Abstract
Curcumin, a natural polyphenol in the spice turmeric, has been found to exhibit anticancer activity. Although curcumin is generally considered an antioxidant, it is also able to elicit apoptosis through the generation of ROS, thereby functioning as a pro-oxidant in cancer cells. The present study investigated the effects of antioxidant pretreatment on curcumin-induced cytotoxicity in the human cancer cell lines A2780, MCF-7, and MDA-MB-231. Cytotoxicity was enhanced by trolox, vitamin C or vitamin E; trolox, a water soluble vitamin E derivative, was the most potent. The combination of curcumin (10 μM) and trolox (10-50 μM) induced apoptosis of cancer cells as evidenced by PARP cleavage and caspase-3 activation. Furthermore, expression of the pro-apoptotic protein Bad was up-regulated and expression of the anti-apoptotic proteins Bcl-2 and Bcl-xl was down-regulated in cells that had been treated with trolox plus curcumin. ROS generation was detected in curcumin-treated cells and was significantly enhanced when cells were treated with trolox plus curcumin. Exogenous catalase or SOD1 did not alter cytotoxicity, while over-expression of either catalase or SOD1 did, pointing to the importance of intracellular hydrogen peroxide generation in cell killing. In conclusion, we demonstrated for the first time that antioxidants such as trolox can potentiate cancer cell killing by curcumin, a finding which may help in the development of novel drug combination therapies.
Key Words: Curcumin, Trolox, Anti-oxidant, Oxidative stress, Apoptosis
Introduction
Curcumin (diferuloylmethane), a natural polyphenol extracted from the spice turmeric, has been used widely in Asian countries as an alternative medicine [1]. This compound has been demonstrated to have anticancer activity in cell culture and animal model systems [1, 2, 3, 4]. It is considered relatively safe in humans as reported from many studies, and doses of up to 8 g per day are well tolerated. Curcumin has been tested in clinical trials [5, 6].
The mechanisms of curcumin's anticancer properties have been extensively studied. Several mechanisms were proposed, including its inhibition of NF-κB [7, 8], AP-1 [9] and COX-2 [10] signaling, and induction of DNA damage [11]. It is believed that curcumin could act as either antioxidant or pro-oxidant, depending on the concentrations used and the experimental circumstances [1]. We recently showed that curcumin acts as a metal ionophore, bringing transient metals, especially Cu (II), into cells, and leading to enhanced cytotoxicity [12]. Studies involving the interaction of curcumin with Cu (II) have indicated pro-oxidant effects, suggesting that the metal complex may be able to enter a redox cycle, similar to the Fenton process [13, 14]. We therefore postulated that curcumin's pro-oxidant activity may contribute to its anticancer effects in our model system. In order to confirm this assumption, vitamin E (α-tocopherol), vitamin C (L-ascorbic acid), and trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), a water soluble derivative of vitamin E [15], were applied to test whether the cytotoxicity induced by curcumin could be attenuated by these antioxidants. To our surprise, neither vitamin E nor trolox attenuated the cytotoxicity induced by curcumin; instead, these compounds significantly enhanced curcumin's killing of cancer cells, with trolox being the most effective. These observations not only indicate new potential mechanisms of curcumin's anticancer activity, but also support a recently emerged notion that antioxidants such as vitamin E and vitamin C could be cytotoxic under certain circumstances [16, 17, 18], which provides the rationale for potential applications of these antioxidants in cancer therapy. The present study was designed to understand and characterize how trolox enhances curcumin's cytotoxicity in cancer cells. We found that trolox enhances curcumin's cytotoxicity through generation of ROS, an action contrary to its typical antioxidant properties.
Materials and Methods
Materials
Curcumin, trolox, vitamin E, vitamin C, superoxide dismutase 1 (SOD1), and catalase were purchased from Sigma-Aldrich (St. Louis, MO). The (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) reagent was purchased from Promega (Madison, WI). Carboxy-H2DCFDA and tert-butyl hydroperoxide (TBHP) were purchased from Invitrogen (Carlsbad, CA). All other reagents were analytical grade and purchased from Sigma-Aldrich (St. Louis, MO).
Cell culture and cell viability assay
MCF-7 and MDA-MB-231 cell lines were purchased from American Type Culture Collection (ATCC) and cultured according to the protocol provided by ATCC. A2780 cells were provided by Dr. Stephen Howell (University of California, San Diego). Cells were routinely cultured in RPMI-1640 medium supplemented with 10% FBS and 100 IU/ml penicillin, and 100 µg/ml streptomycin, at 37°C in a humidified environment containing 5% CO2. For viability assays, 5000 cells per well were seeded in a 96-well tissue culture plate with 100 µl RPMI-1640 medium, and 40% to 50% cell confluence was achieved within 24 hours. At that point, the medium was replaced with 100 µl of fresh medium and the cells were treated with various compounds at different concentrations and durations. Curcumin, trolox, and vitamin E were dissolved in ethanol for the stock and further diluted in cultured medium. Vitamin C and N-acetylcysteine (NAC) were prepared in PBS. After 72 hours of treatment, cell viability was assessed with the MTS reagent as we previously described [19]. Briefly, 20 µl of MTS solution was added to each well and cells were incubated at 37°C for 1 hour. The optical density was recorded at 490 nm using a spectrophotometer and data presented as a percentage of untreated cells cultured under the same conditions. The optical readings at 490 nm of untreated control cells were around 1.
