Abstract
Background
Nesfatin-1, an 82 amino acid peptide derived from the prohormone nucleobindin-2 (NUCB2), is a novel satiety hormone acting through a leptin-independent mechanism in the hypothalamus. The mechanisms by which production of nesfatin-1/NUCB2 is regulated remain unknown.
Methods
Nesfatin-1/NUCB2 mRNA and immunoreactivity were examined in gastric tissue and Min-6 cells by RT-PCR and immunofluorescent staining or Western blotting.
Results
Nesfatin-1/NUCB2 is co-localized with pS6K1, the downstream target of mammalian target of rapamycin (mTOR), in gastric X/A like cells. A parallel relationship between gastric mTOR signaling and nesfatin-1/NUCB2 was observed during changes in energy status. Both mTOR activity and gastric nesfatin-1/NUCB2 were down-regulated by fasting, and returned to basal levels with re-feeding. In high fat diet induced obese mice, gastric mTOR signaling and nesfatin-1/NUCB2 were increased. Inhibition of the gastric mTOR signaling by rapamycin attenuated the expression of gastric nesfatin-1/NUCB2 mRNA and protein in both lean and obese mice. Attenuation of mTOR activity by rapamycin or over-expression of TSC1 or TSC2 reduced the expression of nesfatin-1/NUCB2 in Min-6 cells, suggesting a direct effect of mTOR signaling.
Conclusion
Gastric mTOR is a gastric energy sensor whose activity is linked to the regulation of gastric nesfatin-1/NUCB2.
Key Words: Gastric mTOR, Gastric X/A like cells, Gastric hormones, Nesfatin-1/NUCB2
Introduction
Nesfatin-1, an 82-amino-acid peptide derived from a 396-amino-acid precursor protein nucleobindin 2 (NUCB2), was originally identified in hypothalamic nuclei involved in the regulation of food intake such as the arcuate nucleus, lateral hypothalamus, paraventricular nucleus and supraoptic nucleus [1]. Subsequent studies revealed that nesfatin-1/NUCB2 is also distributed in peripheral tissues including the stomach, pancreatic islets, testis and adipose tissue [2, 3]. In gastric oxyntic mucosa, tenfold higher levels of nesfatin-1/NUCB2 mRNA have been detected relative to the brain [2]. At the cellular level, nesfatin-1/ NUCB2 localizes to ghrelin-containing X/A-like cells, within a pool of cytoplasmic vesicles distinct from those containing ghrelin [2]. Peripheral nesfatin-1 administrated by intravenous injection has been shown to be able to cross the blood-brain-barrier by a non-saturable mechanism [4, 5]. These observations, along with the detection of nesfatin-1/NUCB2 in the circulation, indicate that gastric mucosa is the main source of nesfatin-1/ NUCB2 in the circulation.
Nesfatin-1 inhibits dark phase food intake and induces a reduction in body weight and fat mass upon chronic administration [6]. Human studies indicate that fasting concentrations of nesfatin-1 are negatively correlated with body mass index in non-obese males [7], suggesting that energy status at the organism level may influence the expression and secretion of this peptide. This concept is further supported by the finding that fasting decreases circulating concentrations of nesfatin-1 [7, 8]. These observations suggest that nesfatin-1 is a critical gastrointestinal hormone involved in the regulation of energy homeostasis. Regulation of gastric nesfatin-1/ NUCB2 may therefore provide novel therapeutic strategies for treatment of obesity. A necessary precondition for this goal is an improved understanding of the mechanism by which synthesis of gastric nesfatin-1/NUCB2 is modulated.
Our previous studies demonstrated that gastric mammalian target of rapamycin (mTOR) is altered by changes in energy status such as fasting and obesity, and that alteration in gastric mTOR activity affects the expression and secretion of ghrelin [9]. Since both nesfatin-1/NUCB2 and ghrelin are mainly expressed in X/A like cells in the gastric mucosa, we hypothesized that gastric mTOR regulates nesfatin-1/NUCB2 expression. Here, we present evidence that the mTOR signaling molecule pS6K co-localizes in nesfatin-1/ NUCB2 expressing cells in the gastric oxyntic mucosa and that alteration in mTOR activity is linked to nesfatin-1/NUCB2 production.
Materials and Methods
Materials
Phospho-mTOR (Ser2448) rabbit polyclonal antibody (species cross-reactivity: human, mouse and rat), phospho-S6K1 (Thr389) mouse monoclonal antibody (species cross-reactivity: human, mouse and rat) and rabbit anti-phospho-S6 (ser235, 236) (species cross-reactivity: human, mouse and rat) were from Cell Signaling Technology (Beverly, MA). Rabbit anti-nesfatin-1 (species cross-reactivity: human, mouse and rat) was purchased from Phoenix Pharmaceuticals Inc. (Burlingame, CA). Mouse anti-β-actin antibody (species cross-reactivity: human, mouse, rat, dog and monkey), chicken anti-rabbit fluorescein isothiocyanate-conjugated IgG, and goat antimouse Texas Red-conjugated IgG and rapamycin were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Dimethylsulfoxide was from Sigma Chemical Co. (St. Louis, MO). Aprotinin was purchased from Amersham Biosciences (Pittsburgh, PA). IRDye-conjugated affinity purified anti-rabbit, anti-mouse IgGs were purchased from Rockland (Gilbertsville, PA). Trizol reagent and the reverse transcription (RT) system were from Promega Inc. (Madison, WI).
