Abstract
The objective of this study is to evaluate and verify the effectiveness of plasma treatment for improving adhesive/dentin interfacial bonding by performing micro-tensile bond strength (μTBS) test using the same-tooth controls and varying cross-sectional surface areas. Extracted unerupted human third molars were used by removing the crowns to expose the dentin surface. For each dentin surface, one half of it was treated with a non-thermal argon plasma brush, while another half was shielded with glass slide and used as untreated control. Adper Single Bond Plus adhesive and Filtek Z250 dental composite were then applied as directed. The teeth thus prepared were further cut into micro-bar specimens with cross-sectional size of 1×1 mm2, 1×2 mm2 and 1×3 mm2 for μTBS test. The test results showed that plasma treated specimens gave substantially stronger adhesive/dentin bonding than their corresponding same tooth controls. As compared with their untreated controls, plasma treatment gave statistically significant higher bonding strength for specimens having cross-sectional area of 1×1 mm2 and 1×2 mm2, with mean increases of 30.8% and 45.1%, respectively. Interface examination using optical and electron microscopy verified that plasma treatment improved the quality of the adhesive/dentin interface by reducing defects/voids and increasing the resin tag length in dentin tubules.
Keywords: Plasma treatment, Bonding strength, Resin restoration, Same tooth control
In order to increase the bonding strength and longevity of dental composite restoration, it is essential to modify the physical and chemical properties of dentin surface and get a much compatible adhesive/dentin interface. It has been found that adhesive permeation into dentinal tubules and spaces in collagen fibrils created by etching played an important role in adhesive/dentin bonding strength and durability (1). Addition of hydrophilic monomer into dental adhesives would increase the penetration and improve bonding strength of adhesive to dentin (2). Besides improving the formulation of dental adhesives (3), surface modification of dentin is another effective approach to achieve stronger adhesive/dentin interfacial bonding. In fact, a lot of research work has been done on modification of dentin surface, such as use of collagen cross-linking agents (4), immediate dentin sealing (5), treatment with hydroxyapatite nanorods (6), etc.
As an effective surface modification technique, low-temperature or non-thermal plasmas that are usually created at a reduced pressure in a vacuum chamber have been well adopted for surface treatment/preparation of a variety of natural and synthetic materials to improve their performance. The rapid development of non-thermal atmospheric plasma technology in the past two decades has opened the door for the direct use and applications of gas plasmas in both medical and dental field. Our recent research works (7, 8) have demonstrated that plasma treatment using a non-thermal atmospheric plasma brush was very effective in deactivating cavity-causing oral bacteria and changing the surface properties of human dentin. It was found that the a short plasma treatment could change the chemical structure of the exposed collagen fibrils and increase the dentin surface hydrophilicity, which allowed better adhesive penetration into dental collagen fibrils and enhance the bonding strength at adhesive/dentin interface. The treatment of dentin using non-thermal plasma brush has provided about 60 % stronger adhesive/dentin interface bonding than their untreated controls (7, 8).
Micro-tensile bond strength (μTBS) test has been widely accepted as the most often used testing method for evaluating adhesive/dentin interfacial bonding because it permits the measurement of regional bond strengths within teeth with very few cohesive failures. Sano et al (9) showed that tensile bond strength was inversely related to bonded surface area, which was also confirmed by PHRUKKANON et al. (10). The bonding strength of adhesive/dentin showed the dramatic decrease with the increase of the interface area of specimens (11). The bonding strength of specimens with 1mm2 interface area ranged from 40 to 60 MPa. When the interface area was increased to 2 mm2, the bonding strength decreased to 15–30 MPa. This is probably the result of more defects existing in the large bonding surface area specimens (10).
The bonding strength between restorative materials and dentin is also affected by intrinsic dentin characteristics such as the number of tubules per mm2 (12), the thickness of dentin (13), water content of dentin (14), tooth conditions, etc. To better assess the plasma treatment effects on dentin for adhesive/dentin bonding improvement, it is necessary to collect and compare the adhesive/dentin bonding strength of plasma treated specimens with their untreated same tooth controls. μTBS test using small bonding surface areas makes it possible to take multiple treatments within a single tooth (9). Therefore, the aim of this study is to evaluate and verify the effectiveness of plasma treatment of dentin surfaces for improving adhesive/dentin interfacial bonding by performing μTBS test using the same-tooth controls and varying cross-sectional surface areas.
Material and methods
Tooth preparation
Twelve unerupted human third molars were collected under a protocol approved by the University of Missouri-Kansas City Adult Health Sciences Institutional Review Board and after obtaining patient consent. All tissue samples were non-patient identified and all samples were handled and disposed according to the protocols suggested by Environmental Health and Safety at University of Missouri. The teeth had no caries and were stored in the PBS (pH7.4) with 0.02% sodium azide to inhibit bacteria growth. Each tooth was cut off the root and enamel using an Isomet 5000 diamond saw (Buehler, Lake Bluff, IL, USA) to expose the dentin surface. The surface was polished with 600-grit silicon carbide abrasive paper under wet condition. The dentin surface was further demineralized for 15 s using Scotchbond phosphoric acid gel (3M ESPE, St Paul, MN, USA) and then thoroughly washed using a water spray. Excess water was blot dried from the surface with Kimwipes. Half of a tooth was treated with plasma brush by shielding the other side with a glass slide.
The plasma treatment method using a plasma brush has been described in our previous work (8). The plasma brush was operated at a current level of 6 mA (equivalent to a power level of 2–3 W) using Spellman HV power supply SL60 (Spellman, New York, USA) and an argon flow rate of 3,000 standard cubic centimeters per minute (sccm). Based on our previous work (8), plasma treatment time was set for 30 s to achieve the highest adhesive/dentin interface bonding. After plasma treatment, the dentin surface was rewetted to be visibly moist before applying dental adhesive (Adaper Single Bond Plus, 3M ESPE). The applied dental adhesive was then light-cured for 10 s using Spectrum 800 (Dentsply, Milford, DE, USA). Then a dental composite (Filtek Z250, 3M ESPE) was applied on top of adhesive for three to four times and light cured for 20 s for each application. The tooth-composite bonded sample was stored in distilled water at 37 °C for 24 h before preparing micro-bar specimens for micro-tensile bond strength (μTBS) test. The prepared teeth were sectioned with a diamond saw under water cooling to produce micro-bar specimens with cross-section dimension of ~1×1 mm2, ~1×2 mm2 and ~1×3 mm2. 4 teeth were used for each cross-sectional dimension and the numbers of the microbars obtained from each tooth were summarized in Table 1. The exact dimension of prepared micro-bar specimens were measured using a digital caliper.
Table 1.
