Abstract
Objective
Cardiac catheterization shortly before coronary artery bypass grafting or valve surgery has been associated with increased postoperative acute kidney injury. The relationship between catheterization timing and acute kidney injury following proximal aortic surgery remains unknown.
Methods
Between August 2005 and February 2011, 285 consecutive patients underwent cardiac catheterization prior to elective proximal aortic surgery with cardiopulmonary bypass at a single institution. The association between timing of catheterization and postoperative acute kidney injury (defined as postoperative increase in serum creatinine ≥ 50% of baseline) was assessed using logistic regression analysis.
Results
Of 285 patients, 152 (53%) underwent catheterization on preoperative days 1-3 and 133 (47%) underwent catheterization on preoperative day 4 or before. Acute kidney injury occurred in 88 (31%) patients, three (1.1%) requiring dialysis. Acute kidney injury occurred in 37 (24%) patients catheterized on preoperative days 1-3, and 51 (38%) patients catheterized on preoperative day 4 or before. Catheterization on preoperative day 1-3 was not associated with an increased risk of acute kidney injury relative to catheterization on preoperative day 4 or before (unadjusted odds ratio 0.52, 95% confidence interval 0.31–0.86, P = 0.01; adjusted odds ratio 0.35, 95% confidence interval 0.17–0.73, P = 0.005).
Conclusions
Cardiac catheterization within one to three days of elective proximal aortic surgery appears safe and should be considered acceptable practice for patients at low-risk of acute kidney injury.
INTRODUCTION
Acute kidney injury (AKI) occurs in 18–48% of patients following thoracic aortic surgery and is associated with increased hospital costs, length of stay, and mortality (1-4). Cardiac catheterization is routinely performed prior to elective surgery of the proximal thoracic aorta to define aortic root and coronary anatomy and screen for coronary artery disease requiring concomitant intervention (5). A number of recent observational studies have suggested cardiac catheterization within zero to five days of coronary artery bypass grafting (CABG) or valve surgery leads to an increased risk of postoperative AKI, presumably from the combined insult of contrast-induced acute kidney injury (CI-AKI) and the physiologic stresses of cardiac surgery and cardiopulmonary bypass (CPB) (6-11).
Patients undergoing proximal thoracic aortic procedures represent a distinct cohort of the cardiac surgery population. A high proportion of individuals harbor aortic disease of congenital or hereditary origin, and the majority of operations require deep hypothermic circulatory arrest (DHCA) (12). Further, preoperative cardiac catheterization often includes aortic angiogram in addition to coronary and left ventricular angiography, resulting in higher average contrast volumes than patients undergoing cardiac catheterization for CABG or valve surgery. We therefore sought to assess the relationship between the timing of preoperative cardiac catheterization before elective proximal thoracic aortic surgery and the incidence of postoperative AKI in this disparate patient group theoretically at a greater risk of AKI.
METHODS
Patient population and data collection
The Duke Thoracic Aortic Surgery Database is a prospectively maintained electronic clinical registry of all patients who have undergone a thoracic aortic procedure at Duke University Medical Center (Durham, NC) since July 2005. Preoperative and intraoperative variables are recorded at the time of surgery and postoperative events are ascertained 30 days after hospital discharge. Data accuracy and adjudication of postoperative events are verified by at least two independent surgeon investigators. Long term follow-up and life status is assessed from the medical record and the Social Security Death Index.
This study was approved by the Institutional Review Board of Duke University and the need for individual patient consent was waived. A query of the Duke Thoracic Aortic Surgery Database identified 293 consecutive elective proximal thoracic aortic procedures performed between August 2005 and February 2011 at our institution via median sternotomy with the use of CPB and without the use of intraoperative contrast agents. Retrospective review of individual medical records was undertaken to obtain detailed catheterization and preadmission data. Two patients receiving preoperative dialysis and one patient who died on postoperative day zero were excluded from the study. Of the remaining 290 patients, 285 (98%) underwent preoperative cardiac catheterization and form the basis of this report.
Catheterization and preadmission protocol
Preoperative cardiac catheterization was performed at our institution in 216 (76%) patients and at an outside institution in the remainder. If not already performed prior to referral, cardiac catheterization was arranged by the cardiac surgeon and timing determined by patient convenience as well as comorbidities. Catheterization was not performed on the same day as the operation. The routine catheterization protocol at our institution includes discontinuation of nephrotoxic drugs 48 hours before the procedure, pre-hydration with intravenous normal saline, use of N-acetyl cysteine for patients with serum creatinine ≥ 1.5 mg/dl, use of low volumes of low-osmolar (iopamidol) or iso-osmolar (iodixanol) non-ionic contrast agents, and post-hydration with 1 liter of intravenous normal saline in patients not at risk for volume overload. Patients catheterized the day prior to surgery are frequently admitted for overnight hydration with 1 ml/kg/hr intravenous 0.9% saline up until the time of surgery. Irrespective of catheterization, patients with congestive heart failure (CHF) are routinely preadmitted for volume optimization with intravenous diuretics, and patients with chronic kidney disease are routinely preadmitted for intravenous hydration.
