Abstract
Vitamin D, an anti-inflammatory mediator, has potential benefits for physical and oral health. Although it is produced endogenously, some individuals have a greater need for dietary and supplemental sources. This repeated-measures cross-sectional study assessed associations between total vitamin D intake and periodontal health in older men. Participants were 562 members of the Department of Veterans Affairs Dental Longitudinal Study, mean age 62.9 years, who were examined 1 to 4 times between 1986 and 1998. A calibrated examiner measured probing pocket depth (PPD) and attachment loss (AL) on each tooth. Alveolar bone loss (ABL) was determined from radiographs. Severe periodontal disease was defined as PPD ≥ 5 mm on ≥ 1 tooth and AL ≥ 6 mm at ≥ 2 sites (not on same tooth), and moderate-to-severe alveolar bone loss as ABL ≥ 40% at ≥ 3 sites. Generalized estimating equations were used to compute the odds ratios (OR) and 95% confidence intervals (95% CI) of having periodontal disease by level of vitamin D intake. Total vitamin D intake ≥ 800 IU was associated with lower odds of severe periodontal disease (OR = 0.67, 95% CI = 0.55-0.81) and moderate-to-severe ABL (OR = 0.54, 95% CI = 0.30-0.96) relative to intake < 400 IU/day. Vitamin D intake may protect against periodontal disease progression.
Keywords: periodontal disease, alveolar bone, probing depth, clinical attachment loss, vitamin D intake, aging
Introduction
The importance of nutritional factors to the maintenance of periodontal health has been long recognized (Neiva et al., 2003). A paradigmatic example is the effect of severe vitamin C deficiency on the gingiva (Schifferle, 2009). However, there is a surprising paucity of evidence regarding the relationship of other vitamins to periodontal health at their usual daily levels of intake.
Vitamin D is a fat-soluble vitamin that is obtained from the diet or from endogenous production in the skin when exposed to adequate sunlight (Holick, 2008). The importance of vitamin D to bone health through its regulation of calcium homeostasis has been long recognized (Vieth, 2007). More recently, protective associations of vitamin D with conditions such as hypertension, cardiovascular disease, diabetes, and cancer have been reported (Holick, 2008; Hewison, 2012), some of which may involve vitamin D as a modulator of inflammatory responses.
Mounting evidence suggests that vitamin D may be beneficial to periodontal health. In NHANES III, an inverse relationship was found between serum 25-hydroxyvitamin D3 levels and mean attachment loss in men and women aged 50 yrs and older (Dietrich et al., 2004). A separate analysis of NHANES III data showed a 10% decrease in the odds of bleeding upon probing for each 30 nmol/L increase in serum vitamin D level among non-smokers (Dietrich et al., 2005). Periodontal maintenance patients who took combined dietary supplements of vitamin D and calcium tended to have better periodontal health, with shallower probing depth, less attachment loss, and less alveolar bone loss (Miley et al., 2009; Garcia et al., 2011). Bashutski et al. (2011) conducted a 6-month placebo-controlled trial of parathyroid hormone among patients with severe periodontal disease who underwent open flap debridement surgery. In analyses that also accounted for pre-surgery vitamin D status (deficiency defined as serum 25-hydroxyvitamin-D < 20 ng/mL), they found that, in the placebo group, there was significantly more improvement in clinical periodontal measures if patients were vitamin D-sufficient rather than deficient. In the PTH-treated group, there was significantly more resolution of linear bony defects in the vitamin D-sufficient group compared with the deficient group (Bashutski et al., 2011). However, another report found only calcium intake, and not vitamin D, to be significantly related to reduced periodontal disease progression and tooth loss in men (Krall, 2004).
We used data from a study of aging and oral health in men to determine whether recommended daily intakes of total vitamin D are associated with better periodontal health, as measured by alveolar bone loss, pocket depth, and attachment loss.
Materials & Methods
Study Population
We used data from 562 adult, dentate, male participants in the VA Dental Longitudinal Study (DLS), who had dental examinations between 1986 and 1998. The DLS began in 1968 with 1,231 volunteers drawn from the 2,280 participants in the VA Normative Aging Study (NAS), a closed-panel cohort study of aging among healthy, community-dwelling male veterans. Participants are not patients of the VA health care system. They received their medical and dental care in the private sector. The cohort is almost entirely non-Hispanic white. As part of the NAS and DLS, the men received triennial comprehensive oral and medical examinations. In 1986, a food frequency questionnaire (Willett et al., 1985) was added, providing the first opportunity for the estimation of daily intakes of vitamin D. Our analyses are thus limited to individuals present in 1986 and subsequent years. The Fig. provides the timeline and number of participants at each of the 4 examination visits included in this report. Written informed consent was obtained from all participants. The study was approved by institutional review boards at Boston University Medical Center and the VA Boston Healthcare System. This report complies with STROBE guidelines for observational studies (von Elm et al., 2007).
