Abstract
Short QT Syndrome (SQTS) is a novel clinical entity characterized by markedly rapid cardiac repolarization and lethal arrhythmias. A mutation in the Kir2.1 inward rectifier K+ channel (D172N) causes one form of SQTS (SQT3). Pharmacologic block of Kir2.1 channels may hold promise as potential therapy for SQT3. We recently reported that the anti-malarial drug chloroquine blocks Kir2.1 channels by plugging the cytoplasmic pore domain. In this study, we tested whether chloroquine blocks D172N Kir2.1 channels in a heterologous expression system and if chloroquine normalizes repolarization properties using a mathematical model of a human ventricular myocyte. Chloroquine caused a dose- and voltage-dependent reduction in wild-type (WT), D172N and WT-D172N heteromeric Kir2.1 current. The potency and kinetics of chloroquine block of D172N and WT-D172N Kir2.1 current were similar to WT. In silico modeling of the heterozygous WT-D172N Kir2.1 condition predicted that 3 μM chloroquine normalized inward rectifier K+ current magnitude, action potential duration and effective refractory period. Our results suggest that therapeutic concentrations of chloroquine might lengthen cardiac repolarization in SQT3.
Key Words: Potassium channel, Inward rectifier, Action potential clamp, Channelopathy, Pharmacology
Introduction
Chloroquine is an important therapeutic agent for the prevention and treatment of malaria in many parts of the world. Chloroquine is also used as adjunct therapy for treatment of systemic inflammatory disorders. Despite its widespread use, chloroquine has a narrow margin of safety, primarily as a consequence of its cardiovascular side-effects. Chloroquine prolongs cardiac action potential duration, enhances automaticity and reduces maximum diastolic potential [1, 2, 3]. As a consequence, chloroquine causes prolongation of the QT and QRS intervals on the surface electrocardiogram even at therapeutic concentrations [4, 5]. These cellular and electrocardiographic side-effects are primarily due to chloroquine block of the inward rectifier K+ current (IK1) [2, 3], and to a lesser degree, block of the rapidly activating delayed rectifier, IKr [2, 6].
The Kir2.x subfamily of potassium channels (Kir2.1, 2.2 and 2.3) is responsible for cardiac IK1, although several lines of evidence suggest that Kir2.1 is the primary channel subunit. For example, loss of function mutations in gene encoding Kir2.1 (KCNJ2), cause Andersen-Tawil Syndrome, an autosomal dominant disorder characterized by prolongation of the QT interval and ventricular arrhythmias [7, 8]. A gain of function mutation in KCNJ2 was recently reported to cause one form of Short QT Syndrome (SQT3). Priori and colleagues described a missense mutation (D172N) located within the transmembrane ion conduction pathway of Kir2.1 in a father and daughter with a short QT interval [9]. D172 is a principal binding site for Mg2+ and polyamines which are the endogenous Kir2.1 blockers that impart the characteristic strong inward rectification property [10]. The biophysical consequences of D172 mutations have been well-characterized and include a reduction in the degree of current rectification at depolarized potentials [10]. In silico simulations of the loss of rectification predict a significant amount of repolarizing current during the terminal repolarization phase with resultant shortening of action potential duration [9]. The markedly short action potential duration provides the substrate for atrial and ventricular arrhythmias.
Given the relatively high incidence of sudden death in SQTS, an implantable cardioverter defibrillator (ICD) is considered as primary therapy [11, 12]. However, ICD therapy is expensive and particularly problematic in small children due to technical considerations. Thus, specific pharmacological therapy aimed at the molecular defect in SQTS would be beneficial. Chloroquine may be a potential therapeutic agent for treatment of SQTS3 given its known ability to block Kir2.1. We recently mapped the location of the chloroquine binding site to the cytoplasmic domain of Kir2.1 [13]. Chloroquine blocks Kir2.1 channels by plugging the cytoplasmic conduction pathway, even while polyamines remain bound to deeper sites, such as D172, within the channel vestibule. In this study, we tested experimentally whether chloroquine can effectively block D172N Kir2.1 channels and normalize repolarization duration in silico. The ability of chloroquine to block heterologously expressed D172N channels was recently reported [14].
Materials and Methods
Molecular Biology and Cell Transfection
Kir2.1 cDNA (kind gift of C. Vandenberg) was subcloned into pcDNA3.1(+) plasmid (Invitrogen, La Jolla, CA). Mutations were made using the QuikChange Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA). The mutation was confirmed by direct DNA sequencing. Kir2.1 constructs were expressed in HEK293 cells as described [15]. A total of 5 µg DNA was transfected for inside-out and action potential voltage clamp experiments and 2.5 µg DNA for whole-cell experiments. To mimic the heterozygous state, WT and D172N Kir2.1 were co-expressed together at one-half the amounts of total transfected DNA. Cells were transfected with Lipofectamine 2000 reagent (Invitrogen) according to manufacturer instructions.
