Abstract
The frequency and impact of temporomandibular joint (TMJ) disorders necessitate research in characterizing the joint’s function. The 6 discal attachments have not yet been systematically characterized under tension. Understanding their role in joint function may guide our study of TMJ pathologies, including disc displacement. In the present study, a porcine model was used to characterize the attachments in tension anteroposteriorly and mediolaterally, based on previously identified similarities in the porcine and human masticatory behaviors and discal properties. Tensile stiffness, strength, toughness, and maximum strain were quantified. Collagen alignment was characterized via polarized light and scanning electron microscopy. Anisotropy was demonstrated in all attachments, with the exception of the anterior inferior attachment. Anteroposteriorly, the lateral attachment was stiffest (8.3 MPa) and the anterior superior was least stiff (1.4 MPa). Mediolaterally, the posterior superior attachment was stiffest (16.3 MPa) and the medial was least stiff (1.4 MPa). The greatest strain was observed in the lateral attachment in the mediolateral direction and the posterior superior attachment in the anteroposterior direction. With greatest strains in the most commonly observed directions of disc displacement, it is suggested that compromise in the posterior and lateral attachments contributes to partial lateral and anterior disc displacement.
Keywords: cartilage, extracellular matrix (ECM), microscopy, regenerative medicine, stress analysis, temporomandibular disorders (TMD)
Introduction
The prevalence and severity of temporomandibular joint disorders (TMD) have resulted in increased study of the joint’s components, potential mechanisms in dysfunction, and therapeutic approaches. However, the role of the discal attachments in normal and dysfunctional joint motion is poorly understood. The present study characterizes the discal attachments in a porcine model, which is a useful non-primate model for temporomandibular joint (TMJ) function due to notable similarities to the human TMJ disc and chewing behaviors (Sindelar and Herring, 2005; Kalpakci et al., 2011). Mechanical analysis provides a useful means of understanding tissue functionality, since a tissue needs to be stiff and strong enough to support the loads it experiences through motion. Elucidating the structure and function of the discal attachments will contribute to the understanding of this complex mechanical environment.
Symptoms of TMD affect 20% to 25% of the population (LeResche, 1997), and at least 70% of TMD patients suffer from pathology and/or malpositioning of the TMJ disc (Katzberg et al., 1996; Tasaki et al., 1996). During normal function, the joint undergoes a combination of rotational and translational motions. The disc is situated between the condyle and fossa and distributes loads between these incongruent surfaces. In doing so, the disc experiences a combination of compressive, tensile, and shear forces (Beek et al., 2001a; Tanaka and van Eijden, 2003; Juran et al., 2013). However, in the presence of TMD, the disc’s motion may become uncoordinated and obstructed, and pathologic changes may appear in the disc, necessitating discectomy and functional disc replacements (Wilkes, 1989; Tasaki et al., 1996).
Mechanical characterization of the TMJ disc suggests that the disc may function in a trampoline-like manner to distribute compressive loads and absorb shock. According to this hypothesis, compressive loads are borne by circumferentially aligned collagen fibers placed in tension (Allen and Athanasiou, 2006). The trampoline-like mechanism has been suggested based on the observation that the disc is 100 to 1,000 times stiffer in tension than in compression (Detamore and Athanasiou, 2003). Based on this hypothesis, it is likely that the discal attachments, which secure the disc to the bony joint components, play a role in transmitting load (Scapino et al., 2006). Additionally, it may be hypothesized that the attachments play a role in the progression from normal to abnormal joint motion observed in TMD patients with malpositioned discs.
Biochemical and histological characterization of the discal attachments has demonstrated region- and direction-dependent properties likely related to variability in functional requirements (Willard et al., 2012). Histological characterization demonstrates that much of the attachments’ matrix is continuous with the disc, yielding a seamless transition. However, the attachments show lower glycosaminoglycan (GAG) and collagen content, and higher cellularity, compared with the disc. Biochemical characterization of the 6 discal attachments demonstrates that the attachments are composed primarily of collagen, elastin, and cells, with minimal GAGs. The presence of region- and direction-dependent biochemical content and collagen alignment suggests a unique role for each attachment in load transmission.