Number of the micro-bars and the percentage of each failure mode of the μTBS tested specimens obtained from each tooth with cross-section size of 1×1 mm2, 1×2 mm2, and ×3 mm2.
| Micro-bar cross- section |
Tooth | 1 | 2 | 3 | 4 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Plasma treated |
Control | Plasma treated |
Control | Plasma treated |
Control | Plasma treated |
Control | ||
| 1×1 mm2 | Number of micro-bars | 9 | 5 | 16 | 9 | 7 | 9 | 8 | 5 |
| Interface & mixed failure* (%) | 33 | 60 | 63 | 44 | 29 | 44 | 100 | 100 | |
| Cohesive failure* (%) | 67 | 40 | 27 | 56 | 71 | 56 | 0 | 0 | |
| 1×2 mm2 | Number of micro-bars | 9 | 5 | 8 | 5 | 7 | 8 | 11 | 9 |
| Interface & mixed failure (%) | 33 | 80 | 100 | 100 | 71 | 100 | 82 | 89 | |
| Cohesive failure (%) | 67 | 20 | 0 | 0 | 29 | 0 | 18 | 11 | |
| 1×3 mm2 | Number of micro-bars | 3 | 2 | 2 | 3 | 4 | 3 | 1 | 2 |
| Interface & mixed failure (%) | 33 | 50 | 0 | 67 | 50 | 33 | 100 | 100 | |
| Cohesive failure (%) | 67 | 50 | 100 | 33 | 50 | 67 | 0 | 0 | |
Interface failure: fracture occurred in adhesive with no fractured dentin on the resin and no remnants of resin on the dentin surfaces; Cohesive failure: fracture is completely located in dentin, resin or Zapit; Mixed failure: test specimen shows both interface and cohesive failures.
Micro-tensile bond strength (μTBS) test
The micro-bar specimens were examined and screened using an optical microscope (AMscope, City?, California, CA, USA) for possible defects existing at the adhesive/dentin interface. The specimens that passed the screening were adhered to micro tensile tester (BISCO, Schaumburg, IL, USA) using a cyanoacrylate adhesive (Zapit, Corona, CA, USA) and tested at a 0.5 mm/min strain rate.
Characterization of fractured surfaces
After the μTBS test, optical microscopy was used to examine the fractured surface for failure locations. The mode of failure location was defined as following: Interface failure - fracture occurred in adhesive with no fractured dentin on the resin and no remnants of resin on the dentin surfaces; Cohesive failure - fracture is completely located in dentin, resin or Zapit; Mixed failure - test specimen shows both interface and cohesive failures (15).
The morphology of the fractured surfaces was examined using a scanning electron microscopy (SEM) (Quanta 600, FEI, City?, OR, USA). The fractured bars were fixed on aluminum stubs with the fracture surfaces facing upward. All samples were imaged using the environmental and/or backscatter modes of the microscope. Specimens with cohesive failure of dentin or composite were selected for interface quality observation. In order to see hybrid layer and resin tags, acid-bleach treatment was used by following the procedures reported in previous work (16–19). In brief, 5 M HCl was used to treat the plasma treated and untreated specimens for 60 s and 30 s, respectively. After thoroughly washed with distilled water, the specimens were immersed in a NaOCl solution (chlorine 4.00–4.99%) for 30 min. After rinsing with distilled water, the specimens were dehydrated in ethanol/water mixture with ascending concentrations starting with 50%, 70% and 85% for 15 min each, followed by 95%, 100% and 100% for 30 min each. After drying, the prepared specimens were mounted on aluminum stubs and coated with 5 nm of platinum for SEM examination.