Electrophysiology
Macroscopic currents were recorded in the whole-cell and inside-out configurations of the patch-clamp technique [16] using an Axopatch-200B amplifier (Molecular Devices Corp). Data acquisition and command potentials were controlled by pClamp 8.0 software (Molecular Devices Corp.). Patch pipettes with a resistance of 1-2 MΩ were made from borosilicate capillary glass (WPI, Sarasota, FL). For whole-cell recordings, the internal solution contained (in mM): 100 KCl, 10 HEPES, 5 K4BAPTA, 5 K2ATP and 1 MgCl2; pH 7.2. The standard bath solution contained (in mM): 130 NaCl, 4 KCl, 1.8 CaCl2, 1 MgCl2, 10 HEPES and 10 glucose; pH 7.4. Inside-out patches were recorded using Mg2+- and spermine-free solution on both sides of the patch containing (in mM): 123 KCl, 5 K2EDTA, 7.2 K2HPO4 and 8 KH2PO4; pH 7.2. Action potential voltage clamp recordings were performed in HEK293 cells using the action potential recorded from an isolated feline endocardial ventricular myocyte as the command signal. Currents elicited in response to the action potential voltage clamp were recorded under control conditions, in the presence of chloroquine and after application of 2 mM BaCl2. Kir2.1 currents are represented as Ba2+-subtracted currents elicited by the action potential voltage clamp.
Drugs
Chloroquine (Sigma, St. Louis, MO) was dissolved directly in the external solution at the desired concentration. HEK293 cells were exposed to chloroquine solutions until steady-state effects were achieved. The effects of chloroquine were measured 8-10 minutes after bath application in whole cell experiments and 15 s after application in excised inside-out patches. To determine the concentration-effect relationships, a single cell was exposed to cumulative concentrations of chloroquine.
Data Analysis
Data are present as mean ± SEM (n = number of cells). pClamp 8.0 software (Molecular Devices Corp.) was used to perform nonlinear least-squares kinetic analyses of time-dependent currents. The fractional block of current f was plotted as a function of drug concentration [D] and the data were fit with a Hill equation:
to determine the half-maximal inhibitory concentration IC50 and the Hill coefficient, nh. The voltage dependence of block was measured by fitting fractional unblock as a function of voltage with a Woodhull equation of the form: Iblock/Icontrol=1/(1+[chloroquine]/Kd(0)e-zFV/RT)
where the test potential V, the dissociation constant at 0 mV Kd(0), valence of block z, temperature T, Faraday constant F, and gas constant R.
Action potential modeling
A mathematical model of human left ventricular myocytes [17] was used to simulate the effects of chloroquine on IK1 and action potentials. The model describes IK1 currents by:
with the conductance GK1, the parameters P1 and P2, the potassium reversal voltage EK, the transmembrane voltage Vm, and the extracellular potassium concentration [K+]o. The parameters of the IK1 model were set to resemble measured control WT, D172N and WT-D172N using mathematical optimization methods (Matlab, MathWorks, Inc., Natick, MA, USA). The model parameter temperature was set to 310 K replicating physiologic conditions of the myocyte. Although experimental measures of IK1 were performed at room temperature, no temperature scaling was necessary in the model because of the instantaneous nature of IK1. In the myocyte model, the WT conductance GK1 was adjusted to reproduce original model action potential duration at 90% repolarization (APD90). Currents for D172N and and WT-D172N channels were scaled according to measured data (Fig. 2). The conductances GK1 at various chloroquine concentrations were calculated based on the Hill equation with parameters from Fig. 1B. The simulations with the myocyte models were carried out with the Euler method for numerical solution of ordinary differential equations [18]. The temporal discretization for solving the equations was chosen heterogeneously for various components of the model. The simulation results after the 10th stimulation were analyzed. Action potential duration (APD) was defined as the interval between the time of maximal action potential upstroke velocity and repolarization to the level of 50% (APD50) and 90% of the upstroke amplitude. The effective refractory period (ERP) was measured using a standard S1-S2 stimulation protocol: an 8-pulse conditioning train was delivered at 60 bpm, and the S1-S2 interval was shortened in 2.5 ms decrements. The ERP was defined as the longest S1-S2 interval which failed to elicit an S2 action potential of amplitude > 80% of the preceding S1 action potential.
Fig. 2.
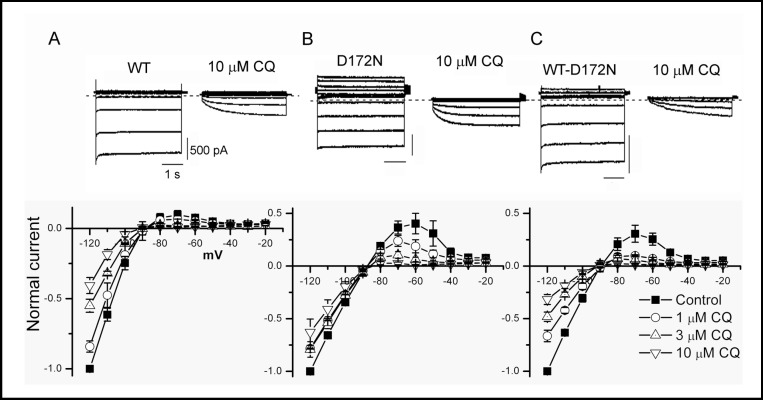
Chloroquine preferentially blocks outward current through WT and mutant Kir2.1 channels. Top panel, WT and mutant Kir 2.1 current elicited by 4 s pulses from a holding potential of −80 mV to test potentials from −120 to −20 mV, applied in 10 mV increments, in absence and presence of chloroquine 10 µM. B, Normalized current-voltage relationship for currents measured at the end of 4 s pulses for control and indicated concentrations of chloroquine.