Initial efforts have been made in identifying functional requirements in the lateral and posterior attachments (Ben Amor et al., 1998; Liu and Herring, 2000; Sun et al., 2002; Tanaka et al., 2002, 2003). It has been suggested that the attachments function to maintain the disc’s position with respect to the condyle. However, the attachments have not been characterized mechanically via a systematic comparison. Preliminary mechanical analysis suggests that these tissues contribute to the TMJ’s complex motion, calling for a more detailed analysis of their structure and function.
Previously, the porcine discal attachments have been described anatomically (Herring et al., 2002). The porcine TMJ shows a substantial retrodiscal tissue which divides into a temporal and condylar attachment, described here as the posterior superior and posterior inferior attachments, respectively (Herring et al., 2002) (Fig. 1). Analyzed histologically, the medial, lateral, and posterior attachments have been described as more “robust” than the anterior attachments (Herring et al., 2002). When the porcine attachments are compared with those of the human, the human retrodiscal tissue shows greater vascularity. Its function during translation may be associated with this vascularity (Rees, 1954), in contrast to the fibro-fatty characteristics of the pig’s retrodiscal tissue. Additionally, the lateral attachment in the porcine TMJ has been described as thinner than that in humans, but its insertions are consistent between the species (Sun et al., 2002). Analysis of the lateral capsule during passive manipulation and mastication has suggested that its function is similar to that of humans, limiting lateral and retrusional movement (Sun et al., 2002). The porcine TMJ provides a useful animal model (Herring, 2003; Kalpakci et al., 2011), but differences in anatomy between humans and pigs should be noted.
Figure 1.
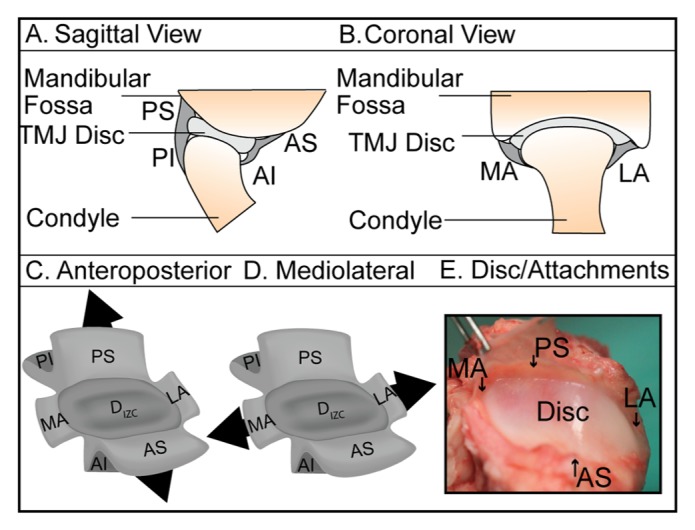
Schematic of TMJ discal attachments. (A) Sagittal and (B) coronal views of the attachments, including the medial (MA) and lateral (LA) attachments, and the posterior and anterior attachments, which bifurcate to form the posterior superior (PS), posterior inferior (PI), anterior superior (AS), and anterior inferior (AI) attachments. Each attachment region was tested in the (C) anteroposterior and (D) mediolateral directions. (E) Excised disc/attachment complex imaged prior to the isolation of specimens for testing.
