% and 16.4% for 2 or 1 annual preventive visits (absolute risk reduction, 2.6%; 95%CI, 0.5% to 5.8%; p = .092). HiR event rates were 16.9% and 22.1% for 2 and 1 preventive visits (absolute risk reduction, 5.2%; 95%CI, 1.8% to 8.4%; p = .002). Increasing RFs increased events (p < .001). Oral health care costs were not increased by any single RF, regardless of prevention frequency (p > .41), but multiple RFs increased costs vs. no (p < .001) or 1 RF (p = .001). For LoR individuals, the association between preventive dental visits and tooth loss was not significantly different whether the frequency was once or twice annually. A personalized medicine approach combining gene biomarkers with conventional risk factors to stratify populations may be useful in resource allocation for preventive dentistry (ClinicalTrials.gov, NCT01584479).
Keywords: comparative effectiveness research, personalized medicine, periodontal disease, interleukin polymorphisms, oral health, health care delivery
Introduction
Health care costs in many countries appear unsustainable (Keehan et al., 2011), and a substantial proportion of those costs may arise from unnecessary services and missed opportunities to prevent chronic diseases (Yong and Olsen, 2010).
Tooth loss in adults is primarily attributable to periodontitis (PD) and dental caries (Murray et al., 1996; Ong et al., 1996; Chrysanthakopoulos, 2011; Mai et al., 2013). Periodontitis is a common chronic inflammatory disease affecting 47% in the U.S., with 8.5% having severe disease (Eke et al., 2012). Caries is also highly prevalent in the majority of adults (Dye et al., 2012). PD destroys bone and connective tissues of the gingiva and is associated with increased systemic inflammation and risk for inflammatory diseases (Blaizot et al., 2009; Sfyroeras et al., 2012). Caries arises from demineralization and destruction of the hard tissues of the tooth caused by bacterial fermentation and acid production. Regular adult dental prophylaxis directed at prevention of PD and caries is one of the most widely used health care services (Wall and Brown, 2003). The approximately 500 million annual dental visits cost more than $100 billion (Chronic Disease Prevention and Health Promotion, 2012), and routine preventive visits account for 76% of dental services (Smithwick, 2012).
Regular control of oral microbial biofilms prevents periodontitis and caries (Axelsson and Lindhe, 1981). Some risk factors, most prominently smoking, diabetes, and certain genetic variations, are associated with more severe and progressive PD (Van Dyke and Sheilesh, 2005), and previous caries experience and levels of cariogenic bacteria are among the risk factors associated with future caries lesions (Fontana and Zero, 2006; Ito et al., 2011). In spite of this information, the present preventive model rests on the tacit assumption that all adults are at equal risk, therefore needing biannual professional preventive measures, as adopted in many health systems. Scant evidence supports this preventive care frequency in adults (Sheiham, 1977; Rosen et al., 2004; Beirne et al., 2007; Clarkson et al., 2009; Ito et al., 2012).
We used pre-defined criteria to stratify adults without a periodontitis diagnosis into high-risk (HiR) and low-risk (LoR) groups for development/progression of PD, based on 3 risk factors, and then questioned whether the frequency of dental visits (mainly once or mainly twice yearly) was associated with tooth loss events equally in the two risk groups. Risk factors used for this stratification were: presence of diabetes (Lalla and Papapanou, 2011), cigarette smoking (Hanioka et al., 2011), and the interleukin-1 (IL-1) genotype (Kornman et al., 1997; Karimbux et al., 2012), all previously associated with adult PD. We used an insurance claims database and contemporaneously collected genetic and health history information to conduct a retrospective cohort study primarily to determine if, in low-risk patients, 2 preventive dental visits annually were superior to 1 relative to long-term tooth loss. We use tooth loss event rates and actual cost history to discuss the potential implications of our findings.
The approach adopted here, based on population stratification with pre-defined risk factors to guide prevention of a highly prevalent chronic disease, may provide a proof-of-principle for approaches aimed at better outcomes and a more cost-effective use of health care resources.
Methods
Study Design
We designed a retrospective cohort study to test associations between the frequency of preventive services and tooth loss events in patients at LoR for periodontitis. The protocol was approved by the institutional review board (IRB) at the University of Michigan, and all participants provided written consent. This investigation complied with the recommendations of the STROBE statement guidelines.
Participants
Participants were recruited from a dental insurance claims database (Delta Dental of Michigan) in a two-stage process defined in the protocol and compliant with the Health Insurance Portability and Accountability Act of 1996 (HIPAA) Privacy and Security Rules. Details of the participant entrance criteria and selection process are in the Appendix. In brief, patients with insurance coverage through one employee group were identified if they met the following criteria: ≥ 15 consecutive yrs of claims data; age 34 through 55 yrs at initial record; no prior diagnosis of early periodontitis; and had received regular preventive care. Claims data were used to identify patients who habitually met criteria for preventive dental visits once (P1: mean 1.0/yr, median 1.1, interquartile range [IQR] 1.0-1.2) or twice (P2: mean 1.8/yr, median 1.8, IQR 1.8-2.0) annually during a six-year index period, although all patients were covered for 2 preventive visits/yr. This employee group and a second employee group located in the Great Lakes Region of Michigan were compared for tooth loss characteristics (Appendix Table 1), including an “irregular care” group that consistently had < 1 preventive visit/yr during the index period and an “early periodontitis” group that qualified for and had > 2 annual preventive visits during that period.