Generation of catalase- and SOD1-overexpression cell lines
Catalase was over-expressed in A2780 cells as described by Bai et al. [20]. Briefly, 1.5× 106 A2780 cells were seeded onto a 100 mm culture dish and reached approximately 60-80% confluence after 24 hours. The expression plasmid vectors pZeoSV2(+) and pZeoSV2(+) containing human catalase cDNA (pZeoSV-CAT) were kindly provided by Dr. Cederbaum (Albany Medical College, New York). A2780 cells were transfected with pZeoSV2(+) and pZeoSV-CAT using the FuGene HD transfection reagent (Roche Molecular Biochemicals, Hoffmann, LA) following the instructions of the manufacturer. After 72 hours of transfection, cells were trypsinized and seeded at a low density onto 100 mm dishes in RPMI medium containing 300 µg/ml Zeocin, a selection reagent. Two weeks after transfection, survival clones were trypsinized and 60 cells were diluted in 10 ml medium and randomly plated into 96-well to achieve one cell per well for further selection. Another two weeks later, single cell clones were transferred to wells of a 24-well plate and cells were constantly kept in the medium containing 300 µg/ml Zeocin. When the cells reached 60% confluence, they were transferred to wells of a 6-well plate. Cells were then transferred to flasks and grown to a large scale. Catalase over-expression in each single clone was measured by Western blot. The clone that expressed the highest catalase level was chosen for the study.
The expression plasmid vector pcDNA3 was purchased from Invitrogen (Carlsbad, CA). We previously cloned human SOD1 cDNA in the pcDNA3 vector [21]. This was used to generate a SOD1 over-expression A2780 line. A2780 cells were transfected with pcDNA3 and pcDNA3-SOD1 vectors using the FuGene HD transfection reagent (Roche Molecular Biochemicals, Hoffmann, LA) following the instructions of the manufacturer. After 48 hours of transfection, cells were treated with 500 µg/ml G418. Two weeks later, surviving clones were trypsinized and split into a 96-well plate as described above.
Cells were constantly kept in the medium containing 500 µg/ml G418. Single cell clones were transferred progressively to a 24-well plate, a 6-well plate, and a flask. Over-expression of SOD1 was confirmed by Western blot. The clone that expressed the highest level of SOD1 was used for the study.
Detection of intracellular ROS
Intracellular ROS generation was analyzed using a fluorescent probe, Carboxy-H2DCFDA, following the manufacturer's protocol. A2780 cells were seeded in a 6-well plate (2.5 ×105/well) and cultured for 24 hours prior to treatment with curcumin (10 µM), trolox (50 µM) or a combination of the two for various time periods (0.5, 1, and 2 hours). Medium was then exchanged for serum-free medium and cells were incubated at 4°C for 10 min. Cells were then washed twice in 4 ml HBSS. ROS indicator H2DCFDA stock (10 mM) was prepared in DMSO and working solution (25 µM) was prepared in warm HBSS (37°C). Cells were incubated with H2DCFDA in HBSS at 37°C for 1 hour, protected from light, and were washed again with HBSS twice at room temperature. Fresh pre-warmed medium was added to cells and the plates were incubated at 37°C for 5 minutes to allow a short recovery time. After recovery, cells were detached by trypsin-EDTA and resuspended in 2 ml complete medium and transferred to a 15 ml tube and collected by centrifugation. Finally, cells were resuspended in 1 ml HBSS and the fluorescent intensity was examined by flow cytometry. For negative controls, cells were not treated but loaded with H2DCFDA dye. Positive controls were obtained by incubating cells with 100 µM TBHP for 1 hour. Fluorescent intensity was measured at 530 nm when the samples were excited at 485 nm.
