Abstract
Purpose.
To determine if there is a relationship between refractive error and ciliary muscle thickness in different muscle regions.
Methods.
An anterior segment optical coherence tomographer was used to measure cycloplegic ciliary muscle thicknesses at 1 mm (CMT1), 2 mm (CMT2), and 3 mm (CMT3) posterior to the scleral spur; maximum (CMTMAX) thickness was also assessed. An autorefractor was used to determine cycloplegic spherical equivalent refractive error (SPHEQ). Apical ciliary muscle fibers were obtained by subtracting corresponding CMT2 values from CMT1 and CMTMAX. Multilevel regression models were used to determine the relationship between ciliary muscle thickness in various regions of the muscle and refractive error.
Results.
Subjects included 269 children with a mean age of 8.71 ± 1.51 years and a mean refractive error of +0.41 ± 1.29 diopters. In linear models with ciliary muscle thicknesses and SPHEQ, SPHEQ was significantly associated only with CMT2 (β = −11.34, P = 0.0008) and CMT 3 (β = −6.97, P = 0.007). When corresponding values of CMT2 were subtracted from CMT1 and CMTMAX, apical fibers at CMT1 (β = 14.75, P < 0.0001) and CMTMAX (β = 18.16, P < 0.0001) had a significant relationship with SPHEQ.
Conclusions.
These data indicated that in children the posterior ciliary muscle fibers are thicker in myopia (CMT2 and CMT3), but paradoxically, the apical ciliary muscle fibers are thicker in hyperopia (CMTMAX and CMT1). This may be the first evidence that hyperopia is associated with a thicker apical ciliary muscle region.
Keywords: ciliary muscle, hyperopia, refractive error, children's vision, myopia
The relationship between the ciliary muscle and refractive error is complex; the posterior region of the ciliary muscle is enlarged in myopia, whereas the anterior, apical region appears to be enlarged in hyperopia.
Introduction
Researchers have investigated multiple avenues aimed at unraveling the mechanisms underlying juvenile myopia; nevertheless, a unifying myopia theory has yet to be described. One promising line of research is related to the ciliary muscle.1,2 Specifically, myopic children are known to have increased accommodative lags,3,4 higher accommodative convergence/stimulus to accommodation (AC/A) ratios,4,5 and thicker ciliary muscles compared with those of nonmyopic subjects.1,2 Currently, it is unclear why the ciliary muscle is thicker in myopes.1,2 Gaining a better understanding of this anatomic anomaly could not only provide valuable information for the treatment and prevention of myopia, but it could also provide insight into other ciliary muscle–related conditions such as accommodative dysfunction, presbyopia, and glaucoma.
When considering a muscle's normal response to stress, it would be logical for one to hypothesize, much like Oliveira and colleagues did,2 that the ciliary muscle should be thicker in hyperopic subjects because they have a greater accommodative workload than that of nonhyperopic subjects.6 An everyday, common example of a normal muscle–stress response is with resistance exercise; subjects who habitually stress their skeletal muscles eventually develop larger, stronger muscles.7 Another less common example of this phenomenon is bladder disease; subjects who have bladder obstruction experience smooth muscle hypertrophy (thickening) from organ stretch.8 The stress experienced by these two very different organs and stressors (workload and stretch) is mechanistically similar and is conserved across organs and organisms.9 However, when considering the ciliary muscle, two studies have not shown evidence for this reaction.1,2 In fact, the ciliary muscle is reportedly thinner in hyperopic subjects who presumably have the largest workload,1,2 a finding that is inconsistent with other muscle types. The thinner ciliary muscle found in hyperopia could be a violation of a basic stress–response relationship that exists in all known muscles, or perhaps there could be structural changes due to the increased workload, which may have been missed because they happened in isolated regions of the ciliary muscle (e.g., different fiber types).
The latter hypothesis may have merit because the ciliary muscle is composed of three different fiber orientations: circular, radial, and longitudinal fibers.10 The longitudinal fibers run anterior and parallel to the sclera.10,11 The circular fibers form an annulus that follows the iris,10 and they are connected to the longitudinal fibers via the radial fibers. This structural relationship allows the muscle to act as a single functional unit.11
In addition to having different orientations, the different regions also have histologic dissimilarities. For example, the longitudinal fibers are known to have fewer mitochondria and a greater number of myofilaments (subunits of a muscle's contractile unit) than those of the circular fibers.11 Also, molecules such as myosin-ATPase and lactate dehydrogenase have been shown to be more active in longitudinal fibers than those in circular fibers; the reverse is true with succinate dehydrogenase.11 These data would suggest that the longitudinal fibers behave like fast phasic fibers, whereas the circular fibers behave more like the slow tonic fibers.11 Overall, these structural differences provide evidence that longitudinal and circular muscle fibers have different functions. Alternatively, it may also be that these structural differences lead to a differential response to workload.