Risk Classification
Patients were classified as “low risk” (LoR) if they never smoked or had not smoked in the previous 10 yrs (questionnaire), had no history of Type I or II diabetes (questionnaire), and were IL-1 genotype-negative (buccal swab samples). They were classified as “high risk” (HiR) if they met any 1 of the 3 criteria. Therefore, tooth loss was compared across four groups designated as HiR-P1, HiR-P2, LoR-P1, and LoR-P2.
Genotyping
Buccal swabs were self-collected by patients and submitted by mail to the University. Samples were genotyped (see Appendix for methods and criteria for positive/negative status) for specific IL-1 single-nucleotide polymorphisms (SNPs) in a CLIA-certified genetics laboratory (Interleukin Genetics, Waltham, MA, USA) and classified as IL-1 genotype-positive or -negative by 2 versions of a genotype test. The primary analysis used genotype version 1, and some secondary analyses used version 2 (identified in the text).
Statistical Analysis
The primary outcome was the 16-year proportion of patients having tooth loss events, identified as ≥ 1 tooth extracted according to American Dental Association Current Dental Terminology (CDT) tooth extraction codes (American Dental Association, 2010), excluding 3rd molars. Secondary analyses used all dental procedure costs submitted by the dentist during the observation interval and periodontal treatment costs including CDT codes for surgical, non-surgical, and local chemotherapeutic procedures for periodontitis treatment (see Appendix). Demographic characteristics were summarized, with means and differences among patient groups assessed by the Wilcoxon Rank-Sum test (for continuous measures) and a chi-squared test of association (for categorical measures). Logistic regression was used to estimate and compare extraction rates among patient groups at each individual time point. Logistic regression was also used to estimate the pattern of extraction rates over time; statistical significance was assessed with an empirical estimate of variance to account for correlation of measures from the same participant. Statistical significance was defined as p < .05.
Results
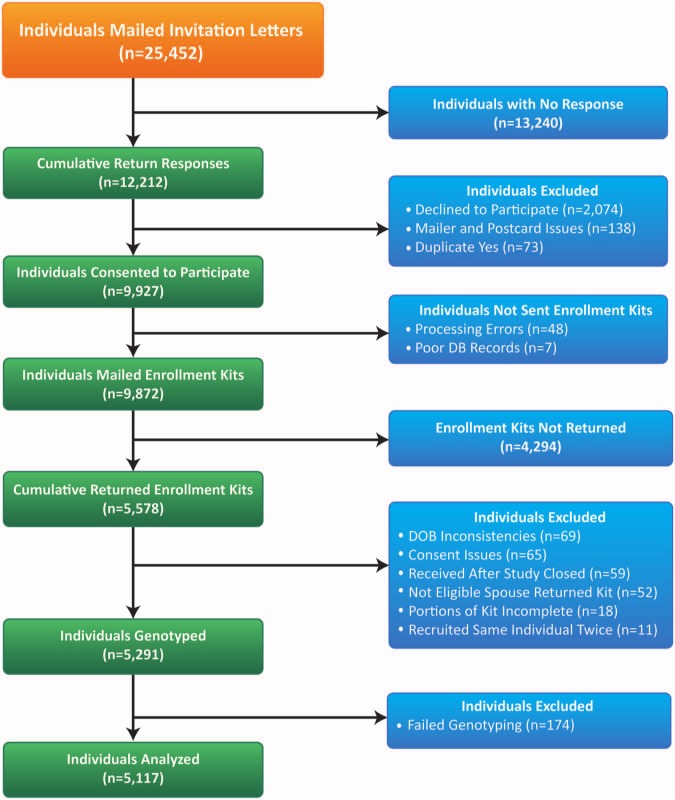
From 25,452 individuals meeting inclusion criteria, 9,927 (0.39 of eligible) consented to participate, and 5,117 (0.515 of consented) returned completed questionnaires (Table) and were successfully genotyped (Fig. 1).
Table.
