Abstract
While proanthocyanidins (PA) are effective in improving collagen’s resistance to collagenolytic degradation, the direct incorporation of PA into an adhesive system is detrimental to the light-curing thereof. Conversely, the use of PA as a primer could circumvent this issue, but little is known about the efficacy of PA in stabilizing collagen when applied in a clinically relevant manner. This study investigated the pre- and post-digestion morphology of an acid-etched dentin collagen layer that underwent PA treatment for time periods on a scale of seconds. The null hypothesis, that there is no difference between the PA-treated and untreated control group, had to be rejected, since it was revealed that the untreated control could not survive 1 hr of exogenous collagenase digestion, while the PA-treated collagen could sustain at least 16 hrs of digestion with no perceptible changes in collagen structure. In addition, the stabilizing effect of the gold-standard cross-linker glutaraldehyde at comparable experimental conditions was found to be almost non-existent within the 5, 15, or 30 sec of cross-linking permitted. Therefore, PA have been proven to be extraordinarily efficient in stabilizing demineralized dentin collagen against enzymatic challenges in a clinically relevant setting, likely due to the non-covalent nature of their interaction with collagen molecules.
Keywords: collagen, dental acid etching, cross-linking reagents, collagenase, scanning electron microscopy, transmission electron microscopy
Introduction
In the adhesive/dentin hybrid layer (Nakabayashi et al., 1982), demineralized collagen and adhesive resin form an entangled network; consequently, improving collagen’s longevity in an enzymatic environment is important to the longevity of dentin bonding. Measures taken in this regard involve the utilization of collagen cross-linkers and collagenase inhibitors. The former approach imparts auxiliary interactions between collagen molecules, enhancing collagen’s chemical and mechanical tenacity as a result. The latter approach elongates collagen’s lifetime by reducing the potency of endogenous and bacterial collagen-digesting enzymes. Indeed, previous studies have demonstrated that dentin collagen treated with cross-linking agents such as glutaraldehyde (GA), genipin, and proanthocyanidins (PA) showed increased tensile strength and elastic modulus, and improved resistance to collagenase digestion (Bedran-Russo et al., 2007; Castellan et al., 2010; Xu and Wang, 2011). Meanwhile, chlorhexidine, an MMP inhibitor, was found to deter the degradation of the dentin hybrid layer if applied to dentin cavities after being acid-etched (Hebling et al., 2005; Carrilho et al., 2007). In light of this, as a group of naturally occurring polyphenolic compounds, PA are of particular interest, because not only are they non-toxic collagen cross-linkers (Han et al., 2003), but also their building blocks, such as catechin and epigallocatechin gallate, have been identified as potent collagenase inhibitors (Demeule et al., 2000; Madhan et al., 2007). Therefore, PA could improve the enzymatic stability of collagen synergistically via both approaches.
The direct incorporation of PA into current adhesive systems, however, incurs the intrinsic complication that PA, well-known radical scavengers (Quideau et al., 2011), could adversely interfere with radical polymerization of adhesive monomers (Green et al., 2010; Epasinghe et al., 2012; Hechler et al., 2012; Liu and Wang, 2012). While the alternative use of PA, namely, as a primer, could potentially avoid this side-effect, previous studies have reported only clinically unfeasible application times varying from 10 min to 1 hr (Bedran-Russo et al., 2007; MacEdo et al., 2009; Castellan et al., 2010; Green et al., 2010). Our understanding is that these long treatments are almost solely for the purpose of detecting the statistically significant effect of PA on collagen’s mechanical properties. Admittedly, some variabilities in mechanical tests, such as specimen shape and dimension, could be reduced by standardizing the protocol. But other variables, including the inter- and intra-tooth differences in tubular structure and orientation, are built-in and impossible to remove. Consequently, researchers had to prolong the treatment time beyond a clinically acceptable point so that the effect of PA on collagen’s mechanical strength could become statistically significant.
