Abstract
Interstitial cells of Cajal (ICCs) are the pacemaking cells in the gastrointestinal muscles that generate the rhythmic oscillations in membrane potential known as slow waves. ICCs also mediate or transduce inputs from the enteric nervous system. Substance P (SubP) is a member of the family of mammalian tachykinin peptides that are predominantly released by enteric neurons. This study assessed the relationship of Na+-leak channel (NALCN) in the SubP-induced depolarization in pacemaking activity in the gastrointestinal tract. The patch-clamp technique for whole-cell recording was used in cultured cluster and single ICCs. Electrophysiological and pharmacological properties of SubP in ICC pacemaking activity were similar to those of NALCN. Reverse-transcription polymerase chain reaction, Western blotting, and immunohistochemistry all showed abundant and localized expression of NALCN messenger RNA and protein in mouse small intestine. NALCN is involved in the SubP-induced depolarization of intestinal pacemaking activity. The protein is a potential target for pharmacological treatment of motor disorders of the gut.
Key Words: Interstitial Cells of Cajal, Na+-leak channel, NALCN, Substance P, Gastrointestinal muscle
Introduction
Interstitial cells of Cajal (ICCs) are the pacemaking cells in the gastrointestinal (GI) muscles that generate the rhythmic oscillations in the membrane potential known as slow waves [1, 2, 3]. Slow waves propagate within ICC networks, are conducted into smooth muscle cells via gap junctions, and initiate phasic contractions by activating Ca2+ entry through L-type Ca2+ channels. The pacemaker activity in the murine small intestine is due mainly to periodic activation of nonselective cation channels (NSCC) [4, 5] or Cl− channels [6, 7]. ICCs also mediate or transduce inputs from the enteric nervous system.
Neurotransmission is mediated not only by the classical neurotransmitters but also by neuropeptide transmitters. The major excitatory neurotransmitters are acetylcholine (ACh) and substance P (SubP), whereas the major inhibitory neurotransmitters are nitric oxide (NO) and vasoactive intestinal polypeptide (VIP) [8, 9]. SubP, neurokinin A, and neurokinin B are members of the family of mammalian tachykinin peptides that are predominantly released by enteric neurons, and which exert a potent contractile effect on GI smooth muscle through tachykinin receptors by modulating ionic channels and by producing second messengers [10, 11, 12]. Tachykinin receptors are distributed in enteric nerves and smooth muscles. In addition, a tachykinin receptor has been immunohisto-chemically identified in the ICCs [13, 14], suggesting that SubP may modulate GI motility by affecting the ICCs. SubP may regulate pacemaker currents through tachykinin NK1 receptor in murine small intestine ICCs [15], and may also activate a NSCC in murine pacemaker ICC [16]. In the mouse brainstem, SubP activates transient receptor potential canonical (TRPC) channels to enhance respiratory rhythm regularity [17].
Recently, the Na+-leak channel (NALCN) has been characterized [18, 19]. NALCN is a member of the 24-transmembrane-spanning (24 TM) ion channel family, which also includes 10 voltage-gated Ca2+ channels (CaVs) and 10 Na+-selective channels (NaV1.1–1.9 and NaX) [20, 21]. NALCN is the only nonselective, noninactivating, voltage-independent channel among the family's 21 members [18]. NALCN forms a background Na+ leak conductance in neurons and is required for normal respiratory rhythm [18]. Also, SubP activates cation current through the NALCN channel complex, which also includes another large protein UNC80 [22].
In this study, we found that NALCN is not required for the basal pacemaking activity in ICCs. However, NALCN is partly involved in the SubP-induced depolarization and the modulation of pacemaking activity in ICCs.
Materials and Methods
Preparation of cells and cell cultures
The experiments and animal care were in accordance with the guiding principles approved by the ethics committee in Pusan National University, South Korea and University of Pennsylvania for the Care and Use of Laboratory Animals. Balb/c and the Nalcn mutant were used in the studies. The Nalcn knockout mutant [18] was backcrossed to C57BL/6J for more than 10 generations. P0 mice (within approximately 24 hr of birth) were anaesthetized with ether and killed by cervical dislocation. The small intestines from 1 cm below the pyloric ring to the caecum were removed and opened along the mesenteric border. Luminal contents were washed away with Krebs-Ringer bicarbonate solution. The tissues were pinned to the base of a Sylgard dish and the mucosa removed by sharp dissection. Small tissue strips of intestine muscle (consisting of both circular and longitudinal muscles) were equilibrated in Ca2+-free Hank's solution (containing, in mM: KCl 5.36, NaCl 125, NaOH 0.34, Na2HCO3 0.44, glucose 10, sucrose 2.9 and HEPES 11, pH 7.4) for 30 min. The cells were then dispersed with an enzyme solution containing collagenase (Worthington Biochemical, Lakewood, NJ, U.S.A., 1.3 mg ml1), bovine serum albumin (BSA, Sigma-Aldrich, St Louis, MO, U.S.A., 2 mg ml1), trypsin inhibitor (Sigma-Aldrich, 2 mg ml1) and ATP (0.27 mg ml1). Cells were plated onto sterile glass coverslips coated with murine collagen (2.5 ?g ml1; Falcon/ BD, Franklin Lakes, NJ, U.S.A.) in a 35 mm culture dish. The cells were then cultured at 37°C in a 95% O2-5% CO2 incubator in a smooth muscle growth medium (SMGM; Clonetics, San Diego, CA, U.S.A.) supplemented with 2% antibiotics/ antimycotics (Gibco, Grand Island, NY, U.S.A.) and murine stem cell factor (SCF; 5 ng ml1; Sigma-Aldrich). All experiments on single cells were performed on cells cultured for 1 day. ICCs were identified immunologically with anti-c-kit antibody (phycoerythrin (PE)-conjugated rat anti-mouse c-kit monoclonal antibody; eBioscience, San Diego, CA, U.S.A.) at a dilution of 1:50 for 20 min.
Patch-clamp experiments
The physiological salt solution used to bathe cultured ICC cells (Na+-Tyrode) contained (in mM): KCl 5, NaCl 135, CaCl2 2, glucose 10, MgCl2 1.2, and HEPES 10, adjusted to pH 7.4 with NaOH. The pipette solution contained (in mM): KCl 140, MgCl2 5, K2ATP 2.7, NaGTP 0.1, creatine phosphate disodium 2.5, HEPES 5, and EGTA 0.1, adjusted to pH 7.2 with KOH. The whole-cell configuration of the patch-clamp techniques was used to record membrane currents (voltage clamp) and potentials (current clamp) from cultured ICCs with an Axopatch I-D and an Axopatch 200B amplifiers (Axon Instruments, Foster, CA, U.S.A.) amplified membrane currents and potentials. The command pulse was applied using an IBM-compatible personal computers and pClamp software (version 6.1 and version 10.0; Axon Instruments). The data were filtered at 5kHz and displayed on an oscilloscope, a computer monitor, and/or with a pen recorder (Gould 2200; Gould, Valley View, OH, U.S.A.). Results were analyzed using pClamp and Origin software (version 6.0, Microcal, U.S.A.). All experiments were performed at 30-33°C.