To the best of our knowledge, there has yet to be a study aimed at correlating the various regions of the ciliary muscle, which contain different fiber types, with refractive error or muscle function. Thus, the purpose of this study was to explore how a child's refractive error is related to general ciliary muscle thickness and thickness in the apical versus posterior muscle fiber regions.
Methods
Subjects
This was a prospective study. First- through fifth-grade children were recruited. This study was approved by The Ohio State University's Biomedical Sciences Institutional Review Board; it was conducted in accordance with the Declaration of Helsinki and was compliant with the Health Insurance Portability and Accountability Act. Each subject's legal guardian provided consent, and subjects also provided written assent prior to testing. Only subjects with a mental disability that would prevent them from completing the testing protocol were excluded.
Biometry
All measurements were completed on the right eye only. One drop of 0.5% proparacaine followed by two drops of 1% tropicamide were administered to the right eye to obtain cycloplegia. Tropicamide was chosen as the cycloplegic agent for this study over cyclopentolate because the effects of tropicamide are much shorter lived and because the two drops result in nonstatistically different refractive error measurements.12,13 Axial length measurements were then taken with an optical biometer (IOLMaster; Carl Zeiss Meditec, Inc., Dublin, CA). Five high-confidence measurements (signal-to-noise ratio > 2.0) were recorded. The mean was used in analysis.
Cycloplegic refractive error and ciliary muscle measurements were taken 25 minutes after the last drop of tropicamide was instilled. Ten refractive error measurements were obtained with a binocular autorefractor/keratometer (WR-5100K; Grand Seiko Co., Ltd., Hiroshima, Japan). The mean cycloplegic spherical equivalent refractive error was used in analysis.
Ciliary muscle images were obtained with an anterior segment optical coherence tomographer (Visante OCT system; Carl Zeiss Meditec, Inc.). This technique is capable of imaging only the muscle and structures such as the ciliary processes are not visible; therefore, only the muscle will be referred to herein.14 All images were made through the nasal sclera in enhanced high-resolution corneal mode, while the subjects fixated on a target that was outside of the machine and directly to the right of the normal, internal fixation target. The external fixation target was a large, bold, black “X” so that subjects could see it while under cycloplegia. Subjects were allowed to turn their heads slightly toward the target in addition to turning their eyes. Thus, the target was not in a position of extreme lateral gaze. Raw images were exported as binary files using commercial software (Visante OCT Image Exporter; Carl Zeiss Meditec).14
Images of the ciliary muscle were then imported into a computing software program (Matlab; The MathWorks, Natick, MA) for extraction of ciliary muscle thickness using a semiautomatic algorithm, which has been previously described by this laboratory.14 In brief, a trained examiner (MDB) marked the location of the scleral spur three separate times, and the mean location of the three selections was used in analysis. Neither the file names nor the identification numbers associated with the files were visible when the examiner was selecting the sclera spur. Images were then transferred to a trained, masked reader (CYK) who had no knowledge of the subject's age, sex, or refractive error for segmentation and measuring. The thickness of the muscle was then measured at 1 mm (CMT1), 2 mm (CMT2), and 3 mm (CMT3) posterior to the scleral spur and maximum (CMTMAX) thickness was also determined (Fig. 1). The algorithm determined these measurements by computing the distance in pixels (mm after image resolution conversion) between the point of maximum height and the base of the ciliary muscle.14 The use of this algorithm has been shown to provide repeatable, efficient, and masked measurements of the ciliary muscle.14
Figure 1.
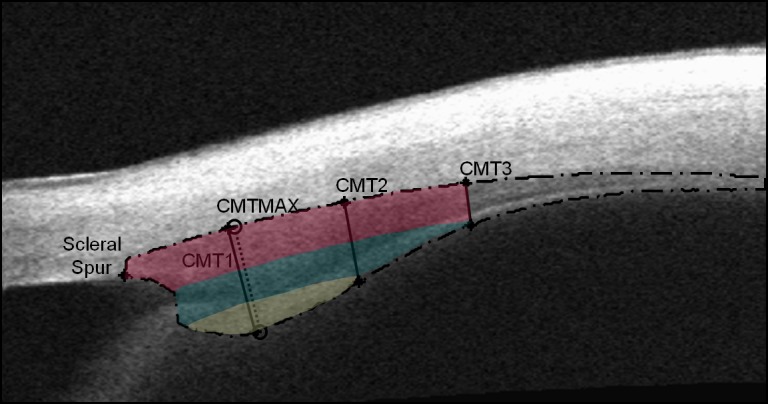
Anterior segment optical coherence tomography image of the ciliary muscle. The long-dashed line indicates the outline of the ciliary muscle. The measurement locations of CMT1, CMT2, and CMT3 are labeled with solid lines. The measurement location of CMTMAX is labeled with a short-dashed line. The image was color-enhanced to show how it would be divided if various CMT measurements were subtracted from other regions. The dark pink shading indicates the thickness of CMT3 throughout the muscle. The thickness of CMT2 is indicated by the addition of the dark pink region to the teal region. Finally, the remaining area shaded yellow would be specific to the region where the apical fibers at CMTMAX and CMT1 would be measured.
