Abstract
Rab proteins are small GTPases required for vesicle trafficking through the secretory and endocytic pathways. Rab GDP-dissociation inhibitor (rab-GDI) regulates Rab protein function and localization by maintaining Rab proteins in the GDP-bound conformation. Two isoforms of rab-GDI are present in most mammalian cells: GDI-1 and GDI-2. It has recently been demonstrated that a Heat shock protein 90 (Hsp90) chaperone complex regulates the interactions between Rab proteins and Rab-GDI-1. The AR42J cell line is derived from rat pancreatic exocrine tumor cells and develops an acinar-like phenotype when treated with dexamethasone (Dex). The aim of the present study was to examine the expression of rab-GDI isoforms and Hsp90 in AR42J cells in the presence or absence of Dex. Rab-GDI: Hsp90 interactions were also examined. Both rab-GDI isoforms were detected in AR42J cells by immunoblotting. In Dex-treated cells, quantitative immunoblotting revealed that rab-GDI-1 expression increased by 28%, although this change was not statistically significant. Rab-GDI-2 levels were unaltered by Dex treatment. Approximately 21% rab-GDI-1 was membrane associated, whereas rab-GDI-2 was exclusively cytosolic. Dex treatment did not affect the subcellular distribution of rab-GDI isoforms. Hsp90 was present in the cytosolic and membrane fractions of AR42J cells and co-immunoprecipitated with cytosolic rab-GDI-1. Moreover, density gradient centrifugation of AR42J cell membranes revealed that Hsp90 and rab-GDI-1 co-localize on low- and high-density membrane fractions, including amylase-containing secretory granules. The Hsp90 inhibitor, geldanamycin, inhibited CCK-8-induced amylase release from these cells in a dose-dependent manner. Our results indicate that as AR42J cells differentiate into acinar-like cells, rab-GDI isoform expression and localization is not significantly altered. Moreover, our findings suggest that Hsp90 regulates agonist-induced secretion in exocrine cells by interacting with rab-GDI-1.
Key Words: Rab proteins, GDI, Hsp90, Ar42J cells, Exocrine, Secretion, Pancreas, Amylase
Introduction
Rab proteins are small GTPases required for vesicle trafficking through the exocytic and endocytic pathways [1, 2]. Over 60 isoforms have been identified and, like other small GTPases, Rab proteins cycle between GTP- and GDP-bound conformations. Rab protein function involves the movement of Rab proteins between various cellular compartments. Several accessory proteins are involved with regulating the localization and guanine nucleotide binding state of Rab proteins including rab-Guanine nucleotide exchange factor, Rab escort protein (REP) and Rab GDP-dissociation inhibitor (rab-GDI) [3, 4, 5, 6, 7, 8].
Rab-GDI regulates Rab protein function and localization by maintaining Rab proteins in the GDP-bound conformation and regulating their cycling between cytosolic and membrane compartments [6, 7, 8, 9]. Two rab-GDI isoforms have been cloned from bovine brain [10] and one from mouse skeletal muscle [11]. Most cytosolic Rab proteins exist as a complex with rab-GDI and these complexes represent a cytosolic pool of Rab proteins that are eventually presented to their target membranes [12, 13]. In contrast, other Rab proteins, like Rab3D, interact exclusively with Rab escort protein (REP) [14].
REP isoforms 1 and 2 are rab accessory proteins that act as chaperone molecules during the lipid modification of Rab proteins and are considered part of the rab GDI superfamily [7, 15]. In most cell types, two rab-GDI isoforms and both REP isoforms are expressed to varying degrees. With respect to rab-GDI isoform expression in exocrine tissues, we have previously demonstrated that in parotid two rab-GDI isoforms (55 and 45 kDa) are present in the cytosol, whereas a 43-kDa isoform is membrane-associated [16]. The 55 and 45-kDa isoforms probably represent rab-GDI-1 and rab-GDI-2, while the membrane-associated isoform has not yet been characterized. Whether or not rab-GDI isoforms are functionally redundant or have specific roles in various cell types remains to be determined.
Structural studies of rab-GDI suggest that the recycling of Rab proteins between the membrane and soluble compartments most likely involves rab-GDI and other rab recycling factors (RRF) [17, 18]. Using synaptosomes, the key components of the RRF have been identified and include Heat shock protein 90 (Hsp90), Hsp70 and cysteine string protein [18]. Moreover, it has been demonstrated that recycling of Rab1b and Rab3A involves the Hsp90 chaperone system [18, 19]. Whether or not Hsp90 interacts with rab-GDI and regulates exocytosis in exocrine cells has not yet been determined.
The AR42J cell line is derived from rat pancreatic exocrine tumor cells and develops an acinar cell-like phenotype when treated with dexamethasone (Dex). Dex-treatment results in an increase in amylase-containing secretory granules and an increase in the number of CCK-8 receptors per cell [20, 21]. CCK-8 induces amylase release from AR42J cells and, therefore, this cell line represents a convenient model for studying the molecular events involved with regulated secretion in exocrine cells. The aim of the present study was to examine the expression and localization of Hsp90 and rab-GDI isoforms in AR42J cells as they develop their secretory phenotype in response to Dex. In addition, we examined the possibility that interactions between rab-GDI and Hsp90 are important with respect to agonist-induced secretion in exocrine cells.
Materials and Methods
Materials
Antisera which recognizes Rab3D was raised in rabbits using recombinant Rab3D as an antigen and was affinity purified using a recombinant Rab3D column. Rab1B and the pan rab-GDI antibodies were obtained from Zymed (Carlsbad, CA). GDI-2 specific antisera from Genway (San Diego, CA). Rab5 antibodies and GDI-1 monoclonal antibody from Synaptic systems (Goettingen, Germany). Hsp90 antibodies from Biovision (Mountain View, CA). Calnexin, MEK1/2 and synataxin 6 antibodies from Cell Signaling Technologies Inc. (Beverly, MA). Geldanamycin, bovine serum albumin (BSA), protein G-agarose and dexamethasone from Sigma-Aldrich (St. Louis, MO). Optiprep from Invitrogen (Carlsbad, CA). Protease inhibitor mix from Calbiochem (San Diego, CA). CCK-8 from American peptide (Sunnyvale, CA).
