Abstract
Introduction
Under normal conditions, hepatocyte growth factor (HGF)-induced activation of its cell surface receptor, the Met tyrosine kinase (TK), is tightly regulated by paracrine ligand delivery, ligand activation at the target cell surface, and ligand activated receptor internalization and degradation. Despite these controls, HGF/Met signaling contributes to oncogenesis and tumor progression in several cancers and promotes aggressive cellular invasiveness that is strongly linked to tumor metastasis.
Area covered
The prevalence of HGF/Met pathway activation in human malignancies has driven rapid growth in cancer drug development programs. The authors review Met structure and function, the basic properties of HGF/Met pathway antagonists now in preclinical and clinical development, as well as the latest clinical trial results.
Expert opinion
Clinical trials with HGF/Met pathway antagonists show that as a class these agents are well tolerated. Although widespread efficacy was not seen in several completed phase 2 studies, promising results have been reported in lung, gastric, prostate and papillary renal cancer patients treated with these agents. The main challenges facing the effective use of HGF/Met-targeted antagonists for cancer treatment are optimal patient selection, diagnostic and pharmacodynamic biomarker development, and the identification and testing of optimal therapy combinations. The wealth of basic information, analytical reagents and model systems available concerning HGF/Met oncogenic signaling will continue to be invaluable in meeting these challenges and moving expeditiously toward more effective disease control.
Keywords: Hepatocyte growth factor and Met
1. Introduction
The MET oncogene was first isolated from a human osteosarcoma-derived cell line on the basis of its transforming activity in vitro, caused by a DNA rearrangement where sequences from the TPR (translocated promoter region) locus on chromosome 1 were fused to MET sequence on chromosome 7 (TPR-MET) 1. A similar gene rearrangement was later found in patients with gastric carcinoma 1. Isolation of the full-length MET proto-oncogene sequence revealed that it encoded a receptor tyrosine kinase (TK) 1 known as Met (or cellular-Met, c-Met). Hepatocyte growth factor (HGF, also known as scatter factor, SF) was discovered independently of Met 2 and is secreted primarily by mesenchymal cells 1, 3, especially fibroblasts and smooth muscle cells 4, 5 and signals through Met in a paracrine manner 6, 7, 8. These and other early studies established that a single receptor transduced multiple biological activities including motility, proliferation, survival and morphogenesis 9–12.
The HGF and Met proteins are processed proteolytically from single chain precursors into mature disulfide linked heterodimers, both genes are widely expressed during development, and deletion of either gene lethally disrupts embryogenesis 9, 10, 12. MET and HGF expression persist throughout adulthood and upregulation of HGF after kidney, liver or heart injury suggests that pathway activation protects against tissue damage and promotes repair and regeneration 13–17.
2. Met: Structure and Function
The MET gene is located on chromosome 7 band 7q21–q31 and spans more than 120 kb in length, consisting of 21 exons separated by 20 introns 18. The primary MET transcript produces a 150 kDa polypeptide 19 that is partially glycosylated to produce a 170 kDa single chain precursor protein. This 170 kDa precursor is further glycosylated to a mass of approximately 190 kDa and then cleaved into a 50 kDa beta chain and 140 kDa alpha chain which are linked via disulfide bonds 20.
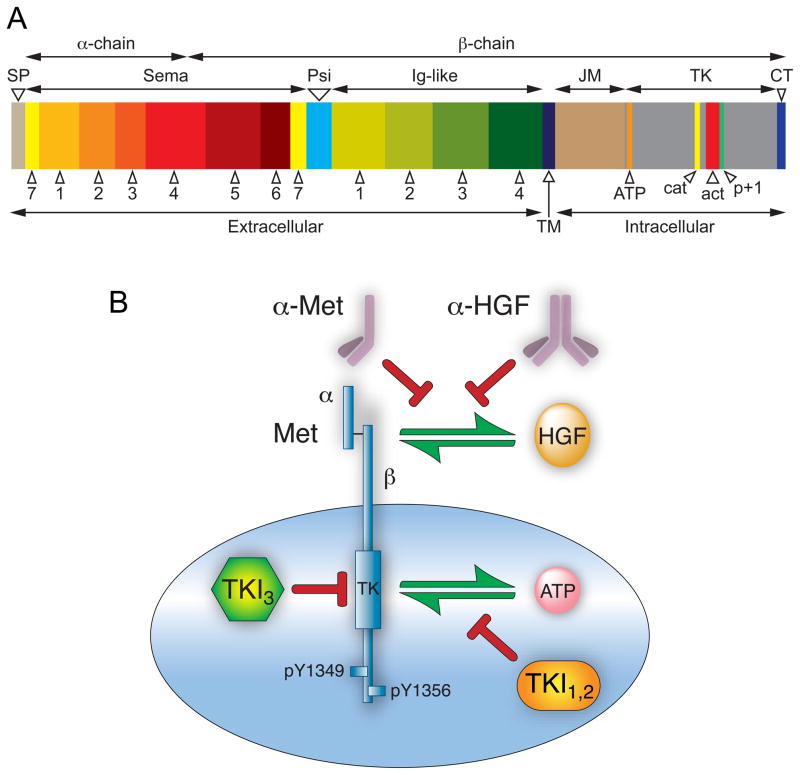
The Met beta chain has seven conserved subdomains which have functional significance and homology with other cell signaling proteins. The amino-terminal semaphorin (or Sema) domain has a 7-bladed beta-propeller fold 21, 22 that serves as a key element for ligand binding, and is also found in the plexin family of semaphorin receptors 23, 24. The presence of the semaphorin domain, as well as the more highly conserved tyrosine kinase domain, places Met in a subfamily of tyrosine kinases that includes Ron and the avian Ron ortholog, Sea 19. Carboxyl-terminal to the Sema domain is the PSI domain, so named because it is found in plexins, semaphorins and integrins 20. Further downstream are four immunoglobulin domains, also referred to as IPT repeats, because they are found in immunoglobulins, plexins and transcription factors 20. The PSI domain is thought to function as a linking module to orient the extracellular fragment of Met for proper ligand binding 25. Although several reports claim that the sema domain is the sole HGF binding domain in Met 21, one report claims that IPT repeats 3 and 4, located closest to the transmembrane domain, also mediate high affinity HGF binding 26 (Figure 1A).
Figure 1. Met domain structure and routes to antagonize the HGF/Met pathway.
A. Schematic of Met domain structure; domain lengths are proportional to number of constituent amino acid residues. Mature Met is a disulfide-linked two chain heterodimer with an extracellular amino terminal α-chain (45 kDa) and a carboxyl terminal β-chain (145 kDa) containing extracellular, transmembrane and intracellular domains. The signal peptide (SP) is not present in mature protein. The extracellular domain contains a sema homology region (Sema) organized in 7 blades; a cysteine-rich region (Psi); and four immunoglobulin-like repeats (Ig-like). The intracellular domain contains juxtamembrane (JM), tyrosine kinase (TK) and carboxyl terminal (CT) domains. Within the TK domain are the ATP binding site (orange), catalytic loop (cat, yellow), activation loop (act, red) and p+1 loop (p+1, green). B. At least three routes of pathway intervention have been followed as selective Met anticancer drug development strategies: 1) fully human monoclonal antibodies that neutralize HGF by binding to one of its two Met binding sites; 2) monovalent (one-armed), monoclonal antibodies designed to bind to Met and inhibit HGF binding; and 3) tyrosine kinase inhibitors classified as type I, type II (TKI1,2) and type III (TKI3) as described in text.
Like all receptor tyrosine kinases, the Met transmembrane domain is thought to consist of a single alpha helix 6. The most amino terminal cytoplasmic subdomain, the juxtamembrane (JM) region, contains two protein phosphorylation sites: S985 and Y1003 (numbered per Swissprot P08581). Phosphorylation of S985 negatively regulates kinase activity and phosphorylation of Y1003 recruits c-Cbl, which monoubiquinates Met and interacts with endophilin, targeting Met for internalization and degradation. A PEST sequence, which may serve as a site for this ubiquitination, is present in the JM domain 27. A specific protein tyrosine phosphatase (PTP-S) is also reported to bind to this region28.
Carboxyl terminal to the JM region is the tyrosine kinase (TK) domain, which shares homology with insulin growth factor I receptors and the Tyro 3 family of immunoregulatory molecules, and lastly, a carboxy-terminal tail region. Upon HGF binding, Met autophosphorylation occurs on tyrosine residues Y1234 and Y1235 within the activation loop of the TK domain, inducing kinase activity, while phosphorylation on Y1349 and Y1356 in the carboxyl terminal region (all numbered per Swissprot P08581) forms a multifunctional docking site for intracellular adaptors that recruit a collection of intracellular signal effectors containing Src homology-2 (SH2) domains and other specific receptor recognition motifs that act as adapters in transmitting signals further downstream 29. An intact multifunctional docking site is required to mediate transformation and induce a metastatic phenotype 30.
Among the adaptor proteins and direct kinase substrates thus far implicated in Met signaling are Grb2, Gab1, phosphatidylinositol 3-kinase (PI3K), phospholipase C-γ (PLCγ), Shc, Src, Shp2, Ship1, and STAT3 9, 11. Gab 1 and Grb2 are considered critical effectors and are among those that interact directly with the receptor; through these, a larger network of adaptor proteins are involved in signaling, presumably contributing to the pleiotropic biological effects elicited by HGF stimulation. In particular, the direct binding of Grb2 directly to the Met docking site through Y1356 links the receptor to the Ras/MAPK pathway regulating cell cycle progression 11. Gab1 is recruited to Met through direct binding and indirectly via Grb2; these interactions initiate branching morphogenesis in several epithelial and vascular endothelial cell types 31. Gab1 is also highly phosphorylated by the Met kinase, resulting in the additional recruitment of PI3K (which is also recruited to Met directly through its p85 subunit), contributing in turn to cell cycle progression, protection from apoptosis, as well as increased cell motility 10.
3. MET Mutations Associated with Cancer
Under normal conditions, HGF-induced Met TK activation is tightly regulated by paracrine ligand delivery, ligand activation at the target cell surface, and ligand activated receptor internalization and degradation. Despite these multiple controls, pathway deregulation occurs in a variety of neoplasms. Among the hundreds of genes upregulated by HGF are those encoding proteases required for HGF and Met processing, as well as MET, creating the potential for its overexpression through persistent ligand stimulation 32. Indeed, MET overexpression is characteristic of several epithelial cell derived cancers and for some is an independent prognostic factor associated with adverse outcome 33. Other mechanisms of oncogenic pathway activation include aberrant paracrine or autocrine ligand production, constitutive kinase activation in the presence or absence of MET gene amplification, and MET gene mutation 12, 34, 35. Missense MET mutations occur in several cancers; the earliest reported mutations were found exclusively in the Met TK domain and were associated with hereditary and sporadic forms of papillary renal cell carcinoma (PRC) 36, 37. Mutations throughout the MET coding sequence were later found in lung cancer and in head and neck cancers 6, 38.
The impact of specific MET mutations has been studied at the molecular, cellular and organism levels. Early cell-based investigations indicated that kinase activity was deregulated in various mutant forms and revealed that these could have distinct biological effects. For example, the PRC-associated mutations D1228H/N and M1250T showed enhanced kinase activity, Ras pathway activation and focus formation, while L1195V and Y1230C more effectively activated PI3K, promoting cell survival, soft agar colony formation and matrix invasion 39, 40. Although mutations that were reconstituted in HGF-producing cells (such as NIH3T3) could not rigorously address the role of ligand binding in oncogenesis, later studies showed that mutations expressed in epithelial cells required added ligand for soft agar colony formation and that colony formation by NIH3T3 bearing Met M1250T could be blocked by ligand binding antagonists 41. PRC-associated MET mutations also have been investigated in mice by engineering changes in the murine MET locus 42. Interestingly, mice harboring D1226N, Y1228C, and both M1248T and L1193V mutations developed sarcomas with high frequency and some lymphomas, whereas the M1248T mice developed carcinomas and lymphomas; no mice developed PRC 42. Furthermore, analogous to the trisomy of chromosome 7 frequently observed in human PRC tumors, trisomy of chromosome 6 (containing the murine MET locus) and preferential duplication of the mutant MET allele was observed in most tumors. Very recently, investigations of two PRC associated Met mutants revealed defect in Met internalization that results in persistent signaling, similar to alterations found in the Met juxtamembrane domain 43. These results independently confirm the oncogenicity of PRC-associated MET mutations in vivo and suggest that distinct mutations influence the types of cancers that develop in mice 42.
Other alterations in the MET coding sequence have been identified in regions encoding the extracellular semaphorin domain (E168D, L229F, S323G, and N375S) and the intracellular JM domain (R988C, T1010I, S1058P, and exon 14 deletions) of non-small cell lung carcinoma (NSCLC)-derived cell lines, in 12.5 % of small lung cell cancer (SCLC) cases, as well as in 8% of samples of lung adenocarcinoma tissues 38, 44–46. Some of these mutations activate proliferation, motility and invasiveness in cultured cells38. As noted earlier, Y1003 is phosphorylated in response to HGF binding and recruits c-Cbl, leading to Met ubiquitination and degradation 1. In Met JM domain mutants missing exon 14, the loss of Y1003 results in Met accumulation at the cell surface and persistent HGF-stimulated signaling that leads, in turn, to increased transforming activity and tumorigenic potential 1.