Fig. 1.
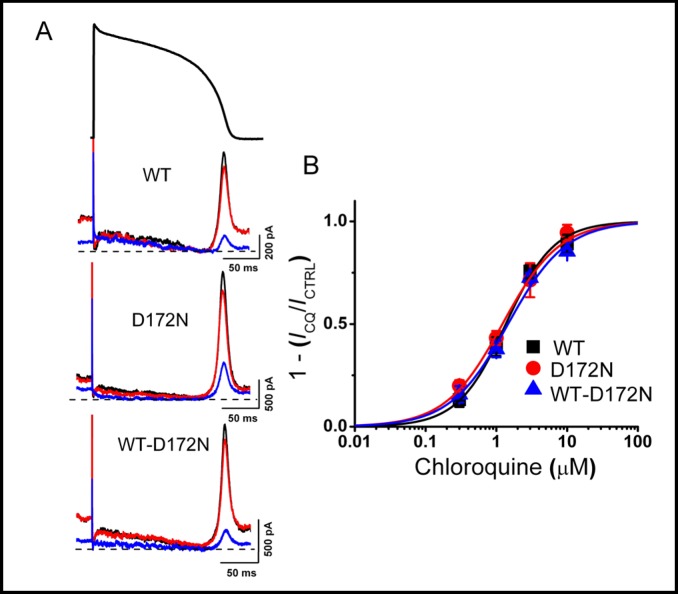
Concentration-response relationships for chlorquine inhibition of WT and mutant Kir2.1 current. A, Representative WT and mutant Kir2.1 currents elicited by action potential command signals as voltage protocol, before (black traces) and after application of 0.3 (red traces) and 3 (blue traces) µM chloroquine. B, Concentration-response relationship for WT and mutant Kir2.1 current inhibition. Steady-state peak-current amplitudes (shown in A) for each concentration of chloroquine normalized to control. Mean values were plotted against chloroquine concentration and fitted with the Hill equation (IC50 was 1.4 ± 0.1 µM with a Hill coefficient of 1.3 for WT, 1.2 ± 0.1 µM with a Hill coefficient of 1.1 for D172N and 1.5 ± 0.2 µM with a Hill coefficient of 1.1 for WT-D172N Kir2.1 current (n = 5 cells).
Results
To determine the effect of chloroquine on WT and mutant Kir2.1 current in a physiologically relevant setting, we used an action potential voltage clamp protocol in the HEK293 cell heterologous expression system. The effect of chloroquine on current elicited by expression of D172N mutant Kir2.1 channels was studied, as well as co-expressed WT and D172N Kir2.1 (WT-D172N) channels to mimic the autosomal dominant nature of SQTS. In control conditions, Kir2.1 current was small during the plateau phase of the action potential, increased rapidly during the terminal phase of repolarization and declined in early diastole (Fig. 1A). D172N and WT-D172N currents were also small during the plateau phase, but were significantly larger during the terminal repolarization and diastolic phases of the action potential (note the differences in scale bars, Fig. 1A). Chloroquine inhibited the peak outward current in a concentration-dependent manner for WT, D172N and WT-D172N Kir2.1 (Fig. 1B). The potency of chloroquine block was not different for WT, D172N and WT-D172N Kir2.1. (IC50 was 1.4 ± 0.1 µM with a Hill coefficient of 1.3 for WT, 1.2 ± 0.1 µM with a Hill coefficient of 1.1 for D172N and 1.5 ± 0.2 µM with a Hill coefficient of 1.1 for WT-D172N Kir2.1 current (n = 5 cells, Fig. 1B).
Next, we measured the effect of chloroquine on WT and mutant Kir2.1 currents elicited by 4 s hyper- and depolarizing voltage steps between −120 and −20 mV using the whole-cell configuration. For WT Kir2.1, large inward currents, but small outward currents were elicited by this voltage protocol, as expected from a strong inward rectifier channel (Fig. 2A). Outward current peaked at −70 mV and measured 0.10 ± 0.03 of the maximum current elicited at −120 m V. Consistent with disrupted rectification, D172N Kir2.1 outward current peaked at −60 mV and measured 0.40 ± 0.10 of the maximum current (Fig. 2B). WT-D172N Kir2.1 current was intermediate, with outward current peaking at −70 mV and representing 0.31 ± 0.08 of maximum current at −120 mV (Fig. 2C). Similar to WT Kir2.1, chloroquine reduced D172N and WT-D172N Kir2.1 current in a voltage-dependent manner, with preferential inhibition of outward current (Fig. 2).
