Abstract
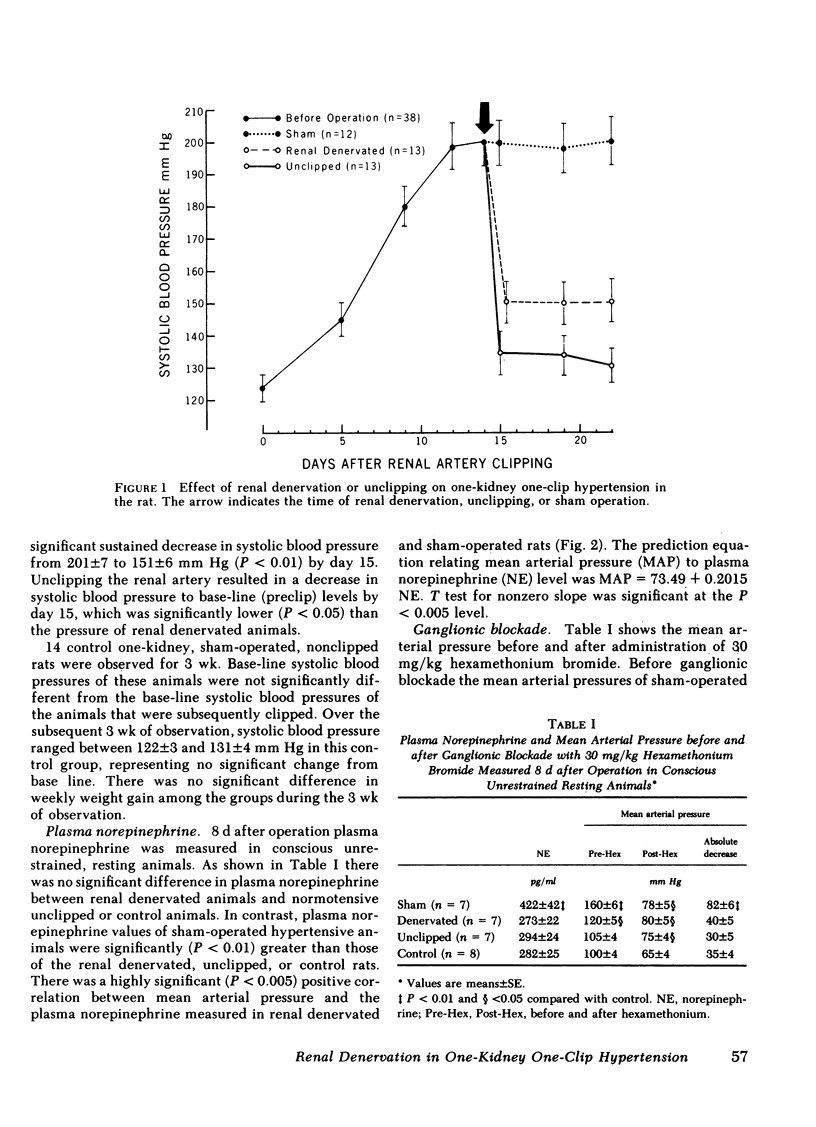
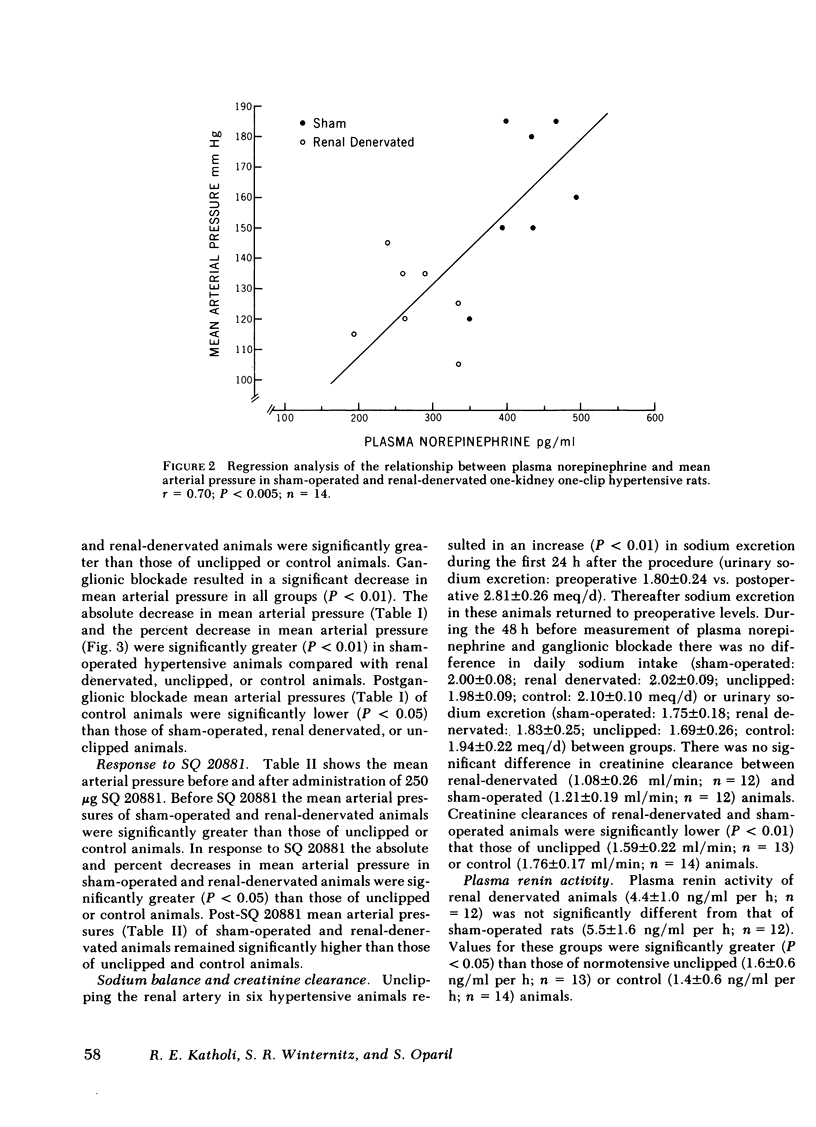
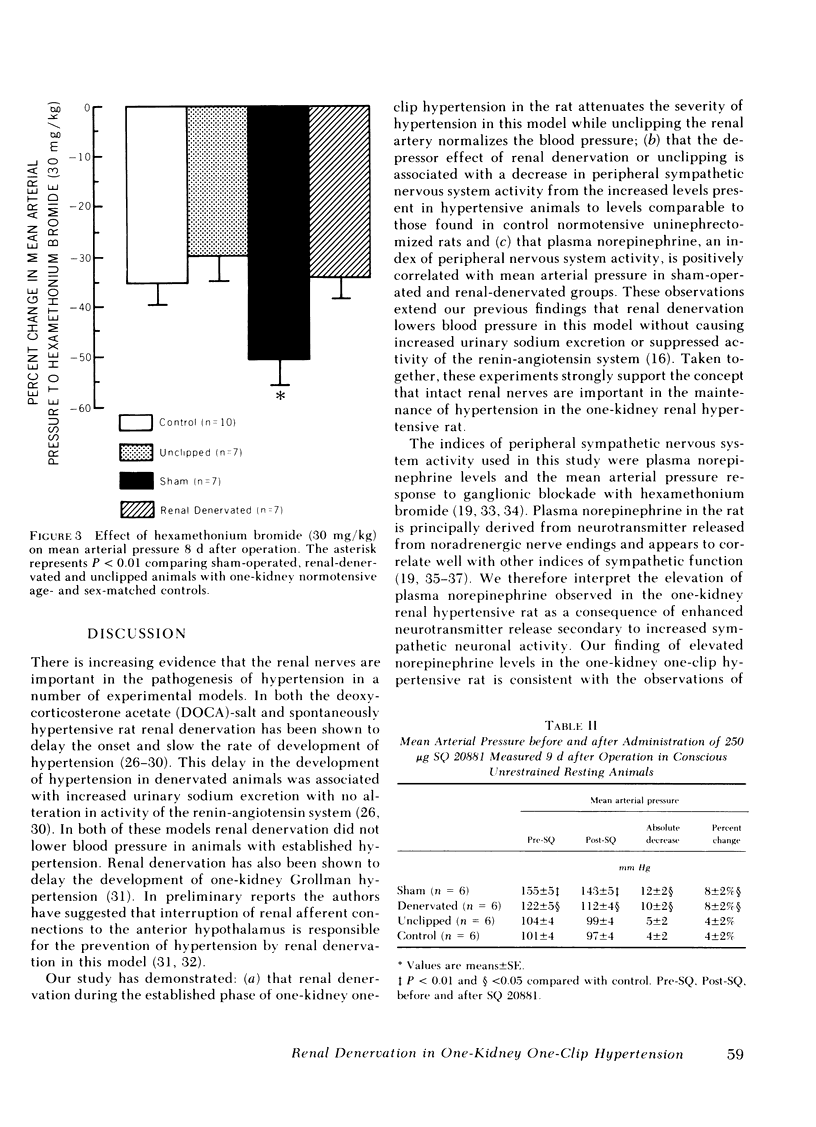
Increased sympathetic nervous system activity has been demonstrated in established one-kidney one-clip hypertension in the rat. We have found that renal denervation in this model results in an attenuation of hypertension, unassociated with alterations in sodium or water balance or renin activity. To determine whether the depressor effect of renal denervation is associated with changes in peripheral sympathetic nervous system activity, sham operation (n = 12), renal denervation (n = 13), or unclipping (n = 13) was carried out 2 wk after the onset of one-kidney one-clip hypertension. Normotensive unine-phrectomized age- and sex-matched rats were used as controls (n = 14). Renal denervation resulted in a significant decrease in systolic blood pressure (201±7 to 151±6 mm Hg), while unclipping lowered systolic blood pressure to normotensive levels (130±6 mm Hg). 8 d after operation plasma norepinephrine and mean arterial pressure before and after ganglionic blockade with 30 mg/kg hexamethonium bromide were measured in conscious, unrestrained, resting animals, as indices of peripheral sympathetic nervous system activity. Plasma norepinephrine was significantly higher in hypertensive sham-operated rats (422±42 pg/ml) compared with normotensive controls (282±25 pg/ml) (P < 0.01). Both renal denervation and unclipping restored plasma norepinephrine to normal levels (273±22 and 294±24 pg/ml, respectively). Ganglionic blockade in hypertensive sham-operated animals resulted in a significantly greater decrease in mean arterial pressure than occurred in renal denervated, unclipped, or control rats. The data suggest that the depressor effect of renal denervation or unclipping in the one-kidney one-clip hypertensive rat is associated with a decrease in peripheral sympathetic nervous system activity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayitey-Smith E., Varma D. R. An assessment of the role of the sympathetic nervous system in experimental hypertension using normal and immunosympathectomized rats. Br J Pharmacol. 