Abstract
Background
P. aeruginosa chronically colonizes the lung in CF patients and elicits a proinflammatory response. Excessive secretion of IL-6 and IL-8 by CF airway cells in response to P. aeruginosa infection in the CF airway is though to contribute to lung injury. Accordingly, the goal of this study was to test the hypothesis that Corr4a and VRT325, investigational compounds that increase ΔF508-CFTR mediated Cl− secretion in human CF airway cells, reduce the pro-inflammatory response to P. aeruginosa.
Methods
IL-6 and IL-8 secretion by polarized CF human airway epithelial cells (CFBE41o-) were measured by multiplex analysis, and ΔF508-CFTR Cl- secretion was measured in Ussing chambers. Airway cells were exposed to P. aeruginosa (PAO1 or PA14) and Corr4a or VRT325.
Results
Corr4a and VRT325 increased ΔF508-CFTR Cl− secretion but did not reduce either constitutive IL-6 or IL-8 secretion, or IL-6 and IL-8 secretion stimulated by P. aeruginosa (PA14 or PAO1).
Conclusions
Corr4a and VRT325 do not reduce the inflammatory response to P. aeruginosa in human cystic fibrosis airway epithelial cells.
Key Words: CFTR, P. aeruginosa, Cystic fibrosis, Drug treatment, DF508-CFTR, Cl secretion
Introduction
Cystic Fibrosis (CF), one of the most common lethal genetic disorders in Caucasians, is an autosomal recessive disorder caused by mutations in the CFTR gene [1, 2, 3]. The most common mutation in CFTR, ΔF508, results in the degradation of CFTR by the proteasome. Thus, unlike wt-CFTR, ΔF508-CFTR is unable to reach the apical plasma membrane and mediate Cl− secretion. Accordingly, CF is characterized by abnormal CFTR mediated Cl−secretion in affected tissues including the lung, pancreas, gastrointestinal tract, liver, and sweat glands. Reduced Cl− and fluid secretion in the lung, hyperabsorption of sodium together with an increased viscosity of mucus all contribute to reduced mucociliary clearance of bacteria from the lung of CF patients [4, 5, 6]. Chronic bacterial infection primarily by P. aeruginosa and an exacerbated and prolonged hyper-inflammatory response result in the accumulation of neutrophils in the CF airways, which is responsible for lung injury and the morbidity and mortality in CF [7, 8].
Close to 80% of CF patients are colonized by highly drug resistant strains of Pseudomonas aeruginosa by the age of 16 [7, 8]. P. aeruginosa induces an intense proinflammatory response in the CF lung, which is characterized by an increase in cytokine secretion, notably IL-6 and IL-8 [9, 10, 11, 12]. These proinflammatory cytokines, as well as many others, play an important role in the innate immune response to P. aeruginosa infection by recruiting macrophages and neutrophils into the lung, which are critical for reducing the bacterial burden.
Recently, a major effort among CF researchers has been to identify drugs to correct defective trafficking of ΔF508-CFTR and increase Cl− secretion. A number of investigational compounds have been identified that increase the expression of ΔF508-CFTR in the apical plasma membrane of human airway epithelial cells, and at least partially restore CFTR mediated Cl− secretion. Notably, Corr4a and VRT325 increase the cell surface expression of ΔF508-CFTR and stimulate CFTR mediated Cl− secretion [13, 14]. Although identification of these and other compounds to correct defective CFTR Cl− secretion is promising, there is little information regarding the effects of these compounds on other phenotypic changes in CF. Ideally, the most efficacious drug for CF patients would enhance ΔF508-CFTR mediated Cl− secretion, reduce the excessive proinflammatory response of airway cells and reduce the bacterial burden in the lungs. Recently, we showed that Corr4a reduced the ability of P. aeruginosa to form antibiotic resistant biofilms on CF airway epithelial cells [15]. However, the effect of Corr4a and VRT325 on the proinflammatory response to P. aeruginosa has not been reported. Thus, goal of this study was to test the hypothesis that Corr4a and VRT325 reduce P. aeruginosa stimulated IL-6 and IL-8 secretion by human CF airway epithelial cells. We report that Corr4a and VRT325 did not reduce either constitutive IL-6 or IL-8 secretion, or IL-6 and IL-8 secretion stimulated by P. aeruginosa (PA14 or PAO1). Thus, Corr4a and VRT325 do not reduce the inflammatory response to P. aeruginosa in human cystic fibrosis airway epithelial cells.