Clinical definitions
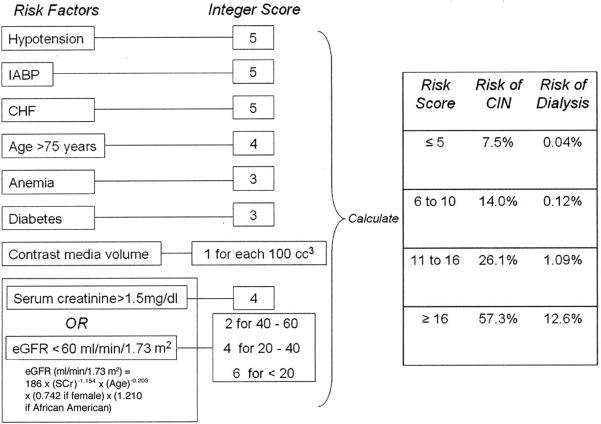
The most recent creatinine level recorded prior to surgery was defined as the baseline. Postoperative creatinine levels were measured at least once daily until hospital discharge. Postoperative AKI was defined by achieving any Acute Dialysis Quality Initiative Workgroup RIFLE (Risk, Injury, Failure, Loss, End-stage renal disease) criteria for acute renal failure (earliest “R = risk” stage: plasma creatinine increase ≥ 1.5 × baseline) based on differences between baseline and peak postoperative serum creatinine level (13). Rates of acute renal failure by Society of Thoracic Surgeons criteria (postoperative serum creatinine ≥ 2× baseline and > 2.0 mg/dl) and new onset dialysis were also recorded. Patient characteristics and comorbidities were defined using Society of Thoracic Surgeons definitions. Estimated glomerular filtration rate was calculated using the Modification of Diet in Renal Disease formula (14). The contrast-induced nephropathy (CIN) risk score was calculated as described by Mehran et al (Figure) (15).
FIGURE.
Scheme to define contrast-induced nephropathy (CIN) risk score. Anemia = baseline hematocrit value <39% for men and <36% for women; CHF = congestive heart failure class III/IV by New York Heart Association classification and/or history of pulmonary edema; eGFR = estimated glomerular filtration rate; hypotension = systolic blood pressure <80 mm Hg for at least 1 h requiring inotropic support with medications or intra-aortic balloon pump (IABP) within 24 h periprocedurally. Reprinted from “Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol 2004 Oct 6;44(7):1393-9” with permission from Elsevier.
Statistical analysis
Preoperative and intraoperative variables were compared between patients undergoing cardiac catheterization greater (long interval) or less than (short interval) 72 hours before surgery. The cutoff between short and long interval catheterization was chosen a priori to capture the post-catheterization period at highest risk of CI-AKI and the preoperative catheterization period associated with postoperative AKI in most prior studies (6-11, 16). Given that CI-AKI occurs within three days of angiography (17), for patients in the short interval catheterization group the period of CI-AKI after catheterization should overlap with surgery and potentially lead to an increased incidence of postoperative AKI. For patients in the long interval catheterization group, CI-AKI should theoretically have resolved prior to surgery and should not contribute to the incidence of postoperative AKI. However, direct assessment of CI-AKI incidence and resolution was unable to be performed given the lack of serial creatinine recordings between catheterization and surgery for most patients. Distribution of baseline characteristics was compared using the parametric chi-square test and the Kruskal-Wallis test for normally and non-normally distributed continuous variables, respectively. Binary variables were compared using the Cochran-Armitage trend test.
The association between timing of catheterization and AKI was assessed using a logistic regression model. In multivariable models, estimates were adjusted for the following risk factors for AKI following cardiac or thoracic aortic surgery: age, sex, body mass index, baseline estimated glomerular filtration rate, hypertension, diabetes mellitus, CHF, CPB duration, use of DHCA, ejection fraction, preoperative hemoglobin, and aprotinin exposure (1-3, 7, 18). Concomitant CABG was further included in the model as a surrogate for coronary artery disease and because patients undergoing CABG surgery have been shown to be at higher risk of AKI with short interval catheterization (6-9, 11). Intravenous hydration within 24 hours of operation was included to adjust for this potential renoprotective adjunct (19) that was highly associated with short interval catheterization. The volume of contrast administered during catheterization was not included given that this data was unavailable for patients catheterized at outside institutions. Underlying assumptions of the logistic regression model were tested and valid unless otherwise indicated (including important interactions: age, sex, and concomitant CABG). P values < 0.05 were considered statistically significant. Calculations were performed using SAS 9.2 (SAS Institute, Cary, NC).