In contrast, we are placing our emphasis on the ability of PA to stabilize collagen against collagenolytic stresses, because dentin collagen in its natural form is already mechanically sound due to the extensive endogenous inter- and intra-fibrillar cross-links (Hulmes, 2008). The impetus for the present work was an interesting observation in our earlier studies (Liu et al., 2013), that is, a demineralized dentin slab (0.8 × 0.8 × 1 mm) was treated with PA for only 10 sec and then subjected to digestion with bacterial collagenase. Although the entire slab appeared to have been digested in 12 hrs, there actually remained a very thin brownish film that could withstand much longer digestion. It was reasoned that this film was the collagen layer into which PA could diffuse and with which they could interact in a time period as short as 10 sec. The implication of this rationalization is that, in a situation where the limitation due to cross-linker diffusion was removed, tens of seconds of PA treatment should be enough to render dentin collagen substantially more resistant to collagenase degradation.
The verification of this speculation has practical significance for the use of PA as a primer because clinically, the demineralized dentin layer we wish to protect is only 8 to 10 µm deep. That is, the layer of demineralized collagen generated by acid-etching, which is also the layer of collagen that PA need to penetrate, is only a few microns thick. With this in mind, this study aimed to examine the stability of the thin acid-etched dentin collagen layer treated with PA for clinically relevant times. The null hypothesis is that there is no difference in morphological structure of the demineralized collagen layer between the PA-treated and PA-untreated groups following bacterial collagenase digestion.
Materials & Methods
Unless otherwise stated, all chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA). PA were donated by MegaNatural (Madera, CA, USA), and glutaraldehyde (GA, 25 wt%) was purchased from Electron Microscopy Sciences (Hatfield, PA, USA).
Specimen Preparation
Twenty non-carious human molars were collected after the patients’ informed consent was obtained under a protocol approved by the University of Missouri-Kansas City Adult Health Sciences Institutional Review Board (IRB). Extracted teeth were stored at 4°C in 0.96% (w/v) phosphate-buffered saline containing 0.002% sodium azide. A water-cooled low-speed diamond saw (Buehler, Lake Bluff, IL, USA) was used to remove the crown from the roots. Additional sections were made to remove all occlusal enamel. A uniform smear layer was created on the dentin surface by means of wet 600-grit silicon carbide sandpaper (Buehler). Further sections were made in the occusal-apical direction at 1-mm [for scanning electron microscopy (SEM)] or 0.5-mm increments [for transmission electron microscopy (TEM)], followed by a single cut parallel to and ~1.5 mm below the abraded dentin surface, to free the slabs from the rest of the dentin block. Slabs for SEM were additionally notched at the middle position from the side opposite the abraded surface, for the purpose of subsequent fracturing to permit visualization of the subsurface dentin.
Scanning Electron Microscopy
The abraded surfaces of notched slabs were etched with 35% phosphoric acid gel (Scotchbond Etchant, 3M-ESPE, St. Paul, MN, USA) for 15 sec and rinsed with water for 10 sec. The slabs were pooled and randomly treated with either PA (3.75 or 15 wt% in de-ionized water) or GA (2.5 or 10 wt% in de-ionized water) for the pre-determined time periods of 5, 15, or 30 sec after being blot-dried. Upon completion of treatment, the slabs were immediately submerged in a copious amount of de-ionized water and then rinsed for an additional 30 min, with water changed every 10 min. Additional slabs received no cross-linking treatment and served as the control. For each cross-linking condition, 3 slabs were prepared, 1 of which was not subject to collagenase digestion, whereas the other 2 underwent 1 or 16 hrs of digestion, respectively, at 37°C with 1 mL of 0.1 wt% collagenase solution that was prepared as described elsewhere (Green et al., 2010). Specimens were prepared in triplicate for each treatment-digestion combination. Subsequently, all slabs were fixed in 2.5% glutaraldehyde buffered with 0.1 M sodium cacodylate for 1 hr, and dehydrated in graded solutions of ethanol (33%, 67%, 85%, 95%, 100%) for 2 hrs each. After being air-dried overnight, the slabs were fractured, mounted on aluminum stubs with conductive tape, and coated with carbon, and the fractured cross-sections were then examined in an FEI/Philips XL30 Field-Emission Environmental SEM (Philips, Eindhoven, the Netherlands).