Western blot analysis
Western blot was performed as previously described [19]. Briefly, cell lysates were prepared with cell lysis buffer, sonicated on ice, and centrifuged at 15,000g for 15 min to remove insoluble materials. Then 10-20 µg of cell lysate from each sample was resolved in 7.5% or 10% SDS PAGE gel, transferred to a PVDF membrane, and blotted with antibodies against human caspase 3, PARP, Bcl-2, Bcl-xl, Bad, and β-actin. All antibodies were from Cell Signaling Technology (Danvers, MA).
Results
Vitamin E, trolox, and vitamin C enhance curcumin's cytotoxicity in cancer cells
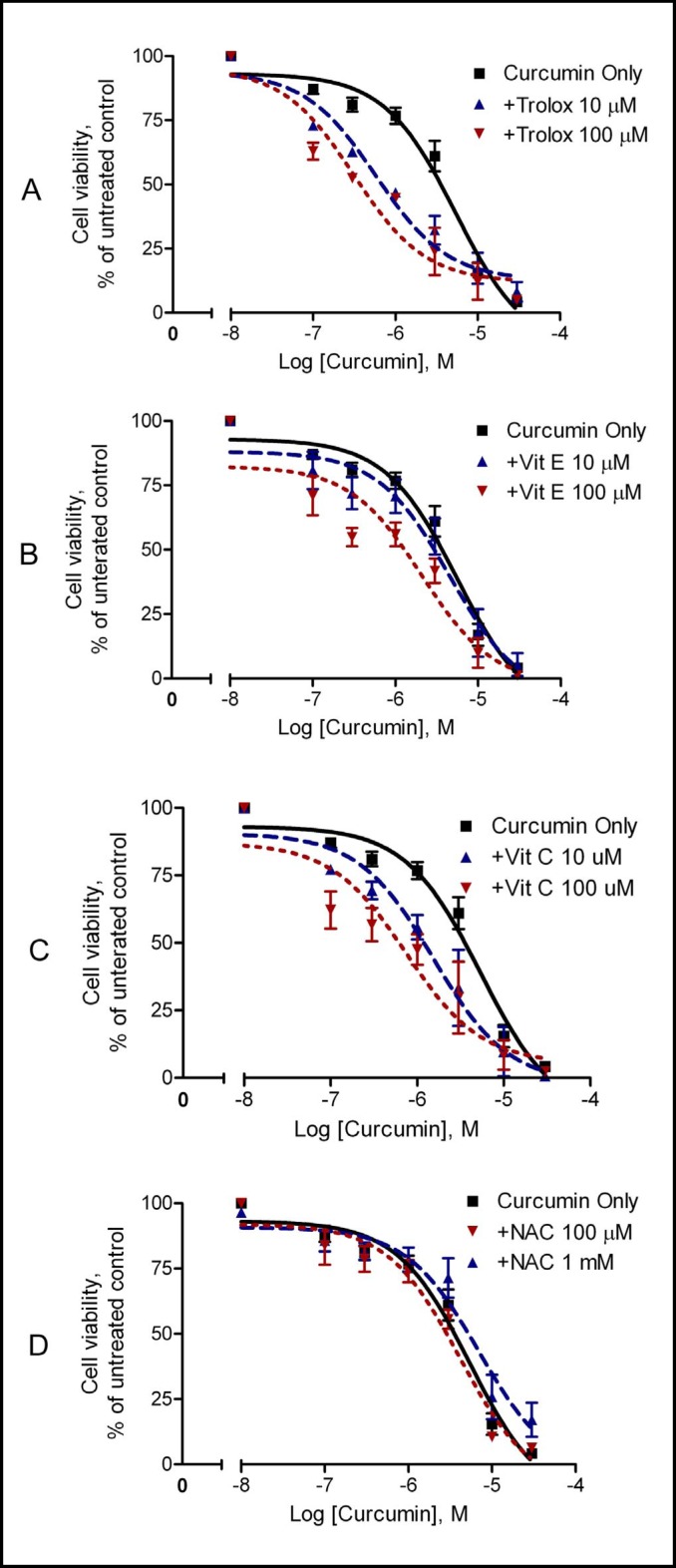
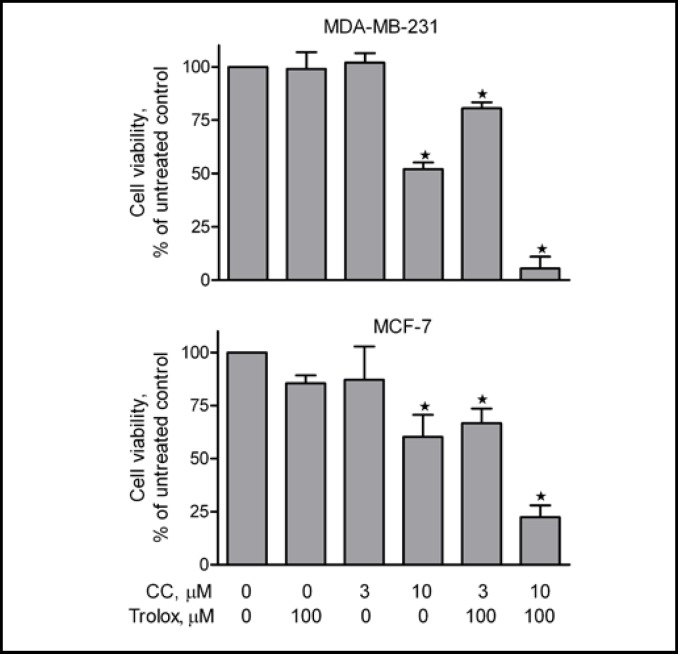
We first examined the effects of vitamin E, trolox, and vitamin C on curcumin-induced cytotoxicity in A2780 cells. Cells were treated with increasing concentrations of curcumin (100 nM-30 µM) in the presence of vitamin E, trolox, or vitamin C for 72 hours. Curcumin itself inhibited cell viability in a concentration-dependent manner, with an IC50 at around 5.4 µM (Table 1, Fig. 1). The inhibitory effect of curcumin on cell viability was significantly enhanced by pretreatment of the cells with trolox (Fig. 1A), vitamin E (Fig. 1B), and vitamin C (Fig. 1C). The enhanced cytotoxicity of curcumin by trolox was also seen in other cancer lines including MCF-7 and MDA-MB-231 (Fig. 2). Notably, trolox was the most effective enhancer of curcumin's cytotoxicity (Table 1). The IC50 of curcumin was reduced about 10-fold, to 0.58 µM in the presence of 10 µM trolox, and 0.31 µM in the presence of 100 µM trolox (Table 1). These results demonstrate that trolox, vitamin E, and vitamin C enhance curcumin's cytotoxicity in a concentration-dependent manner in cancer cells. To understand whether other antioxidants also increase curcumin's cytotoxicity, cells were pretreated with NAC, a widely used antioxidant [22], prior to addition of curcumin. Interestingly, pretreatment of the cells with NAC did not potentiate curcumin's cytotoxicity (Fig. 1D).
Table 1.
The effects of trolox, vitamin E or vitamin C on curcumin-induced cytotoxicity of ovarian cancer cells.
| Curcumin | +Trolox | + Vit E | +Vit C | ||||
|---|---|---|---|---|---|---|---|
| 10 μM | 100 μM | 10 μM | 100 μM | 10 μM | 100 μM | ||
| IC50, (μM) | 5.44 | 0.58 | 0.31 | 3.75 | 2.10 | 1.5 | 0.75 |
A2780 cells were treated with curcumin at increasing concentrations in the presence or absence of 10-100 μM trolox, vitamin E or vitamin C for 72 hours.
Cell viability was analyzed using the MTS assay.
IC50 (μM) values were calculated through nonlinear regression using the one site competition curve (n=3).
Fig. 1.
Effects of vitamin E, trolox, vitamin C, and NAC on curcumin's cytotoxicity in A2780 cells. Cells were pre-treated with 10 µM or 100 µM trolox (A), vitamin E (B), vitamin C (C), or 1 mM NAC (D) for 15 min prior to addition of increasing concentrations of curcumin. Cell viability was analyzed by the MTS assay after 72 hours of treatment. Data (mean ± SE, n = 3-5) are expressed as percentages of the MTS level detected in untreated control cells.
Fig. 2.
Effects of trolox on curcumin's cytotoxicity in MCF-7 and MDA-MB-231 cells. Cells were pre-treated with 100 µM trolox for 15 min prior to addition of increasing concentrations of curcumin. Cell viability was analyzed by the MTS assay after 72 hours of treatment. Data (mean ± SE, n = 3) are expressed as percentages of the MTS level detected in untreated control cells. * P < 0.05, compared with untreated control cells, using one-way ANOVA followed by Dunnett's analysis.
