Abstract
This short review outlines our understanding of cervical cancer precursors, concentrating on the central etiologic role of persistent human papillomavirus (HPV) infection. The stages of cervical carcinogenesis are better understood than for most other major cancers, providing a successful cancer etiology and prevention model.
Introduction and historic context
The association of risk with sexual behavior has been posited since the mid-1800s but the central causal role of human papillomavirus (HPV) infection was identified just 35 years ago (1). Thus, the important preventive impact of cervical cytologic screening (Papanicolaou tests) in the second half of the twentieth century preceded and even advanced etiologic understanding. The accessibility of the cervix for population-wide tissue sampling, the delimited ring of tissue at risk (the cervical transformation zone), and the uniform causal pathway centered on HPV infection, fostered the last few decades of productive interdisciplinary studies and improved preventive strategies (2).
Histological and/or molecular definition
The cervix is the lower third of the uterus, projecting into the anterior aspect of the vagina; with regard to carcinogenesis, it can be viewed topologically as a two-dimensional ring of epithelium. The cervical transformation zone is an area of metaplastic tissue between the squamous epithelium of the vagina and the glandular tissue of the endocervical canal. While the entire anogenital epithelium can be infected by HPV, the cervical transformation zone is especially susceptible to carcinogenesis. Recently, a cell population in the transformation zone with specific morphologic and molecular features was described that may represent the cells of origin of most cervical precancers and cancers (3). Destruction or excision of the entire susceptible cell population when precursor lesions are found is still the mainstay of prevention.
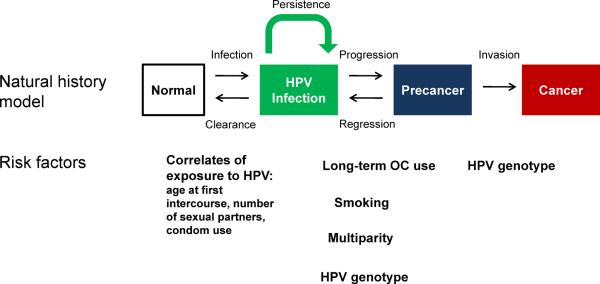
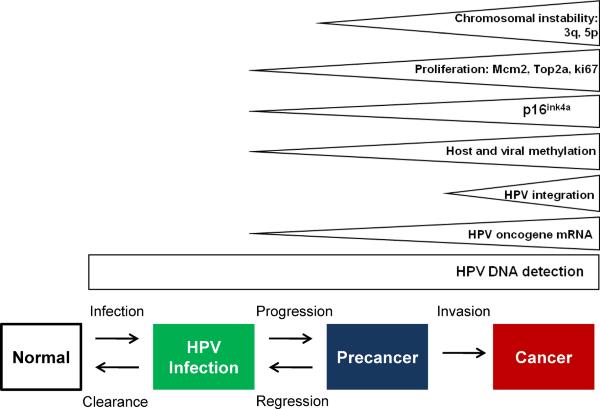
As currently conceived (FIGURE 1), the stages in cervical carcinogenesis include HPV infection; persistence, rather than clearance of the virus, linked to the development of a high-grade precursor lesion or “precancer”; and invasion. These are necessary stages; cervical cancer is virtually impossible in the absence of sexually transmitted HPV infection (4), and in the absence of intermediate progression to precancer. New prevention strategies reviewed below are based on this strong causal model, and the established chain of surrogate endpoints.
Figure 1.
Cervical cancer natural history model
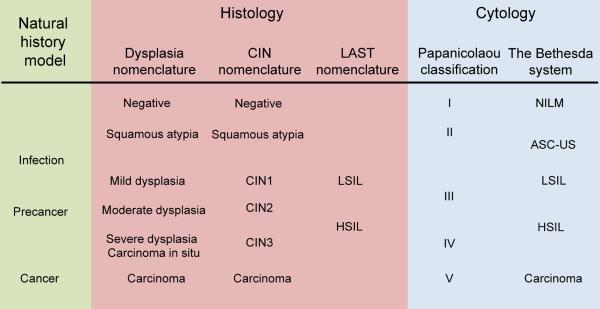
It is interesting to review the historic development of nomenclature for precursors from our current perspective; as the changing terms have slowly adapted to understanding of the key role of HPV infection (5) (FIGURE 2). The nomenclature for the much more common squamous precursors (glandular lesions will be considered only briefly below) has evolved somewhat separately for histology than for cytology. Histology is what defines the underlying neoplastic process and guides treatment, while exfoliative cytology is used in screening that assesses the probable underlying histologic state.
Figure 2.
