Abstract
The steroid hormone 1α,25-dihydroxyvitamin D3 [1α,25-(OH)2D3] is the most active metabolite of vitamin D3 which exerts its control over a multitude of biological processes related to calcium and phosphorus homeostasis, cell proliferation and differentiation, and immune regulation. Unfortunately, the therapeutic application of 1α,25-(OH)2D3 is limited by induction of hypercalcemia. The need for vitamin D compounds with selective biological profiles has stimulated the synthesis of more than three thousand analogs of 1α,25-(OH)2D3. Most of these compounds have structural modifications in the side chain and A-ring; there is also an increasing number of modifications in the CD-rings and limited number in the triene system (seco-B ring). Herein, we report the synthesis and biological evaluation of seco-A-19-nor analogs of 1α,25-dihydroxyvitamin D3, developed to study the role of ring A in the biological activity of 1α,25-(OH)2D3. Interestingly, compounds 2 and 4 show substantial ability to bind the vitamin D receptor and the former is also characterized by selective intestinal calcium transport activity.
Keywords: Vitamin D analogs, 19-Norvitamin D3, Vitamin D receptor, VDR binding, Ring-A-seco analogs
1. Introduction
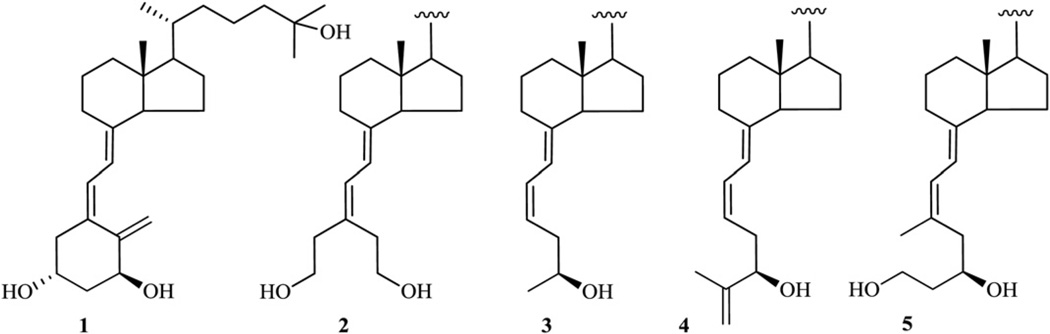
The importance of multiple physiological processes [1] controlled by 1α,25-dihydroxyvitamin D3 [1α,25-(OH)2D3, calcitriol, 1; Fig. 1], the hormonally active metabolite of vitamin D3, has stimulated a very intensive research directed toward developing vitamin D therapeutics that preserve clinically useful activities of 1α,25-(OH)2D3 such as blocking cell proliferation or modulating the immune system but without hypercalcemia and hyperphosphatemia. During last few decades, more than three thousand vitamin D analogs have been synthesized, most of which have focused on side chain modifications, leading to many interesting structures with more rigid side chains [2] as well as widely enlarged [3] or drastically reduced ones [4]. In recent years CD-ring system has also been extensively explored affording numerous analogs with modified hydrindane moiety, along with far-reaching modifications such as removal of the C-ring, D-ring or complete elimination of the CD-ring fragment [5]. The A-ring has been the second, most frequently modified part of the vitamin D scaffold. Thus, alterations at positions C-1, C-2, C-3, C-4 and C-10 [6] have been accomplished, with C-2 [7,8] and C-10 [9] providing the most successful and biologically interesting calcitriol analogs. Although the A-ring modifications have been extensively explored, the closed ring structure has commonly been preserved. The only synthesis of a seco-A-ring calcitriol, namely, 2-nor-1,3-seco-1,25-dihydroxyvitamin D3, was reported by Okamura’s group [10] but without biological evaluation of the analog. Herein, we report the synthesis and biological evaluation of seco-A-ring analogs of 1α,25-dihydroxy-19-norvitamin D3 (2–5, Fig. 1), developed to evaluate the importance of ring A for biological activity of vitamin D compounds. Since it is well-known that the presence of a 1α-hydroxyl group in the vitamin D molecule is crucial for receptor binding [11] and biological potency, the newly designed structures 2–5 possess a hydroxyl group capable of restoring spatial arrangement and acting like the 1α-OH.
Fig. 1.
Chemical structure of 1α,25-dihydroxyvitamin D3 (calcitriol, 1) and its analogs 2–5.
2. Materials and methods
2.1. Preparation of the A-seco-19-norvitamin D3 analogs 2–5
The vitamin D analogs 2–5 were synthesized at the Department of Biochemistry, University of Wisconsin-Madison according to the synthetic routes presented in Schemes 1–5. The spectroscopic and analytical data of all obtained compounds confirmed the assigned structures. The details of the synthesis will be reported elsewhere.