The present study seeks to elucidate structure-function relationships of the porcine discal attachments. In uniaxial tension, the mechanical properties of the attachments are quantified in the anteroposterior and mediolateral directions, yielding region- and direction-dependent comparisons. Scanning electron and polarized light microscopy are used to demonstrate collagen alignment. Results are presented in the context of previously described properties of the TMJ disc, since the 2 tissues are in close proximity. To our knowledge, this is the first evidence of mechanical characterization of several of the attachments. Hypotheses are presented regarding topographical and anisotropic variations in attachment properties, and links to TMD. This work provides structural and functional clues into the role of the attachments in the TMJ, demonstrating a need for their consideration in regenerative strategies and joint-modeling efforts.
Materials & Methods
Specimen Preparation
TMJs were excised en bloc from skeletally mature (3- to 6-month-old) crossbred Yorkshire and Hampshire Sus scrofa (Yosemite Meat Co., Modesto, CA, USA; n = 24) within 48 hrs of sacrifice. A detailed methodology is described in the Appendix.
Tensile Testing
Specimens were isolated and tested in tension anteroposteriorly and mediolaterally (Fig. 1). Samples were elongated at a strain rate of 1%/sec of the gauge length. Young’s modulus, ultimate tensile strength (UTS), maximum strain, and toughness were measured. Please see the Appendix for a detailed testing methodology.
Macroscopic Characterization
Collagen organization was examined in the 6 attachments and the disc’s intermediate zone. Samples were frozen in HistoPrep Frozen Tissue Embedding Media (Fisher Scientific, Fair Lawn, NJ, USA) and cryosectioned at 30 μm in the transverse plane. Sections were fixed in acetone for 20 min, dried, and imaged under polarized light (Olympus, Center Valley, PA, USA). Additional samples were fixed in 3% gluteraldehyde (Sigma, St. Louis, MO, USA) and prepared for scanning electron microscopy (SEM). A detailed cryofracturing methodology is described in the Appendix.
Statistical Analysis
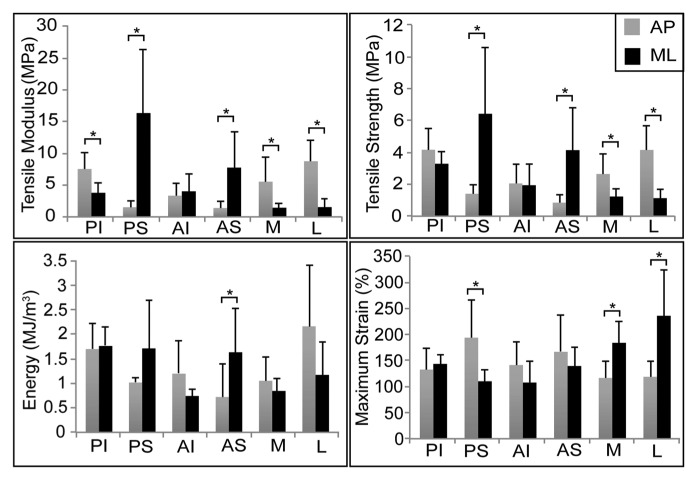
To determine regional differences in each of the measured properties, we performed a one-way analysis of variance across all groups for each property. Tukey’s post hoc analysis was performed when indicated (α = 0.05, Table). To determine the presence of anisotropy, we analyzed data by Student’s t test in each region (α = 0.05, Fig. 2). Data are represented as mean ± standard deviation.
Table.
