Abstract
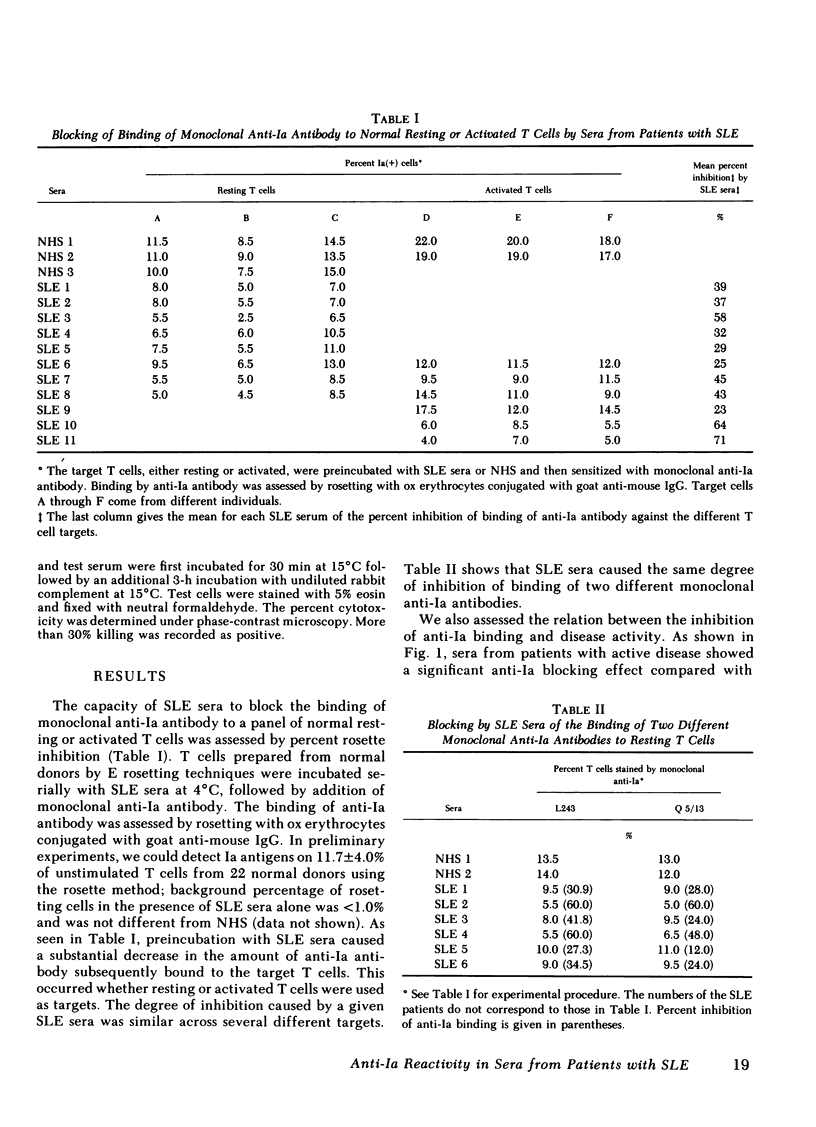
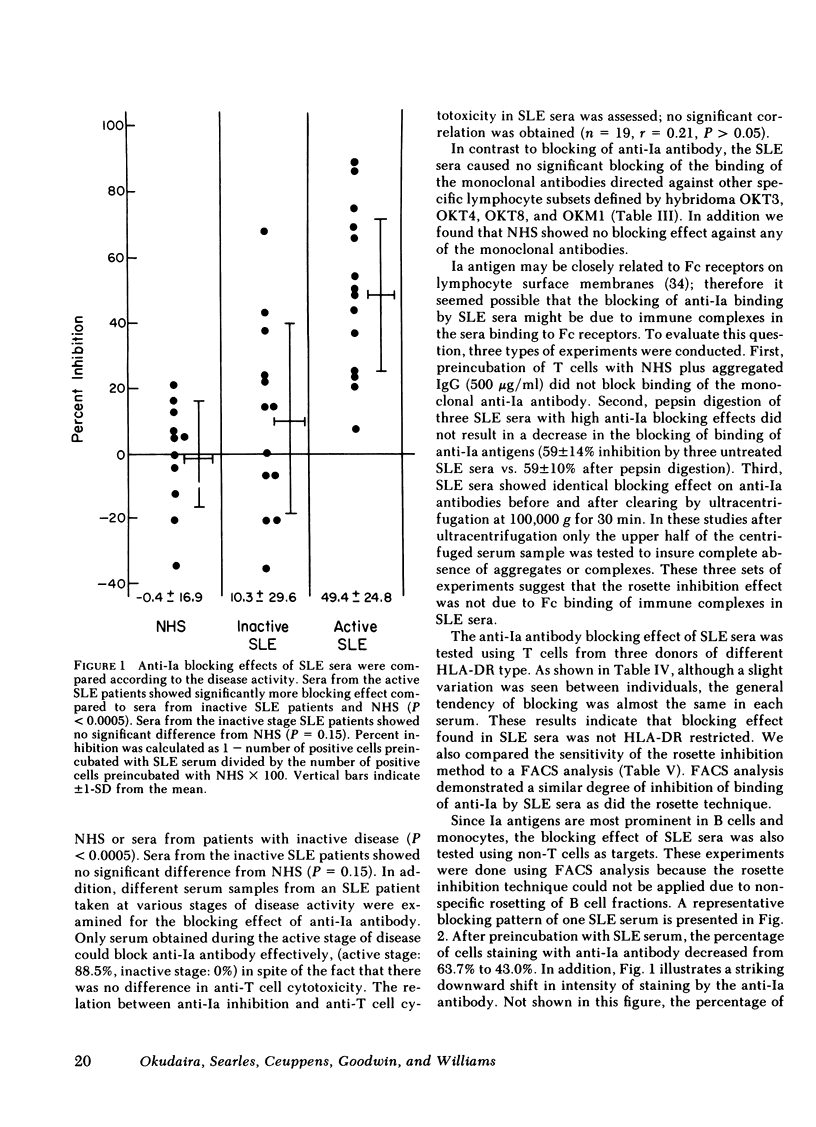
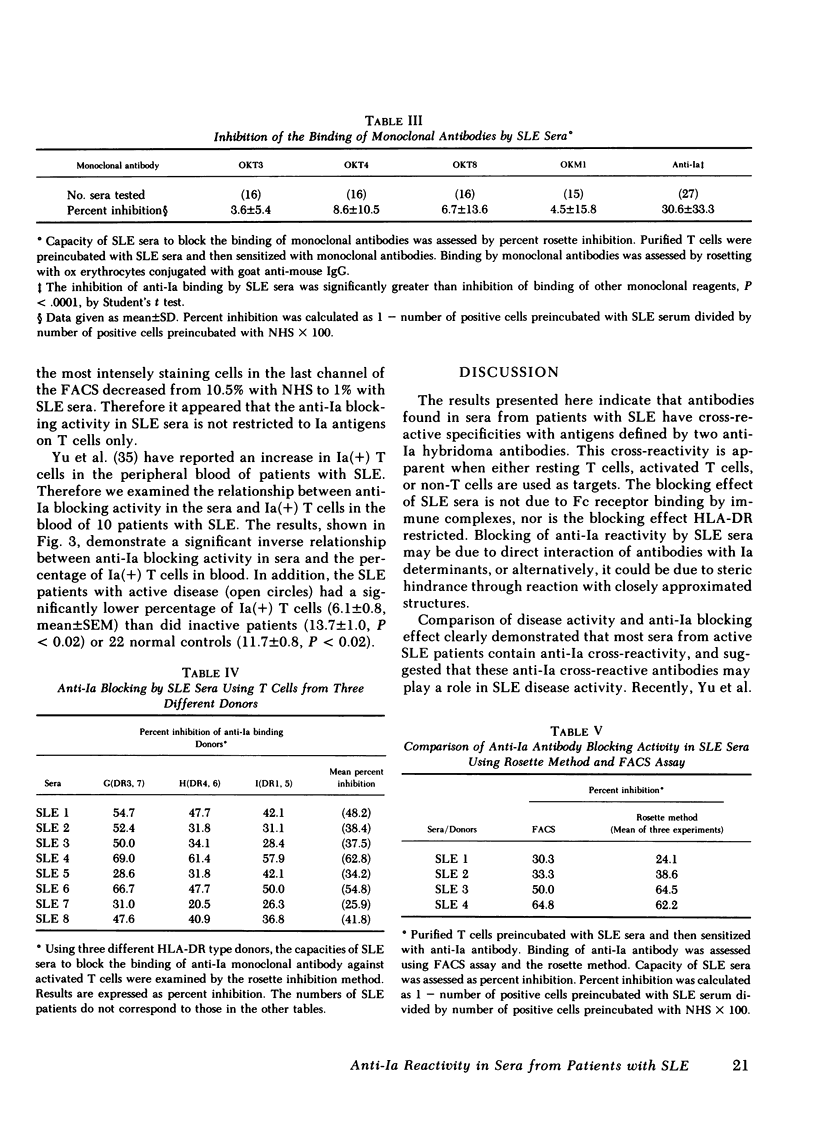
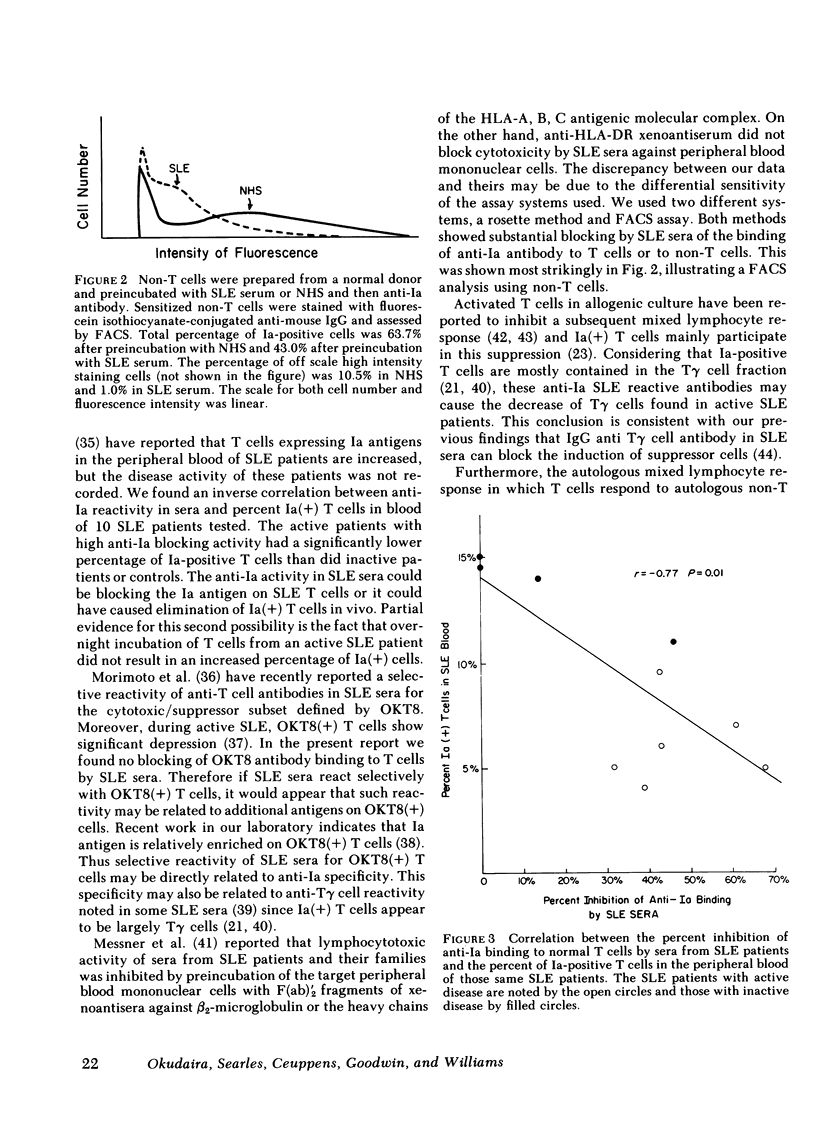
Antileukocyte antibodies in sera from patients with systemic lupus erythematosus (SLE) were characterized by determining cross-reacting specificies with the antigens defined by OKT3, OKT4, OKT8, OKM1 and anti-Ia hybridoma antibodies (Abs). T cells were prepared by sheep erythrocyte (E) rosetting after removal of adherent cells. T cells, or non-T cells, were preincubated with SLE sera at 4°C and then with monoclonal Abs. Binding by specific monoclonal Abs was assessed by two methods: rosetting with ox erythrocytes conjugated with goat anti-mouse IgG and also in the fluorescence-activated cell sorter using fluorescein isothiocyanate-conjugated goat anti-mouse IgG. Using the rosetting method, we found that sera from SLE can block the binding of monoclonal mouse hybridoma anti-Ia Abs to T cells; the blocking of other monoclonal Abs was not consistent. Using fluorescence-activated cell sorter analysis, preincubation with SLE sera lowered the intensity of staining and total percentage of either T or non-T cells stained by monoclonal anti-Ia Abs. Blocking of anti-Ia Abs binding by SLE sera was not histocompatibility leukocyte antigen (HLA)-DR restricted and was not due to Fc receptor binding. These results indicated that antibodies in SLE sera react with structures contiguous to or identical with Ia determinants. Anti-Ia activities in SLE sera correlate with SLE disease activity. In addition, there was a significant negative correlation between anti-Ia blocking activity in the sera and the percentage of Ia-positive T cells in the blood of SLE patients. Antibodies in SLE sera with anti-Ia blocking activity may play an important role in immune dysregulation in SLE patients.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdou N. I., Sagawa A., Pascual E., Hebert J., Sadeghee S. Suppressor T-cell abnormality in idiopathic systemic lupus erythematosus. Clin Immunol Immunopathol. 1976 Sep;6(2):192–199. doi: 10.1016/0090-1229(76)90110-0. [DOI] [PubMed] [Google Scholar]