Terminology of cervical disease categories
The figure shows histological and cytological terminologies of cervical disease categories. CIN=Cervical Intraepithelial Neoplasia (6). LAST=Lower Anogenital Squamous Terminology Standardization (13). LSIL=Low Grade Squamous Intraepithelial Lesion; HSIL=High Grade Squamous Intraepithelial Lesion. NILM=Negative for Intraepithelial Lesion or Malignancy; ASCUS=Atypical Squamous Cells of Undetermined Significance (15).
Fifty years ago, for squamous histology, the cervical cellular abnormalities viewed as the precursor of cervical cancer were termed mild, moderate or severe dysplasia; severe dysplasia was distinguished from the more severe diagnosis of carcinoma in situ. In the late 1960s, Richart proposed the concept of intraepithelial neoplasia (6). CIN3 encompassed severe dysplasia and carcinoma in situ, CIN2 replaced moderate dysplasia, and CIN1 later came to include both the cytologic evidence of HPV infection (koilocytotic atypia) and mild dysplasia. The severity of the diagnosis was based on the degree of replacement of the normal stratified epithelium with mitotically active basal-like epithelium (≤1/3 = CIN1, ≤2/3 = CIN2, >2/3 = CIN3). CIN was viewed as a stepwise progression, with a high probability of transition from the more minor to more serious cancer precursors.
As HPV research demonstrated the high prevalence and transient nature of most cervical HPV infections, it became clear that the notion of inexorable CIN progression was not correct. CIN1 was found to be a poorly reproducible and insensitive histologic diagnosis of acute and mostly transient HPV infection (7). CIN2 was reconsidered as a heterogeneous borderline category between acute HPV infection and the more likely cancer precursor lesions (CIN3). The risk factor profiles (8–10) and HPV genotype distributions (11) in CIN2 and CIN3 are different, and CIN2 is more likely to regress spontaneously compared to CIN3 (12), but current clinical management of CIN2 and CIN3 diagnoses is very similar. The histologic nomenclature did not formally change, however, to a two-stage system (low-grade lesion reflecting acute HPV infection, high-grade lesion representing cancer precursor to be treated) until the Lower Anogenital Squamous Terminology (LAST) conference in 2012 (13). The LAST nomenclature relies on p16 staining to triage CIN2; p16 is a biomarker of disruption by HPV of the Rb pathway (14). CIN2 that is p16-positive is combined with CIN3 to form “High-grade Squamous Intraepithelial Lesion (HSIL)”, representing the immediate precursor to cervical cancer. CIN2 negative for p16 is combined with CIN1 to form “Low-grade Squamous Intraepithelial Lesion (LSIL)”, representing the histologic sign of HPV infection (Figure 2). The LAST nomenclature is new for histology, somewhat controversial, and has not been implemented widely as of yet. In an effort to be forward-looking, we will use this terminology for this review, although many centers still report histology using the CIN, or even the dysplasia, scale.
Cytology nomenclature evolved separately, and was focused practically on which women to refer for colposcopically directed biopsy. The early Pap classification (I, II, III, IV, V) gave the probabilistic prediction of underlying invasive cancer. As experience with cytology increased, an effort was made to describe and predict the actual precursor lesions. The dysplasia/CIS nomenclature was applied, and then replaced by CIN terminology. Importantly, in 1990 and with subsequent revisions, the Bethesda System recognized the low-grade/high-grade distinction, with explicit distinction between acute HPV effect (LSIL) and high-grade precursor (HSIL) (15).
Therefore, if LAST terminology is accepted, squamous histology and cytology will eventually be congruent in the US, and in line with the established stages of cervical carcinogenesis: Negative = normal cervix, LSIL= acute HPV-associated lesions, HSIL= cancer precursors aka precancer, and Cancer (squamous).
The much less common glandular lesions are not as well studied, but are also caused by HPV infection (16). The putative stages, in cervical cytology, are atypical glandular cells (AGC), adenocarcinoma in situ (AIS), and Adenocarcinoma. There is no defined precursor pathway delineated for even rarer histologies, e.g., adenosquamous.
Descriptive epidemiology and etiology
In describing the epidemiology of cervical cancer precursors, there are more easily accessible statistics for cytologic results than for histologic precursors because SEER sites generally do not record CIN3 or lesser lesions, whereas there are large published databases of screening cytologic results (17). It is important to note that the distribution of precursors is affected not just by the prevalence of HPV in the population but also by previous rounds of screening and treatment of HSIL and cancer (HSIL+). Both LSIL and HSIL are detected only when screening is performed. In well-screened populations in the US, approximately 0.5% of screening cytology results are HSIL (18). Much more common are the results (LSIL and the half of equivocal or ASC-US lesions) that reflect the cytologic evidence of acute HPV infection, accounting together for approximately 5% of cytologic results. HPV infection, detected by molecular tests, is much more common still, i.e., only a minority of infections of infections produce even equivocal cytologic abnormalities (17, 19).