Statistical Analysis
Models of General Ciliary Muscle Thickness.
Multilevel regression models were fitted to describe the relationship between ciliary muscle thicknesses (CMTMAX, CMT1, CMT2, and CMT3) and spherical equivalent refractive error (SPHEQ). The following model was fitted for each muscle thickness outcome:
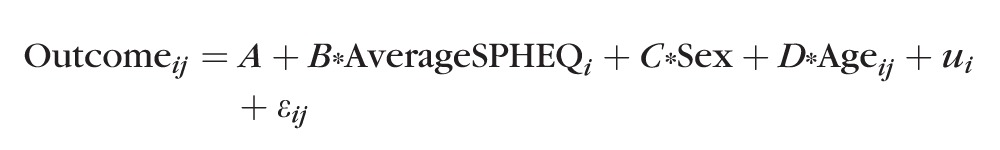 |
In the above model, i indexed the subject and j represented a subject's measure of CMT at age j. The Sex variable was coded “0” for males and “1” for females. As a result of this coding, the parameter estimate for sex is the estimated difference in outcome for a female relative to a male. The term u was a random effect that corrected the intercept (A) for between-subject variation; this term was needed to account for repeated within-subject measures of CMT. The term ε accounts for within-subject model error. Thirty-seven percent of the subjects had two or more observations in the data set, with on average approximately 1 year separating the repeated visits. For these subjects, the above model used their time-varying, actual age, and ciliary muscle thickness measures, rather than a mean age and ciliary muscle thickness measure. The primary focus of this analysis was in the relationship between ciliary muscle thickness and refractive error between subjects; thus, the analysis did not make use of the time-varying refractive error data. A multilevel model was chosen over a strictly cross-sectional analysis because its use provides a better estimate of the relationship between muscle thickness and aging.
All models were centered at age 9 years, so the intercept of the model would be meaningful (i.e., an outcome value for a 9-year-old subject). Centering the model on age affected only the model's intercept; it did not affect estimates of slopes. When subjects with repeated visits were included, the mean of their spherical equivalent refractive error across visits was used. When analyzing CMT values, we allowed for the possibility that the relationship could be fitted by a quadratic model, rather than a simple linear model, by including the square of the mean spherical equivalent refractive error because other studies in our laboratory have determined that some curvature exists in the relationship between CMT and SPHEQ (Bailey MD, et al. IOVS 2012:53;AAO E-Abstract 120615).
Models of Apical Ciliary Muscle Thickness.
The above multilevel regression model was also used to determine if spherical equivalent refractive error was significantly related to the thickness of the apical muscle fibers at CMTMAX or CMT1. Apical muscle fiber thickness indicators were created by subtracting CMT2 from CMTMAX and CMT1. The following is a summary of these equations:
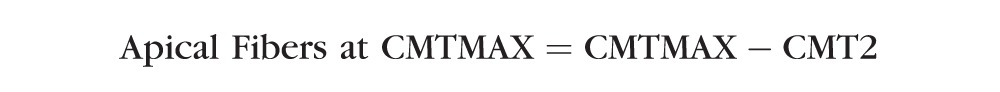 |
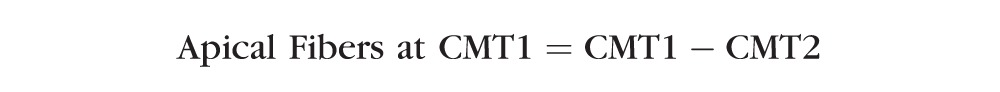 |
In Figure 1, the region of the muscle that appears in yellow is the apical region (i.e., the thickness that is unique to CMTMAX and CMT1). These models were chosen to isolate the apical muscle fibers (circular and some radial fibers) because no circular fibers should be found as far posterior as CMT2 and CMT3. CMT2 was chosen over CMT3 because subtracting CMT2 is more likely to remove more of both the radial and longitudinal fibers and isolate the circular fibers. The subtraction of CMT2 was also chosen over CMT3 because when we evaluated models where CMT3 was subtracted from CMTMAX and CMT1, the models behaved more like the models that included the entire thickness at CMTMAX and CMT1, indicating that the apical fibers may not have been isolated (data not shown). Conversely, when CMT2 was subtracted from CMTMAX and CMT1, the relationship between the thickness of the apical ciliary muscle region and refractive error was very different from the relationship with the entire thickness.