The capacity for JM mutations R988C and T1010I to contribute to oncogenesis has been a topic of debate. First identified by Schmidt and colleagues 47, T1010I was thought to represent a rare polymorphism, owing to lack of disease segregation and failure to induce focus formation or constitutive Met phosphorylation in NIH3T3 cells. Although this potential polymorphism did not stimulate NIH3T3 cell growth in soft agar, it was more active than the wild-type Met in the athymic nude mice tumorigenesis assay, suggesting that it may have effects on tumorigenesis 48 and lead to altered cytoskeletal functions 45. Recently Tyner and colleagues found these variants in a wide variety of malignancies as well as individuals without cancer, suggesting that R988C and T1010I are indeed rare polymorphisms that may predispose an individual toward cancer when combined with an oncogene that drives cellular proliferation 49.
Of interest, another novel germ-line missense mutation P1001S (numbered per Swissprot P08581) in exon 14 that encodes the JM domain of Met has been detected in a patient with gastric carcinoma. P1009S caused colony formation in soft agar, was tumorigenic in athymic nude mice, but appears to be oncogenic by a different mechanism: while Met mutations in the tyrosine kinase domain are constitutively activated, the P1009S Met mutant, after HGF/SF treatment, stays phosphorylated for a significantly longer time (24–48 h) than wild-type Met, but it is not constitutively activated. This suggests that the downregulation of Met, which occurs after receptor activation and tyrosine phosphorylation, may be impaired by this mutation 48. Overall, MET mutation occurs at a lower frequency than most other mechanisms of pathway activation in tumors; nonetheless, mutations provide strong direct evidence of the pathway’s oncogenic potential and may identify patients most likely to benefit from Met-targeted therapeutics.
Consistent with the role of this pathway in organogenesis, oncogenic Met signaling resembles developmental transitions between epithelial and mesenchymal cell types normally regulated by HGF: increased protease production coupled with cell dissociation and motility promotes cellular invasion through extracellular matrices, enabling tumor invasiveness and metastasis. Conversely, silencing the endogenous, overexpressed MET gene in tumor cells suppresses tumor growth and metastasis, and induces the regression of established metastases in mouse models 50. In addition, HGF/Met signaling in vascular endothelial cells stimulates tumor angiogenesis, facilitating tumor growth for cancers that are growth-limited by hypoxia, and independently promoting tumor metastasis. Hypoxia alone upregulates MET expression and enhances HGF signaling in cultured cells and mouse tumor models 51.
4. Pharmacological Inhibitors of the HGF/Met Pathway
The prevalence of HGF/Met pathway activation in human malignancies has driven rapid growth in drug development programs. Agents currently under development as HGF/Met pathway inhibitors can be broadly subdivided into biologicals and low molecular weight synthetic compounds. Biologicals, or protein-based agents, act through a variety of mechanisms and possess target selectivity and pharmacokinetic properties that are predictable and often desirable. Nonetheless, their size typically restricts their action to extracellular events and their complexity impacts drug manufacture, routes of administration and shelf life. Thus it is not surprising that synthetic, low molecular weight TK inhibitors (TKIs) presently outnumber every other class of HGF/Met therapeutic (Figure 1B and Table 1).
Table 1. HGF/Met pathway inhibitors in human clinical trials.
Status of clinical trials uses terms as defined at ClinicalTrials.gov
| Drug | Design | Phase | Status | Patient population | Combinations | ClinicalTrials.gov Identifier |
|---|---|---|---|---|---|---|
| Rilotumumab (AMG 102) | Safety | Ib/II | Active | NSCLC1 | Cisplatin/Etoposide | NCT00791154 |
| Safety/Efficacy | I/II | Active | mCRC2, wt KRAS | Panitumumab | NCT00788957 | |
| Safety/Efficacy | I/II | Active | CRPC3 | Mitoxantrone/Prednisone | NCT00770848 | |
| Safety/Efficacy | II | Active | RCC4 | NCT00422019 | ||
| Safety/Efficacy | II | Active | Advanced Malignant Glioma | NCT00427440 | ||
| Safety/Efficacy | I/II | Active | Locally Advanced/Metastatic EGJ Adenocarcinoma5 | ECXx | NCT00719550 | |
| Safety | I/II | Recruiting | NSCLC | Erlotinib | NCT01233687 | |
| Efficacy/Safety | II | Recruiting | Recurrent Malignant Glioma | Avastin | NCT01113398 | |
| Efficacy | II | Recruiting | Advanced Gastroesophageal Adenocarcinoma | FOLFOXxx | NCT01443065 | |
| Safety | II | Recruiting | Persistent/Recurrent Ovarian Epithelial Cancer, Fallopian Tube Cancer, Primary Peritoneal Cancer | NCT01039207 | ||
| Onartuzumab (MetMAb) | Safety | I | Completed | Advanced Solid Malignancies | Avastin | NCT01068977 |
| Efficacy | II | Active | NSCLC | Erlotinib | NCT00854308 | |
| Efficacy | II | Recruiting | Metastatic/Recurrent, triple-negative Breast Cancer | Bevacizumab and Paclitaxel | NCT01186991 | |
| Efficacy | II | Recruiting | mCRC | FOLFOX/Bevacizumab | NCT01418222 | |
| Safety/Efficacy | III | Not yet recruiting | NSCLC (Met diagnostic-positive) | Erlotinib | NCT01456325 | |
| Ficlatuzumab (AV-299) | Safety/Efficacy | I | Terminated | Glioblastoma | NCT01189513 | |
| Safety/Tolerability | I | Active | Advanced/Metastatic Solid Malignancies | NCT00969410 | ||
| Safety/Efficacy | I/II | Active | NSCLC | Gefitinib | NCT01039948 | |
| Safety | I | Active | Relapsed/Refractory Solid Malignancies | Erlotinib | NCT00725634 | |
|
TAK-701 AMG 337 AMG 208 Tivatinib (ARQ 197) |
Safety/Tolerability/PK | I | Active | Non-Hematologic Malignancies | NCT00831896 | |
| Safety | I | Recruiting | Advanced Solid Malignancies | NCT01253707 | ||
| Safety | I | Recruiting | Advanced Solid Malignancies | NCT00813384 | ||
| Safety/Efficacy | I | Completed | Advanced Solid Malignancies | NCT00612209 | ||
| Bio-availability | I | Completed | Advanced Solid Malignancies | NCT01149720 | ||
| Safety/Efficacy | II | Active | GC6 | NCT01152645 | ||
| Safety/Efficacy | I/II | Recruiting | CRC, wt KRAS | Irinotecan/Cetuximab | NCT01075048 | |
| Safety/Efficacy | II | Recruiting | Advanced/Metastatic NSCLC, KRAS mutation + | Erlotinib | NCT01395758 | |
| Safety | I | Completed | Advanced Solid Malignancies | Gemcitabine | NCT00874042 | |
| Safety/Efficacy | II | Active | HCC7 | NCT00988741 | ||
| Safety | I | Recruiting | Advanced Solid Malignancies | Sorafenib | NCT00827177 | |
| PK# | I | Completed | Healthy volunter | NCT00651638 | ||
| Safety | I | Active | NSCLC | Erlotinib | NCT01251796 | |
| Safety | I | Active | Advanced/Recurrent NSCLC | Erlotinib | NCT01069757 | |
| Safety/Efficacy | III | Recruiting | Non Squamous NSCLC | Erlotinib | NCT01244191 | |
| Safety/Tolerability | I | Completed | Metastatic Solid Malignancies | NCT00302172 | ||
| Efficacy | II | Completed | Unresectable Locally Advanced/Metastatic Pancreatic Adenocarcinoma | Gemcitabine | NCT00558207 | |
| Safety/Efficacy | I | Completed | Advanced Solid Malignancies | Erlotinib | NCT00612703 | |
| Efficacy | II | Withdrawn | Locally Advanced/Metastatic GC | Oxaliplatin/Capecitabine/Irinotecan | NCT01070290 | |
| Safety | I | Recruiting | Advanced Solid Malignancies | Pazopanib | NCT01468922 | |
| Safety | I | Active | HCC (Cirrhotic patients) | Erlotinib | NCT00802555 | |
| Efficacy | II | Completed | Advanced/Metastatic NSCLC | Erlotinib | NCT00777309 | |
| PK | I | Completed | Healthy Volunter | NCT00658554 | ||
| Safety/Tolerability | I | Active | Advanced Solid/Recurrent Malignancies | NCT00609921 | ||
| Efficacy | III | Recruiting | Advanced/Metastatic Non Squamous NSCLC wt EGFR | Erlotinib | NCT01377376 | |
| Safety/Efficacy | II | Active | Refractory Germ Cell Tumors | NCT01055067 | ||
| Safety/Efficacy | I/II | Recruiting | Subjects previously enrolled in other ARQ 197 study | NCT01178411 | ||
| Safety/Efficacy | II | Completed | MiT8 associated with RCC9/ASPS10/CCS11 | NCT00557609 | ||
| Safety/Efficacy | II | Not yet recruiting | Relapsed Multiple Myeloma | NCT01447914 | ||
| BMS777607 | Safety/Efficacy | I/II | Completed | Advanced/Metastatic Solid SCCHN12, PRCC13 Malignancies, CRPC, | NCT00605618 | |
| E7050 | Efficacy | I/II | Recruiting | Advanced Solid Malignancies, Glioblastoma, Unresectable Stage III/IV Melanoma | E7080 | NCT01433991 |
|
EMD 1214063 EMD1204831 Foretinib (GSK1363089) |
Safety | I | Recruiting | Advanced Solid Malignancies | NCT01014936 | |
| Safety | I | Recruiting | Advanced Solid Malignancies | NCT01110083 | ||
| Safety/Efficacy | II | Completed | Recurrent/Metastatic SCCHN | NCT00725764 | ||
| Safety | I | Completed | Advanced Solid Malignancies | NCT00743067 | ||
| Safety/Efficacy | II | Completed | mGC | NCT00725712 | ||
| Bio-availability | I | Completed | Advanced Solid Malignancies | NCT00742261 | ||
| Safety/Efficacy | I | Active | HCC | NCT00920192 | ||
| Safety/Efficacy | II | Active | PRCC | NCT00726323 | ||
| Safety/Efficacy | I/II | Recruiting | Locally Advanced/Metastatic NSCLC | Erlotinib | NCT01068587 | |
| Safety/Efficacy | I/II | Recruiting | HER2 Over-Expressing Metastatic Breast Cancer | Lapatinib | NCT01138384 | |
| Safety/Efficacy | II | Recruiting | ER+/PR+and HER2-Brest Cancer 14 | NCT01147484 | ||
|
INCB28060 LY2801653 MK8033 |
Safety/Tolerability | I | Recruiting | Advanced Solid Malignancies | NCT01072266 | |
| Safety/Tolerability | I | Recruiting | Advanced and/or Metastatic Cancer | NCT01285037 | ||
| Safety | I | Completed | Advanced Solid Malignancies | Omeprazole | NCT00559182 | |
| Safety/Efficacy | I/II | Not yet recruiting | Locally Advanced/Metastatic Solid Malignancies, GC | Ciplastin/Capecitabine | NCT01355302 | |
| Safety/Efficacy | I | Completed | Advanced Solid Malignancies | NCT00921869 | ||
| Safety/Efficacy | I/II | Recruiting | Platinum Resistant, Recurrent/Metastatic SCCHN | Cetuximab | NCT01332266 | |
| Safety/Efficacy | I/II | Recruiting | Locally Advanced/Metastatic HCC | Sorafenib | NCT01271504 | |
| Safety/Efficacy | I | Not yet recruiting | Advanced Solid Malignancies | NCT01428141 | ||
| Safety | I | Recruiting | Advanced Solid Malignancies | NCT00869895 | ||
| Amuvatinib (MP470) | Safety | I | Completed | Advanced Solid Malignancies | NCT00894894 | |
| Safety/Efficacy | I | Completed | Advanced Solid Malignancies | Topotecan/Docetaxel/Erlotinib/Paclitaxel/Carboplatin/Carboplatin/Etoposide | NCT00881166 | |
| Efficacy | II | Recruiting | SCLC | Platinum/Etoposide | NCT01357395 | |
| Safety/PK/PD* | NA | Terminated | Metastatic Solid Malignancies/Lymphoma | NCT00504205 | ||
| MGCD265 | Safety/Efficacy | I/II | Recruiting | Advanced Malignancies/NSCLC | Erlotinib/Docetaxel | NCT00975767 |
| Safety | I | Recruiting | Advanced Solid Malignancies | NCT00697632 | ||
| Safety | I | Completed | Advanced Solid Malignancies | NCT00679133 | ||
| MK-2461 | Safety/Efficacy | I/II | Completed | Advanced Solid Malignancies | NCT00496353 | |
| Safety | I | Completed | Advanced Solid Malignancies | NCT00518739 | ||
|
Crizotinib PF02341066 |
PK | I | Not yet recruiting | Subjects With Impaired Renal Function | NCT01419041 | |
| Safety/PK/PD | I | Not yet recruiting | Advanced Solid Malignancies | Axitinib/Sunitinib/Bevacizumab/Sorafenib | NCT01441388 | |
| Safety/Efficacy | I | Recruiting | ALK+ Tumors15 | NCT01121588 | ||
| PK | I | Completed | Japanese Healthy Male Volunteers | NCT01250730 | ||
| PK | I | Completed | Healthy Volunter | NCT01297595 | ||
| Safety/Efficacy | I/II | Recruiting | NSCLC | Erlotinib | NCT00965731 | |
| PK | I | Completed | Healthy Volunter | NCT01168934 | ||
| Safety/Efficacy | I | Recruiting | NSCLC | PF 00299804 | NCT01121575 | |
| PK^ | I | Completed | Healthy Male Volunter | NCT01082380 | ||
| PK | I | Completed | Healthy Volunter | Ketoconazole | NCT01149785 | |
| PK | I | Completed | Healthy Volunter | Rifampin | NCT01147055 | |
| PK | I | Completed | Healthy Volunter | NCT01154218 | ||
| Safety | I | Recruiting | Advanced Solid Malignancies | NCT00585195 | ||