Materials and Methods
Cell Culture
CFBE41o- (Cystic Fibrosis Bronchial Epithelial cells, clone 41o-) cells homozygous for the ΔF508 mutation were originally isolated from a CF patient by Dr. D. Gruenert [16, 17]. Cells were propagated in tissue polystyrene culture flasks in MEM supplemented with 10% FBS, 50 U/ml penicillin, 50 µg/ml streptomycin, and 2 mM L-glutamine. For cytokine assays and Ussing chamber studies cells were seeded on 12 mm diameter collagen-coated semi-permeable polycarbonate membranes (0.4 µm pore size; Corning Corporation; Corning, NY) at 5 × 105 cells per filter and grown in air-liquid interface at 37°C for 7-10 days to develop polarized monolayers. The media was replaced on the basolateral side of the membranes every 24 hours. To synchronize cells they were serum-starved for the last 24 hours in culture before experiments.
Drug Treatment
The Cystic Fibrosis Foundation Therapeutics Inc. provided Corr4a and VRT325 and stocks were prepared in DMSO. Corr4a (5 or 10 µM) was added to both apical and basolateral sides of cells for 24 hours and then to the basolateral side only for an additional 24 hours. VRT325 (3.3 or 6.7 µM) was added to both apical and basolateral side for 24 hours and then to the basolateral side only for an additional 72 hours. DMSO (vehicle) was added to control cells. These treatment protocols were similar to those published previously and shown to produce a submaximal (lower dose for each compound) or maximal (higher dose for each compound) stimulatory effect on CFTR Cl transport [13, 14].
Transepithelial current measurements
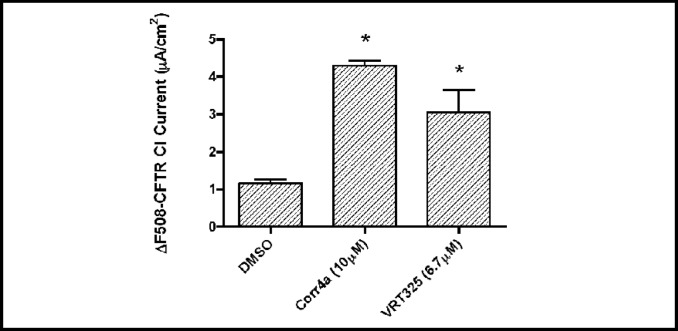
Studies were conducted to confirm that VRT325 and Corr4a enhance ΔF508-CFTR Cl− secretion in CFBE41o- cells. Polarized monolayers of CFBE41o- cells grown on 12-mm-diameter permeable membrane supports as described above were mounted in an Ussing-type chamber (Physiological Instruments, San Diego, CA) and bathed in solutions that were maintained at 37°C and pH 7.4 and stirred using bubbling with 5% CO2-95% air. The apical bath solution contained 5 mM NaCl, 115 mM sodium gluconate, 25 mM NaHCO3, 3.3 mM KH2PO4, 0.8 mM K2HPO4, 1.0 mM MgCl2, 1.0 mM CaCl2, and 10 mM mannitol. The basolateral bath solution contained 120 mM NaCl, 25 mM NaHCO3, 3.3 mM KH2PO4, 0.8 mM K2HPO4, 1.0 mM MgCl2, 1.0 mM CaCl2, and 10 mM glucose. Short-circuit current (Isc) was measured by clamping the transepithelial voltage across the monolayers to 0 mV by using a voltage clamp (model VCC MC6; Physiological Instruments) as described previously [18, 19, 20]. In previous studies we demonstrated that the genistein (50 µM) stimulated and CFTR-172 inhibited current across CFBE41o- cells was mediated by ΔF508-CFTR [19, 20]. Thus, the ΔF508-CFTR Cl− current reported in Figure 1 represents the genistein (50 µM) stimulated Isc that is inhibited by CFTR-172. Current output from the clamp was digitized using an analog-to-digital converter (iWorx, Dover, NH). Data collection and analysis were performed using LabScribe software, version 1.8 (iWorx).