Statistical Tests and Significance Level.
Descriptive statistics such as means and SD values were used to describe general trends in the data. The statistical significance of regression parameters was assessed using F tests. To address the use of multiple comparisons, the significance level for statistical tests was set at P = 0.01. The 0.01 level of significance was chosen over a Bonferroni correction of the standard 0.05 level of significance because a 0.01 level of significance will still provide reasonable power to detect statistical effects.
Results
A total of 270 subjects with a mean age of 8.70 ± 1.51 years (range: 6.15–14.05 years) had complete data for all measurements and were included in analysis. The subjects were 43.0% female. Boys and girls had a mean axial length of 23.46 ± 0.82 and 22.83 ± 0.80 mm, respectively. Additionally, boys and girls had a mean refractive error of 0.44 ± 1.28 diopters (D) and 0.40 ± 1.32 D, respectively. A total of 21 subjects had astigmatic refractive error greater than 1.00 D. Spectacle correction was worn by 51 subjects. No subjects wore contact lenses. The subjects were 78.0% non-Hispanic white, 12.9% African American, 2.7% Asian, 2.7% Hispanic, and 3.7% other. The vast majority of the subjects were healthy, with the following exceptions reported by parents: 3 cases of strabismus, 4 cases of amblyopia, 2 cases of diabetes, 58 cases of seasonal or food-related allergies, 18 cases of asthma, and 7 cases of attention-deficit hyperactivity disorder (ADHD). In one subject, the amblyopic/strabismic eye was the right eye, or the eye that was measured for this study. Because it is unknown how this factor could affect the relationship between refractive error and the ciliary muscle, this subject's data were excluded from all analyses.
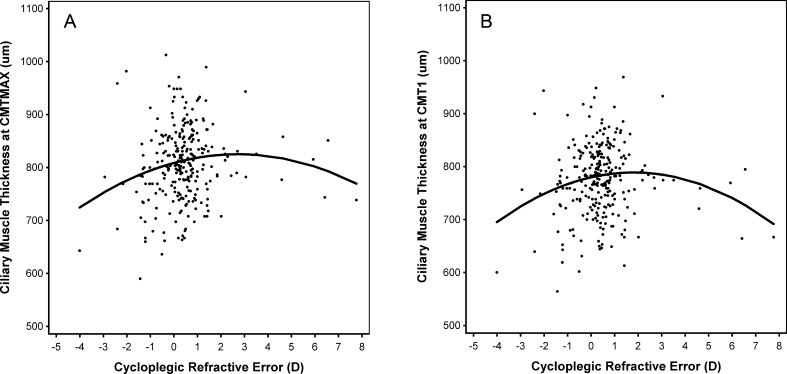
The data for 269 of the 270 subjects were included in the following analyses, tables, and figures, thus providing useful models. Table 1 summarizes ciliary muscle thickness, age, refractive error, and axial length distributions. Tables 2 and 3 provide parameter estimates for the models of the relationship between each CMT measurement and spherical equivalent refractive error. Across all models, sex was not statistically significant; this indicated that a correction of the intercept term for sex was not necessary. Values of CMT did, however, increase with age at all locations (all P ≤ 0.01). In the anterior region of the ciliary muscle (CMTMAX and CMT1) the relationship between CMT and spherical equivalent refractive error was quadratic (Table 2: CMTMAX: P = 0.005 and CMT1: P = 0.0003). Thus, the ciliary muscle is thinner at CMTMAX and CMT1 with larger values of both hyperopia and myopia, which is depicted in Figure 2, where the curve of the best-fit model shows the maximum ciliary muscle thickness occurring at low-to-moderate amounts of myopia. Figure 2 was unadjusted for sex and age.
Table 1. .
Mean Age, Refractive Error, and Ciliary Muscle Thickness of the Study Sample
|
Measurement |
Mean |
SD |
Minimum |
Maximum |
| Age, y | 8.71 | 1.51 | 6.15 | 14.05 |
| CMTMAX, μm | 809.01 | 67.63 | 595.00 | 1007.50 |
| CMT1, μm | 778.46 | 65.15 | 570.00 | 965.00 |
| CMT2, μm | 527.87 | 73.13 | 345.00 | 725.00 |
| CMT3, μm | 280.92 | 55.29 | 125.00 | 447.50 |
| Apical fibers: CMTMAX, μm | 280.88 | 70.60 | 83.33 | 440.00 |
| Apical fibers: CMT1, μm | 250.59 | 60.34 | 45.00 | 390.00 |
| Refractive error, D | +0.41 | +1.29 | −4.01 | +7.76 |
| Axial length, mm | 23.19 | 0.86 | 19.96 | 25.97 |
Table 2. .