Cell Culture
AR42J cells were obtained from the ATCC (Manassas, VA) and cultured in DMEM with 10% FBS, 1mM pyruvate, 1X penstrep and 2 mM glutamine. Cells were cultured in a humidified incubator at 37oC in an atmosphere of 95% air and 5% CO2. For Dex treatment, the cells were seeded onto 6-well plates, T-75 or T-25 flasks. The following day, complete media containing 50 nM Dex was added to the cells. The cells were harvested or assayed 2-3 days after the addition of Dex.
Preparation of Cell Lysates and Subcellular Fractions
AR42J cells were scraped into PBS, washed and resuspended in lysing solution (20 mM Tris-HCl [pH 7.0], 150 mM NaCl, 1X protease inhibitor mix). The cells were freeze-thawed 3X and centrifuged at 120,000 X g for 40 min. The supernatant represented the cytosolic fraction. The pellet was resuspended in an equal volume of lysing solution and represented the membrane fraction.
Immunoblotting of subcellular fractions
An equal volume of 2X SDS sample buffer was added to cell lysates or subcellular fractions. The samples were boiled 3 min and applied to 10% polyacrylamide gels. Following SDS-PAGE, proteins were electroblotted to nitrocellulose membranes (Schleicher and Schuel, NH). The membranes were incubated with 1% BSA/1% goat serum (GS) in TBST (50 mM Tris pH 7.5, 0.15M NaCl, 0.05% Tween-20) for >2 hours to block non-specific binding, followed by incubation with TBST+BSA containing the primary antibody. Blots were washed 4 × 10 min with TBST+BSA and incubated with Horseradish peroxidase-conjugated anti-mouse, anti-chicken or anti-rabbit IgG. Following extensive washing with TBST, bands were visualized by chemiluminescence on a GeneGnome Bioimaging system (Syngene, MD). Band intensity was quantitated using Genetools Analysis software (Syngene, MD).
Immunoprecipitation of rab-GDI and Hsp90
The cytosolic fraction was prepared from Dex-treated AR42J cells grown in T-75 flasks. Cells were scraped into immunoprecipitation buffer (50 mM Tris, pH 7.5, 5 mM MgSO4, 100 mM NaCl, 2 mM DTT, 1X protease inhibitors), lysed and centrifuged at 120,000 X g for 40 min. Aliquots of cytosol were incubated overnight at 4oC with an antibody that recognizes rab-GDI isoform 1 or mouse IgG (30 μg). The next day, 50 μl of protein G-agarose beads were added and the tubes were mixed for three hours. The beads were washed fours times with IP buffer, then boiled in SDS sample buffer for 5 min. The immunoprecipitated proteins were immunoblotted for rab-GDI and Hsp90 as described above.
Density gradient centrifugation
AR42J cells were harvested and gently homogenized using a Tissue Tearor (Glen Mills Inc., NJ). A post-nuclear supernatant (PNS) fraction obtained by centrifugation at 80 X g for 3 min. The PNS (200 μl) was loaded on top of a 10-30% continuous iodixanol gradient (2 ml). The gradient was prepared in 0.25 M sucrose, 10 mM Tris-HCl [pH 7.4] and 1X protease inhibitor mix. Following centrifugation (2 hrs-137,000 x g) in a Beckman TL-100 ultracentrifuge, 150 μl fractions were collected from the top of the gradient. Fractions were assayed for amylase activity and immunoblotted for rab-GDI, Hsp90 and organelle markers.
Amylase and Protein Assays
Protein assays were performed using the BioRad protein assay reagent according to the manufacturer's instructions. For amylase release assays, the cells were seeded in 6-well plates (2-3 × 105 cells/well). The day of the assay, the cells were washed with amylase assay buffer (AAB-98 mM NaCl, 4.8 mM KCl, 2 mM CaCl2, 1.2 mM MgSO4, 0.1% BSA, 0.01% soybean trypsin inhibitor, 25 mM HEPES pH7.5) and incubated with AAB containing no additions or CCK-8 (1 nM) for 40 min at 37oC. The AAB was removed from the wells and replaced with AAB + 1% TX-100. Amylase levels in the media and cell extracts were determined using the Phadebus amylase assay (Pharmacia, Stockholm, Sweden). Amylase release was expressed as Units released or percent total release into the AAB during the 40 min incubation. To examine the effects of geldanamycin on agonist-induced amylase release, AR42J cells were incubated in the presence or absence of increasing concentrations of geldanamycin for 30 min, washed once with amylase assay buffer and incubated in the presence or absence of CCK-8 (1 nM) for 40 min. Amylase release during the 40 min incubation was determined.