Ethical approval
The animals used in this study were handled in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication no. 85-23, revised 1996), and all the experimental protocols were approved by the Animal Care and Use Committee of Peking University.
Animals
Ten to twelve week old male C57BL/6J mice were housed in standard plastic rodent cages and maintained at a regulated environment (24°C, 12:12 hour light:dark cycle with lights on at 7:00 AM). Regular chow and water were available ad libitum. Where indicated, 3-wk-old mice were assigned to receive standard laboratory chow, or a high-fat diet (60% fat, D12451; Research Diets, New Brunswick, NJ) for 12 weeks.
Cell culture
Min-6 cells, a mouse pancreatic islet cell line, were maintained in high-glucose Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% FBS (U.S. Biotechnologies) and 100 units/ml penicillin and 100 units/ml streptomycin (Invitrogen). Cells were passaged weekly after trypsin-EDTA detachment. All studies were performed on Min-6 cells at passages 20-25.
For transient transfection, Min-6 cells were seeded at a density of 1 × 105 cells per well in 12-well plates for 24 h. Cells were then transfected with one of the following constructs: human tuberous sclerosis complex 1 (TSC1, 500ng), rat TSC2 (500ng), and EGFP (500ng) per well using Lipofectamine reagent according to the manufacturer's instruction. Cells were harvested 48 h later and lysed in 100 µl Passive Lysis Buffer. Cell lysates were analyzed by Western blot.
RNA extraction and quantitative real-time PCR analysis
Gastric total RNA was isolated using the trizol reagent. RT was performed using the RT system according to the manufacturer's instruction. PCR was conducted in a 25µL volume containing 2.5µL cDNA, 5 mM MgCl2, 0.2 mM dNTPs, 0.2 µM each primer, 1.25 U AmpliTaq Polymerase and 1µL 800 × diluted SYBRGreen I stock using the Mx3000 multiplex quantitative PCR system (Stratagene, La Jolla, CA). Primers used in this study were:
Mouse nesfatin-1/NUCB2 (accession no. NM 50132)
Sense: 5'-GGA AGA CTG CGG ATG CTC AT-3’ and
Antisense: 5'-ACT CTC TCC GCT CGT GTT CC-3’,
Mouse glyceraldehyde 3-phosphate dehydrogenase (GAPDH (accession no. NM32599)
Sense: 5'-ATG ACA TCA AGA AGG TGG TG-3’ and
Antisense 5'-CAT ACC AGG AAA TGA GCT TG-3'. The r2 value of the primers for nesfatin-1/NUCB2 and GAPDH was 0.9901 and 0.9908 respectively.
mRNA expression was quantified using the comparative cross threshold (CT) method. The CT value of the housekeeping gene GAPDH) was subtracted from the CT value of the target gene to obtain ΔCT. The normalized fold changes of nesfatin-1/NUCB2 mRNA expression were expressed as 2-ΔΔCT, where ΔΔCT equals to ΔCT sample-ΔCT control.
Western blot analysis
Gastric tissue was quickly harvested, rinsed thoroughly with PBS, and then homogenized on ice in lysis buffer (50 mM Tris-HCl; 15 mM EGTA; 100 mM NaCl; 0.1% Triton X-100 supplemented with protease inhibitor cocktail, pH 7.5). After centrifugation for 10 min at 4 °C, the supernatant was used for Western blot analysis. Protein concentration was measured by Bradford's method. A total of 80 µg protein from each sample was loaded onto SDS-PAGE gels. Proteins were transferred to polyvinylidene fluoride membranes. The membranes were incubated for 1 h at room temperature with 5% fat-free milk in Tris buffered saline containing Tween-20, followed by incubation overnight at 4°C with the individual primary antibody. Specific reaction was detected using IRDye-conjugated second antibody and visualized using the Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE). Quantification of image density in pixel was performed by using the Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE).