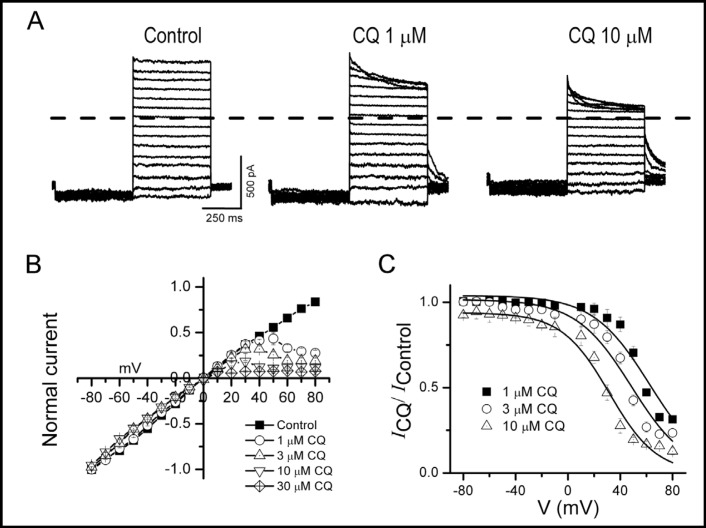
To further characterize the voltage-dependence of chloroquine block of D172N Kir2.1 channels, we used excised inside-out patches where the competing effects of endogenous blockers were absent. Application of chloroquine to the intracellular surface caused a voltage-and dose-dependent reduction in outward currents (Fig. 3A, B), similar to that reported for WT Kir2.1 [13]. We estimated the distance that chloroquine penetrates the channel pore by plotting the fraction of unblocked D172N current at various chloroquine concentrations versus membrane voltage (Fig. 3C). Fitting the data to a Woodhull equation yielded an apparent Kd (at 0 mV) of 34 ± 11 µM with an apparent valence (z) of 1.6 ± 0.3. The apparent valence estimates the ability of a blocker to displace K+ ions across the electrical field and thus is an indirect measure of the distance penetrated by the blocker. These values are similar to those reported for chloroquine block of WT Kir2.1 (Kd, 27 µM and z, 2.1) [13], suggesting that the binding site for chloroquine is not appreciably altered by the D172N mutation.
Fig. 3.
Block of D172N Kir2.1 current by intracellular chloroquine is voltage-dependent. A, Effect of intracellular application of chloroquine on D172N Kir2.1 current recorded in excised inside-out patches. Currents from a single cell elicited by 1 s step depolarization between +40 and −80 mV in control and after application of 1 and 10 µM chloroquine. Dashed lines define zero current levels. B, Averaged steady-state I-V curves in the absence and presence of chloroquine at the indicated concentrations recorded in excised inside-out patches. C, Relative current-voltage relationships obtained for block of D172N Kir2.1 currents by chloroquine at the indicated concentrations. The continuous lines show fits with Woodhull equation, yielding an apparent Kd (at 0 mV) of 34 ± 11 µM with an apparent valence (z) of 1.6 ± 0.3 (n = 5 cells).
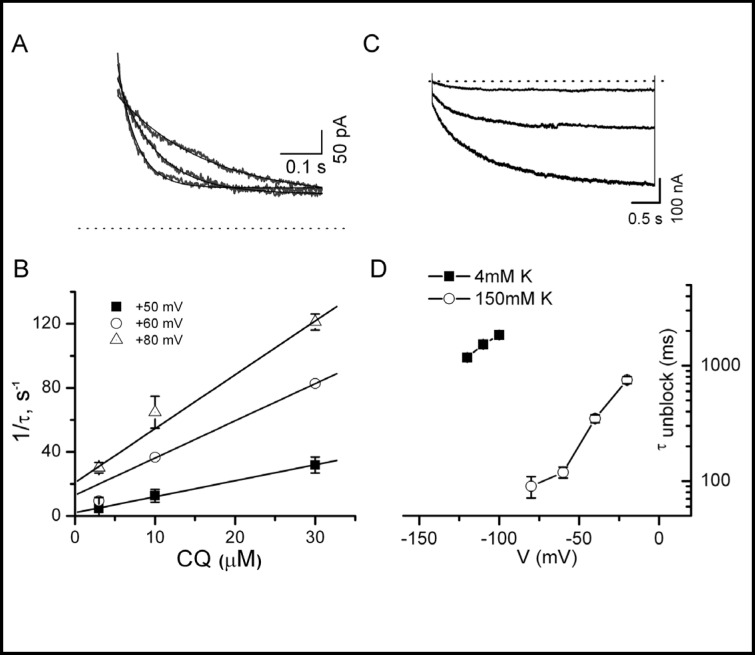
Next, we determined the kinetics of onset and recovery of chloroquine block on D172N Kir2.1. The onset of chloroquine block of D172N Kir2.1 was determined by fitting current traces in Fig. 3 to a monoexponential function and plotting the inverse values (1/τblock) versus chloroquine concentration (Fig. 4B). Representative current traces recorded in inside-out patches elicited by voltage steps to +80, +70 and +60 mV in presence of 10 µM chloroquine and the corresponding monoexponential fits to data are shown in Fig 4A. Like WT Kir2.1, the onset of chloroquine block of D172N was concentration- and voltage-dependent. At a concentration of 10 µM, 1/τblock at +60 mV for D172N Kir2.1 channels was 36 ± 1 s−1 compared to 42 s−1 for WT Kir2.1 [13]. The time constants of chloroquine unblock (τunblock) of D172N Kir2.1 were also measured in excised patches (symmetrical 150 mM K+) and in the whole-cell configuration (4mM external K+) at variable membrane potentials (Fig. 4C and D). Unblock of chloroquine at voltages negative to EK was voltage- and potassium-dependent with a time course similar to that for WT Kir2.1. For example, at 10 µM chloroquine in 4mM external K+, τunblock was 1.8 ± 0.1 s at −100 mV for D172N (n = 4 cells) compared to 1.5 s for WT Kir2.1 [13]. In summary, the kinetics of the onset of chloroquine block and unblock for D172N channels were not appreciably different than that reported for WT Kir2.1.