Patient Demographics
| Measure | 1 Preventive Visit/yr (n = 1,584; %) | 2 Preventive Visits/yr (n = 3,533; %) | p value |
|---|---|---|---|
| Age mean (Interquartile range) | 47 (44, 50) | 47 (44, 50) | .87 |
| Race (White frequency) | 96 | 98 | .17 |
| Female (frequency) | 63 | 67 | .004 |
| Smoking (frequency) | 18 | 19 | .57 |
| Diabetes (frequency) | 9 | 7 | .03 |
| IL-1 genotype (Positive) | 38 | 37 | .44 |
| Other Reported Diseases | |||
| Psoriasis | 4 | 4 | .541 |
| Rheumatoid arthritis | 3 | 3 | .944 |
| Seizure disorders | 1 | 1 | .675 |
| Inflammatory bowel disease | 2 | 3 | .496 |
| Crohn’s disease | 1 | 0 | .086 |
| Ulcerative colitis | 1 | 1 | .238 |
| Kidney disease | 1 | 1 | .965 |
| Sjögren’s Syndrome | 0 | 1 | .494 |
| Organ transplant | 0 | 0 | n/a |
| Cancer or malignancy | 14 | 15 | .326 |
| Consumption of alcohol (responded anything other than “< 1 drink/day”) | 18 | 17 | .849 |
| None of above | 75 | 75 | .900 |
| Osteoarthritis | 20 | 20 | .711 |
| 2 or more yrs of medication | 46 | 49 | .342 |
| Non-steroidal | 11 | 12 | .386 |
| Steroidal | 1 | 1 | .763 |
| Osteoporosis | 11 | 13 | .019 |
| 2 or more yrs of medication | 66 | 75 | .009 |
| Bisphosphonate | 8 | 11 | .008 |
| Hormone replacement therapy | 3 | 4 | .126 |
| Chronic Pain | 21 | 19 | .140 |
| 2 or more yrs of medication | 78 | 83 | .053 |
| Ibuprofen | 10 | 8 | .045 |
| Naproxen | 6 | 6 | .786 |
| Aspirin | 5 | 4 | .029 |
| Steroids | 2 | 2 | .341 |
| Asthma or Chronic Obstructive Pulmonary Disease (COPD) | 13 | 11 | .029 |
| 2 or more yrs of medication | 60 | 61 | .761 |
| Corticosteroids | 2 | 1 | .034 |
| Inhaled steroids | 7 | 6 | .230 |
| Heart Arrhythmia or Heart Condition | 18 | 15 | .014 |
| 2 or more yrs of medication | 55 | 58 | .493 |
| Statins | 10 | 9 | .451 |
| Beta blockers | 7 | 5 | .069 |
| Use of low-dose aspirin | 51 | 48 | .060 |
Numbers in boldface indicate a p value of < .05.
Figure 1.
Patient enrollment. Of a total of 25,452 individuals to whom letters were mailed, 12,212 responses were returned, of which 9,927 were from those consenting to participate in the study. From these individuals, 9,872 were mailed enrollment kits, and 5,578 individuals returned enrollment kits for the study. After exclusion of individuals for specific issues noted above, 5,291 individuals were genotyped. In total, 5,117 individuals had complete questionnaires and genetic information for analysis.
Preventive Visit Frequency Relationship to Event Rate by Risk Classification
Preventive visit frequencies remained consistent with the index period for P2 patients from 7 through 11 yrs (mean 1.8/yr, median 1.8, IQR 1.7-1.8) and from 12 through 16 yrs (mean 1.8/yr, median 1.8, IQR 1.8-2.0). Frequencies for P1 patients remained mainly once annually from 7 through 11 yrs (mean 1.3/yr, median 1.3, IQR 1.0-1.7) and exhibited some drift from the index period at 12 through 16 yrs (mean 1.6/yr, median 1.5, IQR 1.2-1.8). In later years, some P1 and P2 patients had 3 to 4 preventive visits/yr, most likely prompted by disease diagnosis.
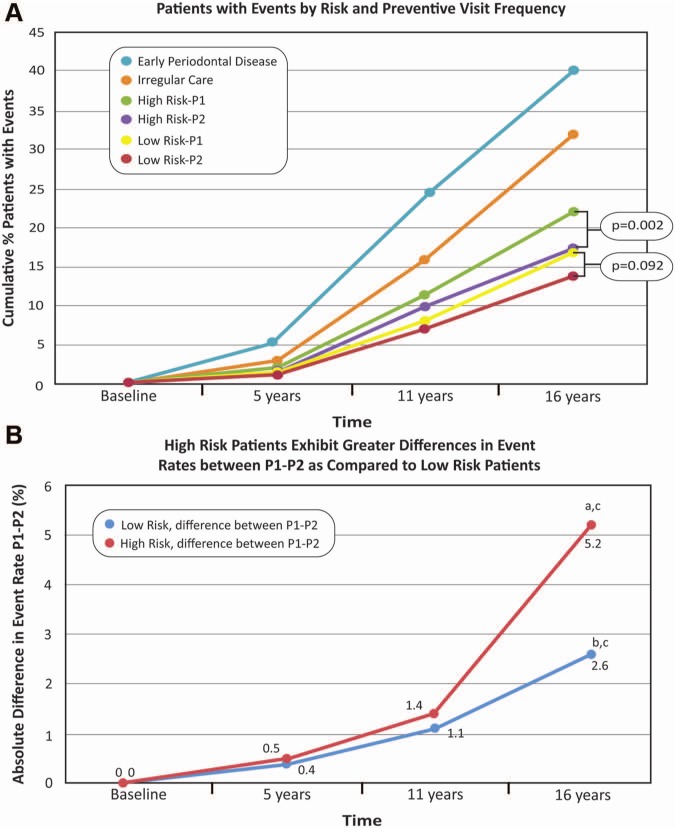
Interactions of risk status and frequency of preventive visits on tooth loss were evident at 16 yrs (Fig. 2A), with a 13.8% cumulative event rate for LoR-P2, in contrast to 22.1% in HiR-P1 patients.
Figure 2.