- Arend W. P., Emmerich T. E., Sturge J. C., Starkebaum G. A. Monocyte-reactive antibodies in patients with systemic lupus erythematosus. Arthritis Rheum. 1977 Jun;20(5):1049–1057. doi: 10.1002/art.1780200503. [DOI] [PubMed] [Google Scholar]
- Breard J., Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody reactive with human peripheral blood monocytes. J Immunol. 1980 Apr;124(4):1943–1948. [PubMed] [Google Scholar]
- Bresnihan B., Jasin H. E. Suppressor function of peripheral blood mononuclear cells in normal individuals and in patients with systemic lupus erythematosus. J Clin Invest. 1977 Jan;59(1):106–116. doi: 10.1172/JCI108607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budman D. R., Merchant E. B., Steinberg A. D., Doft B., Gershwin M. E., Lizzio E., Reeves J. P. Increased spontaneous activity of antibody-forming cells in the peripheral blood of patients with active SLE. Arthritis Rheum. 1977 Apr;20(3):829–833. doi: 10.1002/art.1780200312. [DOI] [PubMed] [Google Scholar]
- Cohen A. S., Canoso J. J. Criteria for the classification of systemic lupus erythematosus--status 1972. Arthritis Rheum. 1972 Sep-Oct;15(5):540–543. doi: 10.1002/art.1780150512. [DOI] [PubMed] [Google Scholar]
- Del Giacco G. S., Leone A. L., Locci F., Manconi P. E., Tognella S., Mantovani G., Grifoni V. Anti-lymphocyte antibodies in systemic lupus erythematosus and in Hodgkin's disease: a comparison by immunofluorescence. Biomedicine. 1976 May;25(3):79–80. [PubMed] [Google Scholar]
- Fu S. M., Chiorazzi N., Wang C. Y., Montrazeri G., Kunkel H. G., Ko H. S., Gottlieb A. B. Ia-bearing T lymphocytes in man. Their identification and role in the generation of allogeneic helper activity. J Exp Med. 1978 Nov 1;148(5):1423–1428. doi: 10.1084/jem.148.5.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves M. F., Verbi W., Festenstein H., Papasteriadis C., Jaraquemada D., Hayward A. "Ia-like" antigens on human T cells. Eur J Immunol. 1979 May;9(5):356–362. doi: 10.1002/eji.1830090504. [DOI] [PubMed] [Google Scholar]