Fig. 4.
Onset of and recovery from chloroquine block of D172N Kir2.1 current is voltage-dependent. A, Representative current traces recorded in inside-out patches elicited by voltage steps to +80, +70 and +60 mV in presence of 10 µM chloroquine. Lines representing monoexponential fits to data are superimposed over raw current traces. B, The time constants of chloroquine block (τ) were derived by fitting current traces (as in A) to a monoexponential function. The rate constant of block (1/τ) is plotted vs. chloroquine concentration for various voltages. C. D172N current traces recorded in whole-cell configuration (4 mM external K+) elicited by hyper-polarizing voltage steps between −120 and −100 mV in the presence of 10 µM chloroquine. D. The time constants of chloroquine unblock (τ unblock) were determined by monoexponential fits to D172N Kir2.1 current elicited by hyperpolarizing voltage steps to membrane potentials negative to the reversal potential for potassium in 4 mM external K+ (whole-cell configuration) and 150 mM symmetrical K+ (excised inside-out patches). Data represent the mean ± SEM of 4 cells.
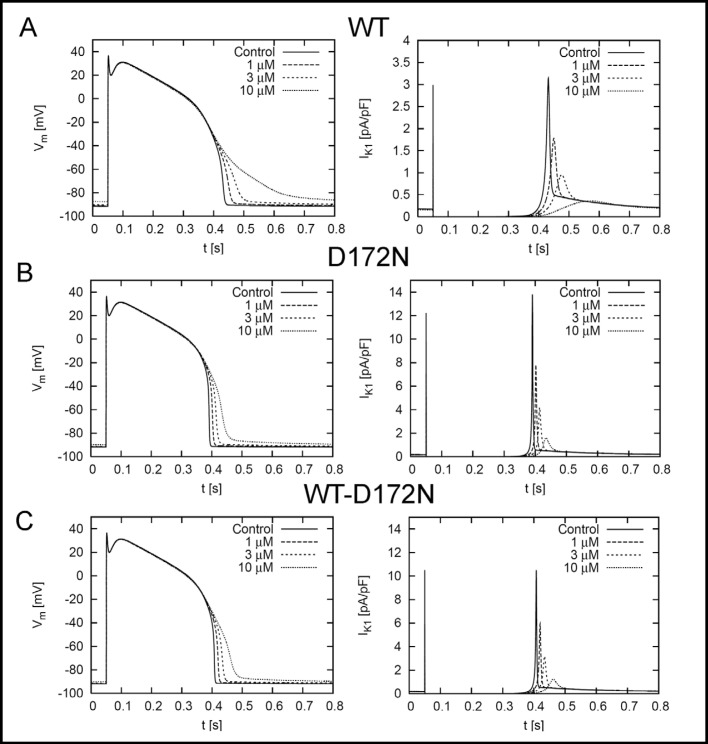
While the action potential voltage clamp protocol provides a reasonable estimate of Kir2.1 current elicited by a physiologically relevant condition (Fig. 1), the greater conductance of D172N and WT-D172N channels would be expected to shorten APD commensurate with the degree of altered rectification. Therefore, we used an in silico approach to further characterize the effects of chloroquine on APD and IK1 for WT and mutant Kir2.1 channels in a simulated human ventricular myocyte. The IK1 conditions were simulated by fits to the I-V relationships of the homozygous (D172N) and heterozygous (WT-D172N) mutant Kir2.1 channels (Fig. 2). Simulated membrane voltage (Vm) for an epicardial human left ventricular myocyte in response to 1 Hz pacing frequency is demonstrated in Fig. 5. Chloroquine caused a dose-dependent prolongation of APD90 and reduced the peak magnitude of IK1 for the WT Kir2.1 condition (Table 1). Under baseline conditions, APD90 was shorter for D172N and WT-D172N conditions (0.342 and 0.358 s, respectively) relative to WT (0.383 s), consistent with the greater conductance of the mutant Kir2.1 channels. The primary effect of the D172N mutation is an alteration in the degree of rectification, with an increase in current magnitude between −80 and −40 mV (Fig. 2). However, WT, D172N and WT-D172N Kir2.1 current fully rectifies at potentials > −20 mV (Fig. 2). Consistent with this observation, in silico modeling revealed that the D172N mutation did not appreciably influence the plateau phase, but exerted its effect predominantly on the terminal phase of repolarization. Therefore, ADP50 for D172N and WTD172N conditions were similar to WT values (0.331, 0.338 and 0.340 s, respectively; Table 1). Similar to WT, chloroquine caused a dose-dependent prolongation of APD90 and reduced the peak magnitude of IK1 for the D172N and WT-D172N simulations. Chloroquine also induced a dose-dependent prolongation in ERP for WT, D172N and WT-D172N conditions (Table 1). ERP was determined by applying extrastimuli at progressively shorter intervals, until the stimulus failed to generate an action potential. ERP is a measure of cellular excitability and defines the minimum time period allowable for propagation of premature stimuli. For the WT-D172N condition, 3 µM chloroquine prolonged ERP from 0.360 to 0.388 s, approaching the WT value at baseline (0.385 s).