Double-labeling immunohistochemistry
C57BL/6J mice were deeply anesthetized using sodium pentobarbital (40mg/kg, ip), perfused transcardially with 20 ml 0.1 M PBS (pH 7.4), followed by 20 ml 4% paraformaldehyde in PBS. The whole stomach was quickly removed and opened along the lesser curvature and rinsed thoroughly with PBS to remove attached debris. The tissues were post-fixed in 4% paraformaldehyde, dehydrated, embedded in wax, and sectioned at 6 µm. Paraffin-embedded sections were dewaxed, rehydrated, and rinsed in PBS. After boiling for 10 min in 0.01 mol/L sodium citrate buffer (pH 6.0), sections were blocked in 5% goat serum in PBS for 1 h at room temperature. Primary antibodies used were mouse anti-pS6K (Thr389) (1:50), rabbit anti-pS6 (ser235, Ser236) (1:100). Slides were incubated with primary antibody overnight at 4°C, then washed in 1X PBS/0.1% Tween-20 three times for 5 minutes each. Secondary antibodies used included chicken anti-rabbit fluorescein isothiocyanate-conjugated IgG (1:100) and goat anti-mouse Texas Red-conjugated IgG (1:100). Gastric tissues were incubated with secondary antibody for 2 hours at room temperature. Controls included substituting primary antibodies with relevant IgGs. Fluorescent signals were observed and photomicrographs taken under a confocal laser-scanning microscope (Leica, Germany).
Statistical analysis
All values are expressed as mean ±SEM. Statistical differences were evaluated by two-way ANOVA and Newman-Student-Keuls test. Comparisons between two groups involved use of the Student's t test using GraphPad Prism software. P value < 0.05 denotes statistical significance. Pearson's correlation analysis was performed to determine the strength of the linear relationship between gastric nesfatin-1/NUCB2 and phosphorylation of S6 induced by different energy status.
Results
Localization of mTOR signaling molecules in gastric mucosa
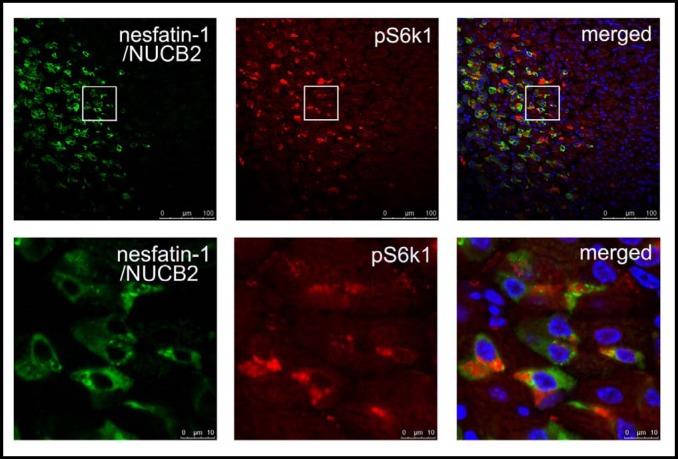
To determine whether mTOR signaling molecules are expressed in gastric nesfatin-1/NUCB2 expressing cells, immunofluorescent staining was used to localize pS6K1 (Thr389), the downstream target of mTOR, and nesfatin-1/NUCB2 in mouse gastric mucosa. Antibody recognizing pS6K1 (Thr389) demonstrated strong positive reactivity in cells also reactive for nesfatin-1/ NUCB2 in the lower 1/3 of the mucosa of the gastric fundus (Fig. 1).
Fig. 1.
Co-localization of pS6K1 with nesfatin-1/NUCB2 in the gastric oxyntic mucosa. Nesfatin-1/NUCB2 is shown in green (left panel), pS6K1 in red (middle panel), merged images in the right panel. Nuclei are counterstained with Hochest (blue).
Reciprocal effects of fasting and obesity on gastric mTOR signaling and nesfatin-1/NUCB2 expression