RNA preparation and reverse transcription-polymerase chain reaction (RT-PCR) in clusters of cultured ICCs
Total RNA was extracted using an RNeasy Mini Kit (Qiagen, Valencia, CA, U.S.A.), and reverse transcription of total RNA was performed by using random hexamer primers and Superscript II-RT (Life Technologies, U.S.A.) according to the manufacturer's instructions. PCR primers were as follows: the PCR amplification with upstream primers NALCN-OF, 5=-ATG TGC TAT GAG ATG GAG AGG CTG-3= for NALCN; and downstream primers NALCN-OR, 5=- TTA AGA GCT GCT GCT GAC TGC TTG-3= for NALCN was performed for 35 cycles under the following conditions: denaturing at 96°C for 20 seconds, annealing at 55°C for 20 seconds, and polymerization at 72°C for 30 seconds. The PCR products predicted as 373 base pairs for NALCN were separated on 1.5% agarose gel by electrophoresis. The identification of the PCR products was confirmed by DNA sequencing.
Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis Western Blotting
Western blotting was performed by using lysates of cultured ICCs. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis by using 6% polyacrylamide gels, transferred to a polyvinylidene difluoride membrane, and analyzed by NALCN antibodies. All procedures used standard methods.
Immunohistochemistry
Cultured ICCs from small intestine of Balb/C mice were used for immunohistochemistry. Cultured ICCs were fixed in cold acetone (4 °C) for 5 min. After fixation, they were washed in phosphate-buffered saline (PBS; 0.01 M, pH 7.4) and immersed in 0.3% Triton X-100 in PBS. After blocking with 1% BSA in 0.01 M PBS for 1 hour at room temperature, they were incubated with a rat monoclonal antibody raised against c-Kit (Ack2; eBioscience) at 0.5 µg/ml, and rabbit polyclonal antibody against NALCN or UNC80 in PBS for 24 hours (4°C). After rinsing in PBS at 4 °C, they were labeled with the fluorescein isothiocyanate (FITC)-coupled donkey anti-rabbit IgG secondary antibody (1:100; Jackson Immunoresearch Laboratories, Bar Harbor, MN, U.S.A.) or Texas red-conjugated donkey anti-rat IgG (1:100, Jackson Immunoresearch Laboratories) for 1 hour at room temperature. Control tissues and sections were prepared by omitting either primary or secondary antibodies from the incubation solutions. For double immunostaining, specimens were incubated with a mixture of antibody raised against NALCN, UNC80, and antibody raised against c-kit for 24h at 4°C. After a thorough wash with PBS, the mixture of labeled secondary antibodies was incubated for 1 hour at room temperature. Tissues were examined with an FV 300 laser scanning confocal microscope (Olympus, Tokyo, Japan) with an excitation wavelength appropriate for FITC (495 nm) and Texas red (590 nm). Final images were constructed with Flow-View software (Olympus).
Statistical analysis
Data are expressed as mean±standard error. Differences between the data were evaluated by Student's t-test. A P-value < 0.05 indicated statistical significance. The n values reported in the text refer to the number of cells used in the patch-clamp experiments.
Results
Effects of SubP and SubP antagonists on pacemaking activity in cultured ICC clusters
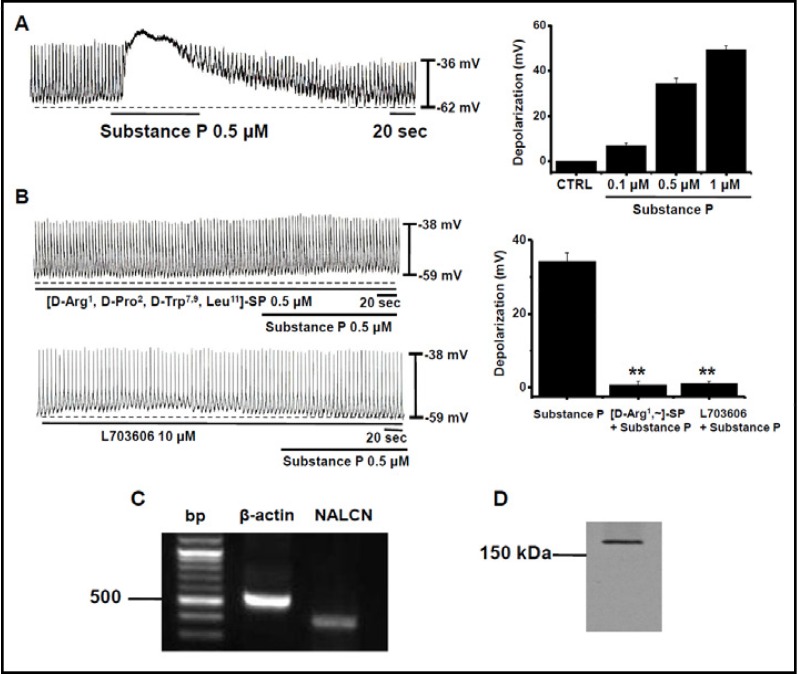
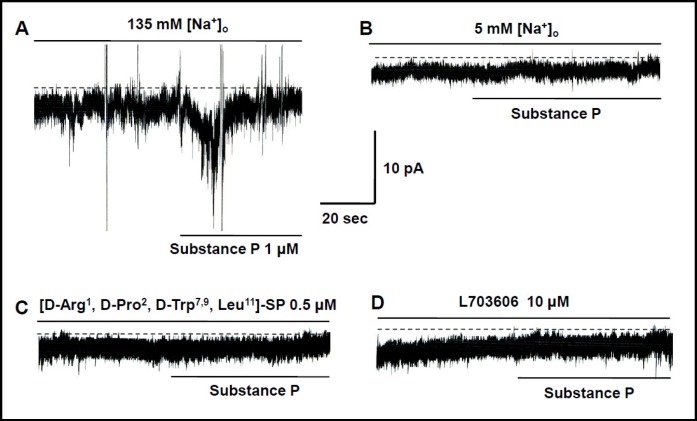
Under current clamp, cells in cultured ICC clusters had a mean resting membrane potential of −58 ± 5 mV and produced electrical pacemaking activity (n = 57). The frequency of this pacemaking was 16 ± 2 cycles per minute and the amplitude was 27 ± 5 mV (n = 57) at 30°C. We first examined the effect of SubP on pacemaking activity. SubP (0.1–1 µM) induced depolarization of pacemaking activity (Fig. 1). The degree of depolarization was by 7 ± 1 mV with 0.1 µM (n = 5), 34 ± 2 mV with 0.5 µM (n = 5), and 49 ± 2 mV with 1 µM (n = 5; Fig. 1A). To identify the receptor types of SubP, we used tachykinin NK1 receptor antagonists. After pretreatment with tachykinin NK1 receptor antagonists ([D-Arg1, D-Pro2, D-Trp7,9, Leu11]-SP 0.5 µM; L703606 10µM), the effects of SubP were not apparent (n=5, Fig. 1B). The degree of depolarization was 34 ± 2 mV with SubP (n = 5), 3 ± 1 mV with ([D-Arg1, D-Pro2, D-Trp7,9, Leu11]-SP, and 3 ± 2 mV with L703606 (n = 5; Fig. 1B). Tachykinin NK1 receptor antagonist itself did not change the resting membrane potential, frequency, and amplitude of pacemaking activity. Given these results concerning depolarization, we were curious about which ion channels were involved in SubP-induced depolarization of pacemaking activity in cultured ICC clusters. Among various ion channels, we focused on the NALCN [18]. In neurons, NALCN forms a nonselective cation channel carrying a small background leak Na+ current at the resting membrane potential, and the channel is also regulated by neuromodulators such as SubP, with antagonist sensitivities to similar to those observed here in the SubP action on the pacemaking activities [22]. To reinforce the candidacy of NALCN, both NALCN mRNA (Fig. 1C) and protein (Fig. 1D) were detected in the cultured ICC clusters by RT-PCR and Western blotting techniques, respectively.