- Hahn B. H., Bagby K. K., Osterland C. K. Abnormalities of delayed hypersensitivity in systemic lupus erythematosus. Am J Med. 1973 Jul;55(1):25–31. doi: 10.1016/0002-9343(73)90146-0. [DOI] [PubMed] [Google Scholar]
- Hirschberg H., Thorsby E. Activation of human suppressor cells in mixed lymphocyte cultures. Scand J Immunol. 1977;6(8):809–815. doi: 10.1111/j.1365-3083.1977.tb02154.x. [DOI] [PubMed] [Google Scholar]
- Horwitz D. A. Impaired delayed hypersensitivity in systemic lupus erythematosus. Arthritis Rheum. 1972 Jul-Aug;15(4):353–359. doi: 10.1002/art.1780150406. [DOI] [PubMed] [Google Scholar]
- Innes J. B., Kuntz M. M., Kim Y. T., Weksler M. E. Induction of suppressor activity in the autologous mixed lymphocyte reaction and in cultures with concanavalin A. J Clin Invest. 1979 Dec;64(6):1608–1613. doi: 10.1172/JCI109622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamoun M., Martin P. J., Hansen J. A., Brown M. A., Siadak A. W., Nowinski R. C. Identification of a human T lymphocyte surface protein associated with the E-rosette receptor. J Exp Med. 1981 Jan 1;153(1):207–212. doi: 10.1084/jem.153.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaszubowski P. A., Goodwin J. S., Williams R. C., Jr Ia antigen on the surface of a subfraction of T cells that bear Fc receptors for IgG. J Immunol. 1980 Mar;124(3):1075–1078. [PubMed] [Google Scholar]
- Ko H. S., Fu S. M., Winchester R. J., Yu D. T., Kunkel H. G. Ia determinants on stimulated human T lymphocytes. Occurrence on mitogen- and antigen-activated T cells. J Exp Med. 1979 Aug 1;150(2):246–255. doi: 10.1084/jem.150.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike T., Kobayashi S., Yoshiki T., Itoh T., Shirai T. Differential sensitivity of functional subsets of T cells to the cytotoxicity of natural T-lymphocytotoxic autoantibody of systemic lupus erythematosus. Arthritis Rheum. 1979 Feb;22(2):123–129. doi: 10.1002/art.1780220204. [DOI] [PubMed] [Google Scholar]