Quantitative Results for Mechanical Characterization
| Modulus (MPa) |
UTS (MPa) |
Energy (MJ/m3) |
Maximum Strain (%) |
|||||
|---|---|---|---|---|---|---|---|---|
| AP | ML | AP | ML | AP | ML | AP | ML | |
| PI | 7.5 ± 2.5bc | 3.8 ± 1.6bc | 4.2 ± 1.3abc | 3.3 ± 0.7bcd | 1.7 ± 0.5ab | 1.8 ± 0.4ab | 133 ± 40bc | 143 ± 18bc |
| PS | 1.5 ± 1.0bc | 16.3 ± 10.1a | 1.4 ± 0.6bcd | 6.4 ± 4.2a | 1.0 ± 0.1ab | 1.7 ± 1.0ab | 194 ± 72ab | 110 ± 23bc |
| AI | 3.3 ± 2.0bc | 4.0 ± 2.8bc | 2.1 ± 1.2bcd | 1.9 ± 1.4bcd | 1.2 ± 0.7ab | 0.7 ± 0.1b | 142 ± 45bc | 107 ± 41c |
| AS | 1.4 ± 1.2c | 7.8 ± 5.7bc | 0.8 ± 0.5d | 4.1 ± 2.7ab | 0.7 ± 0.7b | 1.6 ± 0.9ab | 167 ± 72abc | 139 ± 36bc |
| M | 5.5 ± 3.8bc | 1.4 ± 0.8c | 2.6 ± 1.3bcd | 1.2 ± 0.5cd | 1.0 ± 0.5ab | 0.8 ± 0.3b | 116 ± 32bc | 184 ± 41abc |
| L | 8.8 ± 3.4b | 1.5 ± 1.4c | 4.2 ± 1.5abc | 1.1 ± 0.6bcd | 2.2 ± 1.3a | 1.2 ± 0.7ab | 119 ± 30bc | 236 ± 88a |
The discal attachments demonstrated region-dependent mechanical properties. Data are presented as mean ± standard deviation. A one-way ANOVA is presented across all groups for each mechanical property. Groups not connected by a common letter are significantly different (p < .05).
Figure 2.
Tensile mechanical properties. The discal attachments demonstrated direction-dependent mechanical properties under uniaxial tensile testing (data presented as mean ± standard deviation). Student’s t test is presented for directional variability in each region tested. Groups connected by an asterisk are significantly different from one another (p < .05). All regions, except AI, were direction-dependent in tensile stiffness. The posterior and anterior superior attachments were stiffest in the mediolateral direction. In the anteroposterior direction, the lateral and posterior inferior attachments were stiffest. PI = posterior inferior attachment; PS = posterior superior attachment; AI = anterior inferior attachment; AS = anterior superior attachment; M = medial attachment; L = lateral attachment.
Results
Tensile Properties
Tensile characterization of the 6 discal attachments demonstrated region- and direction-dependent biomechanical properties (Table, Fig. 2). Additionally, the TMJ disc was tested in the intermediate zone in the anteroposterior and mediolateral directions as a control. The tensile modulus was found to be 50.4 ± 13.7 MPa in the anteroposterior direction and 4.1 ± 2.3 MPa in the mediolateral direction.
The greatest tensile stiffness and strength were reported in the posterior superior attachment in the mediolateral direction, followed by the lateral attachment in the anteroposterior direction. The lowest tensile modulus and UTS were reported in the anterior superior attachment in the anteroposterior direction. All attachments, excluding anterior inferior, demonstrated anisotropic tensile properties, with the modulus in the anteroposterior direction being significantly different from that in the mediolateral direction. This was paralleled in UTS for all attachments, excluding both inferior attachments (Fig. 2).
Energy absorbed to peak stress was greatest in the lateral attachment in the anteroposterior direction. The lowest energy was demonstrated by the anterior inferior attachment mediolaterally and the anterior superior attachment anteroposteriorly. The greatest maximum strain was observed in the lateral attachment in the mediolateral direction, followed by the posterior superior attachment in the anteroposterior direction (Table).