Fig. 1.
Effects of SubP and SubP antagonists on pacemaking activity in cultured ICC clusters. (A) Effects of SubP (0.1–1 µM) on pacemaking activity. Population data for the manipulations shown in left are expressed as the degree of depolarization. Values are mean ± SEM. (B) Effects of tachykinin TACR1 receptor antagonists ([D-Arg1, D-Pro2, D-Trp7,9, Leu11]-SP 0.5 µM; L703606 10 µM) on SubP-induced response in pacemaking activity. Population data for the manipulations shown in left is expressed as the degree of depolarization. Values are mean ± SEM. (C) RT-PCR detection of NALCN (373 bp) is expressed in the cultured ICC clusters. (D) NALCN proteins are detected at molecular weight 200 kilodaltons (kDa) using western blotting.
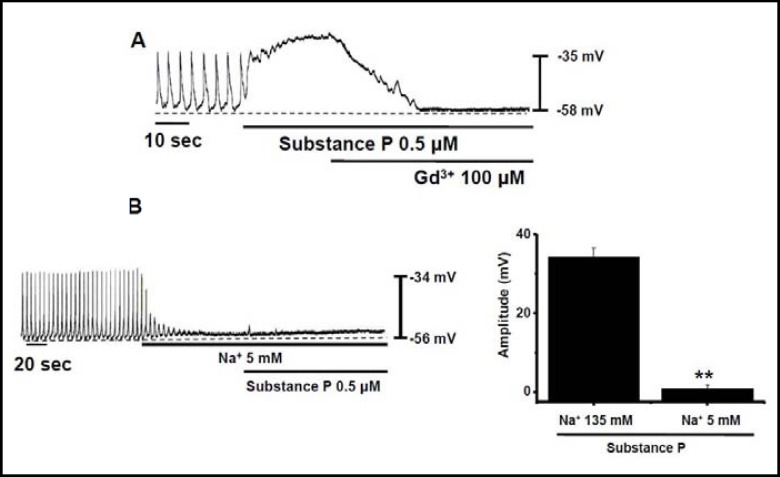
Effects of gadolinium ion and reduced external Na+ on SubP-induced depolarization of pacemaking activity in cultured ICC clusters
In neurons, the basal NALCN current and the SubP-induced NALCN-dependent current in neurons can be blocked by Gd3+ [18, 22]. In the ICC cells under current clamp mode, Gd3+ inhibited the SubP-induced depolarization of pacemaking activity (n = 5; Fig. 2A). Na+ is a major ion passing NALCN into the cells. Reducing external Na+ from normal to 5 mM abolished the pacemaking activity. Under these conditions, SubP did not depolarize the pacemaking activity (Fig. 2B), suggesting that Na+ was the main charge carrier of the pacemaking activity in ICC clusters. Under normal conditions, the amplitude of pacemaking activity was 25 ± 3 mV (n = 5), and at 5 mM of Na+ externally, the amplitude was 2 ± 1 mV (n = 5; Fig. 2B). These results are similar with the characteristics of NALCN [18, 22].
Fig. 2.
Effects of gadolinium ion and reducing external Na+ on the SubP-induced depolarization of pacemaking activity in cultured ICC clusters. (A) Effect of gadolinium ion (Gd3+) on SubP– induced response of pacemaking activity. (B) Effects of SubP in the presence of 5 mM of external Na+ in pacemaking activity. Population data for the manipulations shown in left is expressed as the amplitude of depolarization. Values are mean ± SEM.
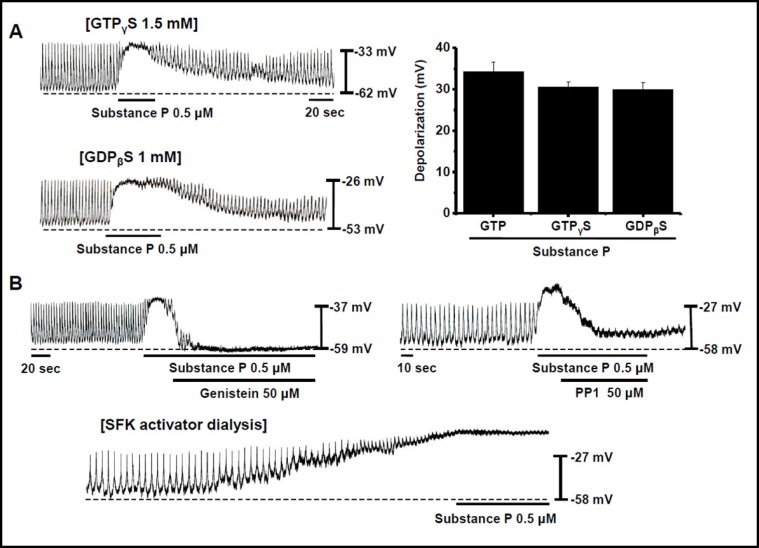
Effects of G proteins and Src family on the SubP-induced depolarization of pacemaking activity in cultured ICC clusters
To investigate the role of G proteins in the SubP-induced depolarization of pacemaking activity in ICC clusters, we applied G proteins in active or inactive states with non-hydrolysable analogues of GTP (GTPyS) or GDP (GDPßS), respectively, by means of patch pipettes. SubP still produced the depolarization of pacemaking activity (Fig. 3A). The degree of depolarization was 34 ± 2 mV with GTP (n = 5), 31 ± 1 mV with GTP S (n = 5), and 30 ± 2 mV with GDPpS (n = 5; Fig. 3A). Thus, the depolarization of pacemaking activity by SubP through G proteins is probably by means of an unconventional mechanism that does not require G-protein stimulation. Some G protein coupled receptors (GPCRs) may also activate the Src family of tyrosine kinases (SFKs) [23, 24]. Bath application of genistein (a phosphotyrosine kinase inhibitor) or PP1 (a SFK inhibitor) inhibited the SubP-induced depolarization of pacemaking activity in ICC clusters (Figs. 3B and 3B), suggesting that SFKs are required for the depolarization of pacemaking activity by SubP in ICC clusters. Also, intracellular dialysis with an SFK activator by means of a patch pipette induced a gradual increase of the depolarization of pacemaking activity (Fig. 3B). After the depolarization plateaued, SubP no longer depolarized the pacemaking activity (Fig. 3B), suggesting that SubP and SFKs activate a common channel. These results suggest that SFK activation is necessary for the depolarization of pacemaking activity by SubP in ICC clusters. The characteristics of NALCN in mouse hippocampal neurons [22] are very similar with the results of this ICC clusters, indicating that NALCN is involved in the SubP-induced depolarization of pacemaking activity in ICC clusters.