Fig. 1.
VRT325 and Corr4a increased the short circuit Cl− current mediated by ΔF508-CFTR. Controls treated with DMSO, the vehicle for the drugs. *P<0.01 versus DMSO. n=5-6/group.
Bacteria
A recent clinical isolate of P. aeruginosa, PA14, and PAO1, a standard laboratory strain of P. aeruginosa, were kindly provided by Dr. George O'Toole in the Department of Microbiology and Immunology at Dartmouth Medical School. PA14 and PAO1 cultures were grown in LB medium at 37oC on a shaker for 18 hours to 5×109 CFU per ml. Bacteria were centrifuged and the pellet was washed twice with MEM. 2×108 CFU of PA14 or PAO1 were added to the apical side of polarized monolayers of CFBE41o- cells for one hour at 37°C. Thereafter, monolayers were washed twice with serum-free MEM containing 100 µg/ml gentamicin and subsequently were incubated with fresh serum-free MEM containing gentamicin on both apical (100 µl) and basolateral sides (1.5 ml) at 37°C for 24 hours. 24 hours after monolayers were treated with PA14 or PAO1 the supernatant was collected from the apical side of the monolayers, centrifuged to eliminate cellular debris, and stored at −80°C until IL-6 and IL-8 levels were measured. Preliminary studies revealed that this treatment protocol (CFU and time of exposure) produced a maximal effect on cytokine secretion with minimal damage to airway epithelial cells.
Multiplex cytokine assays
IL-6 and IL-8 were measured using a Bio-Plex human cytokine multiplex ELISA assay (Bio-Rad, Hercules, CA). Calibration curves from recombinant cytokine standards were prepared with threefold dilution steps in the same matrix as the culture supernatants (RPMI 1640 medium containing 10% FBS). High and low spikes (supernatants from stimulated human dendritic cells) were included to determine cytokine recovery. Standards and spikes were measured in triplicate, samples were measured once, and blank values were subtracted from all readings. All assays were carried out directly in a 96-well filtration plate (Millipore, Billerica, MA) at room temperature and protected from light. Briefly, wells were pre-wetted with 100 µl PBS containing 1% BSA. Beads together with a standard, sample, spikes, or blank were added in a final volume of 100 µl, and incubated together at room temperature for 30 min with continuous shaking. Beads were washed three times with 100 µl PBS containing 1% BSA and 0.05% Tween 20. A cocktail of biotinylated antibodies (IL-6 or IL-8; 50 µl/well) was added to beads for a further 30 min incubation with continuous shaking. Beads were washed three times, and incubated with streptavidin-PE for 10 min. Beads were again washed three times and resuspended in 125 µl of PBS containing 1% BSA and 0.05% Tween 20. The fluorescence intensity of the beads was measured in using the Bio-Plex array reader. Bio-Plex Manager software with five-parametric-curve fitting (Bio-Rad technical note 2861 at www.bio-rad.com) was used for data analysis.
Statistical Analysis
Data were analyzed using Graphpad Prism statistical software version 4.0b and reported as mean ± SEM. Statistical differences between means were compared by one-way ANOVA with Dunnett's post-test using GraphPad Prism (GraphPad Software, San Diego, CA, www.graphpad.com).
Results
Corr4a and VRT325 stimulate ΔF508-CFTR Cl−secretion in CFBE41o- cells
The goal of this study was to test the hypothesis that Corr4a and VRT325, investigational compounds that increase ΔF508-CFTR mediated Cl− secretion in CF airway cells, reduces the pro-inflammatory response to P. aeruginosa. To confirm that Corr4a and VRT325 enhance ΔF508-CFTR mediated Cl− secretion in CFBE41o- cells we conducted Ussing chamber studies. Ussing chamber studies on polarized CFBE41o- cells grown as confluent monolayers in an air-liquid interface culture confirmed that Corr4a and VRT325 increase Cl−secretion (Figure 1). These results are comparable to data reported by other laboratories in several different cell lines and in primary airway cells [13, 14, 21, 22], and indicate that these cells lines are an excellent model to test our hypothesis that VRT325 and Corr4a, by increasing ΔF508-CFTR mediated Cl− secretion, reduce the pro-inflammatory response to P. aeruginosa.