Structural Characterization
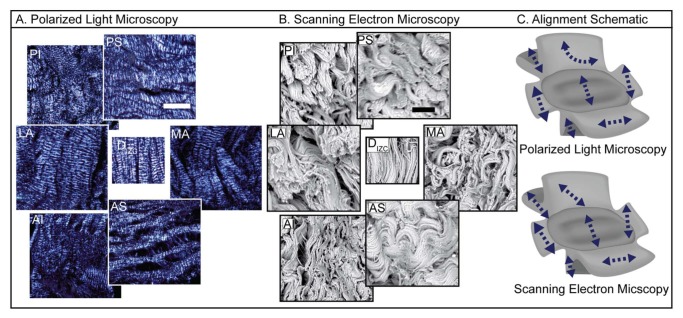
Imaged by polarized light microscopy and scanning electron microscopy, collagen alignment varied regionally in the attachments (Fig. 3). The disc’s intermediate zone (control) demonstrated anteroposterior alignment. The posterior superior and anterior superior attachments demonstrated mediolateral alignment. The posterior superior attachment took a sharp turn toward anteroposterior alignment posteriorly (not shown in its entirety in Figs. 3A and 3B but clearly visible at lower magnification). The posterior inferior, medial, and lateral attachments demonstrated primarily anteroposterior alignment. Together, the anterior and posterior superior, medial, and lateral attachments form ring-like alignment. Schematic diagrams in Fig. 3C represent collagen alignment qualitatively, as viewed in several images at various magnifications (length of arrow corresponds with degree of alignment).
Figure 3.
Matrix organization in the discal attachments. (A) Polarized light microscopy shows ring-like collagen organization in the superior aspect, mirroring collagen organization in the disc’s periphery. The inferior attachments demonstrate primarily anteroposterior organization. Scale bar: 500 μm. (B) Scanning electron microscopy confirms polarized light observations. Scale bar: 10 μm. (C) Schematic reflects overall alignment as observed in several images, at various magnifications (length of arrows qualitatively reflect degree of alignment).
Discussion
This study provides the first systematic tensile characterization of the 6 discal attachments. It was hypothesized that the attachments would demonstrate (1) region-dependent and (2) direction-dependent tensile properties, paralleling respective properties in the disc. The first hypothesis was confirmed, with the posterior attachments demonstrating superior tensile properties compared with the anterior attachments. In confirmation of the second hypothesis, all attachments, with the exception of the anterior inferior, demonstrated directional dependence in stiffness. It is suggested that the superior attachments contribute to load transmission as an extension of the disc, while the posterior inferior, lateral, and medial attachments contribute to load transmission anteroposteriorly. Characterization of the attachments is necessary for an understanding of TMD pathology and for developing functional tissue replacements.
The tensile properties of the superior attachments parallel regional trends observed in the disc and suggest that they function as a continuation of the disc. Mediolaterally, the disc was stiffer and stronger in the posterior band compared with the anterior band (Detamore and Athanasiou, 2003). Similar to the disc, mediolaterally the posterior superior attachment was 110% stiffer and 55% stronger than the anterior superior attachment. Revisiting the ‘trampoline hypothesis,’ the disc acts to transmit load via tensile strains in collagen fibers oriented perpendicular to the axis of compression (Allen and Athanasiou, 2006). Combining the tensile data with the ring-like collagen organization of the attachments, we hypothesize that the attachments contribute to this load transmission via circumferential tensile strains. This study demonstrates that the attachments transition seamlessly with the disc and continue to transmit load in a trampoline-like manner.
Regional variability in biochemical content and tensile properties suggests that the inferior attachments contribute to maintain the disc’s anteroposterior position. In both the human and porcine TMJ, masticatory opening is associated with protrusion, while masticatory closing is associated with retrusion of the condyle (Herring et al., 2002). Previous work in the porcine model has shown collagen content to be highest in the posterior inferior attachment and lowest in the anterior attachments (Willard et al., 2012). In contrast to collagen, elastin staining is the greatest in the anterior inferior and medial attachments, and lowest in the posterior attachments (Griffin and Sharpe, 1962; Willard et al., 2012). In the human TMJ, various mechanisms have been proposed to describe the motion of the disc during translation (Scapino et al., 2006), and it is plausible that both elastin and collagen contribute. Here, the posterior inferior attachment was nearly 400% stiffer than the posterior superior attachment anteroposteriorly. Given that both the human and porcine condyles protrude, variations in collagen and elastin content and tensile properties suggest that the inferior attachments contribute to the disc’s anteroposterior position more than do the superior attachments.