The influence of risk status and frequency of preventive visits on tooth loss events. Panel A demonstrates a 13.8% cumulative event rate for low-risk patients with 2 preventive visits annually (LoR-P2), in contrast to 22.1% in high-risk patients with 1 visit annually (HiR-P1). In low-risk (LoR) patients, 2 preventive visits per yr (LoR-P2) were no better than 1 preventive visit per yr (LoRP1) in reducing the percentage of patients with tooth loss events over 16 yrs. In high-risk patients, 2 preventive visits per yr (HiR-P2) significantly reduced the number of patients who had events, in contrast to 1 visit per yr (HiR-P1; p = .002). The ‘irregular care’ patients are individuals with limited numbers of visits during the indexing period (Table), while the patients considered to have periodontitis during the indexing period were identified according to high-frequency visits of > 14 during the six-year time frame. Panel B shows that the slope of the absolute difference between 1 and 2 preventive visits annually for HiR patients over time differed from 0a (HiR P1-P2 diff; p = .005). At 16 yrs, an additional 2.6/100 LoR patients experienced events as a result of 1 preventive visit per yr in contrast to 2, and the slope of the absolute difference between 1 and 2 preventive visits annually for low-risk patients over time was not different from 0b (LoR P1-P2 diff; p = .36). At 16 yrs, an additional 5.2/100 HiR patients had events as a result of 1 preventive visit per yr, in contrast to 2. In the first 11 yrs of monitoring, HiR patients displayed no difference in the event rate based on the frequency of preventive visits (p = .261). The trends between the 2 slopes were not different (p = .76).c
LoR-P2 patients did not have lower frequency of tooth loss events than did LoR-P1 patients (Fig. 2A; p = .092). At 16 yrs, an additional 2.6/100 LoR patients (16.4/100 for LoR-P1 vs. 13.8/100 for LoR-P2) experienced events associated with one less preventive visit/yr (Fig. 2A; p = .092). Furthermore, the slope of the absolute difference between 1 and 2 visits annually for LoR patients was not different from 0 (Fig. 2B; LoR P1-P2 diff; p = .36).
HiR-P2 patients had significantly lower event rates compared with HiR-P1 patients (Fig. 2A; p = .002). At 16 yrs, an additional 5.2/100 HiR patients (22.1/100 for HiR-P1 vs. 16.9/100 for HiR-P2) had events associated with 1 less preventive visit/yr (Fig. 2A; p = .002). The absolute difference in events between 1 and 2 preventive visits annually for HiR patients over time differed from 0 (Fig. 2B; HiR P1-P2 diff; p = .005).
The mean number of additional teeth lost over 16 yrs associated with approximately 1 less preventive visit annually was 0.127 teeth (p < .001) in HiR patients and 0.082 teeth (p < .001) in LoR patients. The mean cumulative number of teeth lost over 16 yrs in patients who had events ranged from 1.55 to 1.81 in LoR patients and from 1.86 to 2.01 in HiR patients, depending on preventive visit frequency.
Version 2 of the IL-1 genetic test gave results comparable with those in version 1 relative to differences between 1 and 2 preventive visits (Appendix Fig.).
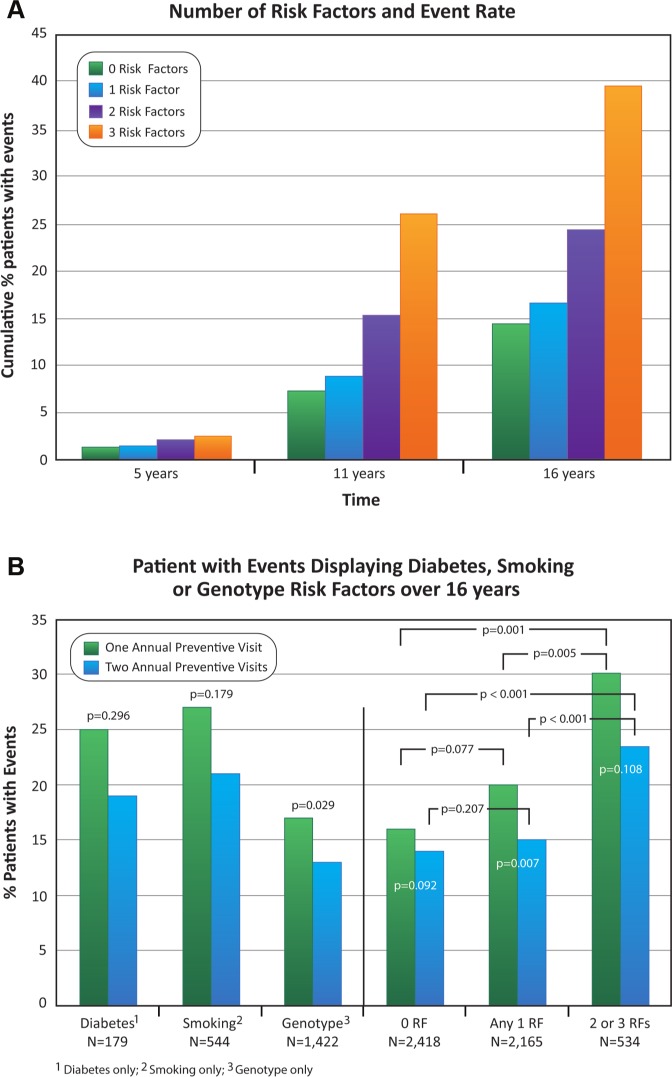
Risk Factor Relationship to Event Rate
The frequency of events in all patients increased with the number of risk factors (Fig. 3A; p < .001 at 11 and 16 yrs). Event rates (Fig. 3B) were not different by preventive visit frequency for patients with diabetes alone (25.3% for P1 vs. 18.8% for P2; p = .296) or smoking alone (26.6% for P1 vs. 21.2% for P2; p = .179), which may reflect sample size, whereas event rates for patients with the IL-1 genotype alone differed by frequency of preventive visits (17.0% for P1 vs. 12.7% for P2; p = .029). In patients with any single risk factor, an event rate of 20.0% was associated with predominantly 1 annual preventive visit, in contrast to an event rate of 15.3% for 2 visits (Fig. 3B; p = .007). The corresponding rates in patients with zero risk factors were 16.4% for P1 and 13.8% for P2; neither rate was significantly different from that in patients with 1 risk factor (Fig. 3B; p = .077 and p = .207, respectively). Patients having 2 or 3 risk factors had higher event rates of 30.1% for P1 and 23.5% for P2; these rates were different compared with 0 (p < .001 for both P1 and P2) and 1 risk factor (p = .005 for P1; p < .001 for P2).