| Safety/Efficacy | III | Recruiting | NSCLC, ALK+ | Pemetrexed/Docetaxel | NCT00932893 | |
| Safety/PK | I/II | Recruiting | Relapsed/Refractory Solid Malignancies or Anaplastic Large Cell Lymphoma (Young Population) | NCT00939770 | ||
| Safety/Efficacy | II | Recruiting | NSCLC, ALK+ | NCT00932451 | ||
| PF02341066 | Bio availability | I | Completed | Healthy Volunter | NCT00939731 | |
| Taste Assessment | I | Completed | Healthy Volunter | NCT01125904 | ||
| Safety | I | Recruiting | NSCLC | PF 00299804 | NCT01441128 | |
| Obtain tissue | Recruiting | NSCLC, ALK+ | NCT01300429 | |||
| Safety/Efficacy | III | Recruiting | Recurrent/Metastatic Non Squamous Cell Carcinoma of the Lung ALK + | NCT01154140 | ||
| PF04217903 | Safety/Efficacy | I | Completed | Advanced Solid Malignancies | NCT00706355 | |
| Cabozantinib (XL184) | Safety/Efficacy | II | Not yet recruiting | mCRPC | NCT01428219 | |
| Safety/Efficacy | II | Not yet recruiting | Carcinoid/Pancreatic Neuroendocrine Tumor | NCT01466036 | ||
| Efficacy | II | Recruiting | Brest Cancer, ER+/PR+and HER2- | NCT01441947 | ||
| Safety/Efficacy | I | Recruiting | mCRPC | NCT01347788 | ||
| Safety | I | Active | Advanced Solid Malignancies | NCT00215605 | ||
| Safety/Efficacy | II | Recruiting | Advanced Solid Malignancies | NCT00940225 | ||
| Efficacy | III | Active | MTC16 | NCT00704730 | ||
| Safety/Efficacy | II | Active | Glioblastoma Multiforme | NCT00704288 | ||
| Safety/Efficacy | I/II | Active | NSCLC | Erlotinib | NCT00596648 | |
| PK | I | Active | Advanced Solid Malignancies | Rosiglitazone | NCT01100619 | |
| Safety | I | Active | Glioblastoma | Temozolomide/Radiation Therapy | NCT00960492 | |
| Safety/Efficacy | II | Active | Grade IV AstrocyticTumor | NCT01068782 | ||
| Safety | I | Completed | Advanced/Metastatic Solid Malignancies (Japanese | NCT01018745 |
PK: Pharmacokinetics
PD: Pharmacodymanics
[14C]PF 02341066
NSCLC: Non Small Cell Lung Cancer
CRC: Colorectal Cancer
CRPC: Castrate Resistant Prostate Cancer
RCC: Renal Cell Carcinoma
EGJ: Gastric or Esophagogastric Junction adenocarcinoma
GC: Gastic Cancer
HCC: Hepatocellular Carcinoma
MiT: Microphthalmia transcription factor associated Tumors
RCC: Renal Cell Carcinoma
ASPS: Alveolar Soft Part Sarcoma
CCS: Clear Cell Sarcoma
PRCC: Papillary Renal-Cell Carcinoma
SCCHN: Squamous Cell Carcinoma for the Head and Neck 14
ER+/PR+and HER2- Brest Cancer: Estrogen-receptor- positive/Progesterone-recepto r-positive and Human Epidermal Growth Factor Receptor 2 negative Breast Cancer
ALK: Anaplastic Lymphoma Kinase
MTC: Metastatic Medullary Thyroid Cancer
ECX: Capecitabine/Epirubicin/Cisplatin
FOLFOX: Oxaliplatin/Folinic Acid/5-fluoro-uracil
4.1. Biological HGF/Met Pathway Antagonists
Biologicals are primarily directed against ligand-receptor binding or related cell-surface events such as receptor clustering, and include: [1] Competitive molecular analogs of HGF; [2] Decoy Met; and [3] mAbs directed against either HGF or Met.
4.1.1. Competitive Molecular Analogs of HGF
Among the competitive HGF analogs NK2, NK4 and uncleavable HGF, anti-tumor efficacy has been demonstrated in preclinical models for the latter two. NK2 is a naturally occurring truncated HGF protein produced by alternative mRNA processing. The potential anti-oncogenic efficacy of NK2 is compromised by its intrinsic motogenic activity, which enhanced HGF-driven metastasis in mouse models 52–56. NK4 is an engineered HGF fragment that has proven to be a complete competitive antagonist of HGF/Met oncogenic signaling in a variety of preclinical models. Adenovirus-mediated stable induction of NK4 has been shown to significantly inhibit in vivo tumor growth and lung metastasis of Lewis lung carcinoma (LLC) and B16F10 melanoma 57 as well as tumor growth in a xenograft model of malignant pleural mesothelioma, without obvious side effects 58. NK4 gene therapy is thus a safe and promising strategy for the treatment of cancer patients, and further validation in clinical trials is needed. Engineered antagonistic HGF forms that resist proteolytic activation or its conformational consequences exploit the requirement for proteolytic cleavage that converts pro-HGF to a biologically active heterodimer 59–62.
4.1.2. Met Decoy Receptor
Truncated soluble forms of the Met ectodomain, e.g. soluble Met Sema domain constructs that sequester HGF and interfere with Met homodimerization, have been shown to suppress HGF-induced tumor cell migration 63, as well as tumor growth and metastasis in mice 64.
4.1.3. Anti-HGF/Met Monoclonal Antibodies
Among HGF/Met-targeted biologicals, the most advanced drug candidates are mAbs directed against either HGF or Met. The majority of these block HGF-Met binding, although for one anti-Met mAb, preclinical studies show decreased Met activation by induction of ectodomain shedding and receptor degradation 65. Neutralizing mAbs against human HGF, such as TAK701, L2G7, AMG102 (rilotumumab) and SCH900105 (also known as AV299) each potently suppressed the growth of tumor xenografts in mice 66–71.
TAK-701, currently in phase I clinical trials in adult patients with advanced nonhematological malignancies, is a humanized anti-HGF neutralizing antibody. In vitro, TAK-701 binds preferably to the mature two-chain form of HGF (Kd of 13 pM), blocking HGF-Met binding (IC50 of 310 pM). In addition, TAK-701 inhibited the intra cellular phosphorylation of Met (IC50 of 0.45 μg/mL) 72 and shows antitumoral activities against various cancer cells with an autocrine dependence on HGF in vivo 73. The first clinical results for TAK-701 from the phase I dose escalation study, showed that TAK-701 was well tolerated up to 20 mg/kg bi-weekly 74. The most common adverse events (AEs) were fatigue, constipation and cough; the most serious treatment-related AEs included ileus, muscular weakness, asthenia, urinary tract infection and dehydration. Pharmacodynamic (PD) analysis showed that in five of seven patients free HGF was not detected while being treated with TAK-701 75 and preliminary analysis of all dose groups indicate dose proportional pharmacokinetics (PK).
Rilotumumab (AMG 102) is a fully human monoclonal antibody that binds to the HGF light chain (Kd of 0.22 nM), also preferably in the context of mature two chain HGF, and blocks HGF-Met binding (IC50 of 2.1 nM). Rilotumumab is under evaluation as monotherapy in phase Ib-II trials respectively in the context of ovarian and renal cancer, as well as in combination with, avastin in glioma, erlotinib in NSCLC, and platinum based chemotherapy in SCLC, mesothelioma, gastric cancer as well as mitoxantrone in prostate cancer. A recent phase Ib trial evaluated AMG102 safety, PK, PD and antitumor activity in combination with bevacizumab at a dose of 3, 10 or 20 mg/kg every two weeks (12 patients) or motesanib (in 2 patients, cohort suspended) in advanced solid tumors. Treatment related AEs among patients receiving rilotumumab plus bevacizumab were generally mild and included fatigue (75%), nausea (58%), constipation (42%), and peripheral edema (42%). PFS ranged from 8 to 122 weeks 76. Rilotumumab monotherapy was not associated with significant antitumor activity in patients with recurrent glioblastoma (GBM) who had previously received bevacizumab compared with bevacizumab-naive patients. Rilotumumab PFS was similar among patients (about 4 weeks) at two different dosages of 10 or 20 mg/kg every two weeks or among patients who had previously received bevacizumab compared with bevacizumab-naive patients. The most common AEs consisted of fatigue (38%), headache (33%), and peripheral edema (23%) 77. A phase II study of the safety and efficacy of rilotumumab in combinaton with Epirubicin, Cisplatin and Capecitabine (ECX) as a first line treatment for unresectable locally advanced or metastatic gastric esophagogastric junction adenocarcinoma in a group of 121 patients. Addition of rilotumumab at a dose of 7.5 mg/kg to ECX appeared to improve PFS, the primary endpoint, especially in patients with high Met expression. Although OS was not a primary endpoint, patients with high Met expression in the rilotumumab treatment arms (n = 27) had a median OS of 11.1 months (hazard ratio = 0.29) vs 5.7 months for patients in the ECX + placebo treatment group (n = 11). The rilotumumab + ECX combination arms showed a modestly higher incidence of AEs including peripheral edema, hematologic toxicities and tromboembolic events 78. A phase II, randomized, double-blinded, placebo-controlled trial compared rilotumumab or ganitumab (AMG 479) with panitumumab (pmab) versus pmab alone in patients with wild-type (WT) K-RAS metastatic colorectal cancer (mCRC). While ganitumab failed to generate any signal in this randomized trial, the arm with rilotumumab did show an increased response rate. Seventy nine percent of patients reported AEs as rash, dermatitis, and hypomagnesemia and a PFS of 5.2 months. Results of biomarker analyses, including Met staining, are ongoing 79. This finding will hopefully lead to further trials investigating the use of HGF/Met inhibitors in colorectal cancer. This is the first study to show promising efficacy of an HGF inhibitor when combined with pmab in pretreated metastatic colorectal cancer patients.
SCH900105 is a humanized anti-HGF IgG1 monoclonal antibody that is now in phase I and II clinical trials. In its first completed trial, SCH900105 treatment was associated with stable disease (SD) in half of the patients, the longest for 34 weeks 68, 69,71. Safety and tolerability studies of SCH900105 in combination with erlotinib or gefitinib are ongoing. SCH900105 dosing (20 mg/kg) increased serum HGF and HGF-drug complex, suggesting target engagement, as seen in rilotumumab trials. Early results suggest clinical activity with the combination of SCH900105 and gefitinib in Asian patients with unresectable NSCLC 80.
h224G11 is a humanized, bivalent anti-Met monoclonal antibody that inhibits Met phosphorylation and dimerization and blocks proliferation, migration, invasion, morphogenesis and angiogenesis in cell-based studies 69, 81, 82.
Onartuzumab (MetMab, formerly OA5D5), is an engineered anti-Met monovalent antibody that blocks ligand binding (IC50 of 2.6 to 8.7 nM in intact cells) and inhibits tumor growth in animal models by more than 95% 83. A phase II study evaluating onartuzumab or placebo in combination with erlotinib in advanced NSCLC showed improved outcome in Met positive, previously treated, advanced stage NSCLC. The study showed that patients whose tumors had high levels of Met, as determined by immunohistochemistry (IHC) staining for Met protein, lived twice as long without their disease getting worse when they received onartuzumab plus erlotinib compared to erlotinib alone. The addition of onartuzumab plus erlotinib in these patients significantly improved PFS and OS, resulting in a near 3-fold reduction in the risk of death 84. A phase III trial restricted to Met positive patients will be activated soon 85. Onartuzumab is also now under clinical evaluation in randomized double-blinded phase II trials in combination with paclitaxel and bevacizumab in triple negative breast cancer or in combination with Folinic acid Oxaliplatin and Fluorouracil (FOLFOX) and bevacizumab in colorectal carcinoma 86.
In addition to its development as a therapeutic, preclinical studies indicate that onartuzumab tagged radioisotopically displays several promising features as an immuno-PET reagent to non-invasively image Met in vivo for diagnostic and prognostic endpoints. Imaging and biodistribution studies showed rapid uptake and delayed clearance of the tracer specifically in tumor xenografts. Radiolabeled onartuzumab biodistribution also appeared to be independent of soluble human Met in the plasma of animals bearing human tumor xenografts, suggesting that the normal level of plasma Met in humans may not interfere with tumor-imaging sensitivity 87. DN30, a murine anti-Met monoclonal antibody that inhibits tumor growth in animal models, also showed promise as an immuno-PET reagent in preclinical studies; a humanized version of DN30 is under development for clinical evaluation 88.