- Kuntz M. M., Innes J. B., Weksler M. E. The cellular basis of the impaired autologous mixed lymphocyte reaction in patients with systemic lupus erythematosus. J Clin Invest. 1979 Jan;63(1):151–153. doi: 10.1172/JCI109270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampson L. A., Levy R. Two populations of Ia-like molecules on a human B cell line. J Immunol. 1980 Jul;125(1):293–299. [PubMed] [Google Scholar]
- Messner R. P., De Horatius R., Ferrone S. Lymphocytotoxic antibodies in systemic lupus erythematosus patients and their relatives: reactivity with the HLA antigenic molecular complex. Arthritis Rheum. 1980 Mar;23(3):265–272. doi: 10.1002/art.1780230301. [DOI] [PubMed] [Google Scholar]
- Morimoto C., Reinherz E. L., Abe T., Homma M., Schlossman S. F. Characteristics of anti-T-cell antibodies in systemic lupus erythematosus: evidence for selective reactivity with normal suppressor cells defined by monoclonal antibodies. Clin Immunol Immunopathol. 1980 Aug;16(4):474–484. doi: 10.1016/0090-1229(80)90189-0. [DOI] [PubMed] [Google Scholar]
- Morimoto C., Reinherz E. L., Schlossman S. F., Schur P. H., Mills J. A., Steinberg A. D. Alterations in immunoregulatory T cell subsets in active systemic lupus erythematosus. J Clin Invest. 1980 Nov;66(5):1171–1174. doi: 10.1172/JCI109948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okudaira K., Nakai H., Hayakawa T., Kashiwado T., Tanimoto K., Horiuchi Y., Juji T. Detection of antilymphocyte antibody with two-color method in systemic lupus erythematosus and its heterogeneous specificities against human T-cell subsets. J Clin Invest. 1979 Nov;64(5):1213–1220. doi: 10.1172/JCI109575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okudaira K., Tanimoto K., Nakamura T., Horiuchi Y. Spontaneously enhanced in vitro immunoglobulin synthesis by B cells in systemic lupus erythematosus. Clin Immunol Immunopathol. 1980 Jul;16(3):267–278. doi: 10.1016/0090-1229(80)90132-4. [DOI] [PubMed] [Google Scholar]