Figure 3.
Frequency of tooth loss events in patients relative to the number of risk factors. Panel A shows that the increasing numbers of risk factors at 5 yrs were not significant (trend p = .168), while from years 11 to 16 the trend was significant with increasing numbers of risk factors (p < .001). In Panel B, event rates were not different by preventive visit frequency for patients with diabetes alone or smoking alone (p = .296 and p = .179, respectively), whereas event rates for patients with the IL-1 genotype alone differed by frequency of preventive visits (p = .029). In patients with any 1 risk factor, 1 annual preventive visit increased the event rate in contrast to 2 (p = .007). Among all patients with 1 preventive visit annually, having any 1 risk factor in contrast to 0 did not increase event rate (p = .077), but having 2 or 3 risk factors increased the event rate in contrast to 0 (p < .001) and 1 risk factor (p = .005). Findings were similar among patients with 2 preventive visits annually, for 1 risk factor in contrast to 0 (p = .207), and for 2 or 3 risk factors in contrast to 0 (p < .001) and 1 risk factor (p < .001).
Preventive Visit Frequency and Risk Factor Relationships to Costs
Total 16-year dental care costs for the 5,117 patients were $40,080,710. The results comparing cost of care are shown in the Appendix. Costs based on visit frequency are shown in Appendix Tables 2 and 3.
Discussion
Among adult regular users of dental services with no prior diagnosis of periodontitis, our study showed that, for LoR patients, as determined by non-smoking, no history of diabetes, and absence of specified IL-1 genotypes, the percentage of patients with tooth loss events over 16 yrs associated with 2 preventive prophylaxis visits annually was not different from the percentage with habitually 1 visit annually. For HiR patients, as indicated by 1 or more of the 3 risk factors, biannual preventive visits were associated with a lower event rate than 1 annual visit. In addition, the percentage of patients with events increased with increasing numbers of risk factors. Analysis of the data also indicated that 2 preventive visits annually may not be sufficient to reduce tooth loss in patients with more than 1 risk factor.
About 8.5% of the adult population develops severe periodontitis (Eke et al., 2012), but treatment prevents disease progression in 75% to 80% of these patients (Lindhe and Nyman, 1984; Tonetti et al., 1998). Smoking, diabetes, and specific IL-1 genotypes are risk factors for periodontitis (Taylor et al., 1998; Meisel et al., 2003), and smoking and IL-1 genotype influence tooth loss post-treatment (Axelsson, 2002; Persson et al., 2003; Eickholz et al., 2008).
The present study showed that population subgroups stratified by previously identified risk factors for PD exhibited different rates of clinical benefit associated with different frequencies of preventive care, and that genetic biomarkers combined with conventional lifestyle and co-morbidity risk factors can improve relevant stratification. In terms of long-term tooth loss, we identified a group associated with greater benefit from 2 preventive dental visits annually and another group where the additional visit was not associated with benefit. The current study did not explicitly address risk factors for dental caries.
We studied 16 consecutive years of claims data from patients during the peak age of incident periodontitis (Eke et al., 2012). For LoR patients, the slope over time of the absolute difference in event rate between 1 and 2 annual preventive visits did not differ from zero, indicating that longer monitoring is unlikely to change the primary outcome. In this real-world population of patients who see dentists regularly and have no history of periodontitis, the tooth loss may be rather low. It would take many years of exposure to 1 less preventive visit/yr for average LoR and HiR patients to lose 1 additional tooth [LoR, 195 yrs = 1/(0.082 teeth/16 yrs); HiR, 126 yrs = 1/(0.127 teeth/16 yrs)]. Different stakeholders must determine what constitutes clinical meaningfulness for the added cost of care; however, 2 preventive visits provided only suggestive evidence of benefit in LoR patients compared with the clear benefit associated with 2 preventive visits vs. 1 in HiR patients. One-visit patients with 2 to 3 RFs had an event rate (30.1%) almost twice that of LoR patients (1 visit, 16.4%; 2 visits, 13.8%), further supporting the value of risk stratification to guide prevention.