Fig. 3.
Effects of G proteins and the Src family kinases on the SubP-induced depolarization of pacemaking activity in cultured ICC clusters. (A) Effect of G proteins on the SubP-induced response of pacemaking activity. Population data for the manipulations shown in left is expressed as the degree of depolarization. Values are mean ± SEM. (B) Effect of the Src family of tyrosine kinases (SFKs) on the SubP-induced response of pacemaking activity. Effects of genistein (a phosphotyrosine kinase inhibitor) or PP1 (an SFK inhibitor) on the SubP-induced response of pacemaking activity. Effect of intracellular dialysis with an SFK activator by means of a patch pipette on pacemaking activity.
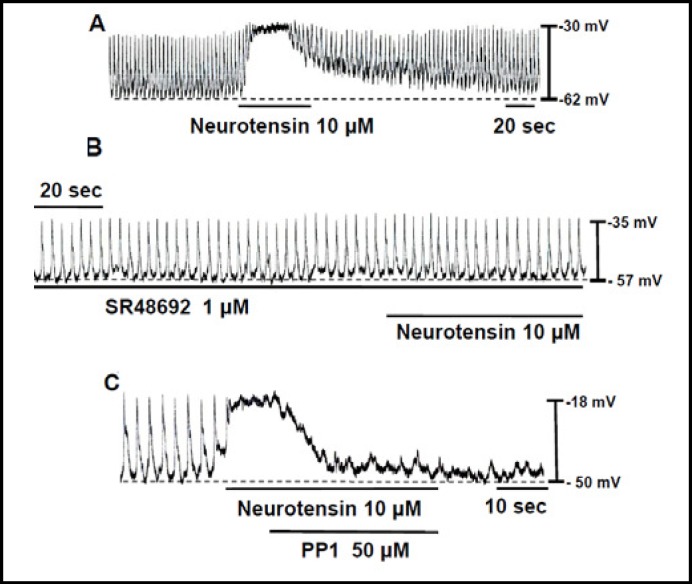
Effects of neurotensin on pacemaking activity in cultured ICC clusters
In neurons, NALCN is also activated by another neuropeptide such as neurotensin (NT). We investigated whether NT had effects on the pacemaking activity in cultured ICC clusters. Under current clamp mode, the addition of NT (10 µM) produced a depolarization of pacemaking activity (Fig. 4A). Under control conditions at the current clamp mode, the resting membrane potential was −59 ± 4 mV and the amplitude of pacemaking activity was 26 ± 4 mV. In the presence of NT, the resting membrane potential and the amplitude of pacemaking activity were −26 ± 4 and 3 ± 2 mV, respectively (n = 5). The depolarization of pacemaking activity by NT in cultured ICC clusters was blocked by an NT receptor antagonist SR48692 (Fig. 4B) and the SFK inhibitor PP1 (Fig. 4C), suggesting that the depolarization of pacemaking activity by NT is similar to that by SubP and it requires SFK activation.
Fig. 4.
Effects of neurotensin (NT) on the pacemaking activity in cultured ICC clusters. (A) Effect of neurotensin (NT) on the pacemaking activity. (B) Effects of a NT receptor antagonist SR48692 and the SFK inhibitor PP1 on the NT-induced response of pacemaking activity.
Effects of SubP in single ICCs
To record the SubP-induced inward currents, we performed whole-cell voltage-clamp recordings in cultured single ICCs. Under a voltage clamp at a holding potential of −60 mV, SubP induced inward currents in the presence of 135 mM of external Na+ (Fig. 5A). Reducing external Na+ from normal to 5 mM abolished the SubP induced inward currents (Fig. 5B), suggesting a crucial role for Na+ in the current inductions. After pretreatment with tachykinin NK1 receptor antagonists ([D-Arg1, D-Pro2, D-Trp7,9, Leu11]-SP 0.5 ìM; L703606 10 µM), the effects of SubP (0.5 µM) was not evident (n=5, Figs. 5C and 5D).
Fig. 5.
Effects of SubP in Single ICCs. (A) Effects of SubP in Single ICCs. Under a voltage clamp at a holding potential of −60 mV, SubP induced inward currents in the presence of 135 mM of external Na+. (B) Effects of SubP in the presence of 5 mM of external Na+ in single ICCs. Reducing external Na+ from normal to 5 mM, abolished the inward currents. (C, D) Effects of tachykinin NK1 receptor antagonists ([D-Arg1, D-Pro2, D-Trp7,9, Leu11]-SP 0.5 µM; L703606 10 µM) in single ICCs.
Expression of NALCN and UNC80 protein in cultured ICCs
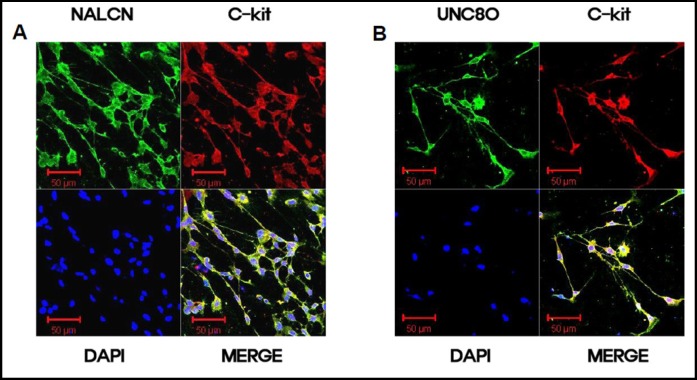
UNC80 is a protein that is associated with the NALCN and binds Src kinases and recruits Src into the channel complex [25]. Double staining with anti-c-kit (a marker of ICCs) and anti-NALCN or anti-UNC80 antibodies showed NALCN and UNC80 immunoreactivity in c-kit–immunopositive ICCs (Fig. 6).