Data analysis
The numbers of micro-bar specimens obtained for each cross-section sizes test were detailed in Table 2. The tensile stress data obtained from μTBS test were used to evaluate the plasma treatment effects on adhesive/dentin bonding strength. The tensile stress data obtained from all test specimens that have the same cross-section size from different teeth was combined and analyzed using Welch’s t-test to account for the possibility of unequal variances after verifying the data distribution normality. Ten sites of the hybrid layer and fifty resin tags shown in SEM images were analyzed using Image J software to calculate the means and standard deviations of the thickness of hybrid layer and the length of the resin tags.
Table 2.
Total number of the micro-bars for each cross-section size and estimation of sample size at a specified confidence level and error using QI Macros.
| Cross-sectional area |
1×1 mm2 | 1×2 mm2 | 1×3 mm2 | |||
|---|---|---|---|---|---|---|
| Plasma treated |
Control | Plasma treated |
Control | Plasma treated |
Control | |
| Total number of micro-bars | 40 | 28 | 35 | 27 | 10 | 10 |
| Predicted sample size | 33 | 21 | 25 | 17 | 18 | 22 |
| Confidence level (%) | 90 | 90 | 90 | 90 | 90 | 90 |
| Error (MPa) | ± 5 | ± 4 | ± 4 | ± 4 | ± 4 | ± 4 |
Results
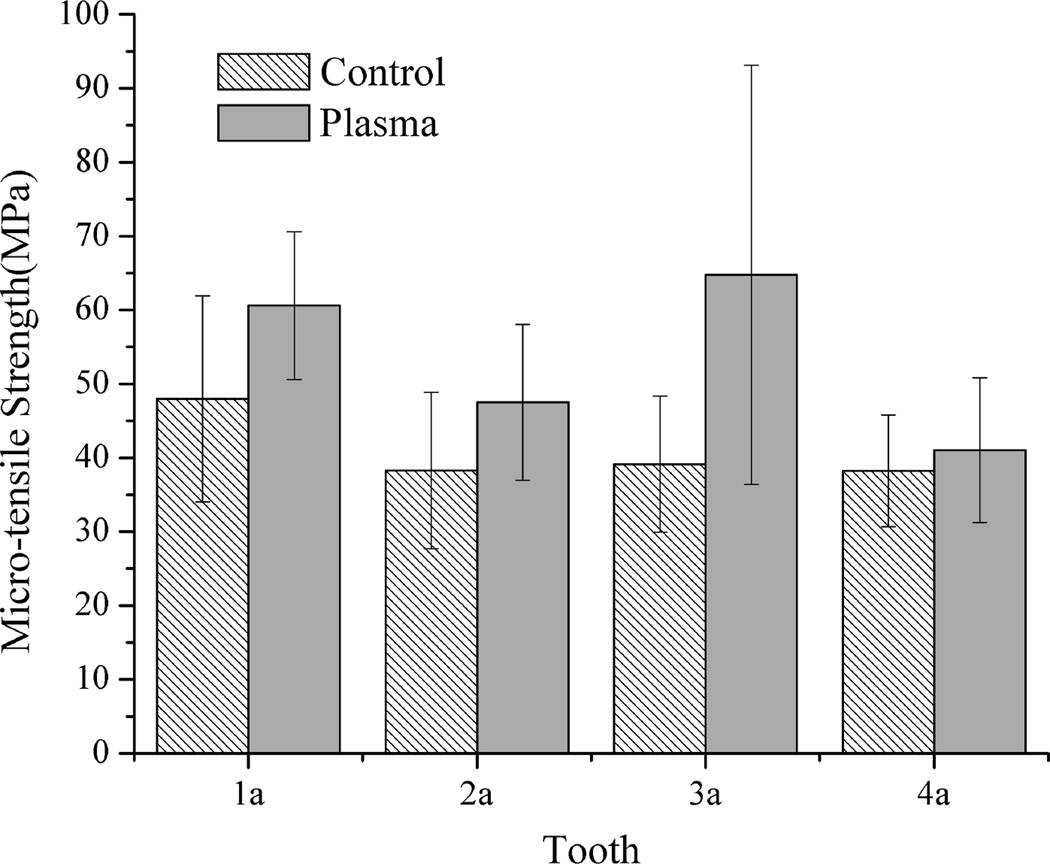
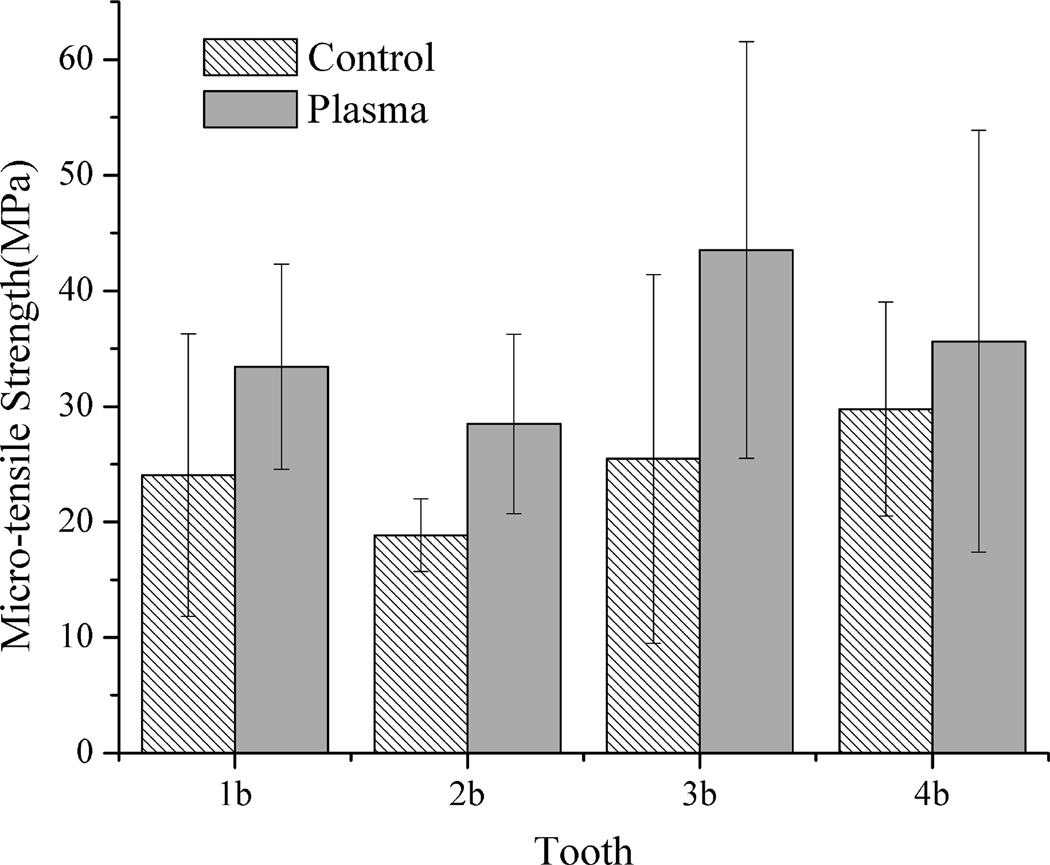
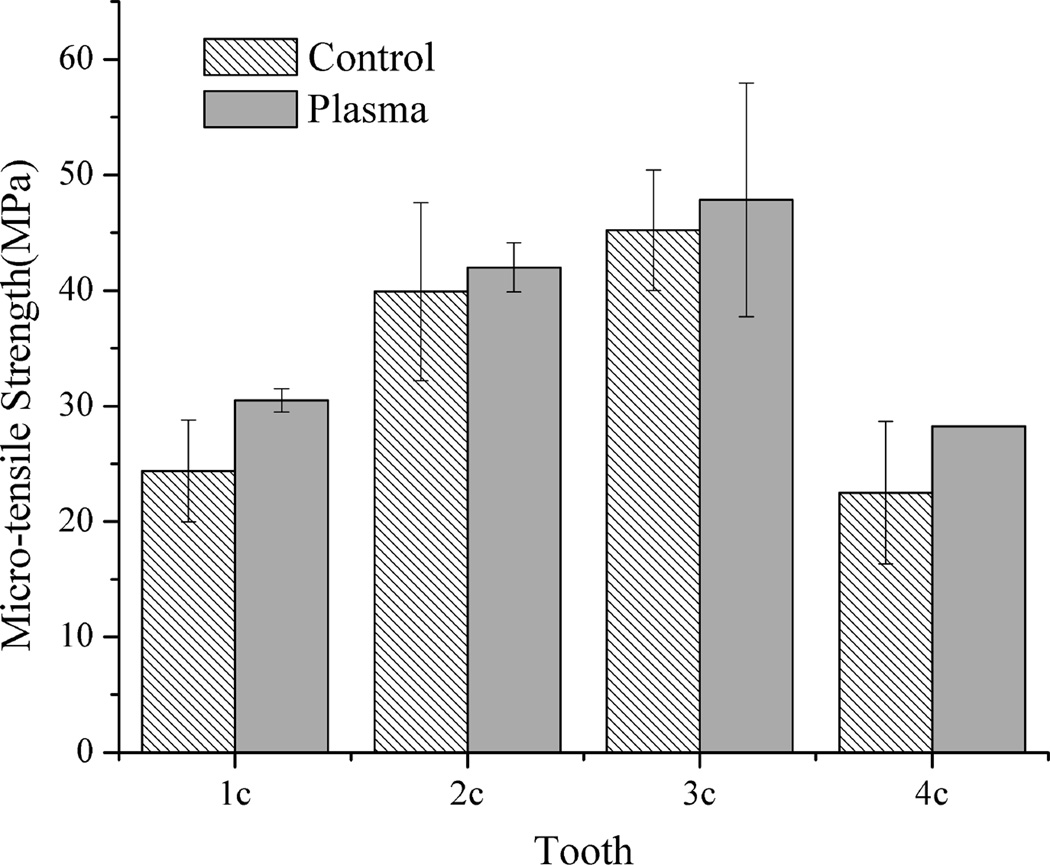
Figs. 1 and 2 show the mean bonding strength of the micro-bar specimens with cross-sectional size of 1×1 mm2, 1×2 mm2, respectively. As shown in Figs. 1 and 2, the plasma-treated specimens with cross-sectional area of 1×1 mm2 and 1×2 mm2 had much higher mean bonding strength than their corresponding untreated same tooth controls. When the specimen cross-sectional area was increased to 1×3 mm2, as shown in Fig. 3, the mean bonding strength of plasma treated specimens was still higher than their corresponding same tooth controls, although the difference became less significant than that of the smaller specimen sizes shown in Figs. 1 and 2. It should be pointed out that, with plasma treatment, the adhesive/dentin bonding strength was improved for all the teeth tested as compared with the same tooth controls. The results shown in Figs. 1–3 also clearly indicate the effects of individual tooth on the measured adhesive/dentin bonding strength for both plasma treated specimens and their untreated controls. It can be seen that individual tooth did have significant effect on the bonding strength of the resulted adhesive/dentin interface for both plasma treated specimens and the untreated controls.