Relationship Between Anterior Ciliary Muscle Thickness and Spherical Equivalent Refractive Error, Where the Best-Fit Model Included Both First-Degree (Linear) and Second-Degree (Quadratic) Refractive Error Terms
|
Predictor |
CMTMAX |
CMT1 |
| Intercept | 805.14 | 780.42 |
| Refractive error, D, linear | 14.18 (P = 0.0009) | 12.52 (P = 0.002) |
| Refractive error, D2, quadratic* | −2.54 (P = 0.005) | −3.15 (P = 0.0003) |
| Age, y | 5.87 (P = 0.01) | 7.32 (P = 0.0007) |
| Sex | 11.10 (P = 0.2) | 2.69 (P = 0.7) |
For CMTMAX and CMT1, both first- and second-degree terms in refractive error were statistically significant. In models with just first-degree terms, refractive error was not statistically significant.
Table 3. .
Relationship Between Posterior Ciliary Muscle Thickness and Spherical Equivalent Refractive Error, Where the Best-Fit Model Included Only a First-Degree Polynomial (Linear) for Refractive Error
|
Predictor |
CMT2 |
CMT3 |
| Intercept | 542.77 | 291.57 |
| Refractive error, D | −11.34 (P = 0.0008) | −6.97 (P = 0.007) |
| Age, y | 16.63 (P < 0.0001) | 12.67 (P < 0.0001) |
| Sex | −12.24 (P = 0.2) | −9.37 (P = 0.2) |
Figure 2.
Model projections of the relationship between anterior ciliary muscle thickness ([A] CMTMAX and [B] CMT1) and spherical equivalent refractive error. Data points represent individual subjects. The solid line represents the model that included both first- and second-degree (quadratic) polynomials. The model was unadjusted for sex and age.
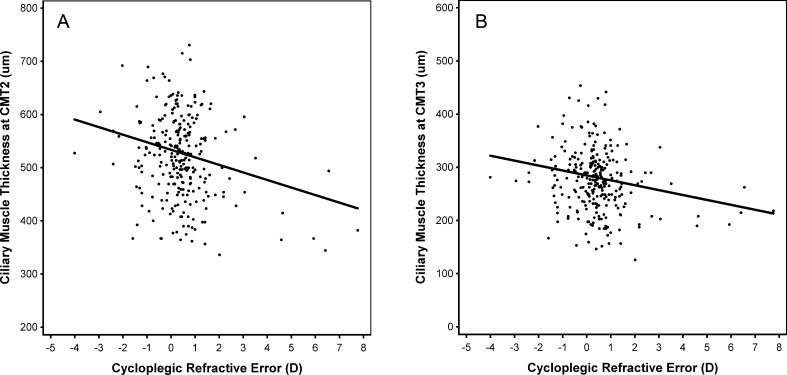
The relationship between spherical equivalent refractive error and ciliary muscle thickness in the posterior region of the muscle, however, was linear (Table 3: CMT2: P = 0.0008; CMT 3: P = 0.007). For both CMT2 and CMT3, the slope was negative, which indicated that a thicker muscle was associated with more negative spherical equivalent refractive error. This relationship is depicted in Figure 3, where the best-fit model shows the maximum ciliary muscle thickness occurring at the highest levels of myopia. Figure 3 was also unadjusted for sex and age.
Figure 3.
Model projections of the relationship between posterior ciliary muscle thickness ([A] CMT2 and [B] CMT3) and spherical equivalent refractive error. Data points represent individual subjects. The solid line represents the model that included only first-degree polynomials. The model was unadjusted for sex and age.
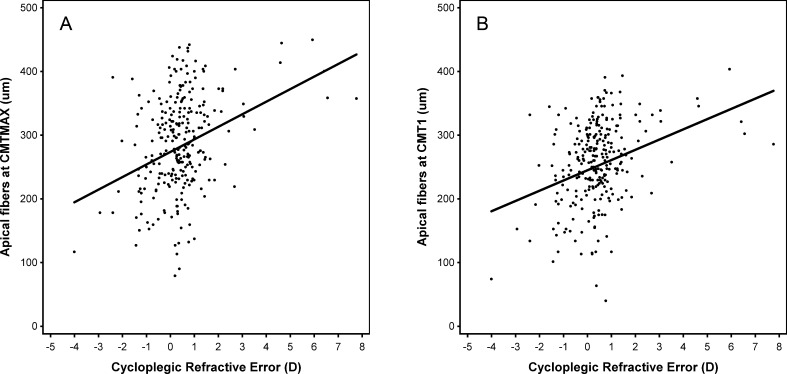
Table 4 provides parameter estimates for the fitted models of the thickness of apical fibers at CMT 1 (CMT1 − CMT2) and the apical fibers at CMTMAX (CMTMAX − CMT2), as a function of spherical equivalent refractive error. Age was statistically significant, with apical fiber thickness decreasing with age. For the apical fibers at CMTMAX, sex was statistically significant (P = 0.003), with thickness greater in females. Apical fibers had a statistically significant linear relationship with spherical equivalent refractive error both at CMT1 (P < 0.0001) and at CMTMAX (P < 0.0001). Overall, these results indicated that thicker apical ciliary muscle fibers were associated with higher amounts of hyperopia. Figure 4 provides the modeled projections of the apical fibers at CMTMAX and CMT1 as a function of spherical equivalent refractive error. Figure 4 was also unadjusted for sex and age.