Curcumin plus trolox induces apoptotic cell death
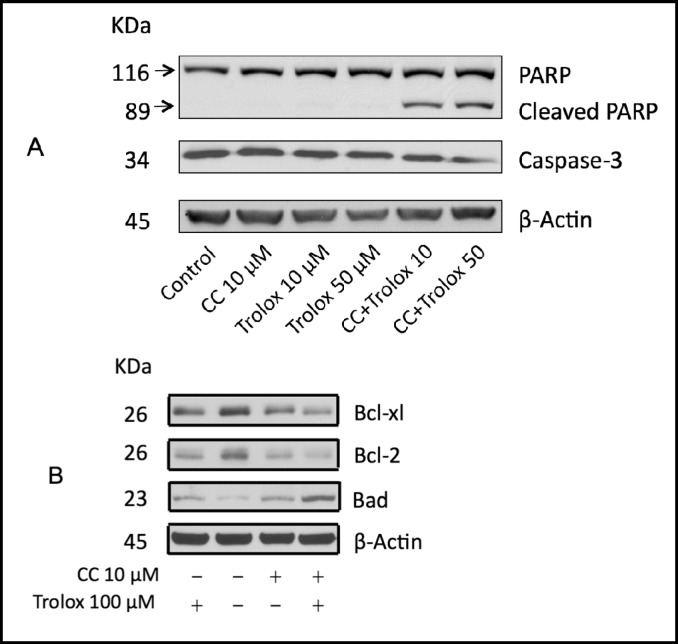
Because trolox was the most effective enhancer of curcumin's inhibitory effect on the viability of A2780 cells, we explored the potential cellular mechanisms by which trolox acts in concert with curcumin to inhibit cancer cell viability. As shown in Fig. 3A, curcumin plus trolox induced cleavage of Poly (ADP-ribose) polymerase 1 (PARP) and activation of caspase 3, indicating that the combination triggers caspase-dependent apoptosis. To further understand how trolox and curcumin could induce apoptotic cell death, we examined expression of several apoptotic mediators in A2780 cells that had been treated with trolox and curcumin. We found that anti-apoptotic Bcl-2 and Bcl-xl protein levels were decreased by the combination treatment, whereas the pro-apoptotic Bad protein levels were increased (Fig. 3B).
Fig. 3.
Curcumin (CC) plus trolox induces apoptosis. A2780 cells were treated with curcumin (10 µM) in the presence or absence of trolox (10 or 50 µM) for 72 hours. Cell lysates were prepared and subjected to Western blot using antibodies against PARP and caspase-3 (A); and antibodies against Bcl-2, Bcl-xl, Bad and Bax (B). β-actin was used as a loading control. Shown are representatives of two separate experiments.
Curcumin plus trolox increases intracellular ROS levels
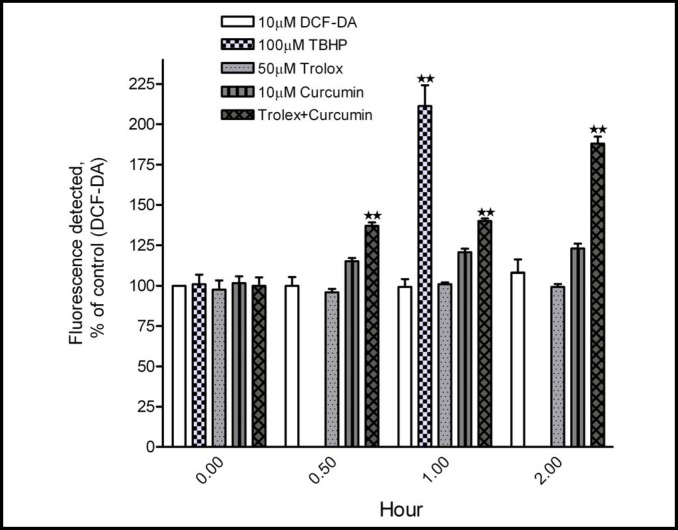
The mechanism underlying the inhibitory effect of curcumin plus trolox on cell viability and the induction of apoptosis was explored. Curcumin has been recognized to have both antioxidant and pro-oxidant properties, depending on the experimental circumstances [1]. Since the addition of trolox, a well-established antioxidant, enhanced curcumin's cytotoxicity, we examined the levels of ROS in A2780 cells. As shown in Fig. 4, curcumin itself moderately increased intracellular ROS levels, as assayed with the H2DCFDA dye. The increase in ROS level was detected after 30 minutes of treatment with curcumin, an effect which persisted for 2 hours. Trolox by itself did not increase ROS levels. When added to cells with curcumin, intracellular ROS levels were significantly enhanced over time, with the highest level being detected after 2 hours of the combination treatment. 100 µM TBHP was used as a positive control of ROS generation and detection.
Fig. 4.
Curcumin plus trolox increases intracellular ROS levels in A2780 cells. A2780 cells were treated with curcumin (10 µM) and trolox (50 µM), alone or in combination for 0.5, 1, and 2 hours. ROS levels were examined by H2DCFDA labeling with flow cytometry. Cells without treatment but with H2DCFDA dye were used as an untreated control. Cells stimulated by TBHP (100 µM) for 1 hour served as a positive control. Data (mean ± SD, n=3) were presented as percentages of the fluorescent level detected in untreated control cells. **, P < 0.001, compared with untreated control cells, using one-way ANOVA followed by Dunnett's analysis.