- Quaranta V., Walker L. E., Pellegrino M. A., Ferrone S. Purification of immunologically functional subsets of human Ia-like antigens on a monoclonal antibody (Q5/13) immunoadsorbent. J Immunol. 1980 Oct;125(4):1421–1425. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Levey R. H., Schlossman S. F. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1588–1592. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody reactive with the human cytotoxic/suppressor T cell subset previously defined by a heteroantiserum termed TH2. J Immunol. 1980 Mar;124(3):1301–1307. [PubMed] [Google Scholar]
- Rode H. N., Uotila M., Gordon J. Regulation of the mixed leukocyte culture reaction by suppressor cells. Eur J Immunol. 1978 Mar;8(3):213–216. doi: 10.1002/eji.1830080314. [DOI] [PubMed] [Google Scholar]
- Sagawa A., Abdou N. I. Suppressor-cell dysfunction in systemic lupus erythematosus. Cells involved and in vitro correction. J Clin Invest. 1978 Oct;62(4):789–796. doi: 10.1172/JCI109190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakane T., Steinberg A. D., Green I. Failure of autologous mixed lymphocyte reactions between T and non-T cells in patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3464–3468. doi: 10.1073/pnas.75.7.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakane T., Steinberg A. D., Reeves J. P., Green I. Studies of immune functions of patients with systemic lupus erythematosus. Complement-dependent immunoglobulin M anti-thymus-derived cell antibodies preferentially inactivate suppressor cells. J Clin Invest. 1979 May;63(5):954–965. doi: 10.1172/JCI109396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlossman S. F., Chess L., Humphreys R. E., Strominger J. L. Distribution of Ia-like molecules on the surface of normal and leukemic human cells. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1288–1292. doi: 10.1073/pnas.73.4.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker J. W., Garotta G., Hausmann B., Trucco M., Ceppellini R. Separation of human cells bearing HLA-DR antigens using a monoclonal antibody rosetting method. Tissue Antigens. 1979 Mar;13(3):212–222. doi: 10.1111/j.1399-0039.1979.tb00786.x. [DOI] [PubMed] [Google Scholar]