Figure.
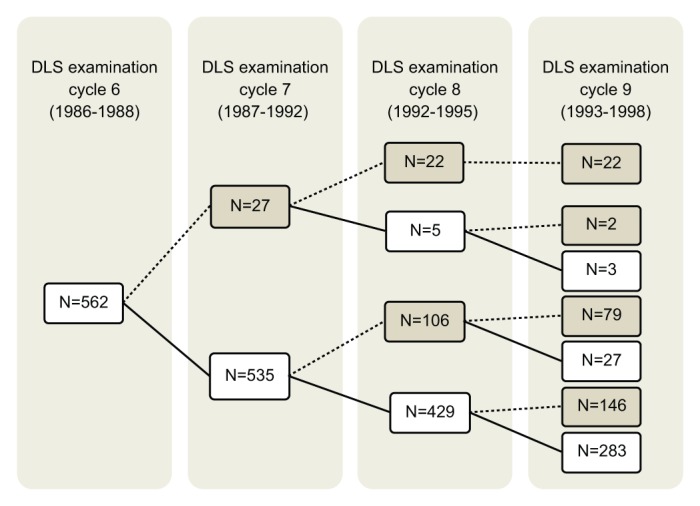
Flowchart of periodontal examination time periods and numbers of men who participated at each examination. Solid lines and numbers in white rectangles indicate numbers of men who did attend an examination cycle, whereas dotted lines and numbers in gray rectangles indicate numbers of men who did not attend. Twenty-two men attended only 1 examination, 81 attended 2 examinations, 176 attended 3 examinations, and 283 men attended all 4. DLS, Dental Longitudinal Study.
Periodontal Health Status
A calibrated periodontist examiner conducted standardized, comprehensive oral examinations, including periodontal probing at 4 sites (buccal, lingual, mesial, and distal) per tooth on all teeth present. The maximum probing pocket depth (PPD) of the 4 sites and the maximum clinical attachment loss (CAL) of the mesial and distal sites per tooth were used for these analyses. Alveolar bone loss (ABL) was measured on the mesial and distal aspects of each tooth by superimposition of a radial ruler onto standardized periapical radiographs (Schei, 1959). The distance from the alveolar crest to the root tip was expressed as the percentage of the distance from the cemento-enamel junction to the root tip and was scored in increments of 20%.
Periodontal disease status was categorized at the person-level based on numbers of teeth with PPD, CAL, or ABL at specified threshold levels. Severe clinical periodontal disease was defined as at least 1 site with PPD ≥ 5 mm and 2 or more sites with AL ≥ 6 mm, not on the same tooth (Eke et al., 2012). Moderate-to-severe ABL was defined as at least 3 proximal sites (i.e., ≥ 2 teeth) with 40% or more bone loss (Gorman et al., 2012). Sensitivity analyses were done with several other threshold levels and definitions, including categorization of ABL based on whole-mouth mean ABL scores (whole mouth mean < 40% or ≥ 40%) after the method of Beck et al. (1996). Third molars were excluded from analyses.
Dietary Intake Assessment
Nutrient intakes were computed from a 126-item Harvard University food frequency questionnaire (Willett et al., 1985) mailed to each participant prior to each examination and reviewed for completeness with participants at the study site by research personnel. Total nutrient intakes at each examination were computed from all sources including foods, supplements, and multivitamins. Vitamin D dose cut-off points used in the analyses were daily intakes of less than 400 IU, equal to or more than 400 IU but less than 800 IU, and 800 IU or more. These cut-off points were based on the dietary recommendations from the Institute of Medicine (IOM) and the International Osteoporosis Foundation (IOF). The Estimated Average Requirement (IOM, 2011), used in assessing the diets of populations, is 400 IU/day. For men ages 51 to 70 yrs and over age 70 yrs, the respective amounts that meet the needs of most individuals are 600 and 800 IU/day (IOM, 2011). However, for older adults beginning at age 60 yrs, the IOF recommends higher intakes, at least 800 to 1000 IU/day (Dawson-Hughes et al., 2010).