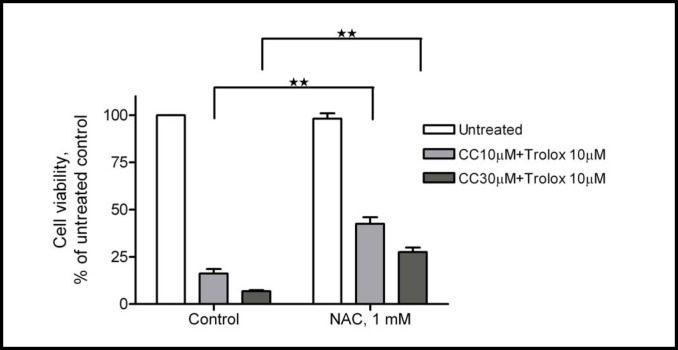
ROS generation induced by curcumin plus trolox on cell viability was further evaluated by using the antioxidant NAC. NAC is an antioxidant that promotes the biosynthesis of glutathione, the major anti-oxidant of the cytoplasm [22]. We found that NAC was able to significantly attenuate the ROS levels (Table 2) and cytotoxicity (Fig. 5), induced by curcumin plus trolox in A2780 cells, supporting the notion that ROS generation is largely responsible for the cytotoxicity.
Table 2.
The effects of NAC on curcumin/trolox-induced ROS levels.
| Untreated | Curcumin +Trolox | NAC | NAC+Curcumin +Trolox | |
|---|---|---|---|---|
| ROS levels | 100 | 185±11 | 107±6 | 135±13* |
A2780 cells were treated with curcumin (10 μM) plus trolox (100 μM) for 2 hours in the presence or absence of 1 mM NAC.
ROS levels were examined by H2DCFDA labeling with flow cytometry.
Cells without treatment but with H2DCFDA dye were used as an untreated control.
Data (mean ± SD, n=3) were presented as percentages of the fluorescent level detected in untreated control cells.
P < 0.05 (column 4 versus column 2), using one-way ANOVA followed by Dunnett's analysis.
Fig. 5.
NAC attenuates curcumin (CC) plus trolox's cytotoxicity. Fifteen minutes prior to addition of curcumin (10 µM) and trolox (50 µM), NAC (1 mM) was added to A2780 cells. Cells were then cultured for 72 hours and cell viability was measured by the MTS assay. Data (mean ± SE, n = 3) are expressed as percentages of the MTS level detected in untreated control cells. **, P < 0.01, using one-way ANOVA followed by Dunnett's analysis.
Exogenous SOD1 and catalase have no effect on cytotoxicity induced by curcumin plus trolox
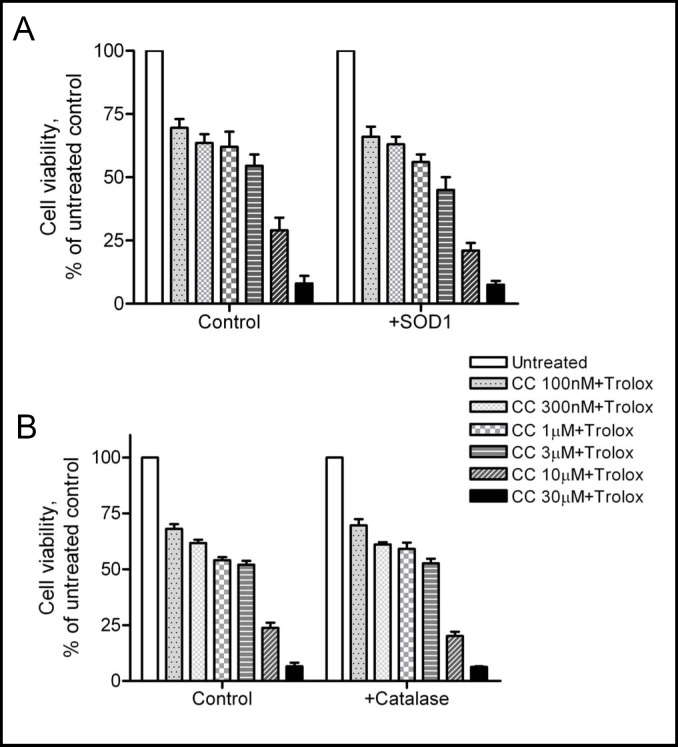
We recently reported that NAC plus copper generates extracellular ROS, which is cytotoxic to cancer cells [23] in a manner similar to that reported for vitamin C [24]. The concept of extracellular and intracellular ROS generation and its influence on cell function has been recognized [25]. To determine whether the cytotoxic effects of curcumin plus trolox occurred through extracellular generation of ROS, we added SOD1 (which converts superoxide to hydrogen peroxide) or catalase (which catalyzes the breakdown of hydrogen peroxide to water) to the medium prior to addition of curcumin and trolox. If ROS was generated in the extracellular space by curcumin plus trolox, the addition of exogenous SOD1 or catalase would be expected to alter their cytotoxicity. As shown in Fig. 6, the addition of exogenous SOD1 or catalase did not enhance or attenuate the toxicity of curcumin and trolox, ruling out a significant effect from extracellular generation of ROS. In contrast, the cytotoxicity induced by 1 mM NAC plus 10 µM Cu (II) was enhanced by exogenous SOD1, and attenuated by exogenous catalase, which is consistent with toxicity due to extracellular ROS generation (data not shown, [23]).
Fig. 6.
Exogenous SOD1 or catalase has no effect on the cytotoxicity induced by curcumin (CC) plus trolox. One unit of SOD1 (A) or catalase (B) was added to A2780 cells prior to addition of curcumin and trolox at indicated concentrations. Cell viability was analyzed by the MTS assay. Data (mean ± SE, n=3) are expressed as percentages of the MTS level detected in untreated control cells.