RESULTS
Patient clinical and procedural characteristics are reported in Table 1 and Table 2. One hundred sixty one (56%) patients required operation for a congenital or hereditary condition (bicuspid aortic valve syndrome: 138 [48%], hereditary connective tissue disease: 22 [8%], coarctation: 1 [0.4%]) whereas 124 (44%) patients suffered from acquired or degenerative disease. In addition to proximal aortic operation, 53 (19%) patients underwent concomitant CABG, and 31 (11%) patients underwent 34 non-CABG concomitant procedures (12 arch vessel bypasses, 7 maze procedures, 6 atrial septal defect repairs, 3 mitral valve replacements, 3 tricuspid valve repairs, 1 mitral valve repair, 1 ascending-descending aortic bypass, and 1 video-assisted thoracoscopic wedge resection).
Table 1.
Clinical features
| Variable | Overall (n = 285) | Cath on preoperative day 1-3 (n = 152) | Cath on preoperative day 4+ (n = 133) | P Value |
|---|---|---|---|---|
| Patient characteristics: | ||||
| - Age (years) | 59 (47-68) | 57 (46-66) | 60 (49-69) | 0.04 |
| - Female sex | 84 (29%) | 44 (29%) | 40 (30%) | 0.84 |
| - White race | 233 (82%) | 128 (84%) | 105 (79%) | 0.54 |
| - Body mass index (kg/m2) | 27 (24-32) | 26 (24-31) | 28 (24-33) | 0.04 |
| Patient comorbidities: | ||||
| - Hypertension | 215 (75%) | 115 (76%) | 100 (75%) | 0.93 |
| - Diabetes | 21 (7%) | 9 (6%) | 12 (9%) | 0.32 |
| - Smoker | 110 (39%) | 59 (39%) | 51 (38%) | 0.94 |
| - Congestive heart failure | 26 (9%) | 8 (5%) | 18 (14%) | 0.02 |
| - Prior myocardial infarction | 15 (5%) | 5 (3%) | 10 (8%) | 0.11 |
| - Prior stroke | 19 (7%) | 10 (7%) | 9 (7%) | 0.95 |
| - Chronic obstructive pulmonary disease | 36 (13%) | 19 (13%) | 17 (13%) | 0.94 |
| - Peripheral vascular disease | 9 (3%) | 4 (3%) | 5 (4%) | 0.59 |
| - Gastroesophageal reflux disease | 68 (24%) | 36 (24%) | 32 (24%) | 0.94 |
| - Chronic alcohol use | 19 (7%) | 9 (6%) | 10 (8%) | 0.59 |
| - Prior aortic surgery | 69 (24%) | 49 (32%) | 20 (15%) | 0.001 |
| - Contrast-induced nephropathy risk score | 2 (1-5) | 2 (1-4.5) | 3 (0-6) | 0.97 |
| Medications: | ||||
| - Chronic steroid use | 4 (1%) | 2 (1%) | 2 (2%) | 0.89 |
| - Preoperative diuretic | 75 (26%) | 35 (23%) | 40 (30%) | 0.18 |
| - Preoperative ACEi/ARB | 110 (39%) | 61 (40%) | 49 (37%) | 0.57 |
| Baseline values: | ||||
| - Ejection fraction | 55 (55-55) | 55 (55-55) | 55 (55-55) | 0.11 |
| - Hemoglobin (g/dl) | 13.9 (12.9-15.0) | 13.9 (13.0-14.9) | 14.1 (12.8-15.1) | 0.70 |
| - Creatinine (mg/dl) | 1.0 (0.8-1.1) | 0.9 (0.8-1.1) | 1.0 (0.9-1.1) | 0.19 |
| > 1.5 | 12 (4%) | 5 (3%) | 7 (5%) | 0.41 |
| -eGFR (ml/min/1.73 m2) | 80 (69-94) | 80 (71-96) | 78 (68-92) | 0.11 |
| > 60 | 248 (87%) | 136 (89%) | 112 (84%) | 0.19 |
| 40 – 60 | 34 (12%) | 14 (9%) | 20 (15%) | 0.13 |
| 20 – 40 | 3 (1%) | 2 (1%) | 1 (0.8%) | 0.64 |
Values expressed as median (interquartile range) or number (percent). ACEi = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker; eGFR = estimated glomerular filtration rate.
Table 2.