Corr4a does not reduce basal or P. aeruginosa simulated IL-6 and IL-8 secretion by CFBE41o-cells
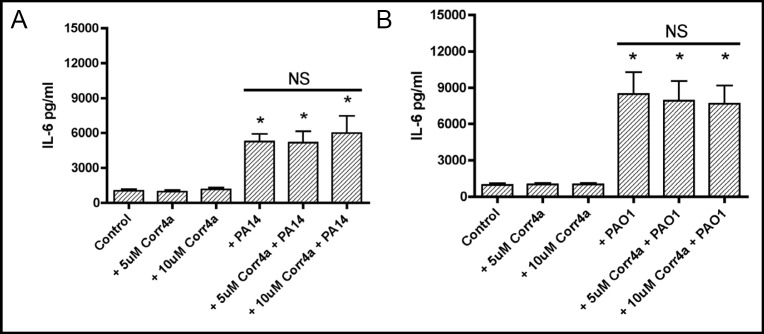
Studies were conducted to test the hypothesis that Corr4a reduces P. aeruginosa simulated IL-6 and IL-8 secretion by CFBE41o- cells. To this end airway cells were grown in air-liquid interface culture and treated with vehicle (DMSO) or Corr4a as described in Methods. P. aeruginosa (PA14 or PAO1) were added to the apical side of polarized CFBE41o- (homozygous for ΔF508/ ΔF508) cells for 1 hour. Subsequently, PA14 or PAO1 were removed by extensive washing with PBS, and the airway cells were cultured for an additional 24 hours in air liquid interface culture in MEM containing gentamicin. IL-6 secretion was measured using a Bio-Plex ELISA assay, as described in Methods.
Corr4a (5 or 10 µM) had no effect on constitutive IL-6 secretion by CFBE41o- cells (Figure 2). As expected PA14 and PAO1 increased IL-6 secretion by CFBE41o-cells treated with vehicle (Figure 2). However, Corr4a (5 or 10 µM) did not reduce the ability of PA14 and PAO1 to stimulate IL-6 secretion (Figure 2). Thus, Corr4a did not reduce the proinflammatory response to P. aeruginosa (PA14 or PAO1) as determined by measuring IL-6 secretion.
Fig. 2.
Corr4a had no effect on constitutive IL-6 secretion (compare Control with +Corr4a (5 and 10 μM)) or on the increase in IL-6 secretion in cells exposed to PA14 (2A: compare +PA14 with both +PA14 + Corr4a groups) or PAO1 (2B: compare +PAO1 with both +PAO1 + Corr4a groups). *P<0.05 versus Control. n=6/group. NS, indicates no statistical difference among these three experimental groups.
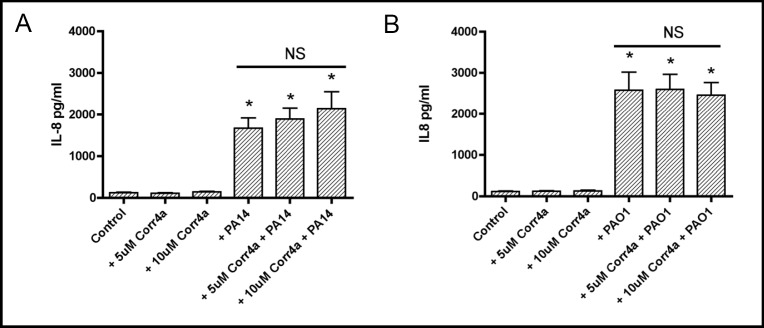
Similar experiments were conducted to examine the effect of Corr4a on IL-8 secretion by polarized CFBE41o-cells. Corr4a (5 or 10 µM) had no effect on constitutive IL-8 secretion (Figure 3). Moreover, Corr4a (5 or 10 µM) did not reduce the ability of PA14 or PAO1 to stimulate IL-8 secretion by CFBE41o- cells (Figure 3). Thus, Corr4a did not reduce the proinflammatory response to P. aeruginosa (PA14 or PAO1) as determined by measuring IL-8 secretion.
Fig. 3.