Transmission Electron Microscopy
Un-notched slabs were acid-etched, cross-linked, digested, and fixed in the same way as above for SEM. Subsequently, specimens were post-fixed with 1% osmium tetroxide for 1.5 hrs and dehydrated in graded solutions of ethanol as above. Then specimens were treated with 1:1 solution of ethanol and propylene oxide for 30 min, followed with 100% propylene oxide for 2 hrs, and finally with 1:1 solution of propylene oxide and epoxy resin (Embed-812, Electron Microscopy Sciences) overnight. After final infiltration with pure epoxy resin, specimens were incubated in an oven at 60°C for 48 hrs. Ultrathin sections were cut with an EM-UC7 ultramicrotome (Leica, Buffalo Grove, IL, USA), stained with 1% phosphotungstic acid, and observed with a CM12 electron microscope (FEI, Hillsboro, OR, USA) at 80 kV accelerating voltage.
Results
Scanning Electron Microscopy
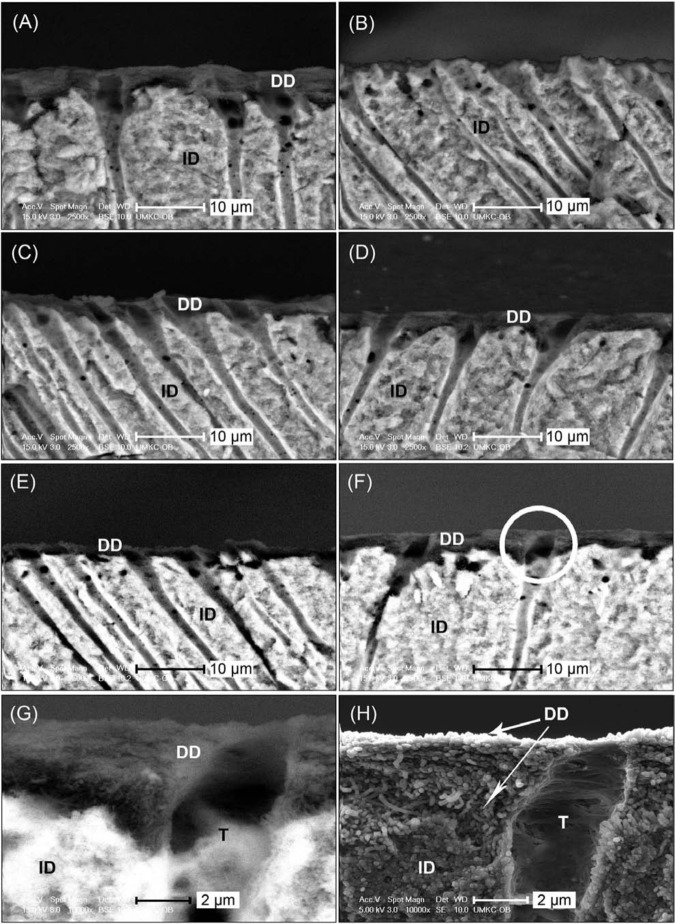
The back-scattered-electron SEM (BSE-SEM) images verified the formation of a demineralized dentin layer following the acid-etching procedure (Fig. 1A). If not treated, this layer of demineralized collagen disappeared completely after 1 hr of bacterial collagenase digestion (Fig. 1B). When treated with PA, regardless of treatment time or PA concentration, the demineralized layer remained evident after both 1 hr (Figs. 1C, 1D) and 16 hrs of digestion (Figs. 1E, 1F). In addition, in secondary-electron SEM (SE-SEM) mode at a higher magnification, the etched-then-PA-cross-linked dentin layer was found to consist of a dense layer of demineralized collagen fibrils (Figs. 1G, 1H) after digestion.