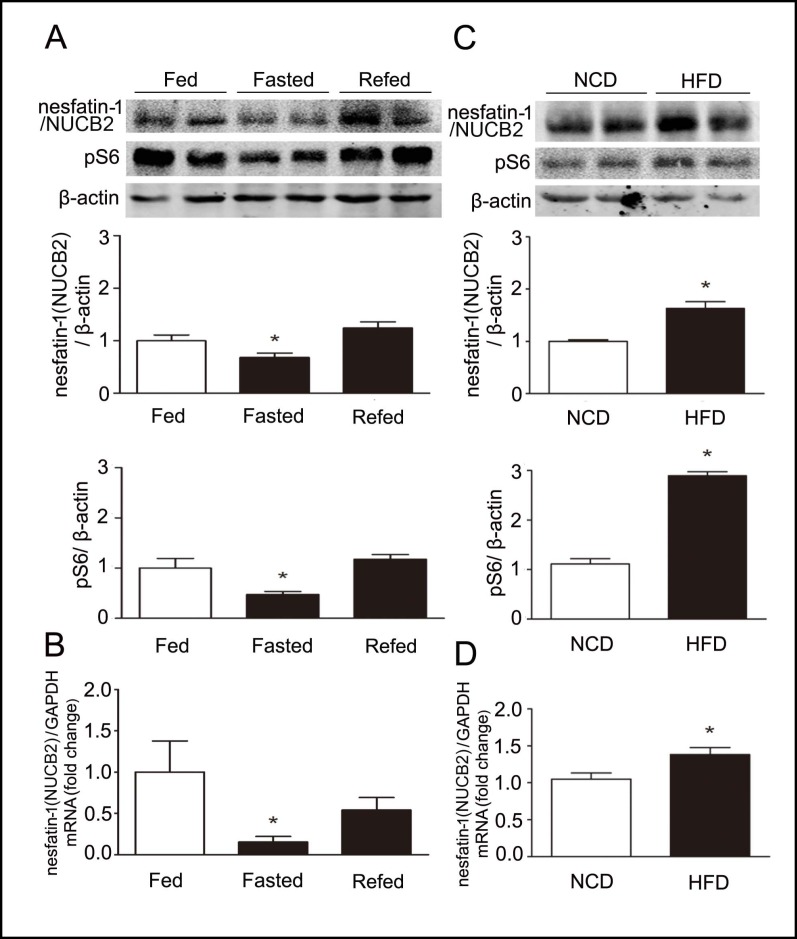
To examine the effect of fasting on mTOR signaling and nesfatin-1/NUCB2, 10-12 week old male C57BL/6J mice were divided into three groups (n=6 for each group), a control group in which animals were fed ad libitum, a fasting group in which mice were fasted for 24h, and a re-fed group in which mice were fasted for 24 h and refed for 2 h. Phosphorylation of S6 protein, a downstream target of mTOR in gastric fundic mucosa, was decreased in fasted mice relative to fed animals (Fig. 2A). Gastric nesfatin-1/NUCB2 mRNA (Fig. 2B) and protein (Fig. 2A) levels were significantly down-regulated in fasted mice relative to control animals. Phosphorylated S6 and gastric nesfatin-1/NUCB2 mRNA and protein levels returned to basal with re-feeding. A significant positive correlation was found between gastric levels of phospho-S6 and nesfatin-1/NUCB2 protein (Pearson's R=0.73, P=0.017).
Fig. 2.
Parallel relationship between gastric mTOR signaling and expression of nesfatin-1/NUCB2 in fasting and obesity. A. Representative Western blots from fed, fasted or refed mice. Gastric nesfatin-1/NUCB2 and phospho-S6K (pS6) were blotted as described in the methods. β-actin was used as loading control. Quantification of image analysis of gastric nesfatin-1/NUCB2 and pS6 was expressed as mean±SEM. B. Results of quantitative PCR analysis of nesfatin-1/NUCB2 were expressed as fold increase from fed condition using GAPDH as loading control. Six stomachs were examined for each condition. *P<0.05 versus mice in the fed condition. C. Representative Western blots from lean (normal chow diet, NCD) or high fat diet (HFD) induced obese mice. Gastric nesfatin-1/NUCB2 and pS6 were examined. β-actin was used as loading control. Quantification of image analysis of gastric nesfatin-1/ NUCB2 and pS6 was expressed as mean±SEM. D. Results of quantitative PCR analysis of nesfatin-1/NUCB2 expression were expressed as fold increase from lean mice using GAPDH as loading control. Six stomachs were examined for each condition. *denotes P<0.05 versus lean mice.
The effects of long-term changes in nutritional status on gastric mTOR signaling and nesfatin-1/NUCB2 expression were examined using C57BL/6J male mice in which obesity had been induced by high fat diet. As shown in Fig. 2C, there were significant increases in gastric pS6 levels in high fat diet induced obese mice relative to lean animals. Similar to changes in mTOR signaling, increases in gastric nesfatin-1/NUCB2 mRNA (Fig. 2D) and protein (Fig. 2C) were observed in obese mice relative to the lean animals. A significant positive correlation was found between the gastric nesfatin-1/NUCB2 and phosphorylation levels of S6 (Pearson's R=0.84, P<0.01).