Corr4a had no effect on constitutive IL-8 secretion (compare Control with +Corr4a (5 and 10 µM)) or on the increase in IL-8 secretion in monolayers exposed to PA14 (3A: compare +PA14 with both +PA14 + Corr4a groups) or PAO1 (3B: compare +PAO1 with both +PAO1 + Corr4a groups). *P<0.05 versus Control. n=6/ group. NS, indicates no statistical difference among these three experimental groups.
VRT325 does not reduce basal or P. aeruginosa simulated IL-6 and IL-8 secretion by CFBE41o-cells
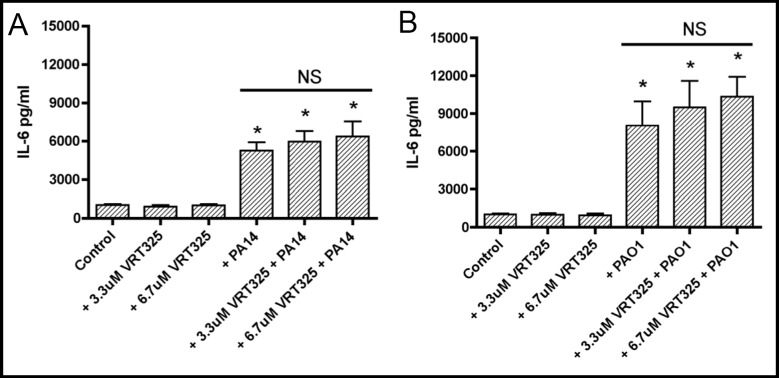
Similar experiments were conducted to test the hypothesis that VRT325 reduces basal and P. aeruginosa induced IL-6 secretion by CFBE41o- cells. VRT325 (3.3 or 6.7 µM) had no effect on constitutive IL-6 secretion. Moreover, VRT325 (3.3 or 6.7 µM) did not reduce the ability of PA14 or PAO1 to stimulate IL-6 secretion by CFBE41o- cells (Figure 4). Thus, VRT325 did not reduce the proinflammatory response to P. aeruginosa (PA14 or PAO1) as determined by measuring IL-6 secretion.
Fig. 4.
VRT325 had no effect on constitutive IL-6 secretion (compare Control with +VRT325 (3.3 and 6.7 µM)) or on the increase in IL-6 secretion in monolayers exposed to PA14 (4A: compare +PA14 with both +PA14 + VRT325 groups) or PAO1 (4B: compare +PAO1 with both +PAO1 + VRT325 groups). *P<0.05 versus Control. n=6/group. NS, indicates no statistical difference among these three experimental groups.
Finally, studies were also conducted to examine the effect of VRT325 on IL-8 secretion by CFBE41o- cells. VRT325 had no effect on constitutive IL-8 secretion (Figure 5). Moreover, VRT325 did not reduce the ability of PA14 or PAO1 to stimulate IL-8 secretion (Figure 5). In fact, VRT (6.7 µM) elicited a statistically significant increase in IL-8 production in cells exposed to PAO1 compared to cells exposed to PAO1 alone (Figure 5). Thus, VRT325 did not reduce the proinflammatory response to P. aeruginosa as determined by measuring IL-8 secretion.
Fig. 5.
VRT325 had no effect on constitutive IL-8 secretion (compare Control with both +VRT325 groups) or on the increase in IL-8 secretion in monolayers exposed to PA14 (5A: compare +PA14 with both +PA14 + VRT325 groups. NS, indicates no statistical difference among these three experimental groups.). Although the 3.3 µM dose of VRT325 had no effect on the PAO1-induced increase in IL-8 secretion, the 6.7 µM dose increased PAO1-stimulated IL-8 secretion (5B: compare +PAO1 with +PAO1 + 6.7 µM VRT325. # over the horizontal line indicates that +PAO1 is significantly different from PAO1 + 6.7 µM VRT325 (P<0.05)). *P<0.05 versus control.
Discussion
The major new finding in this study is that neither Corr4a nor VRT325, investigational compounds that stimulate ΔF508-CFTR Cl− secretion in polarized human CF airway epithelial cells, reduced either constitutive or P. aeruginosa (PA14 and PAO1) stimulated IL-6 and IL-8 secretion. We focused on IL-6 and IL-8 secretion because numerous studies have shown that their secretion by airway cells is enhanced by P. aeruginosa [9, 23, 24, 25, 26, 27] and that excessive IL-6 and IL-8 secretion by CF cells in response to P. aeruginosa is though to contribute to morbidity and mortality in CF.