4.2. Small Synthetic Met Kinase Inhibitors
Most Met TKIs competitively antagonize occupancy of the intracellular ATP binding site, preventing phosphorylation, TK activation and downstream signaling. These inhibitors are known as type I or type II inhibitors depending on whether they bind to the kinase active conformation or an inactive conformation. The majority of inhibitors, Type 1, target the ATP binding site in its active conformation, after tyrosyl residues in the activation loop have been phosphorylated. Type II inhibitors include those that use an additional binding site immediately adjacent to the region occupied by ATP, which is made accessible by an activation loop arrangement that is characteristic of inactive kinases 89. These agents are discussed here starting with preclinical candidates and ending with those now entering phase III clinical trials.
Early studies of Met-targeted TKIs, such as SU11274 (IC50 of 20 nM)45, 90 and PHA665752 (IC50 of 9 nM) 34, established that this class of compounds could potently suppress oncogenesis and provided a platform for improving potency, selectivity and other drug properties. Recently SU11274 was also successfully used as preclinical molecular imaging probe; tumor uptake of [11C] SU11274 correlated with Met content as measured by IHC in xenograft tumor models, suggesting that [11C] SU11274 PET may have utility for non-invasive quantification of Met expression in vivo 91, 92
RP1040 (IC50 of 1.3 nM) 93 and CEP-A (IC50 of 13 nM) 69 are recent preclinical candidates; RP1040 shows good oral availability and displays a half-life of up to 9 h in intact cells 93. CEP-A shows sustainable PD effects in mouse studies, resulting in significant tumor growth inhibition, SD and partial regression 69.
S 49076 is a novel, ATP-competitive tyrosine kinase inhibitor of Met, FGFR1/2/3 and AXL. S 49076 blocks autophosphorylation of these RTKs and their downstream signaling in cells with IC50 values of between 1 and 200 nM depending on the target and cell line. In vivo, oral administration of S 49076 inhibits > 80% tumor growth at 6 mg/kg/day in GTL-16 subcutaneous human gastric carcinoma tumors and in the Met-dependent U87-MG human glioblastoma xenograph model. S 49076 also inhibits FGFR2 autophosphorylation, downstream signaling and tumor growth in FGFR2-dependent SNU-16 gastric tumors, and tumor growth in LS-174T colon carcinoma. Based on these preclinical studies, a phase I study is planned soon to evaluate an oral formulation of S 49076 in patients with advanced solid tumors 94, 95.
Met TKIs now entering phase I clinical trials to establish safety and tolerability include, AMG 337, AMG 208, INCB28060, LY2801653, EMD1214063 and EMD1204831.
AMG 337 is a selective inhibitor of Met, which inhibits multiple mechanisms of Met activation while AMG 208 selectively inhibits both ligand-dependent and ligand-independent Met activation. Phase I results for INCB28060 show that toxicity was manageable and a favorable PK profile supports exploration of a twice-daily dosing schedule. Dose-dependent decreases in Met phosphorylation, monitored by ELISA, are also promising. AEs included mild tremor, fatigue, nausea, diarrhea, indigestion, headache, and agitation 96.
LY2801653 demonstrated dose dependent inhibition of Met phosphorylation in xenograft tumors by IHC, with a long lasting PD effect. LY2801653 displayed potent anti-tumor efficacy in NSCLC, pancreatic, and breast tumor models. Enhanced anti-tumor efficacy was achieved when erlotinib was combined with LY2801653 in NSCLC resistant to EGFR inhibitors 97.
EMD1214063 (IC50 of 1 to 6nM) and EMD1204831 (IC50 of 15nM) are highly selective Met inhibitors when tested in vitro against a large panel of different kinases. In vivo, oral administration of EMD1214063 and EMD1204831 result in a potent inhibition of HGF dependent and independent Met driven tumor xenografts. PK/PD analysis reveal efficient, dose and time dependent inhibition of Met phosphorylation and reduction of tumor derived IL-8 98, 99. Dose-escalation studies designed to explore the safety, tolerability, PK and clinical activity of EMD1214063 and EMD1204831 are now recruiting patients with advanced solid tumors.
JNJ-38877605, a highly Met-selective TKI as determined by comprehensive kinase profiling in vitro, entered phase I in 2008 but the study was terminated early due to increase in serum creatinine levels suggestive of renal toxicity and minimal PD activity. Phase I clinical trials for SGX523, another highly selective Met TKI with structural similarity to JNJ-38877605, were also discontinued after renal toxicity was observed in patients receiving relatively low doses 100.
PF-042793 is a highly selective Met inhibitor with high potency against wild type Met (Ki = 6–7 nM), but was found to be less effective against clinically relevant MET mutations evaluated in cultured cells. Results are awaited for a phase I study testing safety, dose and blood concentration of PF-042793.
A phase I study of MK8033 (IC50 of 1.3 nM), which targets Met and the Met family member Ron, is completed but results are not yet available. Future plans include a trial of this agent in refractory colorectal cancer, with pre- and post-treatment biopsies to evaluate for relevant molecular signatures 101.
Several Met TKIs are in phase I/II clinical trials that further test safety and efficacy. Golvatinib (E7050) targets both Met (IC50 of 6–37nM) and VEGFR2 102. A phase I clinical study will determine the maximum tolerated dose (MTD) of golvatinib given orally in patients with advanced solid tumors. Three phase Ib/II studies will determine the benefit observed in subjects receiving golvatinib in combination with either sorafenib (hepatocellular carcinoma, HCC), E7080 (recurrent glioblastoma or unresectable stage III/IV melanoma) or cetuximab (platinum-resistant squamous cell carcinoma of the head and neck). Phase Ib/II studies will be conducted in two parts: a phase Ib part comprising a dose escalation and an expansion cohort; and a phase II part, which will comprise two cohorts. Approximately 95 patients will be enrolled in each study (10–15 patients in the phase Ib portion and 80 patients in the phase II portion). Patients who experience evidence of clinical benefit may continue golvatinib in combination with the other drug for as long as clinical benefit is sustained and the treatment is well tolerated.
Amuvatinib (MP470) an oral multi-targeted tyrosine kinase inhibitor which inhibits the mutant forms of c-Kit, PDGFR alpha and Met, sensitized glioblastoma cells to radiotherapy in mice 103. When combined with erlotinib, amuvatinib inhibits prostate cancer cell proliferation and tumor xenograft growth. Amuvatinib also disrupts DNA repair, likely through suppression of the homologous recombination protein Rad51 104. Tumor responses in SCLC were observed in a phase Ib clinical study in combination with VP-16 and carboplatin. PK data suggests that co-administration of amuvatinib did not alter exposures of standard of care (SOC) agents VP-16 or carboplatin as measured by overall exposure 105. A phase II clinical trial is recruiting SCLC subjects who have not responded to standard treatment or relapsed after standard treatment. The outcome will measure overall objective response rate (CR or PR), PFS and OS, duration of response, safety and tolerability and metabolites PK and other biomarkers 106.
A phase I/II study of BMS777607 (IC50 of 3.9 nM) in metastatic cancer patients has been completed but results are not yet available 107. BMS-777607 inhibited both ligand stimulated and constitutive Met phosphorylation in preclinical studies with an IC50 of 20 nmol/L and impaired xenograft growth 107. A recent study also found BMS-777607 to be capable of inhibiting Met signaling and suppressing HGF-stimulated prostate cancer cell scattering, migration, invasion, and proliferation 108.
MGCD265, targeting Met, VEGFR1-3, Ron and Tie2, is currently in phase I/II studies in combination with erlotinib or SOC treatments; safety trials have shown a half-life of 20–30 hours with no grade 2 or higher AEs 109. In vivo anti-tumor activity of MGCD265 has been reported in combination with either docetaxel, paclitaxel, or erlotinib in multiple xenograft models including NSCLC models that express an EGFR mutant resistant to erlotinib (T790M). The combination of MGCD265 with either a taxane or erlotinib resulted in improved antitumor activities when compared to treatments with either agent alone. In addition, these combinations were well tolerated in xenograft models and do not show drug-drug interactions, supporting the clinical development of MGCD265 in combination with docetaxel or erlotinib in patients with NSCLC 110.
MK-2461 is a Type 1 inhibitor with similar potency (2.5 – 10 nM) for Met, Flt1 and Ron 111. Nine other kinases, including FGFR1, FGFR2, FGFR3, PDGFRβ, KDR, Flt3, Flt4, TrkA, and TrkB, were found to be 8- to 30-fold less sensitive to MK-2461 than Met 111. MK-2461 has completed phase I/II trials and showed a half-life of approximately 6 hours, few AEs above grade 1 (anorexia, fatigue and nausea), and a best response of SD for six treatment cycles 112.
Foretinib (GSK1363089; formerly XL880) has shown promising results in phase II trials. Foretinib targets Met VEGFR2, Ron and Tie-2. Phase I trials showed a half-life of 60 hours and generally good drug tolerance with the most common AEs of grade 1 or 2 (fatigue, hypertension, nausea, anorexia and vomiting) 113. Foretinib did not show efficacy in a phase II study for advanced or metastatic gastric cancer 114. In a phase II trial of foretinib in PRC, several patients showed SD for at least 10 months; several patients with germline MET mutations experienced PRs by RECIST criteria while others showed 10–30% percent reduction in tumor size 115, 116. Recent preclinical results indicate that foretinib and human EGFR (HER) targeted agents synergistically suppressed the growth of tumor cells with Met and HER1/2 amplification or overexpression 117. A phase I/II study of foretinib in combination with lapatinib in patients with HER 2 over-expressing metastatic breast cancer is now recruiting patients.
Tivantinib (ARQ197) may represent a distinct type of low molecular weight TKI (neither Type I or II; tentatively identified as Type III), as it apparently binds to a region of Met outside of the ATP binding site and impairs kinase activation allosterically. Based on kinase profiling studies in vitro, it is reported to be highly selective for Met (IC50 of 50 nM in vitro), but it binds only to the inactive form 118. It is further noted that in vivo a relatively high dose of tivantinib inhibited tumor growth in the MDA-MB-231 human breast cancer cell-derived mouse tumor model 119, whereas another potent Met inhibitor showed little antitumor activity in this model 120. Although MDA-MB-231 cells express Met 121, it is unclear if the growth of the implanted cells in mice is primarily driven by Met activity. Therefore, it is possible that antitumor activity observed using relatively high doses of tivantinib might be due to unidentified off-target effects and attributing tivantinib clinical activity solely to Met inhibition should be done cautiously. Phase I/II clinical trials demonstrated promise in terms of both tolerability and tumor response. A phase II study of tivantinib enrolled 167 patients with advanced NSCLC of any histology who were previously treated with a single line of chemotherapy; patients were randomized to receive either erlotinib alone or as erlotinib combined with tivantinib. The trial did require availability of tumor tissue for analysis of EGFR, K-RAS, and MET mutations status. PFS improvement was particularly prominent among patients with nonsquamous histology, EGFR wild-type status, and K-RAS mutations treated with tivantinib plus erlotinib; no significant differences in side effects were observed (rash, diarrhea, and some fatigue) between the groups 122. In January 2011, the first patient was enrolled in the phase III trial of tivantinib in combination with erlotinib for patients diagnosed NSCLC who have received one or two prior systemic anti-cancer therapies. The phase III trial is a randomized, double-blinded, controlled study of previously treated patients with locally advanced or metastatic, non-squamous NSCLC who will receive tivantinib plus erlotinib or placebo plus erlotinib. The primary objective is to evaluate OS in the intent-to-treat population (ITT). Secondary endpoints include OS in the subpopulation of EGFR wild type patients, PFS in the ITT population, and further assessment of the safety of tivantinib in combination with erlotinib 123.
Met TKIs furthest in development include Cabozantinib (XL184) and Crizotinib (PF-02341066), both now in phase III clinical trials. Cabozantinib (XL184) is a multikinase inhibitor that potently targets Met, VEGFR2, and Ret and has a half-life of 80–90 hours 124. On average, patients show SD greater than 3 months with several up to 6 months while on treatment 125. In January 2011, cabozantinib was granted orphan drug status by the U.S. FDA for treatment of follicular, medullary, and anaplastic thyroid carcinoma, and metastatic or locally advanced papillary thyroid cancer. Cabozantinib is being evaluated in phase II in solid tumors including metastatic castration-resistant prostate cancer (mCRPC), ovarian cancer 126, melanoma 127, breast cancer, NSCLC 128 and hepatocellular cancer 129, and in a phase I trial in renal cell carcinoma and differentiated thyroid cancer. Remarkable interim data from the phase II study on mCRPC were presented in an oral abstract session in 2011 ASCO Annual Meeting 130, 131. Cabozantinib, at a dose of 40 mg daily, reduced measurable soft-tissue lesions, showed resolution (complete or partial) or stabilization of bone metastases, decreased bone pain, and reduced narcotic use 132–134. Because of the relatively broad target spectrum of this TKI, it is unclear whether these results are attributable to Met inhibition.
Crizotinib (PF-02341066), is a dual Met and Anaplastic Lymphoma Kinase (ALK) inhibitor. In August 2011, the U.S. FDA approved crizotinib for lung cancer patients with ALK gene rearrangments 135. Currently, clinical development of crizotinib is focused primarily on ALK rearrangements in NSCLC. However a recent report describes rapid and durable clinical response to crizotinib in a NSCLC patient with de novo MET amplification and no ALK rearrangement, suggesting that de novo MET amplification may be a primary oncogenic driver in some NSCLC and thus a valid clinical target 136.