1970 Oct;40(2):175–185. doi: 10.1111/j.1476-5381.1970.tb09911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines A. D., Craan A., Chan W., Morgunov N. Tubular secretion and metabolism of dopamine, norepinephrine, methoxytyramine and normetanephrine by the rat kidney. J Pharmacol Exp Ther. 1979 Jan;208(1):144–147. [PubMed] [Google Scholar]
- Bengis R. G., Coleman T. G. Antihypertensive effect of prolonged blockade of angiotensin formation in benign and malignant, one- and two-kidney Goldblatt hypertensive rats. Clin Sci (Lond) 1979 Jul;57(1):53–62. doi: 10.1042/cs0570053. [DOI] [PubMed] [Google Scholar]
- Bomzon L., Wilton P. B., McCalden T. A. Impaired skeletal muscle vasomotor response to infused noradrenaline in baboons with obstructive jaundice. Clin Sci Mol Med. 1978 Jul;55(1):109–112. doi: 10.1042/cs0550109. [DOI] [PubMed] [Google Scholar]
- Calaresu F. R., Kim P., Nakamura H., Sato A. Electrophysiological characteristics of renorenal reflexes in the cat. J Physiol. 1978 Oct;283:141–154. doi: 10.1113/jphysiol.1978.sp012493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dargie H. J., Franklin S. S., Reid J. L. Plasma noradrenaline concentrations in experimental renovascular hypertension in the rat. Clin Sci Mol Med. 1977 May;52(5):477–483. doi: 10.1042/cs0520477. [DOI] [PubMed] [Google Scholar]
- Davis J. O., Freeman R. H., Johnson J. A., Spielman W. S. Agents which block the action of the renin-angiotensin system. Circ Res. 1974 Mar;34(3):279–285. doi: 10.1161/01.res.34.3.279. [DOI] [PubMed] [Google Scholar]
- Davis J. O. The pathogenesis of chronic renovascular hypertension. Circ Res. 1977 May;40(5):439–444. doi: 10.1161/01.res.40.5.439. [DOI] [PubMed] [Google Scholar]
- De Champlain J., Cousineau D., Van Amerigen M. R., Marc-Aurèle J., Yamaguchi N. The role of the sympathetic system in experimental and human hypertension. Postgrad Med J. 1977;53 (Suppl 3):15–30. [PubMed] [Google Scholar]