Results
Effect of Dex on amylase and protein levels in AR42J cells
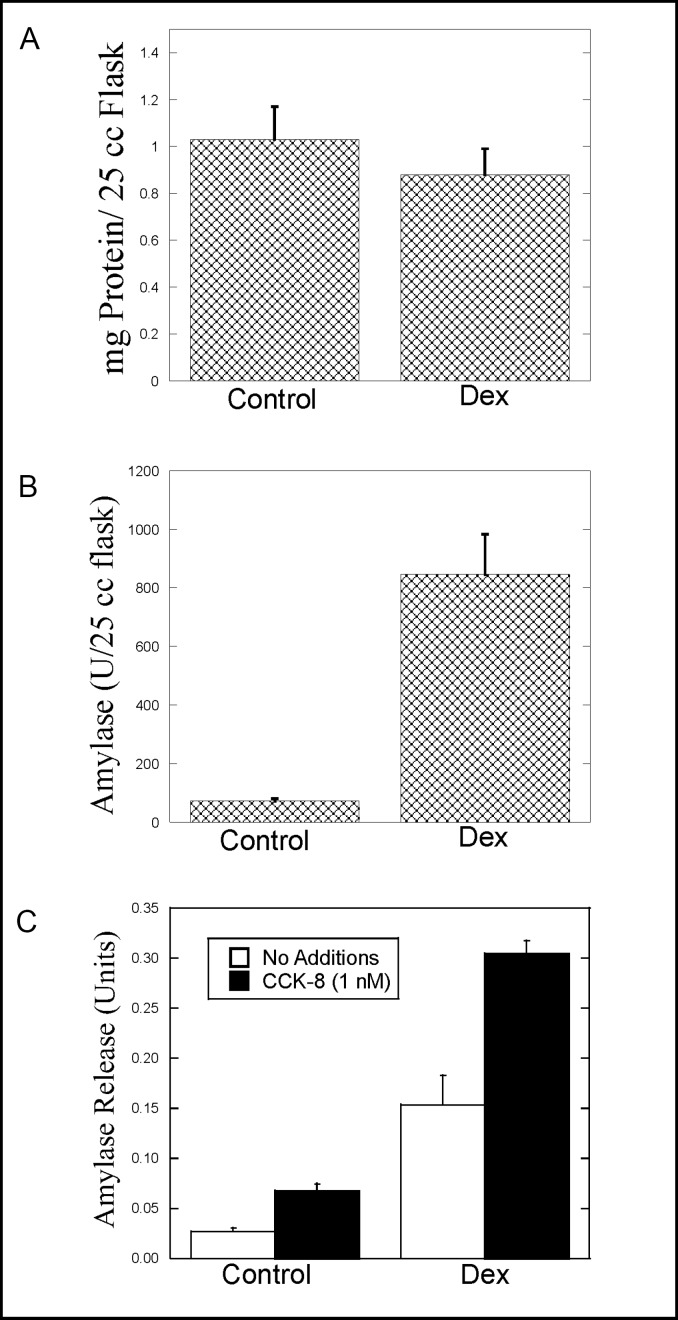
The AR42J cell line was derived from rat pancreatic cancer cells and has been used as a model of exocrine cell structure and function for more than 20 years. Nevertheless, we believed it was necessary to characterize the effects of Dex on AR42J cells in our hands. AR42J cells were cultured in T-25 flasks in the absence or presence of 50 nM Dex for 2-3 days. The cells were harvested and assayed for total protein and amylase levels per flask. Visual inspection of the flask revealed a lower cell density in Dex-treated flasks, consistent with the notion that as the cells differentiate, the rate of cell proliferation decreases. As shown in Fig. 1A, total protein levels in T-25 flasks were consistently lower in Dex-treated flasks. However, this decrease was not statistically significant. As expected, Dex treatment increased amylase expression in AR42J cells (11.7–fold over control) (Fig. 1B).
Fig. 1.
Effect of Dex treatment on AR42J cells. AR42J cells were cultured in T-25 flasks in the absence (Control) or presence (Dex) of 50 nM Dex for 2-3 days. The cells were washed and harvested by scraping into PBS. The cells were then pelleted and resuspended in approximately 0.5 ml of lysing solution. Total protein (Fig. 1A) and amylase (Fig. 1B) levels in the flasks were determined as described in Methods. C. AR42J cells were seeded onto 6-well plates and cultured in the absence (Control) or presence (Dex) of 50 nM Dex for 2-3 days. On the day the cells were assayed, the media was removed and the cells washed with amylase assay buffer (AAB). The cells were then incubated with 1 nM CCK-8 or no additions for 40 min at 37oC. Amylase release from control and Dex-treated cells during the 40 min incubation was measured as described in methods. Values for amylase release are expressed as Amylase Units. Results shown are means ± SEM from at least three experiments.
The effect of Dex on agonist-induced amylase release was examined next using cells grown in 6-well plates. CCK-8-induced amylase release was observed in control and Dex-treated cells (Fig. 1C). However, because of the increase in amylase levels in Dex-treated cells, both basal and CCK-8-induced amylase release were increased with Dex treatment. Interestingly, when CCK-8-induced amylase release in control and Dex-treated cells was compared in terms of fold-over-basal release, the results in control and Dex-treated cells were similar. In control cells, CCK-8-induced amylase release was increased 2.5-fold over basal release, whereas in Dex-treated cells, CCK-8-induced amylase release was increased 2.3-fold.
Effect of Dex on Rab protein and Rab-GDI isoform expression and localization in AR42J cells
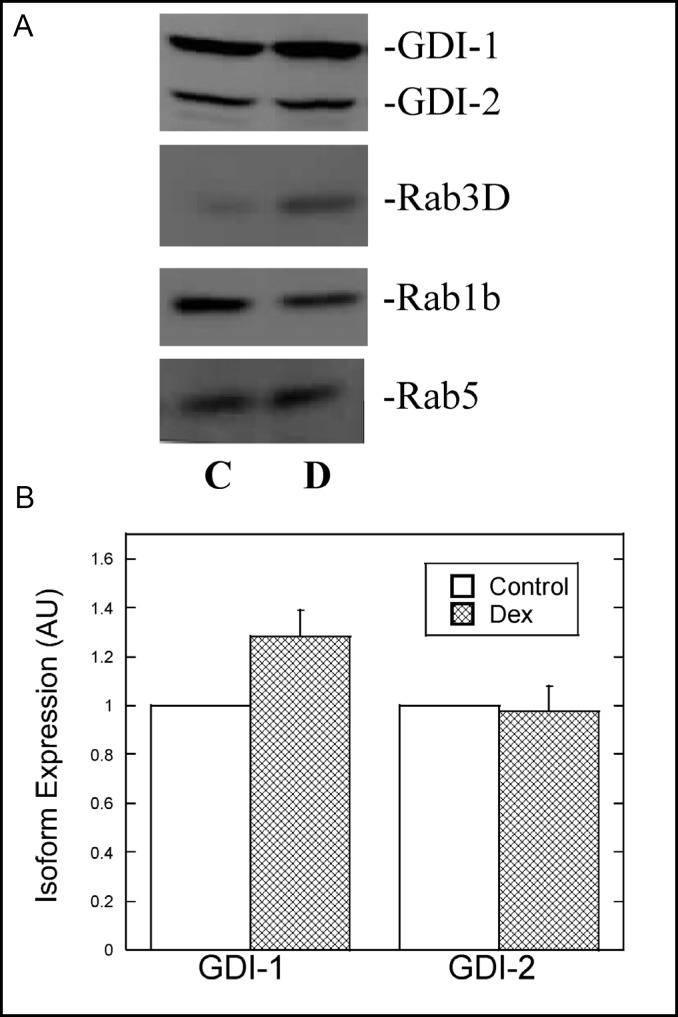
The development of an increased secretory capacity in AR42J cells in response to Dex most likely involves alterations in the expression and localization of specific proteins. We examined the possibility that as AR42J cells differentiate in response to Dex, rab-GDI isoform expression and/or localization will be affected, suggesting a role for one or more rab-GDI isoform in exocrine secretion. We also examined the effects of Dex on the relative levels of various Rab proteins. AR42J cell lysates from control and Dex-treated cells were immunoblotted with a rab-GDI antibody that recognizes rab-GDI isoforms 1 and 2. As shown in Fig 2A, both isoforms were expressed in control and Dex-treated AR42J cells. Moreover, the levels of rab-GDI isoforms in AR42J cells were similar to those observed in rat pancreas when equal amounts of protein were immunoblotted (not shown). Quantitative immunoblotting revealed that Dex treatment resulted in a 28% increase in rab-GDI-1 expression, whereas rab-GDI-2 levels were not altered (Fig. 2B). The relatively small increase in rab-GDI-1 levels was not statistically significant. With respect to the Rab proteins examined, Rab3D levels were increased 60% in the presence of Dex, whereas Rab1b levels decreased 28% (Fig. 2A). Rab5 levels were increased 13% with Dex treatment (Fig. 2A). The Dex-induced increase in Rab3D is consistent with what others have observed [22].