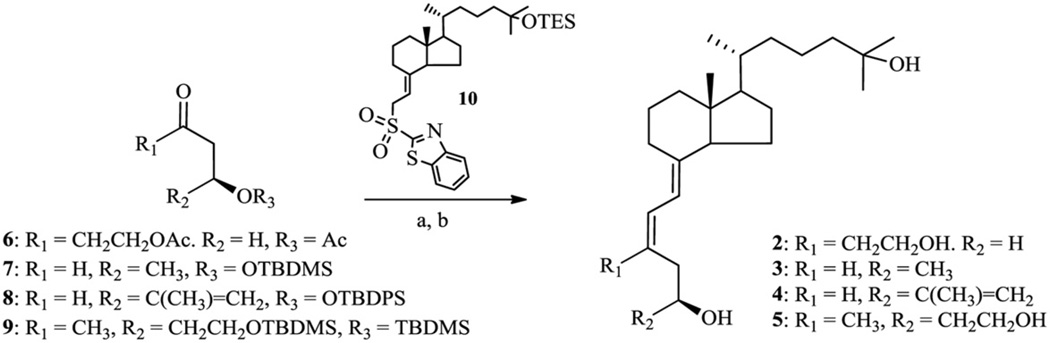
Scheme 1.
(i) (a) 10, LiHMDS, THF, then the ketone 6 in THF; (b) CSA, MeOH, 35% (two steps); (ii) (a) 10, LiHMDS, THF, then the ketone 7–9 in THF; (b) TBAF, THF, for 7: 30% (two steps), 5E:5Z = 1:2.5; for 8: 35% (two steps), 5E:5Z = 1:6; for 9: 31% (two steps), 5E:5Z = 1:12.
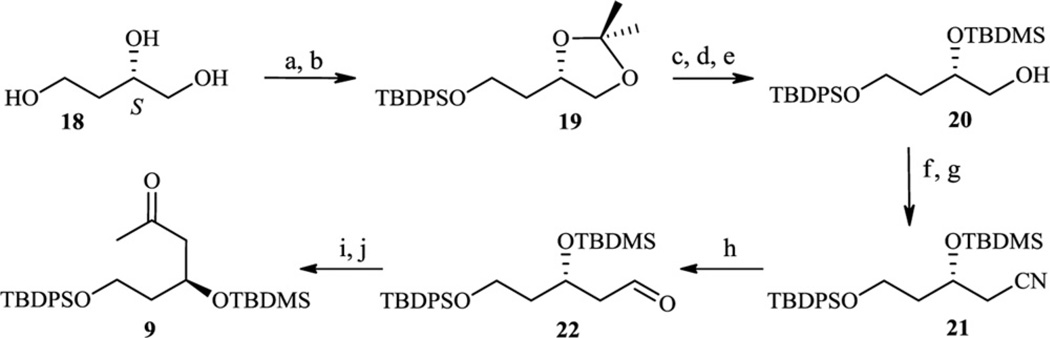
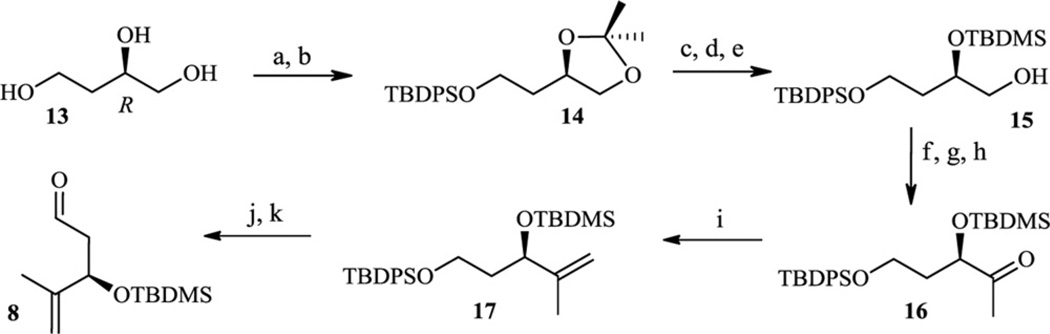
Scheme 5.
(a) CH3C(OCH3)CH3, p-TsOH, DMF, 98%; (b) TBDPSCl, imidazole, DMF, 100%; (c) AcOH, THF, H2O, 92%; (d) TBDMSCl, imidazole, DMF, 95%; (e) AcOH, THF, H2O, 56%; (f) p-TsCl, Pyr, 92%; (g) NaCN, DMSO, 74%; (h) DIBALH, CH2Cl2, 78%; (i) CH3MgBr, THF, 82%; (j) NMO, TPAP, CH2Cl2, 90%.