- De Champlain J., Mueller R. A., Axelrod J. Turnover and synthesis of norepinephrine in experimental hypertension in rats. Circ Res. 1969 Sep;25(3):285–291. doi: 10.1161/01.res.25.3.285. [DOI] [PubMed] [Google Scholar]
- Dorr L. D., Brody M. J. Preliminary observations on the role of the sympathetic nervous system in development and maintenance of experimental renal hypertension. Proc Soc Exp Biol Med. 1966 Oct;123(1):155–158. doi: 10.3181/00379727-123-31429. [DOI] [PubMed] [Google Scholar]
- Douglas J. R., Jr, Johnson E. M., Jr, Heist J. F., Marshall G. R., Needleman P. Is the peripheral sympatho-adrenal nervous system necessary for renal hypertension? J Pharmacol Exp Ther. 1976 Jan;196(1):35–43. [PubMed] [Google Scholar]
- Eide I., Myers M. R., DeQuattro V., Kolloch R., Eide K., Whigham H. Increased hypothalamic noradrenergic activity in one-kidney, one-clip renovascular hypertensive rats. J Cardiovasc Pharmacol. 1980 Nov-Dec;2(6):833–841. doi: 10.1097/00005344-198011000-00012. [DOI] [PubMed] [Google Scholar]
- Engel S. L., Schaeffer T. R., Gold B. I., Rubin B. Inhibition of pressor effects of angiotensin I and augmentation of depressor effects of bradykinin by synthetic peptides. Proc Soc Exp Biol Med. 1972 May;140(1):240–244. doi: 10.3181/00379727-140-36433. [DOI] [PubMed] [Google Scholar]
- Estrugamou M., de la Riva I. J. Cardiovascular reactivity and neurogenic tone in hypertension derived from renal artery stenosis and contralateral nephrectomy in the rat. Acta Physiol Lat Am. 1977;27(5):231–238. [PubMed] [Google Scholar]
- Fink G. D., Brody M. J. Impaired neurogenic control of renal vasculature in renal hypertensive rats. Am J Physiol. 1980 Jun;238(6):H770–H775. doi: 10.1152/ajpheart.1980.238.6.H770. [DOI] [PubMed] [Google Scholar]
- Fink G. D., Brody M. J. Neurogenic control of the renal circulation in hypertension. Fed Proc. 1978 Apr;37(5):1202–1208. [PubMed] [Google Scholar]