Fig. 5.
Left panel, simulated transmembrane voltages of an epicardial myocyte for (A) WT, (B) D172N and (C) WT-D172N simulations at stimulation frequencies of 1 Hz under control conditions and the indicated chloroquine concentrations. The stimulus was applied at t = 50ms. Right panels, simulated IK1 elicited during action potential for WT, D172N and WT-D172N simulations at indicated chloroquine concentrations.
Table 1.
Electrophysiological properties of simulated WT, homozygous (D172N) and heterozygous (WT-D172N) ventricular myocytes. APD90 and APD50, action potential duration at 90% and 50% repolarization, respectively. [CQ], chloroquine concentration. ERP, effective refractory period.
| WT | D172N | WT/D172N | |
|---|---|---|---|
| APD90 (s) | |||
| [CQ] | |||
| 0 | 0.383 | 0.342 | 0.358 |
| 1 | 0.402 | 0.354 | 0.371 |
| 3 | 0.430 | 0.368 | 0.387 |
| 10 | 0.528 | 0.395 | 0.419 |
| APD50 (s) | |||
| [CQ] | |||
| 0 | 0.340 | 0.331 | 0.338 |
| 1 | 0.341 | 0.337 | 0.341 |
| 3 | 0.341 | 0.340 | 0.343 |
| 10 | 0.338 | 0.342 | 0.342 |
| Max IK1 (pA/pF) | |||
| [CQ] | |||
| 0 | 3.15 | 13.80 | 10.51 |
| 1 | 1.78 | 7.80 | 5.94 |
| 3 | 0.95 | 4.18 | 3.18 |
| 10 | 0.36 | 1.59 | 1.21 |
| ERP (s) | |||
| [CQ] | |||
| 0 | 0.385 | 0.340 | 0.360 |
| 1 | 0.405 | 0.355 | 0.373 |
| 3 | 0.425 | 0.370 | 0.388 |
| 10 | 0.377 | 0.393 | 0.415 |
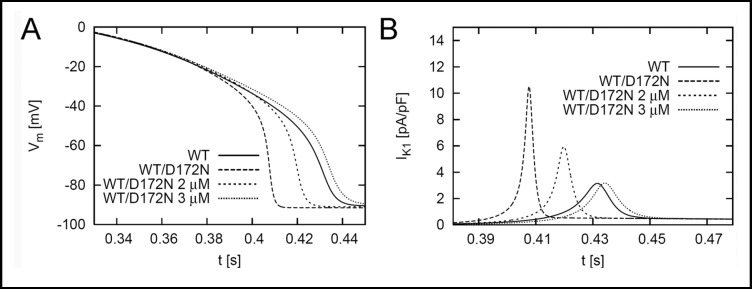
A direct comparison between the effects of chloroquine on the action potential properties of WT and the heterozygous mutant (WT-D172N) is presented in Fig. 6. Chloroquine (3 µM) prolonged WT-D172N APD toward that of WT and reduced IK1 to a similar magnitude as the WT baseline condition. Thus, our in silico modeling predicts that 3 µM chloroquine blocks sufficient IK1 to normalize APD in the heterozygous SQT3 condition and restore normal ventricular excitability, as measured by ERP.
Fig. 6.
Transmembrane voltages and IK1 for WT and heterozygous (WT-D172N) conditions. The effect of 2 and 3 µM chloroquine on WT-D172N is shown.
Discussion
SQTS is a sporadic or autosomal dominant disorder characterized by markedly accelerated cardiac repolarization, ventricular arrhythmias and sudden cardiac death. To date, mutations in 5 different ion channel genes (KCNH2, KCNQ1, KCNJ2, CACNA1C and CACNB2) have been identified to cause SQTS [9, 19, 20, 21]. The risk of ventricular arrhythmias and sudden death is remarkably high in SQTS with cardiac arrest reported as a presenting symptom in 31% of SQTS subjects [22]. While ICD therapy is currently recommended for primary prevention of sudden death, ICD placement is not always practical or technically feasible. Therefore, pharmacological therapy directed at the specific mutant channel underlying the various forms of SQTS is clinically important. To this end, we sought to characterize the blocking effect of chloroquine on D172N mutant channels.