Table 4. .
Models of the Relationship Between Spherical Equivalent Refractive Error and the Apical Fibers at CMT1 or at CMTMAX
|
Predictor |
Apical CMTMAX |
Apical CMT1 |
| Intercept | 261.03 | 236.25 |
| Refractive error, D | 18.16 (P < 0.0001) | 14.75 (P < 0.0001) |
| Age, y | −9.99 (P < 0.0001) | −8.40 (P < 0.0001) |
| Sex | 23.34 (P = 0.003) | 15.01 (P = 0.02) |
Figure 4.
Model projections of the relationship between apical ciliary muscle thickness ([A] apical fibers at CMTMAX and [B] apical fibers at CMT1) and spherical equivalent refractive error. Data points represent individual subjects. The solid line represents the model that included only first-degree polynomials. The model was unadjusted for sex and age.
Discussion
Currently, the refractive error research community is unable to explain why the posterior ciliary muscle is thicker in myopic subjects. The underlying mechanism, which produces this anatomic change, might have clinical implications for myopia treatment and prevention and for other conditions such as presbyopia, glaucoma, and accommodative dysfunction. The current study reports additional information about differences in the ciliary muscle in different types of refractive error that will further fuel investigations. Specifically, the apical ciliary muscle fibers were thicker in hyperopic subjects (Fig. 4), whereas the posterior muscle fibers were thicker in myopic subjects (Fig. 3).
Our primary finding suggests that the apical ciliary muscle fibers are responsive to increased accommodative workload in a manner similar to that of subjects who build skeletal muscle during resistance training. The possibility that the result is related to increased workload is supported by the fact that only 23% of the hyperopic children (subjects with +1.00 spherical equivalent refractive error or higher) in this study had optical correction. This means that the majority of hyperopic subjects used their accommodative muscle if they sought to see clearly at any distance.
Even though the above explanation seems reasonable, validation of this theory would require a study that evaluates how the ciliary muscle responds before and after experimentally induced increases in amounts of workload. For example, one could evaluate the ciliary muscle dimensions before and after assigning patients to a daily regimen of accommodative vision therapy for a period of weeks or months. The effects of workload could also be evaluated in an animal model where the different regions of the ciliary muscle are observed before and after an extended period of experimental stimulation or inhibition of accommodation.
The present study also provides additional evidence for the negative relationship between refractive error and posterior ciliary muscle thickness. Specifically, measurements at the posterior region of the ciliary muscle, CMT2 and CMT3 (Fig. 3), were thicker with increasing amounts of myopia. Oliveira et al.2 were the first to document that the posterior ciliary muscle is thicker in myopia in adults with an ultrasound biomicroscope (Table 5). It was later confirmed by Bailey et al.1 in children with OCT (Table 5). Muftuoglu et al.15 further corroborated this finding by studying subjects with unilateral high axial myopia; they found that the more myopic eye had a thicker ciliary muscle than the less myopic eye (Table 5). They also reported, however, that some subjects appeared to have no difference in ciliary muscle thickness between eyes.15 Together, all of these studies and the present data indicate that more myopic eyes generally have a thicker posterior ciliary muscle. A summary of the above studies can be found in Table 5.
Table 5. .