Fig. 6.
Expression of NALCN and UNC80 proteins in cultured ICCs. (A) Double labeling with NALCN (green) and c-kit (red) antibodies on cultured ICCs. Cultured ICCs show colocalization of NALCN-like and c-kit like immuno-reactivity (bars = 50 µm). (B) Double labeling with UNC80 (green) and c-kit (red) antibodies on cultured ICCs. Cultured ICCs show colocalization of UNC80-like and c-kit like immuno-reactivity (bars = 50 µm).
NALCN is required for the SubP-induced depolarization of pacemaking activity in cultured ICCs
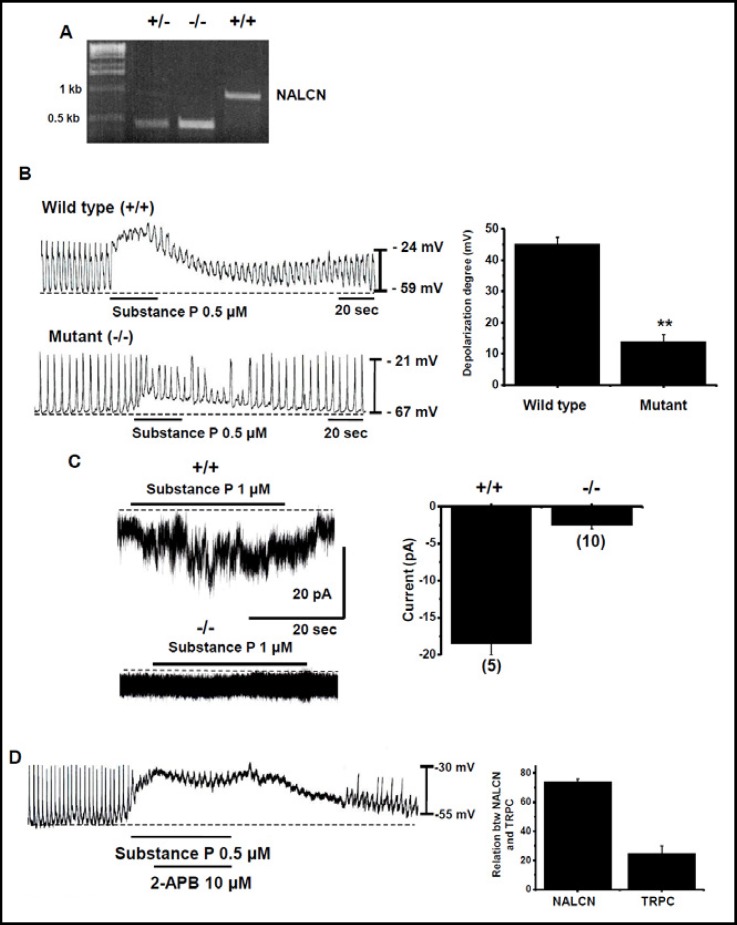
To determine the potential contribution of NALCN in the SubP-induced depolarization of pacemaking activity in cultured ICCs, we used wild type and NALCN−/− mutant mice [18] (Fig. 7A). In the wild type, SubP-induced the depolarization of pacemaking activity in cultured ICC clusters under current clamp mode. The degree of depolarization was 45 ± 5 mV (n = 5; Figs. 7B and 7B). However, in the mutant mice, SubP marginally depolarized the pacemaking activity in cultured ICC clusters. The degree of depolarization was 14 ± 5 mV (n = 5; Figs. 7B and 7B). In single ICCs, under a voltage clamp at a holding potential of −60 mV, SubP induced inward currents in normal mice (n = 5; Fig. 7C). However, in mutant mice, SubP did not induce inward currents (n = 5; Fig. 7C). The currents were 19 ± 4 pA in normal mice and 3 ± 1 pA in mutant ICCs (Fig. 7C). This finding confirmed the dependence of the NALCN in the SubP-induced depolarization on the pacemaking activity in cultured ICCs. In NALCN mutant mice, the SubP-induced depolarization did not disappear completely. Therefore, we investigated the involvement of the TRPC channel. When we applied 2-APB, a TRPC channel blocker, in the SubP-induced depolarization in pacemaking activity, SubP-induced effect was decreased marginally (Fig. 7D). In SubP-induced depolarization in pacemaking activity in cultured ICC clusters, we estimated that NALCN was responsible for 75 ± 2% and TRPC for about 24 ± 5% (Fig. 7D). Therefore, TRPC channel is also involved, albeit partially, in the SubP-induced depolarization in pacemaking activity in cultured ICCs [26].
Fig. 7.
NALCN is required for the SubP-induced depolarization on the pacemaking activity in cultured ICCs. (A) RT-PCR detection of NALCN using tail clips within approximately 10 hours of birth. (B) Effects of SubP in pacemaking activity in wild-type and Nalcn knockout ICCs. Population data for the manipulations shown in left is expressed as the degree of depolarization. Values are mean ± SEM. (C) SubP-activated inward currents in small intestine single ICCs from wild-type and Nalcn mutant mice. Cells were held at −60 mV. Population data for the manipulations shown in left is expressed as the degree of currents. Values are mean ± SEM. (D) Effect of 2-APB on SubP-induced response in pacemaking activity.
Discussion
Our data show that NALCN is involved in the SubP-induced depolarization of pacemaking activity in ICCs. The principle evidences supporting our findings were: (1) inhibition of the SP–induced depolarization by a NALCN blocker Gd3+, (2) suppression of the depolarization by reducing the concentration of a NALCN-permeant ion Na+, (3) modulation of the depolarization by a NALCN activator SFK, (4) identification of NALCN like immunoreactivity in c-kit positive ICCs, (5) inhibition of SubP–induced depolarization in Nalcn knockout, and (6) absence of the SubP-induced inward current in the mutant.
After ICCs were shown to generate a rhythmic pacemaker current [1, 2], ICCs cultured with SCF have been used for studying the pacemaking activity [27, 28]. The cultured ICC clusters are a mixture of smooth muscle cells and ICCs. If NALCN is involved in pacemaking activity, this activity might be inhibited in NALCN mutant mice. However, the basal pacemaking activity was not changed in the mutant, suggesting that NALCN is not required for the basal pacemaking. Instead, our finding that the SubP-induced depolarization is drastically decreased in the NALCN mutant mice (Fig. 7), suggests that the NALCN channel is responsible for the regulation of the pacemaking in ICCs.