Procedural characteristics
| Variable | Overall (n = 285) | Cath on preoperative day 1-3 (n = 152) | Cath on preoperative day 4+ (n = 133) | P Value |
|---|---|---|---|---|
| ASA class: | 0.44 | |||
| -2 | 5 (2%) | 1 (1%) | 4 (3%) | |
| -3 | 224 (79%) | 120 (79%) | 104 (78%) | |
| -4 | 56 (20%) | 31 (20%) | 25 (19%) | |
| Hospital preadmission: | 153 (54%) | 96 (63%) | 57 (43%) | 0.006 |
| - IV hydration w/in 24 hours of operation | 133 (47%) | 114 (75%) | 19 (14%) | < 0.001 |
| - IV diuretic w/in 24 hours of operation | 31 (11%) | 12 (8%) | 19 (14%) | 0.08 |
| Etiology: | ||||
| - Bicuspid aortic valve syndrome | 138 (48%) | 70 (46%) | 68 (51%) | 0.39 |
| - Degenerative atherosclerosis | 94 (33%) | 46 (30%) | 48 (36%) | 0.28 |
| - Hereditary connective tissue disease | 22 (8%) | 18 (12%) | 4 (3%) | 0.005 |
| - Idiopathic | 17 (6%) | 10 (7%) | 7 (5%) | 0.64 |
| - Vasculitis | 11 (4%) | 7 (5%) | 4 (3%) | 0.48 |
| - Pseudoaneurysm | 1 (0.4%) | 0 | 1 (0.8%) | 0.28 |
| - Endocarditis | 1 (0.4%) | 0 | 1 (0.8%) | 0.28 |
| - Coarctation | 1 (0.4%) | 1 (0.6%) | 0 | 0.35 |
| Primary procedure: | 0.36 | |||
| - Root or ascending aorta replacement only | 57 (20%) | 30 (20%) | 27 (20%) | |
| - Root/ascending + hemi-arch | 203 (71%) | 105 (69%) | 98 (74%) | |
| - Root/ascending + total arch | 25 (9%) | 17 (11%) | 8 (6%) | |
| - Concomitant CABG | 53 (19%) | 19 (13%) | 34 (26%) | 0.005 |
| - Other concomitant procedure | 31 (11%) | 12 (8%) | 19 (14%) | 0.08 |
| Operative Characteristics: | ||||
| - Maximum aortic diameter (cm) | 5.5 (5.1-5.9) | 5.5 (5.2-5.8) | 5.5 (5.0-6.0) | 0.76 |
| - Redo sternotomy | 67 (24%) | 45 (30%) | 22 (17%) | 0.01 |
| - Cross-clamp time (min) | 143 (117-168) | 140 (109-169) | 144 (121-168) | 0.28 |
| - Cardiopulmonary bypass time (min) | 209 (183-240) | 209 (183-235) | 209 (184-244) | 0.43 |
| - Deep hypothermic circulatory arrest | 229 (80%) | 123 (81%) | 106 (80%) | 0.80 |
| - Cerebral DHCA time (min) | 17 (14-22) | 17 (14-22) | 17 (15-22) | 0.37 |
| - Systemic DHCA time (min) | 17 (14-23) | 17 (14-23) | 17 (15-22) | 0.69 |
| - Aprotinin use | 63 (22%) | 31 (20%) | 32 (24%) | 0.46 |
Values expressed as median (interquartile range) or number (percent). ASA = American Society of Anesthesiologists; CABG = coronary artery bypass grafting; DHCA = deep hypothermic circulatory arrest; IV = intravenous.
Of the 285 patients included in the analysis, 152 (53%) underwent catheterization on preoperative day 1-3 (127 [45%] on preoperative day 1, 7 [2%] on preoperative day 2, and 18 [6%] on preoperative day 3) and 133 (47%) underwent catheterization on preoperative day 4 or before (median preoperative day: 30, interquartile range [IQR]: 15-54). Data on the volume of contrast administered during catheterization was available for 152 of 152 (100%) short interval catheterization patients and 63 of 133 (47%) long interval catheterization patients. The median overall contrast dose was 1.55 ml/kg (IQR: 1.09-2.13 ml/kg). Short interval catheterization patients received lower contrast volumes than long interval catheterization patients (1.47 ml/kg [IQR: 1.06-2.03] ml/kg vs. 1.81 ml/kg [IQR: 1.22-2.38 ml/kg], P = 0.01).
Unadjusted measures of AKI and mortality are reported in Table 3. AKI by any RIFLE criteria occurred in 88 (31%) individuals. AKI was an early postoperative event and occurred on postoperative days 0-3 in 79 (90%) patients, on postoperative days 4-7 in eight (9%) patients, and after postoperative day seven in one (1%) patient. New onset dialysis occurred in three (1.1%) patients who were catheterized on preoperative day one, six, and 14, respectively. Thirty-day / in-hospital mortality occurred in five (1.8%) patients, of whom three (60%) experienced AKI.
Table 3.