Chemical rescue of ΔF508-CFTR Cl− secretion by other compounds changes IL-8 secretion. For example, MPB-07 (benz(c)quinolizinium), increases ΔF508-CFTR Cl− secretion in IB3-1 and CuFi-1 CF cells and decreases P. aeruginosa (PAO1) stimulation of IL-8 secretion by an unknown mechanism [28]. Moreover, 8-cyclopentyl-1,3-dipropylxanthine (CPX), which increases ΔF508-CFTR Cl− secretion in IB3-1 CF cells, reduces constitutive IL-8 secretion, but enhances IL-8 secretion in response to a clinical isolate of P. aeruginosa [25]. Taken together with the present studies these data illustrate the clinically relevant point that experimental drugs that correct ΔF508-CFTR trafficking and Cl− secretion do not all have similar effects on constitutive or stimulated (by P. aeruginosa) cytokine secretion. The most effective drugs for CF patients would ideally enhance ΔF508-CFTR Cl− secretion and reduce the proinflammatory response.
The inability of Corr4a and VRT325 to reduce basal or P. aeruginosa stimulated IL-6 and IL-8 secretion by CF human airway epithelial cells suggests that they are not optimal drugs for CF patients since elevated levels of cytokines in the CF airway contribute to lung injury [29].
A major goal in CF research is to develop a treatment or a cure for this pernicious disease. As drug candidates are identified and developed it is essential to evaluate the effects of these therapeutic agents on ΔF508-CFTR Cl− secretion as well as on other pathological changes that occur in CF including the ability of P. aeruginosa to colonize the lung, hypersecretion of mucus, enhanced sodium reabsorption, and secretion of proinflammatory cytokines. To this end in a previous study we demonstrated that Corr4a reduced the ability of P. aeruginosa to form antibiotic resistant biofilms on CFBE41o- cells [15]. However, in the present study we demonstrated that, unfortunately, neither Corr4a nor VRT325 reduced either constitutive or stimulated (P. aeruginosa) IL-6 and IL-8 secretion.
Acknowledgements
This study was supported by NIH grants RO1-HL0741745 (BAS) and RO1-DK45881 (BAS) and a Research Development Program grant from the Cystic Fibrosis Foundation (BAS). We thank Dr. Jennifer Bomberger for comments on the manuscript, as well as the Cystic Fibrosis Foundation Therapeutics Inc., and Drs. Robert Bridges and Melissa Ashlock for providing VRT325 and Corr4a.
References
- 1.Guggino WB, Stanton BA. New insights into cystic fibrosis: molecular switches that regulate CFTR. Nat Rev Mol Cell Biol. 2006;7(6):426–36. doi: 10.1038/nrm1949. [DOI] [PubMed] [Google Scholar]
- 2.Davis PB. Cystic fibrosis since 1938. Am J Respir Crit Care Med. 2006;173(5):475–82. doi: 10.1164/rccm.200505-840OE. [DOI] [PubMed] [Google Scholar]
- 3.Boucher RC. Relationship of airway epithelial ion transport to chronic bronchitis. Proc Am Thorac Soc. 2004;1(1):66–70. doi: 10.1513/pats.2306018. [DOI] [PubMed] [Google Scholar]
- 4.Thiagarajah JR, Song Y, Haggie PM, Verkman AS. A small molecule CFTR inhibitor produces cystic fibrosis-like submucosal gland fluid secretions in normal airways. Faseb J, 2004;18(7):875–7. doi: 10.1096/fj.03-1248fje. [DOI] [PubMed] [Google Scholar]
- 5.Mall M, Grubb BR, Harkema JR, O'Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med. 2004;10(5):487–93. doi: 10.1038/nm1028. [DOI] [PubMed] [Google Scholar]