Previously, the posterior inferior attachment was shown to play a role in maintaining the disc’s position during jaw closing (Tanaka et al., 2003), and here we show how this relates contextually to the tensile properties of the other attachments. When the stiffness of the attachments is compared anteroposteriorly vs. mediolaterally, it would appear that the disc is held in place anteroposteriorly by the combination of both inferior attachments, as well as by the lateral and medial attachments. Among these, the higher stiffness values of the posterior inferior and lateral attachments would suggest that not only the posterior inferior attachment, but also the lateral attachment, are primarily responsible for maintaining anteroposterior disc positioning.
Increased stresses in the posterior and lateral regions of the porcine and human disc and associated attachments may be linked to the etiology of disc displacement and degeneration. In tension and compression, the porcine disc’s posterior band has greater properties compared with the anterior band (Detamore and Athanasiou, 2003). Additionally, the lateral region demonstrates greater compressive properties compared with the medial region (Lumpkins and McFetridge, 2009), also observed by Allen and Athanasiou (2006) on the superior surface of the disc. Tensile properties in the attachments were consistent with these findings. While the porcine TMJ provides a useful model for human joint function, known differences exist between the human and pig TMJ. For the human TMJ, finite element analysis of the disc has also demonstrated that, during translation from a closed to a protrusive position, the stress distribution shifts from the central part of the intermediate zone to its lateral side. During this motion, there is a small displacement of the disc in the medial direction (Beek et al., 2001b). The posterior and lateral attachments are likely essential to proper load transmission, and their failure may contribute to disc displacement and downstream degeneration of the disc and cartilage.
Problems involving the posterior attachments have been implicated in pathological conditions afflicting the human TMJ. In the presence of disc displacement, the posterior attachments have shown increased vascularity and cellularity, varied fiber pattern, and decreased elastin (Kurita et al., 1989; de Bont and Stegenga, 1993). Additionally, the posterior attachment has been imaged in efforts to identify the presence of disc displacement and pain (Katzberg and Tallents, 2005). While the posterior attachments have been indicated in the etiology of TMD, this study suggests that the lateral attachment may also play a role.
Evaluated by magnetic resonance imaging and histology, rotational and sideways disc displacements are associated with TMJ pathology, and lateral attachment stretching may be involved in this progression (Katzberg et al., 1988; Tasaki and Westesson, 1993). This is corroborated here by mechanical data, since the lateral attachment had the greatest maximum strain mediolaterally, and therefore is capable of undergoing substantial stretching. Clinically, 85% of patients with partial disc displacement have shown anterolateral or lateral displacement (Tasaki et al., 1996). Additionally, the lateral attachment demonstrates the greatest toughness despite not being the strongest tissue tested. Here it is proposed that the notable stiffness and toughness may be a mechanical adaptation in response to the loading patterns of the joint. A similar adaptation has been suggested in the posterior attachments during disc displacement (de Bont and Stegenga, 1993). Despite potential mechanisms of adaptation, TMD often progresses to disc thinning and perforation. In a cadaver study, 27% of perforations occurred in the posterior superior attachment and 53% occurred in the lateral region of the disc (Helmy et al., 1989). The substantial strain experienced by the lateral attachment and the significant toughness indicate key roles for this attachment in joint motion and the development and progression of pathology.