A Summary of Ciliary Muscle Thickness Studies
|
Study Authors/Number |
Refractive Error Type With Thickest Ciliary Muscle Region |
Age of Subjects |
Refractive Error Range |
Study Design |
|||||
|
CMTMAX |
CMT1 |
CMT2 |
CMT3 |
Apical CMTMAX |
Apical CMT1 |
||||
| Oliveira et al.2/ n = 75 | N/A | N/A | Myopia | Myopia | N/A | N/A | Older adults | −14.75 to +15.50 | Cross-sectional |
| Bailey et al.1/ n = 53 | No relationship | No relationship | Myopia | Myopia | N/A | N/A | Children | −6.00 to +3.00 | Cross-sectional |
| Muftuoglu et al.15/n = 19 | More myopic eye | N/A | N/A | N/A | N/A | N/A | Young adults | −17.38 to −6.25 | Anisometropia |
| Anisomeropic adults*/n = 30 | Myopic subjects, no difference between eyes | Myopic subjects, no difference between eyes | Myopic subjects, no difference between eyes | Myopic subjects, no difference between eyes | Hyperopes | Hyperopes | Young adults | −8.40 to +5.84 | Anisometropia |
| Young adults*/ n = 92 | Moderate myopia (quadratic) | Moderate myopia (quadratic) | Moderate myopia (quadratic) | Moderate myopia (quadratic) | Hyperopes | Hyperopes | Young adults | −10.93 to +6.08 | Cross-sectional |
| Present study | Low to moderate myopia (quadratic) | Low to moderate myopia (quadratic) | Moderate myopia (linear) | Moderate myopia (linear) | Hyperopes | Hyperopes | Children | −4.01 to +7.76 | Mixed |
N/A, not available, measurements were not made/reported. Anisometropia: study included subjects with anisometropia and reported the within-subject relationship between the more and less myopic eyes.
Bailey MD, et al. IOVS 2012;53:AAO E-Abstract 120615.
The current study found that CMTMAX and CMT1 were thinner with larger values of hyperopia and myopia (Fig. 2), and thickest in low-to-moderate levels of myopia. In our previously published study in children, this relationship between CMT1 and refractive error was not significant.1 It is possible that we were not able to detect the quadratic relationship in the original study in children because it included a smaller sample of only 53 subjects, and it had a smaller range of refractive errors.1 The current study has a substantially larger sample size (270 subjects) and this may have made it easier to detect the quadratic relationship. Overall, our data suggest that the different regions of the ciliary muscle have their own intrinsic properties, thus providing evidence for the muscle regions having specialized tasks.
The current study analyzed children who have low amounts of refractive error, and the lack of more subjects with higher levels of refractive error should be considered a limitation of the current study. It is possible that the relationships noted in Figures 2 through 4 are primarily driven by the subjects with more extreme values of refractive error. Although we are unaware of any studies from other laboratories that have evaluated the relationship between refractive error and apical ciliary muscle thickness, we have confirmed this result in two of our other data sets. Both data sets consisted of young adults with a wide range of refractive errors (Bailey MD, et al. IOVS 2012:53:AAO E-Abstract 120615; Table 5).
Also, this work has other potential limitations. Although this study found that, on average, ciliary muscle thickness is related to age, the observed range of thickness for a given age is large. For example, an 11-year-old subject with +4.00 D of hyperopia was observed to have a thicker posterior ciliary muscle (CMT2 = 518 μm; CMT3 = 270 μm) than that of an 11-year-old subject with +1.00 D of hyperopia (CMT2 = 440 μm; CMT3 = 185 μm). Figures 2 through 4 also show a considerable amount of variability when comparing refractive error and ciliary muscle thickness. Clearly, ciliary muscle thickness is related to refractive error, but there may be other factors that influence its dimensions as well.
In addition, it is important to remember that many of the children who appear on the graphs included in this study appear to be emmetropes at the time these data were collected, but will become myopes at some point in the future. We do not currently know when refractive-error–related differences in ciliary muscle thickness occur. It is possible that myopes have a thicker ciliary muscle at birth, but it is also possible that it develops as myopia develops. Also, some of the children who are emmetropic in this sample may have been higher hyperopes at some point in infancy or early childhood but have since undergone emmetropization. If this is the case, the apical region of their muscle may be thicker than that of a child who has always been an emmetrope due to the higher ciliary muscle workload due to hyperopia earlier in life. These are things that need to be verified with a greater amount of longitudinal data.
There could be lots of reasons for the variability in our data, but if workload does influence the dimensions of the apical fibers, then some of the variability could be explained by the amount of near work performed by each subject. In a future investigation, we plan to explore the relationship between how much time a child spends performing near work and how this is related to ciliary muscle thickness, function, and refractive error. Specifically, our laboratory is following the subjects measured in this study longitudinally; once the longitudinal data are available, we will be better able to account for variability, address the above interests, and draw stronger conclusions.
Overall, these data indicated that the posterior region of the ciliary muscle is thicker in myopic subjects, and the apical region of the ciliary muscle is thicker in hyperopic subjects. This is likely the first evidence suggesting that accommodative workload is associated with a specific region of the muscle, which is largely comprised of circular and some radial fibers. In future investigations of this relationship, we will evaluate how the general accommodative workload (i.e., factors such as time spent on near work) influences the structure of the ciliary muscle within refractive error groups. These data may also lead to a more complete view of myopia development and the role of accommodative lag, increased AC/A ratios, and altered crystalline lens growth in myopia development.3–5,16
Acknowledgments
Presented at the annual meeting of the Association for Research in Vision and Ophthalmology, Fort Lauderdale, Florida, May 2012, and in part at the annual meeting of the American Academy of Optometry, Phoenix, Arizona, October 2012.