Statistical Analyses
We performed univariate analyses for baseline variables, and bivariate analyses of repeated data with t tests, analysis of variance (ANOVA), and chi-square tests to explore associations with vitamin D intake. Generalized Estimating Equations (GEE) multivariate regression analyses were used to estimate the odds of having severe periodontal disease or moderate-to-severe ABL by the vitamin D intake strata. A repeated-measures model was specified to account for correlated outcome data, with an autoregressive within-subject correlation matrix and logit link function. Examination cycle (i.e., time) was used as a factor to cluster the observations. Models were adjusted for variables found to be significantly associated in bivariate analyses with vitamin D intakes and periodontal disease outcomes, including age, body mass index, flossing frequency (never/ever), highest education (high school or some college/college graduate), smoking status (no/yes), the presence of diabetes (no/yes), and cardiovascular disease (no/yes). Except for education, which was measured only at the study baseline, the values of vitamin D level and all covariates used in the models were specific to each examination and varied over time. SAS statistical software version 9.1 was used for all analyses, with the level of significance set a priori at 0.05.
Results
Overall, this was a medically healthy cohort of adult men with generally good health behaviors at the baseline examination in 1986 to 1988. The mean age of the total study population at baseline was 62 yrs. Ninety-five percent were non-diabetic, 95% had no history of cardiovascular diseases, and 85% were non-smokers. Thirty-two percent of the men were college graduates. Fifty-eight percent reported flossing at least once per month. The average number of teeth present (excluding 3rd molars) was 22. The overall baseline mean of vitamin D intake was 332 IU/day.
Men with vitamin D intakes ≥ 800 IU/day were less likely to be diabetic or current smokers, and more likely to be college graduates and to floss; however, only education differed significantly among the vitamin D intake categories (Table 1). Severe periodontitis was present in 17% of participants overall at baseline, and 21% had 3 or more sites with ≥ 40% ABL. The prevalence of low vitamin D intake (< 400 IU/day) as well as of severe periodontitis and moderate-to-severe ABL varied by examination cycle, and the lowest disease prevalence tended to occur in the men with vitamin D intake ≥ 800 IU/day (Table 2).
Table 1.
Descriptive Statistics for Dental Longitudinal Study Cohort at Baseline (1986-1988) by Total Vitamin D Intake Level (mean ± SD or %)
| Total Vitamin D Intake (IU/Day) |
||||
|---|---|---|---|---|
| Variable | Less than 400 (N = 407) | ≥ 400 to < 800 (N = 125) | ≥ 800 (N = 30) | |
| Age (yrs) | 61.9 ± 7.5 | 63.4 ± 7.1 | 63.3 ± 7.6 | |
| Number of teeth | 22 ± 7 | 22 ± 7 | 22 ± 7 | |
| Vitamin D intake (IU/day) | 201 ± 90 | 559 ± 106 | 1161 ± 427 | |
| Body mass index (kg/m2) | 26.7 ± 3.4 | 26.7 ± 3.3 | 26.2 ± 3.4 | |
| Diabetes statusa | Yes | 5% | 4% | 3% |
| No | 95% | 96% | 97% | |
| Cardiovascular disease statusa | Yes | 5% | 4% | 7% |
| No | 95% | 96% | 93% | |
| Educational level* | High school or some college | 70% | 63% | 59% |
| College graduate | 30% | 37% | 41% | |
| Current smoking status | Non-smoker | 83% | 86% | 93% |
| Smoker | 17% | 14% | 7% | |
| Flossing frequency | Never | 43% | 41% | 33% |
| At least once/month | 57% | 59% | 67% | |
Determined on physical examination by study physician.
Differences among groups, p < .05, Chi-square test.
Table 2.