5. Conclusion
Preclinical evidence supports the involvement of dysregulated Met signaling in a variety of human cancers. As anti-cancer therapeutics, HGF/Met pathway inhibitors can be divided broadly into biologicals and low molecular weight synthetic TK inhibitors; of these, the latter now outnumber all other inhibitor types. The clinical assessment of these agents is complicated by several factors, ranging from specific biological and pharmacological properties of the investigational agent to the molecular and genetic characteristics of the patients studied in clinical trials. However, substantial progress has been made, and additional trials with adequate control arms in carefully selected patient populations are required to further confirm clinical benefits and assess the potential long-term benefit and the safety of agents that have shown early promising results.
6. Expert Opinion
Clinical trials with HGF/Met pathway antagonists show that as a class these agents are well tolerated. Although widespread efficacy was not seen in several completed phase 2 studies, promising results have been reported in lung, gastric, prostate and PRC patients treated with these agents. An important challenge facing the effective development of targeted agents such as Met pathway inhibitors is identifying those patients most likely to achieve maximal benefit and minimal toxicity from treatment. Success will be dependent on the accurate and timely assessment of the molecular and genetic background in the patients, which include various molecular changes (e.g., MET overexpression, amplification and mutation, and HGF elevation) leading to Met pathway activation and genetic alterations resulting in the activation of other cancer-promoting pathways. Preclinical studies of several Met-targeted agents have included investigating their effectiveness against known MET mutants. For example, selective inhibitors such as PF-2341066/4217903 are active against some MET mutations surrounding the ATP binding site but less active against mutations in the activation loop of the Met kinase domain 89. It is important that clinical trials are designed with this information in mind. An interim report of phase II clinical findings showed that foretinib is active against PRC patients with germline MET mutations compared to those without but with otherwise histologically similar tumor phenotype 116. These results underscore the importance of assessing MET sequence status in patient selection and/or stratification; since it is not yet clear whether the presence of other MET gene abnormalities, such as amplification or chromosome 7 polysomy, indicate sensitivity to Met targeted agents, patients with these features should also be identified.
The use of IHC to assess Met protein abundance in tumor sections also offers promise as an effective patient selection strategy. These specimens are routinely obtained for standard pathological diagnosis and provide important spatial and morphological information. Early findings indicated that great care must be taken in qualifying antibodies for target affinity and selectivity. Phase II clinical trials of the highly selective Met antagonists onartuzumab and rilotumumab, for the treatment of NSCLC and gastroesophgeal cancer, respectively, afforded retrospective assessments of outcome stratified on the basis of tumor Met content as determined by IHC where efficacy appeared to correlate with high Met abundance. Accordingly, a phase III clinical trial of onartuzumab restricted to NSCLC patients with a Met diagnostic-positive status will be activated soon. All patients have to provide tissue specimens or other material to be analyzed for Met expression as a requirement for entry. Patients will be stratified by tumor Met abundance as Met-high or Met-low using a commercially available IHC kit according to a pre-defined scoring system.
As for other tyrosine kinases thought to be valid cancer drug targets, the use of antibodies against sites of Met tyrosyl phosphorylation implicated in kinase activation and effector binding have the potential to be powerful tools for patient selection. However, results of their use in IHC have been mixed. Challenges to developing robust diagnostic assays of this class include [1] the inherent instability of protein phosphorylation during sample procurement and processing, [2] the limited antigenic features that distinguish a phosphorylated site from its unmodified counterpart, [3] the inherent similarity of tyrosyl phosphorylation sites among different receptor TKs that are co-expressed in many cancers, and [4] the general decline of IHC staining quality with storage for fixed/paraffin embedded as well as frozen tissue. An important alternative to IHC are two-site immunoassays of tissue extracts that can provide precise, absolute measurements of Met content and phosphorylation state. These assays lack the morphological information provided by IHC and usually require flash frozen tissue samples procured and processed according to protocols optimized for this purpose. Assays of this type for Met are in development and should complement IHC assessment of Met content with absolute measurements of content and activation state in the near future 137.
PD studies have the potential to dramatically impact the drug development process, even more so now that combinations of highly selective targeted drugs are considered. When used in conjunction with patient selection, proven PD markers can provide a rational basis for clinical study design and interpretation. Several ongoing clinical trials of Met-targeted drugs include ancillary PD marker studies. Some completed studies have linked PD markers to clinical response, such as plasma HGF levels during XL184 treatment 138, similar to changes in HGF levels reported in a study of RCC patients treated with sorafenib 139 or pazobanib 140. Abnormally high plasma HGF concentrations have been associated with advanced disease and poor outcome for several cancers, notably breast and gastric cancer; in a more recent study of RCC patients, serum HGF values correlated directly with clinical stage and tumor grade, and inversely with patient survival 141. Thus circulating HGF levels may be relevant to prognosis in several cancers, suggesting that monitoring plasma HGF levels during treatment may help predict response.
Plasma concentrations of soluble Met (sMet), soluble VEGFR2 (sVEGFR2), VEGF, PIGF, and EPO changed significantly during foretinib dosing 115, 116, 142. Plasma HGF, VEGF, sMet, sVEGFR2 were also examined in response to MGCD265 treatment 109, 143; both studies suggest that these may become useful PD markers. In a clinical study of XL184, modulation of plasma VEGFA, sMet, sVEGFR2, sKIT, and PIGF were also consistent with on-target drug effects 144. A phase Ib, open-label, dose escalation study to evaluate PD markers of crizotanib in combination with VEGF inhibitors in patients with advanced solid tumors will start recruiting patients soon. Detailed analyses of these PD results may help refine the rationale for targeting several kinases implicated in various human cancers.
The emergence of primary and acquired resistance to TKIs from pre-existing or de novo mutations, respectively, should be addressed in the design of future clinical studies. Strategies to overcome this problem include: [1] selecting treatments based on the presence of known susceptibility factors; [2] combining different classes of inhibitors of a single pathway; [3] combining targeted therapeutics with SOC treatments; and [4] combining therapeutics against multiple pathways. An example of the first strategy is the use of MetMAb in NSCLC patients with a Met diagnostic-positive status, with the hope of confirming previous clinical results. Combinations of HGF/Met mAbs and Met TKIs, as in the second strategy, are planned for future trials. A combination of agents with distinct activity profiles might also prevent the development of resistant mutations within the Met pathway and thereby improve clinical benefit. The third design is being used in several current trials of Met antagonists: trials combining AMG102, MetMAb, ARQ197, MK8033, MP470, MGCD265, XL184, or PF02341066 with SOC treatments -chemotherapeutic agents or radiotherapy, are also currently underway. Again, preclinical studies such as those combining AMG102 with temozolomide or docetaxel for the treatment of gastric, prostate, and colorectal cancers provided a sound rationale and guided initial trial design 67.
Examples of the fourth strategy are also abundant and supported by preclinical data 145. Many Met inhibitors are currently tested for safety/efficacy combined with various targeted agents that block other important pathways in cancer. Met-targeted therapies are being evaluated clinically with VEGF or EGFR inhibitors, and early results are promising. AMG102, MetMAb, ARQ197, MGCD265, XL184, and PF02341066 are being used in combination with Erlotinib for the treatment of NSCLC. MK8033, PF02341066 and ARQ197 are also being tested in combination with sorafenib for the treatment of advanced solid tumors. Adding Met-targeted therapies to first-line therapies targeting other pathways may be particularly useful for cancers where Met may participate in the acquisition of resistance and thereby dramatically increase the risk of metastasis. MET gene amplification was detected in 22% of lung cancer specimens that had acquired resistance to gefitinib or erlotinib, and treatment of a lung cancer cell line that had acquired gefitinib resistance through MET amplification with a Met-targeted TKI restored gefitinib sensitivity 146. Following these results in NSCLC, MetMAb and ARQ197 progressed into phase III clinical testing in combination with erlotinib.
In closing, the key to successful development of targeted therapies will be dependent on accurate and timely identification of the critical, dysfunctional nodes in the oncogenic pathways of the individual patient, whose effective inhibition will hopefully result in reversal of the malignant state. The wealth of basic knowledge about HGF/Met biology has enabled an accurate assessment of the pathway’s oncogenic potential and provided the insight needed to develop potent and selective inhibitors and use them with relative safety in humans. Patient selection, of primary importance, will advance, as more robust methods are developed to analyze the many known potential diagnostic biomarkers of pathway activity. Methods that rely on DNA or RNA (e.g. detecting MET gene amplification or mutation) are now faster and more sensitive than those available for quantitating Met protein content and phosphorylation state, but efforts to improve both are underway. Periodic monitoring of molecular changes in patients may be necessary to benefit from a treatment regimen with targeted therapy since patient genotype and phenotype may evolve dynamically under the selective pressure of targeted therapies 147. The need for PD markers that track drug effect and patient response is recognized and clinical PD marker studies currently underway reveal solid candidates. Finally, although the complexity of cancer and the risk of acquired resistance may limit the use of HGF/Met molecular therapeutics as single agents, much evidence suggests that pathway involvement is widespread and critical for metastasis. Thus for HGF/Met pathway inhibitors in particular, combinatorial phase II trials with small, carefully selected patient groups with endpoints that include time-to-first metastasis may be the most expedient path to more effective management of disease progression.
Highlights.
Despite regulation at multiple levels, HGF/Met signaling frequently contributes to oncogenesis, tumor progression and metastasis in a variety of human cancers.
Cancer drug development programs targeting HGF or Met have grown significantly, with > 100 human clinical trials in progress or completed.
HGF/Met pathway antagonists are generally well tolerated; promising results have been reported in lung, gastric, prostate and papillary renal cancer patients.
Challenges facing the effective use of HGF/Met-targeted treatments include patient selection, biomarker development and identifying therapy combinations.
Basic information, reagents and model systems for HGF/Met signaling will be invaluable in meeting these challenges and achieving effective disease control.
Acknowledgments
Web site addresses are provided as an informational resource, not as an endorsement of any product or manufacturer.