At the precursor level, squamous LSIL/HSIL lesions are overwhelmingly more prevalent than glandular AGC/AIS lesions (0.2% of screening results), whereas in the US about 15% of cancers now are adenocarcinoma (20). This reflects that most screen detected precursors are squamous, which are easier to detect, i.e., it shows that squamous cancer precursors are better caught and prevented by screening.
The etiology and descriptive epidemiology of each carcinogenic stage can be viewed separately. With regard to the first stage, acute HPV infection, its epidemiology as just stated is that of a sexually transmitted infectious agent. The behavioral factors influencing risk are those that increase the chance of encountering an infected partner. There is no known innately immune state, although one could theoretically exist in a few individuals, without our knowledge.
HPV infection is a necessary precursor state to cervical cancer. Most HPV infections as detected by molecular (DNA or RNA) assays become undetectable after several months (21, 22). It is not known to which extent the lack of detectability represents viral clearance or persistence in some kind of latent stage (23). The subsequent necessary, and proximal precursor state is HSIL linked to viral persistence. It is not known, by the way, whether long-term persistence precedes the first clonal expansion of (possibly regressing and never diagnosed) HSIL or vice versa. This is a theoretical point that is difficult to study; at the level of detection, persistence is more common than and precedes HSIL. Etiologic co-factors for persistence and progression to HSIL include viral, behavioral, and genetic host factors (FIGURE 1).
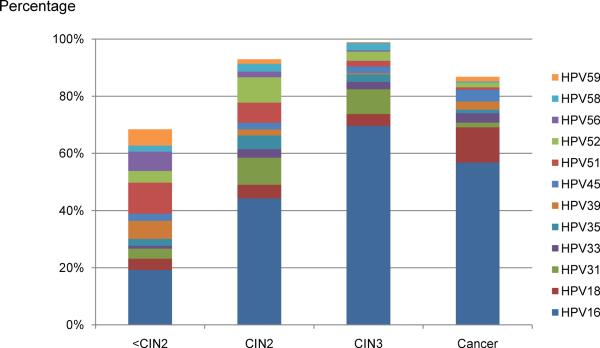
By far the most important viral factor is HPV type (24). The carcinogenic types of HPV are genetically related, and found in several species of the alpha HPV genus. The established carcinogenic types include HPV16, HPV18, HPV31, HPV33, HPV35, HPV39, HPV45, HPV51, HPV52, HPV56, HPV58, HPV59, and HPV68 (25). Another dozen HPV types are possible cervical carcinogens, and might account for a tiny fraction of cases of cancer. The most important HPV type is HPV16, which is responsible for only 20% of infections but which causes 40% of HSIL and half of cervical cancer (see below). HPV18 is next most important, and is also preferentially responsible for adenocarcinoma. HPV18 is underrepresented in cancer precursors compared to its importance in cervical cancer (11). Viral genomic variation is so important for etiology that even subtle variations within viral types (called viral variants) influence risk of progression and invasion, with relative risks stronger than for behavior and genetic factors (26). While many other anogenital HPV types can cause LSIL and even a fraction of HSIL, the types listed above are the ones that can cause cervical cancer. (FIGURE 3)
Figure 3.
Attribution of carcinogenic HPV types to cervical disease categories
Expanded from (11). The type attribution is based on the hierarchical attribution model for carcinogenic genotypes present in multiple infections.
Behavioral factors that approximately double the risk of HSIL among HPV-infected women include multiparity, long-term oral contraceptive use, and smoking (27, 28). These co-factors are firmly established by large case-control studies, and prospective evidence, but it is not known why they are co-factors. Accordingly, multiparity might be a risk factor because of hormonal, trauma, or other mechanisms. The risk-inducing (hormonal?) component of oral contraceptives is likewise unknown (29). It is even uncertain whether smoking acts via an immune-suppressing or genotoxic pathway. Less well-established co-factors for progression to HSIL given HPV infection include chronic cervical inflammation and immunosuppression (e.g., HIV).
Host genetic factors influencing control of infection certainly exist but are poorly understood. The only consistent association is with HLA, supporting the importance of T-cell responses in control of HPV infections and cervical precancers (30).
The natural history of CIN3 in terms of risk factors for invasion is not well understood. An old natural history study of CIN3 progression demonstrated that a minority of CIN3 invaded over the lifetime of patients (31). The factors associated with invasion remain elusive and cannot be directly studied, since prospective observation of CIN3 is unethical. Similarly, it is not understood which mechanisms lead to containment or even regression of CIN3, although immunological factors are presumed to play an important role. There are few identified risk factors for the transition between HSIL and cancer, besides time (age) (32).