- Haber E., Koerner T., Page L. B., Kliman B., Purnode A. Application of a radioimmunoassay for angiotensin I to the physiologic measurements of plasma renin activity in normal human subjects. J Clin Endocrinol Metab. 1969 Oct;29(10):1349–1355. doi: 10.1210/jcem-29-10-1349. [DOI] [PubMed] [Google Scholar]
- Katholi R. E., Naftilan A. J., Oparil S. Importance of renal sympathetic tone in the development of DOCA-salt hypertension in the rat. Hypertension. 1980 May-Jun;2(3):266–273. doi: 10.1161/01.hyp.2.3.266. [DOI] [PubMed] [Google Scholar]
- Katholi R. E., Winternitz S. R., Oparil S. Role of the renal nerves in the pathogenesis of one-kidney renal hypertension in the rat. Hypertension. 1981 Jul-Aug;3(4):404–409. doi: 10.1161/01.hyp.3.4.404. [DOI] [PubMed] [Google Scholar]
- Kline R. L., Kelton P. M., Mercer P. F. Effect of renal denervation on the development of hypertension in spontaneously hypertensive rats. Can J Physiol Pharmacol. 1978 Oct;56(5):818–822. doi: 10.1139/y78-128. [DOI] [PubMed] [Google Scholar]
- Kopin I. J., Lake R. C., Ziegler M. Plasma levels of norepinephrine. Ann Intern Med. 1978 May;88(5):671–680. doi: 10.7326/0003-4819-88-5-671. [DOI] [PubMed] [Google Scholar]
- Liard J. F., Peters G. Role of the retention of water and sodium in two types of experimental renovascular hypertension in the rat. Pflugers Arch. 1973 Nov 26;344(2):93–108. doi: 10.1007/BF00586544. [DOI] [PubMed] [Google Scholar]
- Liard J. F. Renal denervation delays blood pressure increase in the spontaneously hypertensive rat. Experientia. 1977 Mar 15;33(3):339–340. doi: 10.1007/BF02002815. [DOI] [PubMed] [Google Scholar]
- Lowe R. D., Scroop G. C. Effects of angiotensin on the autonomic nervous system. Am Heart J. 1970 Apr;79(4):562–567. doi: 10.1016/0002-8703(70)90264-4. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Gerold M., Thoenen H. Experimental hypertension of the rat: reciprocal changes of norepinephrine turnover in heart and brain-stem. Jpn J Pharmacol. 1970 Dec;20(4):605–607. doi: 10.1254/jjp.20.605. [DOI] [PubMed] [Google Scholar]
- Petty M. A., Reid J. L. Changes in noradrenaline concentration in brain stem and hypothalamic nuclei during the development of renovascular hypertension. Brain Res. 1977 Nov 11;136(2):376–380. doi: 10.1016/0006-8993(77)90814-9. [DOI] [PubMed] [Google Scholar]