Of particular interest is that 2 annual preventive visits may be inadequate in patients with more than 1 risk factor, in that patients with multiple risk factors and 2 preventive visits had event rates more than 50% higher than patients with 0 or 1 risk factor. We speculate that patients with 2 or 3 risk factors may require more than 2 preventive visits, but this database does not permit direct assessment of this hypothesis. In the present study of the 534 patients with 2 or 3 risk factors, 67.6% were IL-1 genotype-positive smokers.
The strengths of our study include a clinically relevant population, an objective primary endpoint, a prospective hypothesis for testing pre-defined risk categories, a long monitoring period, collection of an objective genetic biomarker as part of the risk assessment, and stringently defined a priori criteria for 1 and 2 preventive visits annually.
Some potential limitations need clarification. First, the patients who had mainly 1 preventive visit annually were insured for 2 visits within the same plan as those who had 2 preventive visits annually. We do not know why these patients sought less preventive care. Some patients may have been advised by their dentist that their clinical state did not require biannual preventive care; in others, it may have reflected poorer health behaviors in general. Some objective indicators support the latter assumption, including more frequent histories of heart condition (Table: 18% vs. 15%; p = .014) and asthma or chronic obstructive pulmonary disease (COPD) (13% vs. 11%; p = .029). However, LoR patients with mainly 1 preventive visit did not experience worse outcomes than those with mainly 2 preventive visits, as one might expect if the one-visit patients had poorer health behaviors. Second, the rate of tooth loss was lower than in some previous reports (Dietrich et al., 2007; Holtfreter et al., 2012). The rate observed in our study population is likely to be lower than that in the general population, primarily because of protocol exclusion of patients, based on claims data, for whom the study hypothesis would not be relevant, i.e., diagnosis of periodontitis prior to the monitoring period which qualified patients for > 2 preventive visits annually (Fig. 2A; Early PD), or they sought only symptomatic care with little preventive care (Irregular Care). Analysis of tooth loss data available for both of those populations showed relatively high tooth loss rates (Fig. 2A; Appendix Table 1). We explored the generalizability of the study population by comparing tooth loss rates with those from another employee group in the same payer database (Appendix Table 1). The primary differences between the two employee groups were frequencies of the excluded patient segments. Third, causality cannot be inferred from this, or indeed any, observational study. This limitation can be fully overcome only by the use of randomization with respect to treatment groups. Such a study would likely require several thousand patients randomized to 1 or 2 preventive visits and followed for > 10 yrs.
Fourth, since dentistry has no diagnostic codes, reasons for extractions are not known, and few large databases include dental caries or periodontitis diagnostic data in clinical treatment settings. Since tooth loss in adults is attributable primarily to PD and caries, we used dental extraction codes as an outcome that reflects the cumulative effects of both. Since preventive care is applied at the patient level, not the tooth level, and risk factors used for stratification act at the patient level, the primary outcome used was absolute proportion of individuals with ≥ 1 tooth extraction during the monitoring period. Most patients (approximately 80%) in the sample lost no teeth, so the mean number of teeth lost in the population would not inform an individual’s likelihood of an outcome. Tooth loss events lead to substantial direct costs for tooth replacement and should be an appropriate outcome for the effectiveness of long-term prevention of oral diseases.
Fifth, a six-year index period was used to qualify patients as mainly attending for either 1 or 2 preventive visits annually, with tooth loss monitored during that period and for 10 years following. It is of course not possible for the visit frequency to remain consistent over long time periods, since some patients will develop disease and be advised, or choose, to attend more frequently, and others may have life changes that alter consistency of attendance. In spite of this predictable challenge with long-term studies, the frequency of visits remained very consistent throughout the monitoring period in P2 patients and remained reasonably consistent through 11 years in P1 patients. Although the interquartile ranges of P1 and P2 did not overlap during any five-year period, we should not assume that all patients consistently attended 1 or 2 preventive visits for each of the 16 yrs.
Sixth, although 39% of invited individuals consented to participate, and 51% of those returned complete data, selection biases may have been introduced. No demographic information is available for the original population beyond study inclusion criteria and tooth loss. It is therefore not possible to explore potential biases by comparing the study sample with the original population.
Stratified or personalized medicine aims to achieve better outcomes for patients and optimal use of health care resources by integrating genomic, phenomic, and clinical information to predict disease susceptibility, clinical progression, and responses to prevention and treatment regimens; but practical translation requires sufficient clinical validity and utility to produce value for clinical adoption (Chan and Ginsburg, 2011; Kornman and Duff, 2012). The present study may provide a proof-of-principle that resources could be targeted to selected groups for public health gain in the prevention of a chronic disease. In addition, in this instance, the cost of risk-based population stratification plus stratified intervention was calculated as being lower than in the model where the same prevention regime is applied equally across the population.