Clinical perspective and natural history
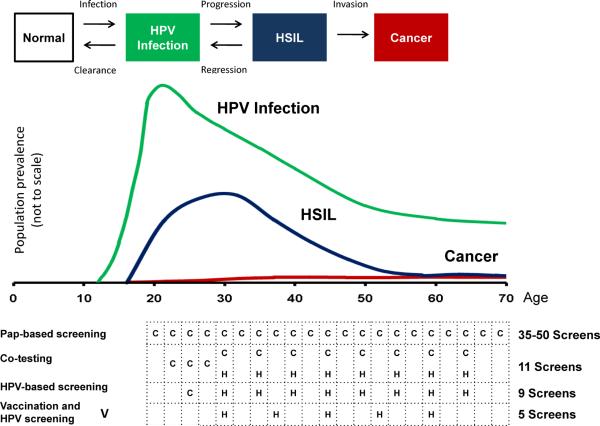
The natural history of a cervical cancer starts with a sexually transmitted carcinogenic HPV infection (FIGURE 4) (22). In the minority of infections, an ASC-US or LSIL cytologic abnormality is evident. Clearance of the HPV infection and associated LSIL is often rapid, with more than half of infections clearing (undetectable using standard DNA/RNA detection methods) within a year, and 90% of infections within approximately 2 years of acquisition. As the rate of clearance slows, the chance of development of HSIL gradually increases, representing the growth of a clonal high-grade lesion. In cohort studies, HSIL is diagnosed up to several years following HPV acquisition. HSIL lesions typically grow laterally around the circumference of the transformation zone, taking many years to decades before invasion, and accounting for the success of screening and secondary prevention of cancer.
Figure 4.
Prevalence of HPV infections, HSIL, and cancer by age
The figure shows the natural history model and the corresponding prevalence of HPV infection, HSIL, and cancer in the population. Data on HPV infections are based on a summary of US-based HPV prevalence studies (50). The age distribution of HSIL is estimated based on data on CIN2 and CIN3 from Kaiser Permanente Northern California Health Maintenance Organization (P. E. Castle, personal communication) and the data on cancers are from the Surveillance, Epidemiology, and End Results 17 database (http://seer.cancer.gov/). Exemplary cervical cancer prevention strategies based on cytology, HPV testing, and HPV vaccination, are shown with the effective total number of screens among screen-negative women per lifetime (47).
The typical time course of the natural history leads to typical ages of each stage (Figure 4) with the peak of HPV acquisition in adolescence and early adulthood, the peak of HSIL around 25–30, and the peak of cancer from 45–60 (33).
There are biomarkers associated with and reflecting each stage in natural history. Biomarkers for HPV-related diseases either measure viral nucleic acids, viral proteins, or cellular factors altered by viral oncogenes (FIGURE 5). The most important biomarker of HPV infection is the detection of HPV DNA. DNA tests, based on hybridization or PCR of a pool of carcinogenic types, have been the mainstay of HPV-related prevention efforts (34). Individual genotyping can predict risk of HSIL, since progression differs strongly by type. HPV16, in particular, predicts a greater prospective risk than other carcinogenic types. It tends to persist slightly longer (23), and predicts a substantially higher risk of HSIL and cancer (35–37). However, for clinical purposes, genotyping alone does not allow sufficient discrimination between transient infections and cancers or HSIL. Increased expression of HPV oncogene mRNA has been evaluated as a marker for the transition from a productive to a transforming infection. p16 is a cellular marker of HPV oncogene activity and has been evaluated for adjudication of ambiguous histology and as a cytological test for triage of women with abnormal screening results (14). Other cellular markers associated with HPV transformation are proliferation markers such as Ki-67, mcm-2, and top2a (38). Expression of HPV oncogenes leads to increasing chromosomal instability, even at the HSIL stage. Several regions, most importantly 3q and 5p, are often altered in HPV-related cancer precursors and can be detected by in-situ hybridization assays. Recently, methylation of late HPV genes has been described in the transition from HPV infection to HSIL (39–41). Disease-specific biomarkers will be important to decide who among women testing positive for HPV needs referral to colposcopy. While there is increasing evidence that HPV oncogene mRNA and p16 could serve these needs, data for other markers is very limited.
Figure 5.
Biomarkers for cervical disease categories
Prospects and implications for screening, detection and prevention
The steps in a conventional cervical screening program are cervical cytologic screening, triage by HPV testing of equivocal (ASC-US) cytologic results, colposcopic referral of women with definitely abnormal or HPV-positive ASC-US results, biopsies of acetowhite lesions (those that turn white on application of acetic acid), and treatment by loop electrical excision procedure (LEEP) of the transformation zone if histologic HSIL is found. HSIL is treated to prevent cervical cancer; there is no specific treatment yet for HPV infection itself. Cervical cancer treatment is beyond the scope of this review of precursors.