Fig. 2.
Effect of Dex on the expression of rab-GDI isoforms and Rab proteins in AR42J cells. A. AR42J cells were grown in the absence (C) or presence (D) of Dex (50 nM) for 3 days. Equal amounts of protein from AR42J cell lysates were immunoblotted for Rab-GDI, Rab1B, Rab3D and Rab5 as described in Methods. B. Relative levels of rab-GDI isoforms in control and Dex-treated cells were determined by quantitative immunoblotting. The data are presented as arbitrary units. Results shown are means ± SEM from at least three experiments.
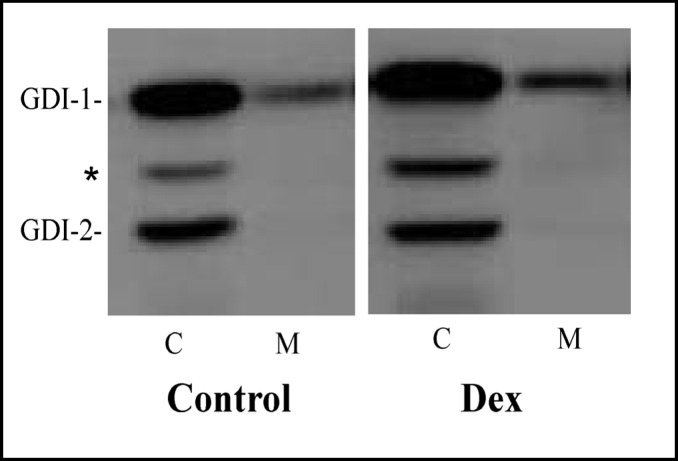
The subcellular localization of rab-GDI isoforms in control and Dex-treated cells was examined next. Rab-GDI-1 was predominantly cytosolic, although approximately 21% of rab-GDI-1 was membrane-associated (Fig. 3). In contrast, rab-GDI-2 was exclusively cytosolic (Fig. 3). Quantitative immuno-blotting revealed that the subcellular distribution of rab-GDI isoforms was not altered by Dex treatment.
Fig. 3.
Effect of Dex on the subcellular localization of rab-GDI isoforms in AR42J cells. AR42J cells were cultured in the absence (Control) or presence of Dex (50 nM) for 3 days. The cells were harvested and cytosol (C) and membrane (M) fractions were prepared and immunoblotted for rab-GDI isoforms using an antibody that recognizes both GDI-1 and GDI-2 as described in Methods. The asterisk indicates a non-specific band that is often observed between the two rab-GDI isoforms and most likely represents amylase.
Hsp90 interacts with rab-GDI in AR42J cells
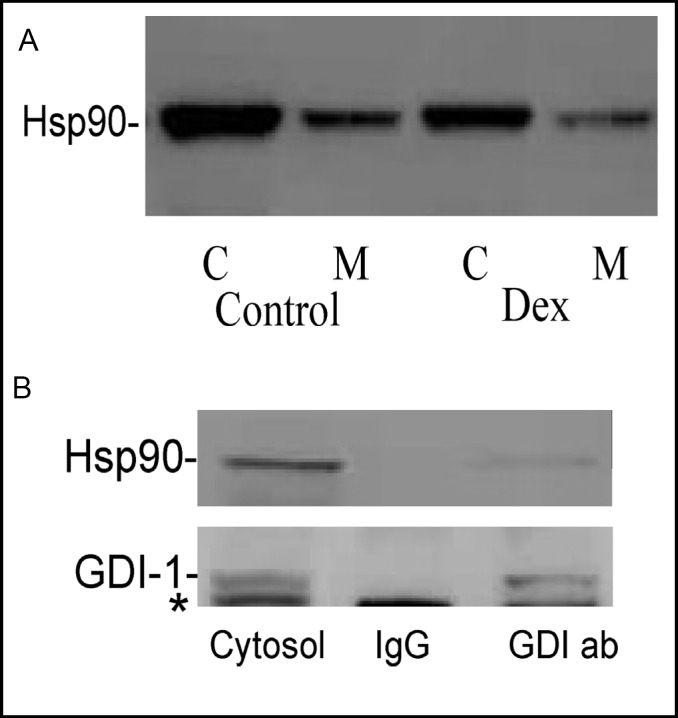
Recent studies have demonstrated that Hsp90 binds to rab-GDI-1 and regulates its ability to interact with Rab proteins [18, 19]. Hsp90 expression and localization in control and Dex-treated AR42J cells were examined. As shown in Fig. 4A, Hsp90 is expressed in AR42J cells and is predominantly cytosolic. Moreover, Dex-treatment caused a decrease in Hsp90 expression.
Fig. 4.