Figure 1.
Comparison of micro-tensile strength of 1×1 mm2 specimens treated with plasma and the untreated same tooth controls
Figure 2.
Comparison of micro-tensile strength of 1×2 mm2 specimens treated with plasma and the untreated same tooth controls
Figure 3.
Comparison of micro-tensile strength of 1×3 mm2 specimens treated with plasma and the untreated same tooth controls
Besides bonding strength, the fracture location of the tested specimens provides information for further evaluation of adhesive/dentin interface quality. The fractured surface of all the tested specimens including both plasma treated specimens and the untreated controls was carefully examined and analyzed under an optical microscope. Table 1 summarizes the number of micro-bar specimens from each individual tooth and the fractured location percentage after the μTBS test for the micro-bar test specimens from each individual tooth. It can been seen from the Table 1 that interface failure was observed more frequently in the specimens obtained from the untreated controls than that in the plasma-treated specimens. The large percentage of cohesive failure was found with tested specimens that have larger cross-sectional size of 1×3 mm2 for both the untreated controls and the plasma-treated ones.
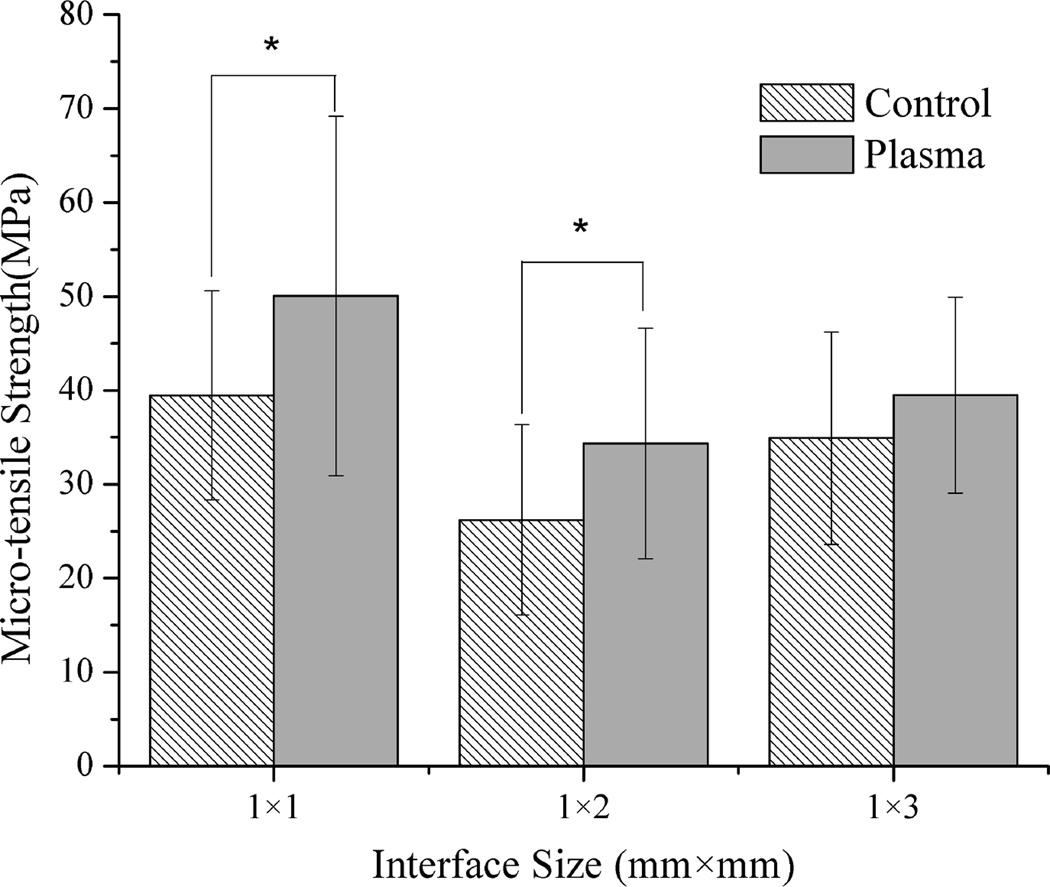
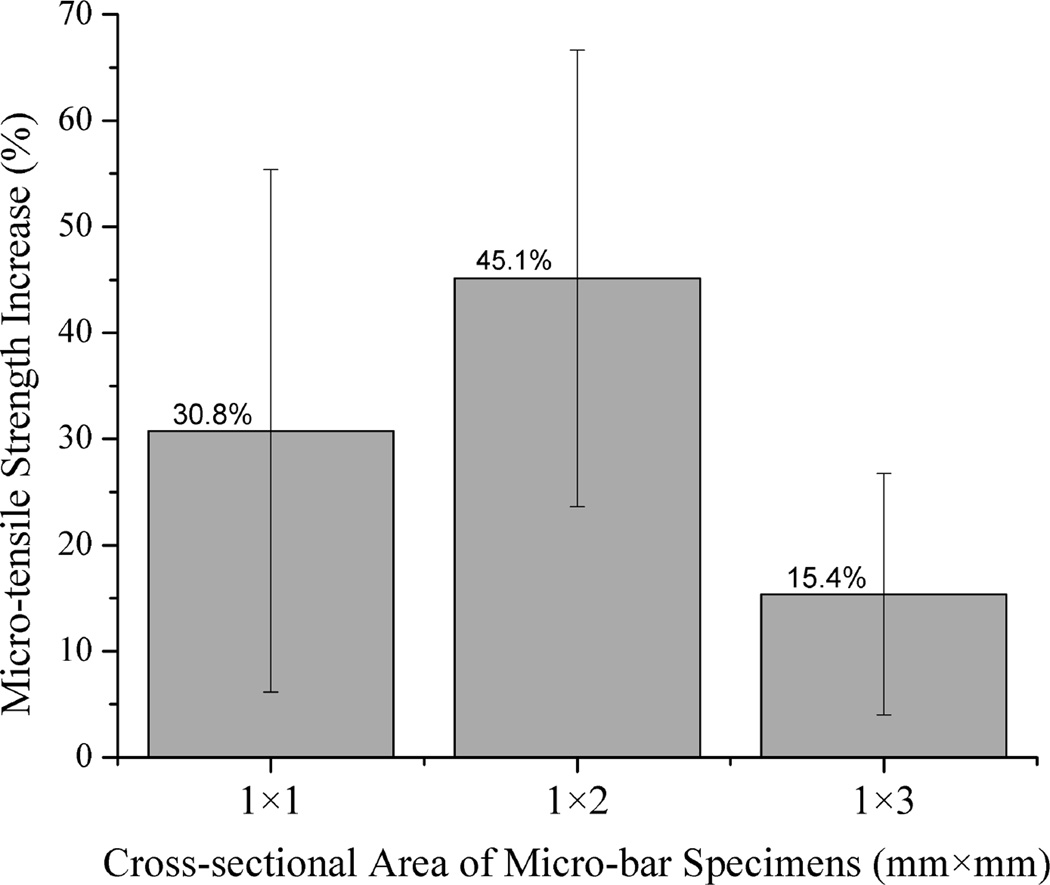
Fig. 4a shows the statistical comparison of μTBS test data obtained from all the test specimens from different teeth with cross-sectional size of 1×1 mm2, 1×2 mm2 and 1×3 mm2. It was noted that the adhesive/dentin bonding strength of plasma treated specimens with both 1×1 mm2 and 1×2 mm2 interface area was significantly higher than that of respective untreated controls (p<0.01). When the interface area was increased to 1×3 mm2, the mean value of the μTBS tensile strength for plasma treated specimens was higher than the corresponding untreated controls, but significant difference was not observed between them. To further show the plasma treatment effect, the percentage increase in μTBS test data due to plasma treatment is presented in Fig. 4b. Regardless of the individual tooth effect and different cross-sectional area, plasma treatment substantially enhanced the adhesive/dentin bonding strength with an average of 15–45 % increase as compared with the untreated controls. It should be noted