New knowledge of the central etiologic role of HPV has led to two preventive strategies, vaccines and HPV-based screening that both act at the cancer precursor stage. Already available HPV vaccines are highly effective prophylactic agents against HPV infection and associated ASC-US, LSIL, and HSIL, but are not therapeutic (42). Therefore, they are best administered to girls prior to onset of sexual activity (e.g., age 11, range 9–14). The bivalent vaccine (GSK) targets the two most important HPV types (HPV16 and HPV18), which together account for approximately 70% of cervical cancers and slightly over half of HSIL (43). The quadrivalent vaccine (Merck) also prevents infections with HPV6 and HPV11, which together cause 90% of genital warts (condyloma acuminatum) (44). It is important to realize that it is not essential that HPV vaccines (whose prophylactic efficacy has already been shown prospectively to last a decade) confer lifelong immunity. Cervical cancers in later life are mainly the result of HPV infections in earlier life. If sufficient population coverage is achieved, the early peak of HPV will be removed, the secondary peak in HSIL will not occur, and cervical cancer will inevitably be substantially reduced.
Despite the promise, vaccine uptake has been variable in wealthy nations, and limited in the low-resource regions that are most in need. The available vaccines are expensive, require a cold chain, and are administered in three doses spanning six months. Thus, for a variety of practical and societal reasons (e.g., opposition to vaccination of young girls against a sexually transmitted agent, fear of vaccination), coverage in the US has been lower than would be optimal from a public health perspective.
Next-generation vaccines are being tested. The closest to release is a nonavalent vaccine (HPV16, HPV18, HPV31, HPV33, HPV45, HPV52, HPV58, and HPV6 and HPV11) that, if as efficacious as the current vaccines, would protect against 90% of cervical cancers as well as condyloma (45). Other novel vaccines are in development, and have been reviewed elsewhere (46). It would be ideal to have a safe, therapeutic anti-HPV vaccine or other treatment, but that is not yet available.
Until prophylactic HPV vaccine coverage is much more extensive, and time has passed to create an immune cohort of women (i.e., primary prevention), screening will remain the mainstay of cervical cancer (secondary) prevention. The main intent of screening is to identify HSIL and treat it to prevent cervical cancer morbidity and mortality.
Screening programs previously built around cytology alone are changing, prompted by our improved understanding of HPV natural history and the advent of HPV testing (17). We have already mentioned the nearly universal use in the US of HPV testing to triage equivocal (ASC-US) cytologic results, the most common cytologic abnormality (up to 5% of screens) (ALTS). The next phase is the incorporation of HPV testing into general screening, permitting the extension of screening intervals (47).
In Figure 4, we show the evolution of screening strategies. In the era before the etiology of cervical cancer was understood, the public health community successfully convinced most US women to participate in annual cytology screening. The annual Pap smear was effective programmatically, given the typically slow growth of HSIL, because repetition overcame the relative insensitivity of each round of screening. Cervical cytology remains a successful example of screening, and can be credited with greatly reducing cervical cancer incidence and mortality. However, more focused screening is now possible.
Specifically, we now know that it typically takes many years for an HPV infection, even if persistent, to cause cervical cancer. We understand that there is a peak in HPV acquisition and LSIL in adolescent and young adult women, a secondary peak in HSIL some years later, and a rise in cancer many years later. Thus, it is not optimal to screen adolescent women, when HPV infection and LSIL are extremely common but the risk of cervical cancer is extremely low. Accordingly, the age of initiation of cytologic screening has been raised to 21, which is still conservative because cervical cancer rates do not start to rise appreciably until ages 25–30. The interval between cytologic screens has been increased to every 3 years. Beginning at age 30, past the peak of acute HPV infection, co-testing with HPV assays and cytology is preferred in the U.S. over cytology alone (47). In some other countries, e.g., the Netherlands, there is a move toward primary screening with HPV testing alone, followed by cytology restricted to the HPV screen-positive women (48). For women testing HPV-negative, Pap-negative, the recommended repeat screening interval is 5 years. This extension recognizes that too-frequent screening with sensitive HPV testing will mainly and non-specifically pick up new HPV infections and associated LSIL, rather than HSIL. Screening now stops for women with normal screening histories at age 65, because new infections are rarely acquired at that age and, even if they are, the latency period until invasive cancer would typically exceed a woman's lifespan (47).
Due to the great sensitivity and negative predictive value (reassurance) provided by HPV testing, it is possible in low-resource regions to consider even more extended and cost-effective HPV-based screening (49). One or 2 rounds of HPV screening at ages 30–45 could theoretically reduce the burden of cervical cancer, with the proviso that there must be clinical resources available to manage the 5–20% or more of women, depending on region, who will test HPV-positive even at those ages.