NALCN is a cation channel carrying a small background leak Na+ current at the resting membrane potential in neurons [18]. NALCN is unique in that its S4 transmembrane segments lack some of the charged residues (K and R) found at every third position in the S4s of the NaV, CaV, and KV channels. In addition, its pore filter regions have an EEKE motif, a mixture between the EEEE found in the CaVs and the DEKA of NaVs [29]. Consistent with these unique structural features, NALCN is the only nonselective, noninactivating, voltage-independent channel among the family's 21 members [18]. NALCN is widely-expressed in the nervous system. In cultured hippocampal neurons, it contributes the major TTX and Cs-resistant Na+ leak at voltages close to the resting membrane potential. Mice with a targeted disruption in Nalcn have severely disrupted respiratory rhythms and die shortly after birth [18]. Mutations in the NALCN homolog genes in Drosophila melanogaster and Caenorhabditis elegans lead to defects in locomotion, anesthetic sensitivity, rhythmic behaviors, and synaptic function [30, 31, 32, 33]. In addition, mutant screening suggests that nalcn genetically interacts with other genes such as unc-79 and unc-80, whose mammalian counterparts are unc79 and unc80, respectively [30, 31, 33]. UNC79 and UNC80 forms a complex with NALCN in the mouse brain [34].
NALCN has various functions in cellular physiology. It is activated by muscarinic receptors in a pancreatic ß-cell line [35]. NALCN is required for the pacemaker activity of the adult central pattern generator neurons in Lymnaea stagnalis [36]. The channel is also regulated by neuromodulators. In cultured hippocampal pyramidal neurons, the effect of SubP on NALCN through TACR1 is independent of G-proteins and requires the SFKs [22]. Besides the above-mentioned functions, this study shows that NALCN is a cation channel involved in the SubP-induced depolarization in the pacemaking activity of the murine small intestine.
ICCs generate the electrical pacemaker activity (slow wave) in GI muscles [1, 2]. Slow waves propagate within ICC networks, are conducted into smooth muscle cells via gap junctions, and initiate phasic muscle contractions (gut motility) by activating Ca2+ entry through L-type Ca2+ channels. The pacemaker activity in the murine small intestine is due mainly to periodic activation of NSCC, TRPC4 or TRPM7 [4, 5] or Cl- channels [6, 7]. Especially, Hwang et al. [37] suggested that anoctamin (ANO) 1/TMEM16A in ICCs is important for the pacemaker activity in GI muscles. Tmem16a encodes ANO1, a Ca2+-activated Cl− channel [38, 39, 40], and immunohistochemical studies have documented expression of ANO1 protein in ICC [41, 42]. Recent findings suggest that the 5-lipoxygenase (5-LOX) inhibitors, NDGA and AA861, are potent blockers of the TRPM7 channel capable of attenuating TRPM7's function [43], making them effective tools for the biophysical characterization and suppression of TRPM7 channel conductance. Therefore, we investigated the effects of 5-LOX inhibitors on pacemaking activity in cultured ICC clusters and single ICC. NDGA and AA861 decreased the amplitude of pacemaker potentials in ICC clusters, but the resting membrane potentials displayed little change. Also, perfusing NDGA and AA861 into the bath reduced both inward current and outward current in TRPM7-like current in single ICC [44]. But, they had no effects on the overexpressed tmen16a, Ca2+-activated Cl− currents in HEK 293 cells [44]. Therefore, the nature of molecular candidates involved in the pacemaing activity in ICCs needs to be further investigated.
Hormones and the enteric nervous system regulate GI mechanical contractions and electrical activity by modulating the slow waves [45]. ICCs could thus be physiological and therapeutic targets for numerous hormones, neurotransmitters, and drugs. In the enteric nervous system, numerous neurotransmitters are released and they regulate GI motility. ICCs are connected to each other to form a network, and they form gap junctions with smooth muscles. Therefore, the pacemaker currents induced by ICCs are directly transmitted to smooth muscles through gap junctions [3]. In addition, the ICCs are closely associated with varicosities of the enteric nerves, which mediate inhibitory and excitatory nerve signals to smooth muscles [3, 46, 47, 48]. Therefore, these cells play an important role as basic regulators of GI motility. Moreover, disruptions to these cells, such as a reduction in their numbers or changes to their morphological patterns, produce pathological motility disorders in the GI tract [49, 50, 51].
SubP increases the slow wave frequency and duration and activates a non selective cation channel in murine pacemaker ICCs [16]. The single channel conductance is approximately 25 pS and, in the on-cell configuration, the activity can occur in a rhythmic fashion. SubP also modulates NSC currents in primary sensory neurons [52] and in human oesophageal smooth muscle [53]. Oh et al. [54] investigated the molecular candidate for the tachykinin-activated NSC currents in sensory neurons. In trasfected HEK293 cells, SubP could evoke TRPC4-mediated inward currents through the NK2 receptors. SubP also activated TRPC3 and/or TRPC7 channels to enhance respiratory rhythm regularity [26]. TRPCs are nonselective cation channels that are permeable to monovalent and divalent ions [55]. The SubP receptor could be functionally linked to TRPC channels [26, 54]. In this study, in Nalcn mutant, the SubP-induced depolarization was not completely abolished (Fig. 7). Therefore, activation of TRPC channels might be responsible for the remaining effects.
ICCs are the pacemakers in GI muscles and also mediate or transduce inputs from the enteric nervous system. Because of the central role of ICCs in GI motility, loss of these cells would be extremely detrimental. Research into the biology of ICCs provides exciting new opportunities to understand the etiology of diseases that have long eluded comprehension. Thus, NALCN could be a new target for pharmacological treatment of GI motility disorders.
Acknowledgements
This work was supported by a Mid-career Researcher Program through NRF grant funded by the MEST to Insuk So (No. 2009-0084587) and NIH grants to DR (5R01NS055293 and 1R01NS074257). We thank Chunlei Cang and Janice Harlow (University of Pennsylvania) for help on patch clamp recording, tissue culture, genotyping and reagent preparation. This study was supported by Seoul National University Hospital Research Fund (03-2011-0310) (I.So).