Figure 1.
Representative SEM images from the fractured cross-sections of control and PA-treated dentin samples. All images were taken with BSE-SEM unless otherwise noted. DD, demineralized dentin; ID, intact dentin; T, dentinal tubule. (A) Undigested control with demineralized dentin identified by its dark color. (B) Control, uncross-linked, after 1 hr of bacterial collagenase digestion, showing the absence of a dark layer, indicating the complete digestion of demineralized dentin. (C) Dentin sample treated with the lowest dose of PA (3.75 wt% PA for 5 sec) and then subjected to 1 hr of bacterial collagenase digestion. (D) Dentin sample treated with the highest dose of PA (15 wt% PA for 30 sec) and then subjected to 1 hr of bacterial collagenase digestion. (E) Dentin sample treated in the same conditions as in (C) but digested with collagenase for 16 hrs. (F) Dentin sample treated in the same conditions as in (D) but digested with collagenase for 16 hrs. (C-F) indicate the persistence of a demineralized layer once treated with PA, regardless of collagenase digestion time. (G) Higher-magnification view of the circled area in (F). (H) SE-SEM image of the same location as in (G), showing typical morphology of PA-treated samples after 16 hrs of digestion.
In comparison, when treated with 2.5 or 10 wt% GA, the layer of demineralized substance remained discernible after 1 hr of bacterial collagenase digestion (Figs. 2A, 2B). However, when the digestion time was extended to 16 hrs, the only condition that allowed the demineralized collagen fibrils to remain was in dentin treated with the highest GA dose for the longest time (10 wt% GA for 30 sec) (Figs. 2C, 2D). Etched dentin that was cross-linked under this set of conditions revealed a “hairy” morphology after 16 hrs of digestion (Figs. 2E, 2F).
Figure 2.
Representative SEM images from the fractured cross-sections of GA-treated dentin samples. All images were taken with BSE-SEM unless otherwise noted. DD, demineralized dentin; ID, intact dentin; T, dentinal tubule. (A) Dentin sample treated with the lowest dose of GA (2.5 wt% GA for 5 sec) and then subjected to 1 hr of digestion with bacterial collagenase. (B) Dentin sample treated with the highest dose of GA (10 wt% GA for 30 sec) and then subjected to 1 hr of collagenase digestion. (2A, 2B) indicate that the GA-treated demineralized dentin could sustain 1 hr of digestion, like its PA-treated counterpart (1C, 1D). (C) Dentin sample treated in the same conditions as in 2(A) but digested with collagenase for 16 hrs, showing that GA-treated collagen generally cannot sustain 16 hrs of digestion. (D) Dentin sample treated in the same conditions as 2(B), which is the only GA-treatment condition in which remnant collagen could barely be observed after 16 hrs of digestion with collagenase. (E) High-magnification view of the circled area in Fig. 2(D). (F) SE-SEM image of the same location as in 2(E), showing the unique “hairy” morphology of highest dose GA-treated demineralized dentin after 16 hrs of digestion with collagenase.