To summarize, we now have the knowledge of cervical cancer precursors and tools to approach the virtual eradication of cervical cancer efficiently. From a primary prevention standpoint, girls should be vaccinated early to prevent the initial peak of carcinogenic HPV infections. For complementary secondary prevention, screening using HPV and cytology co-testing should be focused especially on the age of maximal incidence of easily treatable HSIL (ages 25–40 are critical), prior to the age of highest cervical cancer rates. Eventually, for women who follow the vaccination-screening prevention strategy, it will be possible to assure extremely low risk of cervical cancer in later life, possibly permitting (and this would require the kind of medical record and patient follow-up that does not yet exist in most places) the societal cessation of prevention efforts even earlier than age 65.
Footnotes
The authors report no conflicts of interest in the preparation of this manuscript.
Reference List
- (1).zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342–50. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- (2).Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370:890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- (3).Herfs M, Yamamoto Y, Laury A, Wang X, Nucci MR, McLaughlin-Drubin ME, et al. A discrete population of squamocolumnar junction cells implicated in the pathogenesis of cervical cancer. Proc Natl Acad Sci U S A. 2012;109:10516–21. doi: 10.1073/pnas.1202684109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Bosch FX, Lorincz A, Munoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55:244–65. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Koss LG. Cytologic and histologic manifestations of human papillomavirus infection of the uterine cervix. Cancer Detect Prev. 1990;14:461–4. [PubMed] [Google Scholar]
- (6).Richart RM. Proceedings: An assessment of the biology of cervical intraepithelial neoplasia. Proc Natl Cancer Conf. 1972;7:219–22. [PubMed] [Google Scholar]
- (7).Stoler MH, Schiffman M. Interobserver reproducibility of cervical cytologic and histologic interpretations: realistic estimates from the ASCUS-LSIL Triage Study. JAMA. 2001;285:1500–5. doi: 10.1001/jama.285.11.1500. [DOI] [PubMed] [Google Scholar]
- (8).Castle PE, Stoler MH, Solomon D, Schiffman M. The relationship of community biopsy-diagnosed cervical intraepithelial neoplasia grade 2 to the quality control pathology-reviewed diagnoses: an ALTS report. Am J Clin Pathol. 2007;127:805–15. doi: 10.1309/PT3PNC1QL2F4D2VL. [DOI] [PubMed] [Google Scholar]
- (9).Luhn P, Walker J, Schiffman M, Zuna RE, Dunn ST, Gold MA, et al. The role of cofactors in the progression from human papillomavirus infection to cervical cancer. Gynecol Oncol. 2012 doi: 10.1016/j.ygyno.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Wang SS, Zuna RE, Wentzensen N, Dunn ST, Sherman ME, Gold MA, et al. Human papillomavirus cofactors by disease progression and human papillomavirus types in the study to understand cervical cancer early endpoints and determinants. Cancer Epidemiol Biomarkers Prev. 2009;18:113–20. doi: 10.1158/1055-9965.EPI-08-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Wentzensen N, Schiffman M, Dunn T, Zuna RE, Gold MA, Allen RA, et al. Multiple human papillomavirus genotype infections in cervical cancer progression in the study to understand cervical cancer early endpoints and determinants. Int J Cancer. 2009;125:2151–8. doi: 10.1002/ijc.24528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Castle PE, Schiffman M, Wheeler CM, Solomon D. Evidence for frequent regression of cervical intraepithelial neoplasia-grade 2. Obstet Gynecol. 2009;113:18–25. doi: 10.1097/AOG.0b013e31818f5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Darragh TM, Colgan TJ, Cox JT, Heller DS, Henry MR, Luff RD, et al. The Lower Anogenital Squamous Terminology Standardization Project for HPV-Associated Lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. J Low Genit Tract Dis. 2012;16:205–42. doi: 10.1097/LGT.0b013e31825c31dd. [DOI] [PubMed] [Google Scholar]
- (14).Wentzensen N, von Knebel DM. Biomarkers in cervical cancer screening. Dis Markers. 2007;23:315–30. doi: 10.1155/2007/678793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Solomon D, Davey D, Kurman R, Moriarty A, O'Connor D, Prey M, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002;287:2114–9. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- (16).Castellsague X, Diaz M, de SS, Munoz N, Herrero R, Franceschi S, et al. Worldwide human papillomavirus etiology of cervical adenocarcinoma and its cofactors: implications for screening and prevention. J Natl Cancer Inst. 2006;98:303–15. doi: 10.1093/jnci/djj067. [DOI] [PubMed] [Google Scholar]