Effect of rapamycin on gastric nesfatin-1/NUCB2
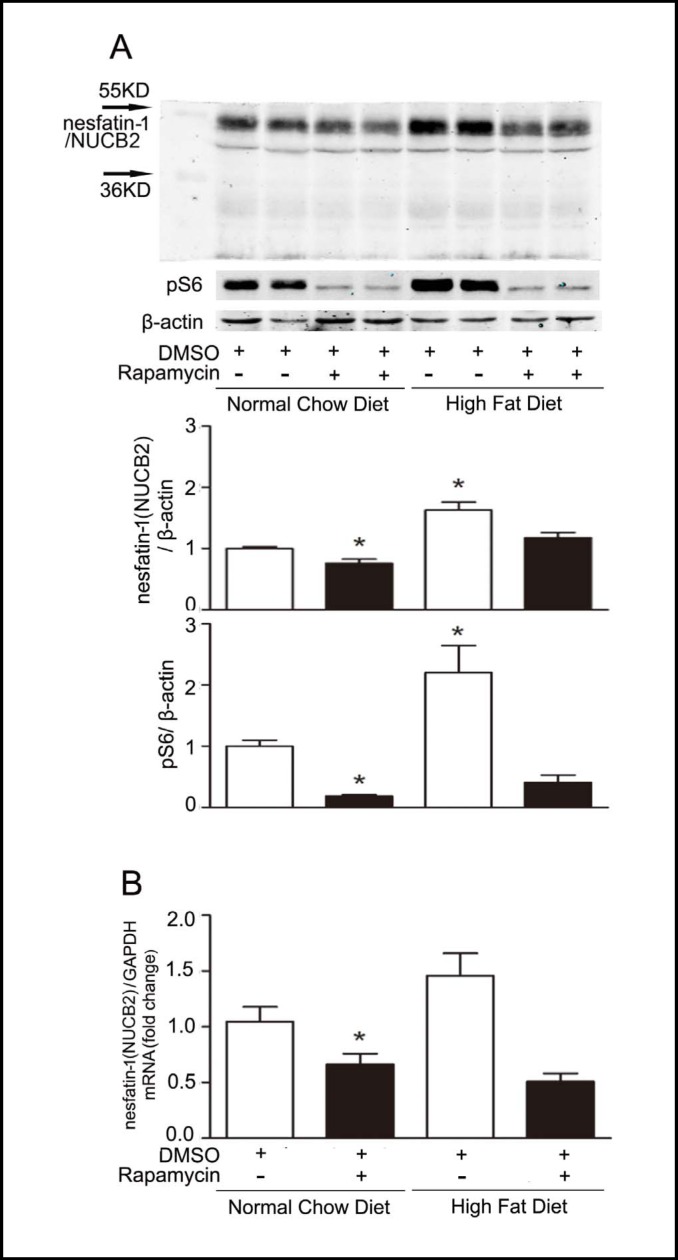
The effects of rapamycin, a well-characterized mTOR inhibitor, were examined in C57BL/6J mice. Ten-twelve week old male C57BL/6J mice were divided into two groups: control dimethylsulfoxide (DMSO) intraperitoneal (ip) injection or rapamycin (1 mg/kg) injection ip for 6 days (n=6 for each group). As shown in Fig. 3A, intraperitoneal injection of rapamycin significantly decreased gastric pS6 (Ser235/236) levels, a marker of mTOR activity. The decrease in gastric mTOR signaling in mice treated with rapamycin was associated with a significant decrease in gastric nesfatin-1/NUCB2 mRNA (Fig. 3B) and protein (Fig. 3A) levels compared with control mice.
Fig. 3.
Rapamycin regulates gastric mTOR signaling and nesfatin-1/NUCB2 expression. A. Representative Western blots from lean and HFD-induced obese C57BL/6J mice that received intraperitoneal injection of DMSO or rapamycin (1mg/kg). Nesfatin-1/NUCB2 and pS6 in gastric mucosa were detected using specific antibodies. β-actin was used as loading control. Quantification of image analysis of gastric nesfatin-1/NUCB2 and pS6 was expressed as mean ±SEM. B. Results of quantitative PCR analysis of nesfatin-1/NUCB2 were expressed as fold increase from control using GAPDH as loading control. Six stomachs were examined for each condition. *P<0.05 versus lean mice receiving DMSO.
The effect of rapamycin on the expression of gastric nesfatin-1/NUCB2 was next investigated in the model of high fat diet-induced obesity. As shown in Fig. 3A, intraperitoneal injection of rapamycin significantly inhibited gastric pS6 (Ser235/236) levels in obese mice. The decrement in the gastric mTOR signaling was accompanied by significant down-regulation of gastric nesfatin-1/NUCB2 mRNA (Fig. 3B) and protein (Fig. 3A) levels in obese mice treated with rapamycin.
In vitro effects of mTOR signaling on nesfatin-1/NUCB2 production
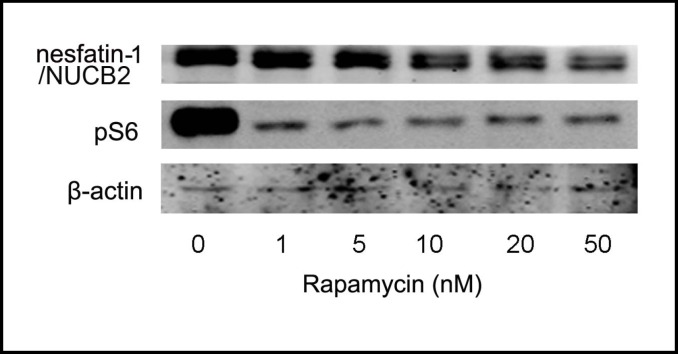
To determine whether mTOR signaling directly affects the expression of nesfatin-1/NUCB2, we first examined the effect of rapamycin using Min-6 cells in which high levels of nesfatin-1/NUCB2 expression were detected. Consistent with in vivo studies, rapamycin significantly attenuated mTOR signaling in Min-6 cells as demonstrated by a decrease in phosphorylation of S6 ribosome protein. This was accompanied by a significant decrease in the expression of nesfatin-1/NUCB2. Rapamycin reduced the expression of nesfatin-1 in a concentration-dependent manner (Fig. 4).
Fig. 4.