References
- 1.Ward SM, Burns AJ, Torihashi S, Sanders KM. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol. 1994;480:91–97. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huizinga JD, Thuneberg L, Klüppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- 3.Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996;111:492–515. doi: 10.1053/gast.1996.v111.pm8690216. [DOI] [PubMed] [Google Scholar]
- 4.Koh SD, Jun JY, Kim TW, Sanders KM. A Ca2+-inhibited non-selective cation conductance contributes to pacemaker currents in mouse interstitial cell of Cajal. J Physiol. 2002;540:803–814. doi: 10.1113/jphysiol.2001.014639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim BJ, Lim HH, Yang DK, Jun JY, Chang IY, Park CS, So I, Stanfield PR, Kim KW. Melastatin-type transient receptor potential channel 7 is required for intestinal pacemaking activity. Gastroenterology. 2005;129:1504–1517. doi: 10.1053/j.gastro.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 6.Huizinga JD, Ye J, Molleman A. High-conductance chloride channels generate pacemaker currents in interstitial cells of Cajal. Gastroenterology. 2002;123:1627–1636. doi: 10.1053/gast.2002.36549. [DOI] [PubMed] [Google Scholar]
- 7.Zhu MH, Kim TW, Ro S, Yan W, Ward SM, Koh SD, Sanders KM. A Ca2+-activated Cl- conductance in interstitial cells of Cajal linked to slow wave currents and pacemaker activity. J Physiol. 2009;587:4905–4918. doi: 10.1113/jphysiol.2009.176206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daniel EE. Physiology and pathophysiology of the interstitial cells of Cajal: from bench to beside III. Interaction of interstitial cells of Cajal with neuromediators: an interim assessment. Am J Physiol. 2001;281:G1329–G1332. doi: 10.1152/ajpgi.2001.281.6.G1329. [DOI] [PubMed] [Google Scholar]
- 9.Sanders KM. G protein-coupled receptors in gastrointestinal physiology IV. Neural regulation of gastrointestinal smooth muscle. Am J Physiol. 1998;275:G1–G7. doi: 10.1152/ajpgi.1998.275.1.G1. [DOI] [PubMed] [Google Scholar]
- 10.Bartho L, Holzer P. Search for a physiological role of substance P in gastrointestinal motility. Neuroscience. 1985;16:1–32. doi: 10.1016/0306-4522(85)90043-0. [DOI] [PubMed] [Google Scholar]
- 11.Mayer EA, Loo DDF, Snape Jr WJ, Sachs G. The activation of calcium and calcium-activated potassium channels in mammalian colonic smooth muscle by substance P. J Physiol. 1990;420:47–71. doi: 10.1113/jphysiol.1990.sp017901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watson SP, Downes CP. Substance P induced hydrolysis of inositol phospholipids in guinea-pig ileum and hypothalamus. Eur J Pharmacol. 1983;93:223–245. doi: 10.1016/0014-2999(83)90144-9. [DOI] [PubMed] [Google Scholar]
- 13.Lavin ST, Southwell BR, Murphy R, Jenkinson KM, Furness JB. Activation of neurokinin 1 receptors on interstitial cells of Cajal of the guinea-pig small intestine by substance P. Histochem Cell Biol. 1998;110:263–271. doi: 10.1007/s004180050288. [DOI] [PubMed] [Google Scholar]
- 14.Vannucchi MG, De Giorgio R, Faussone-Pellegrini MS. NK1 receptor expression in the interstitial cells of Cajal and neurons and tachykinins distribution in rat ileum during development. J Comp Neurol. 1997;383:153–162. doi: 10.1002/(sici)1096-9861(19970630)383:2<153::aid-cne3>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 15.Jun JY, Choi S, Yeum CH, Chang IY, You HJ, Park CK, Kim MY, Kong ID, Kim MJ, Lee KP, So I, Kim KW. Substance P induces inward current and regulates pacemaker currents through tachykinin NK1 receptor in cultured interstitial cells of Cajal of murine small intestine. Eur J Pharmacol. 2004;495:35–42. doi: 10.1016/j.ejphar.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 16.d'Antonio C, Wang B, McKay C, Huizinga JD. Substance P activates a non-selective cation channel in murine pacemaker ICC. Neurogastroenterol Motil. 2009;21:985–e79. doi: 10.1111/j.1365-2982.2009.01318.x. [DOI] [PubMed] [Google Scholar]
- 17.Ben-Mabrouk F, Tryba AK. Substance P modulation of TRPC3/7 channels improves respiratory rhythm regularity and ICAN-dependent pacemaker activity. Eur J Neurosci. 2010;31:1219–1232. doi: 10.1111/j.1460-9568.2010.07156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu B, Su Y, Das S, Liu J, Xia J, Ren D. The neuronal NALCN channel contributes resting sodium permeability and is required for normal respiratory rhythm. Cell. 2007;129:371–383. doi: 10.1016/j.cell.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 19.Nicholls JG, Martin AR, Wallace BG. From Neuron to Brain. IV Edition. Sunderland, MA: Sinauer Associates, Inc.; 2001. [Google Scholar]
- 20.Snutch TP, Monteil A. The sodium “leak“ has finally been plugged. Neuron. 2007;54:505–507. doi: 10.1016/j.neuron.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 21.Yu FH, Yarov-Yarovoy V, Gutman GA, Catterall WA. Overview of molecular relationships in the voltage-gated ion channel superfamily. Pharmacol Rev. 2005;57:387–395. doi: 10.1124/pr.57.4.13. [DOI] [PubMed] [Google Scholar]
- 22.Lu B, Su Y, Das S, Wang H, Wang Y, Liu J, Ren D. Peptide neurotransmitters activate a cation channel complex of NALCN and UNC-80. Nature. 2009;457:741–744. doi: 10.1038/nature07579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by b-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 24.DeFea KA, Vaughn ZD, O'Bryan EM, Nishijima D, Déry O, Bunnett NW. The proliferative and antiapoptotic effects of substance P are facilitated by formation of b-arrestin-dependent scaffolding complex. Proc Natl Acad Sci USA. 2000;97:11086–11091. doi: 10.1073/pnas.190276697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang H, Ren D. UNC80 functions as a scaffold for Src kinases in NALCN channel function. Channels (Austin) 2009;3:161–163. doi: 10.4161/chan.3.3.8853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ben-Mabrouk F, Tryba AK. Substance P modulation of TRPC3/7 channels improves respiratory rhythm regularity and ICAN-dependent pacemaker activity. Eur J Neurosci. 2010;31:1219–1232. doi: 10.1111/j.1460-9568.2010.07156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koh SD, Sanders KM, Ward SM. Spontaneous electrical rhythmicity in cultured interstitial cells of cajal from the murine small intestine. J Physiol. 1998;513:203–213. doi: 10.1111/j.1469-7793.1998.203by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomsen L, Robinson TL, Lee JC, Farraway LA, Hughes MJ, Andrews DW, Huizinga JD. Interstitial cells of Cajal generate a rhythmic pacemaker current. Nat Med. 1998;4:848–851. doi: 10.1038/nm0798-848. [DOI] [PubMed] [Google Scholar]