from Table 1 that only 1–4 specimens with cross-sectional area of 1×3 mm2 could be prepared from a single tooth because of the limited dentin area. Table 2 summarizes the sample sizes used for the statistical analysis and the predicted sample size required to get an accurate estimate of the mean bonding strength at a confidence level 90% using QI Macros. As seen in Table 2, the numbers of the test specimens with both 1×1 mm2 and 1×2 mm2 interface area used in this study were larger than the predicted sample sizes. However, the number of the test specimens with 1×3 mm2 needs to be increased in order to improve the analysis reliability.
Figure 4.
Comprehensive comparison of micro-tensile strength of specimens of different interface size treated with plasma and the untreated controls of all teeth. * represents the significant difference (p<0.01).
The adhesive/dentin interface of the plasma treated specimens and their untreated controls as examined by using an optical microscope. It was noted that the plasma treated specimens had less defects or voids existing at the dentin /adhesive/ composite interface than the untreated same tooth controls. Moreover, the size of the voids at the interface was also smaller for the plasma treated specimens.
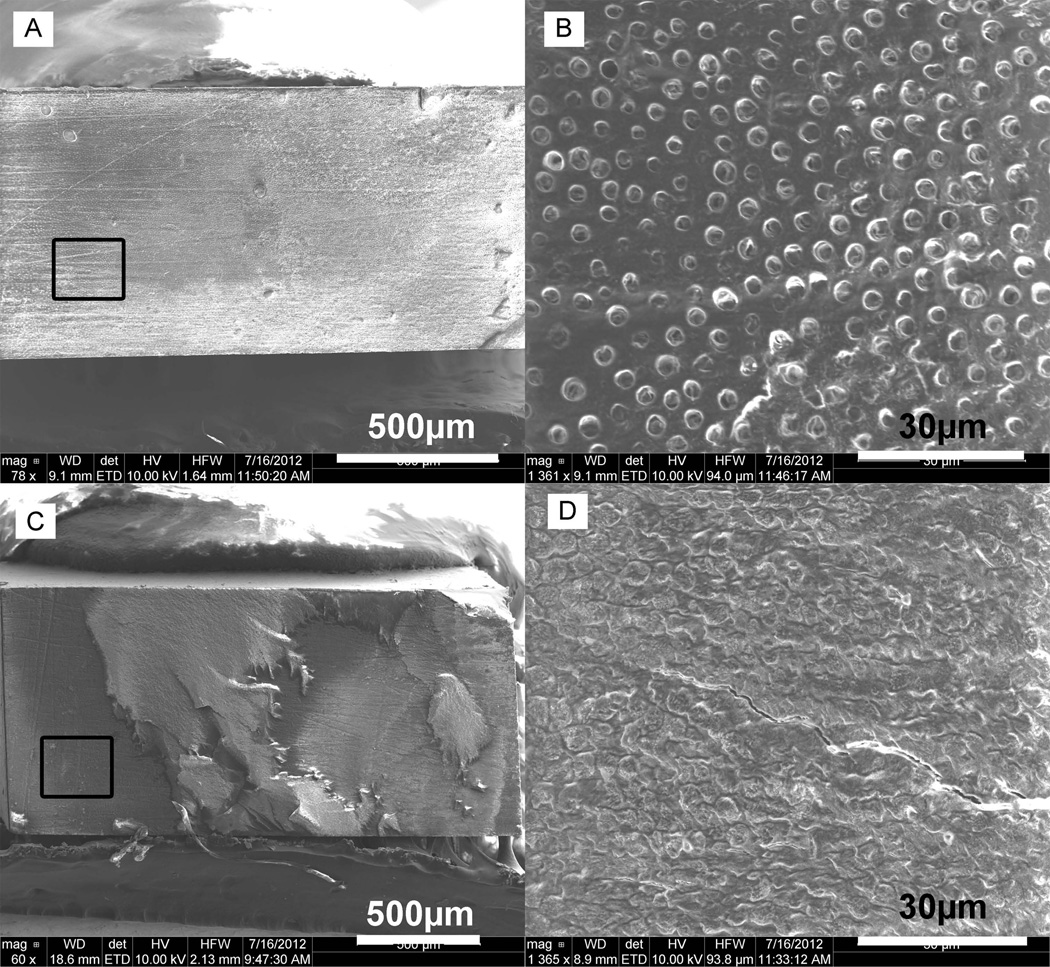
In the backscatter mode SEM images, high atomic number elements backscatter electrons more strongly and appear brighter than low atomic number elements. Fig. 5 shows the representative backscattered images of fracture surfaces of the plasma treated specimens and their untreated same tooth controls after μTBS test. As seen from the images, the composite appeared brighter because of containing Ba and Si elements, while the adhesive and the etched dentin looked darker because they contain only C and O elements. The images of the fractured surfaces revealed that the untreated control specimens mainly failed at the adhesive/dentin interface with dentin exposed, while the plasma treated specimens showed a mixed failure with more cohesive failure of composite and Zapit. Through the SEM images with higher magnifications shown in Fig. 5, the dental tubules can be clearly seen on the exposed dentin surface due to adhesive pull out, while only composite was observed on the fractured surface for plasma treated specimens due to cohesive failure of the composite.