Over-expression of catalase reduces, while over-expression of SOD1 enhances, the cytotoxicity induced by curcumin plus trolox
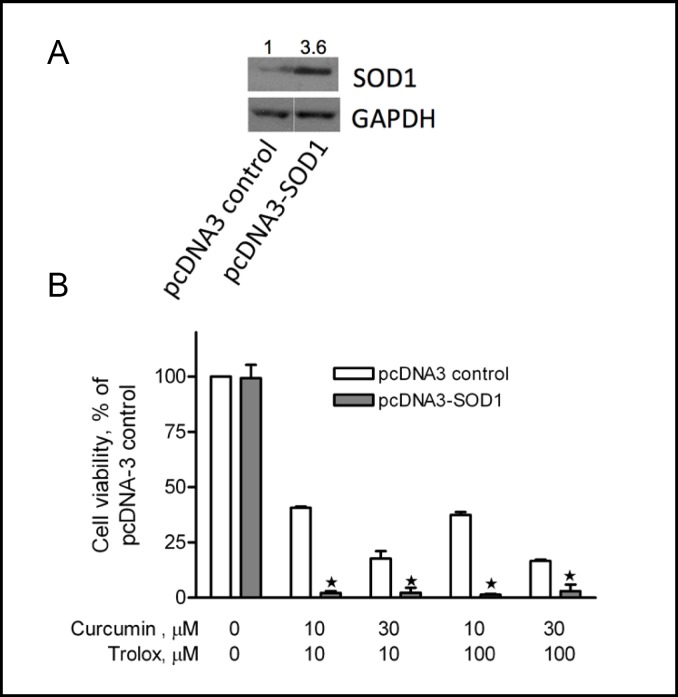
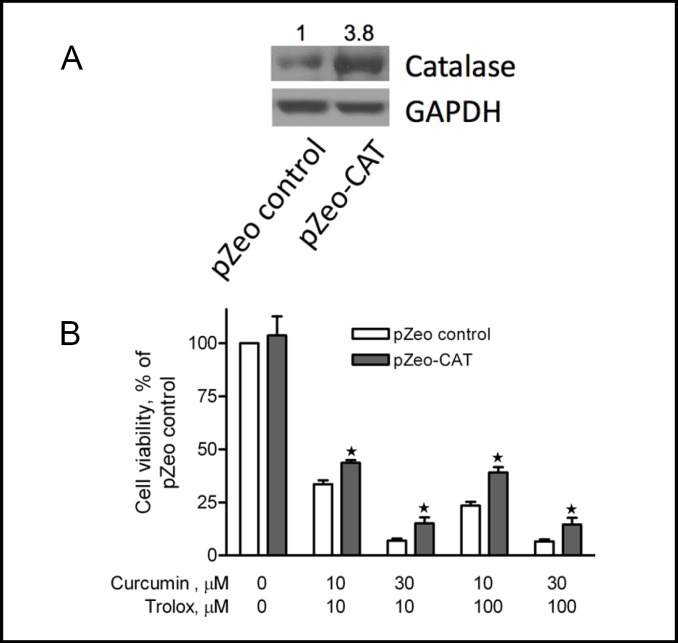
To determine whether intracellular ROS generation by curcumin plus trolox was responsible for the cytotoxicity, we generated stable A2780 cell lines over-expressing catalase or SOD1. Endogenous over-expression of SOD1 (Fig. 7A) significantly enhanced the cytotoxicity induced by curcumin plus trolox (Fig. 7B). Compared to pcDNA3 vector control cells, over-expression of SOD1 dramatically decreased cell viability in cells that were treated with 10 µM curcumin plus 10 µM or 100 µM trolox, and 30 µM curcumin plus 10 µM or 100 µM trolox. In contrast, endogenous over-expression of catalase (Fig. 8A) significantly attenuated the cytotoxicity induced by curcumin plus trolox (Fig. 8B). For instance, in catalase over-expression cells that had been treated with curcumin (10 µM) plus trolox (100 µM), cell viability was significantly higher over that of P-Zeo vector control cells under the same treatment. These data provide evidence suggesting that intracellular ROS generation accounts at least in part for the cytotoxicity induced by curcumin plus trolox. The amplitude of the protection may indicate that either CAT is not able to get a full access to the intracellular compartments where hydrogen peroxides are produced, or the over-expressed CAT does not reach the level required to fully reverse the cytotoxicity.
Fig. 7.
Endogenous over-expression of SOD1 significantly enhances cytotoxicity induced by curcumin and trolox. A2780 cells stably over-expressing the SOD1 gene (pcDNA3-SOD1, with densitometry value shown on top, A) were treated with curcumin (10 or 30 µM) plus trolox (10 or 100 µM) for 72 hours. Cell viability was examined by the MTS assay (B). Cells stably transfected with the empty vector (pcDNA3 control) were used as a control. Data (mean ± SE, n = 3) are expressed as percentages of the MTS level detected in untreated control cells for each cell line. *, P < 0.05, compared with pcDNA3 vector control cells, using one-way ANOVA followed by Dunnett's analysis.
Fig. 8.
Endogenous over-expression of catalase significantly reduces cytotoxicity induced by curcumin and trolox. A2780 cells stably over-expressing the catalase gene (pZeo-CAT, with densitometry value shown on top, A) were treated with curcumin (10 µM and 30 µM) plus trolox (10 or 100 µM) for 72 hours. Cell viability was examined by the MTS assay. Cells transfected with the empty vector (pZeo control) were used as control. Data (mean ± SE, n = 3) are expressed as percentages of the MTS level detected in untreated control cells for each cell line (B). *, P < 0.05, compared with pZeo vector control cells using one-way ANOVA followed by Dunnett's analysis.
Discussion
The present study demonstrated that several “antioxidant” vitamins, including vitamin E, vitamin C, and trolox, significantly enhance curcumin's cytotoxicity in human cancer cells. Trolox, a water soluble vitamin E derivative, was the most effective in suppressing cancer cell viability and inducing apoptotic cell death. We therefore focused on characterizing and exploring the mechanism of trolox's potentiation of curcumin's cytotoxicity. Our findings reveal that trolox and curcumin act in concert to increase intracellular ROS levels, leading to apoptosis of cancer cells.