Tensile and morphologic characterization of the TMJ attachments revealed region- and direction-dependent properties paralleling those of the disc. Tensile properties and collagen alignment suggest that the attachments contribute to the disc’s trampoline-like load transmission. With respect to commonly observed partial lateral displacement, weakening of the lateral attachment may be a primary contributor. In contrast, anterior displacement may be due to weakening of both the posterior and lateral attachments. Building upon this study’s effort to identify axes of loading and functional roles of the attachments, future work may characterize the tissues’ inherent mechanical properties in further depth. Despite bearing many close similarities with the human TMJ, known differences in the porcine model require that a systematic comparison of the properties identified here with those of the human in health and disease be performed. Guided by the findings of this study, additional studies should specifically examine the functional properties of the lateral and posterior attachments in normal human joints, and in pathologic human joints demonstrating disc displacement. Furthermore, for tissue-engineering biomimetic constructs, it is reasonable to consider the significant role the attachments play.
Supplementary Material
Acknowledgments
The authors thank Mr. Andrew Reimer for his technical assistance.
Footnotes
This material is based upon work supported by the National Science Foundation Graduate Research Fellowship (DGE-1148897) and by the National Institutes of Health (R01DE015038).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Allen KD, Athanasiou KA. (2006). Viscoelastic characterization of the porcine temporomandibular joint disc under unconfined compression. J Biomech 39:312-322. [DOI] [PubMed] [Google Scholar]
- Beek M, Aarnts MP, Koolstra JH, Feilzer AJ, van Eijden TM. (2001a). Dynamic properties of the human temporomandibular joint disc. J Dent Res 80:876-880. [DOI] [PubMed] [Google Scholar]
- Beek M, Koolstra JH, van Ruijven LJ, van Eijden TM. (2001b). Three-dimensional finite element analysis of the cartilaginous structures in the human temporomandibular joint. J Dent Res 80:1913-1918. [DOI] [PubMed] [Google Scholar]
- Ben Amor F, Carpentier P, Foucart JM, Meunier A. (1998). Anatomic and mechanical properties of the lateral disc attachment of the temporomandibular joint. J Oral Maxillofac Surg 56:1164-1167. [DOI] [PubMed] [Google Scholar]
- de Bont LG, Stegenga B. (1993). Pathology of temporomandibular joint internal derangement and osteoarthrosis. Int J Oral Maxillofac Surg 22:71-74. [DOI] [PubMed] [Google Scholar]
- Detamore MS, Athanasiou KA. (2003). Tensile properties of the porcine temporomandibular joint disc. J Biomech Eng 125:558-565. [DOI] [PubMed] [Google Scholar]
- Griffin CJ, Sharpe CJ. (1962). Distribution of elastic tissue in the human temporomandibular meniscus especially in respect to “compression” areas. Aust Dent J 7:72-78. [Google Scholar]
- Helmy ES, Bays RA, Sharawy MM. (1989). Histopathological study of human TMJ perforated discs with emphasis on synovial membrane response. J Oral Maxillofac Surg 47:1048-1052. [DOI] [PubMed] [Google Scholar]
- Herring SW. (2003). TMJ anatomy and animal models. J Musculoskelet Neuronal Interact 3:391-394. [PMC free article] [PubMed] [Google Scholar]
- Herring SW, Decker JD, Liu ZJ, Ma T. (2002). Temporomandibular joint in miniature pigs: anatomy, cell replication, and relation to loading. Anat Rec 266:152-166. [DOI] [PubMed] [Google Scholar]