Unadjusted measures of acute kidney injury and mortality
| Criteria | Overall (n = 285) | Cath on preoperative day 1-3 (n = 152) | Cath on preoperative day 4+ (n = 133) | P Value |
|---|---|---|---|---|
| AKI (all RIFLE stages) | 88 (31%) | 37 (24%) | 51 (38%) | 0.01 |
| STS acute renal failure | 18 (6.3%) | 6 (3.9%) | 12 (9.0%) | 0.09 |
| New onset dialysis | 3 (1.1%) | 1 (0.7%) | 2 (1.5%) | 0.60 |
| Mortality (30-day / in-hospital) | 5 (1.8%) | 3 (2.0%) | 2 (1.5%) | 1 |
AKI = acute kidney injury; STS = Society of Thoracic Surgeons; RIFLE = Risk, Injury, Failure, Loss, End-stage renal disease.
The incidence of AKI was significantly lower in patients undergoing catheterization on preoperative days 1-3 compared to patients catheterized on preoperative day 4 of before (24% vs. 38%). After adjusting for known risk factors for AKI and other pertinent imbalances between the patient groups, short interval catheterization was associated with a lower risk of postoperative AKI when compared to long interval catheterization (odds ratio [OR] 0.35, 95% confidence interval [CI] 0.17–0.73, P = 0.005, Table 4). A subgroup analysis was performed by dividing patients into two risk groups based on median CIN risk score. No association between catheterization timing and AKI was identified for low-risk patients (CIN risk score 0-2, OR 0.44, 95% CI 0.15-1.28, P = 0.10). However, short interval catheterization remained associated with a lower risk of postoperative AKI in the higher-risk subgroup (CIN risk score ≥ 3, OR 0.31, 95% CI 0.10-0.93, P = 0.04).
Table 4.
Association between day of catheterization and acute kidney injury
| Cath on preoperative day 1-3 vs. preoperative day 4+ | OR | 95% CI | P Value |
|---|---|---|---|
| AKI (all RIFLE stages) | |||
| - Crude | 0.52 | 0.31-0.86 | 0.01 |
| - Adjusted | 0.35 | 0.17-0.73 | 0.005 |
| Subgroup analysis (Adjusted) | |||
| - CIN risk score ≤ 2 (n=153) | 0.44 | 0.15-1.28 | 0.10 |
| - CIN risk score > 3 (n=132) | 0.31 | 0.10-0.93 | 0.04 |
AKI = acute kidney injury; CI = confidence interval; CIN = contrast-induced nephropathy; RIFLE = Risk, Injury, Failure, Loss, End-stage renal disease; OR = odds ratio.
DISCUSSION
This observational single-institution study of a large contemporary cohort of patients undergoing complex elective proximal thoracic aortic operations found cardiac catheterization within one to three days of surgery was not associated with an increased risk of postoperative AKI.
Although the incidence of postoperative AKI (31%) in our study appears higher than in the comparable studies of CABG or valve surgery (6-11), it is similar to the AKI rates reported in prior studies of complex thoracic aortic operations (1-4). However, the incidence of dialysis (1.1%) in our study remained low, consistent with the expected rates of dialysis in low-risk patients undergoing elective operation. Although AKI is an established risk factor for mortality following cardiac and thoracic aortic operations (1-4, 11), our study is underpowered to re-demonstrate this association in multivariable models given that only five (1.8%) postoperative deaths occurred. Nonetheless, it is assumed from prior studies that postoperative AKI leads to worse patient outcomes, and aggressive efforts should be taken to minimize AKI in the perioperative period.
There were many differences between the short and long interval catheterization groups that we attempted to adjust for in multivariable models. Risk factors for AKI with unequal distribution included age (57 vs. 60 years), body mass index (26 vs. 28 kg/m2) and CHF (5% vs. 14%). A higher proportion of patients with a history of prior aortic surgery (32% vs. 15%), hereditary connective tissue disease (11% vs. 3%), and undergoing redo sternotomy (30% vs. 17%) underwent short interval catheterization, possibly as a result of younger patient age secondary to congenital or hereditary aortic pathology. These differences were likely partially controlled for by adjusting for age and CPB time. A higher proportion of patients undergoing concomitant CABG (26% vs. 13%) underwent long interval catheterization, possibly as a result of comorbidities or due to referral for aortic surgery only after the identification of thoracic aortic pathology upon catheterization for coronary artery disease. As a result of our catheterization protocols, a greater proportion of patients undergoing short interval catheterization were preadmitted to the hospital prior to surgery (63% vs. 43%) and received intravenous hydration within 24 hours of operation (75% vs. 14%). In contrast, a greater proportion of patients undergoing long interval catheterization received intravenous diuretics prior to surgery (14% vs. 8%) given that there were more patients with CHF in this group (14% vs. 5%). Concomitant CABG, preoperative hydration, and CHF were all included in the multivariable models. Lastly, short interval catheterization patients received lower volumes of contrast during catheterization (1.47 ml/kg vs. 1.81 ml/kg), likely as a result of our institutional protocols aimed at minimizing contrast exposure for patients undergoing catheterization shortly before surgery. We were unable to adjust for the effect of contrast volume on postoperative AKI given that this data was missing for 53% of long interval catheterization patients. However, exposure to greater contrast volumes in the long interval group would not be predicted to increase the rate of AKI given that kidney recovery presumably occurred in the interval between catheterization and surgery.