- Reid J. L., Kopin I. J. The effects of ganglionic blockade, reserpine and vinblastine on plasma catecholamines and dopamine-beta-hydroxylase in the rat. J Pharmacol Exp Ther. 1975 Jun;193(3):748–756. [PubMed] [Google Scholar]
- Roizen M. F., Moss J., Henry D. P., Kopin I. J. Effects of halothane on plasma catecholamines. Anesthesiology. 1974 Nov;41(5):432–439. doi: 10.1097/00000542-197411000-00005. [DOI] [PubMed] [Google Scholar]
- Roizen M. F., Weise V., Moss J., Kopin I. J. Plasma catecholamines: arterial-venous difference and the influence of body temperature. Life Sci. 1975 Apr 1;16(7):1133–1143. doi: 10.1016/0024-3205(75)90197-6. [DOI] [PubMed] [Google Scholar]
- Touw K. B., Haywood J. R., Shaffer R. A., Brody M. J. Contribution of the sympathetic nervous system to vascular resistance in conscious young and adult spontaneously hypertensive rats. Hypertension. 1980 Jul-Aug;2(4):408–418. doi: 10.1161/01.hyp.2.4.408. [DOI] [PubMed] [Google Scholar]
- Vandongen R., Tunney A., Martinez P. Effect of the converting-enzyme inhibitor SQ 14 225 (captopril) in early one-kidney, one-clip hypertension in the rat. Clin Sci (Lond) 1981 Apr;60(4):387–392. doi: 10.1042/cs0600387. [DOI] [PubMed] [Google Scholar]
- Volicer L., Scheer E., Hilse H., Visweswaram D. The turnover of norepinephrine in the heart during experimental hypertension in rats. Life Sci. 1968 May 1;7(9):525–532. doi: 10.1016/0024-3205(68)90057-x. [DOI] [PubMed] [Google Scholar]
- Winternitz S. R., Katholi R. E., Oparil S. Role of the renal sympathetic nerves in the development and maintenance of hypertension in the spontaneously hypertensive rat. J Clin Invest. 1980 Nov;66(5):971–978. doi: 10.1172/JCI109966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Champlain J., Krakoff L., Axelrod J. Interrelationships of sodium intake, hypertension, and norepinephrine storage in the rat. Circ Res. 1969 May;24(5 Suppl):75–92. [PubMed] [Google Scholar]