- 6.Salinas D, Haggie PM, Thiagarajah JR, Song Y, Rosbe K, Finkbeiner WE, Nielson DW, Verkman AS. Submucosal gland dysfunction as a primary defect in cystic fibrosis. Faseb J. 2005;19(3):431–3. doi: 10.1096/fj.04-2879fje. [DOI] [PubMed] [Google Scholar]
- 7.Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med. 2003;168(8):918–51. doi: 10.1164/rccm.200304-505SO. [DOI] [PubMed] [Google Scholar]
- 8.Epelman S, Bruno TF, Neely GG, Woods DE, Mody CH. Pseudomonas aeruginosa exoenzyme S induces transcriptional expression of proinflammatory cytokines and chemokines. Infect Immun. 2000;68(8):4811–4. doi: 10.1128/iai.68.8.4811-4814.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carrabino S, Carpani D, Livraghi A, Di Cicco M, Costantini D, Copreni E, Colombo C, Conese M. Dysregulated interleukin-8 secretion and NF-kappaB activity in human cystic fibrosis nasal epithelial cells. J Cyst Fibros. 2006;5(2):113–9. doi: 10.1016/j.jcf.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Fu Z, Bettega K, Carroll S, Buchholz KR, Machen TE. Role of Ca2+ in responses of airway epithelia to Pseudomonas aeruginosa, flagellin, ATP, and thapsigargin. Am J Physiol Lung Cell Mol Physiol. 2007;292(1):L353–64. doi: 10.1152/ajplung.00042.2006. [DOI] [PubMed] [Google Scholar]
- 11.Machen TE. Innate immune response in CF airway epithelia: hyperinflammatory? Am J Physiol Cell Physiol. 2006;291(2):C218–30. doi: 10.1152/ajpcell.00605.2005. [DOI] [PubMed] [Google Scholar]
- 12.Courtney JM, Ennis M, Elborn JS. Cytokines and inflammatory mediators in cystic fibrosis. J Cyst Fibros. 2004;3(4):223–31. doi: 10.1016/j.jcf.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Pedemonte N, Lukacs GL, Du K, Caci E, Zegarra-Moran O, Galietta LJ, Verkman AS. Small-molecule correctors of defective δF508-CFTR cellular processing identified by high-throughput screening. J Clin Invest. 2005;115(9):2564–71. doi: 10.1172/JCI24898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Goor F, Straley KS, Cao D, González J, Hadida S, Hazlewood A, Joubran J, Knapp T, Makings LR, Miller M, Neuberger T, Olson E, Panchenko V, Rader J, Singh A, Stack JH, Tung R, Grootenhuis PD, Negulescu P. Rescue of δF508-CFTR trafficking and gating in human cystic fibrosis airway primary cultures by small molecules. Am J Physiol Lung Cell Mol Physiol. 2006;290(6):L1117–30. doi: 10.1152/ajplung.00169.2005. [DOI] [PubMed] [Google Scholar]
- 15.Moreau-Marquis, Bomberger S, Anderson JM, Gregory G, Swiatecka-Urban A, Ye S, O'Toole GA, Stanton BA. The δF508-CFTR Mutation Results in Increased Biofilm Formation by P. aeruginosa by Increasing Iron Availability. Am J Physiol Lung. 2007;295(1):L25–37. doi: 10.1152/ajplung.00391.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruscia E, Sangiuolo F, Sinibaldi P, Goncz KK, Novelli G, Gruenert DC. Isolation of CF cell lines corrected at δF508-CFTR locus by SFHR-mediated targeting. Gene Ther. 2002;9(11):683–5. doi: 10.1038/sj.gt.3301741. [DOI] [PubMed] [Google Scholar]
- 17.Cozens AL, Yezzi MJ, Kunzelmann K, Ohrui T, Chin L, Eng K, Finkbeiner WE, Widdicombe JH, Gruenert DC. CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells. Am J Respir Cell Mol Biol. 1994;10(1):38–47. doi: 10.1165/ajrcmb.10.1.7507342. [DOI] [PubMed] [Google Scholar]
- 18.Hentchel-Franks K, Lozano D, Eubanks-Tarn V, Cobb B, Fan L, Oster R, Sorscher E, Clancy JP. Activation of airway Cl-secretion in human subjects by adenosine. Am J Respir Cell Mol Biol. 2004;31(2):140–6. doi: 10.1165/rcmb.2004-0012OC. [DOI] [PubMed] [Google Scholar]