Supplementary Material
Footnotes
Additional Contributions:The authors greatly appreciate support on data management from Susan Burhop, Shari Sidener, and Carol VanHuysen from the Michigan Institute for Clinical and Health Research. We acknowledge the organizational support of Min Oh, Anna Galloro, Hily’e Pittman, and Lea Franco from the Michigan Center for Oral Health Research. The authors appreciate Dr. Stephen Eklund and Ms. Karen Green from Delta Dental of Michigan for assistance in providing patient populations from the claims database. The authors thank Dr. Xiaodong Wu, Ms. Karen Shaver, and the Interleukin Laboratory staff for their assistance in generation of the genotyping data. We also thank Professor Thomas Kocher and Birte Holtfreter of the University of Greiswald, Germany, for providing perspectives on tooth loss rates in different populations. We appreciate Drs. Peter Libby and Robert Bagramian for critically reading the manuscript.
Funding:This study was supported by the National Institutes of Health (NIH) Grant Number UL 1RR024986 and by the Renaissance Health Services Corp.
Conflicts of Interest:All authors provided disclosure according to ICJME guidelines for the following: WVG has institutional grant support or consulted with the following organizations: National Institutes of Health, Colgate-Palmolive Co., Amgen, Inc., OraPharma, Inc., Organogenesis, Inc. Sunstar Corp., Geistlich Pharma, and Medtronic. KSK is a full-time employee, shareholder, and officer of Interleukin Genetics; and LDS is a full-time employee and shareholder of Interleukin Genetics. GWD is a consultant for Interleukin Genetics and is a member of their Scientific Advisory Board. TMB and AKC have no disclosures to declare.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- American Dental Association (2010). The ADA Practical Guide to Dental Procedure Codes, 2011-12. Vol 1 Chicago, IL: American Dental Association. [Google Scholar]
- Axelsson P. (2002). Role of genetic and hereditary factors. In: Diagnosis and risk prediction of periodontal diseases. Carol Stream, IL: Quintessence, pp. 146-163. [Google Scholar]
- Axelsson P, Lindhe J. (1981). Effect of controlled oral hygiene procedures on caries and periodontal disease in adults. Results after 6 years. J Clin Periodontol 8:239-248. [DOI] [PubMed] [Google Scholar]
- Beirne P, Clarkson JE, Worthington HV. (2007). Recall intervals for oral health in primary care patients. Cochrane Database Syst Rev 4:CD004346. [DOI] [PubMed] [Google Scholar]
- Blaizot A, Vergnes JN, Nuwwareh S, Amar J, Sixou M. (2009). Periodontal diseases and cardiovascular events: meta-analysis of observational studies. Int Dent J 59:197-209. [PubMed] [Google Scholar]
- Chan IS, Ginsburg GS. (2011). Personalized medicine: progress and promise. Annu Rev Genomics Hum Genet 12:217-244. [DOI] [PubMed] [Google Scholar]
- Chronic Disease Prevention and Health Promotion (2012). URL Accessed November 23, 2012 at: http://www.cdc.gov/oralhealth/topics/periodontal_disease.htm.
- Chrysanthakopoulos NA. (2011). Reasons for extraction of permanent teeth in Greece: a five-year follow-up study. Int Dent J 61:19-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson JE, Amaechi BT, Ngo H, Bonetti D. (2009). Recall, reassessment, and monitoring. In: Detection, assessment, diagnosis and monitoring of caries. Pitts NN, editor. Basel: Karger, pp. 188-198. [DOI] [PubMed] [Google Scholar]
- Dietrich T, Maserejian NN, Joshipura KJ, Krall EA, Garcia RI. (2007). Tobacco use and incidence of tooth loss among US male health professionals. J Dent Res 86:373-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye BA, Li X, Beltran-Aguilar ED. (2012). Selected oral health indicators in the United States, 2005-2008. NCHS Data Brief 96:1-8. [PubMed] [Google Scholar]
- Eickholz P, Kaltschmitt J, Berbig J, Reitmeir P, Pretzl B. (2008). Tooth loss after active periodontal therapy. 1: patient-related factors for risk, prognosis, and quality of outcome. J Clin Periodontol 35:165-174. [DOI] [PubMed] [Google Scholar]
- Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ. (2012). Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res 91:914-920. [DOI] [PubMed] [Google Scholar]
- Fontana M, Zero DT. (2006). Assessing patients’ caries risk. J Am Dent Assoc 137:1231-1239. [DOI] [PubMed] [Google Scholar]
- Hanioka T, Ojima M, Tanaka K, Matsuo K, Sato F, Tanaka H. (2011). Causal assessment of smoking and tooth loss: a systematic review of observational studies. BMC Public Health 11:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtfreter B, Demmer RT, Bernhardt O, Papapanou PN, Schwahn C, Kocher T, et al. (2012). A comparison of periodontal status in the two regional, population-based studies of SHIP and INVEST. J Clin Periodontol 39:1115-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito A, Hayashi M, Hamasaki T, Ebisu S. (2011). Risk assessment of dental caries by using classification and regression trees. J Dent 39:457-463. [DOI] [PubMed] [Google Scholar]