Periodontal Disease Prevalence at 4 Examination Cycles in Dental Longitudinal Study Cohort, 1986-1998, by Total Vitamin D Intake Level
| Total Vitamin D Intake (IU/Day) |
|||
|---|---|---|---|
| Periodontal Disease Measure | Less than 400 | ≥ 400 to < 800 | ≥ 800 |
| Baseline (1986-1988), N study participants | 407 | 125 | 30 |
| % Participants with severe periodontal diseasea | 16% | 24% | 10% |
| % Participants with moderate-to-severe ABLb | 20% | 26% | 20% |
| First follow-up (1987-1992), N study participants | 396 | 118 | 29 |
| % Participants with severe periodontal disease | 28% | 26% | 14% |
| % Participants with moderate-to-severe ABL | 22% | 26% | 22% |
| Second follow-up (1992-1995), N study participants | 357 | 130 | 43 |
| % Participants with severe periodontal disease | 39% | 39% | 34% |
| % Participants with moderate-to-severe ABL | 22% | 13% | 12% |
| Third follow-up (1993-1998), N study participants | 226 | 116 | 29 |
| % Participants with severe periodontal disease | 36% | 25% | 27% |
| % Participants with moderate-to-severe ABL | 23% | 15% | 12% |
Attachment loss ≥ 6 mm on ≥ 2 sites (not on same tooth) and pocket depth ≥ 5 mm on ≥ 1 site.
Alveolar bone loss ≥ 40% on ≥ 3 sites.
After adjustment for age, body mass index (BMI), education, diabetes, CVD, smoking, and flossing, the GEE models indicated that total vitamin D intake was inversely associated with odds of severe periodontitis and moderate-to-severe ABL (Table 3). Each 100 IU increment in daily total vitamin D intake was independently associated with reduced odds of severe periodontal disease (OR = 0.97, 95% CI = 0.96-0.98) and moderate-to-severe ABL (OR = 0.95, 95% CI = 0.91-0.99). The odds of severe periodontitis among men consuming ≥ 800 IU/day were 0.67 (95% CI, 0.55-0.81) relative to those of men consuming < 400 IU/day. The odds of moderate-to-severe ABL among men consuming ≥ 800 IU/day were 0.54 (95% CI, 0.30-0.96) relative to those of men consuming < 400 IU/day. When the alternate definition of moderate-to-severe ABL (whole mouth mean ≥ 40%) was used, the odds of disease were 0.30 (p < .01) among the men consuming ≥ 800 IU/day vitamin D relative to the < 400 IU/day category (data not shown).
Table 3.
Odds Ratios and 95% Confidence Intervals (95% CI) from Generalized Estimation Equation Multivariate Analyses for the Association of Total Vitamin D Intake and Periodontal Disease Outcomes Measured up to 4 Times between 1986 and 1998
| Total Vitamin D Intake Odds Ratio (95% CI) |
||||
|---|---|---|---|---|
| Categorical |
||||
| Periodontal Disease Outcome | Continuousa | Less than 400 IU/Dayb | ≥ 400 but < 800 IU/Day | ≥ 800 IU/Day |
| Severe periodontal diseasec | 0.97 (0.96, 0.98) | 1.0 | 1.00 (0.71, 1.41) | 0.67 (0.55, 0.81) |
| Moderate-to-severe ABLd | 0.95 (0.91, 0.99) | 1.0 | 0.80 (0.52, 1.21) | 0.54 (0.30, 0.96) |
Models controlled for age, body mass index, number of teeth, college education (yes/no), smoking status (yes/no), diabetes (yes/no), cardiovascular disease (yes/no), and flossing frequency (never, ever).
Per each 100 IU/day.
Reference group.
Attachment loss ≥ 6 mm on ≥ 2 sites (not on same tooth) and pocket depth ≥ 5 mm on ≥ 1 site.
Alveolar bone loss ≥ 40% on ≥ 3 sites.
Discussion
We examined the associations of total vitamin D intake with severe periodontal disease and moderate-to-severe alveolar bone loss and found evidence of a significant protective relationship of vitamin D intake on these measures of periodontal disease. Our findings support earlier work showing an inverse relationship of periodontal disease indicators with vitamin D intake or status (Miley et al., 2009; Bashutski et al., 2011). Use of combined calcium (1,000 mg/day) and vitamin D (400 IU/day) oral supplements by individuals attending a periodontal disease maintenance program was associated with lower probing depths compared with depths in those not taking supplements (Miley et al., 2009). Over follow-up, the supplement users tended to have less periodontal disease (attachment level, bleeding on probing, and alveolar crest height) than non-users, although the differences were not statistically significant (p > .05) at the one-year time point (Garcia et al., 2011). Among patients with severe periodontal disease undergoing periodontal surgery, a sufficient habitual vitamin D status, as determined by baseline serum 25(OH)D level, was associated with greater improvements in attachment loss and pocket depth over a 6-month follow-up. However, vitamin D supplementation begun at the time of surgery had little effect in the vitamin-deficient group over a 6-month period (Bashutski et al., 2011). Those results, together with our findings, support the notion that continual vitamin D sufficiency, whether derived from diet or endogenous production, is important to the maintenance of periodontal health.