A number of cellular mechanisms have been described to account for curcumin's anticancer action, including the inhibition of intracellular signaling pathways such as NF-κB, AP-1, Cox-2, cyclin D1 and mTOR, and induction of DNA damage [1]. In spite of its initially recognized antioxidant property, curcumin has been found to be able to work as a pro-oxidant under certain circumstances [1]. Several studies demonstrated that curcumin by itself significantly increased ROS generation, and induction of oxidative stress constitutes a critical mechanism for its anticancer action [2, 26, 27]. We thus rationalized that the addition of antioxidants would attenuate curcumin's cytotoxicity. To our surprise, the addition of antioxidants such as trolox, vitamin E, and vitamin C did not attenuate curcumin's cytotoxic effects but in fact enhanced it, inducing apoptosis likely via increasing in generation of ROS. These observations add novel insight into our understanding of curcumin's anticancer action. That these antioxidants, when added with curcumin, resulted in greater oxidant generation provides a scaffold for new combination therapies.
Another “antioxidant”, vitamin C, induces oxidative stress and a loss of cancer cell viability [28] which is greater in the presence of the “antioxidant” NAC [17]. To understand whether the combination of curcumin plus trolox produced its effects through ROS generated inside or outside the cell, we utilized two enzymes, SOD1 and catalase in our studies. The addition of either exogenous catalase or SOD1 (which are not able to cross cell membranes) had no effect on the cytotoxicity of the curcumin/trolox combination, indicating that ROS was not generated in the extracellular space. In contrast, manipulation of the cellular expression of either SOD1 or catalase altered cytotoxicity, SOD1 over-expression enhancing it and catalase over-expression decreasing it. Together these results suggest the essential role of endogenously generated hydrogen peroxide in cell killing by this drug combination. We suspect that potentiation of curcumin's cytotoxicity by trolox, vitamin C and vitamin E is due to their being electron donors, which allow for redox cycling of curcumin, though direct experimental evidence to support this hypothesis has yet to be generated. Consideration of the combined effects of redox active drugs may provide novel drug combinations with enhanced anticancer activity.
In conclusion, we demonstrated for the first time that antioxidants such as trolox, vitamin E, and vitamin C can potentiate cancer cell killing by curcumin, findings which may help in the development of novel drug combination therapies against cancer.
Acknowledgements
This work was supported in part by grants from the American Cancer Society (CNE-117557); the Susan G. Komen for the Cure Foundation (KG081083); the NIH OK-INBRE program (3P20RR016478-09S2); and the Oklahoma Center for the Advancement of Science and Technology (HR09-025).
Abbreviations
- MTS
(3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium)
- NAC
(N-acetylcysteine); PARP (Poly (ADP-ribose) polymerase)
- ROS
(reactive oxygen species)
- SOD1
(superoxide dismutase 1)
- TBHP
(tertbutyl hydroperoxide
- Trolox
(6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid)
References
- 1.Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: From ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65:1631–1652. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Q, Wang Y, Xu K, Lu G, Ying Z, Wu L, Zhan J, Fang R, Wu Y, Zhou J. Curcumin induces apoptosis in human lung adenocarcinoma a549 cells through a reactive oxygen species-dependent mitochondrial signaling pathway. Oncol Rep. 2010;23:397–403. [PubMed] [Google Scholar]
- 3.Sun ZJ, Chen G, Zhang W, Hu X, Liu Y, Zhou Q, Zhu LX, Zhao YF. Curcumin dually inhibits both mammalian target of rapamycin and nuclear factor-kappab pathways through a crossed phosphatidylinositol 3-kinase/akt/ ikappab kinase complex signaling axis in adenoid cystic carcinoma. Mol Pharmacol. 2011;79:106–118. doi: 10.1124/mol.110.066910. [DOI] [PubMed] [Google Scholar]
- 4.Huang TY, Tsai TH, Hsu CW, Hsu YC. Curcuminoids suppress the growth and induce apoptosis through caspase-3-dependent pathways in glioblastoma multiforme (gbm) 8401 cells. J Agric Food Chem. 2010;58:10639–10645. doi: 10.1021/jf1016303. [DOI] [PubMed] [Google Scholar]
- 5.Epelbaum R, Schaffer M, Vizel B, Badmaev V, Bar-Sela G. Curcumin and gemcitabine in patients with advanced pancreatic cancer. Nutr Cancer. 2010;62:1137–1141. doi: 10.1080/01635581.2010.513802. [DOI] [PubMed] [Google Scholar]
- 6.Park J, Conteas CN. Anti-carcinogenic properties of curcumin on colorectal cancer. World J Gastrointest Oncol. 2010;2:169–176. doi: 10.4251/wjgo.v2.i4.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jobin C, Bradham CA, Russo MP, Juma B, Narula AS, Brenner DA, Sartor RB. Curcumin blocks cytokine-mediated nf-kappa b activation and proinflammatory gene expression by inhibiting inhibitory factor i-kappa b kinase activity. J Immunol. 1999;163:3474–3483. [PubMed] [Google Scholar]