Hsp90 is expressed in AR42J cells and associates with cytosolic rab-GDI-1. A. Hsp90 expression and localization in AR42J cells in the absence (Control) or presence (Dex) of Dex was examined. Cytosol (C) and membrane (M) fractions were prepared from control and Dex-treated cells and immunoblotted for Hsp90 as described in Methods. B. Immunoprecipitation of Rab-GDI-1 from AR42J cell cytosol. Cytosol was prepared from four T-75 flasks of Dex-treated AR42J cells. The cytosol was incubated overnight with a 30 μg GDI-1-specific antibody (GDI ab-right lane) or rabbit IgG (IgG-middle lane), followed by a three hr incubation with protein G-agarose beads. The beads were washed and boiled into SDS sample buffer as described in Methods. The cytosol (left lane) and immunoprecipitates were immunoblotted for Hsp90 and rab-GDI. The asterisk in the GDI-1 blot is a non-specific band and most likely represents amylase.
To examine the possibility that rab-GDI binds Hsp90 in the cytosol of AR42J cells, cytosolic proteins were immunoprecipitated using a rab-GDI-1 specific monoclonal antibody. As shown in Fig. 4B, rab-GDI-1 was immunoprecipitated from the cytosol with the rab-GDI-specific antibody, but not IgG. Interestingly, a small amount of Hsp90 co-immuno-precipitated with rab-GDI, indicating that these proteins interact with one another in AR42J cell cytosol.
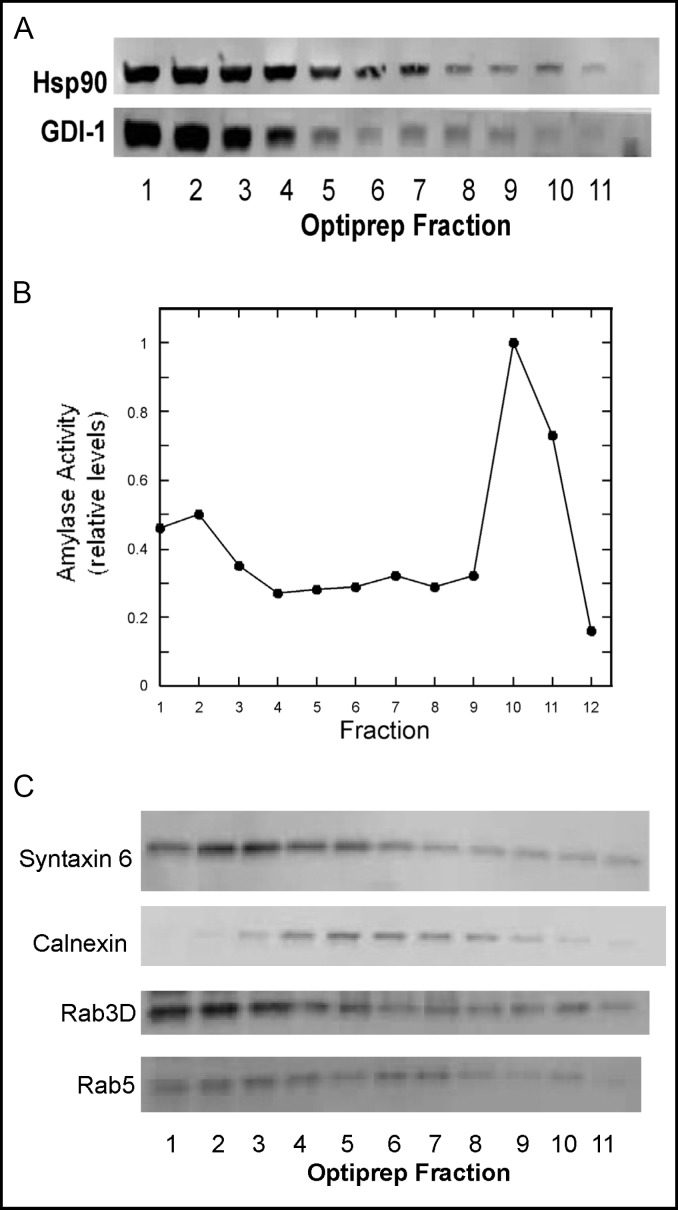
Next, we wanted to determine whether or not rab-GDI-1 and Hsp90 co-localize to the same membrane structures in AR42J cells using immunocytochemistry. However, because both proteins are predominantly cytosolic, the relatively small amount of membrane-associated rab-GDI-1 and Hsp90 could not be visualized by this method. Instead, we examined the membrane association of rab-GDI-1 and Hsp90 by density gradient centrifugation. PNS were prepared from Dex-treated AR42J cells and fractionated on a 10-30% continuous iodixanol gradient as described in Methods. As shown in Fig. 5A, the majority of rab-GDI-1 and Hsp90 was present in the first three low density fractions which most likely contain cytosolic proteins. However, Hsp90 was present throughout the gradient and appeared to peak in fractions 5-7, as well as in fraction 10. Rab-GDI-1 was also present throughout the fractions and peaked in fractions 7-9. Amylase activity was used as a marker for secretory granules and peaked in fraction 10 (Fig. 5B). The amylase activity in the fractions 1-3 represents soluble amylase that was released during the homogenization process.
Fig. 5.
Rab-GDI-1 and Hsp90 co-localize on AR42J cell membrane fractions. A. AR42J cells were treated with Dex for 2 days and harvested into lysing buffer. A post-nuclear supernatant (PNS) was prepared and applied to a 10-30% continuous iodixanol gradient as described in Methods. Fractions (150 μl) were collected from the top of the gradient and immunoblotted for rab-GDI-1 and Hsp90. B. Amylase levels in the fractions were determined and the data were normalized to 1, which represents the maximum level of amylase activity. C. Immunoblotting of gradient fractions for Rab3D, Rab5, Calnexin and syntaxin 6.
The distribution of Rab5, Rab3D and marker proteins in the gradient fractions was also examined. Rab3D was present in the cytosol (Fig. 5C-fractions 1-3) and co-localized with amylase activity on high-density fractions (Fig. 5C-fractions 9-11) which most likely represent secretory granules. A peak in Rab3D levels was also observed in fractions 5-7. We have previously observed a similar distribution of Rab3D in rat parotid [23]. Rab5 levels peaked in fractions 6-7 which most likely represents an endosomal compartment (Fig. 5C). Calnexin and syntaxin 6 were used as markers for the endoplasmic reticulum and Golgi, respectively. Calnexin levels peaked in fractions 5-7 (Fig. 5C), whereas syntaxin 6 was present in the cytosol and low density membrane fractions (Fig. 5C-fractions 4-6). MEK1/2 was used as a cytoplasmic marker and was localized to fractions 1-3 (not shown).