Transmission Electron Microscopy
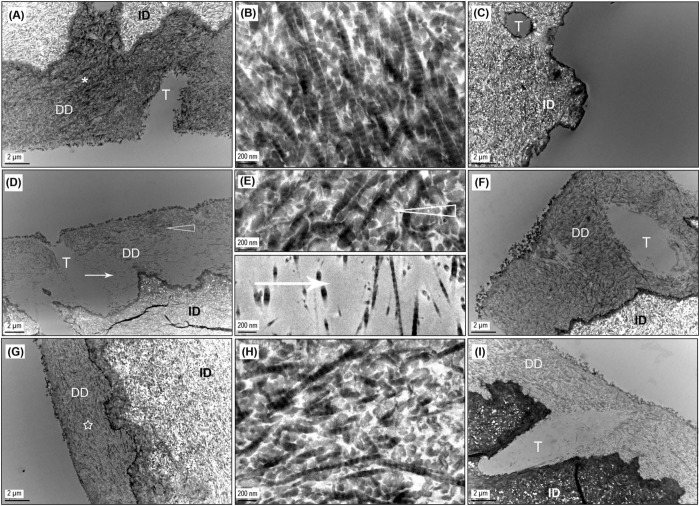
A layer of demineralized collagen (several microns thick) was obvious in the undigested control (Fig 3A), which showed the characteristic 67-nm banding pattern of collagen fibrils (Fig. 3B). Following bacterial collagenase digestion for 1 hr, the control demineralized dentin matrix disappeared, whereas the underlying mineralized dentin remained unchanged (Fig. 3C). With PA cross-linking, the demineralized collagen layer was present after 1 hr of digestion, regardless of treatment conditions (Figs. 3D, 3F). Interestingly, acid-etched dentin treated with the lowest PA dose for the shortest time (3.75 wt% for 5 sec) revealed voids within the demineralized layer (Fig. 3D). A high-magnification view of the void area demonstrated a scarce distribution of collagen fibrils (Fig. 3E). After 16 hrs of digestion, all PA-treated samples featured a similar morphology, i.e., a thick and dense demineralized collagen layer covering the entire surface of intact dentin (Figs. 3G, 3I), with collagen fibrils maintaining their characteristic cross-banding pattern (Fig. 3H).
Figure 3.
Representative TEM images of control and PA-treated specimens. DD, demineralized dentin; ID, intact dentin; T, dentinal tubule. (A) Undigested control with demineralized dentin layer. (B) High-magnification view of the demineralized dentin depicted by the asterisk in 3(A), showing the characteristic banding pattern of collagen fibrils. (C) Control, uncross-linked, after 1 hr of bacterial collagenase digestion, showing the depletion of demineralized collagen. (D) Dentin sample treated with the lowest dose of PA (3.75 wt% PA for 5 sec) and then subjected to 1 hr of collagenase digestion, the unusual and only instance where voids (arrow) were seen within sound demineralized collagen (open arrowhead) following digestion. (E) High-magnification view of the areas indicated by open arrowhead and arrow in 3(D), showing a more scarce distribution of collagen fibrils in voids than in the sound demineralized dentin. (F) Dentin sample treated with the highest dose of PA (15 wt% PA for 30 sec) and then subjected to 1 hr of digestion with collagenase, representing the more typical morphology of PA-treated demineralized layer following 1 hr of digestion. (G) Dentin sample treated in the same conditions as in 3(D) but digested with collagenase for 16 hrs. The inconsistency with 3(D) is attributed to the inter-specimen difference caused by non-uniform dispersion of PA in the demineralized layer under the lowest dose condition. (H) High-magnification view of the area indicated by a star in 3(G), showing that demineralized collagen remained intact after 16 hrs of collagenase digestion once cross-linked by PA. (I) Dentin sample treated in the same conditions as 3(F) but digested for 16 hrs, exhibiting an unchanged morphology compared with that after 1 hr of digestion with collagenase (3F), which is typical for PA-treated samples.
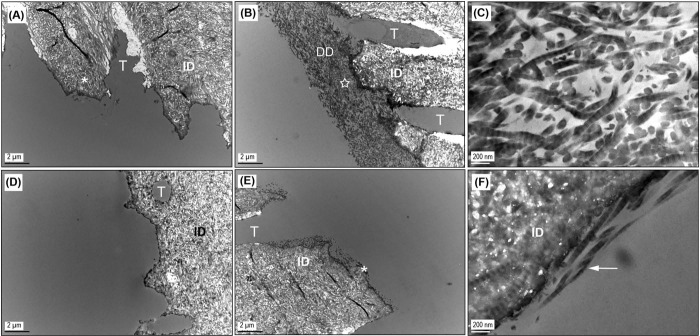
For demineralized dentin cross-linked with GA, collagenase challenge up to 1 hr left no trace of demineralized collagen in specimens treated at the lowest concentration (2.5 wt%) for 5 sec (Fig. 4A), while that of samples under other GA treatment conditions remained intact (Figs. 4B, 4C). This observation is slightly inconsistent with the SEM result (Fig. 2A) and will be discussed later. When the digestion was extended to 16 hrs, complete destruction of the demineralized collagen layer was noted in all GA-treated samples (Fig. 4D) except in the case of the highest dose (Fig. 4E), where small clusters of collagen fibrils could be sporadically located along the edge of intact dentin (Fig. 4F), despite the disappearance of a bulk demineralized layer.