2.2. In vitro studies
2.2.1. Measurement of binding to the rat recombinant vitamin D receptor
The competition binding assays were performed using 1α,25-(OH)2[26,27-3H]D3 as described previously [12]. The experiment was in duplicate.
2.2.2. Measurement of cellular differentiation
Human promyelocytic leukemia HL-60 cells (obtained from ATTC) were plated at 1.2 × 105 cells/mL and incubated. Eighteen hours after plating the compounds tested were added, and after four days the cells were harvested and the nitro blue tetrazolium (NBT) reduction assay was performed. This method is described in detail elsewhere [13].
2.2.3. Transcriptional assay
The transcriptional activity was measured in ROS 17/2.8 (bone) cells that were stably transfected with the 24-hydroxylase (24OHase) gene promoter upstream of the luciferase reporter gene [14]. Cells were given a range of doses. Sixteen hours after dosing, the cells were harvested and luciferase activities were measured using a luminometer. Each experiment was performed twice, each time in duplicate.
2.3. In vivo studies
Bone calcium mobilization and intestinal calcium transport studies were performed in male, weanling Sprague-Dawley rats, as described previously [7].
3. Results and discussion
3.1. Chemical synthesis of 2–5
Structures 2–5 were accomplished by modified Julia coupling reaction of the known thiazoline sulfone 10 [7] with acyclic aldehydes/ketones 6–9 (Scheme 1). This particular method of coupling was chosen for its high E/Z stereoselectivity that was reported for coupling of allylic sulfones with aldehydes or α,β-unsaturated aldehydes with alkylsulfones [15]. The desired 5Z-geometrical isomers were obtained with 2.5–12 times higher yield than their 5E-counterparts. Removal of the protecting groups in the obtained products by treatment with camphorsulfonic acid or tetrabutylammonium fluoride gave the expected A-seco-19-norvitamin D analogs 2–5 which were purified and separated from their minor 5E-isomers by reversed-phase HPLC.
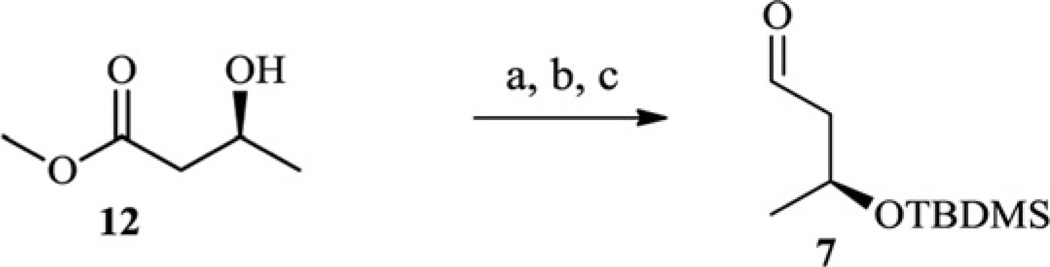
The acyclic fragments 6–9 were synthesized from commercially available substrates according to the routes presented in Schemes 2–5.
Scheme 2.
(a) TBAF, THF, 54%; (b) PCC, CH2Cl2, 68%.
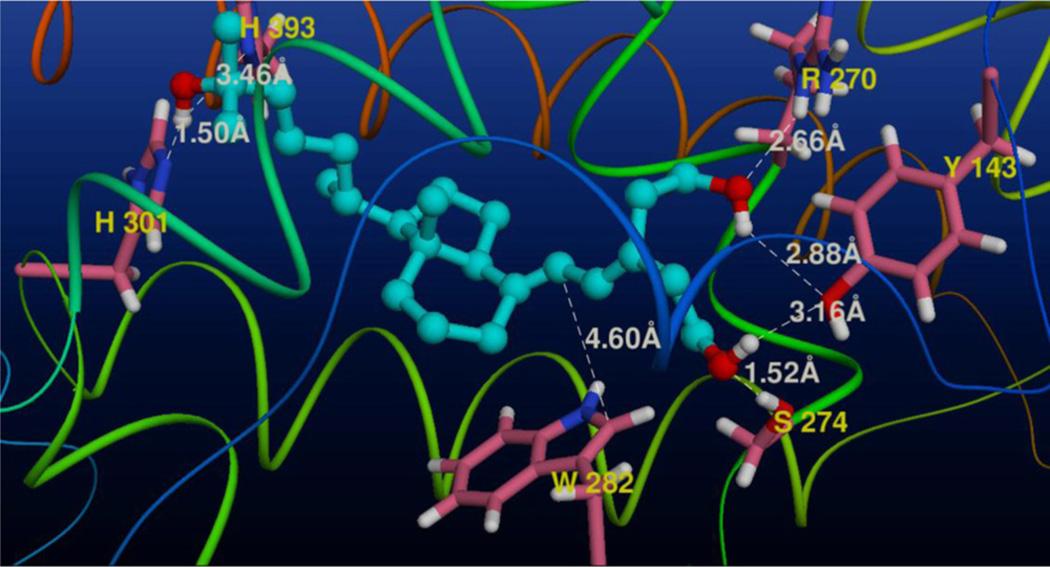
3.2. Docking of analog 2 to the ligand binding pocket of the rVDR (Fig. 2)
Fig. 2.