Hence, our findings suggest that rab-GDI-1 and Hsp90 co-localize on high and low density membranes in AR42J cells and both appear to be associated with secretory granules, albeit to varying degrees. These results indicate that Hsp90 may bind to and regulate membrane-associated rab-GDI-1 function in AR42J cells.
Hsp90 regulates CCK-8-induced amylase release from AR42J cells
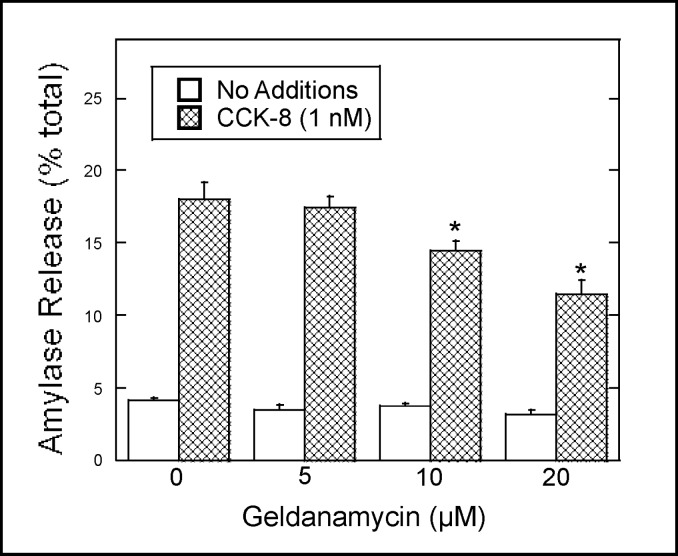
The role of Hsp90 in CCK-8-induced amylase release from Dex-treated AR42J cells was examined using geldanamycin, a specific inhibitor of Hsp90. AR42J cells were preincubated with increasing concentrations of geldanamycin and then treated with the secretagogue CCK-8 for 40 min. Amylase release during the 40 min incubation was determined as described in Methods. As shown in Fig. 6, geldanamycin caused a dose-dependent decrease in CCK-8-induced amylase release. At 20 μM, a 40% decrease in CCK-8-induced amylase release was observed, whereas basal amylase release was not affected (Fig. 6). These results indicate that Hsp90 regulates agonist-induced amylase release in AR42J cells.
Fig. 6.
Geldanamycin inhibits CCK-8-induced amylase release. AR42J cells were plated in 6-well plates (3 × 105 cells/well) and treated with Dex (50 nM) for three days. The cells were washed with AAB and incubated in the presence or absence of the indicated concentration of geldanamycin for 30 min, washed once with AAB and incubated with no additions or CCK-8 (1 nM) for 40 min. Values for amylase release are expressed as percentage of the total cellular amylase present at the start of the 40 min incubation that was released into the medium. Results shown are means ± SEM from four experiments.
Discussion
Previous studies have demonstrated that Dex enhances the secretory phenotype of AR42J cells by inducing an increase in cellular amylase levels, the total number of secretory granules in the cytoplasm and the number of surface CCK-receptors [20, 21]. Consistent with what others have found, Dex-treatment resulted in increased amylase levels and reduced proliferation in AR42J cells under our cell culture conditions. Both basal and CCK-8-induced amylase release were increased in Dex-treated cells. However, it should be noted that the fold increase in CCK-8-induced amylase release over basal release was similar in Dex-treated and untreated cells (approx. 2.5-fold over basal). Hence, Dex treatment does not increase the proportion of stored amylase released in response to CCK-8.
Because it is possible to induce the secretory phenotype in these cells, this model has been used to examine the role of various proteins in regulated exocytosis by examining their expression and localization in response to Dex treatment. For example, Rab3D is a protein believed to be involved with secretion in numerous exocrine cell types. In AR42J cells, Dex treatment results in an increase in Rab3D levels and alters its localization [22]. Rab-GDI and Hsp90 are proteins that regulate Rab protein function and localization. In the present study, we examined the effect of Dex-induced differentiation on the expression and localization of rab-GDI and Hsp90 in AR42J cells.
We hypothesized that if rab-GDI is involved with regulated exocytosis in exocrine cells, than we should observe an increase in the expression levels of one or both rab-GDI isoforms in AR42J cells in response to Dex treatment. Quantitative immunoblotting revealed a small (28%), non-significant increase in rab-GDI-1 levels in Dex-treated AR42J cells. In contrast to these findings, when undifferentiated Ntera2 cells are induced to differentiate into neuron-like cells (NT2N), an almost 5-fold increase in rab-GDI-1 expression is observed [24]. Alterations in rab-GDI isoforms were also examined in NB1 cells which differentiate into neuronal-like cells in response to cAMP [25]. As NB1 cells differentiate into neuronal cells, rab-GDI isoform mRNA levels are not altered, whereas the levels of Rab3A mRNA are increased [25]. This is similar to what we observed in AR42J cells in the present study, i.e., Dex-induced differentiation results in an increase in Rab3 protein expression [22, present study], while rab-GDI isoform levels remain relatively unaltered. Taken together, our findings suggest that a pronounced increase in rab-GDI expression is not required for AR42J cells to acquire the Dex-induced increase in secretory capacity and that the levels of both rab-GDI isoforms are adequate with respect to the ability of these cells to package and sort the increased amount of amylase protein into secretory granules.
Hsp90 is actually a family of proteins that are abundant in all eukaryotic cells and regulate the activity and function of numerous client proteins [26, 27]. Hsp90 is essential for cell viability and contains an ATP-binding site. Recent studies have demonstrated that Hsp90, along with other proteins, forms a chaperone complex with rab-GDI-1 and regulates its ability to extract Rab1b and Rab3A from membranes [18, 19]. Moreover, ER-to-Golgi traffic was inhibited using Hsp90-specific inhibitors suggesting that the rab-GDI-1: Hsp90 complex plays an important role in regulating Rab-mediated membrane trafficking events [19]. In the present study, we have demonstrated that Hsp90 is expressed in AR42J cells and is present in the cytosolic and membrane fractions. As mentioned previously, the AR42J cell line is derived from rat pancreatic exocrine tumor cells. Others have demonstrated that Hsp90 expression levels are higher in pancreatic carcinoma cells when compared with normal pancreas [28]. Consistent with these findings, we found that Dex-treatment resulted in a decrease in Hsp90 levels in AR42J cells, i.e., as these cells changed from a proliferative to a more differentiated phenotype, Hsp90 expression decreased.