Figure 4.
Representative TEM images of GA-treated samples. DD, demineralized dentin; ID, intact dentin; T, dentinal tubule. (A) Dentin sample treated with the lowest dose of GA (2.5 wt% GA for 5 sec) and then subjected to 1 hr of bacterial collagenase digestion, showing the disappearance of demineralized collagen. The apparent inconsistency with the SEM result (Fig. 2A) was attributed to inter-specimen differences at the lowest dose condition. (B) Dentin sample treated with the highest dose of GA (10 wt% GA for 30 sec) and subjected to 1 hr of digestion with collagenase, representing all other GA-treated dentin except the lowest dose following 1 hr of digestion. (C) High-magnification view of the spot indicated by a star in 4(B). (D) Dentin sample treated in the same conditions as 4(A) but digested with collagenase for 16 hrs. (E) Dentin sample treated in the same conditions as 4(B) but digested with collagenase for 16 hrs, showing that GA-treated samples could not sustain 16 hrs of digestion. (F) High-magnification view of the area depicted by an asterisk in 4(E), emphasizing the remaining collagen fibrils (arrow) as seen in 2(F).
Discussion
Following acid-etching, the generation of demineralized collagen on top of intact dentin was verified by the dark-colored layer, as seen in the BSE-SEM image (Fig. 1A), as well as by the characteristic banding pattern of its constituent fibrils, as seen in TEM (Figs. 3A, 3B). Given the high concentration of collagenase with which we chose to challenge the samples, which was 2 to 6 orders of magnitude higher than the MMP-8 level found in human oral fluids (Liede et al., 1999; Chen et al., 2000; Mäntylä et al., 2003), it comes as no surprise that the untreated demineralized collagen disappeared completely after 1 hr of digestion (Figs. 1B, 3C). In contrast, its PA-treated counterpart sustained the digestion (Figs. 1C, 1D, 3D, 3E), regardless of PA concentration or treatment time. Therefore, the null hypothesis was rejected.
To put PA’s collagen-stabilizing capability into perspective, we undertook comparative studies using the more established cross-linker: glutaraldehyde. Samples were treated with comparable concentrations of GA and subjected to the same length of digestion (1 hr). Results showed that, like PA-treated samples, a layer of demineralized substance is also present regardless of GA dose (Figs. 2A, 2B). The TEM results are consistent with SEM (Figs. 4B, 4C) with one exception: that is, for the lowest GA dose, TEM indicated complete removal of the demineralized layer (Fig. 4A) after 1 hr of digestion, but SEM showed otherwise (Fig. 2A). It is speculated that this discrepancy is due to the inter-specimen difference caused by the extremely short treatment time (5 sec), during which uniform dispersion of the cross-linking agent can hardly be attained.