- 19.Swiatecka-Urban A, Moreau-Marquis S, Maceachran DP, Connolly JP, Stanton CR, Su JR, Barnaby R, O'toole GA, Stanton BA. Pseudomonas aeruginosa inhibits endocytic recycling of CFTR in polarized human airway epithelial cells. Am J Physiol Cell Physiol. 2006;290(3):C862–72. doi: 10.1152/ajpcell.00108.2005. [DOI] [PubMed] [Google Scholar]
- 20.MacEachran DP, Ye S, Bomberger JM, Hogan DA, Swiatecka-Urban A, Stanton BA, O'Toole GA. The Pseudomonas aeruginosa secreted protein PA2934 decreases apical membrane expression of the cystic fibrosis transmembrane conductance regulator. Infect Immun. 2007;75(8):3902–12. doi: 10.1128/IAI.00338-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loo TW, Bartlett MC, Clarke DM. Correctors promote folding of the CFTR in the endoplasmic reticulum. Biochem J. 2008;413(1):29–36. doi: 10.1042/BJ20071690. [DOI] [PubMed] [Google Scholar]
- 22.Varga K, Goldstein RF, Jurkuvenaite A, Chen L, Matalon S, Sorscher EJ, Bebok Z, Collawn JF. Enhanced cell-surface stability of rescued δF508 cystic fibrosis transmembrane conductance regulator (CFTR) by pharmacological chaperones. Biochem J. 2008;410(3):555–64. doi: 10.1042/BJ20071420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DiMango E, Ratner AJ, Bryan R, Tabibi S, Prince A. Activation of NF-kappaB by adherent Pseudomonas aeruginosa in normal and cystic fibrosis respiratory epithelial cells. J Clin Invest. 1998;101(11):2598–605. doi: 10.1172/JCI2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonfield TL, Konstan MW, Berger M. Altered respiratory epithelial cell cytokine production in cystic fibrosis. J Allergy Clin Immunol. 1999;104(1):72–8. doi: 10.1016/s0091-6749(99)70116-8. [DOI] [PubMed] [Google Scholar]
- 25.Eidelman O, Srivastava M, Zhang J, Leighton X, Murtie J, Jozwik C, Jacobson K, Weinstein DL, Metcalf EL, Pollard HB. Control of the proinflammatory state in cystic fibrosis lung epithelial cells by genes from the TNF-alphaR/ NFkappaB pathway. Mol Med. 2001;7(8):523–34. [PMC free article] [PubMed] [Google Scholar]
- 26.Becker MN, Sauer MS, Muhlebach MS, Hirsh AJ, Wu Q, Verghese MW, Randell SH. Cytokine secretion by cystic fibrosis airway epithelial cells. Am J Respir Crit Care Med. 2004;169(5):645–53. doi: 10.1164/rccm.200207-765OC. [DOI] [PubMed] [Google Scholar]
- 27.Tchilibon S, Zhang J, Yang Q, Eidelman O, Kim H, Caohuy H, Jacobson KA, Pollard BS, Pollard HB. Amphiphilic pyridinium salts block TNF alpha/NF kappa B signaling and constitutive hypersecretion of interleukin-8 (IL-8) from cystic fibrosis lung epithelial cells. Biochem Pharmacol. 2005;70(3):381–93. doi: 10.1016/j.bcp.2005.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dechecchi MC, Nicolis E, Bezzerri V, Vella A, Colombatti M, Assael BM, Mettey Y, Borgatti M, Mancini I, Gambari R, Becq F, Cabrini G. MPB-07 reduces the inflammatory response to Pseudomonas aeruginosa in cystic fibrosis bronchial cells. Am J Respir Cell Mol Biol. 2007;36(5):615–24. doi: 10.1165/rcmb.2006-0200OC. [DOI] [PubMed] [Google Scholar]
- 29.Perez A, Issler AC, Cotton CU, Kelley TJ, Verkman AS, Davis PB. CFTR inhibition mimics the cystic fibrosis inflammatory profile. Am J Physiol Lung Cell Mol Physiol. 2007;292(2):L383–95. doi: 10.1152/ajplung.00403.2005. [DOI] [PubMed] [Google Scholar]