View of the three-dimensional structure of ligand binding cavity of the rat VDR with the docked analog 2. The five amino acids (Tyr 143, Arg 270, Ser 274, His 301 and His 393) forming the shortest hydrogen bonds (the Å distances are marked in white) with the ligand are depicted; also the Trp 282 residue is shown.
Taking into account the selective intestinal activity of the synthesized analog 2 (see below) we decided to model its complex with rat vitamin D receptor (rVDR). Surprisingly, this 4-nor-A-seco analog, possessing only two hydroxyethyl fragments attached to C-5, and lacking the stereogenic centers in the ring A, bound vitamin D receptor only 10 times weaker than the natural hormone. We docked compound 2 into the modeled full-length [118–423] ligand binding pocket (LBP) of the rVDR and it was found this ligand anchored to the LBP similarly to 1α,25-(OH)2D3 (1) in its crystalline complex with the hVDR [14].
Analysis of the modeled complex revealed that flexibility of the side chain and both hydroxyalkyl substituents at C-5 allowed the ligand hydroxyl groups to form hydrogen bonds with the same set of neighboring amino acids that was found in the crystalline 1-hVDR complex [16]. Thus, A-ring hydroxyls of 2 contacted R270, Y143 and S274, with the last contact being the strongest. The side chain 25-hydroxyl group was positioned between histidines 301 and 393, creating strong hydrogen bonds with both residues. Moreover, indole ring of tryptophan 282 was positioned parallel to the plane of the ligand 5,7-diene moiety at a distance (ca. 4.6Å) allowing π–π interactions.
3.3. Biological evaluation of the synthesized analogs 2–5
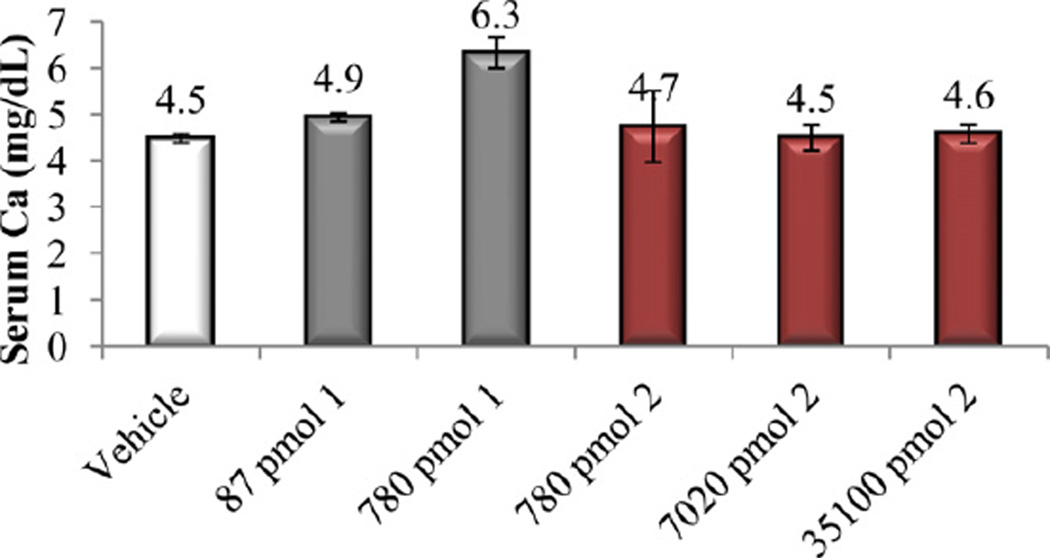
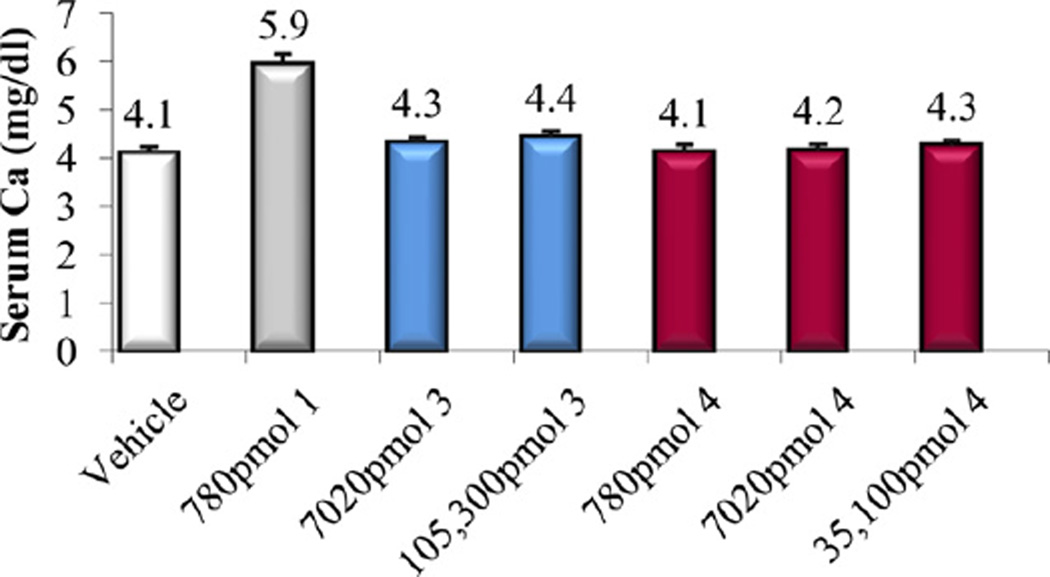
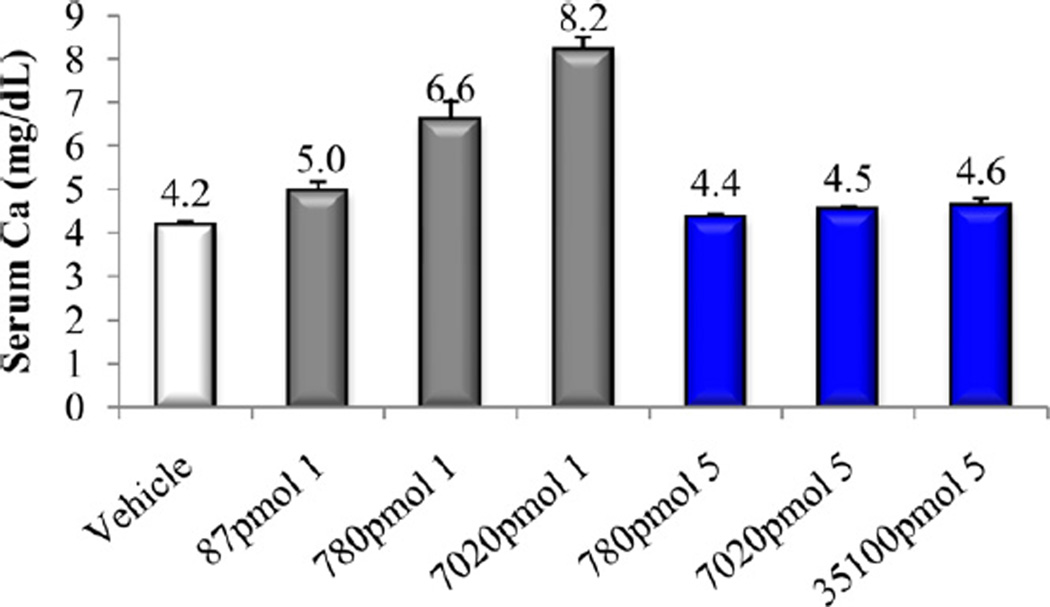
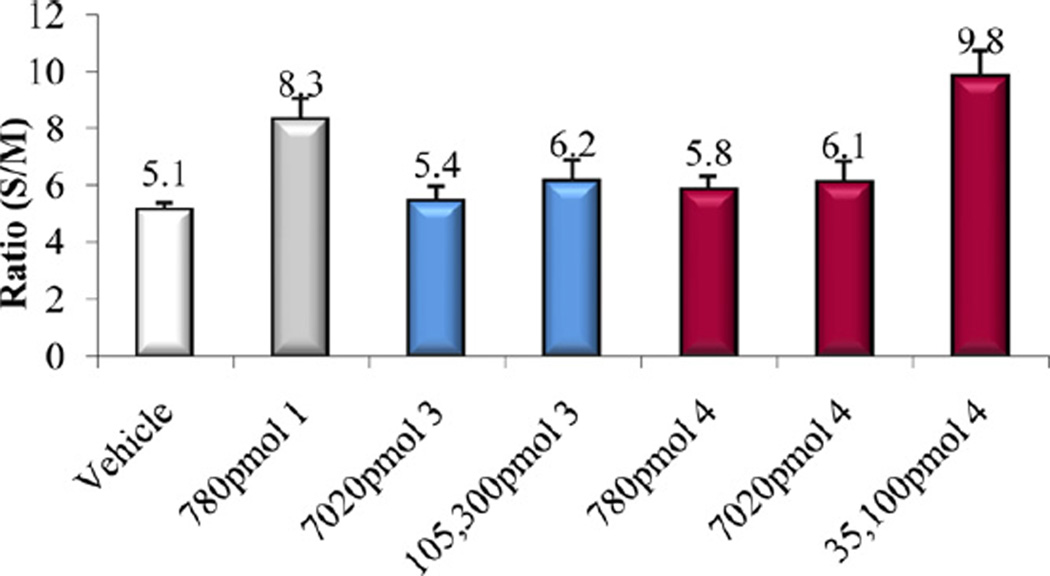
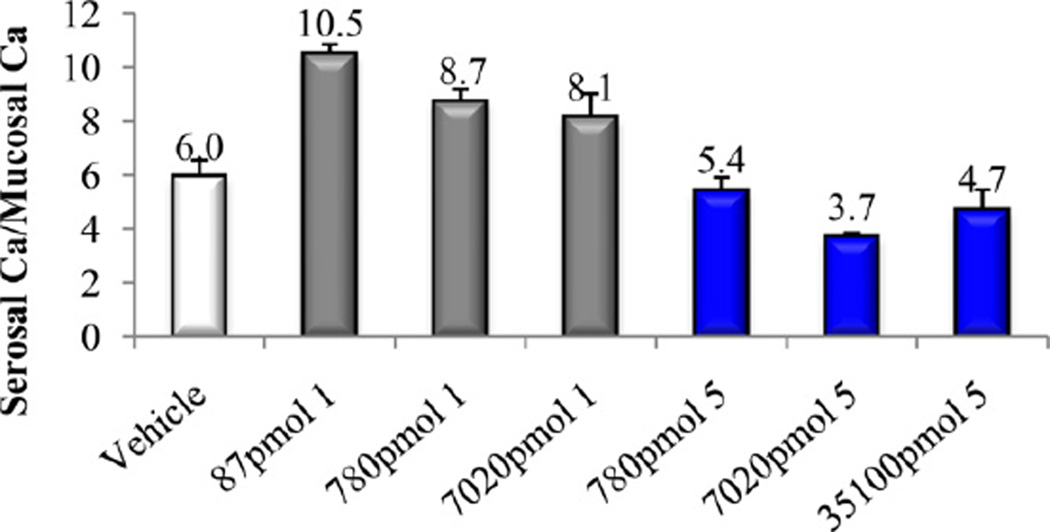
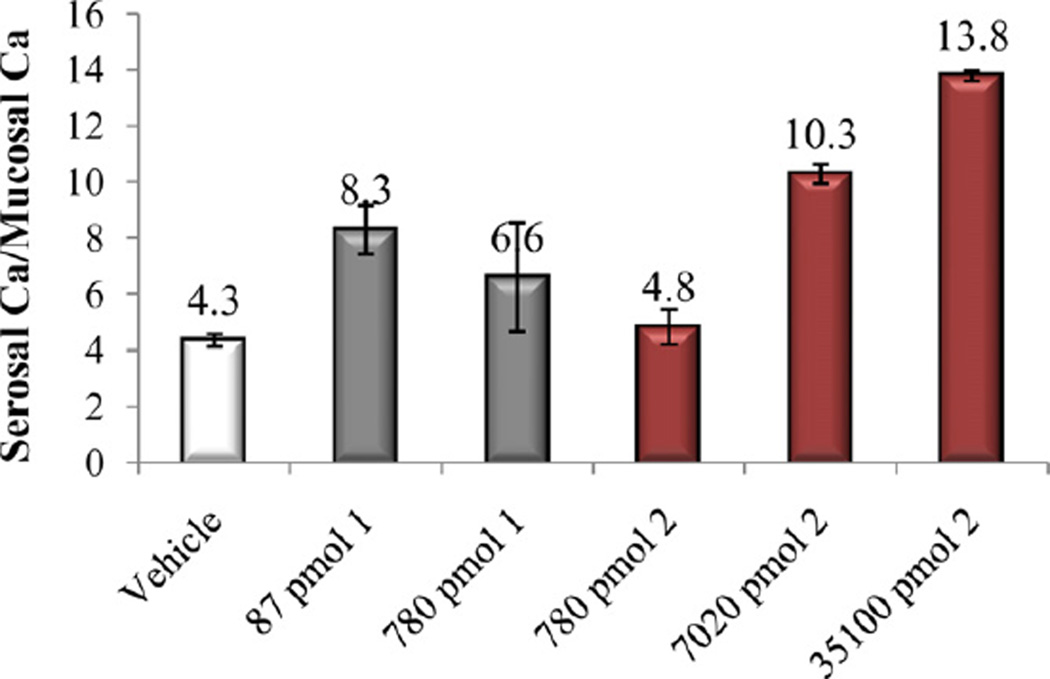
In vitro and in vivo biological activities of the vitamin D analogs 2–5 were tested. Compound 2 and 4 retained ca. 10% and 30%, respectively, of the 1α,25-(OH)2D3 affinity for VDR. Analogs 3 and 5 were poor binders to VDR since their activity was two and four orders of magnitude lower than that of the natural hormone (Table 1). A similar trend was observed in ability to cause differentiation of promyleocytic leukemia cells into monocytes. Analog 4 showed 8% activity compared to 1α,25-(OH)2D3, whereas