Six retrospective studies assessing the relationship between the timing of preoperative cardiac catheterization and the incidence of AKI following CABG or valve surgery reported an increased risk of AKI with short interval catheterization. In three studies, this risk was restricted to patients undergoing catheterization within 24 hours of surgery (7, 8, 10). The remaining studies reported that catheterization within five days of surgery (6, 11) or during the same admission as surgery (9) were associated with increased post-surgical AKI. As a result, the various study authors have proposed surgery should be delayed for as long as seven days following catheterization, or until after hospital discharge and readmission.
The explanation for our disparate study findings is likely related to the unique features of proximal thoracic aortic surgery patients, the inclusion of a purely elective patient population, and the absence of patients undergoing same-day catheterization. Many proximal thoracic aortic surgery patients suffer from aortopathy of congenital or hereditary disease and therefore present for surgery at a younger age and harbor few comorbidities. The median age of patients in our study was nearly a decade younger than patients in the comparable studies of CABG and valve surgery (59 years vs. 65-68 years), and the median CIN risk score was two, placing them at the lowest risk of developing CI-AKI after catheterization (15). Although 31% of patients developed AKI after surgery, only 1.1% progressed to kidney failure requiring dialysis, suggesting when AKI did occur in this low-risk patient cohort it was typically mild and transient.
Our study was further restricted to patients undergoing elective surgery, and all emergent and urgent procedures were excluded. In all but one prior study (11) patients undergoing urgent procedures were included in the analysis and were more likely to undergo short interval catheterization (6-10). Hence, the association between AKI and short interval catheterization was likely partially confounded by unstable patients at higher risk of AKI undergoing expedited surgery shortly after catheterization.
Same-day cardiac catheterization and surgery is not offered at our institution for patients undergoing elective proximal thoracic aortic procedures. Ranucci and colleagues found that the risk of AKI with short interval catheterization was restricted only to those undergoing surgery on the same-day as catheterization (7). In all other studies, same-day catheterization patients were not assessed independently and were grouped together with patients catheterized on preoperative day one or before (6, 8-11). Hence, it is possible that the increased risk of AKI associated with short interval catheterization is due entirely to same-day catheterization, and no such patients were included in our study. Conversely, two reports from the Mayo Clinic found amongst carefully selected patients at low-risk of AKI, cardiac catheterization on the same-day as elective valve surgery was not associated with postoperative AKI (20, 21).
The largest study to examine the relationship between catheterization timing and postoperative AKI was recently published by Mehta and colleagues from our institution and found amongst 2441 patients undergoing elective, isolated CABG that cardiac catheterization on preoperative days zero to five was associated with increased postoperative AKI (11). Numerous features of the Mehta study are consistent with our study findings. First, the CABG patients included in the Mehta study were older (median age 66) and sicker (33% with CHF, 33% with diabetes) than the proximal aortic patients included in our study and are therefore at greater risk of CI-CKI after catheterization (15). Second, the risk of AKI with short interval catheterization reported by Mehta was quite modest and bordered on non-significance, likely due to the exclusion of all urgent and emergent procedures (OR 1.11, 95% CI 1.02-1.20, P = 0.02, when day of catheterization was treated as a continuous variable from ≤ one to ≥ five days). Third, same-day catheterizations were included in the Mehta study but were not assessed as an independent group. However, when day of catheterization was treated as a categorical variable the increased risk of AKI with short interval catheterization was again restricted only to patients undergoing catheterization on preoperative days zero to one (OR 1.74, 95% CI 1.13-3.68; reference = catheterization on preoperative day five or before). Hence, the overriding conclusion from this and other studies may be to avoid short interval catheterization for CABG and other high-risk patients, but that short interval, or even same-day, catheterization may be well tolerated by patients at low-risk of CI-AKI who are undergoing elective surgery.
Paradoxically, our study reports a lower risk of postoperative AKI for patients undergoing short interval catheterization, as opposed to equivalence between the short and long interval catheterization groups. Upon subgroup analysis, the “benefit” of short interval catheterization was lost in low-risk patients, but persisted in higher-risk patients. We can only conclude that this is due to greater preoperative kidney protection and medical optimization in patients undergoing short interval catheterization, and that these benefits are more pronounced in higher-risk patients and outweigh the insult of CI-AKI. Although we adjusted for preoperative hydration in the multivariable models, unmeasured confounders related to hospital preadmission, such as medical optimization, blood pressure and glycemic control, and careful review and discontinuation of nephrotoxic medications, likely account for the reduced risk of AKI in short interval catheterization patients (22). However, as this remains a speculative hypothesis that is untested in our study, we refrain from recommending short interval catheterization for high-risk patients and instead suggest that long interval catheterization followed by hospital preadmission for aggressive preoperative renal protection may lead to optimal results.