- Ito A, Hayashi M, Hamasaki T, Ebisu S. (2012). How regular visits and preventive programs affect onset of adult caries. J Dent Res 91(7 Suppl):52S-58S. [DOI] [PubMed] [Google Scholar]
- Karimbux NY, Saraiya VM, Elangovan S, Allareddy V, Kinnunen T, Kornman KS, et al. (2012). Interleukin-1 gene polymorphisms and chronic periodontitis in adult whites: a systematic review and meta-analysis. J Periodontol 83:1407-1419. [DOI] [PubMed] [Google Scholar]
- Keehan SP, Sisko AM, Truffer CJ, Poisal JA, Cuckler GA, Madison AJ, et al. (2011). National health spending projections through 2020: economic recovery and reform drive faster spending growth. Health Aff (Millwood) 30:1594-1605. [DOI] [PubMed] [Google Scholar]
- Kornman KS, Duff GW. (2012). Personalized medicine: will dentistry ride the wave or watch from the beach? J Dent Res 91(7 Suppl):8S-11S. [DOI] [PubMed] [Google Scholar]
- Kornman KS, Crane A, Wang HY, di Giovine FS, Newman MG, Pirk FW, et al. (1997). The interleukin-1 genotype as a severity factor in adult periodontal disease. J Clin Periodontol 24:72-77. [DOI] [PubMed] [Google Scholar]
- Lalla E, Papapanou PN. (2011). Diabetes mellitus and periodontitis: a tale of two common interrelated diseases. Nat Rev Endocrinol 7:738-748. [DOI] [PubMed] [Google Scholar]
- Lindhe J, Nyman S. (1984). Long-term maintenance of patients treated for advanced periodontal disease. J Clin Periodontol 11:504-514. [DOI] [PubMed] [Google Scholar]
- Mai X, Wactawski-Wende J, Hovey KM, LaMonte MJ, Chen C, Tezal M, et al. (2013). Associations between smoking and tooth loss according to the reason for tooth loss: the Buffalo OsteoPerio Study. J Am Dent Assoc 144:252-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisel P, Siegemund A, Grimm R, Herrmann FH, John U, Schwahn C, et al. (2003). The interleukin-1 polymorphism, smoking, and the risk of periodontal disease in the population-based SHIP study. J Dent Res 82:189-193. [DOI] [PubMed] [Google Scholar]
- Murray H, Locker D, Kay EJ. (1996). Patterns of and reasons for tooth extractions in general dental practice in Ontario, Canada. Community Dent Oral Epidemiol 24:196-200. [DOI] [PubMed] [Google Scholar]
- Ong G, Yeo JF, Bhole S. (1996). A survey of reasons for extraction of permanent teeth in Singapore. Community Dent Oral Epidemiol 24:124-127. [DOI] [PubMed] [Google Scholar]
- Persson GR, Matuliene G, Ramseier CA, Persson RE, Tonetti MS, Lang NP. (2003). Influence of interleukin-1 gene polymorphism on the outcome of supportive periodontal therapy explored by a multi-factorial periodontal risk assessment model (PRA). Oral Health Prev Dent 1:17-27. [PubMed] [Google Scholar]
- Rosen B, Olavi G, Birkhed D, Edvardsson S, Egelberg J. (2004). Effect of different frequencies of preventive maintenance treatment on dental caries: five-year observations in general dentistry patients. Acta Odontol Scand 62:282-288. [DOI] [PubMed] [Google Scholar]
- Sfyroeras GS, Roussas N, Saleptsis VG, Argyriou C, Giannoukas AD. (2012). Association between periodontal disease and stroke. J Vasc Surg 55:1178-1184. [DOI] [PubMed] [Google Scholar]
- Sheiham A. (1977). Is there a scientific basis for six-monthly dental examinations? Lancet 2:442-444. [DOI] [PubMed] [Google Scholar]
- Smithwick CL. (2012). Dental benefits; a guide to managed plans. 3rd ed. Brookfield, WI, USA: International Foundation of Employee Benefit Plans. [Google Scholar]
- Taylor GW, Burt BA, Becker MP, Genco RJ, Shlossman M, Knowler WC, et al. (1998). Non-insulin dependent diabetes mellitus and alveolar bone loss progression over 2 years. J Periodontol 69:76-83. [DOI] [PubMed] [Google Scholar]
- Tonetti MS, Muller-Campanile V, Lang NP. (1998). Changes in the prevalence of residual pockets and tooth loss in treated periodontal patients during a supportive maintenance care program. J Clin Periodontol 25:1008-1016. [DOI] [PubMed] [Google Scholar]
- Van Dyke TE, Sheilesh D. (2005). Risk factors for periodontitis. J Int Acad Periodontol 7:3-7. [PMC free article] [PubMed] [Google Scholar]
- Wall TP, Brown LJ. (2003). Recent trends in dental visits and private dental insurance, 1989 and 1999. J Am Dent Assoc 134:621-627. [DOI] [PubMed] [Google Scholar]
- Yong PL, Olsen LA. (2010). The healthcare imperative: lowering costs and improving outcomes: Workshop Series Summary. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.