the other compounds were approximately two (2, 3) and four (5) orders of magnitude less active than the hormone. Moreover, vitamins 2–4 showed two–three orders of magnitude lower transcriptional activity, compared to 1α,25-(OH)2D3. When tested in vivo, none of the new compounds 2–5 possessed any ability to mobilize calcium from bone, even given at a high doses (Figs. 3, 5 and 7). Analogs 3 and 5 did not support intestinal calcium transport (Figs. 6 and 8), whereas 2-methylene compound 4 showed some low intestinal activity (Fig. 6). Interestingly, compound 2 possessed selective activity in inducing intestinal calcium transport, significantly increasing when it was administered at higher doses (Fig. 4).
Table 1.
Relative VDR binding properties, HL-60 differentiation activities and transcriptional activities of the vitamin D compounds.a
| Compound | VDR binding |
HL-60 cell differentiation |
24-OHase transcription |
|---|---|---|---|
| 1 | 100 | 100 | 100 |
| 2 | 10 | 1 | 0.5 |
| 3 | 2 | 2 | 0.13 |
| 4 | 30 | 8 | 1 |
| 5 | 0.01 | 0.01 | – |
The activity of 1α,25-(OH)2D3 is normalized to 100.
Fig. 3.
Bone calcium mobilization activity of calcitriol (1) and compound 2.
Fig. 5.