- Juran CM, Dolwick MF, McFetridge PS. (2013). Shear mechanics of the TMJ disc: relationship to common clinical observations. J Dent Res 92:193-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalpakci KN, Willard VP, Wong ME, Athanasiou KA. (2011). An interspecies comparison of the temporomandibular joint disc. J Dent Res 90:193-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzberg RW, Tallents RH. (2005). Normal and abnormal temporomandibular joint disc and posterior attachment as depicted by magnetic resonance imaging in symptomatic and asymptomatic subjects. J Oral Maxillofac Surg 63:1155-1161. [DOI] [PubMed] [Google Scholar]
- Katzberg RW, Westesson PL, Tallents RH, Anderson R, Kurita K, Manzione JV, Jr, et al. (1988). Temporomandibular joint: MR assessment of rotational and sideways disk displacements. Radiology 169:741-748. [DOI] [PubMed] [Google Scholar]
- Katzberg RW, Westesson PL, Tallents RH, Drake CM. (1996). Anatomic disorders of the temporomandibular joint disc in asymptomatic subjects. J Oral Maxillofac Surg 54:147-153. [DOI] [PubMed] [Google Scholar]
- Kurita K, Westesson PL, Sternby NH, Eriksson L, Carlsson LE, Lundh H, et al. (1989). Histologic features of the temporomandibular joint disk and posterior disk attachment: comparison of symptom-free persons with normally positioned disks and patients with internal derangement. Oral Surg Oral Med Oral Pathol 67:635-643. [DOI] [PubMed] [Google Scholar]
- LeResche L. (1997). Epidemiology of temporomandibular disorders: implications for the investigation of etiologic factors. Crit Rev Oral Biol Med 8:291-305. [DOI] [PubMed] [Google Scholar]
- Liu ZJ, Herring SW. (2000). Masticatory strains on osseous and ligamentous components of the temporomandibular joint in miniature pigs. J Orofac Pain 14:265-278. [PubMed] [Google Scholar]
- Lumpkins SB, McFetridge PS. (2009). Regional variations in the viscoelastic compressive properties of the temporomandibular joint disc and implications toward tissue engineering. J Biomed Mater Res A 90:784-791. [DOI] [PubMed] [Google Scholar]
- Rees LA. (1954). The structure and function of the mandibular joint. Br Dent J 96:125-133. [Google Scholar]
- Scapino RP, Obrez A, Greising D. (2006). Organization and function of the collagen fiber system in the human temporomandibular joint disk and its attachments. Cells Tissues Organs 182:201-225. [DOI] [PubMed] [Google Scholar]
- Sindelar BJ, Herring SW. (2005). Soft tissue mechanics of the temporomandibular joint. Cells Tissues Organs 180:36-43. [DOI] [PubMed] [Google Scholar]
- Sun Z, Liu ZJ, Herring SW. (2002). Movement of temporomandibular joint tissues during mastication and passive manipulation in miniature pigs. Arch Oral Biol 47:293-305. [DOI] [PubMed] [Google Scholar]
- Tanaka E, van Eijden T. (2003). Biomechanical behavior of the temporomandibular joint disc. Crit Rev Oral Biol Med 14:138-150. [DOI] [PubMed] [Google Scholar]
- Tanaka E, Del Pozo R, Sugiyama M, Tanne K. (2002). Biomechanical response of retrodiscal tissue in the temporomandibular joint under compression. J Oral Maxillofac Surg 60:546-551. [DOI] [PubMed] [Google Scholar]
- Tanaka E, Hanaoka K, Tanaka M, Van Eijden T, Iwabe T, Ishino Y, et al. (2003). Viscoelastic properties of bovine retrodiscal tissue under tensile stress-relaxation. Eur J Oral Sci 111:518-522. [DOI] [PubMed] [Google Scholar]
- Tasaki MM, Westesson PL. (1993). Temporomandibular joint: diagnostic accuracy with sagittal and coronal MR imaging. Radiology 186:723-729. [DOI] [PubMed] [Google Scholar]
- Tasaki MM, Westesson PL, Isberg AM, Ren YF, Tallents RH. (1996). Classification and prevalence of temporomandibular joint disk displacement in patients and symptom-free volunteers. Am J Orthod Dentofacial Orthop 109:249-262. [DOI] [PubMed] [Google Scholar]
- Wilkes CH. (1989). Internal derangements of the temporomandibular joint. Pathological variations. Arch Otolaryngol Head Neck Surg 115:469-477. [DOI] [PubMed] [Google Scholar]
- Willard VP, Arzi B, Athanasiou KA. (2012). The attachments of the temporomandibular joint disc: a biochemical and histological investigation. Arch Oral Biol 57:599-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.