- Suciu-Foca N., Buda J. A., Thiem T., Reemtsma K. Impaired responsiveness of lymphocytes in patients with systemic lupus erythematosus. Clin Exp Immunol. 1974 Nov;18(3):295–301. [PMC free article] [PubMed] [Google Scholar]
- Sármay G., Ivanyi J., Gergely J. The involvement of a preformed cytoplasmic receptor pool in the reexpression of Fc receptors following their interaction with various antibodies. Cell Immunol. 1980 Dec;56(2):452–464. doi: 10.1016/0008-8749(80)90120-3. [DOI] [PubMed] [Google Scholar]
- Terasaki P. I., Mottironi V. D., Barnett E. V. Cytotoxins in disease. Autocytotoxins in lupus. N Engl J Med. 1970 Oct 1;283(14):724–728. doi: 10.1056/NEJM197010012831403. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Jr, Froelich C. J., Kilpatrick K., Crowe W. E., Levinson J. E. T gamma subset specificity of lymphocyte reactive factors in juvenile rheumatoid arthritis and systemic lupus erythematosus sera. Arthritis Rheum. 1981 Apr;24(4):585–591. doi: 10.1002/art.1780240403. [DOI] [PubMed] [Google Scholar]
- Wilson B. S., Indiveri F., Pellegrino M. A., Ferrone S. DR (Ia-like) antigens on human melanoma cells. Serological detection and immunochemical characterization. J Exp Med. 1979 Mar 1;149(3):658–668. doi: 10.1084/jem.149.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiman K., Curman B., Forsum U., Klareskog L., Malmnäs-Tjernlund U., Rask L., Trägårdh L., Peterson P. A. Occurrence of Ia antigens on tissues on non-lymphoid origin. Nature. 1978 Dec 14;276(5689):711–713. doi: 10.1038/276711a0. [DOI] [PubMed] [Google Scholar]
- Winfield J. B., Winchester R. J., Wernet P., Fu S. M., Kunkel H. G. Nature of cold-reactive antibodies to lymphocyte surface determinants in systemic lupus erythematosus. Arthritis Rheum. 1975 Jan-Feb;18(1):1–8. doi: 10.1002/art.1780180101. [DOI] [PubMed] [Google Scholar]