Bone calcium mobilization activity of calcitriol (1) and compounds 3 and 4.
Fig. 7.
Bone calcium mobilization activity of calcitriol (1) and compound 5.
Fig. 6.
Intestinal calcium transport activity of calcitriol (1) and compounds 3 and 4.
Fig. 8.
Intestinal calcium transport activity of calcitriol (1) and compound 5.
Fig. 4.
Intestinal calcium transport activity of calcitriol (1) and compound 2.
Opening of the ring A in vitamin D compounds causes large decrease in both (in vitro and in vivo) biological activities of the analogs. An entropy penalty for immobilization of flexible, acyclic fragments in the ligand binding pocket can be, at least partially, responsible for this effect. Additionally, loss of hydrophobic interactions with VDR, like in the case of 2 and 3 might also play a role.
Scheme 3.
(a) TBDMSCl, imidazole, DMF, 76%; (b) DIBALH, CH2Cl2, 62%; (c) PCC, CH2Cl2, 51%.
Scheme 4.
(a) CH3C(OCH3)CH3, p-TsOH, DMF, 98%; (b) TBDPSCl, imidazole, DMF, 100%; (c) AcOH, THF, H2O, 92%; (d) TBDMSCl, imidazole, DMF, 95%; (e) AcOH, THF, H2O, 56%; (f) PCC, CH2Cl2, 92%; (g) CH3MgBr, THF, 75%; (h) (COCl)2, DMSO, TEA, 82%; (i) Ph3PCH3Br, n-BuLi, THF, 60%; (j) KOH, MeOH, 55%; (k) PCC, CH2Cl2, 88%.