Footnotes
Declaration of interest
This work was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Bibliography
- 1*.Peschard P, Park M. From Tpr-Met to Met, tumorigenesis and tubes. Oncogene. 2007;26:1276–85. doi: 10.1038/sj.onc.1210201. Review of the cloning and characterization of the MET oncogene from its discovery to the present day. [DOI] [PubMed] [Google Scholar]
- 2*.Nakamura T, Sakai K, Nakamura T, Matsumoto K. Hepatocyte growth factor twenty years on: Much more than a growth factor. J Gastroenterol Hepatol. 2011;26:188–202. doi: 10.1111/j.1440-1746.2010.06549.x. Review of the purification, cloning and characterization of HGF from its discovery to the present day. [DOI] [PubMed] [Google Scholar]
- 3.Sonnenberg E, Meyer D, Weidner KM, Birchmeier C. Scatter factor/hepatocyte growth factor and its receptor, the c-met tyrosine kinase, can mediate a signal exchange between mesenchyme and epithelia during mouse development. The Journal of Cell Biology. 1993;123:223–35. doi: 10.1083/jcb.123.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim ES, Salgia R. MET pathway as a therapeutic target. J Thorac Oncol. 2009 Apr;4:444–7. doi: 10.1097/JTO.0b013e31819d6f91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naldini L, Weidner KM, Vigna E, Gaudino G, et al. Scatter factor and hepatocyte growth factor are indistinguishable ligands for the MET receptor. EMBO J. 1991 Oct;10:2867–78. doi: 10.1002/j.1460-2075.1991.tb07836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma PC, Maulik G, Christensen J, Salgia R. c-Met: structure, functions and potential for therapeutic inhibition. Cancer Metastasis Rev. 2003;22:309–25. doi: 10.1023/a:1023768811842. [DOI] [PubMed] [Google Scholar]
- 7.Stoker M, Gherardi E, Perryman M, Gray J. Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature. 1987 May 21–27;327:239–42. doi: 10.1038/327239a0. [DOI] [PubMed] [Google Scholar]
- 8.Bottaro DP, Rubin JS, Faletto DL, Chan AML, Kmiecik TE, et al. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science. 1991;251:802–4. doi: 10.1126/science.1846706. [DOI] [PubMed] [Google Scholar]
- 9.Corso S, Comoglio PM, Giordano S. Cancer therapy: can the challenge be MET? Trends Mol Med. 2005;11:284–92. doi: 10.1016/j.molmed.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Rosario M, Birchmeier W. How to make tubes: signaling by the Met receptor tyrosine kinase. Trends Cell Biol. 2003;13:328–35. doi: 10.1016/s0962-8924(03)00104-1. [DOI] [PubMed] [Google Scholar]
- 11.Zhang YW, Vande Woude GF. HGF/SF-met signaling in the control of branching morphogenesis and invasion. J Cell Biochem. 2003;88:408–17. doi: 10.1002/jcb.10358. [DOI] [PubMed] [Google Scholar]
- 12.Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. NatRevMol Cell Biol. 2003;4:915–25. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int. 2006;69:213–7. doi: 10.1038/sj.ki.5000054. [DOI] [PubMed] [Google Scholar]
- 14.Borowiak M, Garratt AN, Wustefeld T, Strehle M, et al. Met provides essential signals for liver regeneration. ProcNatlAcadSciUSA. 2004;101:10608–13. doi: 10.1073/pnas.0403412101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huh CG, Factor VM, Sanchez A, Uchida K, Conner EA, Thorgeirsson SS. Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair. ProcNatlAcadSciUSA. 2004;101:4477–82. doi: 10.1073/pnas.0306068101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morishita R, Aoki M, Hashiya N, Yamasaki K, et al. Therapeutic angiogenesis using hepatocyte growth factor (HGF) CurrGene Ther. 2004;4:199–206. doi: 10.2174/1566523043346453. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto K, Nakamura T. Hepatocyte growth factor: Renotropic role and potential therapeutics for renal diseases. Kidney International. 2001;59:2023–38. doi: 10.1046/j.1523-1755.2001.00717.x. [DOI] [PubMed] [Google Scholar]
- 18.Yamashita J, Ogawa M, Yamashita S, Nomura K, et al. Immunoreactive hepatocyte growth factor is a strong and independent predictor of recurrence and survival in human breast cancer. Cancer Res. 1994;54:1630–3. [PubMed] [Google Scholar]
- 19.Maulik G, Shrikhande A, Kijima T, Ma PC, et al. Role of the hepatocyte growth factor receptor, c-Met, in oncogenesis and potential for therapeutic inhibition. Cytokine Growth Factor Rev. 2002;13:41–59. [Google Scholar]
- 20.Sattler M, Ma PC, Salgia R. Therapeutic targeting of the receptor tyrosine kinase Met. Cancer Treat Res. 2004;119:121–38. doi: 10.1007/1-4020-7847-1_7. [DOI] [PubMed] [Google Scholar]
- 21.Gherardi E, Youles ME, Miguel RN, Blundell TL, et al. Functional map and domain structure of MET, the product of the c-met protooncogene and receptor for hepatocyte growth factor/scatter factor. Proc NatlAcadSci USA. 2003;100:12039–44. doi: 10.1073/pnas.2034936100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niemann HH, Jager V, Butler PJ, van den Heuvel J, et al. Structure of the human receptor tyrosine kinase met in complex with the Listeria invasion protein InlB. Cell. 2007 Jul 27;130:235–46. doi: 10.1016/j.cell.2007.05.037. [DOI] [PubMed] [Google Scholar]
- 23.Antipenko A, Himanen JP, van Leyen K, Nardi-Dei V, et al. Structure of the semaphorin-3A receptor binding module. Neuron. 2003 Aug 14;39:589–98. doi: 10.1016/s0896-6273(03)00502-6. [DOI] [PubMed] [Google Scholar]
- 24.Love CA, Harlos K, Mavaddat N, Davis SJ, et al. The ligand-binding face of the semaphorins revealed by the high-resolution crystal structure of SEMA4D. Nat Struct Biol. 2003 Oct;10:843–8. doi: 10.1038/nsb977. [DOI] [PubMed] [Google Scholar]
- 25.Kozlov G, Perreault A, Schrag JD, Park M, et al. Insights into function of PSI domains from structure of the Met receptor PSI domain. Biochem Biophys Res Commun. 2004 Aug 13;321:234–40. doi: 10.1016/j.bbrc.2004.06.132. [DOI] [PubMed] [Google Scholar]
- 26.Basilico C, Arnesano A, Galluzzo M, Comoglio PM, et al. A high affinity hepatocyte growth factor-binding site in the immunoglobulin-like region of Met. JBiolChem. 2008;283:21267–77. doi: 10.1074/jbc.M800727200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crepaldi T, Pollack AL, Prat M, Zborek A, et al. Targeting of the SF/HGF receptor to the basolateral domain of polarized epithelial cells. J Cell Biol. 1994 Apr;125:313–20. doi: 10.1083/jcb.125.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villa-Moruzzi E, Puntoni F, Bardelli A, Vigna E, et al. Protein tyrosine phosphatase PTP-S binds to the juxtamembrane region of the hepatocyte growth factor receptor Met. Biochem J. 1998 Nov 15;336:235–9. doi: 10.1042/bj3360235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Comoglio PM, Boccaccio C. Scatter factors and invasive growth. Seminars in Cancer Biology. 2001;11:153–65. doi: 10.1006/scbi.2000.0366. [DOI] [PubMed] [Google Scholar]
- 30.Giordano S, Bardelli A, Zhen Z, Menard S, et al. A point mutation in the MET oncogene abrogates metastasis without affecting transformation. Proc NatlAcadSci USA. 1997;94:13868–72. doi: 10.1073/pnas.94.25.13868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gu H, Neel BG. The “Gab” in signal transduction. Trends Cell Biol. 2003;13:122–30. doi: 10.1016/s0962-8924(03)00002-3. [DOI] [PubMed] [Google Scholar]
- 32.Zang T, Zhuang L, Zhang Z, Xin D, et al. Expression of beta-catenin in renal cell carcinoma. Chin Med J (Engl) 2001;114:152–4. [PubMed] [Google Scholar]
- 33.2012 http://www.vai.org/met/
- 34.Smolen GA, Sordella R, Muir B, Mohapatra G, et al. Amplification of MET may identify a subset of cancers with extreme sensitivity to the selective tyrosine kinase inhibitor PHA-665752. Proceedings of the National Academy of Sciences. 2006;103:2316–21. doi: 10.1073/pnas.0508776103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lengyel E, Prechtel D, Resau JH, Gauger K, et al. C-Met overexpression in node-positive breast cancer identifies patients with poor clinical outcome independent of Her2/neu. IntJ Cancer. 2005;113:678–82. doi: 10.1002/ijc.20598. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt C, Bladt F, Goedecke S, Brinkmann V, et al. Scatter factor/hepatocyte growth factor is essential for liver development. Nature. 1995;373:699–702. doi: 10.1038/373699a0. [DOI] [PubMed] [Google Scholar]
- 37*.Dharmawardana PG, Giubellino A, Bottaro DP. Hereditary papillary renal carcinoma type I. CurrMolMed. 2004;4:855–68. doi: 10.2174/1566524043359674. Review of earliest reported mutations found exclusively in the Met TK domain and associated with hereditary and sporadic forms of papillary renal cell carcinoma. [DOI] [PubMed] [Google Scholar]
- 38*.Lengyel E, Sawada K, Salgia R. Tyrosine kinase mutations in human cancer. CurrMol Med. 2007;7:77–84. doi: 10.2174/156652407779940486. Review of mutations throughout the MET coding sequence later found in lung cancer and in head and neck cancers. [DOI] [PubMed] [Google Scholar]
- 39.Bardelli A, Longati P, Gramaglia D, Basilico C, et al. Uncoupling signal transducers from oncogenic MET mutants abrogates cell transformation and inhibits invasive growth. Proc NatlAcadSci USA. 1998;95:14379–83. doi: 10.1073/pnas.95.24.14379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giordano S, Maffe A, Williams TA, Artigiani S, et al. Different point mutations in the met oncogene elicit distinct biological properties. FASEB J. 2000;14:399–406. doi: 10.1096/fasebj.14.2.399. [DOI] [PubMed] [Google Scholar]
- 41.Michieli P, Basilico C, Pennacchietti S, Maffe A, et al. Mutant Met-mediated transformation is ligand-dependent and can be inhibited by HGF antagonists. Oncogene. 1999;18:5221–31. doi: 10.1038/sj.onc.1202899. [DOI] [PubMed] [Google Scholar]
- 42*.Graveel CR, London CA, Vande Woude GF. A mouse model of activating Met mutations. Cell Cycle. 2005:4518–20. doi: 10.4161/cc.4.4.1590. Renal cell carcinoma-associated MET TK domain mutations investigated in mice by engineering changes in the murine MET locus. [DOI] [PubMed] [Google Scholar]
- 43.Joffre C, Barrow R, Menard L, Calleja V, et al. A direct role for Met endocytosis in tumorigenesis. Nat Cell Biol. 2011 Jul;13:827–37. doi: 10.1038/ncb2257. [DOI] [PubMed] [Google Scholar]
- 44.Kong-Beltran M, Seshagiri S, Zha J, Zhu W, et al. Somatic mutations lead to an oncogenic deletion of met in lung cancer. Cancer Res. 2006;66:283–9. doi: 10.1158/0008-5472.CAN-05-2749. [DOI] [PubMed] [Google Scholar]
- 45.Ma PC, Kijima T, Maulik G, Fox EA, et al. c-MET mutational analysis in small cell lung cancer: novel juxtamembrane domain mutations regulating cytoskeletal functions. Cancer Res. 2003;63:6272–81. [PubMed] [Google Scholar]
- 46.Ma PC, Jagadeeswaran R, Jagadeesh S, Tretiakova MS, et al. Functional Expression and Mutations of c-Met and Its Therapeutic Inhibition with SU11274 and Small Interfering RNA in Non-Small Cell Lung Cancer. Cancer Research. 2005;65:1479–88. doi: 10.1158/0008-5472.CAN-04-2650. [DOI] [PubMed] [Google Scholar]
- 47.Schmidt L, Junker K, Nakaigawa N, Kinjerski T, et al. Novel mutations of the MET proto-oncogene in papillary renal carcinomas. Oncogene. 1999;18:2343–50. doi: 10.1038/sj.onc.1202547. [DOI] [PubMed] [Google Scholar]
- 48.Lee JH, Han SU, Cho H, Jennings B, et al. A novel germ line juxtamembrane Met mutation in human gastric cancer. Oncogene. 2000;19:4947–53. doi: 10.1038/sj.onc.1203874. [DOI] [PubMed] [Google Scholar]
- 49.Tyner JW, Fletcher LB, Wang EQ, Yang WF, et al. MET receptor sequence variants R970C and T992I lack transforming capacity. Cancer Res. Aug 1;70:6233–7. doi: 10.1158/0008-5472.CAN-10-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Corso S, Migliore C, Ghiso E, De Rosa G, et al. Silencing the MET oncogene leads to regression of experimental tumors and metastases. Oncogene. 2008 Jan 24;27:684–93. doi: 10.1038/sj.onc.1210697. [DOI] [PubMed] [Google Scholar]
- 51.Pennacchietti S, Michieli P, Galluzzo M, Mazzone M, et al. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell. 2003;3:347–61. doi: 10.1016/s1535-6108(03)00085-0. [DOI] [PubMed] [Google Scholar]
- 52.Stahl SJ, Wingfield PT, Kaufman JD, Pannell LK, et al. Functional and biophysical characterization of recombinant human hepatocyte growth factor isoforms produced in Escherichia coli. Biochemical Journal. 1997;326:763–72. doi: 10.1042/bj3260763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu Y, Merlino G. Constitutive c-Met signaling through a nonautocrine mechanism promotes metastasis in a transgenic transplantation model. Cancer Res. 2002 May 15;62:2951–6. [PubMed] [Google Scholar]
- 54.Toyoda M, Takayama H, Horiguchi N, Otsuka T, et al. Overexpression of hepatocyte growth factor/scatter factor promotes vascularization and granulation tissue formation in vivo. FEBS Lett. 2001 Nov 30;509:95–100. doi: 10.1016/s0014-5793(01)03126-x. [DOI] [PubMed] [Google Scholar]
- 55.Otsuka T, Horiguchi N, Kanda D, Kosone T, et al. Overexpression of NK2 inhibits liver regeneration after partial hepatectomy in mice. World J Gastroenterol. 2005 Dec 21;11:7444–9. doi: 10.3748/wjg.v11.i47.7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Montesano R, Soriano JV, Malinda KM, Ponce ML, et al. Differential effects of hepatocyte growth factor isoforms on epithelial and endothelial tubulogenesis. Cell Growth and Differentiation. 1998;9:355–65. [PubMed] [Google Scholar]
- 57.Kishi Y, Kuba K, Nakamura T, Wen J, et al. Systemic NK4 gene therapy inhibits tumor growth and metastasis of melanoma and lung carcinoma in syngeneic mouse tumor models. Cancer Sci. 2009 Jul;100:1351–8. doi: 10.1111/j.1349-7006.2009.01184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suzuki Y, Sakai K, Ueki J, Xu Q, et al. Inhibition of Met/HGF receptor and angiogenesis by NK4 leads to suppression of tumor growth and migration in malignant pleural mesothelioma. Int J Cancer. Oct 15;127:1948–57. doi: 10.1002/ijc.25197. [DOI] [PubMed] [Google Scholar]