Supported by the American Optometric Foundation William C. Ezell Fellowship and Arene T. Wray Fellowship (ADP); National Science Foundation Grant DMS 1216742 (C-YK); National Eye Institute Grant R24-EY014792 (LTS); Ohio Lions Eye Research Foundation (MDB); and National Center for Research Resources Award Number KL2 RR025754, funded by the Office of the Director, National Institutes of Health (OD) (MDB). The authors alone are responsible for the writing of the paper and the content, which does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. The Ohio State University has filed a nonprovisional patent application on the behalf of the authors (MDB, C-YK): U.S. Utility Patent Application No. 13/757243, filed February 1, 2013, entitled “Detection and measurement of tissue images.”
Disclosure: A.D. Pucker, None; L.T. Sinnott, None; C.-Y. Kao, P; M.D. Bailey, P
References
- 1. Bailey MD, Sinnott LT, Mutti DO. Ciliary body thickness and refractive error in children. Invest Ophthalmol Vis Sci. 2008; 49: 4353–4360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Oliveira C, Tello C, Liebmann JM, Ritch R. Ciliary body thickness increases with increasing axial myopia. Am J Ophthalmol. 2005; 140: 324–325 [DOI] [PubMed] [Google Scholar]
- 3. Mutti DO, Mitchell GL, Hayes JR, et al. Accommodative lag before and after the onset of myopia. Invest Ophthalmol Vis Sci. 2006; 47: 837–846 [DOI] [PubMed] [Google Scholar]
- 4. Gwiazda J, Thorn F, Held R. Accommodation, accommodative convergence, and response AC/A ratios before and at the onset of myopia in children. Optom Vis Sci. 2005; 82: 273–278 [DOI] [PubMed] [Google Scholar]
- 5. Mutti DO, Jones LA, Moeschberger ML, Zadnik K. AC/A ratio, age, and refractive error in children. Invest Ophthalmol Vis Sci. 2000; 41: 2469–2478 [PubMed] [Google Scholar]
- 6. McBrien NA, Millodot M. The effect of refractive error on the accommodative response gradient. Ophthalmic Physiol Opt. 1986; 6: 145–149 [PubMed] [Google Scholar]
- 7. Bird SP, Tarpenning KM, Marino FE. Designing resistance training programmes to enhance muscular fitness: a review of the acute programme variables. Sports Med. 2005; 35: 841–851 [DOI] [PubMed] [Google Scholar]
- 8. Boberg L, Poljakovic M, Rahman A, Eccles R, Arner A. Role of Rho-kinase and protein kinase C during contraction of hypertrophic detrusor in mice with partial urinary bladder outlet obstruction. BJU Int. 2012; 109: 132–140 [DOI] [PubMed] [Google Scholar]
- 9. Kim S, Iwao H. Molecular and cellular mechanisms of angiotensin II-mediated cardiovascular and renal diseases. Pharmacol Rev. 2000; 52: 11–34 [PubMed] [Google Scholar]
- 10. Remington LA, McGill EC. Clinical Anatomy of the Visual System. Boston: Butterworth-Heinemann; 1998: ix [Google Scholar]
- 11. Ebersberger A, Flugel C, Lutjen-Drecoll E. Ultrastructural and enzyme histochemical studies of regional structural differences within the ciliary muscle in various species [in German]. Klin Monatsbl Augenheilkd. 1993; 203: 53–58 [DOI] [PubMed] [Google Scholar]
- 12. Mutti DO, Zadnik K, Egashira S, Kish L, Twelker JD, Adams AJ. The effect of cycloplegia on measurement of the ocular components. Invest Ophthalmol Vis Sci. 1994; 35: 515–527 [PubMed] [Google Scholar]
- 13. Egashira SM, Kish LL, Twelker JD, Mutti DO, Zadnik K, Adams AJ. Comparison of cyclopentolate versus tropicamide cycloplegia in children. Optom Vis Sci. 1993; 70: 1019–1026 [DOI] [PubMed] [Google Scholar]
- 14. Kao CY, Richdale K, Sinnott LT, Grillott LE, Bailey MD. Semiautomatic extraction algorithm for images of the ciliary muscle. Optom Vis Sci. 2011; 88: 275–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Muftuoglu O, Hosal BM, Zilelioglu G. Ciliary body thickness in unilateral high axial myopia. Eye. 2009; 23: 1176–1181 [DOI] [PubMed] [Google Scholar]
- 16. Mutti DO, Mitchell GL, Sinnott LT, et al. Corneal and crystalline lens dimensions before and after myopia onset. Optom Vis Sci. 2012; 89: 251–262 [DOI] [PMC free article] [PubMed] [Google Scholar]