Figure 5.
Representative backscattered SEM micrographs of the fractured surfaces of (A, B) untreated specimens, and (C, D) plasma treated specimens form tooth 2b with the 1×2 mm2 interface area at different magnifications.
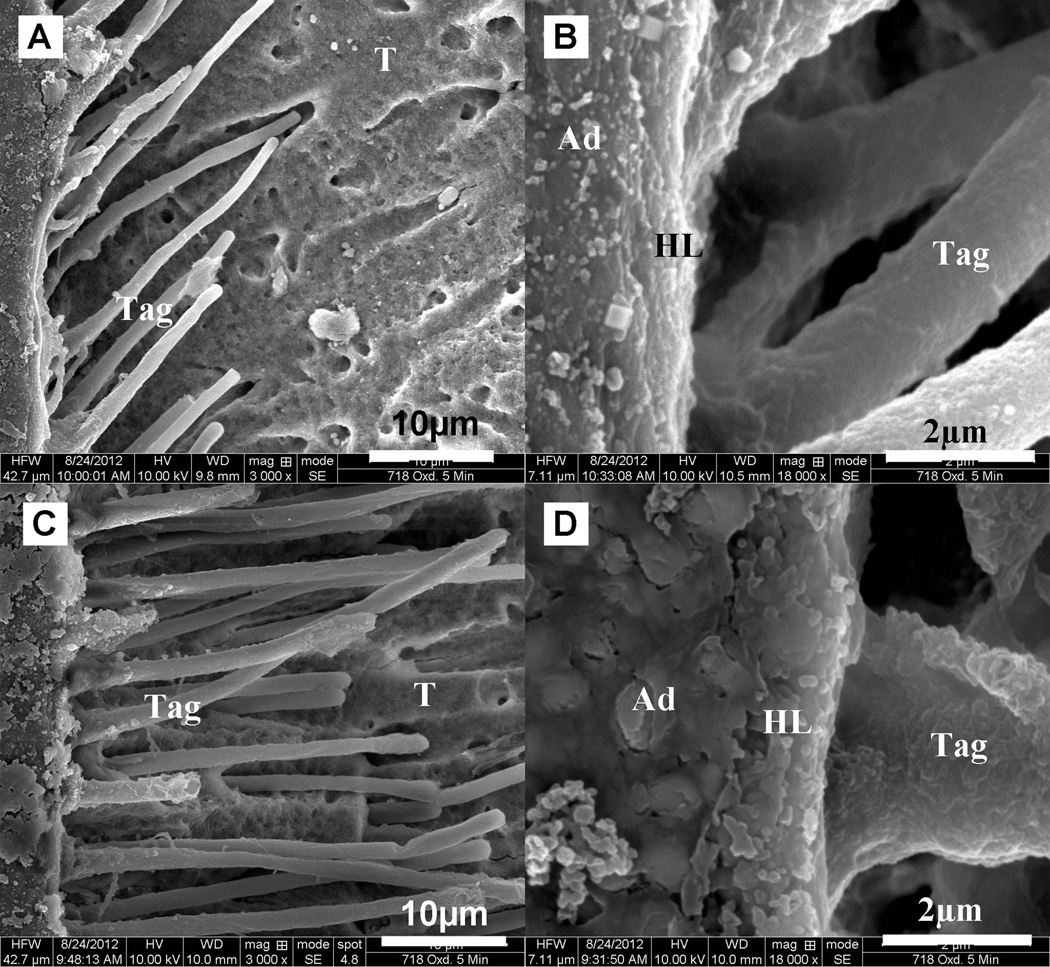
The quality of hybrid layer and resin tag were examined using SEM with the microbar test specimens prepared by the acid-bleach technique (16–19). As seen from the typical SEM images shown in Fig. 6 for plasma treated specimens and the untreated same tooth controls, the adhesive/collagen fibril hybrid layer and adhesive resin tags penetrated into tubules were clearly observed. Through Image J software the thickness of hybrid layer for plasma treated specimens was 1.92±0.15µm, which was apparently much thicker than that of 1.13±0.15µm for untreated specimens. It was also noted the length of resin tags in plasma treated specimens was 38.47±10.80 µm, which was much longer than the length of 20.17±5.50 µm in the untreated specimens.
Figure 6.
Representative SEM images of acid-bleach treatment interface of (A, B) untreated specimens, and (C, D) plasma-treated test specimens from tooth 3a with the 1×3 mm2 interface area
Discussion
Plasma treatment using inert gas argon has been widely used to modify biomaterial surface (19, 20). When electronically activated into plasma state, argon plasmas consist of many energetic and chemically reactive species including high energy electrons, ionic species, electronically excited neutrals, and free radicals, etc. These active species in argon plasmas can react with the treated surface and thus modify the surface chemistry and properties without affecting the bulk material properties (21, 22). It has been reported that, after treated with argon plasma, the water contact angle of collagen surface was significantly decreased (23, 24). Dentin surface contains a fibrous collagen network after removing drilling debris and some surface minerals by acid etching. Our recent results (7, 8) showed that, after argon plasma treatment, the hydrophilicity of the dentin surface was increased and an increased number of carbonyl groups were found on the surface. Surface hydrophilicity increase will improve the penetration of hydrophilic monomer components (e.g. hydroxyethylmethacrylate, HEMA) of dental adhesive into collagen fibrils and dentin tubules. After light curing, the penetrated monomers polymerize and provide micromechanical retention of resin to the dentin surface (1). Another reason for enhancing the adhesive/dentin bonding strength is that plasma treatment could introduce activated sites, such as free radicals or peroxides, to the dentin surface, which would initiate polymerization of adhesive monomers and graft resin onto the collagen fibrils through covalent chemical bonding. Our previous FT-IR result has shown that plasma treated dentin surface could induce HEMA graft polymerization on dentin collagen fibrils (7). It is indubitable that the covalent bond formation between HEMA and dentin collagen fibrils would lead to stronger adhesive/dentin bonding strength.
Our results shown in Figs. 1, 2 and 4 have evidently indicated that argon plasma treatment has significantly increased the bonding strength measured using micro-bar test specimens with cross-sectional area of both 1×1 mm2 and 1×2 mm2. The mean bonding strength of plasma treated specimens with 1×2 mm2 interface area has been found showing 45% increase as compared with untreated specimens. It was also noted that (Table 1) the percentage of interface failure in the plasma treated specimens was less than that in the untreated specimens, indicating the adhesive/dentin bonding strength of the plasma treated specimens was even stronger than the measured data by μTBS test. Examination of the fractured surfaces using an optical microscope and SEM showed that (Table 1, Fig. 5) plasma treated specimens had more cohesive failure than the untreated controls. The SEM images shown in Fig. 6 also revealed that plasma treatment made the adhesive wet the dentin surface better and penetrate deeper into the tubules with longer resin tags when compared with the untreated controls.