Our data demonstrate that the potency for chloroquine block of WT is similar to that of D172N and WT-D172N Kir2.1 (IC50 1.4, 1.2 and 1.5 µM). These values are qualitatively similar to that reported by El Harchi and colleagues (2.5-3 µM) for WT and D172N Kir2.1 heterologously expressed in CHO cells [14]. We further characterized the biophysical properties of chloroquine block of D172N channels and determined that the rate of onset and recovery from chloroquine block were similar to that observed in WT Kir2.1. Moreover, the estimated distance that chloroquine penetrated the D172N conduction pathway was qualitatively similar to that of WT Kir2.1. These experiments are relevant given that mutations within the Kir2.1 ion pore can affect blocker energetics over the entire distance of the ion conduction pathway [23]. Our findings confirm that the blocking properties of chloroquine are similar between WT and D172N Kir2.1 channels.
Similar to data presented by Priori and colleagues [9], our in silico modeling predicts that the most terminal phase of repolarization is affected in the heterozygous state (WT-D172N) resulting in shortening of APD90, but not APD50. Moreover, our simulations predict that chloroquine produces a dose-dependent reduction in IK1 with resultant prolongation of APD90 and ERP. The simulations suggest that chloroquine concentrations ∼3 µM are sufficient to normalize APD90, peak IK1 and ERP. Interestingly, plasma concentrations are typically in the range of 2-3 µM following administration of therapeutic doses of chloroquine [24]. This range of concentrations would also be expected to affect IKr, although to a lesser degree than IK1 [2]. The combination of IK1 and IKr blockade might result in a greater degree of APD prolongation than that predicted by our simulations. Clearly, there are limitations inherent in this study which precludes the direct comparison of our experimental findings with the use of chloroquine for treatment of SQT3. However, our results and those of El Harchi and colleagues [14] provide a proof of principle foundation for a carefully controlled clinical trial.
Acknowledgements
The authors wish to thank Miguel Angel Flores-Virgen for technical assistance. This work was supported by a SEP-CONACYT (México) grant CB-2008-01-105941 (J.S-Ch), FOMIX (Mexico) No 2008-01-82948 (J.S-Ch) and NHLBI/NIH grant HL075536 (M.T-F). We gratefully acknowledge the support of the Richard A. and Nora Eccles Harrison Endowment and the Nora Eccles Treadwell Foundation.
References
- 1.Harris L, Downar E, Shaikh NA, Chen T. Antiarrhythmic potential of chloroquine: New use for an old drug. Can J Cardiol. 1988;4:295–300. [PubMed] [Google Scholar]
- 2.Sanchez-Chapula JA, Salinas-Stefanon E, Torres-Jacome J, Benavides-Haro DE, Navarro-Polanco RA. Blockade of currents by the antimalarial drug chloroquine in feline ventricular myocytes. J Pharmacol Exp Ther. 2001;297:437–445. [PubMed] [Google Scholar]
- 3.Benavides-Haro DE, Sanchez-Chapula JA. Chloroquine blocks the background potassium current in guinea pig atrial myocytes. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:311–318. doi: 10.1007/s002109900185. [DOI] [PubMed] [Google Scholar]
- 4.Bustos M, Gay F, Diquet B, Thomare P, Warot D. The pharmacokinetics and electrocardiographic effects of chloroquine in healthy subjects. Trop Med Parasitol. 1994;45:83–86. [PubMed] [Google Scholar]
- 5.Sowunmi A, Salako LA, Walker O, Ogundahunsi OA. Clinical efficacy of mefloquine in children suffering from chloroquine-resistant plasmodium falciparum malaria in nigeria. Trans R Soc Trop Med Hyg. 1990;84:761–764. doi: 10.1016/0035-9203(90)90067-o. [DOI] [PubMed] [Google Scholar]
- 6.Sanchez-Chapula JA, Navarro-Polanco RA, Culberson C, Chen J, Sanguinetti MC. Molecular determinants of voltage dependent herg k+ channel block. J Biol Chem. 2002;277:23587–23595. doi: 10.1074/jbc.M200448200. [DOI] [PubMed] [Google Scholar]
- 7.Plaster NM, Tawil R, Tristani-Firouzi M, Canun S, Bendahhou S, Tsunoda A, Donaldson MR, Iannaccone ST, Brunt E, Barohn R, Clark J, Deymeer F, George AL, Jr, Fish FA, Hahn A, Nitu A, Ozdemir C, Serdaroglu P, Subramony SH, Wolfe G, Fu YH, Ptacek LJ. Mutations in kir2.1 cause the developmental and episodic electrical phenotypes of andersen's syndrome. Cell. 2001;105:511–519. doi: 10.1016/s0092-8674(01)00342-7. [DOI] [PubMed] [Google Scholar]