Rapamycin regulates mTOR signaling and nesfatin-1/ NUCB2 expression in vitro. Representative Western blot of nesfatin-1/NUCB2, pS6 and β-actin in Min-6 cells exposed to varying concentrations of rapamycin. Experiments were repeated for four times.
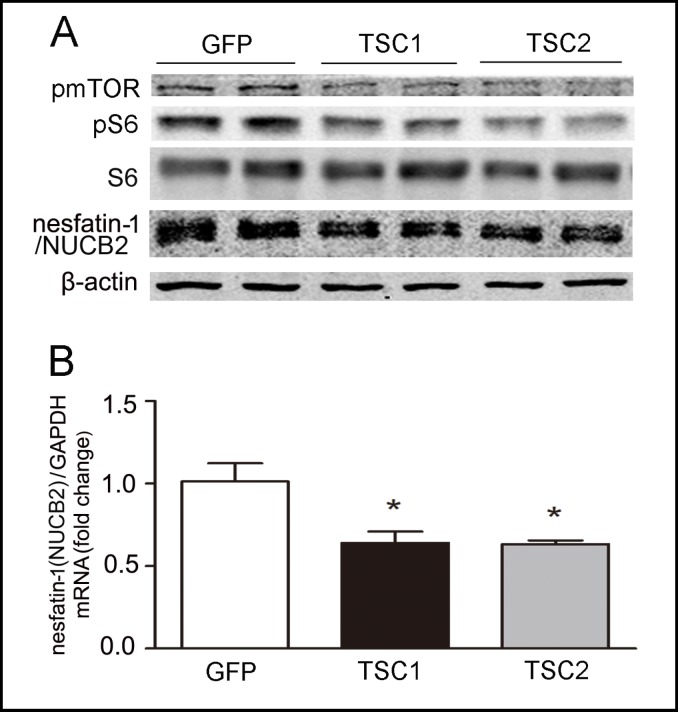
To further confirm the effect of mTOR signaling on nesfatin-1/NUCB2 expression, we inhibited mTOR activity by overexpression of its negative regulators: the tuberous sclerosis complex (TSC) gene products TSC1 and TSC2. TSC1 and TSC2 are tumor suppressor genes that are mutated in the disease TSC. TSC1 and TSC2 form a tight complex in the cell to negatively regulate mTOR activity, and the presence of both proteins is required for the TSC1/2 heterodimer to function [10].
As shown in Fig. 5, overexpression of TSC1 or TSC2 significantly decreased nesfatin-1/NUCB2 expression at mRNA (Fig. 5B) and protein (Fig. 5 A) levels. Both TSC1 and TSC2 function to inhibit mTOR signaling (Fig. 5 A).
Fig. 5.
TSC1 and TSC2 regulated mTOR signaling and nesfatin-1/NUCB2. Effects of TSC1 and TSC2 on mTOR signaling and nesfatin-1/NUCB2 expression were examined in cultured Min-6 cells. Cells were transfected with 500ng of one of the following plasmids: EGFP, human TSC1 (Myc-TSC1), rat TSC2 (HATSC2). A. Representative Western blots of nesfatin-1/NUCB2, pmTOR, and pS6 were showed. Results were repeated four times. B. Results of quantitative PCR analysis of nesfatin-1/ NUCB2 were expressed as fold increase from control using GAPDH as loading control. *P<0.05 versus control.
Discussion
The major finding of the current study is that mTOR signaling is present in gastric endocrine cells expressing nesfatin-1/NUCB2 and that the mTOR signaling pathway regulates the expression of this hormone. This conclusion is supported by the following distinct observations: 1) the mTOR signaling molecule pS6K1 co-localizes with nesfatin-1/NUCB2 immunoreactivity in gastric mucosa; 2) there exists a parallel relationship between gastric mTOR activity and expression of nesfatin-1/NUCB2 during changes in energy status such as fasting and obesity; 3) rapamycin inhibits gastric mTOR signaling which is associated with a significant decrease in the expression of nesfatin-1/NUCB2 in both lean and obese mice; 4) inhibition of mTOR signaling in Min-6 cells by treatment with rapamycin or overexpression of either TSC1 or TSC2 significantly attenuates the expression of nesfatin-1/NUCB2.
Nesfatin-1 is a potent anorexigenic peptide hormone whose primary sites of action are believed to be the hypothalamus and the hindbrain. Administration of nesfatin-1 into the third and fourth brain ventricles induces an anorexigenic response in rodents [6]. The underlying mechanisms of nesfatin-1 satiety effects are independent of leptin [11, 12]. Several pathways involved in the regulation of food intake have been reported to be influenced by nesfatin-1, including activation of the anorexigenic CRF receptor 2 [6], melanocortin 3/4 [1] and oxytocin signaling pathways [12, 13], and suppression of the neuropeptide Y orexigenic pathway [14]. The differential action sites, along with lack of information on the nesfatin-1 receptor, pose a significant challenge to defining a therapeutic strategy for obesity targeting the actions of nesfatin-1. An alternative approach is to target the production of nesfatin-1/NUCB2. To this end, it is critical to determine the primary source of nesfatin-1 and the molecular mechanisms by which production of nesfatin-1/NUCB2 is regulated.