We have demonstrated that rab-GDI-1 and Hsp90 form a complex in the cytosol of AR42J cells by co-immunoprecipitation experiments, suggesting that Hsp90 regulates rab-GDI function in exocrine cells. In addition, using membrane gradient centrifugation, we found that Hsp90 and rab-GDI-1 co-localize on membrane fractions in AR42J cells. Moreover, Hsp90 and, to some extent, rab-GDI-1 are both present in high-density, amylase-positive membrane fractions which most likely represent secretory granules. These findings raise the interesting possibility that rab-GDI-1: Hsp90 interactions may regulate the function and activation of membrane associated Rab proteins involved with regulated secretion.
We used the Hsp90-specific inhibitor geldanamycin to examine the role of Hsp90 in regulated exocytosis in AR42J cells. It has previously been demonstrated that geldanamycin stabilizes Hsp90: rab-GDI-1 complexes and inhibits the recycling of at least two rab proteins, Rab1B and Rab3A [18, 19]. Geldanamycin partially inhibited CCK-8-induced amylase releases in a dose dependent-manner, indicating that Hsp90 activity is required for agonist-induced secretion from AR42J cells. Consistent with our findings, preincubation with geldanamycin caused a decrease in calcium-induced neurotransmitter release from intact synaptosomes [18]. However, inhibition of Hsp90 with geldanamycin did not affect regulated exocytosis or the cycling of Rab3A between secretory granules and cytosol in PC12 cells [29]. Why inhibition of regulated secretion would be observed in some cell systems but not others with geladanmicin is unclear. Rab-GDI and Hsp90 are for the most part ubiquitous cellular proteins and one would expect that their interactions would be similar in various secretory cell types. Further studies are required in other secretory cell types to help clarify the role of Hsp90 in regulated secretion.
How does geldanamicin inhibit CCK-8-induced secretion in AR42J cells? One possibility is that inhibition of Hsp90 stabilizes membrane-associated complexes involving Hsp90, rab-GDI-1 and Rab proteins on secretory granules. Proteomic analysis of secretory granule membranes obtained from pancreatic acinar cells has revealed that several Rab proteins are present on these membranes [30]. These Rab isoforms include Rab3D, Rab8A, Rab 11A, Rab14 and Rab27b. Interestingly, unlike what is observed in pancreas, we did not detect Rab27b expression in AR42J cells (unpublished observations). Geldanamicin may interfere with the Hsp90: rab-GDI-1 mediated recycling of one or more of these Rab proteins during exocytosis. For example, it has recently been demonstrated that knock-down of Rab8 in AR42J cells resulted in a decrease in CCK-8-induced secretion [31]. Geldanamicn may interfere with Rab-GDI-1: Rab8 interactions, thereby affecting agonist-induced secretion.
The fact that Hsp90 and rab-GDI-1 interact with one another in the cytosol and co-localize on membrane fractions in AR42J cells suggests that rab-GDI-1: Hsp90 complexes may play an important role in regulating Rab protein function and recycling, as well as regulated secretion. However, because Hsp90 binds to and regulates the activity of many client proteins, we cannot rule out the possibility that the function of Hsp90 in regulated exocytosis may involve interactions with proteins other than rab-GDI-1. Nevertheless, we believe that this is the first study to demonstrate a role for Hsp90 in regulated secretion in exocrine cells.
In conclusion, we have demonstrated that the expression and subcellular distribution of rab-GDI isoforms 1 and 2 are not significantly altered as AR42J cells differentiate into a more acinar cell-like phenotype. We have also demonstrated that Hsp90 interacts with rab-GDI-1 and regulates agonist-induced amylase release from AR42J cells, suggesting that these proteins may form a complex that regulates Rab protein function and localization in exocrine cells.
Abbreviations
GDI (GDP dissociation inhibitor); Dex (Dexamethasone); Hsp90 (heat shock protein 90); REP (Rab escort protein); CCK-8 (Cholecystokinin octa-peptide).
Acknowledgements
This work was suported by Contract Grant Sponsor: NIH-NIGMS, Contract Grant Number: 1 R15 GM076034-01 and Contract Grant Sponsor: PSC-CUNY.