Clinical Implications
Given the small number of centers that specialize in thoracic aortic procedures, a patient requiring a thoracic aortic intervention is likely to be referred away from their community hospital to a regional center of excellence. Short interval catheterization allows these patients to undergo catheterization and surgery within a single extended episode of care. The benefits of this practice include reduced costs of stay and resource consumption for patients who are kept in the hospital between catheterization and surgery, and reduced travel and lodging expenses and increased convenience for patients who would otherwise be discharged home between catheterization and surgery. Nonetheless, for patients at high-risk of CI-AKI, such as those with baseline renal dysfunction, diabetes, congestive heart failure, and age greater than 75, long interval catheterization remains prudent pending further study. In all patients, aggressive CI-AKI prophylaxis and perioperative kidney protection should be employed and may negate the harmful effects of short interval catheterization and reduce postoperative AKI.
Study limitations
Our data is observational and therefore inherently limited by a selection bias, as patients selected for long interval catheterization were older and harbored more risk factors for AKI. Although logistic regression was used to adjust for these differences, unmeasured confounders related to patient selection and perioperative renal protection likely account for the higher rate of AKI observed in the long interval catheterization group, as it is unlikely that short interval catheterization itself is renoprotective. We are also unable to adjust for the volume of contrast administered during catheterization, as this data was missing from 53% of the long interval catheterization patients who were not catheterized at our institution. Intraoperative and postoperative risk factors for AKI, such as hypotension on bypass and vasopressor use (10), were also not recorded given that these variables are unable to influence the decision regarding timing of catheterization. Our data is further limited by the constraints of any observational retrospective study and results should be viewed as hypothesis generating and inference regarding causation made with caution.
Conclusions
Despite the use of high contrast volumes during catheterization and the complexity of the subsequent operations, cardiac angiography with aggressive CI-AKI prophylaxis can be performed safely within one to three days of elective proximal thoracic aortic surgery and should be considered acceptable practice for patients at low-risk of AKI.
ULTRA-MINI ABSTRACT.
In this large single institution series of elective proximal aortic operations, cardiac catheterization on preoperative day 1-3 was not associated with an increased risk of postoperative acute kidney injury relative to catheterization on preoperative day 4 or before. This suggests catheterization shortly before elective proximal aortic surgery is an acceptable practice.
Acknowledgments
Funding source: Thoracic Surgery Foundation for Research and Education (Beverly, MA) Research Fellowship (to NDA), National Institutes of Health (Bethesda, MD) grants T32-HL069749 (to JBW), U01-HL088953 (to JBW) and T32-CA093245 (to SDB).
Footnotes
Disclosures: None
REFERENCES
- 1.Augoustides JG, Pochettino A, Ochroch EA, Cowie D, Weiner J, Gambone AJ, et al. Renal dysfunction after thoracic aortic surgery requiring deep hypothermic circulatory arrest: definition, incidence, and clinical predictors. J Cardiothorac Vasc Anesth. 2006 Oct;20(5):673–7. doi: 10.1053/j.jvca.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 2.Arnaoutakis GJ, Bihorac A, Martin TD, Hess PJ, Jr., Klodell CT, Ejaz AA, et al. RIFLE criteria for acute kidney injury in aortic arch surgery. J Thorac Cardiovasc Surg. 2007 Dec;134(6):1554–60. doi: 10.1016/j.jtcvs.2007.08.039. discussion 60-1. [DOI] [PubMed] [Google Scholar]
- 3.Englberger L, Suri RM, Greason KL, Burkhart HM, Sundt TM, 3rd, Daly RC, et al. Deep hypothermic circulatory arrest is not a risk factor for acute kidney injury in thoracic aortic surgery. J Thorac Cardiovasc Surg. 2011 Feb;141(2):552–8. doi: 10.1016/j.jtcvs.2010.02.045. [DOI] [PubMed] [Google Scholar]
- 4.Augoustides JG, Pochettino A, McGarvey ML, Cowie D, Weiner J, Gambone AJ, et al. Clinical predictors for mortality in adults undergoing thoracic aortic surgery requiring deep hypothermic circulatory arrest. Ann Card Anaesth. 2006 Jul;9(2):114–9. [PubMed] [Google Scholar]