References
- 1.Feldman D, Pike JW, Glorrieux FH, editors. Vitamin D. second ed. Burlington: Elsevier Academic Press; 2005. [Google Scholar]; DeLuca HF. Evolution of our understanding of vitamin D. Nutrition Reviews. 2008;66(Suppl. 2):S73–S87. doi: 10.1111/j.1753-4887.2008.00105.x. [DOI] [PubMed] [Google Scholar]
- 2.Yamada S, Shimizu M, Yamamoto K. Structure–function relationships of vitamin D including ligand recognition by vitamin D receptor. Medicinal Research Reviews. 2003;23:89–115. doi: 10.1002/med.10023. [DOI] [PubMed] [Google Scholar]
- 3.Inaba Y, Yamamoto K, Yoshimoto N, Matsunawa M, Uno S, Yamada S, Makishima M. Vitamin D3 derivatives with adamantane or lactone ring side chains are cell type-selective vitamin D receptor modulators. Molecular Pharmacology. 2007;71:1298–1311. doi: 10.1124/mol.106.032318. [DOI] [PubMed] [Google Scholar]
- 4.Glebocka A, Sicinski RR, Plum LA, DeLuca HF. Synthesis and biological activity of 2-(3′-hydroxypropylidene)-1α-hydroxy-19-norvitamin D analogues with shortened alkyl side chains. Journal of Medicinal Chemistry. 2011;54:6832–6842. doi: 10.1021/jm200743p. [DOI] [PubMed] [Google Scholar]
- 5.Verlinden L, Eelen G, Bouillon R, Vandewalle M, DeClercq P, Verstuyf A. Analogs of calcitriol. In: Feldman D, Pike JW, Adams JS, editors. Vitamin D. third ed. San Diego, CA: Elsevier Academic Press; 2011. pp. 1461–1487. [Google Scholar]
- 6.Glebocka A, Chiellini G. A-ring analogs of 1,25-dihydroxyvitamin D3. Archives of Biochemistry and Biophysics. 2012;523:48–57. doi: 10.1016/j.abb.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Glebocka A, Sicinski RR, Plum LA, Dame MC, DeLuca HF. New 2-alkylidene 1α,25-dihydroxy-19-norvitamin D3 analogues of high intestinal activity: synthesis and biological evaluation of 2-(3′-alkoxypropylidene) and 2-(3′-hydroxypropylidene) derivatives. Journal of Medicinal Chemistry. 2006;49:2909–2920. doi: 10.1021/jm051082a. [DOI] [PubMed] [Google Scholar]
- 8.Okano T, Tsugawa N, Masuda S, Takeuchi A, Kobayashi T, Takita Y, Nishii Y. Regulatory activities of 2β-(3′-hydroxypropoxy)-1α,25-dihydroxyvitamin D3, a novel synthetic vitamin D3 derivative, on calcium. Biochemical and Biophysical Research Communications. 1989;163:1444–1449. doi: 10.1016/0006-291x(89)91140-6. [DOI] [PubMed] [Google Scholar]
- 9.Perlman KL, Sicinski RR, Schnoes HK, DeLuca HF. 1α,25-Dihydroxy-19-norvitamin D3, a novel vitamin D-related compound with potential therapeutic activity. Tetrahedron Letters. 1990;31:1823–1824. [Google Scholar]
- 10.Barrack AS, Gibbs RA, Okamura WH. Potential inhibitors of vitamin D metabolism: an oxa analogue of vitamin D. Journal of Organic Chemistry. 1988;53:1790–1796. [Google Scholar]
- 11.DeLuca HF, Paaren HE, Schnoes HK. Vitamin D and calcium metabolism. Topics in Current Chemistry. 1979;83:1–65. doi: 10.1007/BFb0019662. [DOI] [PubMed] [Google Scholar]
- 12.Dame MC, Pierce EA, Prahl JM, Hayes CE, DeLuca HF. Monoclonal antibodies to the porcine intestinal receptor for 1,25-dihydroxyvitamin D3: interaction with distinct receptor domains. Biochemistry. 1986;25:4523–4534. doi: 10.1021/bi00364a011. [DOI] [PubMed] [Google Scholar]
- 13.Ostrem VC, Lau WF, Lee SH, Perlman K, Prahl J, Schnoes HK, DeLuca HF, Ikekawa N. Induction of monocytic differentiation of HL-60 cells by 1,25-dihydroxyvitamin D analogs. Journal of Biological Chemistry. 1987;262:14164–14171. [PubMed] [Google Scholar]
- 14.Arbour NC, Ross TK, Zierold C, Prahl JM, DeLuca HF. A highly sensitive method for large-scale measurements of 1,25-dihydroxyvitamin D. Analytical Biochemistry. 1998;255:148–154. doi: 10.1006/abio.1997.2439. [DOI] [PubMed] [Google Scholar]
- 15.Charette AB, Berthelette C, St-Martin D. An expedient approach to E,Z-dienes using the Julia olefination. Tetrahedron Letters. 2001;42:5149–5153. [Google Scholar]
- 16.Rochel N, Wurtz JM, Mitschler A, Klaholz B, Moras D. The crystal structure of the nuclear receptor for vitamin D bound to its natural ligand. Molecular Cell. 2000;5:173–179. doi: 10.1016/s1097-2765(00)80413-x. [DOI] [PubMed] [Google Scholar]