While we did not measure serum vitamin D levels, or examine the association of vitamin D with attachment loss in women, our findings are generally consistent with cross-sectional NHANES analyses which found that higher serum 25(OH)D levels correlated with less attachment loss in both genders ages 50 yrs and older (Dietrich et al., 2004). The less severe pocket depth and attachment loss were attributed to anti-inflammatory effects of vitamin D (Dietrich et al., 2004, 2005; Miley et al., 2009). In contrast, Liu et al. (2009) found that plasma 25(OH)D levels were higher among individuals with aggressive periodontitis compared with control individuals.
Alveolar bone loss (ABL) is a distinctive cumulative sign of periodontal disease (Albander, 1990). In our analyses, individuals were considered as having moderate-to-severe ABL if they had at least 3 proximal sites with ≥ 40% ABL. Based on this case definition, we found that those consuming ≥ 800 IU/day of total vitamin D had lower odds of having periodontal disease compared with those consuming less than 400 IU/day. The medical literature regards vitamin D deficiency as a risk factor for osteoporosis, which may also affect the jaws (van Schoor et al., 2008; Amano et al., 2009).
Strengths of our study include the use of a cohort that has been well-characterized with respect to both medical and dental outcomes and risk factors, and multivariate GEE models. However, limitations of the study design and analysis exist. The absence of women in the cohort and the lack of racial and ethnic diversity limit our ability to generalize these results to broader populations. In addition, vitamin D intake was estimated from food frequency questionnaires, which are sufficient for ranking individuals according to intake level of a particular nutrient, but may not provide accurate estimates of absolute intakes. Serum 25-hydroxyvitamin D level represents the contributions from both endogenous production and diet and is a more accurate measure of vitamin D status than either component by itself. Endogenous production stemming from sunlight exposure is the major source of serum 25-hydroxyvitamin D. Data on the length of sunlight exposure and the latitude at which such exposure occurs might be useful as a surrogate measure for vitamin D status, and the lack of such exposure information is another limitation of our study. However, for segments of the population who get less sun exposure or are less efficient in generating endogenous vitamin D, such as the elderly and populations living in northern climates, the dietary and supplemental sources of vitamin D take on greater importance. Moreover, since serum vitamin D measurements are not routinely made in clinical care, a patient’s best information concerning his or her vitamin D status comes from determining how much vitamin D is obtained from diet and supplements.
It is estimated that more than 40% of the US adult population is vitamin D-deficient, based on serum 25-hydroxyvitamin D levels ≤ 20 ng/mL or 50 nmol/L (Forrest and Stuhldreher, 2011). Among older well-educated white men, such as the participants in this study, the prevalence is somewhat lower, approximately 30%. Additional factors such as poor health status, obesity, and cardiovascular disease risk factors increase the chance of deficiency. Thus, the prevalence of vitamin D deficiency may be higher in populations that are more diverse in terms of ethnicity and disease status. Correcting vitamin D deficiency may have a large beneficial impact on chronic disease prevalence. Our findings suggest that maintenance of vitamin D intake near or above the recommendations, whether through diet or supplementation, could be a safe, effective, and inexpensive method of reducing periodontal disease prevalence. Adherence to the daily recommendation of vitamin D intake for older adults of at least 800 IU/Day may not only have great impact on improving periodontal health but may also provide recognized benefits in terms of bone health.