Since the PA- and GA-treated samples were not significantly different after 1 hr of digestion, we extended the digestion time to 16 hrs, to better gauge the stabilizing capacity of PA. As a result, the superiority of PA became more striking. The PA-treated demineralized collagen withstood 16 hrs of digestion (Figs. 1E, 1F) with its banding pattern intact (Fig. 3I) regardless of PA concentration or treatment time, whereas the GA-treated collagen was completely (Figs. 2C, 4D) or almost completely gone (Figs. 2D, 4E), depending upon GA dose. Of all the GA dosages studied, only the highest dose condition (10 wt% GA for 30 sec) cross-linked enough collagen that could barely survive 16 hrs of digestion (Figs. 2D, 4E). Under this particular condition, the remaining demineralized tissue presents a unique “hairy” morphology (Fig. 2F), different from that of PA-treated dentin (Fig. 1H). With the aid of TEM (Fig. 4F), it can be inferred that the remnant “hair” must be the small fraction of collagen that does become adequately cross-linked by GA at the highest concentration for the longest time, and it consequently survived 16 hrs of digestion, as did PA-treated collagen. This superior efficiency of PA treatment was further corroborated by the results of a semi-quantitative analysis (see Appendix) using a mass spectroscopic method (Nimptsch et al., 2011)). The work in the Appendix showed a large decrease in the release of collagen peptide fragments from PA cross-linked dentin specimens.
The consequence of too little cross-linking by GA was manifested in PA-treated samples examined by TEM. More specifically, at the lowest dose of PA (3.75 wt% for 5 sec), collagenase digestion for 1 hr induced apparent voids in the otherwise sound demineralized layer (Figs. 3D, 3E), but, contrary to expectations, such voids are not seen after longer digestion periods (Fig. 3G). Considering the previously discussed inconsistency found between SEM and TEM for the GA-treated samples at the lowest dose (2.5 wt% for 5 sec), it is concluded that the ideal PA treatment time should be 15 to 30 sec.
In summary, upon treatment with 3.75 to 15 wt% of PA for 15 to 30 sec, etched dentin presents no perceptible morphologic change after a long period of bacterial collagenase digestion (16 hrs) at a concentration several orders of magnitude higher than physiologically exists. The reason for the outstanding efficiency of PA in stabilizing collagen is likely related to the non-covalent nature of PA cross-linking (Oh et al., 1980; Baxter et al., 1997). Since the rate of non-covalent interactions is primarily limited by the diffusion of reagents, and this limitation is greatly diminished in adhesive dental practice due to the minute thickness (8-10 µm) of the demineralized collagen layer, PA have shown to be very promising priming agents. One shortcoming of grape-seed extract PA treatment is that it stains dentin reddish-brown. Therefore, it is our future plan to isolate PA components or synthesize PA-mimicking compounds that inherit the cross-linking ability of PA while exhibiting less intense color.
Supplementary Material
Footnotes
This investigation was supported by R15-DE021023 from the National Institutes of Health, Bethesda, MD 20892, USA.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Baxter NJ, Lilley TH, Haslam E, Williamson MP. (1997). Multiple interactions between polyphenols and a salivary proline-rich protein repeat result in complexation and precipitation. Biochemistry 36:5566-5577. [DOI] [PubMed] [Google Scholar]
- Bedran-Russo AK, Pereira PN, Duarte WR, Drummond JL, Yamauchi M. (2007). Application of crosslinkers to dentin collagen enhances the ultimate tensile strength. J Biomed Mater Res B Appl Biomater 80:268-272. [DOI] [PubMed] [Google Scholar]
- Carrilho MR, Geraldeli S, Tay F, de Goes MF, Carvalho RM, Tjäderhane L, et al. (2007). In vivo preservation of the hybrid layer by chlorhexidine. J Dent Res 86:529-533. [DOI] [PubMed] [Google Scholar]