- (17).Katki HA, Kinney WK, Fetterman B, Lorey T, Poitras NE, Cheung L, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12:663–72. doi: 10.1016/S1470-2045(11)70145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Davey DD, Neal MH, Wilbur DC, Colgan TJ, Styer PE, Mody DR. Bethesda 2001 implementation and reporting rates: 2003 practices of participants in the College of American Pathologists Interlaboratory Comparison Program in Cervicovaginal Cytology. Arch Pathol Lab Med. 2004;128:1224–9. doi: 10.5858/2004-128-1224-BIARRP. [DOI] [PubMed] [Google Scholar]
- (19).Porras C, Wentzensen N, Rodriguez AC, Morales J, Burk RD, Alfaro M, et al. Switch from cytology-based to human papillomavirus test-based cervical screening: implications for colposcopy. Int J Cancer. 2012;130:1879–87. doi: 10.1002/ijc.26194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Wang SS, Sherman ME, Hildesheim A, Lacey JV, Jr., Devesa S. Cervical adenocarcinoma and squamous cell carcinoma incidence trends among white women and black women in the United States for 1976–2000. Cancer. 2004;100:1035–44. doi: 10.1002/cncr.20064. [DOI] [PubMed] [Google Scholar]
- (21).Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998;338:423–8. doi: 10.1056/NEJM199802123380703. [DOI] [PubMed] [Google Scholar]
- (22).Rodriguez AC, Schiffman M, Herrero R, Wacholder S, Hildesheim A, Castle PE, et al. Rapid clearance of human papillomavirus and implications for clinical focus on persistent infections. J Natl Cancer Inst. 2008;100:513–7. doi: 10.1093/jnci/djn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Rositch AF, Koshiol J, Hudgens MG, Razzaghi H, Backes DM, Pimenta JM, et al. Patterns of persistent genital human papillomavirus infection among women worldwide: A literature review and meta-analysis. Int J Cancer. 2012 doi: 10.1002/ijc.27828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Schiffman M, Herrero R, Desalle R, Hildesheim A, Wacholder S, Rodriguez AC, et al. The carcinogenicity of human papillomavirus types reflects viral evolution. Virology. 2005;337:76–84. doi: 10.1016/j.virol.2005.04.002. [DOI] [PubMed] [Google Scholar]
- (25).Cogliano V, Baan R, Straif K, Grosse Y, Secretan B, El GF. Carcinogenicity of human papillomaviruses. Lancet Oncol. 2005;6:204. doi: 10.1016/s1470-2045(05)70086-3. [DOI] [PubMed] [Google Scholar]
- (26).Schiffman M, Rodriguez AC, Chen Z, Wacholder S, Herrero R, Hildesheim A, et al. A population-based prospective study of carcinogenic human papillomavirus variant lineages, viral persistence, and cervical neoplasia. Cancer Res. 2010;70:3159–69. doi: 10.1158/0008-5472.CAN-09-4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Appleby P, Beral V, Berrington de GA, Colin D, Franceschi S, Goodill A, et al. Carcinoma of the cervix and tobacco smoking: collaborative reanalysis of individual data on 13,541 women with carcinoma of the cervix and 23,017 women without carcinoma of the cervix from 23 epidemiological studies. Int J Cancer. 2006;118:1481–95. doi: 10.1002/ijc.21493. [DOI] [PubMed] [Google Scholar]
- (28).Smith JS, Green J, Berrington de GA, Appleby P, Peto J, Plummer M, et al. Cervical cancer and use of hormonal contraceptives: a systematic review. Lancet. 2003;361:1159–67. doi: 10.1016/s0140-6736(03)12949-2. [DOI] [PubMed] [Google Scholar]
- (29).Chung SH, Franceschi S, Lambert PF. Estrogen and ERalpha: culprits in cervical cancer? Trends Endocrinol Metab. 2010;21:504–11. doi: 10.1016/j.tem.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Madeleine MM, Johnson LG, Smith AG, Hansen JA, Nisperos BB, Li S, et al. Comprehensive analysis of HLA-A, HLA-B, HLA-C, HLA-DRB1, and HLA-DQB1 loci and squamous cell cervical cancer risk. Cancer Res. 2008;68:3532–9. doi: 10.1158/0008-5472.CAN-07-6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).McCredie MR, Sharples KJ, Paul C, Baranyai J, Medley G, Jones RW, et al. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol. 2008;9:425–34. doi: 10.1016/S1470-2045(08)70103-7. [DOI] [PubMed] [Google Scholar]
- (32).Schiffman M, Rodriguez AC. Heterogeneity in CIN3 diagnosis. Lancet Oncol. 2008;9:404–6. doi: 10.1016/S1470-2045(08)70110-4. [DOI] [PubMed] [Google Scholar]