- 5.Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE, Jr., et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010 Apr 6;121(13):e266–369. doi: 10.1161/CIR.0b013e3181d4739e. [DOI] [PubMed] [Google Scholar]
- 6.Del Duca D, Iqbal S, Rahme E, Goldberg P, de Varennes B. Renal failure after cardiac surgery: timing of cardiac catheterization and other perioperative risk factors. Ann Thorac Surg. 2007 Oct;84(4):1264–71. doi: 10.1016/j.athoracsur.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 7.Ranucci M, Ballotta A, Kunkl A, De Benedetti D, Kandil H, Conti D, et al. Influence of the timing of cardiac catheterization and the amount of contrast media on acute renal failure after cardiac surgery. Am J Cardiol. 2008 Apr 15;101(8):1112–8. doi: 10.1016/j.amjcard.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Medalion B, Cohen H, Assali A, Vaknin Assa H, Farkash A, Snir E, et al. The effect of cardiac angiography timing, contrast media dose, and preoperative renal function on acute renal failure after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2010 Jun;139(6):1539–44. doi: 10.1016/j.jtcvs.2009.08.042. [DOI] [PubMed] [Google Scholar]
- 9.Kramer RS, Quinn RD, Groom RC, Braxton JH, Malenka DJ, Kellett MA, et al. Same admission cardiac catheterization and cardiac surgery: is there an increased incidence of acute kidney injury? Ann Thorac Surg. 2010 Nov;90(5):1418–23. doi: 10.1016/j.athoracsur.2010.04.029. discussion 23-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hennessy SA, LaPar DJ, Stukenborg GJ, Stone ML, Mlynarek RA, Kern JA, et al. Cardiac catheterization within 24 hours of valve surgery is significantly associated with acute renal failure. J Thorac Cardiovasc Surg. 2010 Nov;140(5):1011–7. doi: 10.1016/j.jtcvs.2010.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta RH, Honeycutt E, Patel UD, Lopes RD, Williams JB, Shaw LK, et al. Relationship of the time interval between cardiac catheterization and elective coronary artery bypass surgery with postprocedural acute kidney injury. Circulation. 2011 Sep 13;124(11 Suppl):S149–55. doi: 10.1161/CIRCULATIONAHA.110.011700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elefteriades JA. Natural history of thoracic aortic aneurysms: indications for surgery, and surgical versus nonsurgical risks. Ann Thorac Surg. 2002 Nov;74(5):S1877–80. doi: 10.1016/s0003-4975(02)04147-4. discussion S92-8. [DOI] [PubMed] [Google Scholar]
- 13.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004 Aug;8(4):R204–12. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med. 2006 Jun 8;354(23):2473–83. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 15.Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004 Oct 6;44(7):1393–9. doi: 10.1016/j.jacc.2004.06.068. [DOI] [PubMed] [Google Scholar]
- 16.Solomon R, Dauerman HL. Contrast-induced acute kidney injury. Circulation. 2010 Dec 7;122(23):2451–5. doi: 10.1161/CIRCULATIONAHA.110.953851. [DOI] [PubMed] [Google Scholar]
- 17.McCullough PA, Stacul F, Becker CR, Adam A, Lameire N, Tumlin JA, et al. Contrast-Induced Nephropathy (CIN) Consensus Working Panel: executive summary. Rev Cardiovasc Med. 2006;7(4):177–97. Fall. [PubMed] [Google Scholar]
- 18.Mori Y, Sato N, Kobayashi Y, Ochiai R. Acute kidney injury during aortic arch surgery under deep hypothermic circulatory arrest. J Anesth. 2011 Aug 17; doi: 10.1007/s00540-011-1210-8. [DOI] [PubMed] [Google Scholar]
- 19.Marathias KP, Vassili M, Robola A, Alivizatos PA, Palatianos GM, Geroulanos S, et al. Preoperative intravenous hydration confers renoprotection in patients with chronic kidney disease undergoing cardiac surgery. Artif Organs. 2006 Aug;30(8):615–21. doi: 10.1111/j.1525-1594.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- 20.Brown ML, Holmes DR, Tajik AJ, Sarano ME, Schaff HV. Safety of same-day coronary angiography in patients undergoing elective valvular heart surgery. Mayo Clin Proc. 2007 May;82(5):572–4. doi: 10.4065/82.5.572. [DOI] [PubMed] [Google Scholar]
- 21.Greason KL, Englberger L, Suri RM, Park SJ, Rihal CS, Pislaru SV, et al. Safety of same-day coronary angiography in patients undergoing elective aortic valve replacement. Ann Thorac Surg. 2011 Jun;91(6):1791–6. doi: 10.1016/j.athoracsur.2011.02.064. [DOI] [PubMed] [Google Scholar]
- 22.Brown JR, Thompson CA. Contrast-induced acute kidney injury: the at-risk patient and protective measures. Curr Cardiol Rep. 2010 Sep;12(5):440–5. doi: 10.1007/s11886-010-0129-2. [DOI] [PubMed] [Google Scholar]