Nesfatin-1, an 82 amino acid peptide with a predicted molecular mass of 9.7 kDa, was originally detected in the hypothalamus [1]. However, subsequent studies failed to detect mature peptide in protein extracts from the hypothalamus [15]. Instead, nesfatin-1 is present in cerebrospinal fluid [1]. These findings suggest a peripheral source of central nesfatin-1. This concept is supported by the work of Stengel et al. in which nesfatin-1 mRNA, protein and immunoreactivity were detected in the gastric mucosa [2]. Interestingly, the expression level of nesfatin-1/NUCB2 in the stomach was found to be 10 fold higher relative to levels of the brain [2]. These findings, together with the demonstration that peripheral nesfatin-1 administrated by intravenous injection has been shown to be able to cross the blood-brain-barrier by a non-saturable mechanism [4, 5], suggest that gastric nesfatin-1 may be the main source of central nesfatin-1.
Although studies of the peripheral effects of nesfatin-1 on food intake have been less consistent, one investigation reported that nesfatin-1 as well as nesfatin-124–53 reduced the dark-phase food intake after intaperitoneal injection in ad libitum-fed mice through leptin-independent mechanisms [11]. The peripheral effects of nesfatin-1 on food intake are likely mediated via the vagus nerve. In vitro studies showed that nesfatin-1 activates Ca2+ influx in primary cultured nodose ganglion neurons from mice, while mice pretreated with capsaicin demonstrated no reduction in food intake to intraperitoneal injection of nefatin-1 24-53 [16].
In the gastric mucosa, nesfatin-1/NUCB2 immuno-reactivity has been reported to be present in endocrine cells in the basal 1/3 of the gastric epithelia [2]. Our data also demonstrate an expression pattern of nesfatin-1/ NUCB2 in the gastric oxyntic mucosa. At the cellular level, nesfatin-1/NUCB2 has been detected mainly in ghrelin-expressing X/A like cells, while a small proportion of nesfatin-1/NUCB2 expressing cells at the basal 1/3 of the oxyntic glands coexpress somatostain or histidine decarboxylase [2]. Ghrelin-expressing X/A like cells may therefore be the primary locus of nesfatin-1 production. We have previously reported that the presence of mTOR signaling molecules in ghrelin-expressing X/A like cells [9]. Consistent with this observation, our present study demonstrates the co-localization of nesfatin-1/NUCB2 and pS6K1 immunoreactivity in the gastric oxyntic mucosa.
Extensive evidence demonstrates that mTOR signaling is a critical mechanism linking energy status at the organism level with cellular activities such as protein expression, cell survival and proliferation. mTOR activity is linked to the development of cancer, diabetes, and obesity. Significant elevation of mTOR signaling is observed in liver and skeletal muscle of insulin-resistant obese rats maintained on a high-fat diet. In contrast, absence of ribosomal protein S6 kinase 1 protects against diet induced obesity and improves insulin sensitivity in mice [17]. Our previous and present studies provide further evidence supporting the concept that mTOR signaling may be involved in the modulation of energy metabolism by selectively regulating the production of gastric hormones [9]. It is worth noting that differential regulation of ghrelin and nesfatin-1/NUCB2 occurs upon inhibition of gastric mTOR signaling. While inhibition of gastric mTOR signaling stimulates the production of ghrelin, it decreases the expression of nesfatin-1/NUCB2 in X/A like cells. The selective action of mTOR signaling on the expression of ghrelin and nesfatin-1/NUCB2 indicates a specific interaction of this signaling pathway with the orexigenic mechanisms recruited during alteration in energy supply. Since our previous study demonstrated that gastric mTOR signaling is reciprocally linked with the energy status at the organism level, we propose that decrease in the energy supply during fasting leads to the inhibition of gastric mTOR which functions to restore the energy balance by increasing the production of ghrelin and reducing the nesfatin-1/NUCB2 expression.