- (33).Gustafsson L, Adami HO. Natural history of cervical neoplasia: consistent results obtained by an identification technique. Br J Cancer. 1989;60:132–41. doi: 10.1038/bjc.1989.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Schiffman M, Wentzensen N, Wacholder S, Kinney W, Gage JC, Castle PE. Human papillomavirus testing in the prevention of cervical cancer. J Natl Cancer Inst. 2011;103:368–83. doi: 10.1093/jnci/djq562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Chen HC, You SL, Hsieh CY, Schiffman M, Lin CY, Pan MH, et al. Prevalence of genotype-specific human papillomavirus infection and cervical neoplasia in Taiwan: a community-based survey of 10,602 women. Int J Cancer. 2011;128:1192–203. doi: 10.1002/ijc.25685. [DOI] [PubMed] [Google Scholar]
- (36).Khan MJ, Castle PE, Lorincz AT, Wacholder S, Sherman M, Scott DR, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst. 2005;97:1072–9. doi: 10.1093/jnci/dji187. [DOI] [PubMed] [Google Scholar]
- (37).Kjaer SK, Frederiksen K, Munk C, Iftner T. Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence. J Natl Cancer Inst. 2010;102:1478–88. doi: 10.1093/jnci/djq356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Sahasrabuddhe VV, Luhn P, Wentzensen N. Human papillomavirus and cervical cancer: biomarkers for improved prevention efforts. Future Microbiol. 2011;6:1083–98. doi: 10.2217/fmb.11.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Clarke MA, Wentzensen N, Mirabello L, Ghosh A, Wacholder S, Harari A, et al. Human papillomavirus DNA methylation as a potential biomarker for cervical cancer. Cancer Epidemiol Biomarkers Prev. 2012;21:2125–37. doi: 10.1158/1055-9965.EPI-12-0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Mirabello L, Sun C, Ghosh A, Rodriguez AC, Schiffman M, Wentzensen N, et al. Methylation of human papillomavirus type 16 genome and risk of cervical precancer in a Costa Rican population. J Natl Cancer Inst. 2012;104:556–65. doi: 10.1093/jnci/djs135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Wentzensen N, Sun C, Ghosh A, Kinney W, Mirabello L, Wacholder S, et al. Methylation of HPV18, HPV31, and HPV45 Genomes and Cervical Intraepithelial Neoplasia Grade 3. J Natl Cancer Inst. 2012;104:1738–49. doi: 10.1093/jnci/djs425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Hildesheim A, Herrero R, Wacholder S, Rodriguez AC, Solomon D, Bratti MC, et al. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: a randomized trial. JAMA. 2007;298:743–53. doi: 10.1001/jama.298.7.743. [DOI] [PubMed] [Google Scholar]
- (43).Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter D, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301–14. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- (44).Munoz N, Manalastas R, Jr., Pitisuttithum P, Tresukosol D, Monsonego J, Ault K, et al. Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine in women aged 24–45 years: a randomised, double-blind trial. Lancet. 2009;373:1949–57. doi: 10.1016/S0140-6736(09)60691-7. [DOI] [PubMed] [Google Scholar]
- (45).Van de Velde N, Boily MC, Drolet M, Franco EL, Mayrand MH, Kliewer EV, et al. Population-level impact of the bivalent, quadrivalent, and nonavalent human papillomavirus vaccines: a model-based analysis. J Natl Cancer Inst. 2012;104:1712–23. doi: 10.1093/jnci/djs395. [DOI] [PubMed] [Google Scholar]
- (46).Schiller JT, Lowy DR. Understanding and learning from the success of prophylactic human papillomavirus vaccines. Nat Rev Microbiol. 2012;10:681–92. doi: 10.1038/nrmicro2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain J, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62:147–72. doi: 10.3322/caac.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Arbyn M, Ronco G, Anttila A, Meijer CJ, Poljak M, Ogilvie G, et al. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine. 2012;30(Suppl 5):F88–F99. doi: 10.1016/j.vaccine.2012.06.095. [DOI] [PubMed] [Google Scholar]
- (49).Qiao YL, Sellors JW, Eder PS, Bao YP, Lim JM, Zhao FH, et al. A new HPV-DNA test for cervical-cancer screening in developing regions: a cross-sectional study of clinical accuracy in rural China. Lancet Oncol. 2008;9:929–36. doi: 10.1016/S1470-2045(08)70210-9. [DOI] [PubMed] [Google Scholar]
- (50).Smith JS, Melendy A, Rana RK, Pimenta JM. Age-specific prevalence of infection with human papillomavirus in females: a global review. J Adolesc Health. 2008;43:S5–25. S25. doi: 10.1016/j.jadohealth.2008.07.009. [DOI] [PubMed] [Google Scholar]