- 59.Naka D, Ishii T, Yoshiyama Y, Miyazawa K, et al. Activation of hepatocyte growth factor by proteolytic conversion of a single chain form to a heterodimer. J Biol Chem. 1992 Oct 5;267:20114–9. [PubMed] [Google Scholar]
- 60.Lokker NA, Mark MR, Luis EA, Bennett GL, et al. Structure-function analysis of hepatocyte growth factor: identification of variants that lack mitogenic activity yet retain high affinity receptor binding. EMBO J. 1992;11:2503–10. doi: 10.1002/j.1460-2075.1992.tb05315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hartmann G, Naldini L, Weidner KM, Sachs M, et al. A functional domain in the heavy chain of scatter factor/hepatocyte growth factor binds the c-Met receptor and induces cell dissociation but not mitogenesis. Proc Natl Acad Sci U S A. 1992 Dec 1;89:11574–8. doi: 10.1073/pnas.89.23.11574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gak E, Taylor WG, Chan AM, Rubin JS. Processing of hepatocyte growth factor to the heterodimeric form is required for biological activity. FEBS Lett. 1992 Oct 12;311:17–21. doi: 10.1016/0014-5793(92)81356-q. [DOI] [PubMed] [Google Scholar]
- 63.Kong-Beltran M, Stamos J, Wickramasinghe D. The Sema domain of Met is necessary for receptor dimerization and activation. Cancer Cell. 2004;6:75–84. doi: 10.1016/j.ccr.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 64.Michieli P, Mazzone M, Basilico C, Cavassa S, et al. Targeting the tumor and its microenvironment by a dual-function decoy Met receptor. Cancer Cell. 2004;6:61–73. doi: 10.1016/j.ccr.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 65.Petrelli A, Circosta P, Granziero L, Mazzone M, et al. Ab-induced ectodomain shedding mediates hepatocyte growth factor receptor down-regulation and hampers biological activity. Proc Natl Acad Sci U S A. 2006 Mar 28;103:5090–5. doi: 10.1073/pnas.0508156103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Burgess T, Coxon A, Meyer S, Sun J, et al. Fully Human Monoclonal Antibodies to Hepatocyte Growth Factor with Therapeutic Potential against Hepatocyte Growth Factor/c-Met-Dependent Human Tumors. Cancer Research. 2006;66:1721–9. doi: 10.1158/0008-5472.CAN-05-3329. [DOI] [PubMed] [Google Scholar]
- 67.Kim KJ, Wang L, Su YC, Gillespie GY, et al. Systemic anti-hepatocyte growth factor monoclonal antibody therapy induces the regression of intracranial glioma xenografts. ClinCancer Res. 2006;12:1292–8. doi: 10.1158/1078-0432.CCR-05-1793. [DOI] [PubMed] [Google Scholar]
- 68.Meetze AK, Boudrow A, Venkataraman S, Medicherla S, et al. Preclinical efficacy and pharmacodynamics of SCH 900105 (AV-299) and anti-HGF antibody in an intracranial glioblastoma. AACR-NCI-EORTC International Conference Molecular Target and Cancer Therapeutics; Boston, MA. 2009. [Google Scholar]
- 69.Ramanathan RK, Payumo FC, Papadopoulos PK, Weiss GJ, et al. A Phase 1 first-in-human study of SCH 900105, an antihepatocyte growth factor (HGF) monoclonal antibody, in subjects with advanced solid cancer. AACR-NCI-EORTC International Conference Molecular Target and Cancer Therapeutics; Boston, MA. 2009. [Google Scholar]
- 70.Okamoto W, Okamoto I, Tanaka K, Hatashita E, et al. TAK-701, a humanized monoclonal antibody to hepatocyte growth factor, reverses gefitinib resistance induced by tumor-derived HGF in non-small cell lung cancer with an EGFR mutation. Mol Cancer Ther. Oct 9;:2785–92. doi: 10.1158/1535-7163.MCT-10-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meetze AK, Boudrow A, Connoly K, Huang R, et al. Anti-tumor activity of SCH 900105 (AV299), an anti-HGF antibody in non-small cell lung cancer models. AACR-NCI-EORTC International Conference Molecular Target and Cancer Therapeutics; Boston, MA. 2009. [Google Scholar]
- 72.Kitahara O, Nishizawa S, Ito Y, Inaoka T, et al. In vitro profiles of TAK-701, a humanized anti-hepatocyte growth factor neutralizing antibody, and the antitumor activity against U-87MG GBM with anti-angiogenic activity. American Association for Cancer Research (AACR) Annual Meeting; Denver, CO. 2009. [Google Scholar]
- 73.Hori A, Kitahara O, Ito Y, Nishizawa S, et al. Monotherapeutic and combination antitumor activities of TAK-701, a humanized anti-hepatocyte growth factor neutralizing antibody, against multiple types of cancer. American Association for Cancer Research (AACR) Annual Meeting; Denver, CO. 2009. [Google Scholar]
- 74.Jones SF, Cohen RB, Bendell JC, Denlinger CS, et al. Safety, tolerability, and pharmacokinetics of TAK-701, a humanized anti-hepatocyte growth factor (HGF) monoclonal antibody, in patients with advanced nonhematologic malignancies: First-inhuman phase I dose-escalation study. American Society of Clinical Oncology (ASCO) Annual Meeting; Chicago, IL. 2010. [Google Scholar]
- 75.2012 http://investor.millennium.com/phoenix.zhtml?c=80159&p=irol-newsArticle&ID=1435576&highlight=
- 76.Rosen PJ, Sweeney CJ, Park DJ, Beaupre DM, et al. A phase Ib study of AMG 102 in combination with bevacizumab or motesanib in patients with advanced solid tumors. Clin Cancer Res. May 1;16:2677–87. doi: 10.1158/1078-0432.CCR-09-2862. [DOI] [PubMed] [Google Scholar]
- 77.Wen PY, Schiff D, Cloughesy TF, Raizer JJ, et al. A phase II study evaluating the efficacy and safety of AMG 102 (rilotumumab) in patients with recurrent glioblastoma. Neuro Oncol. Apr;13:437–46. doi: 10.1093/neuonc/noq198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78*.Ivenson T, Davidenko I, et al. Safety and efficacy of epirubicin, cisplastin and capecitabine (ECX) plus rilutumab(R) as first-line treatment for unresectable locally advanced (LA) or metastatic (M) gastric or esophagogastric junction (EGJ) adenocarcinoma. European Multidisciplinary Cancer Congress; Stockholm. 2011. Evidence of increased OS in gastric/gastroesphageal cancer patients treated with a combination of SOC therapy combined with a selective HGF antagonist. [Google Scholar]
- 79.Eng C, Van Cutsem E, Nowara E, Swieboda-Sadlej A, et al. A randomized, phase Ib/II trial of rilotumumab (AMG 102; ril) or ganitumab (AMG 479; gan) with panitumumab (pmab) versus pmab alone in patients (pts) with wild-type (WT) KRAS metastatic colorectal cancer (mCRC): Primary and biomarker analyses. American Society of Clinical Oncology (ASCO) Annual Meeting; Chicago, IL. 2011. [Google Scholar]
- 80.Tan K, Park K, Lim M, Ahn M, et al. Phase Ib study of ficlatuzumab (formerly AV-299), an anti-hepatocyte growth factor (HGF) monoclonal antibody (MAb) in combination with gefitinib (G) in Asian patients (pts) with NSCLC. American Society of Clinical Oncology (ASCO) Annual Meeting; Chicago, IL. 2011. [Google Scholar]
- 81.Corvaïa N, Gonzalez A, Boute N, Beau-Larvor C, et al. First bivalent fully antagonist anti-c-Met antibody targeting the c-Met receptor: I) in vitro mechanism of action. American Association for Cancer Research (AACR) Annual Meeting; Denver, CO. 2009. [Google Scholar]
- 82.Gordon MS, Mendelson DS, Sweeney C, Erbeck N, et al. Interim results from a first-in-human study with AMG102, a fully human monoclonal antibody that neutralizes hepatocyte growth factor (HGF), the ligand to c-Met receptor, in patients (pts) with advanced solid tumors. American Society of Clinical Oncology (ASCO) Annual Meeting; Chicago, IL. 2007. [Google Scholar]
- 83.Martens T, Eckerich C, et al. World Federation of Neuro-Oncology. Edinburgh; United Kingdom: 2005. Inhibition of intracerebral glioblastoma growth by treatment with a novel one-armed anti-Met antibody. [Google Scholar]
- 84**.Spigel DR, Ervin TJ, Ramlau RM, Daniel DB, et al. Final efficacy results from OAM4558g, a randomized phase II study evaluating MetMAb or placebo in combination with erlotinib in advanced NSCLC. American Society of Clinical Oncology (ASCO) Annual Meeting; Chicago, IL. 2011. Demonstration of significant increase in PFS and OS in NSCLC patients with high Met expression treated with a combination of erlotinib and a selective Met antagonist. [Google Scholar]
- 85.2012 http://www.ascopost.com/articles/october-15-2011-supplement/research-increasingly-points-to-the-role-of-molecular-diversity-in-metastatic-lung-cancer/
- 86.Spigel DR, Waterhouse D, Shipley D, Lane CM, et al. Phase II trial of modified FOLFOX6, bevacizumab, and cetuximab in first-line metastatic colorectal cancer treatment. American Society of Clinical Oncology (ASCO), Gastrointestinal Cancers Symposium; San Francisco, CA. 2009. [Google Scholar]
- 87.Merchant M, Marik J, Peng J, Williams SP, et al. Proof of concept of immuno-PET molecular imaging of met using 76Br- and 89Zr-labeled MetMAb. American Society of Clinical Oncology (ASCO) Annual Meeting; Chicago, IL. 2011. [Google Scholar]
- 88.Perk LR, Stigter-van Walsum M, Visser GW, Kloet RW, et al. Quantitative PET imaging of Met-expressing human cancer xenografts with 89Zr-labelled monoclonal antibody DN30. Eur J Nucl Med Mol Imaging. 2008;10:1857–67. doi: 10.1007/s00259-008-0774-5. [DOI] [PubMed] [Google Scholar]
- 89.Bellon SF, Kaplan-Lefko P, Yang Y, Zhang Y, Moriguchi J, et al. c-Met inhibitors with novel binding mode show activity against several hereditary papillary renal cell carcinoma-related mutations. J Biol Chem. 2008 Feb 1;283:2675–83. doi: 10.1074/jbc.M705774200. [DOI] [PubMed] [Google Scholar]
- 90.Sattler M, Pride YB, Ma P, Gramlich JL, et al. A novel small molecule met inhibitor induces apoptosis in cells transformed by the oncogenic TPR-MET tyrosine kinase. Cancer Res. 2003;63:5462–9. [PubMed] [Google Scholar]
- 91.Ma PC, Tang Z, et al. In vivo PET imaging of MET receptor in human cancer using [11C]SU11274. American Association for Cancer Research (AACR) Annual Meeting; Washington, D.C. 2010. [Google Scholar]
- 92.Wu C, Tang Z, Fan W, Zhu W, et al. In vivo positron emission tomography (PET) imaging of mesenchymal-epithelial transition (MET) receptor. J Med Chem. 2010;53:139–46. doi: 10.1021/jm900803q. [DOI] [PubMed] [Google Scholar]
- 93.Viswanadha S, Muthuppalaniappan M, Varanasi KVS, et al. Pharmacological and pharmacokinetic characterization of RP1040, a novel and potent c-Met tyrosine kinase inhibitor. AACR-NCI-EORTC International Conference Molecular Target and Cancer Therapeutics; Boston, MA. 2009. [Google Scholar]
- 94.Burbridge MF, Saunier C, et al. Preclinical molecular stratification of human tumors sensitive or resistant to S 49076, a novel MET / FGFR / AXL kinase inhibitor. AACR-NCI-EORTC International Conference Molecular Target and Cancer Therapeutics; San Francisco, CA. 2011. [Google Scholar]
- 95.Burbridge MF, Bossard C, Saunier C, Bourrier C, et al. S 49076, a novel, potent, MET / FGFR / AXL kinase inhibitor with wide antitumor activity. AACR-NCI-EORTC International Conference Molecular Target and Cancer Therapeutics; San Francisco, CA. 2011. [Google Scholar]
- 96.Donehower RC, Sacrdina M, Hill M, Bowman J, et al. A phase I dose-escalation study of INCB028060, an inhibitor of c-MET receptor tyrosine kinase, in patients with advanced solid tumors. American Society of Clinical Oncology (ASCO), Annual Meeting; Chicago, IL. 2011. [Google Scholar]
- 97.Yang WJ, Credille K, Gao H, Uhlik M, et al. LY2801653, an orally available small molecule inhibitor of c-Met, demonstrated broad antitumor efficacy in patients derived xenograft models. American Association for Cancer Research (AACR) Annual Meeting; Washington, DC. 2010. [Google Scholar]
- 98.Bladt F, Blaukat A, Dorsch D, Fittschen C, et al. Preclinical characterization of EMD1214063, a potent and highly selective inhibitor of the c-Met kinase in Phase I clinical trials. American Association for Cancer Research (AACR) Annual Meeting; Washington, DC. 2010. [Google Scholar]
- 99.Bladt F, Blaukat A, Dorsch D, Fittschen C, et al. Identification and preclinical characterization of EMD1204831 - a selective c-Met kinase inhibitor in clinical phase I. American Association for Cancer Research (AACR) Annual Meeting; Orlando, FL. 2011. [Google Scholar]
- 100.Diamond S, Boer J, Maduskuie TP, Jr, Falahatpisheh N, et al. Species-specific metabolism of SGX523 by aldehyde oxidase and the toxicological implications. Drug Metab Dispos. 2010 Aug;38:1277–85. doi: 10.1124/dmd.110.032375. [DOI] [PubMed] [Google Scholar]
- 101.2012 http://www.mskcc.org/mskcc/html/66036.cfm.
- 102.Nakagawa T, Tohyama O, Yamaguchi A, Matsushima T, et al. E7050: a dual c-Met and VEGFR-2 tyrosine kinase inhibitor promotes tumor regression and prolongs survival in mouse xenograft models. Cancer Sci. Jan;101:210–5. doi: 10.1111/j.1349-7006.2009.01343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Qi W, Cooke LS, Stejskal A, Riley C, et al. MP470, a novel receptor tyrosine kinase inhibitor, in combination with Erlotinib inhibits the HER family/PI3K/Akt pathway and tumor growth in prostate cancer. BMC Cancer. 2009;9:142. doi: 10.1186/1471-2407-9-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Welsh JW, Mahadevan D, Ellsworth R, Cooke L, et al. The c-Met receptor tyrosine kinase inhibitor MP470 radiosensitizes glioblastoma cells. Radiat Oncol. 2009;4:69. doi: 10.1186/1748-717X-4-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sankhala KK, Tolcher AW, Mita MM, Gordon MS, et al. Amuvatinib (MP-470), an oral dual inhibitor of mutant kinases and DNA repair: Final results from a 100-patient, 5-arm phase Ib trial in combination with five standard of care (SOC) anticancer regimens. American Society of Clinical Oncology (ASCO), Annual Meeting; Chicago, IL. 2011. [Google Scholar]
- 106.2012 http://clinicaltrials.gov/ct2/show/NCT01357395.