Examination of the micro-bar test specimens using an optical microscope indicated plasma treatment led to less voids or defects at the adhesive/dentin interface than the untreated controls. The voids or defects at the interface, which have also been observed in the previous work (25), would affect the bond strength measured data by μTBS test. Finite-element analyses have shown the voids or defects in the adhesive/dentin interface induced the propagation of fracture (26, 27). A large number of voids at interface were associated with low bond strength. The voids also would expose collagen fibrils and result in an increased risk for collagen degradation and dentine sensitivity. In contrast, the increased surface hydrophilicity of dentin due to plasma treatment enhanced the diffusion and penetration of adhesive monomers into the collagen fibril network and tubules on dentin surface, which reduce the number of defects and voids at the adhesive/dentin interface and thus improve interface quality.
As noticed in Figs. 3 and 4, for test specimens with an interface area of 1×3 mm2, the mean values of the measured bonding strength are clearly higher for the plasma treated specimens than those for the untreated controls, although statistically significant difference was not found between the plasma treated and untreated specimens. As shown in Fig. 6, however, examination of the adhesive/dentin interface with SEM showed that the plasma treated specimens had a thicker hybrid layer and longer resin tags than the untreated controls. This result indicates that plasma treatment provides an improved adhesive/dentin interface quality, which attributes to enhanced interfacial bonding strength (28).
There might be two reasons for no significant difference found within the specimens having 1×3 mm2 interface area. First, it is probably due to the less number of test specimens. As shown in Tables 1 and 2, because of the larger cross-section area, only ~ 6 specimens, i.e. ~ 3 plasma treated and ~ 3 untreated, can be prepared from one single tooth, while there are 13–20 specimens with 1×2 mm2 interface area and 13–25 specimens with 1×1 mm2 interface area. With 4 teeth used for micro-bars with 1×3 mm2 surface area, only 10 plasma treated test specimens were obtained along with 10 test specimens as their untreated controls. To achieve statistical significance, a larger sample size is necessary and more teeth will be needed. According to the analysis using the Sample Size function of QI Macros shown in Table 2, the number of samples with 1×1 mm2 or 1×2 mm2 interface area used in this study resulted in an accurate bonding strength with 90% confidence level and 4 MPa margin of error. To get the same confidence level analysis result, 8 more plasma treated and 12 more untreated specimens, equal to 4 – 5 more teeth, would be needed for 1×3 mm2 interface area for the given standard deviation. Second, it is hard to measure the true ultimate bonding strength because of a large number of cohesive failures in composite, denting, and/or Zapit as summarized in Table 1. PASHLEY et al. reported that large bonded surface areas would produce cohesive failure at relatively low bond strengths (11). SANO et al. also found that when the cross-sectional area of bonded specimens is reduced, the number of cohesive failures fell down (9). Therefore, use of specimens with 1×3 mm2 interface area might not be suitable for evaluating plasma treatment effects.
In this study, we were able to evaluate plasma treatment effects using the same tooth controls. It is known that tubule size, orientation and water content of dentin vary widely from tooth to tooth (29), which significantly influence the adhesive/dentin bonding strength. Different tubule size and orientation will lead to difference in adhesive permeability (1). Water content of dentin is another factor that affects interface bonding because dental adhesive systems are very sensitive to the water content on dentin surface (30). An under-wet surface will lead to the collapse of collagen fibrils. On the other hand, an over-wet surface will lead to adhesive phase separation (31). As shown in Figs. 1–3, even with the untreated controls, the adhesive/dentin bonding strength varied from tooth to tooth. By using the same tooth controls, in this study, we were able to evaluate the plasma treatment effectiveness in improving adhesive/dentin interface quality and the resultant interfacial bonding strength by eliminating the effects of individual teeth that usually have the huge difference in composition, tubule size and orientation, etc.
Our experimental study evidently demonstrated the effects and effectiveness of plasma treatment on improving adhesive/dentin interface bonding by using the same tooth controls and varying the interface area of the microbar test specimens. The experimental results showed that, using micro-bar test specimens with interface areas of 1×1 mm2 and 1×2 mm2, the adhesive/dentin bonding strengths were significantly enhanced by non-thermal argon plasma treatment. Microscopic examination of the fractured surfaces using both optical microscopy and SEM revealed that plasma treatment could reduce the interface defects/voids and thus improve the interface quality, which could be attributed to the increased hydrophilicity on dentin surface by plasma treatment. On the other hand, microbar test specimens with an interface area of 1×3 mm2 were found not being suitable for evaluating plasma treatment effects using the same tooth controls because of a small quantity of specimens from each single tooth and a large number of cohesive failures of the test specimens. In conclusion, the experimental results from this study further demonstrate that non-thermal argon plasma treatment is very effective in improving adhesive/dentin interface bonding strength, which is crucial for increasing longevity of dental composite restorations.