In summary, our study demonstrates that gastric nesfatin-1/NUCB2 is regulated by mTOR. Gastric mTOR signaling selectively affects the production of nesfatin-1/ NUCB2 and ghrelin. These observations suggest the existence of a fuel-sensing pathway within gastric X/A like endocrine cells. Gastric mTOR may provide a potential target for the development of novel therapeutics for obesity.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (81030012, 30890043, 30971434, 30821001, 81170795 and 30971085), the Major National Basic Research Program of P. R. China (No. 2010CB912504), the Program for New Century Excellent Talents in University (NCET-10-0183), and National Institute of Health grant (RO1DK043225)
References
- 1.Oh IS, Shimizu H, Satoh T, Okada S, Adachi S, Inoue K, Eguchi H, Yamamoto M, Imaki T, Hashimoto K, Tsuchiya T, Monden T, Horiguchi K, Yamada M, Mori M. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature. 2006;443:709–712. doi: 10.1038/nature05162. [DOI] [PubMed] [Google Scholar]
- 2.Stengel A, Goebel M, Yakubov I, Wang L, Witcher D, Coskun T, Tache Y, Sachs G, Lambrecht NW. Identification and characterization of nesfatin-1 immunoreactivity in endocrine cell types of the rat gastric oxyntic mucosa. Endocrinology. 2009;150:232–238. doi: 10.1210/en.2008-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang AQ, Li XL, Jiang CY, Lin L, Shi RH, Chen JD, Oomura Y. Expression of nesfatin-1/NUCB2 in rodent digestive system. World J Gastroenterol. 2010;16:1735–1741. doi: 10.3748/wjg.v16.i14.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan W, Hsuchou H, Kastin AJ. Nesfatin-1 crosses the blood-brain barrier without saturation. Peptides. 2007;28:2223–2228. doi: 10.1016/j.peptides.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Price TO, Samson WK, Niehoff ML, Banks WA. Permeability of the blood-brain barrier to a novel satiety molecule nesfatin-1. Peptides. 2007;28:2372–2381. doi: 10.1016/j.peptides.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 6.Stengel A, Goebel M, Wang L, Rivier J, Kobelt P, Monnikes H, Lambrecht NW, Tache Y. Central nesfatin-1 reduces dark-phase food intake and gastric emptying in rats: differential role of corticotropin-releasing factor2 receptor. Endocrinology. 2009;150:4911–4919. doi: 10.1210/en.2009-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsuchiya T, Shimizu H, Yamada M, Osaki A, Oh IS, Ariyama Y, Takahashi H, Okada S, Hashimoto K, Satoh T, Kojima M, Mori M. Fasting concentrations of nesfatin-1 are negatively correlated with body mass index in non-obese males. Clin Endocrinol (Oxf) 2010;73:484–490. doi: 10.1111/j.1365-2265.2010.03835.x. [DOI] [PubMed] [Google Scholar]
- 8.Ogiso K, Asakawa A, Amitani H, Nakahara T, Ushikai M, Haruta I, Koyama K, Amitani M, Harada T, Yasuhara D, Inui A. Plasma nesfatin-1 concentrations in restricting-type anorexia nervosa. Peptides. 2011;32:150–153. doi: 10.1016/j.peptides.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Xu G, Li Y, An W, Li S, Guan Y, Wang N, Tang C, Wang X, Zhu Y, Li X, Mulholland MW, Zhang W. Gastric mammalian target of rapamycin signaling regulates ghrelin production and food intake. Endocrinology. 2009;150:3637–3644. doi: 10.1210/en.2009-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brugarolas J, Lei K, Hurley RL, Manning BD, Reiling JH, Hafen E, Witters LA, Ellisen LW, Kaelin WG., Jr Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 2004;18:2893–2904. doi: 10.1101/gad.1256804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimizu H, Oh IS, Hashimoto K, Nakata M, Yamamoto S, Yoshida N, Eguchi H, Kato I, Inoue K, Satoh T, Okada S, Yamada M, Yada T, Mori M. Peripheral administration of nesfatin-1 reduces food intake in mice: the leptin-independent mechanism. Endocrinology. 2009;150:662–671. doi: 10.1210/en.2008-0598. [DOI] [PubMed] [Google Scholar]
- 12.Maejima Y, Sedbazar U, Suyama S, Kohno D, Onaka T, Takano E, Yoshida N, Koike M, Uchiyama Y, Fujiwara K, Yashiro T, Horvath TL, Dietrich MO, Tanaka S, Dezaki K, Oh IS, Hashimoto K, Shimizu H, Nakata M, Mori M, Yada T. Nesfatin-1-regulated oxytocinergic signaling in the paraventricular nucleus causes anorexia through a leptin-independent melanocortin pathway. Cell Metab. 2009;10:355–365. doi: 10.1016/j.cmet.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Yosten GL, Samson WK. The anorexigenic and hypertensive effects of nesfatin-1 are reversed by pretreatment with an oxytocin receptor antagonist. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1642–1647. doi: 10.1152/ajpregu.00804.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Price CJ, Samson WK, Ferguson AV. Nesfatin-1 inhibits NPY neurons in the arcuate nucleus. Brain Res. 2008;1230:99–106. doi: 10.1016/j.brainres.2008.06.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stengel A, Taché Y. Nesfatin-1-an emerging new player in the brain-gut, endocrine, and metabolic axis. Endocrinology. 2011;152:4033–4038. doi: 10.1210/en.2011-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwasaki Y, Nakabayashi H, Kakei M, Shimizu H, Mori M, Yada T. Nesfatin-1 evokes Ca2+ signaling in isolated vagal afferent neurons via Ca2+ influx through N-type channels. Biochem Biophys Res Commun. 2009;390:958–962. doi: 10.1016/j.bbrc.2009.10.085. [DOI] [PubMed] [Google Scholar]
- 17.Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, Thomas G. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]