- 107.Schroeder GM, An Y, Cai ZW, Chen XT, et al. Discovery of N-(4-(2-amino-3-chloropyridin-4-yloxy)-3-fluorophenyl)-4-ethoxy-1-(4-fluor ophenyl)-2-oxo-1,2-dihydropyridine-3-carboxamide (BMS-777607), a selective and orally efficacious inhibitor of the Met kinase superfamily. J Med Chem. 2009 Mar 12;52:1251–4. doi: 10.1021/jm801586s. [DOI] [PubMed] [Google Scholar]
- 108.Dai Y, Siemann DW. BMS-777607, a small-molecule met kinase inhibitor, suppresses hepatocyte growth factor-stimulated prostate cancer metastatic phenotype in vitro. Mol Cancer Ther. Jun 9;:1554–61. doi: 10.1158/1535-7163.MCT-10-0359. [DOI] [PubMed] [Google Scholar]
- 109.Hong D, LoRusso P, Kurzroc R, Maroun C, et al. Phase I study of MGCD265 administered intermittently to patients with advanced malignancies (Study 265–102). American Society of Clinical Oncology (ASCO), Annual Meeting; Chicago, IL. 2009. [Google Scholar]
- 110.Besterman JM, Fournel M, Dupont I, Bonfils C, et al. Potent preclinical antitumor activity of MGCD265, an oral Met/VEGFR kinase inhibitor in phase II clinical development, in combination with taxanes or erlotinib. American Society of Clinical Oncology (ASCO), Annual Meeting; Chicago, IL. 2010. [Google Scholar]
- 111.Pan BS, Chan GK, Chenard M, Chi A, et al. MK-2461, a novel multitargeted kinase inhibitor, preferentially inhibits the activated c-Met receptor. Cancer Res. 2010 Feb 15;70:1524–33. doi: 10.1158/0008-5472.CAN-09-2541. [DOI] [PubMed] [Google Scholar]
- 112.Camacho LH, Moulder SL, LoRusso PM, Blumenschein GR, et al. First in human phase I study of MK-2461, a small molecule inhibitor of c-Met, for patients with advanced solid tumors. American Society of Clinical Oncology (ASCO), Annual Meeting; Chicago, IL. 2008. [Google Scholar]
- 113.Eder JP, Health E, Appleman L, Shapiro G, et al. Phase I experience with c-MET inhibitor XL880 administered orally to patients (pts) with solid tumors. American Society of Clinical Oncology (ASCO), Annual Meeting; Chicago, IL. 2007. [Google Scholar]
- 114.Jhawer M, Kindler HL, Wainberg Z, Ford J, et al. Assessment of two dosing schedules of GSK1363089 (GSK089), a dual MET/VEGFR2 inhibitor, in metastatic gastric cancer (GC): interim results of a multicenter phase II study. American Society of Clinical Oncology (ASCO), Annual Meeting; Chicago, IL. 2009. [Google Scholar]
- 115.Ross RW, Stein M, Sarantopoulos J, Eisenberg T, et al. A phase II study of the c-Met RTK inhibitor XL880 in patients (pts) with papillary renal-cell carcinoma (PRC). American Society of Clinical Oncology (ASCO), Annual Meeting; Chicago, IL. 2007. [Google Scholar]
- 116**.Srinivasan R, Choueiri TK, Vaishampayan U, Rosenberg JE, et al. A phase II study of the dual MET/VEGFR2 inhibitor XL880 in patients (pts) with papillary renal carcinoma (PRC). American Society of Clinical Oncology (ASCO), Annual Meeting; Chicago, IL. 2008. Demonstration of RECIST partial response in HPRC patients (with germline MET TK domain mutations) to a multikinase inhibitor targeting Met. [Google Scholar]
- 117.Liu L, Shi H, Liu Y, Anderson A, et al. Synergistic effects of foretinib with HER-targeted agents in MET and HER1- or HER2-coactivated tumor cells. Mol Cancer Ther. Mar;10:518–30. doi: 10.1158/1535-7163.MCT-10-0698. [DOI] [PubMed] [Google Scholar]
- 118.Eathiraj S, Palma R, Volckova E, Hirschi M, et al. Discovery of a novel mode of protein kinase inhibition characterized by the mechanism of inhibition of human mesenchymal-epithelial transition factor (c-Met) protein autophosphorylation by ARQ 197. J Biol Chem. Jun 10;286:20666–76. doi: 10.1074/jbc.M110.213801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Munshi N, Jeay S, Li Y, Chen CR, et al. ARQ 197, a novel and selective inhibitor of the human c-Met receptor tyrosine kinase with antitumor activity. Mol Cancer Ther. 2010 Jun;9:1544–53. doi: 10.1158/1535-7163.MCT-09-1173. [DOI] [PubMed] [Google Scholar]
- 120.Zou HY, Li Q, Lee JH, Arango ME, et al. An orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms. Cancer Res. 2007 May 1;67:4408–17. doi: 10.1158/0008-5472.CAN-06-4443. [DOI] [PubMed] [Google Scholar]
- 121.Matteucci E, Bendinelli P, Desiderio MA. Nuclear localization of active HGF receptor Met in aggressive MDA-MB231 breast carcinoma cells. Carcinogenesis. 2009 Jun;30:937–45. doi: 10.1093/carcin/bgp080. [DOI] [PubMed] [Google Scholar]
- 122.Schiller JH, Akerley WL, Brugger W, Ferrari D, et al. Results from ARQ 197–209: A global randomized placebo-controlled phase II clinical trial of erlotinib plus ARQ 197 versus erlotinib plus placebo in previously treated EGFR inhibitor-naive patients with locally advanced or metastatic non-small cell lung cancer (NSCLC). American Society of Clinical Oncology (ASCO), Annual Meeting; Chicago, IL. 2010. [Google Scholar]
- 123.2012 http://www.daiichisankyo.com/news/detail/003880.html.
- 124.Salgia R, Honk DS, Camacho LH, Ng CS, et al. A phase I dose-escalation study of the safety and pharmacokinetics (PK) of XL184, a VEGFR and MET kinase inhibitor, administered orally to patients (pts) with advanced malignancies. American Society of Clinical Oncology (ASCO), Annual Meeting; Chicago, IL. 2007. [Google Scholar]
- 125.Salgia R, Sherman S, Hong DS, Ng CS, et al. A phase I study of XL184, a RET, VEGFR2, and MET kinase inhibitor, in patients (pts) with advanced malignancies, including pts with medullary thyroid cancer (MTC). American Society of Clinical Oncology (ASCO), Annual Meeting; Chicago, IL. 2008. [Google Scholar]
- 126.Vergote I, Sella A, Bedell C, Ramondetta L, et al. Phase 2 study of XL184 in a cohort of ovarian cancer patients (pts) with measurable soft tissue disease. AACR-NCI-EORTC International Conference Molecular Target and Cancer Therapeutics; Berlin, Germany. 2010. [Google Scholar]
- 127.Nuchushtan H, Edelman G, Jerusalem G, Gordon M, et al. Phase 2 results of XL184 in a cohort of patients (pts) with advanced melanoma. AACR-NCI-EORTC International Conference Molecular Target and Cancer Therapeutics; Berlin, Germany. 2010. [Google Scholar]
- 128.Yasenchak C, Nackaerts K, Awada S, Gadgeel S, et al. Phase 2 results of Cabozantinib (XL184) in a cohort of patients (pts) with advanced non-small cell lung cancer (NSCLC). AACR-NCI-EORTC International Conference Molecular Target and Cancer Therapeutics; Berlin, Germany. 2010. [Google Scholar]
- 129.Van Cutsem E, Su WC, Davis J, Haas N, et al. Phase 2 study of XL184 in a cohort of patients (pts) with hepatocellular carcinoma (HCC). AACR-NCI-EORTC International Conference Molecular Target and Cancer Therapeutics; Berlin, Germany. 2010. [Google Scholar]
- 130.http://chicago2011.asco.org/ASCODailyNews/Abstract4516.aspx
- 131**.Hussain M, Smith MR, Sweeney C, Corn PG, et al. Cabozantinib (XL184) in metastatic castration-resistant prostate cancer (mCRPC): Results from a phase II randomized discontinuation trial. American Society of Clinical Oncology (ASCO), Annual Meeting; Chicago, IL. 2011. Demonstration of RECIST partial response in metastatic CaP patients treated with a multikinase inhibitor that targets Met. [Google Scholar]
- 132.Smith DC, Smith MR, Small EJ, Sweeney C, et al. Phase II study of XL184 in a cohort of patients (pts) with castration-resistant prostate cancer (CRPC) and measurable soft tissue disease. American Society of Clinical Oncology (ASCO), Genitourinary Cancers Symposium; Orlando, FL. 2011. [Google Scholar]
- 133.Basch E, Bennett AV, Scher HI. Cabozantinib (XL184) reduces pain symptoms in patients (pts) with castration resistant prostate cancer (CRPC) and bone metastases: Results from a phase 2 non-randomized expansion cohort; AACR-NCI-EORTC International Conference Molecular Target and Cancer Therapeutics; San Francisco, CA. 2011. [Google Scholar]
- 134.Lee RJB, Saylor P, et al. Efficacy and Tolerability of Cabozantinib at Lower Dose: A Dose Finding Study in Men with Castration-Resistant Prostate Cancer and Bone Metastases. AACR-NCI-EORTC International Conference Molecular Target and Cancer Therapeutics; Berlin, Germany. 2011. [Google Scholar]
- 135.http://www.cancer.gov/cancertopics/druginfo/fda-crizotinib
- 136.Ou SH, Kwak EL, Siwak-Tapp C, Dy J, et al. Activity of crizotinib (PF02341066), a dual mesenchymal-epithelial transition (MET) and anaplastic lymphoma kinase (ALK) inhibitor, in a non-small cell lung cancer patient with de novo MET amplification. J Thorac Oncol. 2011 May;6:942–6. doi: 10.1097/JTO.0b013e31821528d3. [DOI] [PubMed] [Google Scholar]
- 137.Peach ML, Tan N, Choyke S, Giubellino A, et al. Directed discovery of agents targeting the Met tyrosine kinase domain by virtual screening. J Med Chem. 2009;52:943–951. doi: 10.1021/jm800791f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Muller T, DePrimo S, McGrath G, Yu P, et al. Pharmacodynamic and correlative biomarker analyses in clinical trials of XL184, and oral, potent inhibitor of MET, VEGFR2, and RET. AACR-NCI-EORTC International Conference Molecular Target and Cancer Therapeutics; Boston, MA. 2009. [Google Scholar]
- 139.Pena C, Shan M, Bukowski RM, Escudier B, et al. Plasma biomarkers predicting outcome in patients with advanced RCC: results from the sorafenib phase II TARGET trial. AACR-NCI-EORTC International Conference Molecular Target and Cancer Therapeutics; Boston, MA. 2009. [Google Scholar]
- 140.Heymach JV, Fritsche HA, Gornet TG, Liu Y, et al. Lower baseline levels of plasm hepatocyte growth factor, IL-6, IL08 are correlated with greater tumor shrinkage in renal cell carcinoma patients treated with pazopanib. AACR-NCI-EORTC International Conference Molecular Target and Cancer Therapeutics; Boston, MA. 2009. [Google Scholar]
- 141.Tanimoto S, Fukumori T, El-Moula G, Shiirevnyamba A, et al. Prognostic significance of serum hepatocyte growth factor in clear cell renal cell carcinoma: comparison with serum vascular endothelial growth factor. J Med Invest. 2008 Feb;:55106–11. doi: 10.2152/jmi.55.106. [DOI] [PubMed] [Google Scholar]
- 142.Cecchi F, Liu Y, Gagnon RC, Kallender H, et al. ShedMET (sMet), VEGFA, and sVEGFR2 are markers of foretinib treatment in metastatic gastric cancer patients. AACR-NCI-EORTC International Conference Molecular Target and Cancer Therapeutics; Boston, MA. 2009. [Google Scholar]
- 143.Kollmannsberger CK, Hurwitz H, Vlahovic G, Maroun C, et al. Phase I study of daily administration of MGCD265 to patients with advanced malignancies (study 265–101). American Society of Clinical Oncology (ASCO) Annual Meeting; Chicago, IL. 2009. [Google Scholar]
- 144.DePrimo S, Wu B, Huang S, Bautista S, et al. Correlative tumor molecular profiling and plasma biomarker analysis in a phase II study of XL184 in patients with progressive or recurrent glioblastoma multiforme (GBM). American Society of Clinical Oncology (ASCO), Annual Meeting; Chicago, IL. 2009. [Google Scholar]
- 145.Yano S, Wang W, Li Q, Matsumoto K, et al. Hepatocyte growth factor induces gefitinib resistance of lung adenocarcinoma with epidermal growth factor receptor-activating mutations. Cancer Res. 2008 Nov 15;68:9479–87. doi: 10.1158/0008-5472.CAN-08-1643. [DOI] [PubMed] [Google Scholar]
- 146.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007 May 18;316:1039–43. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 147.Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011 Mar 23;3:75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]