- 8.Tristani-Firouzi M, Jensen JL, Donaldson MR, Sansone V, Meola G, Hahn A, Bendahhou S, Kwiecinski H, Fidzianska A, Plaster N, Fu YH, Ptacek LJ, Tawil R. Functional and clinical characterization of kcnj2 mutations associated with lqt7 (andersen syndrome) J Clin Invest. 2002;110:381–388. doi: 10.1172/JCI15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Priori SG, Pandit SV, Rivolta I, Berenfeld O, Ronchetti E, Dhamoon A, Napolitano C, Anumonwo J, di Barletta MR, Gudapakkam S, Bosi G, Stramba-Badiale M, Jalife J. A novel form of short qt syndrome (sqt3) is caused by a mutation in the kcnj2 gene. Circ Res. 2005;96:800–807. doi: 10.1161/01.RES.0000162101.76263.8c. [DOI] [PubMed] [Google Scholar]
- 10.Lu Z. Mechanism of rectification in inward-rectifier k+ channels. Annu Rev Physiol. 2004;66:103–129. doi: 10.1146/annurev.physiol.66.032102.150822. [DOI] [PubMed] [Google Scholar]
- 11.Borggrefe M, Wolpert C, Antzelevitch C, Veltmann C, Giustetto C, Gaita F, Schmipf R. Short qt syndrome. Genotype-phenotype correlations. J Electrocardiol. 2005;38:75–80. doi: 10.1016/j.jelectrocard.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brugada R, Hong K, Cordeiro JM, Dumaine R. Short qt syndrome. CMAJ. 2005;173:1349–1354. doi: 10.1503/cmaj.050596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodriguez-Menchaca AA, Navarro-Polanco RA, Ferrer-Villada T, Rupp J, Sachse FB, Tristani-Firouzi M, Sanchez-Chapula JA. The molecular basis of chloroquine block of the inward rectifier kir2.1 channel. Proc Natl Acad Sci U S A. 2008;105:1364–1368. doi: 10.1073/pnas.0708153105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El Harchi A, McPate MJ, Zhang YH, Hancox JC: Action potential clamp and chloroquine sensitivity of mutant kir2.1 channels responsible for variant 3 short qt syndrome. J Mol Cel Cardiol 2009. [DOI] [PMC free article] [PubMed]
- 15.Kubo Y, Murata Y. Control of rectification and permeation by two distinct sites after the second transmembrane region in kir2.1 k+ channel. J Physiol. 2001;531:645–660. doi: 10.1111/j.1469-7793.2001.0645h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 17.Iyer V, Mazhari R, Winslow RL. A computational model of the human left-ventricular epicardial myocyte. Biophys J. 2004;87:1507–1525. doi: 10.1529/biophysj.104.043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Press WH, Flannery BP, Teukolsky SA, Vetterling WT. 2nd edn. Cambridge: Cambridge University Press; 1992. Numerical Recipes in C -The Art of Scientific Computing. [Google Scholar]
- 19.Bellocq C, van Ginneken AC, Bezzina CR, Alders M, Escande D, Mannens MM, Baro I, Wilde AA. Mutation in the kcnq1 gene leading to the short qt-interval syndrome. Circulation. 2004;109:2394–2397. doi: 10.1161/01.CIR.0000130409.72142.FE. [DOI] [PubMed] [Google Scholar]
- 20.Brugada R, Hong K, Dumaine R, Cordeiro J, Gaita F, Borggrefe M, Menendez TM, Brugada J. Pollevick GD, Wolpert C, Burashnikov E, Matsuo K, Wu YS, Guerchicoff A, Bianchi F, Giustetto C, Schimpf R, Brugada P, Antzelevitch C. Sudden death associated with short-qt syndrome linked to mutations in herg. Circulation. 2004;109:30–35. doi: 10.1161/01.CIR.0000109482.92774.3A. [DOI] [PubMed] [Google Scholar]
- 21.Antzelevitch C, Pollevick GD, Cordeiro JM, Casis O, Sanguinetti MC, Aizawa Y, Guerchicoff A, Pfeiffer R, Oliva A, Wollnik B, Gelber P, Bonaros EP, Jr, Burashnikov E, Wu Y, Sargent JD, Schickel S, Oberheiden R, Bhatia A, Hsu LF, Haissaquerre M, Schimpf R, Borggrefe M, Wolpert C. Loss-of-function mutations in the cardiac calcium channel underlie a new clinical entity characterized by st-segment elevation, short qt intervals, and sudden cardiac death. Circulation. 2007;115:442–449. doi: 10.1161/CIRCULATIONAHA.106.668392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giustetto C, Di Monte F, Wolpert C, Borggrefe M, Schimpf R, Sbragia P, Leone G, Maury P, Anttonen O, Haissaquerre M, Gaita F. Short qt syndrome: Clinical findings and diagnostic-therapeutic implications. Eur Heart J. 2006;27:2440–2447. doi: 10.1093/eurheartj/ehl185. [DOI] [PubMed] [Google Scholar]
- 23.Robertson JL, Palmer LG, Roux B. Longpore electrostatics in inward-rectifier potassium channels. J Gen Physiol. 2008;132:613–632. doi: 10.1085/jgp.200810068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mzayek F, Deng H, Mather FJ, Wasilevich EC, Liu H, Hadi CM, Chansolme DH, Murphy HA, Melek BH, Tenaglia AN, Mushatt DM, Dreisbach AW, Lertora JJ, Krogstadt DJ. Randomized dose-ranging controlled trial of aq-13, a candidate antimalarial, and chloroquine in healthy volunteers. PLoS Clin Trials. 2007;2:e6. doi: 10.1371/journal.pctr.0020006. [DOI] [PMC free article] [PubMed] [Google Scholar]