- 8.Singh S, Aggarwal BB. Activation of transcription factor nf-kappa b is suppressed by curcumin (diferuloylmethane) [corrected] J Biol Chem. 1995;270:24995–25000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 9.Huang TS, Lee SC, Lin JK. Suppression of c-jun/ap-1 activation by an inhibitor of tumor promotion in mouse fibroblast cells. Proc Natl Acad Sci USA. 1991;88:5292–5296. doi: 10.1073/pnas.88.12.5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandur SK, Ichikawa H, Pandey MK, Kunnumakkara AB, Sung B, Sethi G, Aggarwal BB. Role of pro-oxidants and antioxidants in the anti-inflammatory and apoptotic effects of curcumin (diferuloylmethane) Free Radic Biol Med. 2007;43:568–580. doi: 10.1016/j.freeradbiomed.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu HF, Yang JS, Lai KC, Hsu SC, Hsueh SC, Chen YL, Chiang JH, Lu CC, Lo C, Yang MD, Chung JG. Curcumin-induced DNA damage and inhibited DNA repair genes expressions in mouse-rat hybrid retina ganglion cells (n18) Neurochem Res. 2009;34:1491–1497. doi: 10.1007/s11064-009-9936-5. [DOI] [PubMed] [Google Scholar]
- 12.Lou JR, Zhang XX, Zheng J, Ding WQ. Transient metals enhance cytotoxicity of curcumin: Potential involvement of the NF-kappaB and mtor signaling pathways. Anticancer Res. 2010;30:3249–3255. [PubMed] [Google Scholar]
- 13.Ahsan H, Hadi SM. Strand scission in DNA induced by curcumin in the presence of cu(ii) Cancer Lett. 1998;124:23–30. doi: 10.1016/s0304-3835(97)00442-4. [DOI] [PubMed] [Google Scholar]
- 14.Ahsan H, Parveen N, Khan NU, Hadi SM. Pro-oxidant, anti-oxidant and cleavage activities on DNA of curcumin and its derivatives demethoxycurcumin and bisdemethoxycurcumin. Chem Biol Interact. 1999;121:161–175. doi: 10.1016/s0009-2797(99)00096-4. [DOI] [PubMed] [Google Scholar]
- 15.Diaz Z, Colombo M, Mann KK, Su H, Smith KN, Bohle DS, Schipper HM, Miller WH., Jr Trolox selectively enhances arsenic-mediated oxidative stress and apoptosis in apl and other malignant cell lines. Blood. 2005;105:1237–1245. doi: 10.1182/blood-2004-05-1772. [DOI] [PubMed] [Google Scholar]
- 16.Chinery R, Brockman JA, Peeler MO, Shyr Y, Beauchamp RD, Coffey RJ. Antioxidants enhance the cytotoxicity of chemotherapeutic agents in colorectal cancer: A p53-independent induction of p21waf1/cip1 via c/ebpbeta. Nat Med. 1997;3:1233–1241. doi: 10.1038/nm1197-1233. [DOI] [PubMed] [Google Scholar]
- 17.Childs A, Jacobs C, Kaminski T, Halliwell B, Leeuwenburgh C. Supplementation with vitamin c and n-acetyl-cysteine increases oxidative stress in humans after an acute muscle injury induced by eccentric exercise. Free Radic Biol Med. 2001;31:745–753. doi: 10.1016/s0891-5849(01)00640-2. [DOI] [PubMed] [Google Scholar]
- 18.Duarte TL, Almeida GM, Jones GD. Investigation of the role of extracellular h2o2 and transition metal ions in the genotoxic action of ascorbic acid in cell culture models. Toxicol Lett. 2007;170:57–65. doi: 10.1016/j.toxlet.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Ding WQ, Liu B, Vaught JL, Yamauchi H, Lind SE. Anticancer activity of the antibiotic clioquinol. Cancer Res. 2005;65:3389–3395. doi: 10.1158/0008-5472.CAN-04-3577. [DOI] [PubMed] [Google Scholar]
- 20.Bai J, Rodriguez AM, Melendez JA, Cederbaum AI. Overexpression of catalase in cytosolic or mitochondrial compartment protects hepg2 cells against oxidative injury. J Biol Chem. 1999;274:26217–26224. doi: 10.1074/jbc.274.37.26217. [DOI] [PubMed] [Google Scholar]
- 21.Ding WQ, Vaught JL, Yamauchi H, Lind SE. Differential sensitivity of cancer cells to docosahexaenoic acid-induced cytotoxicity: The potential importance of down-regulation of superoxide dismutase 1 expression. Mol Cancer Ther. 2004;3:1109–1117. [PubMed] [Google Scholar]
- 22.Arakawa M, Ito Y. N-acetylcysteine and neurodegenerative diseases: Basic and clinical pharmacology. Cerebellum. 2007;1-7 doi: 10.1080/14734220601142878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng J, Lou JR, Zhang XX, Benbrook DM, Hanigan MH, Lind SE, Ding WQ. N-acetylcysteine interacts with copper to generate hydrogen peroxide and selectively induce cancer cell death. Cancer Lett. 2010;298:186–194. doi: 10.1016/j.canlet.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Q, Espey MG, Sun AY, Lee JH, Krishna MC, Shacter E, Choyke PL, Pooput C, Kirk KL, Buettner GR, Levine M. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc Natl Acad Sci U S A. 2007;104:8749–8754. doi: 10.1073/pnas.0702854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukai T, Ushio-Fukai M. Superoxide dismutases: Role in redox signaling, vascular function and diseases. Antioxid Redox Signal. 2011;15:1583–606. doi: 10.1089/ars.2011.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanchez Y, Simon GP, Calvino E, de Blas E, Aller P. Curcumin stimulates reactive oxygen species production and potentiates apoptosis induction by the antitumor drugs arsenic trioxide and lonidamine in human myeloid leukemia cell lines. J Pharmacol Exp Ther. 2010;335:114–123. doi: 10.1124/jpet.110.168344. [DOI] [PubMed] [Google Scholar]
- 27.Thayyullathil F, Chathoth S, Hago A, Patel M, Galadari S. Rapid reactive oxygen species (ros) generation induced by curcumin leads to caspase-dependent and -independent apoptosis in l929 cells. Free Radic Biol Med. 2008;45:1403–1412. doi: 10.1016/j.freeradbiomed.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 28.Varadharaj S, Watkins T, Cardounel AJ, Garcia JG, Zweier JL, Kuppusamy P, Natarajan V, Parinandi NL. Vitamin c-induced loss of redox-dependent viability in lung microvascular endothelial cells. Antioxid Redox Signal. 2005;7:287–300. doi: 10.1089/ars.2005.7.287. [DOI] [PubMed] [Google Scholar]