- Castellan CS, Pereira PN, Grande RH, Bedran-Russo AK. (2010). Mechanical characterization of proanthocyanidin-dentin matrix interaction. Dent Mater 26:968-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HY, Cox SW, Eley BM, Mäntylä P, Rönkä H, Sorsa T. (2000). Matrix metalloproteinase-8 levels and elastase activities in gingival crevicular fluid from chronic adult periodontitis patients. J Clin Periodontol 27:366-369. [DOI] [PubMed] [Google Scholar]
- Demeule M, Brossard M, Pagé M, Gingras D, Béliveau R. (2000). Matrix metalloproteinase inhibition by green tea catechins. Biochim Biophys Acta 1478:51-60. [DOI] [PubMed] [Google Scholar]
- Epasinghe DJ, Yiu CK, Burrow MF, Tay FR, King NM. (2012). Effect of proanthocyanidin incorporation into dental adhesive resin on resin-dentine bond strength. J Dent 40:173-180. [DOI] [PubMed] [Google Scholar]
- Green B, Yao X, Ganguly A, Xu C, Dusevich V, Walker MP, et al. (2010). Grape seed proanthocyanidins increase collagen biodegradation resistance in the dentin/adhesive interface when included in an adhesive. J Dent 38:908-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Jaurequi J, Tang BW, Nimni ME. (2003). Proanthocyanidin: a natural crosslinking reagent for stabilizing collagen matrices. J Biomed Mater Res A 65:118-124. [DOI] [PubMed] [Google Scholar]
- Hebling J, Pashley DH, Tjäderhane L, Tay FR. (2005). Chlorhexidine arrests subclinical degradation of dentin hybrid layers in vivo. J Dent Res 84:741-746. [DOI] [PubMed] [Google Scholar]
- Hechler B, Yao X, Wang Y. (2012). Proanthocyanidins alter adhesive/dentin bonding strengths when included in a bonding system. Am J Dent 25:276-280. [PMC free article] [PubMed] [Google Scholar]
- Hulmes DJ. (2008). Collagen diversity, synthesis and assembly. In: Collagen: structure and mechanics, Fratzl P. editor. Potsdam, Germany: Springer, pp. 15-41. [Google Scholar]
- Liede KE, Haukka JK, Hietanen JH, Mattila MH, Rönkä H, Sorsa T. (1999). The association between smoking cessation and periodontal status and salivary proteinase levels. J Periodontol 70:1361-1368. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang Y. (2012). Effect of proanthocyanidins and photo-initiators on photo-polymerization of a dental adhesive. J Dent 41:71-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Chen M, Yao X, Xu C, Zhang Y, Wang Y. (2013). Enhancement in dentin collagen’s biological stability after proanthocyanidins treatment in clinically relevant time periods. Dent Mater 29:485-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacEdo GV, Yamauchi M, Bedran-Russo AK. (2009). Effects of chemical cross-linkers on caries-affected dentin bonding. J Dent Res 88:1096-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhan B, Krishnamoorthy G, Rao JR, Nair BU. (2007). Role of green tea polyphenols in the inhibition of collagenolytic activity by collagenase. Int J Biol Macromol 41:16-22. [DOI] [PubMed] [Google Scholar]
- Mäntylä P, Stenman M, Kinane DF, Tikanoja S, Luoto H, Salo T, et al. (2003). Gingival crevicular fluid collagenase-2 (MMP-8) test stick for chair-side monitoring of periodontitis. J Periodontal Res 38:436-439. [DOI] [PubMed] [Google Scholar]
- Nakabayashi N, Kojima K, Masuhara E. (1982). The promotion of adhesion by the infiltration of monomers into tooth substrates. J Biomed Mater Res 16:265-273. [DOI] [PubMed] [Google Scholar]
- Nimptsch A, Schibur S, Ihling C, Sinz A, Riemer T, Huster D, et al. (2011). Quantitative analysis of denatured collagen by collagenase digestion and subsequent MALDI-TOF mass spectrometry. Cell Tissue Res 343:605-617. [DOI] [PubMed] [Google Scholar]
- Oh HI, Hoff JE, Armstrong GS, Haff LA. (1980). Hydrophobic interaction in tannin-protein complexes. J Agric Food Chem 28:394-398. [Google Scholar]
- Quideau S, Deffieux D, Douat-Casassus C, Pouységu L. (2011). Plant polyphenols: chemical properties, biological activities, and synthesis. Angew Chem Int Ed Engl 50:586-621. [DOI] [PubMed] [Google Scholar]
- Xu C, Wang Y. (2011). Cross-linked demineralized dentin maintains its mechanical stability when challenged by bacterial collagenase. J Biomed Mater Res B Appl Biomater 96:242-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.