- 29.Lee JH, Cribbs LL, Perez-Reyes E. Cloning of a novel four repeat protein related to voltage-gated sodium and calcium channels. FEBS Lett. 1999;445:231–236. doi: 10.1016/s0014-5793(99)00082-4. [DOI] [PubMed] [Google Scholar]
- 30.Humphrey JA, Hamming KS, Thacker CM, Scott RL, Sedensky MM, Snutch TP, Morgan PG, Nash HA. A putative cation channel and its novel regulator: Cross-species conservation of effects on general anesthesia. Curr Biol. 2007;17:624–629. doi: 10.1016/j.cub.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 31.Jospin M, Watanabe S, Joshi D, Young S, Hamming K, Thacker C, Snutch TP, Jorgensen EM, Schuske K. UNC-80 and the NCA ion channels contribute to endocytosis defects in synaptojanin mutants. Curr Biol. 2007;17:1595–1600. doi: 10.1016/j.cub.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 32.Pierce-Shimomura JT, Chen BL, Mun JJ, Ho R, Sarkis R, McIntire SL. Genetic analysis of crawling and swimming locomotory pattern in C. elegans. Proc Natl Acad Sci USA. 2008;105:20982–20987. doi: 10.1073/pnas.0810359105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yeh E, Ng S, Zhang M, Bouhours M, Wang Y, Wang M, Hung W, Aoyagi K, Melnik-Martinez K, Li M, Liu F, Schafer WR, Zhen M. A putative cation channel, NCA-1, and a novel protein, UNC-80, transmit neuronal activity in C. elegans. PLoS Biol. 2008;6:e55. doi: 10.1371/journal.pbio.0060055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu B, Zhang Q, Wang H, Wang Y, Nakayama M, Ren D. Extracellular calcium controls background current and neuronal excitability via an UNC79-UNC80-NALCN cation channel complex. Neuron. 2010;68:488–499. doi: 10.1016/j.neuron.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swayne LA, Mezghrani A, Varrault A, Chemin J, Bertrand G, Dalle S, Bourinet E, Lory P, Miller RJ, Nargeot J, Monteil A. The NALCN ion channel is activated by M3 muscarinic receptors in a pancreatic beta-cell line. EMBO Rep. 2009;10:873–880. doi: 10.1038/embor.2009.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu TZ, Feng ZP. A sodium leak current regulates pacemaker activity of adult central pattern generator neurons in Lymnaea stagnalis. PLoS One. 2011;6:e18745. doi: 10.1371/journal.pone.0018745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hwang SJ, Blair PJ, Britton FC, O'Driscoll KE, Hennig G, Bayguinov YR, Rock JR, Harfe BD, Sanders KM, Ward SM. Expression of anoctamin 1/ TMEM16A by interstitial cells of Cajal is fundamental for slow wave activity in gastrointestinal muscles. J Physiol. 2009;587:4887–4904. doi: 10.1113/jphysiol.2009.176198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322:590–594. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- 39.Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell. 2008;134:1019–1029. doi: 10.1016/j.cell.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, Raouf R, Shin YK, Oh U. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008;455:1210–1215. doi: 10.1038/nature07313. [DOI] [PubMed] [Google Scholar]
- 41.Espinosa I, Lee CH, Kim MK, Rouse BT, Subramanian S, Montgomery K, Varma S, Corless CL, Heinrich MC, Smith KS, Wang Z, Rubin B, Nielsen TO, Seitz RS, Ross DT, West RB, Cleary ML, van de Rijn M. A novel monoclonal antibody against DOG1 is a sensitive and specific marker for gastrointestinal stromal tumors. Am J Surg Pathol. 2008;32:210–218. doi: 10.1097/PAS.0b013e3181238cec. [DOI] [PubMed] [Google Scholar]
- 42.Gomez-Pinilla PJ, Gibbons SJ, Bardsley MR, Lorincz A, Pozo MJ, Pasricha PJ, Van de Rijn M, West RB, Sarr MG, Kendrick ML, Cima RR, Dozois EJ, Larson DW, Ordog T, Farrugia G. Ano1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1370–G1381. doi: 10.1152/ajpgi.00074.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen HC, Xie J, Zhang Z, Su LT, Yue L, Runnels LW. Blockade of TRPM7 channel activity and cell death by inhibitors of 5-lipoxygenase. PLoS One. 2010;5:e11161. doi: 10.1371/journal.pone.0011161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim BJ, Nam JH, Kim SJ. Effects of transient receptor potential channel blockers on pacemaker activity in interstitial cells of Cajal from mouse small intestine. Mol Cells. 2011;32:153–160. doi: 10.1007/s10059-011-1019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Olsson C, Holmgren S. The control of gut motility. Comp Biochem Physiol. 2001;128:481–503. doi: 10.1016/s1095-6433(00)00330-5. [DOI] [PubMed] [Google Scholar]
- 46.Publicover NG, Hammond EM, Sanders KM. Amplification of nitric oxide signaling by interstitial cells isolated from canine colon. Proc Natl Acad Sci. 1993;90:2087–2091. doi: 10.1073/pnas.90.5.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ward SM, Beckertt EA, Wang X, Baker F, Khoyi M, Sanders KM. Interstitial cells of Cajal mediate choliner gic neurotransmission from enteric motor neurons. J Neurosci. 2000;20:1393–1403. doi: 10.1523/JNEUROSCI.20-04-01393.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ward SM, Ordog T, Koh SD, Baker SA, Jun JY, Amberg G, Monaghan K, Sanders KM. Pacemaking in interstitial cells of Cajal depends upon calcium handling by endoplasmic reticulum and mitochondria. J Physiol. 2000;525:355–361. doi: 10.1111/j.1469-7793.2000.t01-1-00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jain D, Khalid M, Manish T, Culpepper-Morgan J, Proctor DD. Role of interstitial cells of Cajal in motility disorders of the bowel. Am J Gastroenterol. 2003;98:618–624. doi: 10.1111/j.1572-0241.2003.07295.x. [DOI] [PubMed] [Google Scholar]
- 50.Sanders KM, Ordog T, Koh SD, Torihashi S, Ward SM. Development and plasticity of interstitial cells of Cajal. Neurogastroenterol Motil. 1999;11:311–338. doi: 10.1046/j.1365-2982.1999.00164.x. [DOI] [PubMed] [Google Scholar]
- 51.Vanderwinden JM, Rumessen JJ. Interstitial cells of Cajal in human gut and gastrointestinal disease. Microsc Res Tech. 1999;47:344–360. doi: 10.1002/(SICI)1097-0029(19991201)47:5<344::AID-JEMT6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 52.Inoue K, Nakazawa K, Inoue K, Fujimori K. Nonselective cation channels coupled with tachykinin receptors in rat sensory neurons. J Neurophysiol. 1995;73:736–742. doi: 10.1152/jn.1995.73.2.736. [DOI] [PubMed] [Google Scholar]
- 53.Kovac JR, Chrones T, Preiksaitis HG, Sims SM. Tachykinin receptor expression and function in human esophageal smooth muscle. J Pharmacol Exp Ther. 2006;318:513–520. doi: 10.1124/jpet.106.104034. [DOI] [PubMed] [Google Scholar]
- 54.Oh EJ, Gover TD, Cordoba-Rodriguez R, Weinreich D. Substance P evokes cation currents through TRP channels in HEK293 cells. J Neurophysiol. 2003;90:2069–2073. doi: 10.1152/jn.00026.2003. [DOI] [PubMed] [Google Scholar]
- 55.Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]