References
- 1.Jordens I, Marsman M, Kuijl C, Neefjes J. Rab proteins, connecting transport and vesicle fusion. Traffic. 2005;6(12):1070–1077. doi: 10.1111/j.1600-0854.2005.00336.x. [DOI] [PubMed] [Google Scholar]
- 2.Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci USA. 2006;103(32):11821–11827. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burton J, De Camilli P. A novel mammalian guanine nucleotide exchange factor (GEF) specific for rab proteins. Adv Second Messenger Phosphoprotein Res. 1994;29:109–119. doi: 10.1016/s1040-7952(06)80010-8. [DOI] [PubMed] [Google Scholar]
- 4.Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol Rev. 2001;81(1):153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- 5.Collins RN. “Getting it on“-GDI displacement and small GTPase membrane recruitment. Mol Cell. 2003;12(5):1064–1066. doi: 10.1016/s1097-2765(03)00445-3. [DOI] [PubMed] [Google Scholar]
- 6.Wu S-K, Zeng K, Wilson IA, Balch WE. Structural insights into the function of the rab-GDI superfamily. TIBS. 1996;21:472–476. doi: 10.1016/s0968-0004(96)10062-1. [DOI] [PubMed] [Google Scholar]
- 7.Alory C, Balch WE. Molecular evolution of the Rab-escort-protein/guanine-nucleotide-dissociation-inhibitor superfamily. Mol Biol Cell. 2003;14(9):3857–3867. doi: 10.1091/mbc.E03-04-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pfeffer SR. Rab GDP dissociation inhibitor: Putting rab GTPases in the right place. J Biol Chem. 1995;270:17057–17059. doi: 10.1074/jbc.270.29.17057. [DOI] [PubMed] [Google Scholar]
- 9.Wu YW, Tan KT, Waldmann H, Goody RS, Alexandrov K. Interaction analysis of prenylated Rab GTPase with Rab escort protein and GDP dissociation inhibitor explains the need for both regulators. Proc Natl Acad Sci USA. 2007;104(30):12294–12299. doi: 10.1073/pnas.0701817104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishimura N, Nakamura H, Takai Y, Sano K. Molecular cloning and characterization of two rab GDI species from rat brain. J Biol Chem. 1994;269:14191–14198. [PubMed] [Google Scholar]
- 11.Shisheva A, Sudhof TC, Czech MP. Cloning, characterization and expression of a novel GDP dissociation inhibitor isoform from skeletal muscle. Mol Cell Biol. 1994;14:3459–3468. doi: 10.1128/mcb.14.5.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Regazzi R, Kikuchi A, Takai Y, Wollheim CB. The small GTP-binding proteins in the cytosol of insulin secreting cells are complexed to GDP dissociation inhibitor proteins. J Biol Chem. 1992;267:17512–17519. [PubMed] [Google Scholar]
- 13.Yang C, Slepnev VI, Goud B. Rab proteins form in vivo complexes with two isoforms of the GDP-dissociation inhibitor protein. J Biol Chem. 1994;269:31891–31899. [PubMed] [Google Scholar]
- 14.Raffaniello RD, Raufman J-P. Cytosolic Rab3D is associated with Rab escort protein (REP), not rab GDP-dissociation inhibitor (GDI), in dispersed chief cells from guinea pig stomach. J Cell Biochem. 1999;72:540–548. [PubMed] [Google Scholar]
- 15.Desnoyers L, Anant JS, Seabra MC. Geranylation of Rab proteins. Biochem Soc Trans. 1996;24(3):699–703. doi: 10.1042/bst0240699. [DOI] [PubMed] [Google Scholar]
- 16.Chan S, Lin J, Raffaniello RD. Rab-GDP dissociation inhibitor (GDI) expression and localization in dispersed parotid acini from rat. Gastroenterology. 1998;114(supplement):A1134. [Google Scholar]
- 17.Luan P, Balch WE, Emr SD, Burd CG. Molecular dissection of guanine nucleotide dissociation inhibitor function in vivo. Rab-independent binding to membranes and role of Rab recycling factors. J Biol Chem. 1999;274(21):14806–14817. doi: 10.1074/jbc.274.21.14806. [DOI] [PubMed] [Google Scholar]
- 18.Sakisaka T, Meerlo T, Matteson J, Plutner H, Balch WE. Rab-alphaGDI activity is regulated by a Hsp90 chaperone complex. EMBO J. 2002;21(22):6125–6135. doi: 10.1093/emboj/cdf603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen CY, Balch WE. The Hsp90 chaperone complex regulates GDI-dependent Rab recycling. Mol Biol Cell. 2006;17:3494–3507. doi: 10.1091/mbc.E05-12-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Logsdon CD, Moessner J, Williams JA, Goldfine ID. Glucocorticoids increase amylase mRNA levels, secretory organelles, and secretion in pancreatic acinar AR42J cells. J Cell Biol. 1985;100(4):1200–1208. doi: 10.1083/jcb.100.4.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Logsdon CD. Glucocorticoids increase cholecystokinin receptors and amylase secretion in pancreatic acinar AR42J cells. J Biol Chem. 1986;261(5):2096–2101. [PubMed] [Google Scholar]
- 22.Piiper A, Leser J, Lutz MP, Beil M, Zeuzem S. Subcellular distribution and function of Rab3A-D in pancreatic acinar AR42J cells. Biochem Biophys Res Commun. 2001;287(3):746–751. doi: 10.1006/bbrc.2001.5651. [DOI] [PubMed] [Google Scholar]
- 23.Chan D, Lin J, Raffaniello RD. Expression and localization of rab escort protein isoforms in parotid acinar cells from rat. J. Cell Physiol. 2000;185:339–347. doi: 10.1002/1097-4652(200012)185:3<339::AID-JCP4>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 24.Erdman RA, Maltese WA. Different Rab GTPases associate preferentially with alpha or beta GDP-dissociation inhibitors. Biochem Biophys Res Comm. 2001;282:4–9. doi: 10.1006/bbrc.2001.4560. [DOI] [PubMed] [Google Scholar]
- 25.Nishimura N, Goji J, Nakamura H, Orita S, Takai Y, Sano K. Cloning of a brain-type isoform of human Rab GDI and its expression in human neuroblastoma cell lines and tumor specimens. Cancer Res. 1995;55(22):5445–5450. [PubMed] [Google Scholar]
- 26.Picard DL. Heat-shock protein 90, a chaperone for folding and regulation. Cell Mol Life Sci. 2002;59(10):1640–1648. doi: 10.1007/PL00012491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pratt WB, Toft DO. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med. 2003;228(2):111–133. doi: 10.1177/153537020322800201. [DOI] [PubMed] [Google Scholar]
- 28.Ogata M, Naito Z, Tanaka S, Moriyama Y, Asano G. Overexpression and localization of heat shock proteins mRNA in pancreatic carcinoma. J Nippon Med Sch. 2000;67(3):177–185. doi: 10.1272/jnms.67.177. [DOI] [PubMed] [Google Scholar]
- 29.Handley MTW, Haynes LP, Burgoyne RD. Differential dynamics of Rab3A and Rab27A on secretory granules. J Cell Sci. 2007;120(6):973–984. doi: 10.1242/jcs.03406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen X, Walker AK, Strahler JR, Simon ES, Tomanicek-Volk SL, Nelson BB, Hurley MC, Ernst SA, Williams JA, Andrews PC. Organelle Proteomics: Analysis of pancreatic zymogen granule membranes. Molec Cellular Proteomics. 2006;5:306–312. doi: 10.1074/mcp.M500172-MCP200. [DOI] [PubMed] [Google Scholar]
- 31.Faust F, Gomez-Lazaro M, Borta H, Agricola B, Schrader M. Rab8 is involved in zymogen granule formation in pancreatic acinar AR42J cells. Traffic. 2008;9(6):964–979. doi: 10.1111/j.1600-0854.2008.00739.x. [DOI] [PubMed] [Google Scholar]