Acknowledgments
This study was supported, in part, by the US National Institute of Health (NIH) under grant numbers of 5R01DE021431 and 5R44DE019041. The authors would thank Drs. Thomas E. Coyle D.D.S., John A. Johnson D.D.S., and Timothy T. Coyle, D.D.S., M.D. from Coyle & Johnson Oral and Maxillofacial Surgery for providing the extracted human teeth.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Pashley DH, Carvalho RM. Dentine permeability and dentine adhesion. J. Dent. 1997;25:355–372. doi: 10.1016/s0300-5712(96)00057-7. [DOI] [PubMed] [Google Scholar]
- 2.Eick J, Robinson S, Cobb C, Chappell R, SPENCER P. The dentinal surface: its influence on dentinal adhesion. 2. Quintessence Int. (Berlin, Germany; 1985) 1992;23:43. [PubMed] [Google Scholar]
- 3.Kunio I, Yoshinori K, Takeshi E. A review of the developments of self-etching primers and adhesives —Effects of acidic adhesive monomers and polymerization initiators on bonding to ground, smear layer-covered teeth. Dent. Mater. J. 2011;30:769–789. doi: 10.4012/dmj.2011-110. [DOI] [PubMed] [Google Scholar]
- 4.Fang M, Liu R, Xiao Y, Li F, Wang D, Hou R, Chen J. Biomodification to dentin by a natural crosslinker improved the resin–dentin bonds. J. Dent. 2012;40:458–466. doi: 10.1016/j.jdent.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Magne P, So WS, Cascione D. Immediate dentin sealing supports delayed restoration placement. J. Prosthet. Dent. 2007;98:166–174. doi: 10.1016/S0022-3913(07)60052-3. [DOI] [PubMed] [Google Scholar]
- 6.Sadat-Shojai M, Atai M, Nodehi A, Khanlar LN. Hydroxyapatite nanorods as novel fillers for improving the properties of dental adhesives: Synthesis and application. Dent. Mater. 2010;26:471–482. doi: 10.1016/j.dental.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Yu QS, Li H, Ritts AC, Yang B, Chen M, Hong L, Wang Y. Non-thermal atmospheric plasma treatment for deactivation of oral bacteria and improvement of dental composite restoration. In: Machala Z, Hensel K, Akishev Y, editors. Plasma for Bio-Decontamination, Medicine and Food Security. Netherlands: Springer; 2012. pp. 215–228. [Google Scholar]
- 8.Ritts AC, Li H, Yu Q, Xu C, Yao X, Hong L. Dentin surface treatment using a non-thermal argon plasma brush for interfacial bonding improvement in composite restoration. Eur. J. Oral Sci. 2010;118:510–516. doi: 10.1111/j.1600-0722.2010.00761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sano H, Shono T, Sonoda H, Takatsu T, Ciucchi B, Carvalho R. Relationship between surface area for adhesion and tensile bond strength — Evaluation of a micro-tensile bond test. Dent. Mater. 1994;10:236–240. doi: 10.1016/0109-5641(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 10.Phrukkanon S, Burrow MF, TYAS MJ. The influence of cross-sectional shape and surface area on the microtensile bond test. Dent. Mater. 1998;14:212–221. doi: 10.1016/s0109-5641(98)00034-7. [DOI] [PubMed] [Google Scholar]
- 11.Pashley DH, Sano H, Ciucchi B, Yoshiyama M, Carvalho RM. Adhesion testing of dentin bonding agents: a review. Dent. Mater. 1995;11:117–125. doi: 10.1016/0109-5641(95)80046-8. [DOI] [PubMed] [Google Scholar]
- 12.Marshall GW, Marshall SJ, Kinney JH, Balooch M. The dentin substrate: structure and properties related to bonding. J. Dent. 1997;25:441–458. doi: 10.1016/s0300-5712(96)00065-6. [DOI] [PubMed] [Google Scholar]
- 13.Tagami J, Tao L, Pashley DH. Correlation among dentin depth, permeability, and bond strength of adhesive resins. Dent. Mater. 1990;6:45–50. doi: 10.1016/0109-5641(90)90044-f. [DOI] [PubMed] [Google Scholar]
- 14.Pereira PNR, Okuda M, Sano H, Yoshikawa T, Burrow MF, Tagami J. Effect of intrinsic wetness and regional difference on dentin bond strength. Dent. Mater. 1999;15:46–53. doi: 10.1016/s0109-5641(99)00013-5. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Spencer P, Hager C, Bohaty B. Comparison of interfacial characteristics of adhesive bonding to superficial versus deep dentine using SEM and staining techniques. J. Dent. 2006;34:26–34. doi: 10.1016/j.jdent.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Shin TP, Yao X, Huenergardt R, Walker MP, Wang Y. Morphological and chemical characterization of bonding hydrophobic adhesive to dentin using ethanol wet bonding technique. Dent. Mater. 2009;25:1050–1057. doi: 10.1016/j.dental.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakabayashi N, Takarada K. Effect of HEMA on bonding to dentin. Dent. Mater. 1992;8:125–130. doi: 10.1016/0109-5641(92)90067-m. [DOI] [PubMed] [Google Scholar]
- 18.Guo X, Spencer P, Wang Y, Ye Q, Yao X, Williams K. Effects of a solubility enhancer on penetration of hydrophobic component in model adhesives into wet demineralized dentin. Dent. Mater. 2007;23:1473–1481. doi: 10.1016/j.dental.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang N, Yang P, Leng Y, Wang J, Sun H, Chen J. Surface modification of biomaterials by plasma immersion ion implantation . Surf. Coat. Tech. 2004;186:218–226. [Google Scholar]
- 20.Chu PK, Chen J, Wang L, Huang N. Plasma-surface modification of biomaterials. Mat. Sci. Eng. R. 2002;36:143–206. [Google Scholar]
- 21.Milinchuk V. Photoradiation chemistry of polymers. Nucl. Instrum. Meth. B. 1995;105:24–29. [Google Scholar]
- 22.Prat R, Shi MK, Clouet F. Interactions of Cold Plasmas with Polymers and Their Model Molecules: Degradation vs. Functionalzation. J. Macromol. Sci. A. 1997;34:471–488. [Google Scholar]
- 23.García JL, Asadinezhad A, Pachernik J, LehockÝ M, Junkar I, Humpolíček P. Cell proliferation of HaCaT keratinocytes on collagen films modified by argon plasma treatment. Molecules. 2010;15:2845–2856. doi: 10.3390/molecules15042845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rafat M, Griffith M, Hakim M, Muzakare L, Li F, Khulbe KC. Plasma surface modification and characterization of collagen-based artificial cornea for enhanced epithelialization. J. Appl. Polym. Sci. 2007;106:2056–2064. [Google Scholar]
- 25.Mollica F, De Santis R, Ambrosio L, Nicolais L, Prisco D, Rengo S. Mechanical and leakage behaviour of the dentin–adhesive interface. J. Mater. Sci.-Mater. M. 2004;15:485–492. doi: 10.1023/b:jmsm.0000021125.40282.ba. [DOI] [PubMed] [Google Scholar]
- 26.Van Noort R, Noroozi S, Howard I, Cardew G. A critique of bond strength measurements. J. Dent. 1989;17:61–67. doi: 10.1016/0300-5712(89)90131-0. [DOI] [PubMed] [Google Scholar]
- 27.Van Noort R, Cardew G, Howard I, Noroozi S. The effect of local interfacial geometry on the measurement of the tensile bond strength to dentin. J. Dent. Res. 1991;70:889–893. doi: 10.1177/00220345910700050501. [DOI] [PubMed] [Google Scholar]
- 28.Hashimoto M, Ohno H, Endo K, Kaga M, Sano H, Oguchi H. The effect of hybrid layer thickness on bond strength: demineralized dentin zone of the hybrid layer. Dent. Mater. 2000;16:406–411. doi: 10.1016/s0109-5641(00)00035-x. [DOI] [PubMed] [Google Scholar]
- 29.Schilke R, Lisson Ja, Bau O, Geurtsen W. Comparison of the number and diameter of dentinal tubules in human and bovine dentine by scanning electron microscopic investigation. Arch. Oral Biol. 2000;45:355–361. doi: 10.1016/s0003-9969(00)00006-6. [DOI] [PubMed] [Google Scholar]
- 30.da Silveira Pereira GD, Paulillo L, De Goes MF, Dos Santos Dias CT. How wet should dentin be? Comparison of methods to remove excess water during moist bonding. J. Adhes. Dent. 2001;3:257–264. [PubMed] [Google Scholar]
- 31.Spencer P, Wang Y. Adhesive phase separation at the dentin interface under wet bonding conditions. J. Biomed. Mater. Res. 2002;62:447–456. doi: 10.1002/jbm.10364. [DOI] [PubMed] [Google Scholar]