Footnotes
This work was supported by the National Institute of Dental and Craniofacial Research (K24 DE00419). The Dental Longitudinal Study and Normative Aging Study are components of the Massachusetts Veterans Epidemiology Research and Information Center, supported by the US Department of Veterans Affairs Cooperative Studies Program. Dr. Garcia was a recipient of a Veterans Affairs Career Development Award in Health Services Research from the VA HSR&D Service. The views expressed in this paper are those of the authors and do not necessarily represent the views of the US Department of Veterans Affairs.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Albander JM. (1990). A 6 year study on the pattern of periodontal disease progression. J Clin Periodontol 17(7 Pt 1):467-471. [DOI] [PubMed] [Google Scholar]
- Amano Y, Komiyama K, Makishima M. (2009). Vitamin D and periodontal disease. J Oral Sci 51:11-20. [DOI] [PubMed] [Google Scholar]
- Bashutski JD, Eber RM, Kinney JS, Benavides E, Maitra S, Braun TM, et al. (2011). The impact of vitamin D status on periodontal surgery outcomes. J Dent Res 90:1007-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck J, Garcia R, Heiss G, Vokonas P, Offenbacher S. (1996). Periodontal disease and cardiovascular disease. J Periodontol 67(10 Suppl):1123-1137. [DOI] [PubMed] [Google Scholar]
- Dawson-Hughes B, Mithal A, Bonjour JP, Boonen S, Burckhardt P, Fuleihan GE, et al. (2010). IOF position statement: vitamin D recommendations for older adults. Osteoporos Int 21:1151-1154. [DOI] [PubMed] [Google Scholar]
- Dietrich T, Joshipura KJ, Dawson-Hughes B, Bischoff-Ferrari HA. (2004). Association between serum concentration of 25-hydroxyvitamin D3 and periodontal disease in the US population. Am J Clin Nutr 80:108-113. [DOI] [PubMed] [Google Scholar]
- Dietrich T, Nunn M, Dawson-Hughes B, Bischoff-Ferrari HA. (2005). Association between serum concentration of 25-hydroxyvitamin D3 and gingival inflammation. Am J Clin Nutr 82:575-580. [DOI] [PubMed] [Google Scholar]
- Eke PI, Page RC, Wei L, Thornton-Evans G, Genco RJ. (2012). Update of the case definitions for population-based surveillance of periodontitis. J Periodontol 83:1449-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest KY, Stuhldreher WL. (2011). Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res 31:48-54. [DOI] [PubMed] [Google Scholar]
- Garcia MN, Hildebolt CF, Miley DD, Dixon DA, Couture RA, Spearie CL, et al. (2011). One-year effects of vitamin D and calcium supplementation on chronic periodontitis. J Periodontol 82:25-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman A, Kaye EK, Apovian CA, Fung TT, Nunn M, Garcia RI. (2012). Overweight and obesity predict time to periodontal disease progression in men. J Clin Periodontol 39:107-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewison M. (2012). An update on vitamin D and human immunity. Clin Endocrinol (Oxf) 76:315-325. [DOI] [PubMed] [Google Scholar]
- Holick MF. (2008). The vitamin D deficiency pandemic and consequences for nonskeletal health: mechanisms of action. Mol Aspects Med 29:361-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IOM (Institute of Medicine) (2011). Dietary reference intakes for calcium and vitamin D. Washington, DC: The National Academies Press; Accessed on 6/4/2013 at: http://www.iom.edu/Reports/2010/Dietary-Reference-Intakes-for-Calcium-and-Vitamin-D/DRI-Values.aspx. [Google Scholar]
- Krall EA. (2004). Nutrition and teeth. In: Nutritional aspects of osteoporosis. 2nd ed. Burckhardt P, Dawson-Hughes B, Heaney RP, editors. Burlington, MA: Elsevier Academic Press, pp 153-162. [Google Scholar]
- Liu K, Meng H, Tang X, Xu L, Zhang L, Chen Z, et al. (2009). Elevated plasma calcifediol is associated with aggressive periodontitis. J Periodontol 80:1114-1120. [DOI] [PubMed] [Google Scholar]
- Miley DD, Garcia MN, Hildebolt CF, Shannon WD, Couture RA, Anderson Spearie CL, et al. (2009). Cross-sectional study of vitamin D and calcium supplementation effects on chronic periodontitis. J Periodontol 80:1433-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neiva RF, Steigenga J, Al-Shammari KF, Wang HL. (2003). Effects of specific nutrients on periodontal disease onset, progression and treatment. J Clin Periodontol 30:579-589. [DOI] [PubMed] [Google Scholar]
- Schei O. (1959). Alveolar bone loss as related to oral hygiene and age. J Periodontol 30:7-16. [Google Scholar]
- Schifferle RE. (2009). Periodontal disease and nutrition: separating the evidence from current fads. Periodontol 2000 50: 78-89. [DOI] [PubMed] [Google Scholar]
- van Schoor NM, Visser M, Pluijm SM, Kuchuk N, Smit JH, Lips P. (2008). Vitamin D as a risk factor for osteoporotic fractures. Bone 42:260-266. [DOI] [PubMed] [Google Scholar]
- Vieth R. (2007). Vitamin D toxicity, policy, and science. J Bone Miner Res 22(Suppl 2):V64-V68. [DOI] [PubMed] [Google Scholar]
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative (2007). Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 335:806-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, et al. (1985). Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 122:51-65. [DOI] [PubMed] [Google Scholar]


